Rural Community Perceptions and Interests in Pharmacogenomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Symposium Cohort
2.3. Community Pharmacy Cohort
2.4. Analysis of Data
3. Results
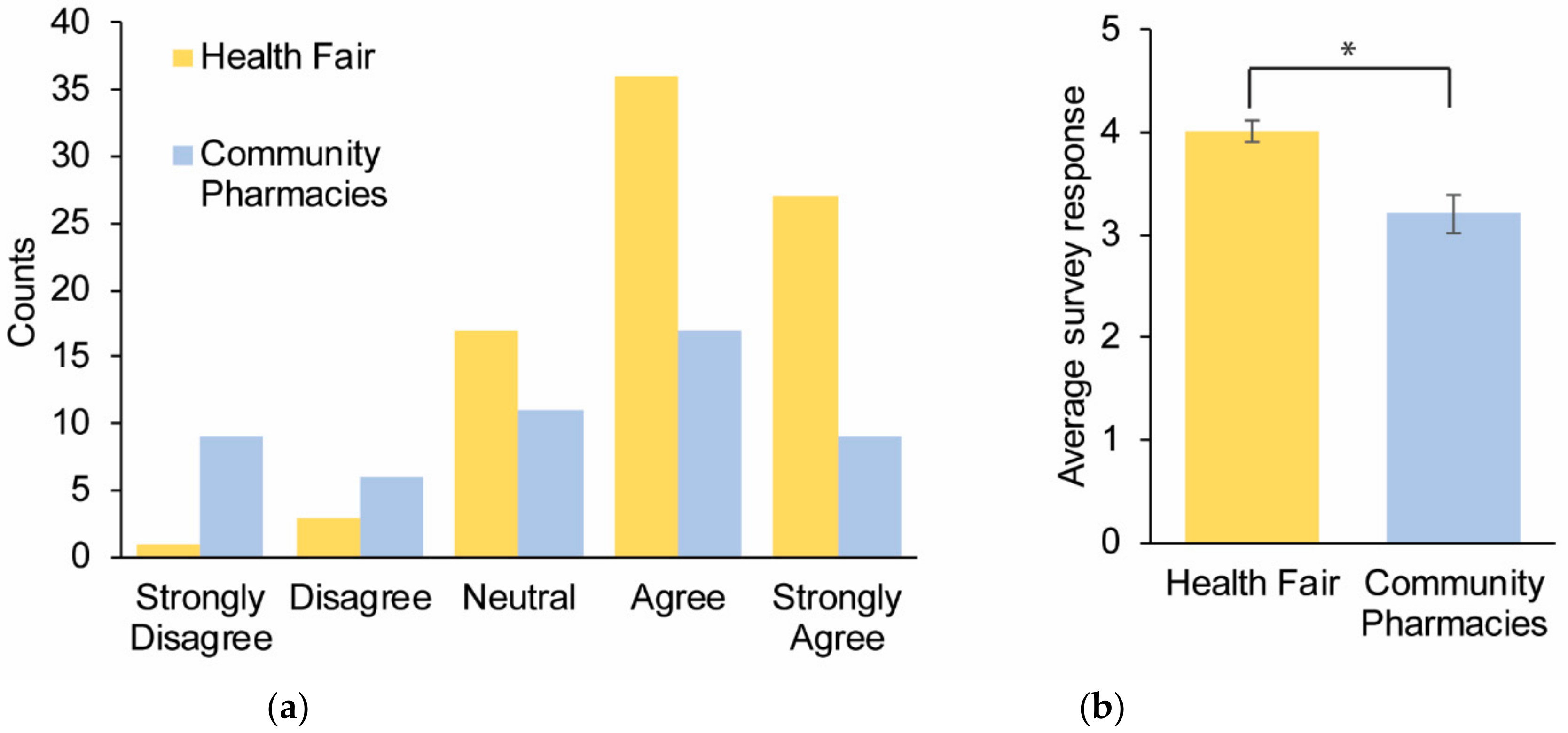
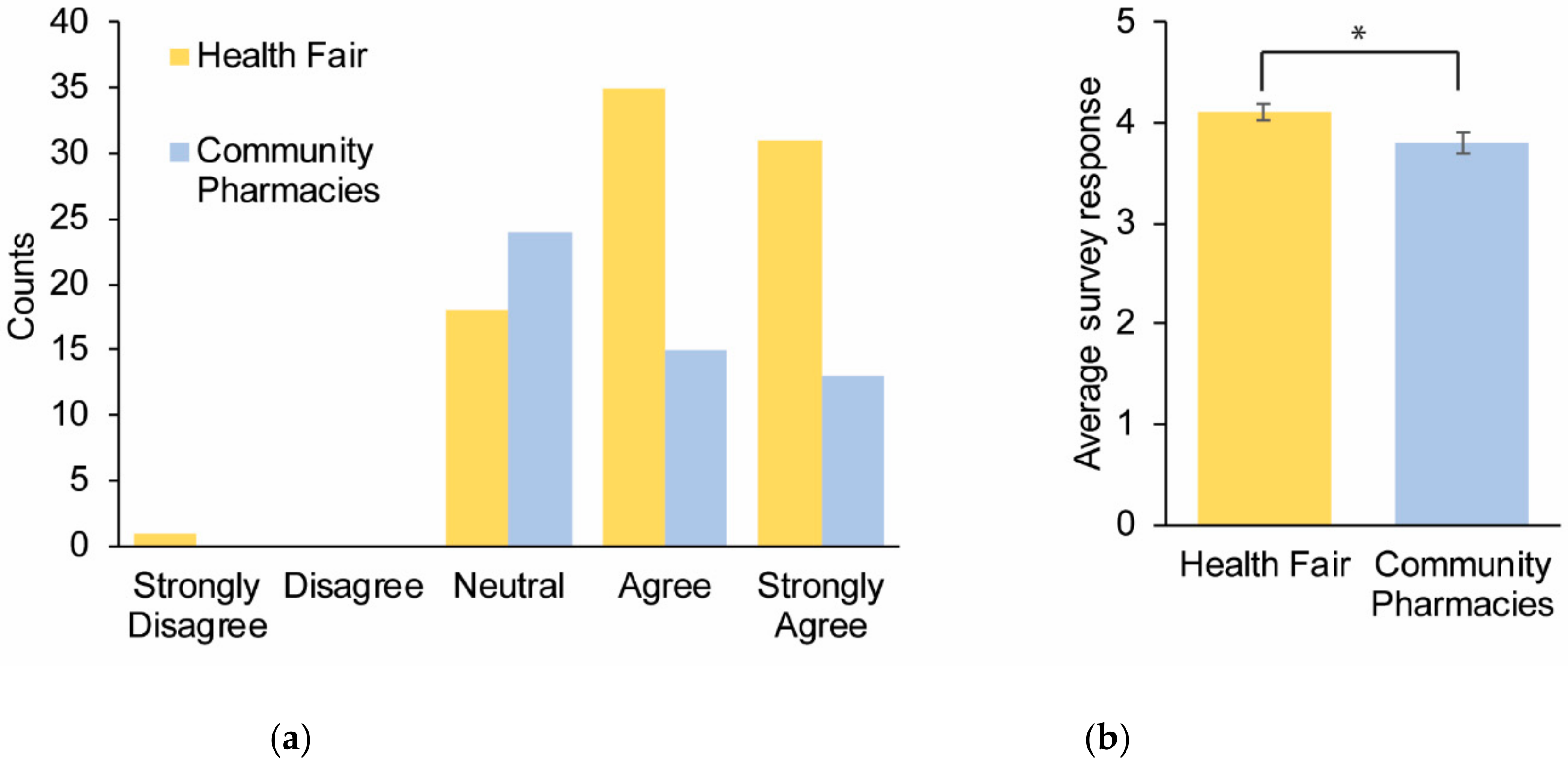
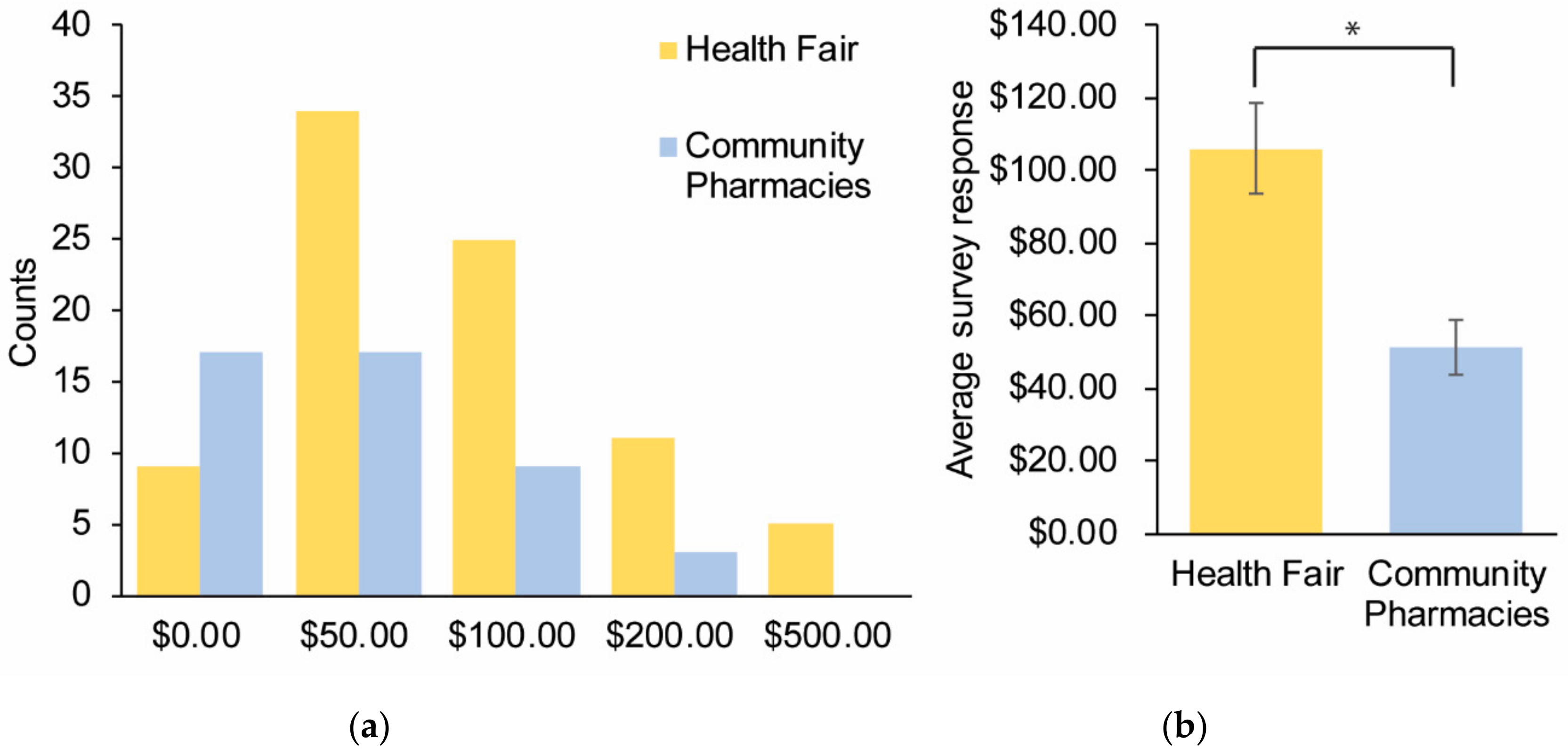
Survey Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gross, T.; Daniel, J. Overview of pharmacogenomic testing in clinical practice. Ment. Health Clin. 2018, 8, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.E.; Relling, M.V. Moving towards individualized medicine with pharmacogenomics. Nature 2004, 429, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Evans, W.E. Pharmacogenomics in the clinic. Nature 2015, 526, 343–350. [Google Scholar] [CrossRef]
