A Mixed Methods Evaluation of a Randomized Control Trial to Evaluate the Effectiveness of the Pure Prairie Living Program in Type 2 Diabetes Participants
Abstract
1. Introduction
2. Materials and Methods
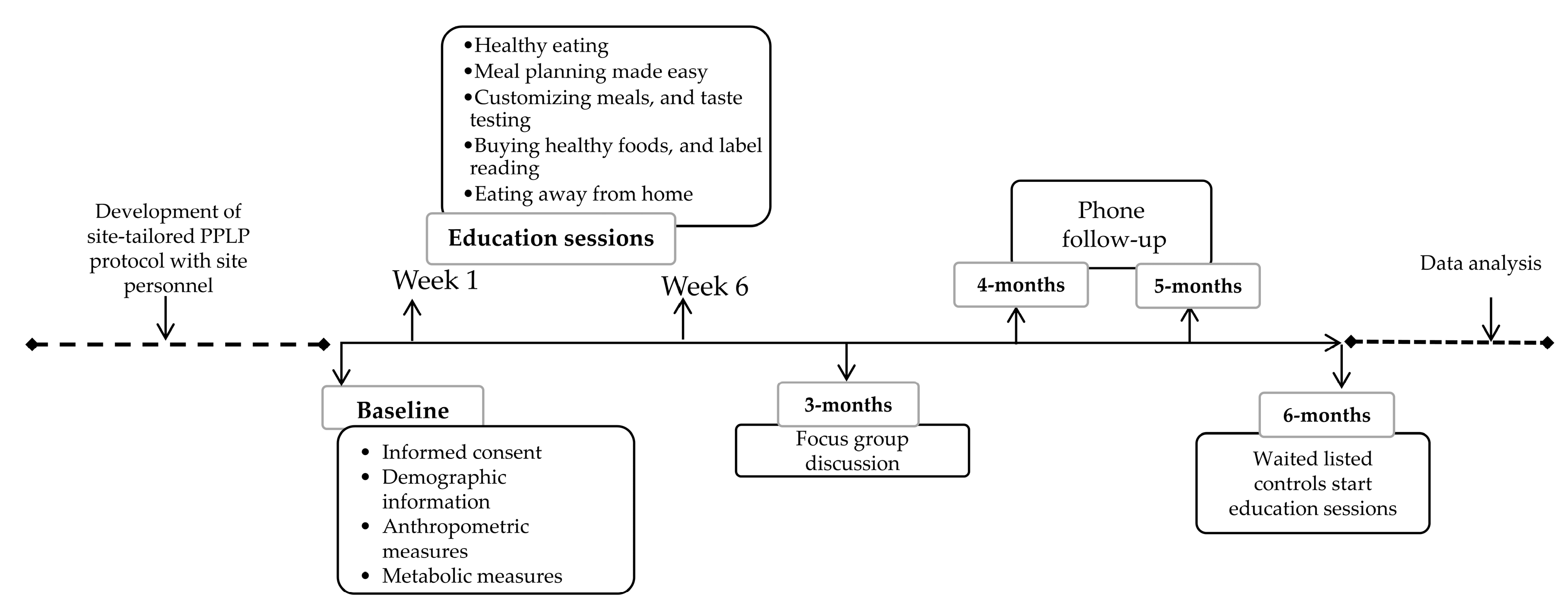
2.1. Study Design
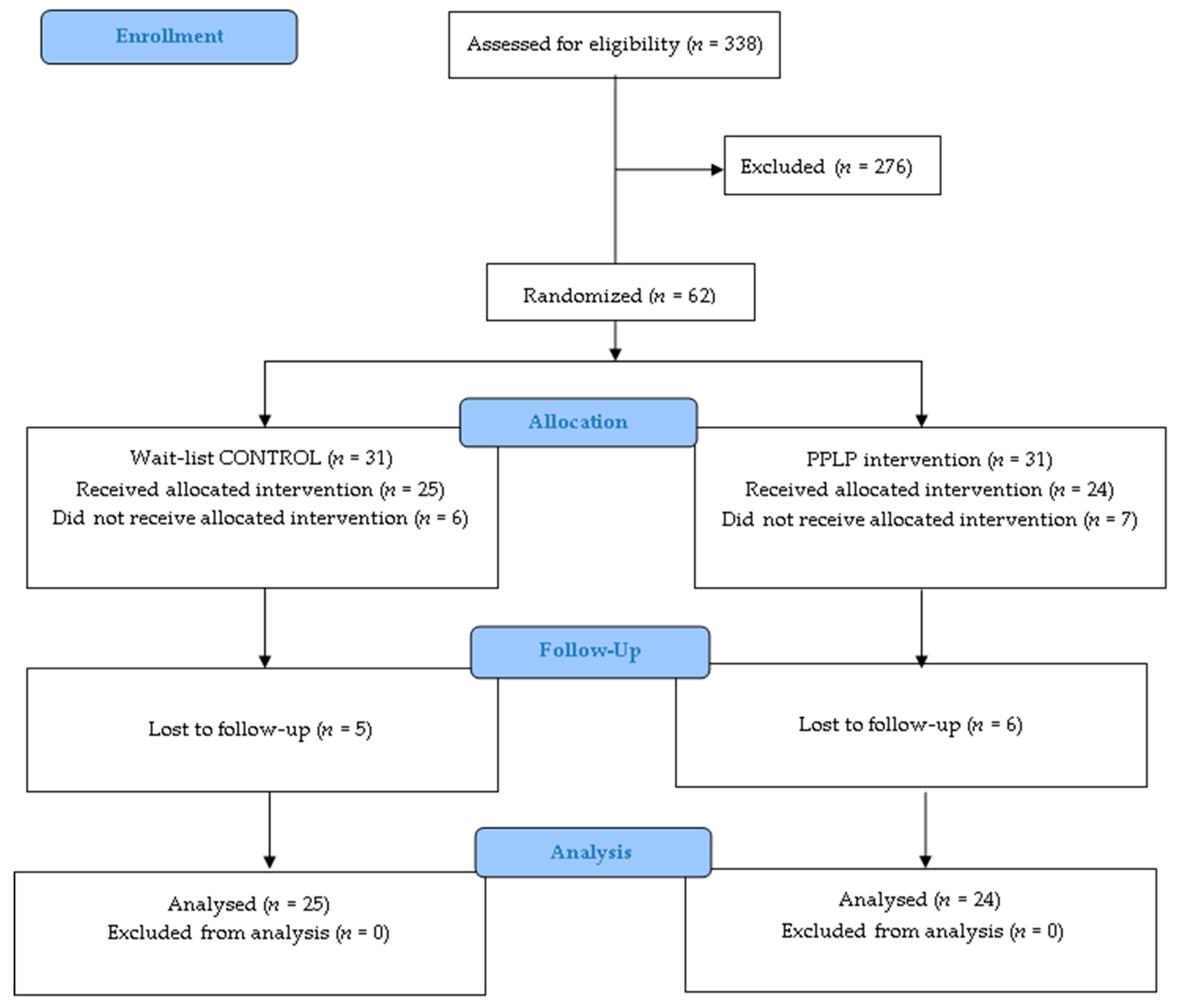
2.2. Participants: Eligibility and Recruitment
2.3. Study Setting
2.4. Study Intervention
2.4.1. Intervention Study Arm
- Education sessions: Participants received a copy of the nutrition education presentations each week. The PPLP curriculum is based on the PANDA program which has been described elsewhere [17]. Briefly, the presentations included information to enhance knowledge of healthy eating based on practical information derived from Eating Well with Canada’s Food Guide [19] and Diabetes Canada Clinical Practice Guidelines for Nutrition Therapy [14]. Participants learned to apply principles of menu planning, grocery shopping, portion control, label reading, and making healthy choices when eating out. A detailed summary of intervention activity content, presentations of group-based educational sessions, and support materials can be found at www.pureprairie.ca/resources [20].
- PPLP workbook: Participants were provided with a workbook that guided them through the educational sessions. It provided support, positive reinforcement of concepts, and the opportunity for skill acquisition through the practice of goal setting, self-monitoring, and problem solving. This aligned with the principles of the social cognitive theory theoretical model.
- Pure Prairie Eating Plan: Participants were provided with a copy of the Pure Prairie Eating Plan (PPEP). This four-week menu plan book meets the DC nutrition therapy guidelines [14] and is guided by the principles of the 4-A framework. Its overall goal is to facilitate solutions to some of the barriers that participants face when trying to adhere to the recommendations. Additional information on the PPEP is available at www.pureprairie.ca [21].
