The Interlinked Rising Epidemic of Insufficient Sleep and Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Role of Sleep Disturbances in the Development of Diabetes
3.2. Sleep Duration and Risk of Developing Diabetes
3.3. The Relationship Between Sleep Quality and Diabetes: The Role of Sleep Apnea
3.4. Diabetes and Prevalence of Sleep Disorders in Individuals Living with Diabetes
4. Discussion
4.1. Mechanism of Development of Diabetes in Sleep Disorder
4.2. Aspects of Insufficient Sleep (Quality and Quantity) Leading to Diabetes
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended amount of sleep for a healthy adult: A joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [PubMed]
- Hernandez, A.; Philippe, J.; Jornayvaz, F. Sleep and diabetes. Rev. Med. Suisse 2012, 8, 1198–1200. [Google Scholar] [PubMed]
- CDC—Data and Statistics—Sleep and Sleep Disorders. 2017. Available online: https://www.cdc.gov/sleep/data_statistics.html (accessed on 15 February 2018).
- Garvey, J.F.; Pengo, M.F.; Drakatos, P.; Kent, B.D. Epidemiological aspects of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 920–929. [Google Scholar] [PubMed]
- Zizi, F.; Jean-Louis, G.; Brown, C.D.; Ogedegbe, G.; Boutin-Foster, C.; McFarlane, S.I. Sleep duration and the risk of diabetes mellitus: Epidemiologic evidence and pathophysiologic insights. Curr. Diabetes Rep. 2010, 10, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- CDC Press Releases. CDC. 2016. Available online: https://www.cdc.gov/media/releases/2017/p0718-diabetes-report.html (accessed on 15 February 2018).
- Bakri, M.A.A.; Alharbi, M.A.T.; Alzaher, A.A.M.; Alzhrani, A.M.A.; Alkaf, W.A.; Alkaf, M.A.; Binnwejim, M.S.; Altemani, A.F.; Rebh, Z.Y.A.; Almusallam, L.A. Short night sleeping is associated with higher risk of diabetes in older adults. Int. J. Community Med. Public Health 2018, 5, 2164–2169. [Google Scholar] [CrossRef]
- Knutson, K.L.; Van Cauter, E. Associations between sleep loss and increased risk of obesity and diabetes. Ann. N. Y. Acad. Sci. 2008, 1129, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Ma, H.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Mallon, L.; Broman, J.-E.; Hetta, J. High incidence of diabetes in men with sleep complaints or short sleep duration: A 12-year follow-up study of a middle-aged population. Diabetes Care 2005, 28, 2762–2767. [Google Scholar] [CrossRef] [PubMed]
- Gangwisch, J.E.; Heymsfield, S.B.; Boden-Albala, B.; Buijs, R.M.; Kreier, F.; Pickering, T.G.; Rundle, A.G.; Zammit, G.K.; Malaspina, D. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 2007, 30, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Mokhlesi, B. Obstructive sleep apnea and diabetes: A state of the art review. Chest 2017, 152, 1070–1086. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Long, Y.S.; Gozal, D.; Epstain, P.N. Beta-cell death and proliferation after intermittent hypoxia: Role of oxidative stress. Free Radic. Biol. Med. 2009, 46, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Khalyfa, A.; Wang, Y.; Carreras, A.; Hakim, F.; Neel, B.A.; Brady, M.J.; Qiao, Z.; Hirotsu, C.; Gozal, D. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int. J. Obes. 2014, 38, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, H.; Peltonen, M.; Partinen, M.; Seppä, J.; Saaristo, T.; Korpi-Hyövälti, E.; Oksa, H.; Puolijoki, H.; Saltevo, J.; Vanhala, M.; et al. Sleep duration is associated with an increased risk for the prevalence of type 2 diabetes in middle-aged women–The FIN-D2D survey. Sleep Med. 2008, 9, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Araujo, A.B.; McKinlay, J.B. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006, 29, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Tsai, Y.H.; Yeh, M.C. Associations between sleep duration and type 2 diabetes in Taiwanese adults: A population-based study. J. Formos. Med. Assoc. 2016, 115, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Wu, J.S.; Yang, Y.C.; Shih, C.C.; Wang, R.H.; Lu, F.H.; Chang, C.J. Sleep duration is a potential risk factor for newly diagnosed type 2 diabetes mellitus. Metabolism 2011, 60, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Beihl, D.A.; Liese, A.D.; Haffner, S.M. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann. Epidemiol. 2009, 19, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M.; Newman, A.B.; Resnick, H.E.; Redline, S.; Baldwin, C.M.; Nieto, F.J. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch. Intern. Med. 2005, 165, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; D’elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Facco, F.L.; Kramer, J.; Ho, K.H.; Zee, P.C.; Grobman, W.A. Sleep disturbances in pregnancy. Obs. Gynecol. 2010, 115, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Larcher, S.; Benhamou, P.Y.; Pépin, J.L.; Borel, A.L. Sleep habits and diabetes. Diabetes Metab. 2015, 41, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Seixas, A.A.; Gyamfi, L.; Newsome, V.; Ranger-Murdock, G.; Butler, M.; Rosenthal, D.M.; Zizi, F.; Youssef, I.; McFarlane, S.I.; Jean-Louis, G. Moderating effects of sleep duration on diabetes risk among cancer survivors: Analysis of the National Health Interview Survey in the USA. Cancer Manag. Res. 2018, 10, 4575–4580. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Murase, K.; Tabara, Y.; Gozal, D.; Smith, D.; Minami, T.; Tachikawa, R.; Tanizawa, K.; Oga, T.; Nagashima, S.; et al. Impact of sleep characteristics and obesity on diabetes and hypertension across genders and menopausal status: The Nagahama study. Sleep 2018, 41. [Google Scholar] [CrossRef] [PubMed]
- Facco, F.L.; Grobman, W.A.; Reid, K.J.; Parker, C.B.; Hunter, S.M.; Silver, R.M.; Basner, R.C.; Saade, G.R.; Pien, G.W.; Manchanda, S.; et al. Objectively measured short sleep duration and later sleep midpoint in pregnancy are associated with a higher risk of gestational diabetes. Am. J. Obstet. Gynecol. 2017, 217, 447.e1–447.e13. [Google Scholar] [CrossRef] [PubMed]
- Kachi, Y.; Ohwaki, K.; Yano, E. Association of sleep duration with untreated diabetes in Japanese men. Sleep Med. 2012, 13, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Enquobahrie, D.; Frederick, I.O.; Abetew, D.; Williams, M.A. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: A pilot study. BMC Womens Health 2010, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Rafalson, L.; Donahue, R.P.; Stranges, S.; Lamonte, M.J.; Dmochowski, J.; Dorn, J.; Trevisan, M. Short sleep duration is associated with the development of impaired fasting glucose: The Western New York Health Study. Ann. Epidemiol. 2010, 20, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Song, Y.; Hollenbeck, A.; Blair, A.; Schatzkin, A.; Chen, H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care 2010, 33, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.H.; Muldoon, M.F.; Jennings, J.R.; Buysse, D.J.; Flory, J.D.; Manuck, S.B. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep 2008, 31, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Lee, J.S.; Park, H.S.; Baik, S.H.; Choi, D.S.; Kim, S.M. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int. J. Obes. 2008, 32, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Hayashino, Y.; Fukuhara, S.; Suzukamo, Y.; Okamura, T.; Tanaka, T.; Ueshima, H.; HIPOP-OHP Research Group. Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in Japan: The High-risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) Study. BMC Public Health 2007, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Despres, J.P.; Bouchard, C.; Tremblay, A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia 2007, 50, 2298–2304. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Ryden, A.M.; Mander, B.A.; Van Cauter, E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch. Intern. Med. 2006, 166, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Heier, M.; Loewel, H. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia 2005, 48, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Bjorkelund, C.; Bondyr-Carlsson, D.; Lapidus, L.; Lissner, L.; Månsson, J.; Skoog, I.; Bengtsson, C. Sleep disturbances in midlife unrelated to 32-year diabetes incidence. The prospective Population Study of Women in Gothenburg. Diabetes Care 2005, 28, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Roost, M.; Engstrom, G.; Hedblad, B.; Berglund, G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care 2004, 27, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, N.; Takatsuka, N.; Shimizu, H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care 2004, 27, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Ayas, N.T.; White, D.P.; Al-Delaimy, W.K.; Manson, J.E.; Stampfer, M.J.; Speizer, F.E.; Patel, S.; Hu, F.B. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 2003, 26, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, K.J.; Austin, D.; Skatrud, J.B.; Young, T. Association of sleep apnea and type II diabetes: A population-based study. Am. J. Respir. Crit. Care Med. 2005, 172, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Tasali, E.; Mokhlesi, B.; Van Cauter, E. Obstructive sleep apnea and type 2 diabetes: Interacting epidemics. Chest 2008, 133, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Trento, M.; Broglio, F.; Riganti, F.; Basile, M.; Borgo, E.; Kucich, C.; Passera, P.; Tibaldi, P.; Tomelini, M.; Cavallo, F.; et al. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008, 45, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.R.; Herdegen, J.; Fogelfeld, L.; Shott, S.; Mazzone, T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch. Intern. Med. 2005, 165, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Muraki, I.; Wada, H.; Tanigawa, T. Sleep apnea and type 2 diabetes. J. Diabetes Investig. 2018, 9, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Nagayoshi, M.; Punjabi, N.M.; Selvin, E.; Pankow, J.S.; Shahar, E.; Iso, H.; Folsom, A.R.; Lutsey, P.L. Obstructive sleep apnea and incident type 2 diabetes. Sleep 2016, 25, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Al-Delaimy, W.K.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Snoring as a risk factor for type II diabetes mellitus: A prospective study. Am. J. Epidemiol. 2002, 155, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Chattu, V.K.; Sakhamuri, S.; Kumar, R.; Spence, D.W.; BaHammam, A.; Pandi-Perumal, S.R. Insufficient sleep: Is it time to recognizeit as a major non-communicable disease? Sleep Sci. 2018, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Holmbäck, U.; Cauter, E.V. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.; Abel, S.L.; Loving, R.T.; Dailey, G.; Shadan, F.F.; Cronin, J.W.; Kripke, D.F.; Kline, L.E. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J. Clin. Sleep Med. 2008, 4, 538–543. [Google Scholar] [PubMed]