- Holden, C.; Bignell, L.; Mukhopadhyay, S.; Jones, C. The public perception of the facilitators and barriers to implementing personalized medicine: A systematic review. Per. Med. 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, S.; Haynes, K.; Zayac, C.; Sprague, J.E.; Bernhardt, B.; Pyeritz, R. Community pharmacists’ attitudes towards clinical utility and ethical implications of pharmacogenetic testing. Per. Med. 2013, 10, 793–800. [Google Scholar] [CrossRef]
- McCullough, K.B.; Formea, C.M.; Berg, K.D.; Burzynski, J.A.; Cunningham, J.L.; Ou, N.N.; Rudis, M.I.; Stollings, J.L.; Nicholson, W.T. Assessment of the pharmacogenomics educational needs of pharmacists. Am. J. Pharm. Educ. 2011, 75. [Google Scholar] [CrossRef]
- Owusu Obeng, A.; Fei, K.; Levy, K.D.; Elsey, A.R.; Pollin, T.I.; Ramirez, A.H.; Weitzel, K.W.; Horowitz, C.R. Physician-reported benefits and barriers to clinical implementation of genomic medicine: A multi-site IGNITE-network survey. J. Pers. Med. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Elewa, H.; Alkhiyami, D.; Alsahan, D.; Abdel-Aziz, A. A survey on the awareness and attitude of pharmacists and doctors towards the application of pharmacogenomics and its challenges in Qatar. J. Eval. Clin. Pract. 2015, 21, 703–709. [Google Scholar] [CrossRef]
- Obara, T.; Abe, S.; Satoh, M.; Gutierrez Ubeda, S.R.; Yoshimachi, S.; Goto, T. Awareness regarding clinical application of pharmacogenetics among Japanese pharmacists. Pharmgenom. Pers. Med. 2015, 8, 35–41. [Google Scholar] [CrossRef]
- Accreditation Standards and Key Elements for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree. Available online: https://www.acpe-accredit.org/pdf/Standards2016FINAL.pdf (accessed on 20 May 2020).
