Abstract
This study was conducted to examine predictive factors of deep vein thrombosis (DVT) in gynecologic cancer survivors with lower extremity edema (LEE). In the current single-center, retrospective study, there was a total of 315 eligible patients, including 80 patients with DVT and 235 without DVT. They were therefore divided into two groups: the DVT group (n = 80) and the non-DVT group (n = 235). Then, baseline and clinical characteristics of the patients were compared between the two groups. In our study, distant organ metastasis, advanced stage, lymphadectomy, and amount of intraoperative blood loss had a positive predictive value for the occurrence of DVT in gynecologic cancer survivors presenting LEE. In conclusion, our results indicate that it is necessary to consider the possibility of LEE arising from DVT in gynecologic cancer survivors with advanced-stage cancer, distant organ metastasis, lymphadectomy, and intraoperative blood loss over 1500 mL.
1. Introduction
The relationship between venous thrombosis and malignancy was first described by Trousseau in 1865. Since then, it has been advocated by multiple clinical, pathologic, and laboratory studies [1,2]. According to Virchow, there is a triad of risk factors that contribute to venous thromboembolism; these include venous stasis, endothelial injury, and hypercoagulable states [3]. Patients with cancer are vulnerable to thrombosis arising from hematologic and biochemical abnormalities. For example, ovarian cancer cells are capable of forming and degrading thrombin. In addition, gynecologic malignancies are characterized by increased fibrinolytic activity. Furthermore, patients receiving surgery, chemotherapy, or radiotherapy are at increased risks of developing thrombosis [4,5].
According to epidemiological studies, patients with advanced-stage cancer are at increased risks of developing idiopathic venous thrombosis or thromboembolism. This deserves special attention [6].
Venous thromboembolism (VTE) is a leading cause of death in patients with cancer; it comprises deep vein thrombosis (DVT) and pulmonary embolism (PE) [7]. Its incidence of postoperative venous thromboembolism in ovarian cancer is reported to be 13.2% (DVT 71.6%, PE 24.3%, both 4.1%) [8]. In more detail, without prophylaxis, the incidence of VTE is estimated at approximately 10–40% [9]. With prophylaxis, it is estimated at 1.14% in patients diagnosed with gynecological disease, 0.7% in those undergoing laparoscopic gynecological surgery, 0.3% in those undergoing urogynecological surgery, and 4% in those with gynecological malignancies [10,11,12,13]. It remains problematic, however, that most published studies have evaluated symptomatic cases rather than asymptomatic ones as the latter could be frequently neglected without efficient methods for detecting VTE. Indeed, approximately 50% of total patients with VTE are presumed to be silent cases [14]. It can therefore be inferred that the actual incidence of postoperative VTE might be higher than compared to published reports [14].
Lower extremity edema (LEE) commonly occurs in patients with advanced-stage cancer, and it often poses a diagnostic dilemma for clinicians because of its non-specific symptoms. The diagnosis can be confirmed by performing a difference in circumference (>2 cm) and/or volume (>200 mL) between the affected and unaffected extremity. A variety of etiologic factors are involved in its pathogenesis, which include lymph blockage from tumor progression or anticancer treatment, DVT, hypoalbuminemia, renal or cardiac failure, and thyroid dysfunction [15]. Moreover, lymphedema is also considered the most likely cause of the LEE and this may obscure signs and symptoms of DVT [16]. It would therefore be mandatory to make a correct diagnosis of DVT, which is essential for lowering the risk of PE [17,18].
Various modalities are used to diagnose DVT. Of these, venography is considered a “gold standard”, but it has disadvantages, such as invasiveness, high cost, requirement of technical expertise, pain, unavailability for cases of allergy or renal insufficiency, difficulty of interpretation, and high inter- and intra-observer variability [19]. Alternative imaging modalities, such as computed tomography (CT) and magnetic resonance (MR) venography, are therefore used. But their applicability is also limited [19]. Currently, non-invasive diagnostic modalities are used to rule out suspected symptomatic DVT, and these include pretest probability estimation, D-dimer, and ultrasonography [19,20,21]. Moreover, compression vein ultrasonography with color Doppler flow or duplex ultrasonography is also recommended [22].
