Association Between Back Muscle Strength and Proprioception or Mechanoreceptor Control Strategy in Postural Balance in Elderly Adults with Lumbar Spondylosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
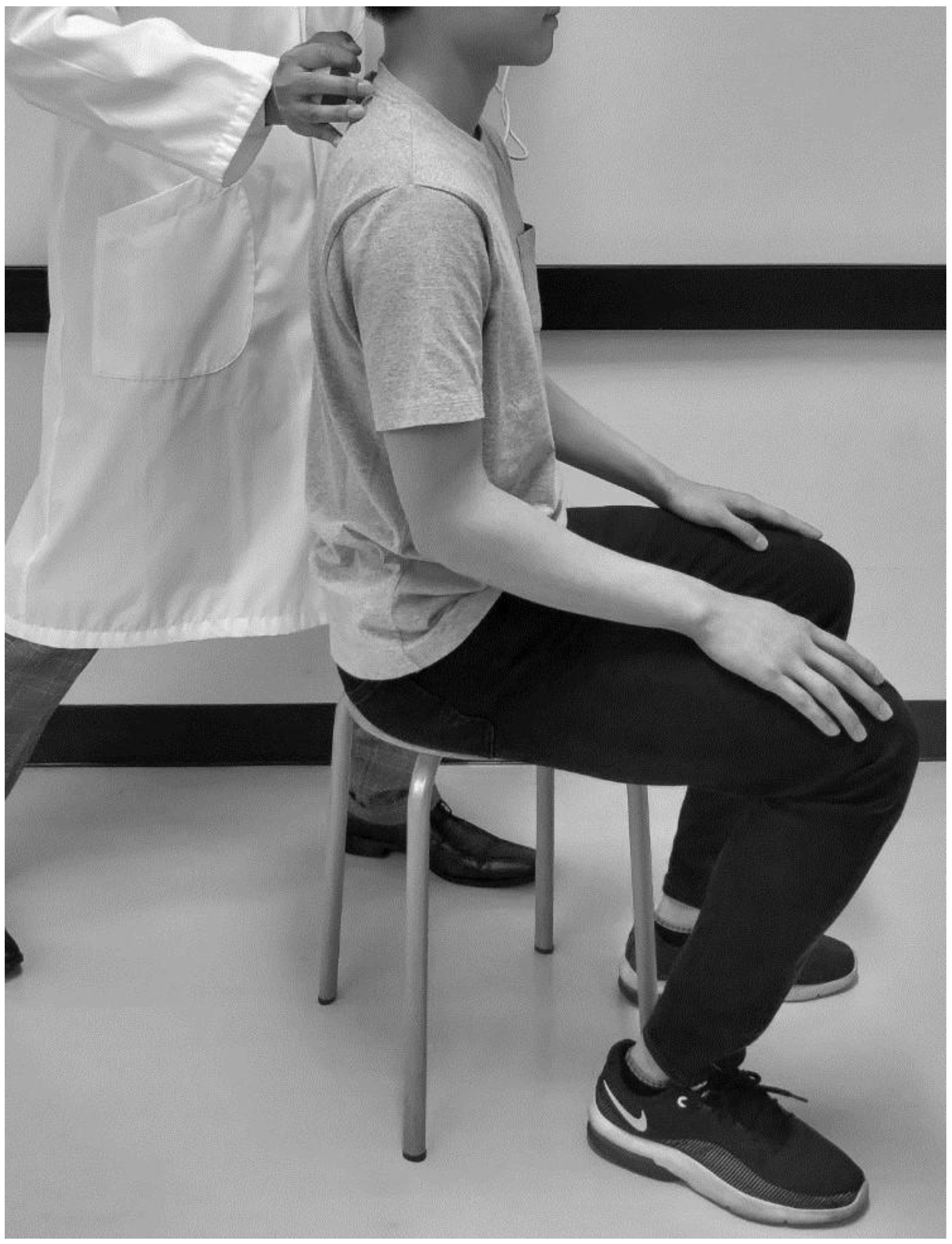
2.2. Back Muscle Strength Assessment
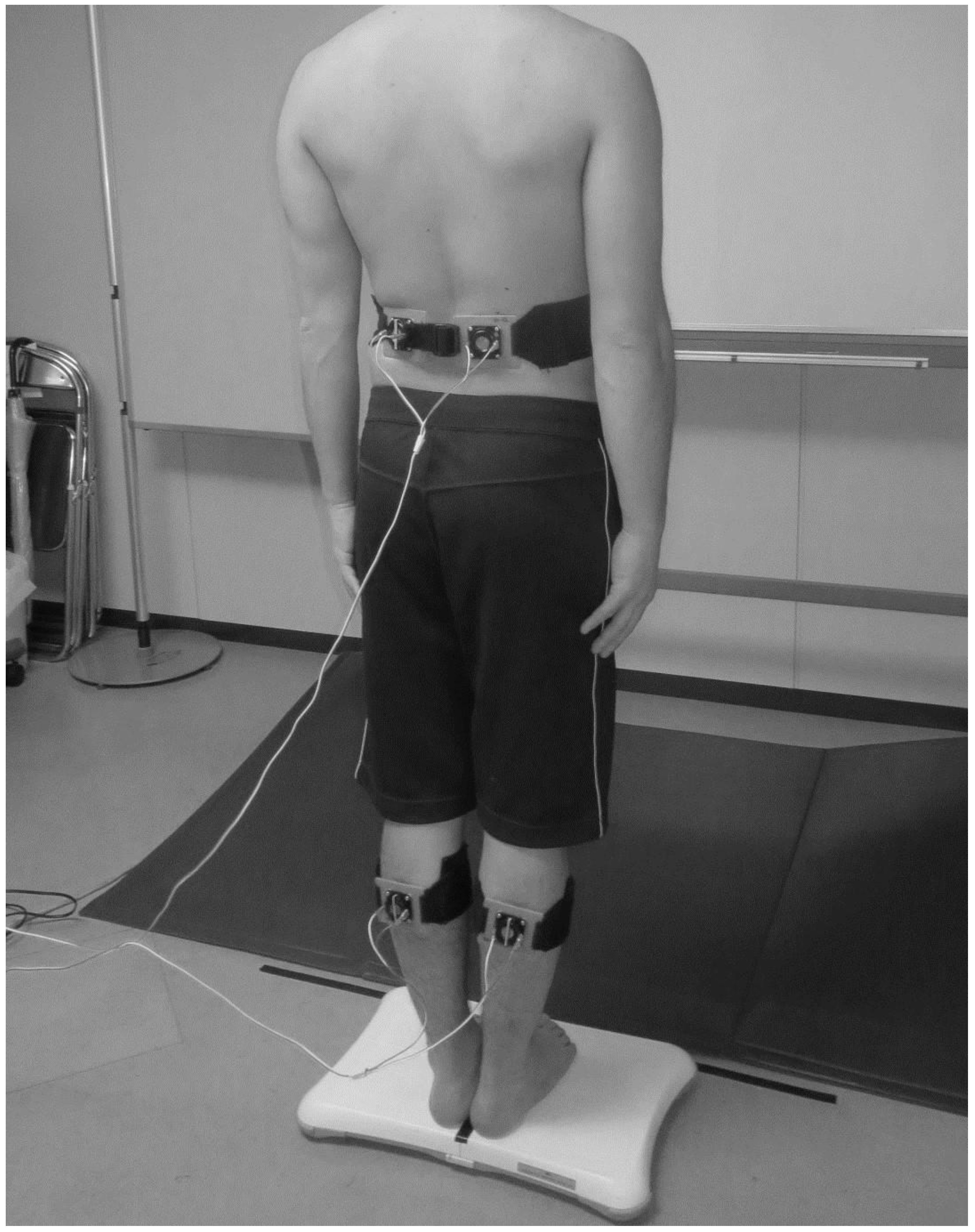
2.3. Muscle Vibration
2.4. Low Back Pain Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johansson, R.; Magnusson, M. Human postural dynamics. Crit. Rev. Biomed. Eng. 1991, 18, 413–437. [Google Scholar] [PubMed]
- Allum, J.H.; Bloem, B.R.; Carpenter, M.G.; Hulliger, M.; Hadders-Algra, M. Proprioceptive control of posture: Review of new concepts. Gait Posture 1998, 8, 214–242. [Google Scholar] [CrossRef]
- Morasso, P.G.; Schieppati, M. Can muscle stiffness alone stabilize upright standing? J. Neurophysiol. 1999, 82, 1622–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part I: The physiologic basis of functional joint stability. J. Athl. Train. 2002, 37, 71–79. [Google Scholar] [PubMed]
- Ito, T.; Sakai, Y.; Morita, Y.; Yamazaki, K.; Igarashi, K.; Nishio, R.; Sato, N. Proprioceptive weighting ratio for balance control in static standing is reduced in elderly patients with non-specific low back pain. Spine 2018, 43, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Brumagne, S.; Cordo, P.; Verschueren, S. Proprioceptive weighting changes in persons with low back pain and elderly persons during upright standing. Neurosci. Lett. 2004, 366, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Brumagne, S.; Janssens, L.; Janssens, E.; Goddyn, L. Altered postural control in anticipation of postural instability in persons with recurrent low back pain. Gait Posture 2008, 28, 657–662. [Google Scholar] [CrossRef]
- Brumagne, S.; Lysens, R.; Swinnen, S.; Verschueren, S. Effect of paraspinal muscle vibration on position sense of the lumbosacral spine. Spine 1999, 24, 1328–1331. [Google Scholar] [CrossRef]
- Mok, N.W.; Brauer, S.G.; Hodges, P.W. Hip strategy for balance control in quiet standing is reduced in people with low back pain. Spine 2004, 29, E107–E112. [Google Scholar] [CrossRef]
- Moseley, G.L.; Hodges, P.W. Are the changes in postural control associated with low back pain caused by pain interference? Clin. J. Pain 2005, 21, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Bard, C.; Fleury, M.; Teasdale, N.; Paillard, J.; Nougier, V. Contribution of proprioception for calibrating and updating the motor space. Can. J. Physiol. Pharmacol. 1995, 73, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part II: The role of proprioception in motor control and functional joint stability. J. Athl. Train. 2002, 37, 80–84. [Google Scholar] [PubMed]
