Tuberculosis Skin Test Screening in the National Tuberculosis Program of Trinidad and Tobago
Abstract
1. Introduction
2. Experimental Section
2.1. Setting
2.2. Study Design
2.3. Participants
2.4. Data Collection
2.5. Administration of Tuberculin Skin Test (TST)
2.6. Interpretation of TST Reaction
2.7. TB Screening Types
- TB suspect screening—for persons displaying signs or symptoms indicative of active TB disease.
- TB contact screening—for persons who have been in contact with a known infectious case of TB.
- Preplacement (baseline) screening—required for entry to healthcare institutions or immigration purposes, especially for migration to countries like UK, USA, Canada, certain European countries, Australia, and New Zealand [9].
- Periodic/annual screening—for healthcare personnel at high risk of exposure to TB.
2.8. Data Analysis
3. Results
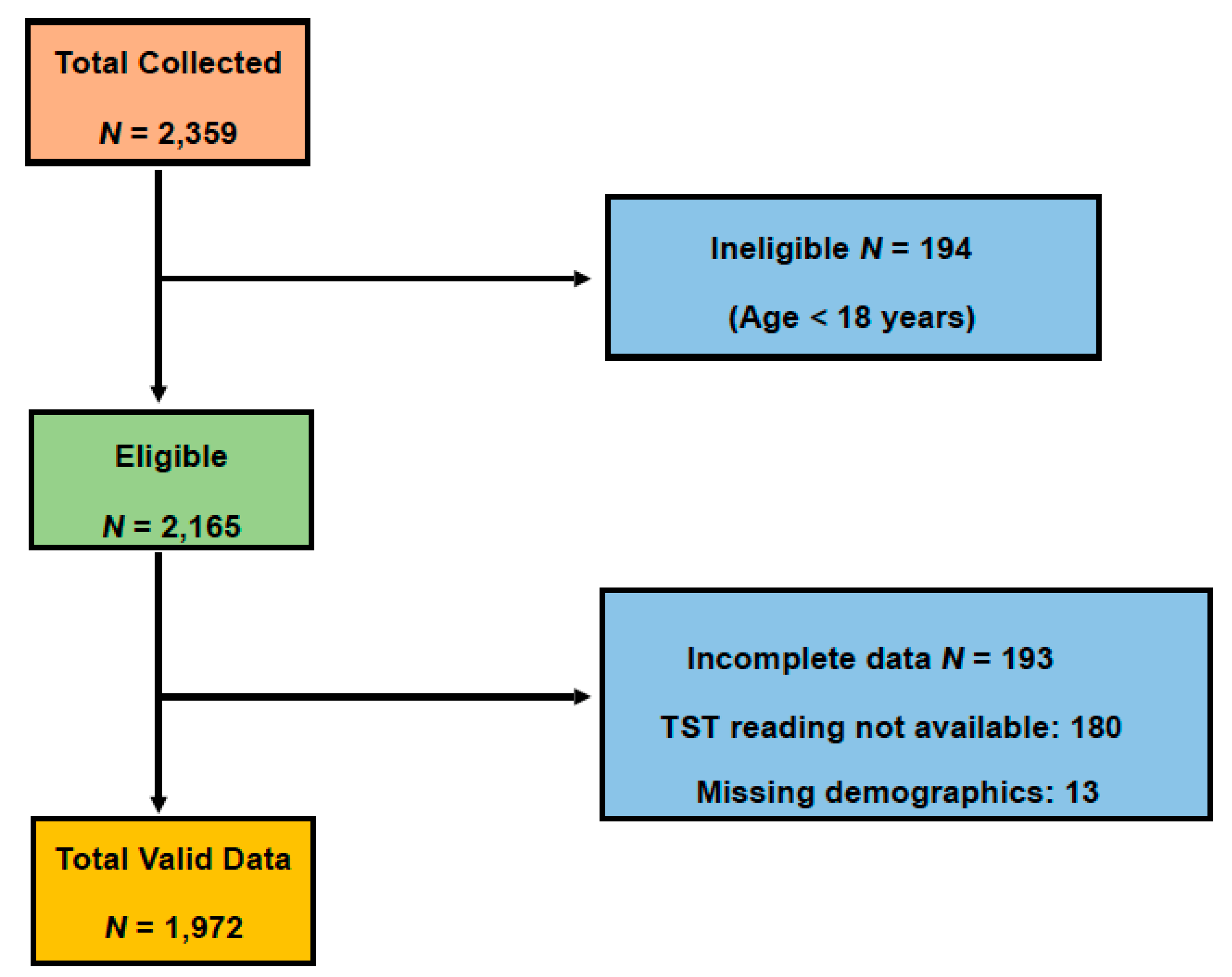
3.1. Subjects Selection
3.2. Demographic, Health, and Screening Details
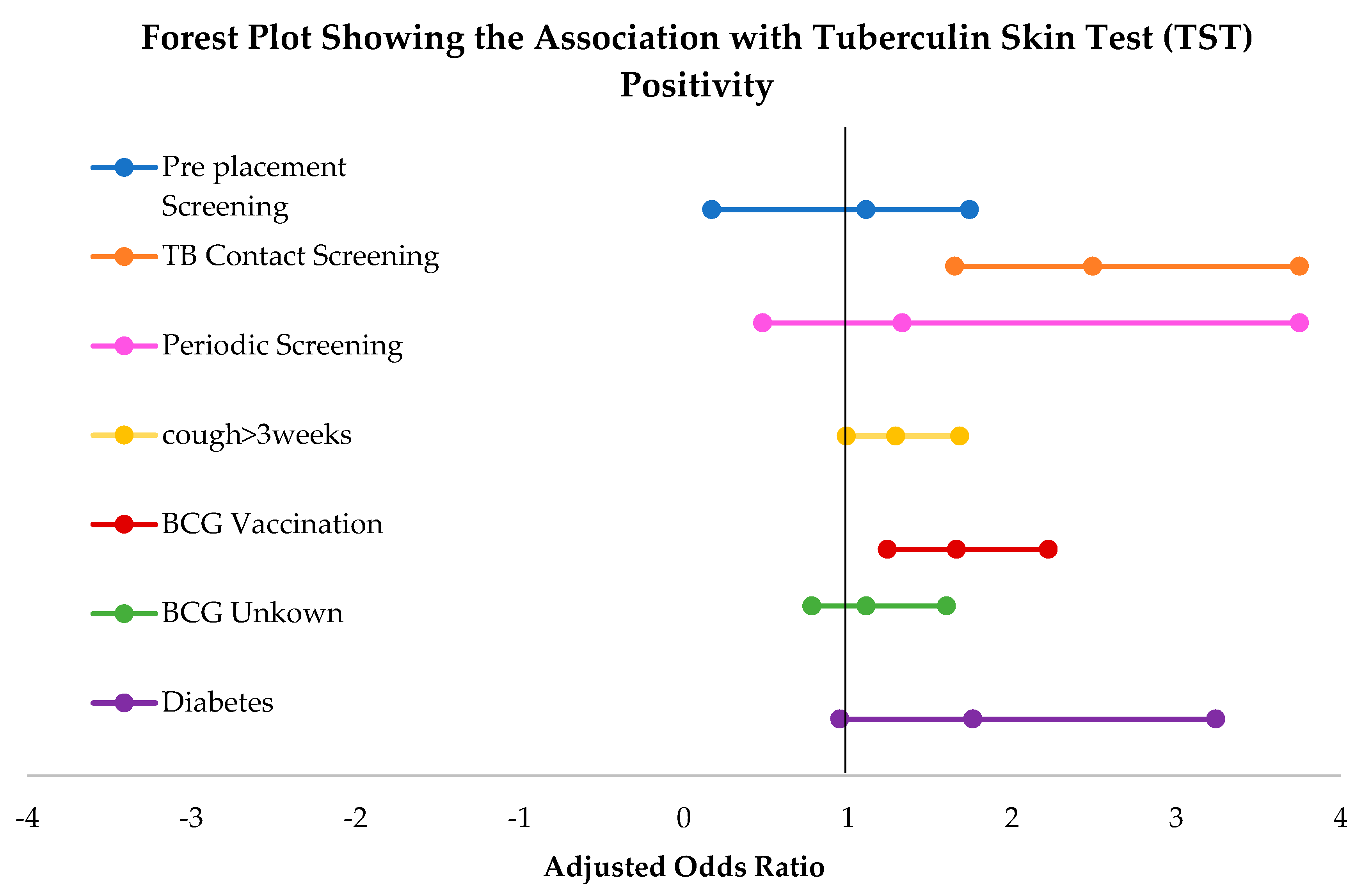
3.3. Univariate and Multivariate Analysis of Tuberculin Skin Test (TST) Positivity
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report. 2019. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 20 April 2020).