According to the guidelines from American College of Chest Physicians (ACCP) and American College of Obstetricians and Gynecologists (ACOG), appropriate preventive interventions are recommended based on diverse levels of postoperative risks of developing VTE [9,23]. Nevertheless, there is a paucity of evidence that advocates for the necessity to stratify patients undergoing gynecological surgery according to the level of risks of developing VTE [24,25,26]. In these patients, several postoperative risk factors of developing VTE have been suggested. These include body mass index (BMI) 30 or 40 kg/m2, operation time >180 minutes, cancer surgery, and blood transfusions of 2000 mL [19,27,28]. But this cannot be generalized, because the corresponding studies have failed to efficiently assess risk factors of developing VTE.
Given the above background, we conducted this single-center, retrospective study to examine predictive factors of the DVT.
2. Materials and Methods
2.1. Study Patients and Setting
We analyzed a total of 580 patients (n = 580) with gynecologic cancer who had been treated at our medical institution between January 2012 and December 2018.
Inclusion criteria for the current study were as follows:
- Gynecologic cancer survivors who are living with, through, and beyond cancer since the diagnosis of cancer, receiving continuous treatment, endeavoring to reduce the risk of recurrence, and managing chronic disease
- Women with a swollen leg
- Women with confirmed radiological evidence of DVT
- Women with metastasis to the lower extremities
- Women with Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 [29]
Exclusion criteria for the current study were as follows:
- Pregnant women (n = 0)
- Women receiving any perioperative prophylaxis or anti-coagulation therapies (n = 15)
- Women who were preoperatively diagnosed with DVT or PE (n = 10)
- Women who were lost-to-follow-up (n = 25)
- Women who are deemed to be ineligible for study participation according to our judgment (n = 0).
The current study was approved by the Institutional Review Board (IRB) of our medical institution (KUCH 2019-08-027).
2.2. Patient Evaluation and Criteria
In our series, we performed a retrospective review of the medical records and thereby analyzed baseline and clinical characteristics of the patients. These include age, the type of malignancy (e.g., cervical cancer, endometrial cancer, and ovarian cancer), TNM stage at initial diagnosis, regional lymph node or distant organ metastasis, duration of disease (the time elapsing from the diagnosis of cancer to the evaluation of edema), BMI, co-morbidities (e.g., diabetes mellitus, hypertension, hyperlipidemia, atrial fibrillation, heart failure, and rheumatic disease), treatment modalities (e.g., surgery, chemotherapy, radiotherapy, and combination of more than two regimens), circumference of the lower extremity measured 10 cm above or below the upper border of the patella, and D-dimer levels.
The anatomical location of the DVT was divided into proximal (the inferior vena cava [IVC], iliac, femoral, and popliteal veins) or distal (the anterior, posterior tibial, peroneal, and muscular veins).
Depending on the presence of DVT on CT venography, the patients were divided into two groups: the DVT group and the non-DVT group (Figure 1). Then, baseline and clinical characteristics of the patients were compared between the two groups.
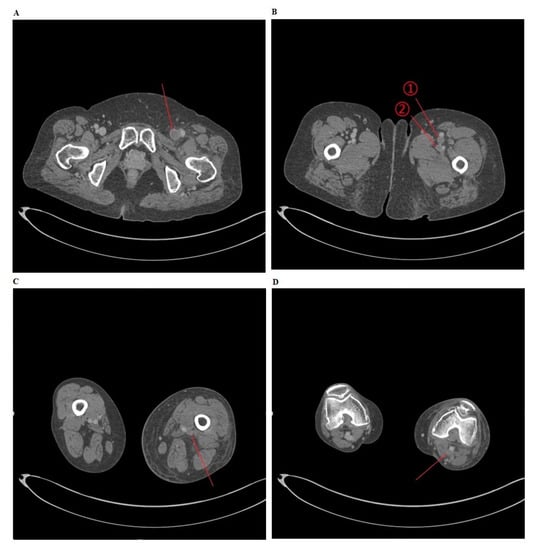
Figure 1.
Deep vein thrombosis (DVT) on computed tomography (CT) venography.
To consider the possibility of lower extremity edema arising from DVT, a CT venography was performed for (A) the common femoral vein, (B) ① the superficial femoral vein ② the deep femoral vein, (C) the popliteal vein, and (D) the popliteal vein.