- Pallayova, M.; Donic, V.; Tomori, Z. Beneficial effects of severe sleep apnea therapy on nocturnal glucose control in persons with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2008, 81, e8–e11. [Google Scholar] [CrossRef] [PubMed]
- Pamidi, S.; Tasali, E. Obstructive sleep apnea and type 2 diabetes: Is there a link? Front. Neurol. 2012, 3, 126. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.D.; Sanders, M.H.; Millman, R.; Zammit, G.; Borradaile, K.E.; Newman, A.B.; Wadden, T.A.; Kelley, D.; Wing, R.R.; Sunyer, F.X.; et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009, 32, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Akhter, N.; Eldeirawi, K.; Christman, J.W.; Carley, D.W.; Herdegen, J.J. Prevalence of type 2 diabetes in patients with obstructive sleep apnea in a multi-ethnic sample. J. Clin. Sleep Med. 2009, 5, 215–221. [Google Scholar] [PubMed]
- Rasche, K.; Keller, T.; Hader, C.; Leidag, M.; Prinz, C. Impact of obstructive sleep apnoea on type 2 diabetes and vice versa. Eur. Endocrinol. 2013, 9, 107. [Google Scholar] [PubMed]
- Menke, A.; Orchard, T.J.; Imperatore, G.; Bullard, K.M.; Mayer-Davis, E.; Cowie, C.C. The prevalence of type 1 diabetes in the United States. Epidemiology 2013, 24, 773–774. [Google Scholar] [CrossRef] [PubMed]
- Mondini, S.; Guilleminault, C. Abnormal breathing patterns during sleep in diabetes. Ann. Neurol. 1985, 17, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Rajan, P.; Greenberg, H. Obstructive sleep apnea as a risk factor for type 2 diabetes mellitus. Nat. Sci. Sleep 2015, 7, 113–125. [Google Scholar] [PubMed]
- Zhu, B.; Bronas, U.G.; Quinn, L.; Kapella, M.C.; Park, C.G.; Collins, E.G.; Ruggiero, L.; Fritschi, C. 0886 relationships between sleep and self-care in adults with type 2 diabetes. Sleep 2018, 41, A329. [Google Scholar] [CrossRef]
- Thorp, A.A.; Schlaich, M.P. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef] [PubMed]
- Clarenbach, C.F.; West, S.D.; Kohler, M. Is obstructive sleep apnea a risk factor for diabetes? Discov. Med. 2011, 12, 17–24. [Google Scholar] [PubMed]
- Martínez Cerón, E.; Casitas Mateos, R.; García-Río, F. Sleep apnea-hypopnea syndrome and type 2 diabetes. A reciprocal relationship? Arch. Bronconeumol. 2015, 51, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Pallayova, M.; Lazurova, I.; Donic, V. Hypoxic damage to pancreatic beta cells--the hidden link between sleep apnea and diabetes. Med. Hypotheses 2011, 77, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Doumit, J.; Prasad, B. Sleep apnea in type 2 diabetes. Diabetes Spectr. 2016, 29, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Punjabi, N.M.; Aurora, R.N. Obstructive sleep apnea and type 2 diabetes in older adults. Clin. Geriatr. Med. 2015, 31, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Webb, W.B.; Agnew, H.W. Are we chronically sleep deprived? Bull. Psychon. Soc. 1975, 6, 47–48. [Google Scholar] [CrossRef]
- Kent, B.D.; Grote, L.; Bonsignore, M.R.; Saaresranta, T.; Verbraecken, J.; Lévy, P.; Sliwinski, P.; Tkacova, R.; Kvamme, J.A.; Fietze, I.; et al. Sleep apnoea severity independently predicts glycaemic health in nondiabetic subjects: The ESADA study. Eur. Respir. J. 2014, 44, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.D.; McNicholas, W.T.; Ryan, S. Insulin resistance, glucose intolerance and diabetes mellitus in obstructive sleep apnoea. J. Thorac. Dis. 2015, 7, 1343. [Google Scholar] [PubMed]