- Jagger, A.; Reiter-Karam, S.; Hamada, Y.; Getahun, H. National policies on the management of latent tuberculosis infection: Review of 98 countries. Bull. World Health Organ. 2018, 96, 173–184F. [Google Scholar] [CrossRef] [PubMed]
- Getahun, H.; Matteelli, A.; Abubakar, I.; Aziz, M.A.; Baddeley, A.; Barreira, D.; Boon, S.D.; Gutierrez, S.M.B.; Bruchfeld, J.; Burhan, E.; et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur. Respir. J. 2015, 46, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.M.G.J.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed]
- Comstock, G.W.; Livesay, V.T.; Woolpert, S.F. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am. J. Epidemiol. 1974, 99, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. Available online: https://apps.who.int/iris/bitstream/handle/10665/260233/9789241550239-eng.pdf (accessed on 20 April 2020).
- Holden, M.; Dubin, M.R.; Diamond, P.H. Frequency of negative intermediate-strength tuberculin sensitivity in patients with active tuberculosis. N. Engl. J. Med. 1971, 285, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am. J. Respir. Crit. Care Med. 2000, 161, 1376–1395. [Google Scholar]
- The Ministry of Health—Trinidad and Tobago. National Tuberculosis control Programme. Available online: http://www.health.gov.tt/sitepages/default.aspx?id=284 (accessed on 20 April 2020).
- Fact Sheets. Available online: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.htm (accessed on 20 April 2020).
- Muture, B.N.; Keraka, M.N.; Kimuu, P.K.; Kabiru, E.W.; Ombeka, V.O.; Oguya, F. Factors associated with default from treatment among tuberculosis patients in Nairobi province, Kenya: A case control study. BMC Public Health 2011, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.; Bavan, L.; Gajari, K.; Paget, S.N.J.; Baussano, I. Active screening at entry for tuberculosis among new immigrants: A systematic review and meta-analysis. Eur. Respir. J. 2010, 35, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Kranzer, K.; Afnan-Holmes, H.; Tomlin, K.; Golub, J.E.; Shapiro, A.E.; Schaap, A.; Corbett, E.L.; Lönnroth, K.; Glynn, J.R. The benefits to communities and individuals of screening for active tuberculosis disease: A systematic review. Int. J. Tuberc. Lung Dis. 2013, 17, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, R.W.; Yates, T.A.; Zenner, D.; White, P.J.; Abubakar, I.; Hayward, A. Pre-entry screening programmes for tuberculosis in migrants to low-incidence countries: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 1240–1249. [Google Scholar] [CrossRef]