2.3. Statistical Analysis
All data was expressed as mean ± standard deviation. Statistical analysis was done using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA). To compare the baseline and clinical characteristics of the patients between the two groups, we performed a Mann-Whitney U-test. In addition, we also performed the χ2-test to identify the correlations between categorical variables and the incidence of DVT. Furthermore, univariate and multiple logistic regression analyses were also performed to identify significant correlations between risk factors of developing DVT and adjusted or unadjusted variables. Their results were expressed as odds ratios (ORs) with 95% confidence intervals (95% CIs). A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristics of the Patients
A total of 315 patients met inclusion/exclusion criteria. These include 80 patients with DVT and 235 without DVT. They were therefore divided into two groups: the DVT group (n = 80) and the non-DVT group (n = 235). The study flow chart is shown in Figure 2. In addition, baseline characteristics of the patients are represented in Table 1 and Table 2.
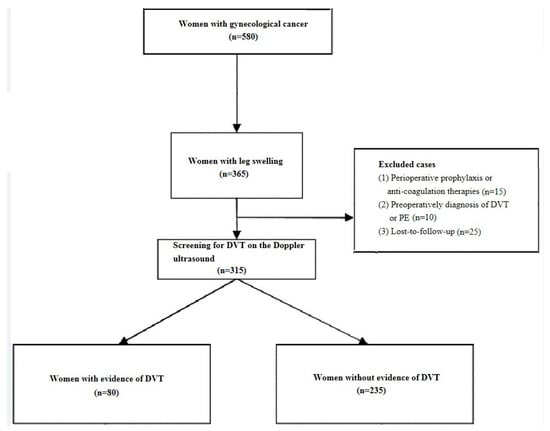
Figure 2.
The study flow chart. Note: DVT, deep vein thrombosis; PE, pulmonary embolism.

Table 1.
Baseline characteristics of the patients.

Table 2.
Patient characteristics in each group.
3.2. Location of the DVT
For the location of the DVT, there were 25 cases (31.3%) in the iliac vein, 20 cases (25.0%) in the femoral vein, 15 cases (18.8%) in the popliteal vein, 15 cases (18.8%) in the peroneal vein, F and 5 cases (6.3%) in the IVC.
3.3. Predictive Factors of the DVT
As shown in Table 3, the incidence of DVT had no significant correlation with the treatment modalities and co-morbidities. In addition, there were no significant differences in the circumference of the lower extremity, regional lymph node involvement, and D-dimer levels between the two groups. But distal organ metastasis, advanced-stage cancer, lymphadectomy, operation time ≥3 hours, and amount of intraoperative blood loss ≥1500 mL were significantly more prevalent in the DVT group as compared to the non-DVT group (p < 0.05).

Table 3.
Predictive factors of deep vein thrombosis.
3.4. Results of Univariate and Multivariate Analyses of Possible Predictive Factors
We performed both univariate and multivariate analyses of predictive factors, such as BMI, distant organ metastasis, advanced stage, lymphadectomy, operation time ≥3 hours, and amount of intraoperative blood loss ≥1500 mL, showing a significant difference between the DVT group and the non-DVT group. This showed that distant organ metastasis, advanced stage, lymphadectomy, and amount of intraoperative blood loss ≥1500 mL were found to be significant predictive factors (Table 4).

Table 4.
Results of univariate and multivariate analyses of possible predictive factors.
4. Discussion
VTE, presenting as DVT and PE, is a major cause of morbidity and mortality [30]. It is known that patients with malignancy are at a 6-fold greater risk of developing VTE and those with gynecologic cancer are at the greatest risk of developing VTE among all malignancies [31].
VTE is the second cause of mortality in patients with gynecologic cancer, and it has been reported that the risk of DVT and the incidence of PE were estimated at 17–40% and 1–26% in women undergoing gynecologic surgery [9,32].
It has been suggested that various risk factors are involved in cancer-related VTE [33]. Such risk factors are evaluated based on two well-known instruments. The Caprini risk assessment model (RAM), originally developed for surgical patients, aims to promote the derivation of risk factors of developing VTE. To do this, individual risk factors are summed and patients are divided into four categories accordingly: “low risk” (0–1 points), “moderate risk” (2 points), “high risk” (3–4 points), and “highest risk” (≥5 points) [34]. Moreover, Rogers Jr. et al. developed a predictive model of VTE through a logistic regression analysis of data obtained from the Patient Safety in Surgery (PSS) study, thus termed as the Rogers RAM. To do this, individual risk factors are summed and patients are divided into three categories accordingly: “low risk” (1–6 points), “moderate risk” (7–10 points), and “high risk” (>10 points) [35]. Blom et al. conducted a prospective study to estimate the incidence of VTE in 66,329 patients with cancer, thus reporting that it was 12.4/1000 patients within 6 months since the diagnosis of cancer and it was relatively higher when compared to normal healthy individuals [36]. Both thrombin formation arising from the pro-coagulant effects of tumor cells and venous compression leading to stasis are inevitable in patients with cancer [37,38]. Furthermore, cancer-treatment related factors, such as prolonged treatment period, immobilization, radiotherapy, and chemotherapy, may also raise the risk of thromboembolic events in patients with cancer [39].