2.4.2. Wait-List Control Group
2.4.3. Study Outcomes
2.4.4. Anthropometric Measures
2.4.5. Metabolic Measures
2.4.6. Demographic Characteristics and Additional Self-Reported Data
2.4.7. Qualitative Assessment
2.4.8. Sample Size
2.4.9. Statistical Method
3. Results
3.1. Quantitative Outcomes
3.1.1. Baseline Characteristics
3.1.2. Changes in HbA1c (Primary Outcome)
3.1.3. Intervention Effects on Diet Adherence and Self-Efficacy (Secondary Outcomes)
3.1.4. Intervention Effects on Anthropometric and Metabolic Parameters (Exploratory Outcomes)
3.1.5. Intervention Effects on Quality of Life (Exploratory Outcomes)
3.2. Qualitative Outcomes
3.2.1. Facilitators and Barriers
Facilitators
Barriers
3.2.2. Experience Evaluation
Satisfaction
Limitations
3.2.3. HCP Perspective
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haslam, D. Weight management in obesity–past and present. Int. J. Clin. Pract. 2016, 70, 206–217. [Google Scholar] [CrossRef]
- Carpenter, R.S. National nutrition conference for defense. JAMA 1941, 116, 2598–2599. [Google Scholar]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635. [Google Scholar] [CrossRef]
- Yang, G.; Badeanlou, L.; Bielawski, J.; Roberts, A.J.; Hannun, Y.A.; Samad, F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E211–E224. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected US state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global economic burden of diabetes in adults: Projections from 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef]
- Jardim, T.V.; Mozaffarian, D.; Abrahams-Gessel, S.; Sy, S.; Lee, Y.; Liu, J.; Huang, Y.; Rehm, C.; Wilde, P.; Micha, R. Cardiometabolic disease costs associated with suboptimal diet in the united states: A cost analysis based on a microsimulation model. PLoS Med. 2019, 16, e1002981. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.Y. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can. J. Diabetes 2013, 37, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 104, 1. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, J.R.; Lyra, R.; Aguilar-Salinas, C.A.; Cavalcanti, S.; Escaño, F.; Tambasia, M.; Duarte, E. Treatment of type 2 diabetes in latin america: A consensus statement by the medical associations of 17 latin american countries. Pan. Am. J. Public Health 2010, 28, 463–471. [Google Scholar] [CrossRef][Green Version]
- Handelsman, Y.; Bloomgarden, Z.T.; Grunberger, G.; Umpierrez, G.; Zimmerman, R.S.; Bailey, T.S.; Blonde, L.; Bray, G.A.; Cohen, A.J.; Dagogo-Jack, S. American association of clinical endocrinologists and American college of endocrinology–clinical practice guidelines for developing a diabetes mellitus comprehensive care plan–2015. Endocr. Pract. 2015, 21, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.R.; Anders, S.; Chan, C.; Bell, R.C. Communicating diabetes best practices to clients: A preliminary investigation of educator perspectives. Health Promot. Pract. 2012, 13, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.B.T.; Wee, W.K.; Ananthanarayanan, J.A.; Soh, Y.H.; Goh, L.M.L.; Tham, D.K.T.; Wong, M.L. Correlates, facilitators and barriers of healthy eating among primary care patients with prediabetes in Singapore—A mixed methods approach. Nutrients 2019, 11, 1014. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Chan, C.B.; Dworatzek, P.D.; Freeze, C.; Williams, S.L. Nutrition therapy. Can. J. Diabetes 2018, 42, S64–S79. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Bell, R. Pure Prairie Eating Plan: Fresh Food, Practical Menus and a Healthy Lifestyle; Division of Human Nutrition, University of Alberta: Edmonton, AB, Canada, 2013. [Google Scholar]
- Soria-Contreras, D.C.; Bell, R.C.; McCargar, L.J.; Chan, C.B. Feasibility and efficacy of menu planning combined with individual counselling to improve health outcomes and dietary adherence in people with type 2 diabetes: A pilot study. Can. J. Diabetes 2014, 38, 320–325. [Google Scholar] [CrossRef]
- Asaad, G.; Soria-Contreras, D.C.; Bell, R.C.; Chan, C.B. Effectiveness of a lifestyle intervention in patients with type 2 diabetes: The physical activity and nutrition for diabetes in alberta (PANDA) trial. Healthcare 2016, 4, 73. [Google Scholar] [CrossRef]
- Garfield, S.A.; Malozowski, S.; Chin, M.H.; Narayan, K.V.; Glasgow, R.E.; Green, L.W.; Hiss, R.G.; Krumholz, H.M. Considerations for diabetes translational research in real-world settings. Diabetes Care 2003, 26, 2670–2674. [Google Scholar] [CrossRef]
- Health Canada. Eating Well with Canada’s Food Guide: A Resource for Educators and Communicators; Publications Health Canada: Ottawa, ON, Canada, 2011. [Google Scholar]
- Pure Prairie Resources. Available online: http://pureprairie.ca/resources/ (accessed on 24 April 2020).
- Pure Prairie Eating Plan. Available online: http://pureprairie.ca (accessed on 24 April 2020).