- Butler, A.A.; Lord, S.R.; Rogers, M.W.; Fitzpatrick, R.C. Muscle weakness impairs the proprioceptive control of human standing. Brain Res. 2008, 1242, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Van der Esch, M.; Steultjens, M.; Harlaar, J.; Knol, D.; Lems, W.; Dekker, J. Joint proprioception, muscle strength, and functional ability in patients with osteoarthritis of the knee. Arthritis Rheumatol. 2007, 57, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Claeys, K.; Brumagne, S.; Dankaerts, W.; Kiers, H.; Janssens, L. Decreased variability in postural control strategies in young people with non-specific low back pain is associated with altered proprioceptive reweighting. Eur. J. Appl. Physiol. 2011, 111, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sakai, Y.; Nakamura, E.; Yamazaki, K.; Yamada, A.; Sato, N.; Morita, Y. Relationship between paraspinal muscle cross-sectional area and relative proprioceptive weighting ratio of older persons with lumbar spondylosis. J. Phys. Ther. Sci. 2015, 27, 2247–2251. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Sakai, Y.; Yamazaki, K.; Igarashi, K.; Sato, N.; Yokoyama, K.; Morita, Y. Proprioceptive change impairs balance control in older patients with low back pain. J. Phys. Ther. Sci. 2017, 29, 1788–1792. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Sakai, Y.; Yamazaki, K.; Nishio, R.; Ito, Y.; Morita, Y. Postural strategy in elderly, middle-aged, and young people during local vibratory stimulation for proprioceptive inputs. Geriatrics 2018, 3, 93. [Google Scholar] [CrossRef] [Green Version]
- Mientjes, M.I.; Frank, J.S. Balance in chronic low back pain patients compared to healthy people under various conditions in upright standing. Clin. Biomech. 1999, 14, 710–716. [Google Scholar] [CrossRef]
- Newcomer, K.L.; Laskowski, E.R.; Yu, B.; Johnson, J.C.; An, K.N. Differences in repositioning error among patients with low back pain compared with control subjects. Spine 2000, 25, 2488–2493. [Google Scholar] [CrossRef]