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef]
- CPIC Guidelines. Available online: https://cpicpgx.org/guidelines/ (accessed on 20 May 2020).
- Unertl, K.M.; Jaffa, H.; Field, J.R.; Price, L.; Peterson, J.F. Clinician perspectives on using pharmacogenomics in clinical practice. Per. Med. 2015, 12, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Rahawi, S.; Naik, H.; Blake, K.V.; Owusu Obeng, A.; Wasserman, R.M.; Seki, Y.; Funanage, V.L.; Oishi, K.; Scott, S.A. Knowledge and attitudes on pharmacogenetics among pediatricians. J. Hum. Genet. 2020, 65, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Luzum, J.A.; Luzum, M.J. Physicians’ attitudes toward pharmacogenetic testing before and after pharmacogenetic education. Pers. Med. 2016, 13, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.B.; O’Daniel, J.M.; Tindall, G.M.; Lipkus, I.R.; Agans, R. Survey of US public attitudes toward pharmacogenetic testing. Pharmacogenom. J. 2012, 12, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.B.; Tindall, G.; O’Daniel, J.M. Public perspectives about pharmacogenetic testing and managing ancillary findings. Genet. Test. Mol. Biomark. 2011, 16, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, S.B.; Coffin, T.B.; Fullerton, S.M.; Ralston, J.; Jarvik, G.P.; Larson, E.B. Getting off the bus closer to your destination: Patients’ views about pharmacogenetic testing. Perm J. 2015, 19, 21–27. [Google Scholar] [CrossRef]
- Dorfman, E.H.; Brown Trinidad, S.; Morales, C.T.; Howlett, K.; Burke, W.; Woodahl, E.L. Pharmacogenomics in diverse practice settings: Implementation beyond major metropolitan areas. Pharmacogenomics 2015, 16, 227–237. [Google Scholar] [CrossRef]
- McKillip, R.P.; Borden, B.A.; Galecki, P.; Ham, S.A.; Patrick-Miller, L.; Hall, J.P.; Hussain, S.; Danahey, K.; Siegler, M.; Sorrentino, M.J.; et al. Patient perceptions of care as influenced by a large institutional pharmacogenomic implementation program. Clin. Pharmacol. Ther. 2017, 102, 106–114. [Google Scholar] [CrossRef]
- Olander, M.; Waring, S.; Stenehjem, D.D.; Taran, A.; Raneli, P.L.; Brown, J. Primary care clinicians attitudes and knowledge of pharmacogenetics in a large.; multi-state.; healthcare system. Innov. Pharm. 2018, 9. [Google Scholar] [CrossRef]
- Lee, Y.M.; McKillip, R.P.; Borden, B.A.; Klammer, C.E.; Ratain, M.J.; O’Donnell, P.H. Assessment of patient perceptions of genomic testing to inform pharmacogenomic implementation. Pharmacogenet. Genom. 2017, 27, 179–189. [Google Scholar] [CrossRef]
- Flesch, R. A new readability yardstick. J. Appl. Psychol. 1948, 32, 221–233. [Google Scholar] [CrossRef]
- Patel, H.N.; Ursan, I.D.; Zueger, P.M.; Cavallari, L.H.; Pickard, A.S. Stakeholder views on pharmacogenomic testing. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2014, 34, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.A.; Hulick, P.J.; Wake, D.T.; Wang, C.; Sereika, A.W.; Yu, K.D.; Glaser, N.S.; Dunnenberger, H.M. Patient perspectives following pharmacogenomics results disclosure in an integrated health system. Pharmacogenomics 2018, 19, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lewis, C.M.; Traylor, M. Pharmacogenetic testing through the direct-to-consumer genetic testing company 23andMe. BMC Med. Genom. 2017, 10. [Google Scholar] [CrossRef]
- Chua, E.W.; Kennedy, M.A. Current state and future prospects of direct-to-consumer pharmacogenetics. Front Pharmacol. 2012, 3. [Google Scholar] [CrossRef]
- Moen, M.; Lamba, J. Assessment of healthcare students’ views on pharmacogenomics at the University of Minnesota. Pharmacogenomics 2012, 13, 1537–1545. [Google Scholar] [CrossRef]