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Morse, J.M.; Barrett, M.; Mayan, M.; Olson, K.; Spiers, J. Verification strategies for establishing reliability and validity in qualitative research. Int. J. Qual. Methods 2002, 1, 13–22. [Google Scholar] [CrossRef]
- Sharma, A.M. M, M & M: A mnemonic for assessing obesity. Obes. Rev. 2010, 11, 808–809. [Google Scholar]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Lindström, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemiö, K.; Hämäläinen, H.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Laakso, M. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the finnish diabetes prevention study. Lancet 2006, 368, 1673–1679. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Pippi, R.; Aiello, C.; Sbroma Tomaro, E.; Ranucci, C.; Buratta, L.; Bini, V.; Marchesini, G.; De Feo, P.; Fanelli, C. Effect of short term intensive lifestyle intervention on hepatic steatosis indexes in adults with obesity and/or type 2 diabetes. J. Clin. Med. 2019, 8, 851. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.A. Fifty years of behavioral/lifestyle interventions for overweight and obesity: Where have we been and where are we going? Obesity 2017, 25, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhuo, X.; Bardenheier, B.; Rolka, D.B.; Gregg, W.E.; Hong, Y.; Wang, G.; Albright, A.; Zhang, P. Cost-effectiveness of the 2014 US preventive services task force (USPSTF) recommendations for intensive behavioral counseling interventions for adults with cardiovascular risk factors. Diabetes Care 2017, 40, 640–646. [Google Scholar] [CrossRef]
- Zhuo, X.; Zhang, P.; Barker, L.; Albright, A.; Thompson, T.J.; Gregg, E. The lifetime cost of diabetes and its implications for diabetes prevention. Diabetes Care 2014, 37, 2557–2564. [Google Scholar] [CrossRef]
- Diabetes Canada Clinical Practice Guideliness Expert Committee. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can. J. Diabetes 2018, 42, S1–S325. [Google Scholar]
- Chrvala, C.A.; Sherr, D.; Lipman, R.D. Diabetes self-management education for adults with type 2 diabetes mellitus: A systematic review of the effect on glycemic control. Patient Educ. Couns. 2016, 99, 926–943. [Google Scholar] [CrossRef]
- Manns, B.J.; Tonelli, M.; Zhang, J.; Campbell, D.J.; Sargious, P.; Ayyalasomayajula, B.; Clement, F.; Johnson, J.A.; Laupacis, A.; Lewanczuk, R. Enrolment in primary care networks: Impact on outcomes and processes of care for patients with diabetes. CMAJ 2012, 184, E144–E152. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Ivers, N.M.; Grimshaw, J.M.; Moher, D.; Turner, L.; Galipeau, J.; Halperin, I.; Vachon, B.; Ramsay, T.; Manns, B. Effectiveness of quality improvement strategies on the management of diabetes: A systematic review and meta-analysis. Lancet 2012, 379, 2252–2261. [Google Scholar] [CrossRef]
- Kahn, R.; Davidson, M.B. The reality of type 2 diabetes prevention. Diabetes Care 2014, 37, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Mestre, S. Transferring to primary care the lifestyle-based type 2 diabetes prevention programmes. Med. Clin. 2019, 153, 323. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, N.; Kim, K.W.; Lee, M.; Lee, Y.H.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef]
- Brownson, R.C.; Fielding, J.E.; Maylahn, C.M. Evidence-based public health: A fundamental concept for public health practice. Annu. Rev. Public Health 2009, 30, 175–201. [Google Scholar] [CrossRef]
- Janke, E.A.; Ramirez, M.L.; Haltzman, B.; Fritz, M.; Kozak, A.T. Patient’s experience with comorbidity management in primary care: A qualitative study of comorbid pain and obesity. Prim. Health Care. Res. 2016, 17, 33–41. [Google Scholar] [CrossRef]
- Cradock, K.A.; ÓLaighin, G.; Finucane, F.M.; Gainforth, H.L.; Quinlan, L.R.; Ginis, K.A.M. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 18. [Google Scholar] [CrossRef]
- Murphy, M.E.; Byrne, M.; Galvin, R.; Boland, F.; Fahey, T.; Smith, S.M. Improving risk factor management for patients with poorly controlled type 2 diabetes: A systematic review of healthcare interventions in primary care and community settings. BMJ Open 2017, 7, e015135. [Google Scholar] [CrossRef]
- Hayward, R.A. Excessive testing of adults with type 2 diabetes. BMJ 2015, 351, H6549. [Google Scholar] [CrossRef]