The deep veins distributed in the lower extremities are classified into two categories: the proximal (the IVC, iliac, femoral, and popliteal veins) and the distal territory (the anterior, posterior tibial, peroneal, and muscular veins). Of note, proximal DVT is more frequently associated with PE and recurrence when compared to the distal one [40,41]. Our results showed that DVT occurred most frequently (31.3%) in the iliac vein. In association with this, Kahn et al. showed that thrombus was present in the common femoral vein and/or iliac vein in 25% of patients with symptomatic DVT of the lower extremities [42].
Our results showed that advanced-stage cancer was significantly more prevalent in the DVT group as compared to the non-DVT one. Presumably, this might be due to an increased immobility of the patients with advanced-stage cancer, which is in agreement with a previous report showing that patients with advanced-stage cancer are at increased risks of developing DVT [43].
In the current study, distant organ metastasis was significantly more prevalent in the DVT group as compared to the non-DVT one, as previously described [44]. But there was no significant difference in the incidence of regional lymph node metastasis between the two groups, which is not in agreement with a previous published study [45]. Involvement of metastases in the risk of DVT has been well described in the literature. Higher risks of DVT in association with distant or regional lymph node metastases may be explained by the process of metastatic dissemination. That is, there is a close association between the presence of metastasis and increased hypercoagulability because the hemostatic system might play an important role in metastatic capacity in malignancies. In more detail, intrusion of tumor cells into the blood or lymphatic fluid is an essential factor for distant metastasis. Distant metastasis is therefore followed by the interaction between tumor cells and the hemostatic system. This leads to the speculation that hypercoagulabulability may already exist in patients with regional spread of cancer [44,45].
We found that the presence of DVT had no significant correlation with the types of treatment modalities. This is in agreement with previous reports describing a lack of statistical significance in it [46,47].
In our series, despite a lack of statistical significance, the duration of cancer was shorter in the DVT group as compared to the non-DVT group. But this is not in agreement with previous published studies showing that risk factors of developing VTE include anatomical sites, biological characteristics, stage, and duration of cancer [48,49].
Lymphadenectomy is commonly used not only to assess lymph node status and the stage of gynecologic malignancies, but also to treat patients with gynecologic cancer [50,51]. But it is often accompanied by complications, such as hemorrhage, hematoma, and lymphocele [52,53]. Of these, lymphocele is one of the most common postoperative complications in that it leads to the occurrence of VTE by venous compression [54,55]. According to a review of the literature, VTE occurred after lymphadectomy at an estimated incidence of 0.8–25% [56,57,58]. This is also seen in our results. We found that lymphadectomy had a significant correlation with the occurrence of VTE.
A substantial amount of blood loss increases the risk of transfusion during perioperative period, and transfusion has been shown to be associated with the postoperative occurrence of VTE in gynecologic surgeries [59,60]. Our results also showed that the amount of intraoperative blood loss was a significant predictive factor of DVT. To summarize, distant organ metastasis, advanced stage, lymphadectomy, and amount of intraoperative blood loss had a positive predictive value for the occurrence of DVT in gynecologic cancer survivors presenting LEE. But our results cannot be generalized, because we retrospectively analyzed a small series of patients at a single, secondary medical institution. The possibility of selection bias could not therefore be completely ruled out. Further large-scale, multi-center studies are therefore warranted to establish our results.
5. Conclusions
It is such a potentially life-threatening condition that more than 90% of total patients with DVT of the lower extremities develop PE [61]. Clinicians should consider the possibility of LEE arising from DVT in these cases, which should be confirmed with imaging modalities such as CT venography.
Author Contributions
Conceptualization, T.H.K.; Data curation, T.H.K.; Formal analysis, S.P.; Funding acquisition, H.-J.K.; Investigation, J.K. and D.K.K.; Project administration, H.-J.K., D.K.K. and T.H.K.; Writing-original draft, J.K.; Writing-review & editing, T.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A3B04031900).