- Sosa, L.E.; Njie, G.J.; Lobato, M.N.; Bamrah, S.M.; Buchta, W.; Casey, M.L.; Goswami, N.D.; Gruden, M.; Hurst, B.J.; Khan, A.R.; et al. Tuberculosis Screening, Testing, and Treatment of U.S. Health Care Personnel: Recommendations from the National Tuberculosis Controllers Association and CDC, 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, D.M.; Leonard, M.K.; LoBue, P.A.; Cohn, D.L.; Daley, C.L.; Desmond, E.; Keane, J.; Lewinsohn, D.A.; Loeffler, A.M.; Mazurek, G.H.; et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin. Infect. Dis. 2017, 64, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Zwerling, A.; Behr, M.; Verma, A.; Brewer, T.; Menzies, D.; Pai, M. The BCG World Atlas: A Database of Global BCG Vaccination Policies and Practices. PLoS Med. 2011, 8, e1001012. [Google Scholar] [CrossRef] [PubMed]
- Altink, H. ‘Fight TB with BCG’: Mass Vaccination Campaigns in the British Caribbean, 1951–6. Med. Hist. 2014, 58, 475–497. [Google Scholar] [CrossRef] [PubMed]
- Pooransingh, S.; Sakhamuri, S. Need for BCG Vaccination to Prevent TB in High-Incidence Countries and Populations. Emerg. Infect. Dis. 2020, 26, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Turner, M.O.; Elwood, R.K.; Schulzer, M.; FitzGerald, J.M. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax 2002, 57, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Cobelens, F.; Egwaga, S.; Ginkel, T.; Muwinge, H.; Matee, M.; Borgdorff, M. Tuberculin Skin Testing in Patients with HIV Infection: Limited Benefit of Reduced Cutoff Values. Clin. Infect. Dis. 2006, 43, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Aabye, M.G.; Ravn, P.; PrayGod, G.; Jeremiah, K.; Mugomela, A.; Jepsen, M.; Faurholt, D.; Range, N.; Friis, H.; Changalucha, J.; et al. The impact of HIV infection and CD4 cell count on the performance of an interferon gamma release assay in patients with pulmonary tuberculosis. PLoS ONE 2009, 4, e4220. [Google Scholar] [CrossRef] [PubMed]
- Linas, B.P.; Wong, A.Y.; Freedberg, K.A.; Horsburgh, C.R., Jr. Priorities for screening and treatment of latent tuberculosis infection in the United States. Am. J. Respir. Crit. Care Med. 2011, 184, 590–601. [Google Scholar] [CrossRef] [PubMed]
- CDC. Nationwide Shortage of Tuberculin Skin Test Antigens: CDC Recommendations for Patient Care and Public Health Practice. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 552–553. [Google Scholar] [CrossRef] [PubMed]
| Variable | Count |
|---|---|
| Gender | |
| Male | 829 (42.0%) |
| Female | 1143 (58.0%) |
| Age group | |
| 18–39 years | 961 (48.7%) |
| 40–59 years | 620 (31.4%) |
| 60 years and above | 391 (19.8%) |
| Cough >3 weeks | |
| Yes | 401 (20.3%) |
| No | 1571 (79.7%) |
| BCG Vaccination | |
| Yes | 390 (19.8%) |
| No | 1348 (68.4%) |
| Unknown | 234 (11.9%) |
| HIV infection | |
| Yes | 20 (1.0%) |
| No | 675 (34.2%) |
| Unknown | 1277 (64.8%) |
| Diabetes | |
| Yes | 50 (2.5%) |
| No | 1922 (97.5%) |
| Cancer | |
| Yes | 2 (0.1%) |
| No | 1970 (99.9%) |
| Organ Transplant | |
| Yes | 4 (0.2%) |
| No | 1968 (99.8%) |
| Screening Type | |
| TB suspect screening | 596 (30.2%) |
| Pre-placement screening | 295 (15.0%) |
| Contact screening | 164 (8.3%) |
| Periodic screening | 28 (1.4%) |
| Unknown | 889 (45.1%) |
| Variable | TST Positive N = 384 | TST Negative N = 1588 | * p Value |
|---|---|---|---|
| TB Screening Type | |||