- Cardona-Morrell, M.; Rychetnik, L.; Morrell, S.L.; Espinel, P.T.; Bauman, A. Reduction of diabetes risk in routine clinical practice: Are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health 2010, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Linmans, J.J.; Spigt, M.G.; Deneer, L.; Lucas, A.E.; de Bakker, M.; Gidding, L.G.; Linssen, R.; Knottnerus, J.A. Effect of lifestyle intervention for people with diabetes or prediabetes in real-world primary care: Propensity score analysis. BMC Fam. Pract. 2011, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Pascale, V.; Finelli, R.; Visco, V.; Giannotti, R.; Massari, A.; Morisco, C.; Ciccarelli, M.; Illario, M.; Iaccarino, G. We are what we eat: Impact of food from short supply chain on metabolic syndrome. J. Clin. Med. 2019, 8, 2061. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodrigo, C.; Gianzo-Citores, M.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Aranceta-Bartrina, J. Lifestyle patterns and weight status in spanish adults: The anibes study. Nutrients 2017, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, M.L.; García-Vigara, A.; Hidalgo-Mora, J.J.; García-Pérez, M.-Á.; Tarín, J.; Cano, A. Mediterranean diet and health: A systematic review of epidemiological studies and intervention trials. Maturitas 2020, 136, 25–37. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A. Food in the anthropocene: The eat–lancet commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Lee, M.W.; Lee, M.; Oh, K.J. Adipose tissue-derived signatures for obesity and type 2 diabetes: Adipokines, batokines and micrornas. J. Clin. Med. 2019, 8, 854. [Google Scholar] [CrossRef]
| Demographic Variables | CON | PPLP | |||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | t | df | p | |
| Age (years) | 61.3 (9.4) | 57.7 (11.8) | 1.14 | 45 | 0.257 |
| Diabetes diagnosis (years) | 8.4 (7.1) | 5.8 (5.7) | 1.41 | 42 | 0.166 |
| N(%) | N(%) | X2 | df | pValue | |
| Gender | 2.481 | 1 | 0.115 | ||
| Male | 15 (60) | 9 (37.5) | |||
| Female | 10 (40) | 15 (62.5) | |||
| Ethnicity | 0.327 | 1 | 0.568 | ||
| White | 23 (92) | 20 (87) | |||
| Other | 2 (8) | 3 (13) | |||
| Education | 0.10 | 1 | 0.921 | ||
| High school or less | 8 (32) | 8 (33) | |||
| More than high school | 17 (68) | 16 (67) | |||
| Employment Status | 0.10 | 1 | 0.921 | ||
| Working | 12 (48) | 10 (42) | |||
| Other | 13 (52) | 14 (58) | |||
| Household annual income | 0.016 | 1 | 0.900 | ||
| <$59,999 | 10 (42) | 10 (43.5) | |||
| >$60,000 | 14 (58) | 13 (56.5) | |||
| Annual income | |||||
| <Meet needs | 7 (28) | 6 (25) | 0.9 | - | - |
| >Meet needs | 18 (72) | 18 (75) | - | - | |
| Anthropometric Characteristics | CON | PPLP | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | t | df | p | ||
| Weight (kg) | ||||||
| Male | 105.7 (16.4) | 118.3 (46.7) | −0.96 | 22 | 0.345 | |
| Female | 90.1 (22.3) | 92.7 (25.2) | −0.267 | 22 | 0.792 | |
| BMI (kg/m2) | ||||||
| Male | 33 (4) | 37(11) | 22 | 0.219 | ||
| Female | 34 (5) | 35 (8) | −0.502 | 22 | 0.621 | |
| Waist circumference (cm) | ||||||
| Male | 114.3 (11.7) | 121.3 (28) | −0.861 | 22 | 0.398 | |
| Female | 104.5 (17) | 108.5 (13) | −0.659 | 23 | 0.516 | |
| SBP (mmHg) | ||||||
| Male | 141 (12) | 126 (13) | 2.7 | 22 | 0.013 | |
| Female | 142 (20) | 138 (21) | 0.417 | 23 | 0.680 | |
| DBP (mmHg) | ||||||
| Male | 82 (10) | 78 (10) | 0.780 | 22 | 0.444 | |
| Female | 79 (9) | 79 (8) | 0.033 | 22 | 0.974 | |
| Fat mass (%) | ||||||
| Male | 31.0 (4.8) | 33.5 (11.7) | −0.710 | 21 | 0.486 | |
| Female | 39.4 (5.2) | 40.7 (8.3) | −0.416 | 20 | 0.682 | |
| Fat mass (kg) | ||||||
| Male | 32.8 (9.4) | 36.9 (19.2) | −0.710 | 21 | 0.486 | |
| Female | 34.9 (10.4) | 36.7 (15.9) | −0.305 | 20 | 0.763 | |
| Fat free mass (kg) | ||||||
| Male | 70.9 (8.5) | 65.9 (9.2) | 1.3 | 21 | 0.206 | |
| Female | 52.9 (13.4) | 49.9 (7.4) | 0.673 | 20 | 0.509 | |
| Metabolic Characteristics | Mean (SD) | Mean (SD) | t | df | p | |
| HbA1c (%) | ||||||
| Male | 6.9 (0.9) | 7.5 (1.1) | −1.40 | 22 | 0.175 | |
| Female | 7.3 (1.2) | 6.7 (0.6) | 1.38 | 22 | 0.179 | |
| Triglycerides (mmol/L) | ||||||
| Male | 2.2 (1.2) | 3.4 (1.6) | −1.84 | 22 | 0.078 | |
| Female | 3.0 (1.1) | 2.8 (1.1) | 0.435 | 23 | 0.667 | |
| Total Cholesterol (mmol/L) | ||||||
