Association between Brachial-Ankle Pulse Wave Velocity and Microalbuminuria and to Predict the Risk for the Development of Microalbuminuria Using Brachial-Ankle Pulse Wave Velocity Measurement in Type 2 Diabetes Mellitus Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Measurement of Pulse Wave Velocity
2.3. Ethics
2.4. Physical and Laboratory Measurements
2.5. Statistical Analysis
3. Results
Baseline and Clinical Characteristics of the Study Subjects
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef]
- World Health Organization. Diabetes: Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 9 September 2019).
- Juutilainen, A.; Lehto, S.; Ronnemaa, T.; Pyorala, K.; Laakso, M. Type 2 diabetes as a “coronary heart disease equivalent”: An 18-year prospective population-based study in Finnish subjects. Diabetes Care 2005, 28, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Erlinger, T.P. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004, 110, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Mogensen, C.E. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N. Engl. J. Med. 1984, 310, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, D.P. The link between microalbuminuria, endothelial dysfunction and cardiovascular disease in diabetes. Cardiovasc. J. S. Afr. 2002, 13, 194–199. [Google Scholar] [PubMed]
- Mattock, M.B.; Morrish, N.J.; Viberti, G.; Keen, H.; Fitzgerald, A.P.; Jackson, G. Prospective study of microalbuminuria as predictor of mortality in NIDDM. Diabetes 1992, 41, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Arnlov, J.; Evans, J.C.; Meigs, J.B.; Wang, T.J.; Fox, C.S.; Levy, D.; Benjamin, E.J.; D’Agostino, R.B.; Vasan, R.S. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: The Framingham Heart Study. Circulation 2005, 112, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, A.H.; Ahmed, S.; El Nahas, M. Microalbuminuria: Marker or maker of cardiovascular disease. Nephron Exp. Nephrol. 2011, 119, 10. [Google Scholar] [CrossRef] [PubMed]
- Dabla, P.K. Renal function in diabetic nephropathy. World J. Diabetes 2010, 1, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Nah, D.Y.; Lee, C.G.; Bae, J.H.; Chung, J.W.; Rhee, M.Y.; Kim, J.H.; Kim, Y.S.; Kim, Y.K.; Lee, M.M. Subclinical renal insufficiency range of estimated glomerular filtration rate and microalbuminuria are independently associated with increased arterial stiffness in never treated hypertensives. Korean Circ. J. 2013, 43, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chen, M.F.; Lin, L.Y.; Liau, C.S.; Lee, Y.T.; Su, T.C. Insulin resistance is the major determinant for microalbuminuria in severe hypertriglyceridemia: Implication for high-risk stratification. Intern. Med. 2008, 47, 1091–1097. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wallace, T.M.; Matthews, D.R. The assessment of insulin resistance in man. Diabet. Med. 2002, 19, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Pi-Sunyer, F.X.; Li, C.I.; Davidson, L.E.; Li, T.C.; Chen, W.; Lin, C.C.; Huang, C.Y.; Lin, W.Y. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010, 211, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, H.; Yamashina, A.; Arai, T.; Hirose, K.; Koji, Y.; Chikamori, T.; Hori, S.; Yamamoto, Y.; Doba, N.; Hinohara, S. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—A survey of 12517 subjects. Atherosclerosis 2003, 166, 303–309. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Li, Y.; Sheng, C.S.; Huang, Q.F.; Wang, J.G. Quantification of the Interrelationship between Brachial-Ankle and Carotid-Femoral Pulse Wave Velocity in a Workplace Population. Pulse 2016, 3, 253–262. [Google Scholar] [CrossRef]
- Miyano, I.; Nishinaga, M.; Takata, J.; Shimizu, Y.; Okumiya, K.; Matsubayashi, K.; Ozawa, T.; Sugiura, T.; Yasuda, N.; Doi, Y. Association between brachial-ankle pulse wave velocity and 3-year mortality in community-dwelling older adults. Hypertens. Res. 2010, 33, 678–682. [Google Scholar] [CrossRef]
- Suzuki, E.; Kashiwagi, A.; Nishio, Y.; Egawa, K.; Shimizu, S.; Maegawa, H.; Haneda, M.; Yasuda, H.; Morikawa, S.; Inubushi, T.; et al. Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care 2001, 24, 2107–2114. [Google Scholar] [CrossRef]
- Turin, T.C.; Kita, Y.; Rumana, N.; Takashima, N.; Kadota, A.; Matsui, K.; Sugihara, H.; Morita, Y.; Nakamura, Y.; Miura, K.; et al. Brachial-ankle pulse wave velocity predicts all-cause mortality in the general population: Findings from the Takashima study, Japan. Hypertens. Res. 2010, 33, 922–925. [Google Scholar] [CrossRef]
- Aso, K.; Miyata, M.; Kubo, T.; Hashiguchi, H.; Fukudome, M.; Fukushige, E.; Koriyama, N.; Nakazaki, M.; Minagoe, S.; Tei, C. Brachial-ankle pulse wave velocity is useful for evaluation of complications in type 2 diabetic patients. Hypertens. Res. 2003, 26, 807–813. [Google Scholar] [CrossRef]