- Ip, M.; Mokhlesi, B. Sleep and glucose intolerance/diabetes mellitus. Sleep Med. Clin. 2007, 2, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Gershon, A.S.; Hawker, G.; Tomlinson, G.; Leung, R.S. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am. J. Respir. Crit. Care Med. 2014, 190, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; García-Río, F. Sleep apnoea, insulin resistance and diabetes: The first step is in the fat. Eur. Respir. J. 2017, 49, 1700179. [Google Scholar] [CrossRef] [PubMed]
- Utpat, K.; Desai, U.; Joshi, J.M. Obstructive sleep apnea and diabetes mellitus: A bitter combo. Indian J. Sleep Med. 2018, 13, 48–52. [Google Scholar]
- AlDabal, L.; BaHammam, A.S. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir. Med. J. 2011, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- McLain, J.M.; Alami, W.H.; Glovak, Z.T.; Cooley, C.R.; Burke, S.J.; Collier, J.J.; Baghdoyan, H.A.; Karlstad, M.D.; Lydic, R. Sleep fragmentation delays wound healing in a mouse model of type 2 diabetes. Sleep 2018, 41. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.G.; Milic, M. Sympathetic nerves and hypertension in stress, sleep apnea, and caregiving. Curr. Opin. Nephrol. Hypertens. 2017, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.T.; Menna-Barreto, L. Diabetes and sleep: A complex cause-and-effect relationship. Diabetes Res. Clin. Pract. 2011, 91, 129–137. [Google Scholar] [CrossRef] [PubMed]
- McMullan, C.J.; Schernhammer, E.S.; Rimm, E.B.; Hu, F.B.; Forman, J.P. Melatonin secretion and the incidence of type 2 diabetes. JAMA 2013, 309, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A.; Rutter, G.A. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Urphy, A.M.; Thomas, A.; Crinion, S.J.; Kent, B.D.; Tambuwala, M.M.; Fabre, A.; Pepin, J.L.; Roche, H.M.; Arnaud, C.; Ryan, S. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur. Respir. J. 2017, 49, 1601731. [Google Scholar]
- Gruber, A.; Horwood, F.; Sithole, J.; Ali, N.J.; Idris, I. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc. Diabetol. 2006, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Rutters, F.; Besson, H.; Walker, M.; Mari, A.; Konrad, T.; Nilsson, P.M.; Balkau, B.; Dekker, J.M. The association between sleep duration, insulin sensitivity, and β-cell function: The EGIR-RISC study. J. Clin. Endocrinol. Metab. 2016, 101, 3272–3280. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, N.M.; Shahar, E.; Redline, S.; Gottlieb, D.J.; Givelber, R.; Resnick, H.E. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am. J. Epidemiol. 2004, 160, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Knutson, K.; Leproult, R.; Tasali, E.; Cauter, E.V. Sleep loss: A novel risk factor for insulin resistance and Type 2 diabetes. J. Appl. Physiol. 2005, 99, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Taub, L.F.; Redeker, N.S. Sleep disorders, glucose regulation, and type 2 diabetes. Biol. Res. Nurs. 2008, 9, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Munkhaugen, J.; Hjelmesæth, J.; Otterstad, J.E.; Helseth, R.; Sollid, S.T.; Gjertsen, E.; Gullestad, L.; Perk, J.; Moum, T.; Husebye, E.; et al. Managing patients with prediabetes and type 2 diabetes after coronary events: Individual tailoring needed—A cross-sectional study. BMC Cardiovasc. Disord. 2018, 18, 160. [Google Scholar] [CrossRef] [PubMed]