- Coriolan, S.; Arikawe, N.; Moscati, A.; Zhou, L.; Dym, S.; Donmez, S.; Garba, A.; Falbaum, S.; Loewy, Z.; Lull, M.; et al. Pharmacy students’ attitudes and perceptions toward pharmacogenomics education. Am. J. Health Syst. Pharm. 2019, 76, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.; Shiekh, M.; Mehra, V.; Vrbicky, K.; Layle, S.; Olson, M.C.; Maciel, A.; Cullors, A.; Garces, J.A.; Lukowiak, A.A. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: A randomized clinical trial demonstrating clinical utility. J. Psychiatry Res. 2018, 96, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Greden, J.F.; Parikh, S.V.; Rothschild, A.J.; Thase, M.E.; Dunlop, B.W.; DeBattista, C.; Conway, C.R.; Forester, B.P.; Mondimore, F.M.; Shelton, R.C.; et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large.; patient- and rater-blinded, randomized, controlled study. J. Psychiatry Res. 2019, 111, 59–67. [Google Scholar] [CrossRef]
- Hippman, C.; Nislow, C. Pharmacogenomic testing: Clinical evidence and implementation challenges. J. Pers. Med. 2019, 9. [Google Scholar] [CrossRef]


| Strongly Disagree | Disagree | Neutral | Agree | Strongly Agree | |
|---|---|---|---|---|---|
| (1) I understand what pharmacogenomics is | 1 | 2 | 3 | 4 | 5 |
| (2) I am currently taking a prescription medication | 1 | 2 | 3 | 4 | 5 |
| (3) I am interested in pharmacogenomics | 1 | 2 | 3 | 4 | 5 |
| (4) I believe that pharmacogenomics could help me | 1 | 2 | 3 | 4 | 5 |
| (5) I believe pharmacogenomics testing is valuable | 1 | 2 | 3 | 4 | 5 |
| (6) I would be willing to pay up to $____ out of pocket | $0 | $50 | $100 | $200 | $500 |
| (7) Any concerns you may have? | |||||
| Healthcare Students | Pharmacy Patrons | p Value | |
|---|---|---|---|
| (1) I understand what pharmacogenomics is | 4.0 ± 0.11 | 3.2 ± 0.19 | 0.001 |
| (2) I am currently taking a prescription medication | 52% | 85% | 0.0003 |
| (3) I am interested in pharmacogenomics | 4.0 ± 0.09 | 3.5 ± 0.15 | 0.008 |
| (4) I believe that pharmacogenomics could help me | 4.1 ± 0.09 | 3.8 ± 0.11 | 0.021 |
| (5) I believe pharmacogenomics testing is valuable | 4.5 ± 0.07 | 4.1 ± 0.12 | 0.003 |
| (6) I would be willing to pay up to $____ out of pocket | $105 ± 12 | $51 ± 7 | 0.0009 |
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | |
|---|---|---|---|---|---|---|
| 1.00 | 0.01 | 0.24 | 0.21 | 0.16 | 0.06 | Q1 |
| 1.00 | 0.00 | 0.01 | 0.01 | 0.04 | Q2 | |
| 1.00 | 0.31 | 0.17 | 0.06 | Q3 | ||
| 1.00 | 0.41 | 0.07 | Q4 | |||
| 1.00 | 0.09 | Q5 | ||||
| 1.00 | Q6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stegelmeier, J.; Nartker, C.; Barnes, C.; Rayo, H.; Hoover, R.; Boyle, J.; O’Connor, S.; Barrott, J. Rural Community Perceptions and Interests in Pharmacogenomics. Healthcare 2020, 8, 159. https://doi.org/10.3390/healthcare8020159
Stegelmeier J, Nartker C, Barnes C, Rayo H, Hoover R, Boyle J, O’Connor S, Barrott J. Rural Community Perceptions and Interests in Pharmacogenomics. Healthcare. 2020; 8(2):159. https://doi.org/10.3390/healthcare8020159
Chicago/Turabian StyleStegelmeier, John, Christopher Nartker, Charles Barnes, Hugo Rayo, Rebecca Hoover, Julia Boyle, Shanna O’Connor, and Jared Barrott. 2020. "Rural Community Perceptions and Interests in Pharmacogenomics" Healthcare 8, no. 2: 159. https://doi.org/10.3390/healthcare8020159
APA StyleStegelmeier, J., Nartker, C., Barnes, C., Rayo, H., Hoover, R., Boyle, J., O’Connor, S., & Barrott, J. (2020). Rural Community Perceptions and Interests in Pharmacogenomics. Healthcare, 8(2), 159. https://doi.org/10.3390/healthcare8020159