- Chang, L.H.; Lin, H.D.; Kwok, C.F.; Won, J.G.; Chen, H.S.; Chu, C.H.; Hwu, C.M.; Kuo, C.S.; Jap, T.S.; Shih, K.C.; et al. The combination of the ankle brachial index and brachial ankle pulse wave velocity exhibits a superior association with outcomes in diabetic patients. Intern. Med. 2014, 53, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Osonoi, T.; Takahara, M.; Saitou, M.; Matsuoka, T.A.; Yamasaki, Y.; Shimomura, I. Clinical utility of brachial-ankle pulse wave velocity in the prediction of cardiovascular events in diabetic patients. Cardiovasc. Diabetol. 2014, 13, 14–128. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Yun, W.J.; Kim, H.Y.; Lee, Y.H.; Kweon, S.S.; Rhee, J.A.; Choi, J.S.; Shin, M.H. Association between albuminuria, carotid atherosclerosis, arterial stiffness, and peripheral arterial disease in Korean type 2 diabetic patients. Kidney Blood Press Res. 2010, 33, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Hashimoto, J.; Morito, R.H.; Hanazawa, T.; Aikawa, T.; Hara, A.; Shintani, Y.; Metoki, H.; Inoue, R.; Asayama, K.; et al. Association of microalbuminuria with brachial-ankle pulse wave velocity: The Ohasama study. Am. J. Hypertens. 2008, 21, 413–418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seo, J.Y.; Kim, M.K.; Choi, B.Y.; Kim, Y.M.; Cho, S.I.; Shin, J. Elevated brachial-ankle pulse wave velocity is independently associated with microalbuminuria in a rural population. J. Korean Med. Sci. 2014, 29, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Ohishi, M.; Onishi, M.; Ito, N.; Takeya, Y.; Maekawa, Y.; Rakugi, H. Cut-off value of brachial-ankle pulse wave velocity to predict cardiovascular disease in hypertensive patients: A cohort study. J. Atheroscler. Thromb. 2013, 20, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Savage, S.; Estacio, R.O.; Jeffers, B.; Schrier, R.W. Urinary albumin excretion as a predictor of diabetic retinopathy, neuropathy, and cardiovascular disease in NIDDM. Diabetes Care 1996, 19, 1243–1248. [Google Scholar] [CrossRef]
- Yamashina, A.; Tomiyama, H.; Takeda, K.; Tsuda, H.; Arai, T.; Hirose, K.; Koji, Y.; Hori, S.; Yamamoto, Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens. Res. 2002, 25, 359–364. [Google Scholar] [CrossRef]
- MedCalc Software bvba. MedCalc Software bvba; 17.9.7; MedCalc Software bvba Ostend: Acacialaan, Ostend, Belgium, 2017. [Google Scholar]
- Kim, B.J.; Lee, H.A.; Kim, N.H.; Kim, M.W.; Kim, B.S.; Kang, J.H. The association of albuminuria, arterial stiffness, and blood pressure status in nondiabetic, nonhypertensive individuals. J. Hypertens. 2011, 29, 2091–2098. [Google Scholar] [CrossRef]