- Janssens, L.; Brumagne, S.; Polspoel, K.; Troosters, T.; McConnell, A. The effect of inspiratory muscles fatigue on postural control in people with and without recurrent low back pain. Spine 2010, 35, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, I.; Jantti, P.; Aalto, H. Postural control in elderly subjects. Age Ageing 1990, 19, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Bacciu, D.; Chessa, S.; Gallicchio, C.; Micheli, A.; Pedrelli, L.; Ferro, E.; Fortunati, L.; Rosa, D.; Palumbo, F.; Vozzi, F.; et al. A learning system for automatic Berg Balance Scale score estimation. Eng. Appl. Artif. Intell. 2017, 66, 60–74. [Google Scholar] [CrossRef]
- Clark, R.A.; Bryant, A.L.; Pua, Y.; McCrory, P.; Bennell, K.; Hunt, M. Validity and reliability of the Nintendo Wii Balance Board for assessment of standing balance. Gait Posture 2010, 31, 307–310. [Google Scholar] [CrossRef]
- Young, W.; Ferguson, S.; Brault, S.; Craig, C. Assessing and training standing balance in older adults: A novel approach using the ‘Nintendo Wii’ Balance Board. Gait Posture 2011, 33, 303–305. [Google Scholar] [CrossRef]
- Talbot, W.H.; Darian-Smith, I.; Kornhuber, H.H.; Mountcastle, V.B. The sense of flutter-vibration: Comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J. Neurophysiol. 1968, 31, 301–334. [Google Scholar] [CrossRef] [Green Version]
- Mountcastle, V.B.; LaMotte, R.H.; Carli, G. Detection thresholds for stimuli in humans and monkeys: Comparison with threshold events in mechanoreceptive afferent nerve fibers innervating the monkey hand. J. Neurophysiol. 1972, 35, 122–136. [Google Scholar] [CrossRef]
- Shakoor, N.; Foucher, K.C.; Wimmer, M.A.; Mikolaitis-Preuss, R.A.; Fogg, L.F.; Block, J.A. Asymmetries and relationships between dynamic loading, muscle strength, and proprioceptive acuity at the knees in symptomatic unilateral hip osteoarthritis. Arthritis Res. Ther. 2014, 16, 455. [Google Scholar] [CrossRef] [Green Version]
- Ageberg, E.; Roberts, D.; Holmström, E.; Fridén, T. Balance in single-limb stance in patients with anterior cruciate ligament injury: Relation to knee laxity, proprioception, muscle strength, and subjective function. Am. J. Sports Med. 2005, 33, 1527–1535. [Google Scholar] [CrossRef]
- Ryan, L. Mechanical stability, muscle strength and proprioception in the functionally unstable ankle. Aust. J. Physiother. 1994, 40, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Willems, T.; Witvrouw, E.; Verstuyft, J.; Vaes, P.; De Clercq, D. Proprioception and muscle strength in subjects with a history of ankle sprains and chronic instability. J. Athl. Train. 2002, 37, 487–493. [Google Scholar] [PubMed]
- Araneda, J.E.; Solorza, E.M. Plantar cutaneous sensibility and dynamic balance in healthy elderly of the community: Relational study. Fisioter. Pesqui. 2013, 20, 310–315. [Google Scholar]