Acknowledgments
We greatly appreciate Seihee Yoon for a critical reading of this manuscript.
Conflicts of Interest
The authors report no conflicts of interest related to this work.
References
- Noble, S.; Pasi, J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br. J. Cancer 2010, 102, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, S.; Ono, R.; Fukuda, K.; Sakayori, M.; Awano, N.; Kondo, K. Trousseau’s syndrome: Cancer-associated thrombosis. Jpn. J. Clin. Oncol. 2016, 46, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Barber, E.L.; Clarke-Pearson, D.L. Prevention of venous thromboembolism in gynecologic oncology surgery. Gynecol. Oncol. 2017, 144, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.C.; Tham, K.F.; Razvi, K.; Oei, P.L.; Lim, F.K.; Roy, A.C.; Prasad, R.N. Hemostatic and fibrinolytic status in patients with ovarian cancer and benign ovarian cysts: Could D-dimer and antithrombin III levels be included as prognostic markers for survival outcome? Clin. Appl. Thromb. Hemost. 2001, 7, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, W.; Ali, Z.; Amjad, W.; Alirhayim, Z.; Farooq, H.; Qadir, S.; Khalid, F.; Al-Mallah, M.H. Venous Thromboembolism in Cancer: An Update of Treatment and Prevention in the Era of Newer Anticoagulants. Front. Cardiovasc. Med. 2016, 3, 24. [Google Scholar] [CrossRef]
- Elyamany, G.; Alzahrani, A.M.; Bukhary, E. Cancer-associated thrombosis: An overview. Clin. Med. Insights Oncol. 2014, 8, 129–137. [Google Scholar] [CrossRef]
- Cohen, A.T.; Nandini, B.; Wills, J.O.; Ota, S. VTE prophylaxis for the medical patient: Where do we stand?—A focus on cancer patients. Thromb. Res. 2010, 125, S21–S29. [Google Scholar] [CrossRef]
- Gunderson, C.C.; Thomas, E.D.; Slaughter, K.N.; Farrell, R.; Ding, K.; Farris, R.E.; Lauer, J.K.; Perry, L.J.; McMeekin, D.S.; Moore, K.N. The survival detriment of venous thromboembolism with epithelial ovarian cancer. Gynecol. Oncol. 2014, 134, 73–77. [Google Scholar] [CrossRef]
- Geerts, W.H.; Pineo, G.F.; Heit, J.A.; Bergqvist, D.; Lassen, M.R.; Colwell, C.W.; Ray, J.G. Prevention of venous thromboembolism: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004, 126, 338S–400S. [Google Scholar] [CrossRef]
- Suzuki, N.; Yoshioka, N.; Ohara, T.; Yokomichi, N.; Nako, T.; Yahagi, N.; Igarashi, S.; Kobayashi, Y.; Yoshimatsu, M.; Takizawa, K.; et al. Risk factors for perioperative venous thromboembolism: A retrospective study in Japanese women with gynecologic diseases. Thromb. J. 2010, 8, 17. [Google Scholar] [CrossRef]
- Nick, A.M.; Schmeler, K.M.; Frumovitz, M.M.; Soliman, P.T.; Spannuth, W.A.; Burzawa, J.K.; Coleman, R.L.; Wei, C.; dos Reis, R.; Ramirez, P.T. Risk of thromboembolic disease in patients undergoing laparoscopic gynecologic surgery. Obstet. Gynecol. 2010, 116, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.R.; Frick, A.C.; Paraiso, M.F.; Barber, M.D. Risk of deep venous thrombosis and pulmonary embolism in urogynecologic surgical patients. Am. J. Obstet. Gynecol. 2010, 203, 510.e1–510.e4. [Google Scholar] [CrossRef] [PubMed]
- Peedicayil, A.; Weaver, A.; Li, X.; Carey, E.; Cliby, W.; Mariani, A. Incidence and timing of venous thromboembolism after surgery for gynecological cancer. Gynecol. Oncol. 2011, 121, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Li, Z.; Zhai, Z.; Liu, C.; Wang, S.; Guo, S.; Zhang, Z. Predicting of Venous Thromboembolism for Patients Undergoing Gynecological Surgery. Medicine 2015, 94, e1653. [Google Scholar] [CrossRef]
- Rockson, S.G. Lymphedema. Am. J. Med. 2001, 110, 288–295. [Google Scholar] [CrossRef]