- Zhao, Q.-h.; Wang, Q.; Zhuang, X.-m.; Wang, P.; Zhang, G.-h. Association between microalbuminuria and brachial-ankle pulse wave velocity in patients with risk factors of cardiovascular disease. Chin. J. Clin. 2012, 6, 570–574. [Google Scholar]
- Yokoyama, H.; Hirasawa, K.; Aoki, T.; Ishiyama, M.; Koyama, K. Brachial-ankle pulse wave velocity measured automatically by oscillometric method is elevated in diabetic patients with incipient nephropathy. Diabet Med. 2003, 20, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; Ferramosca, E.; Ratti, C. Arterial stiffness in chronic kidney disease: The usefulness of a marker of vascular damage. Int. J. Nephrol. 2011, 734832, 23. [Google Scholar] [CrossRef] [PubMed]
- Deckert, T.; Feldt-Rasmussen, B.; Borch-Johnsen, K.; Jensen, T.; Kofoed-Enevoldsen, A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989, 32, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Dinneen, S.F.; Gerstein, H.C. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch. Intern. Med. 1997, 157, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Kuritzky, L. Monitoring and managing urinary albumin excretion: Practical advice for primary care clinicians. Postgrad Med. 2009, 121, 51–60. [Google Scholar] [CrossRef] [PubMed]
- McKenna, K.; Thompson, C. Microalbuminuria: A marker to increased renal and cardiovascular risk in diabetes mellitus. Scott. Med. J. 1997, 42, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R. Microalbuminuria in type 2 diabetics: An important, overlooked cardiovascular risk factor. J. Clin. Hypertens. 2004, 6, 134–141. [Google Scholar] [CrossRef]
- Wang, D.Z.; Tang, Q.; Hua, Q. Prediction of coronary artery disease using pulse wave velocity and retinal artery lesions. Tohoku J. Exp. Med. 2011, 225, 17–22. [Google Scholar] [CrossRef][Green Version]
- Yokoyama, H.; Aoki, T.; Imahori, M.; Kuramitsu, M. Subclinical atherosclerosis is increased in type 2 diabetic patients with microalbuminuria evaluated by intima-media thickness and pulse wave velocity. Kidney Int. 2004, 66, 448–454. [Google Scholar] [CrossRef]
- Barbato, A.; D’Elia, L.; Perna, L.; Molisso, A.; Iacone, R.; Strazzullo, P.; Galletti, F. Increased Microalbuminuria Risk in Male Cigarette Smokers: Results from the “Olivetti Heart Study” after 8 Years Follow-Up. Kidney Blood Press Res. 2019, 44, 33–42. [Google Scholar] [CrossRef]