| Male | 3.6 (0.7) | 3.9 (1.2) | −0.759 | 22 | 0.456 | |
| Female | 4.4 (0.9) | 4.3 (1.3) | 0.163 | 23 | 0.872 | |
| LDL-C (mmol/L) | ||||||
| Male | 1.7 (0.7) | 1.6 (1.5) | 0.172 | 13 | 0.866 | |
| Female | 2.0 (0.5) | 1.7 (0.7) | 0.865 | 18 | 0.399 | |
| HDL-C (mmol/L) | ||||||
| Male | 1.0 (0.2) | 0.9 (0.1) | 0.997 | 22 | 0.330 | |
| Female | 1.3 (0.4) | 1.1 (0.2) | 0.965 | 23 | 0.345 | |
| Anthropometric Characteristics | Control Group | PPLP Group | Mean Differences (95% CI) between Group × Time | |||||
|---|---|---|---|---|---|---|---|---|
| Mean Change (95%) from Baseline | Mean Change (95%) from Baseline | |||||||
| 3-Month | 6-Month | 3-Month | 6-Month | 3-Month | p Value | 6-Month | p Value | |
| Weight (kg) | −0.660 (−1.81 to 0.48) | −0.55 (−2.23 to 1.13) | −1.56 (−2.87 to −0.24) * | −2.43 (−3.63 to −1.23) ** | −0.89 (−2.58 to 0.79) | 0.290 | −1.88 (−3.92 to 0.154) | 0.069 |
| BMI (kg/m2) | −0.20 (−0.59 to 0.19) | −0.24 (−0.81 to 0.32) | −0.70 (−1.2 to −0.14) * | −0.90 (−1.41 to −0.39) * | −0.50 (−1.14 to 0.13) | 0.118 | −0.65 (−1.40 to 0.09) | 0.084 |
| WC (cm) | −1.05 (−3.42 to 1.32) | 4.19 (1.9 to 6.4) ** | 3.39 (0.64 to 6.14) * | 2.10 (−1.89 to 6.09) | 4.44 (0.93 to 7.96) | 0.015 * | −2.09 (−6.31 to 2.13) | 0.322 |
| SBP (mmHg) | −11.9 (−19.1 to −4.8) * | −9.5 (−16.5 to −2.4) * | −8.72 (−15.89 to 1.56) * | −5.80 (−13.75 to 2.15) | 3.25 (−6.71 to 13.23) | 0.512 | 3.70 (−6.49 to 13.90) | 0.465 |
| DBP (mmHg) | −5.0 (−9.2 to −0.8) * | −2.2 (−7.1 to 2.6) | −2.50 (−6.39 to 1.39) | −5.00 (−9.31 to −0.69) * | 2.54 (−3.23 to 8.32) | 0.378 | −2.72 (−9.08 to 3.64) | 0.390 |
| Fat mass (%) | −0.24 (−1.36 to 0.87) | 0.28 (−0.42 to 1.00) | 0.51 (−1.14 to 2.17) | 1.37 (−0.70 to 3.45) | 0.75 (−1.09 to 2.61) | 0.412 | 1.08 (−1.08 to 3.25) | 0.308 |
| Fat mass (kg) | −0.47 (−1.49 to 0.54) | 0.14 (−0.92 to 1.20) | −0.30 (−1.76 to 1.16) | 0.30 (−1.38 to 1.98) | 0.17 (−1.48 to 1.83) | 0.216 | 0.15 (−1.69 to 2.00) | 0.863 |
| Fat free mass (kg) | −0.13 (−1.49 to 1.21) | −0.45 (−1.63 to 0.71) | −1.59 (−2.81 to −0.37) * | −2.92 (−4.83 to −1.01) * | −1.45 (−3.32 to 0.41) | 0.122 | −2.46 (−4.54 to −0.38) | 0.021 * |
| Metabolic Characteristics | ||||||||
| HbA1c (%) | −0.04 (−0.27 to 0.17) | −0.09 (−0.41 to 0.22) | −0.15 (−0.38 to 0.08) | 0.06 (−0.26 to 0.38) | −0.10 (−0.41 to 0.21) | 0.510 | 0.15 (−0.28 to 0.59) | 0.474 |
| Triglycerides (mmol/L) | −0.05 (−0.43 to 0.33) | −0.17 (−0.58 to 0.23) | −0.17 (−0.73 to 0.37) | −0.44 (−1.15 to 0.27) | −0.12 (−0.76 to 0.50) | 0.686 | −0.26 (−1.04 to 0.50) | 0.484 |
| Total Cholesterol (mmol/L) | 0.02 (−0.30 to 0.34) | −0.10 (−0.42 to 0.27) | 0.18 (−0.46 to 0.84) | −0.40 (−1.01 to 0.21) | 0.16 (−0.49 to 0.83) | 0.616 | −0.29 (−0.92 to 0.33) | 0.346 |
| LDL-C (mmol/L) | −0.05 (−0.35 to 0.24) | 0.07 (−0.11 to 0.26) | −0.01 (−0.48 to 0.46) | −0.16 (−0.57 to 0.24) | 0.04 (−0.46 to 0.54) | 0.862 | −0.24 (−0.60 to 0.12) | 0.183 |
| HDL-C (mmol/L) | 0.02 (−0.04 to 0.09) | 0.04 (−0.04 to 0.12) | 0.05 (−0.13 to 0.24) | −0.02 (−0.07 to 0.01) | 0.0. (−0.14 to 0.21) | 0.714 | −0.06 (−0.16 to 0.02) | 0.165 |
| Diet Quality and Adherence Variables | Control Group | PPLP Group | Mean Differences (95% CI) between Group × Time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean Change (95%) from Baseline | Mean (SD) | Mean Change (95%) from Baseline | |||||||
| Baseline | 3-Month | 6-Month | Baseline | 3-Month | 6-Month | 3-Month | p Value | 6-Month | p Value | |
| PDAQ (Score 7 max) | 3.5 (0.9) | 0.3 (−0.1 to 0.7) | 0.3 (−0.03 to 0.8) | 3.7 (0.9) | 0.4 (0.1 to 0.8) * | 0.5 (0.06 to 0.9) * | −0.3 (−0.9 to 0.2) | 0.271 | −0.1 (−0.9 to 0.5) | 0.605 |
| DSES (Score 8 max) | 7.0 (1.8) | 0.04 (−0.7 to 0.8) | 0.68 (−0.3 to 1.6) | 6.2 (1.7) | 0.7 (−0.03 to 1.5) | 0.3 (−0.5 to 1.2) | −0.03 (−1.09 to 1.01) | 0.940 | 0.5 (−0.4 to 1.5) | 0.277 |
| EQ-VAS (Score 100 max) | 68 (18.7) | 7.3 (−0.1 to 14.8) * | 7.5 (3.1 to 11.8) * | 67.8 (15.2) | 5.3 (0.4 to 10.2) * | 3.9 (−2.5 to 10.4) | 3.4 (−5.0 to 11.8) | 0.416 | 5.3 (−5.4 to 16.2) | 0.321 |
| Facilitators and Barriers | |
|---|---|
| Themes | Example Quotes |
| 1. Knowledge acquired | |
| Healthy eating | I spend a lot more time thinking about my menu for the week, versus just grabbing whatever out of the fridge. So when I go shopping, I do it with more intent, when I’m doing my cooking for the week, I do it with more intent [PPLP-5B]. |