- Borman, P. Lymphedema diagnosis, treatment, and follow-up from the view point of physical medicine and rehabilitation specialists. Turk. J. Phys. Med. Rehabil. 2018, 64, 179–197. [Google Scholar] [CrossRef]
- Levine, M.N. Managing thromboembolic disease in the cancer patient: Efficacy and safety of antithrombotic treatment options in patients with cancer. Cancer Treat. Rev. 2002, 28, 145–149. [Google Scholar] [CrossRef]
- Zacharski, L.R.; Ornstein, D.L.; Mamourian, A.C. Low-molecular-weight heparin and cancer. Semin. Thromb. Hemost. 2000, 26, 69–77. [Google Scholar] [CrossRef]
- Bates, S.M.; Jaeschke, R.; Stevens, S.M.; Goodacre, S.; Wells, P.S.; Stevenson, M.D.; Kearon, C.; Schunemann, H.J.; Crowther, M.; Pauker, S.G.; et al. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e351S–e418S. [Google Scholar] [CrossRef]
- Geersing, G.J.; Zuithoff, N.P.; Kearon, C.; Anderson, D.R.; Ten, C.A.J.; Elf, J.L.; Bates, S.M.; Hoes, A.W.; Kraaijenhagen, R.A.; Oudega, R.; et al. Exclusion of deep vein thrombosis using the Wells rule in clinically important subgroups: Individual patient data meta-analysis. BMJ 2014, 348, g1340. [Google Scholar] [CrossRef]
- Tovey, C.; Wyatt, S. Diagnosis, investigation, and management of deep vein thrombosis. BMJ 2003, 326, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Needleman, L.; Cronan, J.J.; Lilly, M.P.; Merli, G.J.; Adhikari, S.; Hertzberg, B.S.; DeJong, M.R.; Streiff, M.B.; Meissner, M.H. Ultrasound for Lower Extremity Deep Venous Thrombosis: Multidisciplinary Recommendations From the Society of Radiologists in Ultrasound Consensus Conference. Circulation 2018, 137, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Committee on Practice Bulletins—Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 84: Prevention of deep vein thrombosis and pulmonary embolism. Obstet. Gynecol. 2007, 110, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.; Arya, R.; Beyer-Westendorf, J.; Douketis, J.; Hull, R.; Elalamy, I.; Imberti, D.; Zhai, Z. Evaluation of unmet clinical needs in prophylaxis and treatment of venous thromboembolism in at-risk patient groups: Pregnancy, elderly and obese patients. Thromb. J. 2019, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Kotaska, A. Venous thromboembolism prophylaxis may cause more harm than benefit: An evidence-based analysis of Canadian and international guidelines. Thromb. J. 2018, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Le, S.S.; McGee, M.; Emed, J.D. Knowledge of venous thromboembolism (VTE) prevention among hospitalized patients. J. Vasc. Nurs. 2008, 26, 109–117. [Google Scholar]
- Sandadi, S.; Lee, S.; Walter, A.; Gardner, G.J.; Abu-Rustum, N.R.; Sonoda, Y.; Brown, C.L.; Jewell, E.; Parameswaran, R.; Barakat, R.R.; et al. Incidence of venous thromboembolism after minimally invasive surgery in patients with newly diagnosed endometrial cancer. Obstet. Gynecol. 2012, 120, 1077–1083. [Google Scholar] [CrossRef]
- Kumar, S.; Al-Wahab, Z.; Sarangi, S.; Woelk, J.; Morris, R.; Munkarah, A.; Dowdy, S.C.; Mariani, A.; Cliby, W. Risk of postoperative venous thromboembolism after minimally invasive surgery for endometrial and cervical cancer is low: A multi-institutional study. Gynecol. Oncol. 2013, 130, 207–212. [Google Scholar] [CrossRef]
- Marin-Barrera, L.; Muñoz-Martin, A.J.; Rios-Herranz, E.; Garcia-Escobar, I.; Beato, C.; Font, C.; Oncala-Sibajas, E.; Revuelta-Rodriguez, A.; Areses, M.C.; Rivas-Jimenez, V.; et al. A Case-Control Analysis of the Impact of Venous Thromboembolic Disease on Quality of Life of Patients with Cancer: Quality of Life in Cancer (Qca) Study. Cancers 2020, 12, 75. [Google Scholar] [CrossRef]