- Aurora, R.N.; Punjabi, N.M. Obstructive sleep apnoea and type 2 diabetes mellitus: A bidirectional association. Lancet Respir. Med. 2013, 1, 329–338. [Google Scholar] [CrossRef]
- Shankar, A.; Syamala, S.; Kalidindi, S. Insufficient rest or sleep and its relation to cardiovascular disease, diabetes and obesity in a national, multiethnic sample. PLoS ONE 2010, 5, e14189. [Google Scholar] [CrossRef] [PubMed]
- Holliday, E.G.; Magee, C.A.; Kritharides, L.; Banks, E.; Attia, J. Short sleep duration is associated with risk of future diabetes but not cardiovascular disease: A prospective study and meta-analysis. PLoS ONE 2013, 8, e82305. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Deliens, G.; Gilson, M.; Peigneux, P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep 2015, 38, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Tahrani, A.A.; Ali, A.; Stevens, M.J. Obstructive sleep apnoea and diabetes: An update. Curr. Opin. Pulm. Med. 2013, 19, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Masoodi, S.R.; Shoib, S. Obstructive sleep apnea in Type 2 diabetes and impact of continuous positive airway pressure therapy on glycemic control. Indian J. Endocrinol. Metab. 2017, 21, 106. [Google Scholar] [PubMed]
- Yoda, K.; Inaba, M.; Hamamoto, K.; Yoda, M.; Tsuda, A.; Mori, K.; Imanishi, Y.; Emoto, M.; Yamada, S. Association between poor glycemic control, impaired sleep quality, and increased arterial thickening in type 2 diabetic patients. PLoS ONE 2015, 10, e0122521. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, E.A.; Rother, K.I.; Cizza, G. Interacting epidemics? Sleep curtailment, insulin resistance, and obesity. Ann. N. Y. Acad. Sci. 2012, 1264, 110–134. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Yamakawa, T.; Takahashi, K.; Suzuki, J.; Shinoda, M.M.; Sakamaki, K.; Danno, H.; Tsuchiya, H.; Waseda, M.; Takano, T.; et al. Association of usual sleep quality and glycemic control in type 2 diabetes in Japanese: A cross sectional study. Sleep and Food Registry in Kanagawa (SOREKA). PLoS ONE 2018, 13, e0191771. [Google Scholar] [CrossRef] [PubMed]
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2019, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; van Egmond, L.; Chapman, C.D.; Cedernaes, J.; Benedict, C. Aiding sleep in type 2 diabetes: Therapeutic considerations. Lancet Diabetes Endocrinol. 2018, 6, 60–68. [Google Scholar] [CrossRef]
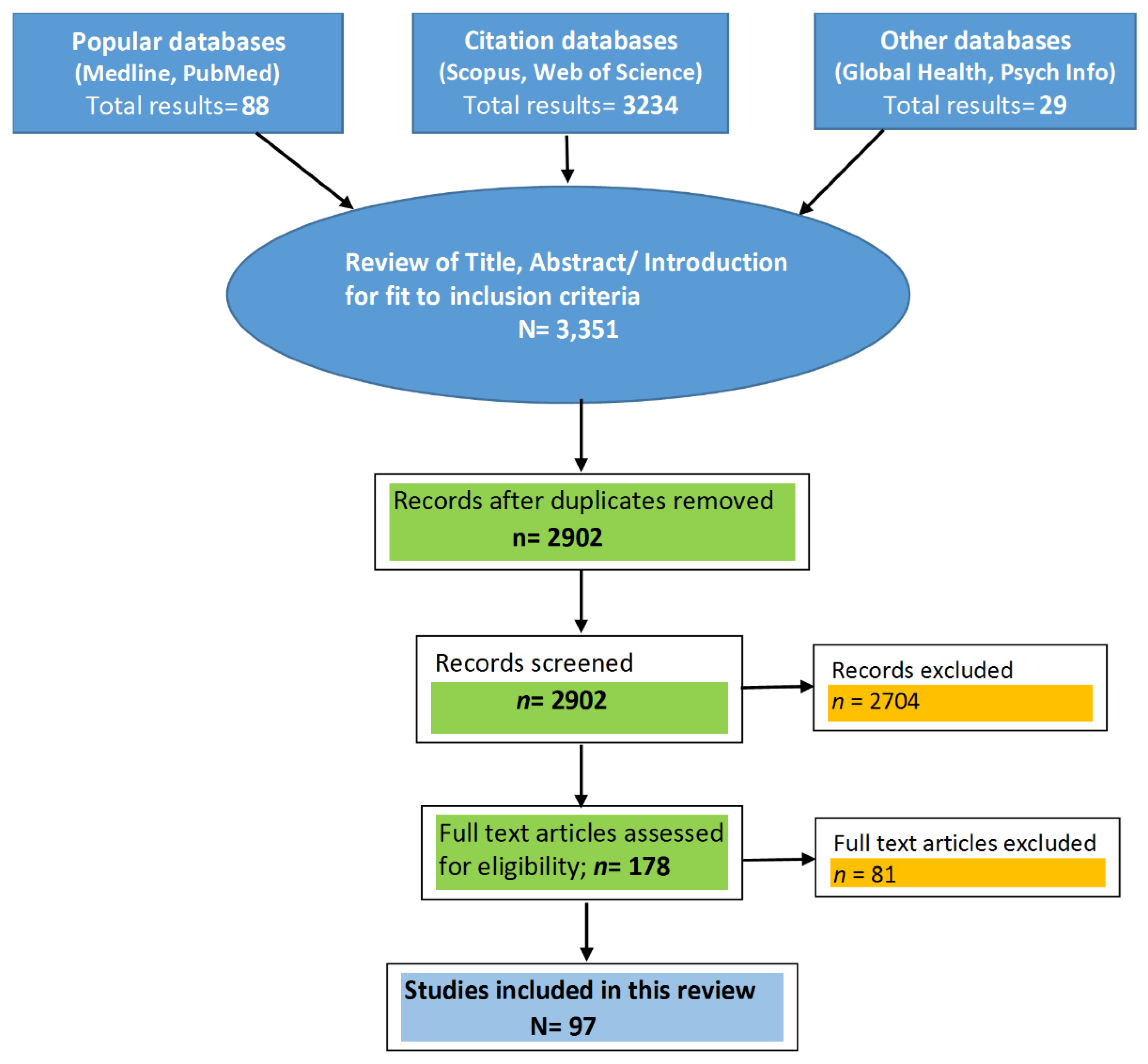
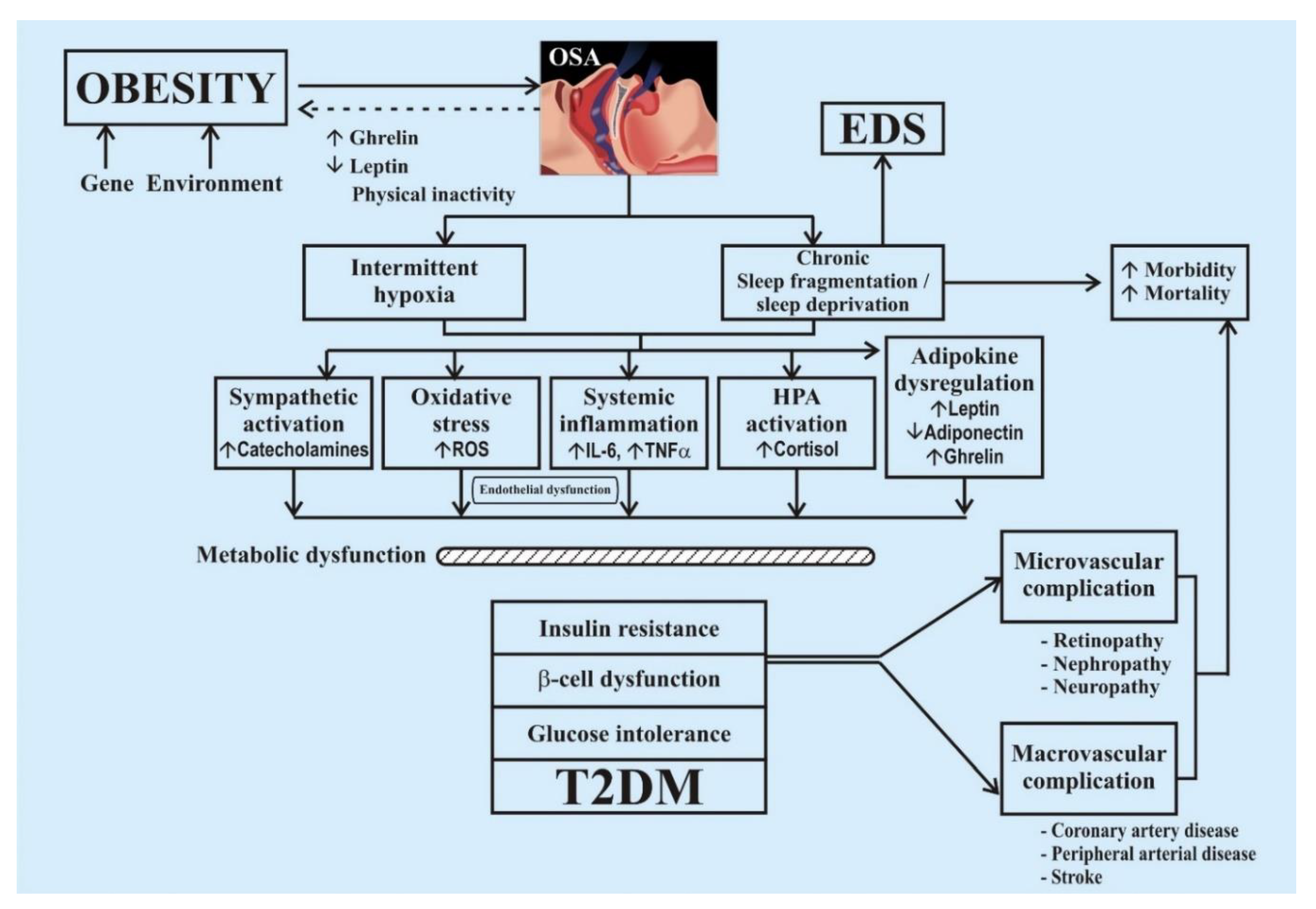
| No. | Reference | Country | Year | Target Population | Type of Study | Sample Description (Sample (N); Males (M); Females (F); Age Range (AR)) | Study Duration | Inference/Major Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Seixas et al. [25] | USA | 2018 | General population | National Health Interview Survey with face-face interviews and questionnaires | N = 236,406; M = 45%; F = 55%; AR = 18–85 years | 10 years (survey data) (2004–2013) | Both short sleep and long sleep were associated with diabetes mellitus. Among cancer survivors, short sleep was associated with higher self-reported diabetes. |
| 2 | Matsumoto et al. [26] | Japan | 2018 | Community participants | Cross-sectional | N = 7051 | 1 year | Sleep-disordered breathing (SDB) was associated with a higher risk of diabetes in premenopausal women and postmenopausal women but not in men. SDB and obesity were independently associated with diabetes. |