| Label reading | I had no idea, uh, one thing I really benefited from was reading labels. I, um, I had no idea how much salt was in processed foods, uh, and sugars, and that was a real benefit to me [PPLP-1B]. |
| Physical Activity | I think awareness of activity level … is very beneficial because it, it did make you realize that you have days where you might walk fifteen hundred steps, which is barely enough to keep you alive [PPLP-7B]. |
| 2. Motivation | |
| Health | My biggest thing was to try and keep control on the diabetes with food and that because I don’t want to go any further with medications. I’m scared of going on insulin [PPLP-1A]. |
| I’m getting older and I was afraid I was gonna get my legs cut off and I was gonna go blind and therefore I needed to find something to, to make sure I’m going down the right path [PPLP-7B]. | |
| Family | I just wanna be able to keep control so that I can stay healthy and that, because you know, like uh, I’m waiting…my son’s getting married, hopefully within the next ten years I’ll be a grandfather, I’d like to be pretty healthy to play with the grandkids and stuff like that [PPLP-1A]. |
| Group support | I think what worked well for me was coming every week and being, like I said, accountable, and then hearing other people doing really well, and then feeling guilty that I didn’t do really well and then the next week I felt…well I gotta…they’re all doing it, I gotta step up and be accountable [PPLP-2B]. |
| 3. Barriers | |
| Mental | I’ve been able to go and buy the food and that, but it’s uh, for me it’s just a lot of laziness at times when I’ll be driving home and thinking it’s easier to stop and buy A&W and pick up a burger than trying to go through the hassle of making something at home and that. So, I still eat out way too much than I should and that [PPLP-4A]. I find that you know, to cook it right before I have to eat it, I hahaha…I don’t do that you know. Like, I just want it ready so I can just take it out and you know, don’t spend the hour cooking … we do get lazy and guilt-ridden and uh you know whiny and that sort of thing [PPLP-3A]. I’ve been really depressed and, so it’s been hard to think of…to take care of myself [PPLP-5A]. |
| Mechanical/metabolic | Having MS [multiple sclerosis] and being immobile, I, my health really depends on what I eat, like I can’t control my weight or anything with movement, so I can’t exercise or burn calories that way [PPLP-6A]. |
| Monetary | Sometimes you don’t get home from work and you haven’t had the time to plan so I, like I often felt like I need to take time to plan out the meals, to get the groceries, to…but if you don’t have the time to get ahead of yourself like that, then the time is working against you [PPLP-2B]. Financially, like I haven’t really had much control over the food that’s been coming into my home [PPLP-3B]. Buying fresh produce and, and uh…and fresh, fresh ingredients is much more expensive [PPLP-5B]. |
| Experience Evaluation | |
|---|---|
| Themes | Example Quotes |
| 1. Satisfaction | Practical and easy to understand and when you take a look at other, uh, books and programs, it becomes very complicated, but this one was, was laid out, I think in, um easy to understand and very practical terms [PPLP2A]. I find the best benefit is that…giving you the alternatives, like… saying don’t eat this, don’t do this…here is something you can…have instead…[PPLP5B] I just enjoyed all the sessions. I learned a lot, you know like I went for…to a dietitian, and it was one day, you know like what can you…learn all this stuff in one day so this is spread over time and you know with the group session, that’s what I really like about it [PPLP4A]. This made me very conscious of my overall lifestyle[PPLP-2A]. |
| 2. Limitations | Honestly the paperwork. Like the recording of everything, I didn’t do it. I, I do enough paperwork at work that when I, you know, spend…to come home and spend another twenty minutes, half an hour on that when I’m already doing lots of other work. This was a deterrent. If this was an app, it would be beneficial [PPLP-5B]. |