- Kesieme, E.; Kesieme, C.; Jebbin, N.; Irekpita, E.; Dongo, A. Deep vein thrombosis: A clinical review. J. Blood Med. 2011, 2, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Khalil, J.; Bensaid, B.; Elkacemi, H.; Afif, M.; Bensaid, Y.; Kebdani, T.; Benjaafar, N. Venous thromboembolism in cancer patients: An underestimated major health problem. World J. Surg. Oncol. 2015, 13, 204. [Google Scholar] [CrossRef]
- Abdol Razak, N.B.; Jones, G.; Bhandari, M.; Berndt, M.C.; Metharom, P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; McCrae, K.R. Risk stratification strategies for cancer-associated thrombosis: An update. Thromb. Res. 2014, 133, S35–S38. [Google Scholar] [CrossRef]
- Caprini, J.A. Thrombosis risk assessment as a guide to quality patient care. Dis. Mon. 2005, 51, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.J.; Kilaru, R.K.; Hosokawa, P.; Henderson, W.G.; Zinner, M.J.; Khuri, S.F. Multivariable predictors of postoperative venous thromboembolic events after general and vascular surgery: Results from the patient safety in surgery study. J. Am. Coll. Surg. 2007, 204, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.W.; Vanderschoot, J.P.; Oostindiër, M.J.; Osanto, S.; Van Der Meer, F.J.M.; Rosendaal, F.R. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: Results of a record linkage study. J. Thromb. Haemost. 2006, 4, 529–535. [Google Scholar] [CrossRef]
- Prandoni, P.; Falanga, A.; Piccioli, A. Cancer and venous thromboembolism. Lancet Oncol. 2005, 6, 401–410. [Google Scholar] [CrossRef]
- Young, A.; Chapman, O.; Connor, C.; Poole, C.; Rose, P.; Kakkar, A.K. Thrombosis and cancer. Nat. Rev. Clin. Oncol. 2012, 9, 437–449. [Google Scholar] [CrossRef]
- Ailawadi, M.; Del, P.G. A comparison of thromboembolic prophylaxis in gynecologic oncology patients. Int. J. Gynecol. Cancer 2001, 11, 354–358. [Google Scholar] [CrossRef]
- Meignan, M.; Rosso, J.; Gauthier, H.; Brunengo, F.; Claudel, S.; Sagnard, L.; d’Azemar, P.; Simonneau, G.; Charbonnier, B. Systematic lung scans reveal a high frequency of silent pulmonary embolism in patients with proximal deep venous thrombosis. Arch. Intern. Med. 2000, 160, 159–164. [Google Scholar] [CrossRef]
- Douketis, J.D.; Kearon, C.; Bates, S.; Duku, E.K.; Ginsberg, J.S. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA 1998, 279, 458–462. [Google Scholar] [CrossRef]
- Kahn, S.R.; Shrier, I.; Julian, J.A.; Ducruet, T.; Arsenault, L.; Miron, M.J.; Roussin, A.; Desmarais, S.; Joyal, F.; Kassis, J.; et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann. Intern. Med. 2008, 149, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.A.; Iyengar, T.D.; Napiorkowski, B.E.; Rubin, S.C.; Mikuta, J.J. The clinical course of deep vein thrombosis in patients with gynecologic cancer. Gynecol. Oncol. 2002, 84, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Gale, A.J.; Gordon, S.G. Update on tumor cell procoagulant factors. Acta Haematol. 2001, 106, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Dickmann, B.; Ahlbrecht, J.; Ay, C.; Dunkler, D.; Thaler, J.; Scheithauer, W.; Quehenberger, P.; Zielinski, C.; Pabinger, I. Regional lymph node metastases are a strong risk factor for venous thromboembolism: Results from the Vienna Cancer and Thrombosis Study. Haematologica 2013, 98, 1309–1314. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Z.; Niu, X.M.; Li, Z.M.; Chen, Z.W.; Jian, H.; Ai, X.H.; Cheng, B.J.; Liao, M.L.; Lu, S. Clinical analysis of postoperative venous thromboembolism risk factors in lung cancer patients. J. Surg. Oncol. 2012, 106, 736–741. [Google Scholar] [CrossRef]