| TB suspect screening | 111 (18.6%) | 485 (81.4%) | 0.577 |
| Pre-placement screening | 38 (12.9%) | 257 (87.1%) | <0.001 |
| TB contact screening | 52 (31.7%) | 112 (68.3%) | <0.001 |
| Periodic screening | 5 (17.9%) | 23 (82.1%) | 0.99 |
| Unknown | 178 (20.0%) | 711 (80.0%) | 0.607 |
| Gender | |||
| Male | 196 (23.6%) | 633 (76.4%) | <0.001 |
| Female | 188 (16.4%) | 955 (83.6%) | |
| Age group | |||
| 18–39 years | 134 (13.9%) | 827 (86.1%) | <0.001 |
| 40–59 years | 153 (24.7%) | 467 (75.3%) | <0.001 |
| 60 years and above | 97 (24.8%) | 294 (75.2%) | <0.001 |
| Cough >3weeks | |||
| Yes | 99 (24.7%) | 302 (75.3%) | 0.004 |
| No | 285 (18.1%) | 1286 (81.9%) | |
| BCG Vaccination | |||
| Yes | 117 (30.0%) | 273 (70.0%) | <0.001 |
| No | 223 (16.5%) | 1125 (83.5%) | <0.001 |
| Unknown | 44 (18.8%) | 190 (81.2%) | 0.860 |
| HIV infection | |||
| Yes | 0 (0.0%) | 20 (100.0%) | 0.021 |
| No | 122 (18.1%) | 553 (81.9%) | 0.281 |
| Unknown | 262 (20.5%) | 1015 (79.5%) | 0.122 |
| Diabetes | |||
| Yes | 17 (34.0%) | 33 (66.0%) | 0.017 |
| No | 367 (19.1%) | 1555 (80.9%) |
| Variables | Category | Univariate Model | # Multivariate Model |
|---|---|---|---|
| OR 95% CI | OR 95% CI | ||
| TB Screening | Pre-placement | 0.65 (0.43–0.96) | 1.11 (0.71–1.74) |
| TB contact | 2.03 (1.38–2.99) | 2.49 (1.65–3.75) | |
| Periodic | 0.95 (0.35–2.95) | 1.33 (0.48–3.75) | |
| TB suspect * | Ref | Ref | |
| Cough for >3 weeks | Yes | 1.48 (1.14–1.92) | 1.29 (0.99–1.68) |
| No * | Ref | Ref | |
| BCG Vaccination | Yes | 2.16 (1.67–2.80) | 1.66 (1.24–2.22) |
| Unknown | 1.17 (0.82–1.67) | 1.11 (0.78–1.60) | |
| No * | Ref | Ref | |
| Diabetes | Yes | 2.18 (1.20–3.96) | 1.76 (0.95–3.24) |
| No * | Ref | Ref |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chattu, V.K.; Sakhamuri, S.; Motilal, S.; Pounder, L.J.; Persad, V.K.; Pierre, N.; Persad, S.; Pooran, N.; Pottinger, A.M. Tuberculosis Skin Test Screening in the National Tuberculosis Program of Trinidad and Tobago. Healthcare 2020, 8, 236. https://doi.org/10.3390/healthcare8030236
Chattu VK, Sakhamuri S, Motilal S, Pounder LJ, Persad VK, Pierre N, Persad S, Pooran N, Pottinger AM. Tuberculosis Skin Test Screening in the National Tuberculosis Program of Trinidad and Tobago. Healthcare. 2020; 8(3):236. https://doi.org/10.3390/healthcare8030236
Chicago/Turabian StyleChattu, Vijay Kumar, Sateesh Sakhamuri, Shastri Motilal, Liam J. Pounder, Vasishma Kanita Persad, Neelmani Pierre, Shivannie Persad, Nikesha Pooran, and Akua Mosi Pottinger. 2020. "Tuberculosis Skin Test Screening in the National Tuberculosis Program of Trinidad and Tobago" Healthcare 8, no. 3: 236. https://doi.org/10.3390/healthcare8030236
APA StyleChattu, V. K., Sakhamuri, S., Motilal, S., Pounder, L. J., Persad, V. K., Pierre, N., Persad, S., Pooran, N., & Pottinger, A. M. (2020). Tuberculosis Skin Test Screening in the National Tuberculosis Program of Trinidad and Tobago. Healthcare, 8(3), 236. https://doi.org/10.3390/healthcare8030236