| 3. Improvements Support group | I think it would be nice that a core group has been established. If you’re feeling like it’s just, uh, overwhelming, or you’re just, you’re not doing as well as you anticipated, to be able to be a part of an evening group that does something, uh nutrition or whatever the program might be. And so that gives you that little bit of an extra boost [PPLP-2A]. |
| Hands on activities | Active cooking classes for next [PPLP-6B]. |
| Active PA | Set up an exercise program for you that would be sort of matched to um your goals, yeah, and your abilities [PPLP-2B]. |
| Examples of HCPs Views of the Intervention |
| “I would rank it high, um I, I think…I was personally very pleased. I would rank it five. Like very high. Uh, I thought everything…I thought it was a…in my opinion, it was a quality um program” [PPLP-HCP1]. “I’d say probably about a four [PPLP-HCP2].” |
| “When we’re talking to a lot of our patients regarding diabetes, we talk a lot about um either exchanging…like the carb choices or um grams of carbohydrate and um I know the Pure Prairie Eating Plan cookbook is based on Canada’s Food Guide. That to me makes better sense in a lot of ways and it’s easier, it’s just wrapping our heads around thinking a little bit differently” [PPLP-HCP1]. |
| “I think, because there was uh, like [RD] and I, there was two of us kind of working on implementing it, that was helpful cause we um tend to be on the same page with a lot of things. And she could take some tasks and I could take some tasks and we could kind of get it done together“ [PPLP-HCP2]. “Uh well a lot of things went well, um I think just, [RD] and I were able to work together very well um with your help and [Research member’s] help…. It was a lot of work, but I think it really worked out well. That we really had, I think there was a lot of agreement, with how um things should look, how the presentation should, how the slides should look, how the presentation, how the slides um should be presented. And I think it was nice to have the support from the team, uh the U of A, um our, our girls out front received everybody here” [PPLP-HCP1]. |
| Examples of HCPs Challenges of the Intervention |
| “The calls were a lot of work haha. A lot more work than we anticipated… For the recruitment and for the follow-ups, yeah… it was a lot of administrative time on our part, and it took away from the rest of our patient time ”[PPLP-HCP2]. |
| “Definitely trying to schedule the boardrooms, cause we’re in competition with all of our regular classes plus our mental health classes plus there’s board meetings, there’s um, Alberta Health Services uses our board rooms for some of their programming too, so there’s…it’s a challenge… to, to find it, and especially cause it…when you need the same board room consistently at the same time for several weeks in a row…that was the big challenge. We could, you know, here and there you can always find an empty boardroom, but um the multiple weeks in a row was very challenging to get the space” [PPLP-HCP2]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Archundia-Herrera, M.C.; Subhan, F.B.; Sakowsky, C.; Watkins, K.; Chan, C.B. A Mixed Methods Evaluation of a Randomized Control Trial to Evaluate the Effectiveness of the Pure Prairie Living Program in Type 2 Diabetes Participants. Healthcare 2020, 8, 153. https://doi.org/10.3390/healthcare8020153
Archundia-Herrera MC, Subhan FB, Sakowsky C, Watkins K, Chan CB. A Mixed Methods Evaluation of a Randomized Control Trial to Evaluate the Effectiveness of the Pure Prairie Living Program in Type 2 Diabetes Participants. Healthcare. 2020; 8(2):153. https://doi.org/10.3390/healthcare8020153
Chicago/Turabian StyleArchundia-Herrera, M. Carolina, Fatheema B. Subhan, Cathy Sakowsky, Karen Watkins, and Catherine B. Chan. 2020. "A Mixed Methods Evaluation of a Randomized Control Trial to Evaluate the Effectiveness of the Pure Prairie Living Program in Type 2 Diabetes Participants" Healthcare 8, no. 2: 153. https://doi.org/10.3390/healthcare8020153
APA StyleArchundia-Herrera, M. C., Subhan, F. B., Sakowsky, C., Watkins, K., & Chan, C. B. (2020). A Mixed Methods Evaluation of a Randomized Control Trial to Evaluate the Effectiveness of the Pure Prairie Living Program in Type 2 Diabetes Participants. Healthcare, 8(2), 153. https://doi.org/10.3390/healthcare8020153