- Dutia, M.; White, R.H.; Wun, T. Risk assessment models for cancer-associated venous thromboembolism. Cancer 2012, 118, 3468–3476. [Google Scholar] [CrossRef]
- Carrier, M.; Le, G.G.; Wells, P.S.; Fergusson, D.; Ramsay, T.; Rodger, M.A. Systematic review: The Trousseau syndrome revisited: Should we screen extensively for cancer in patients with venous thromboembolism? Ann. Intern. Med. 2008, 149, 323–333. [Google Scholar] [CrossRef]
- Khorana, A.A. Targeted prophylaxis in cancer: The evidence accumulates. Intern. Emerg. Med. 2013, 8, 187–189. [Google Scholar] [CrossRef]
- Abdelazim, I.A.; Abu-Faza, M.; Zhurabekova, G.; Shikanova, S.; Karimova, B.; Sarsembayev, M.; Starchenko, T.; Mukhambetalyeva, G. Sentinel Lymph Nodes in Endometrial Cancer Update 2018. Gynecol. Minim. Invasive Ther. 2019, 8, 94–100. [Google Scholar] [CrossRef]
- Matsuo, K.; Machida, H.; Mariani, A.; Mandelbaum, R.S.; Glaser, G.E.; Gostout, B.S.; Roman, L.D.; Wright, J.D. Adequate pelvic lymphadenectomy and survival of women with early-stage epithelial ovarian cancer. J. Gynecol. Oncol. 2018, 29, e69. [Google Scholar] [CrossRef]
- Achouri, A.; Huchon, C.; Bats, A.S.; Bensaid, C.; Nos, C.; Lécuru, F. Complications of lymphadenectomy for gynecologic cancer. Eur. J. Surg. Oncol. 2013, 39, 81–86. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.W.; Kim, S.H.; Kim, Y.T.; Kim, J.H. An analysis of the risk factors and management of lymphocele after pelvic lymphadenectomy in patients with gynecologic malignancies. Cancer Res. Treat. 2004, 36, 377–383. [Google Scholar] [CrossRef]
- Neagoe, O.C.; Ionica, M.; Mazilu, O. The role of pelvic lymphocele in the development of early postoperative complications. Medicine 2018, 97, e12353. [Google Scholar] [CrossRef]
- Tsuda, N.; Ushijima, K.; Kawano, K.; Takemoto, S.; Nishio, S.; Sonoda, G.; Kamura, T. Prevention of lymphocele development in gynecologic cancers by the electrothermal bipolar vessel sealing device. J. Gynecol. Oncol. 2014, 25, 229–235. [Google Scholar] [CrossRef]
- Graul, A.; Latif, N.; Zhang, X.; Dean, L.T.; Morgan, M.; Giuntoli, R.; Burger, R.; Kim, S.; Ko, E. Incidence of Venous Thromboembolism by Type of Gynecologic Malignancy and Surgical Modality in the National Surgical Quality Improvement Program. Int. J. Gynecol. Cancer 2017, 27, 581–587. [Google Scholar] [CrossRef]
- Cohen, A.; Lim, C.S.; Davies, A.H. Venous Thromboembolism in Gynecological Malignancy. Int. J. Gynecol. Cancer 2017, 27, 1970–1978. [Google Scholar] [CrossRef]
- Frost, J.A.; Webster, K.E.; Bryant, A.; Morrison, J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst. Rev. 2015, CD007585. [Google Scholar] [CrossRef]
- Thurn, L.; Wikman, A.; Lindqvist, P.G. Postpartum blood transfusion and hemorrhage as independent risk factors for venous thromboembolism. Thromb. Res. 2018, 165, 54–60. [Google Scholar] [CrossRef]
- Richards, T.; Musallam, K.M.; Nassif, J.; Ghazeeri, G.; Seoud, M.; Gurusamy, K.S.; Jamali, F.R. Impact of Preoperative Anaemia and Blood Transfusion on Postoperative Outcomes in Gynaecological Surgery. PLoS ONE 2015, 10, e0130861. [Google Scholar] [CrossRef]
- Lee, J.S.; Moon, T.; Kim, T.H.; Kim, S.Y.; Choi, J.Y.; Lee, K.B.; Kwon, Y.J.; Song, S.H.; Kim, S.H.; Kim, H.O.; et al. Deep Vein Thrombosis in Patients with Pulmonary Embolism: Prevalence, Clinical Significance and Outcome. Vasc. Spec. Int. 2016, 32, 166–174. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).