- Feodoroff, M.; Harjutsalo, V.; Forsblom, C.; Thorn, L.; Waden, J.; Tolonen, N.; Lithovius, R.; Groop, P.H. Smoking and progression of diabetic nephropathy in patients with type 1 diabetes. Acta Diabetol. 2016, 53, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.A.; Ajose, A.O.; Adedeji, A.T.; Adegoke, A.O.; Jimoh, K.A. Microalbuminuria, Other Markers of Nephropathy and Biochemical Derangementsin Type 2 Diabetes Mellitus: Relationships and Determinants. Ghana Med. J. 2017, 51, 56–63. [Google Scholar] [PubMed]
- Chatzikyrkou, C.; Menne, J.; Izzo, J.; Viberti, G.; Rabelink, T.; Ruilope, L.M.; Rump, C.; Mertens, P.R.; Haller, H. Predictors for the development of microalbuminuria and interaction with renal function. J. Hypertens. 2017, 35, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Maahs, D.M.; Wadwa, R.P.; Pyle, L.; Rewers, M.; Eckel, R.H.; Snell-Bergeon, J.K. Plasma triglycerides predict incident albuminuria and progression of coronary artery calcification in adults with type 1 diabetes: The Coronary Artery Calcification in Type 1 Diabetes Study. J. Clin. Lipidol. 2014, 8, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.N.; Baird, B.C.; Leypoldt, J.K.; Cheung, A.K.; Goldfarb-Rumyantzev, A.S. The association of lipid levels with mortality in patients on chronic peritoneal dialysis. Nephrol. Dial. Transplant. 2006, 21, 2881–2892. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, H.C.; Ahn, S.V.; Hur, N.W.; Choi, D.P.; Park, C.G.; Suh, I. Prevalence of Dyslipidemia among Korean Adults: Korea National Health and Nutrition Survey 1998–2005. Diabetes Metab J. 2012, 36, 43–55. [Google Scholar] [CrossRef]
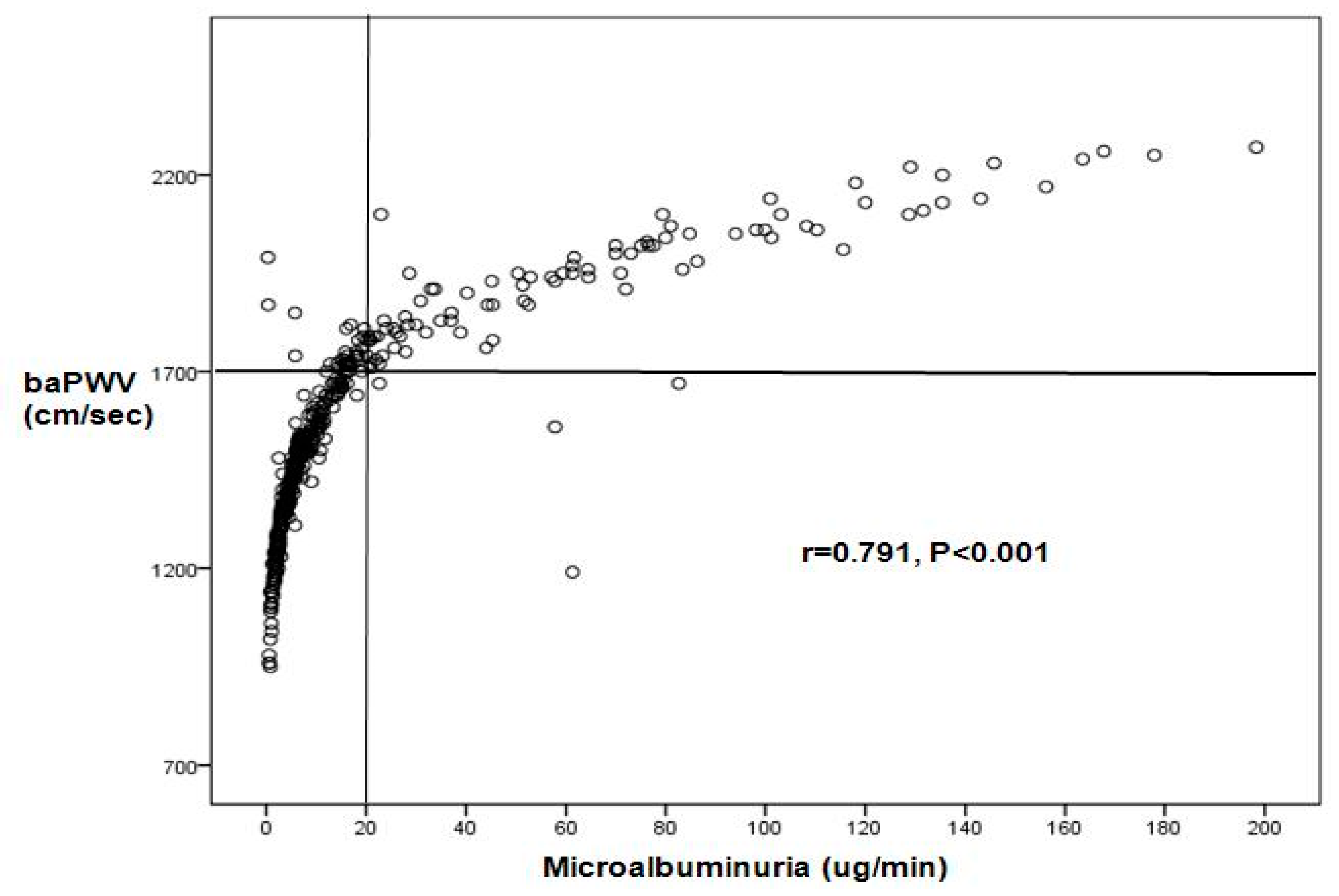
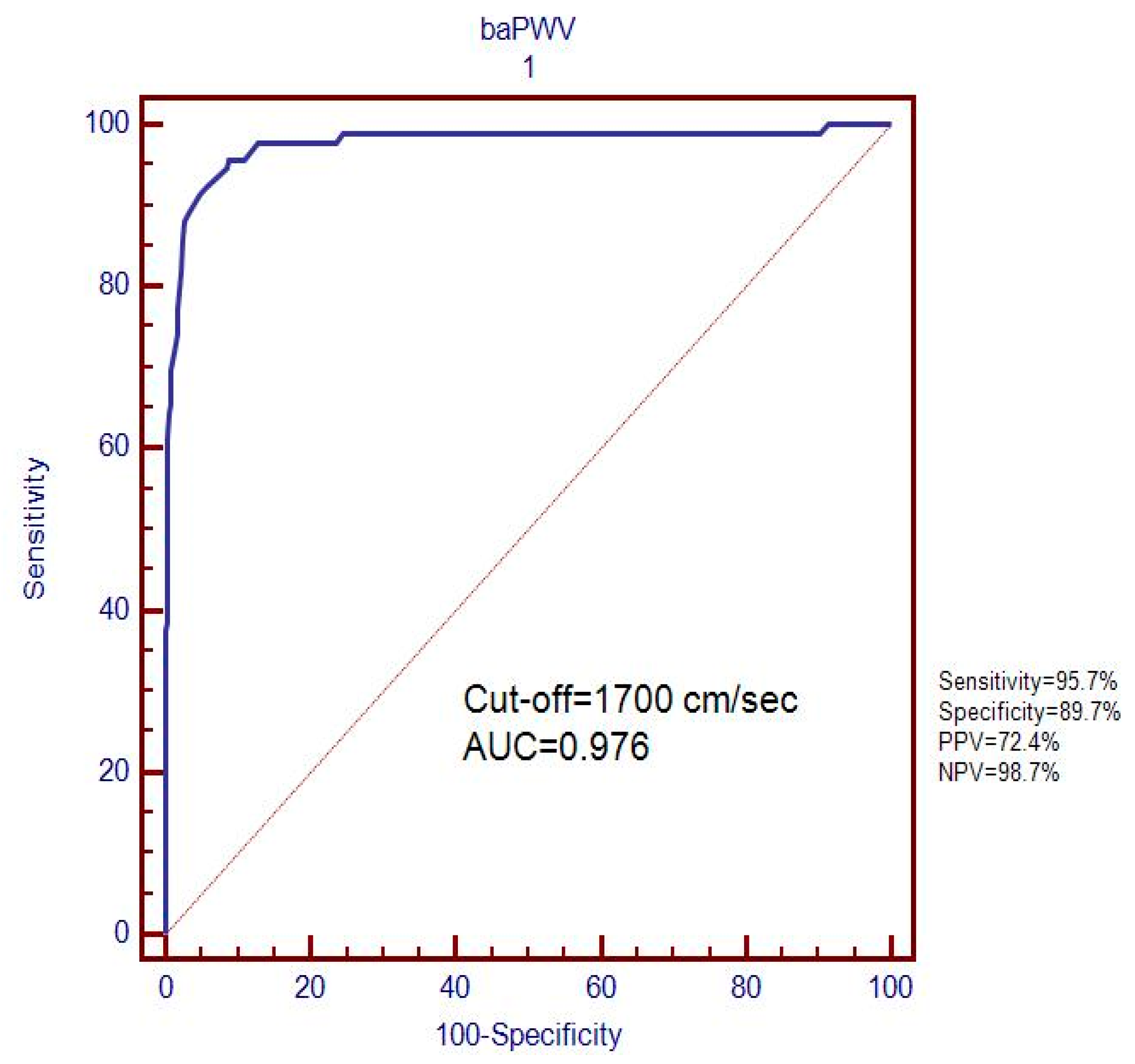
| Variables | Normoalbuminuria (n = 331) (%) | Microalbuminuria (n = 93) (%) | p-Value |
|---|---|---|---|
| Male, n (%) | 173 (52.3) | 57 (61.3) | 0.077 |
| Age, years | 58.2 ± 12.2 | 59.5 ± 13.0 | 0.369 |
| HTN, n (%) | 124 (37.5) | 37 (38.7) | 0.385 |
| H-Chol, n (%) | 141 (42.6) | 36 (38.7) | 0.291 |
| Smoking, n (%) | 47 (14.2) | 25 (26.9) | 0.004 |
| DM duration, Years | 7.5 ± 5.7 | 10.4 ± 8.8 | 0.001 |
| Height (cm) | 161 ± 9 | 163 ± 10 | 0.073 |
| Weight (kg) | 63.7 ± 11.3 | 66.6 ± 12.8 | 0.036 |
| BMI | 24.5 ± 3.9 | 24.8 ± 3.3 | 0.389 |
| SBP, mmHg | 125 ± 16 | 125 ± 18 | 0.956 |
| DBP, mmHg | 77 ± 10 | 76 ± 11 | 0.522 |
| PP, mmHg | 47 ± 10 | 48±11 | 0.53 |
| Variables | Normoalbuminuria (n = 331) (%) | Microalbuminuria (n = 93) (%) | p-Value |
|---|---|---|---|
| FBS (mg/dl) | 136 ± 54 | 138 ± 48 | 0.722 |
| HbA1c (%) | 7.7 ± 2.0 | 8.1 ± 2.1 | 0.107 |