| 3 | Facco et al. [27] | USA | 2017 | Nulliparous women during pregnancy (16 0/7 and 21 6/7 weeks’ gestation) | Prospective Cohort Study | N = 782; F ≥ 18 years | <year till the delivery | Short sleep duration (<7 h) and a later sleep midpoint are proven to increase the risk of gestational diabetes. |
| 4 | Lin et al. [18] | Taiwan | 2016 | Secondary data from the Nutrition and Health Survey | Cross-sectional | N = 1533; M = 733; F = 800; AR = 19–64 years | 3 years (2005–2008) | Risk of diabetes among 19–44 years with ≤5 h of sleep was 5.24-fold higher than who reported 7–8.9 h of sleep at night. |
| 5 | Kachi et al. [28] | Japan | 2012 | Routine Health Assessments data | Cross-sectional | N = 20,744; M = 20,744; AR = 30–64 years | 2003–2007 | Men sleeping for ≤5 h and ≥8 h were more likely to have untreated diabetes compared to those who had 7 h sleep. |
| 6 | Qui et al. [29] | USA | 2010 | Pregnant women (<20 weeks gestation) | Prospective Cohort | N = 1290; F = 1290; AR ≥ 18 years | 2003–2006 | Short sleep duration is strongly associated with glucose intolerance and gestational diabetes. |
| 7 | Facco et al. [23] | USA | 2010 | Nulliparous women in pregnancy (6–20 weeks of gestation) | Prospective cohort study | N = 189; F = 189; AR ≥ 18 years | 2007–2008 (16 months) | Short sleepers had glucose intolerance during pregnancy. |
| 8 | Rafalson et al. [30] | USA | 2010 | Participants with cardiovascular disease but no history of diabetes | Nested Case-Control Study | N = 1455; AR = 35–79 years | 6 years (1996–2001) | Short sleepers had an increased risk of impaired fasting glucose due to insulin resistance. |
| 9 | Xu et al. [31] | USA | 2010 | 164,399 without diabetes and 10,143 participants with diabetes diagnosed after 2000 | Prospective study | N = 174,542; AR = 50–71 years | 2000–2006 | Day napping and a short duration of sleep showed a positive association with diabetes. |
| 10 | Hall et al. [32] | USA | 2008 | Adult Health and Behavior Project Registry | Cross-sectional Community-based cohort study | N = 1214; M = 568; F = 646 AR = 30–54 years | 2006 | Short and long sleepers were at 45% increased risk of having metabolic syndrome compared to those with 7–8 h of sleep |
| 11 | Choi et al. [33] | South Korea | 2008 | Korean Health and Nutrition Survey | Cross-sectional | N = 4222; M = 1822; F = 2400; AR ≥ 20 years | 1 year | Short and long sleep durations - increase the risk of metabolic syndrome compared to those with 7 hrs of sleep. |
| 12 | Tuomilehto et al. [16] | Finland | 2008 | FIN-D2D survey is a population-based survey | Population-based cross sectional study | N = 2800; M = 1366; F = 1434; AR = 45–74 years | 2 years (2004–2005) | Short (<6 h) or long (>8 h) sleep duration increased the risk of type 2 diabetes in middle-aged women but not in men. |
| 13 | Hayashino et al. [34] | Japan | 2007 | High-risk and Population Strategy for Occupational Health Promotion Study | Cohort study | N = 6509; AR = 19–69 years | 6 years (1999–2004) | Among healthy adult subjects, the risk of diabetes was linked to difficulty initiating sleep. |
| 14 | Gangwisch et al. [12] | USA | 2007 | National Health and Nutrition Examination Survey | Multivariate longitudinal analyses | N = 8992 AR = 32–86 years | 10 years (1982–1992) | Short sleep is a risk factor for diabetes. The association between long sleep duration and diabetes due to some unmeasured confounder like poor quality of sleep. |