- Melzer, I.; Benjuya, N.; Kaplanski, J.; Alexander, N. Association between ankle muscle strength and limit of stability in older adults. Age Ageing 2009, 38, 119–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | Elderly Adults with Lumbar Spondylosis (n = 24) | Healthy Young Adults (n = 24) | P |
|---|---|---|---|
| Age (years) | 73.5 (65–85) | 22 (19–24) | 0.001 |
| Sex (men) | 12 (50) | 12 (50) | 1.000 |
| Height (cm) | 158.6 ± 9.6 | 165.6 ± 6.3 | 0.004 |
| Weight (kg) | 59.6 ± 10.6 | 55.6 ± 8.0 | 0.151 |
| BMI (kg/m2) | 23.7 (16.5–30.9) | 20.3 (17.1–26.7) | 0.001 |
| VAS (cm) | 0.5 (0–5) | 0 (0–0) | 0.001 |
| Back muscle strength (N/kg) | 2.9 ± 0.6 | 3.2 ± 0.6 | 0.110 |
| Variables | Elderly Adults with Lumbar Spondylosis (n = 24) | Healthy Young Adults (n = 24) | P |
|---|---|---|---|
| GS with 30 Hz (cm) | 0.82 (0.43–1.73) | 0.67 (0.27–1.3) | 0.030 |
| GS with 60 Hz (cm) | 0.84 (0.43–1.67) | 0.69 (0.18–1.57) | 0.274 |
| GS with 240 Hz (cm) | 0.87 (0.45–1.62) | 0.52 (0.28–1.57) | 0.002 |
| LM with 30 Hz (cm) | 0.63 (0.44–1.2) | 0.61 (0.28–1.28) | 0.578 |
| LM with 60 Hz (cm) | 0.70 (0.46–1.43) | 0.64 (0.32–1.13) | 0.080 |
| LM with 240 Hz (cm) | 0.74 ± 0.21 | 0.56 ± 0.15 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, T.; Sakai, Y.; Ito, Y.; Yamazaki, K.; Morita, Y. Association Between Back Muscle Strength and Proprioception or Mechanoreceptor Control Strategy in Postural Balance in Elderly Adults with Lumbar Spondylosis. Healthcare 2020, 8, 58. https://doi.org/10.3390/healthcare8010058
Ito T, Sakai Y, Ito Y, Yamazaki K, Morita Y. Association Between Back Muscle Strength and Proprioception or Mechanoreceptor Control Strategy in Postural Balance in Elderly Adults with Lumbar Spondylosis. Healthcare. 2020; 8(1):58. https://doi.org/10.3390/healthcare8010058
Chicago/Turabian StyleIto, Tadashi, Yoshihito Sakai, Yohei Ito, Kazunori Yamazaki, and Yoshifumi Morita. 2020. "Association Between Back Muscle Strength and Proprioception or Mechanoreceptor Control Strategy in Postural Balance in Elderly Adults with Lumbar Spondylosis" Healthcare 8, no. 1: 58. https://doi.org/10.3390/healthcare8010058
APA StyleIto, T., Sakai, Y., Ito, Y., Yamazaki, K., & Morita, Y. (2020). Association Between Back Muscle Strength and Proprioception or Mechanoreceptor Control Strategy in Postural Balance in Elderly Adults with Lumbar Spondylosis. Healthcare, 8(1), 58. https://doi.org/10.3390/healthcare8010058