| BUN (mg/dL) | 16.3 ± 5.1 | 19.3 ± 8.8 | 0.002 |
| Creatinine (mg/dL) | 0.99 ± 0.18 | 1.14 ± 0.72 | 0.05 |
| Total cholesterol (mg/dL) | 179 ± 41 | 184 ± 45 | 0.369 |
| Triglyceride (mg/dL) | 160 ± 113 | 158 ± 78 | 0.864 |
| HDL-cholesterol (mg/dL) | 46.3 ± 13.1 | 45.4 ± 12.7 | 0.551 |
| LDL-cholesterol (mg/dL) | 103 ± 33 | 109 ± 40 | 0.138 |
| Uric Acid | 4.9 ± 1.4 | 5.2 ± 1.8 | 0.154 |
| Microalbumin (μg/min) | 6.5 ± 4.8 | 66.9 ± 43.0 | 0.001 |
| baPWV (cm/sec) | 1459.15 ± 200.44 | 1981.74 ± 171.47 | 0.001 |
| Variable | Regression Coefficient | SE | AOR | 95% CI | p-Value |
|---|---|---|---|---|---|
| baPWV * | 2.389 | 0.449 | 10.899 | 4.518–26.292 | 0.0001 |
| Smoking | |||||
| No | - | - | Ref [1] | - | - |
| Yes | 1.747 | 0.873 | 5.736 | 1.036–31.755 | 0.045 |
| Creatinine (mg/dl) * | 1.909 | 0.984 | 6.745 | 0.980-46.432 | 0.052 |
| LDL-cholesterol (mg/dl) * | 0.017 | 0.008 | 1.017 | 1.001–1.033 | 0.035 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-K.; Acharya, D.; Nah, D.-Y.; Rhee, M.-Y.; Yoo, S.-J.; Lee, K. Association between Brachial-Ankle Pulse Wave Velocity and Microalbuminuria and to Predict the Risk for the Development of Microalbuminuria Using Brachial-Ankle Pulse Wave Velocity Measurement in Type 2 Diabetes Mellitus Patients. Healthcare 2019, 7, 111. https://doi.org/10.3390/healthcare7040111
Kim B-K, Acharya D, Nah D-Y, Rhee M-Y, Yoo S-J, Lee K. Association between Brachial-Ankle Pulse Wave Velocity and Microalbuminuria and to Predict the Risk for the Development of Microalbuminuria Using Brachial-Ankle Pulse Wave Velocity Measurement in Type 2 Diabetes Mellitus Patients. Healthcare. 2019; 7(4):111. https://doi.org/10.3390/healthcare7040111
Chicago/Turabian StyleKim, Byong-Kyu, Dilaram Acharya, Deuk-Young Nah, Moo-Yong Rhee, Seok-Ju Yoo, and Kwan Lee. 2019. "Association between Brachial-Ankle Pulse Wave Velocity and Microalbuminuria and to Predict the Risk for the Development of Microalbuminuria Using Brachial-Ankle Pulse Wave Velocity Measurement in Type 2 Diabetes Mellitus Patients" Healthcare 7, no. 4: 111. https://doi.org/10.3390/healthcare7040111
APA StyleKim, B.-K., Acharya, D., Nah, D.-Y., Rhee, M.-Y., Yoo, S.-J., & Lee, K. (2019). Association between Brachial-Ankle Pulse Wave Velocity and Microalbuminuria and to Predict the Risk for the Development of Microalbuminuria Using Brachial-Ankle Pulse Wave Velocity Measurement in Type 2 Diabetes Mellitus Patients. Healthcare, 7(4), 111. https://doi.org/10.3390/healthcare7040111