| 15 | Chaput et al. [35] | Canada | 2007 | Quebec Family Study | Cross-sectional | N = 740; M = 323; F = 417; AR = 21–64 years | 3 years (1989–2001) | Sleep of <6 h resulted in impaired glucose tolerance (IGT). Short- and long-duration sleep times are associated with T2DM/IGT in adults. |
| 16 | Yaggi et al. [17] | USA | 2006 | Massachusetts Male Aging Study without diabetes | Cohort study | N = 1709 (1139); AR = 40–70 years | 18 years (1987–2004) | Short and long sleep durations are proved to increase the risk of T2DM. |
| 17 | Knutson et al. [36] | USA | 2006 | Volunteers with type 2 diabetes | Cross-sectional | N = 161; M = 42; F = 119; AR = 57 years (average) | 2006 | Both sleep duration and quality are significant predictors of HbA1c, which is crucial for glycemic control. |
| 18 | Meisinger et al. [37] | Germany | 2005 | MONICA Augsburg surveys—general population | Cross-sectional | N = 8269; M = 4140; F = 4129; AR = 25–74 years | 12 years (1984–1995) | Difficulty maintaining sleep was associated with an increased risk of type 2 diabetes in men and women. |
| 19 | Bjorkelund et al. [38] | Sweden | 2005 | Women | Prospective study | N = 661; F = 661 | 32 years | Sleep problems and developing diabetes were not linked in this 32-year follow-up study of middle-aged women. Obesity, known to cause increased risk of T2DM, was associated with sleep problems. |
| 20 | Mallon et al. [11] | Sweden | 2005 | A random sample of 2663 subjects | Cohort study | N = 2663 (1170); M = 550; F = 620; AR = 45–65 years | 12 years (1983–1995) | Difficulty in sleep maintenance and short sleep duration increases T2DM in men. |
| 21 | Gottleib et al. [21] | USA | 2005 | Sleep Heart Health Study | Cross-sectional | N = 1486; M = 722; F = 764; AR = 53–93 years | 1995–1998 | Subjects sleeping 6 h or less had adjusted odds ratio for Diabetes of 2.51 and 1.66, respectively. Sleep duration <6 h or >9 h is associated with an increased prevalence of DM and IGT. |
| 22 | Nilsson et al. [39] | Sweden | 2004 | Prospective population-based study | Cohort study | N = 6599 | 14.8 ± 2.4 years | Sleep disturbances are proven to increase the risk of T2DM. |
| 23 | Kawakami et al. [40] | Japan | 2004 | Male employees of the company | Prospective study | N = 2649; M = 2649 | 8 years (1984–1992) | Sleep disturbances resulted in 2–3 times increase in the risk of T2DM. |
| 24 | Ayas et al. [41] | USA | 2003 | Nurses Health Study (without diabetes) | Cohort study | N = 70,026; AR = 40–65 years | 10 years | Sleep restriction may be an independent risk factor for developing T2DM. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chattu, V.K.; Chattu, S.K.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Interlinked Rising Epidemic of Insufficient Sleep and Diabetes Mellitus. Healthcare 2019, 7, 37. https://doi.org/10.3390/healthcare7010037
Chattu VK, Chattu SK, Burman D, Spence DW, Pandi-Perumal SR. The Interlinked Rising Epidemic of Insufficient Sleep and Diabetes Mellitus. Healthcare. 2019; 7(1):37. https://doi.org/10.3390/healthcare7010037
Chicago/Turabian StyleChattu, Vijay Kumar, Soosanna Kumary Chattu, Deepa Burman, David Warren Spence, and Seithikurippu R. Pandi-Perumal. 2019. "The Interlinked Rising Epidemic of Insufficient Sleep and Diabetes Mellitus" Healthcare 7, no. 1: 37. https://doi.org/10.3390/healthcare7010037
APA StyleChattu, V. K., Chattu, S. K., Burman, D., Spence, D. W., & Pandi-Perumal, S. R. (2019). The Interlinked Rising Epidemic of Insufficient Sleep and Diabetes Mellitus. Healthcare, 7(1), 37. https://doi.org/10.3390/healthcare7010037






