Rational Use of Antibiotics in Neonates: Still in Search of Tailored Tools
Abstract
1. Introduction: Why a Review on the Rational Use of Antibiotics in Neonates is Valuable
2. When to Prescribe Antibiotics in Neonates
3. What Antibiotic Regimen to Prescribe in Neonates
4. How to Prescribe Antibiotics in Neonates
4.1. Beta-Lactam Antibiotics
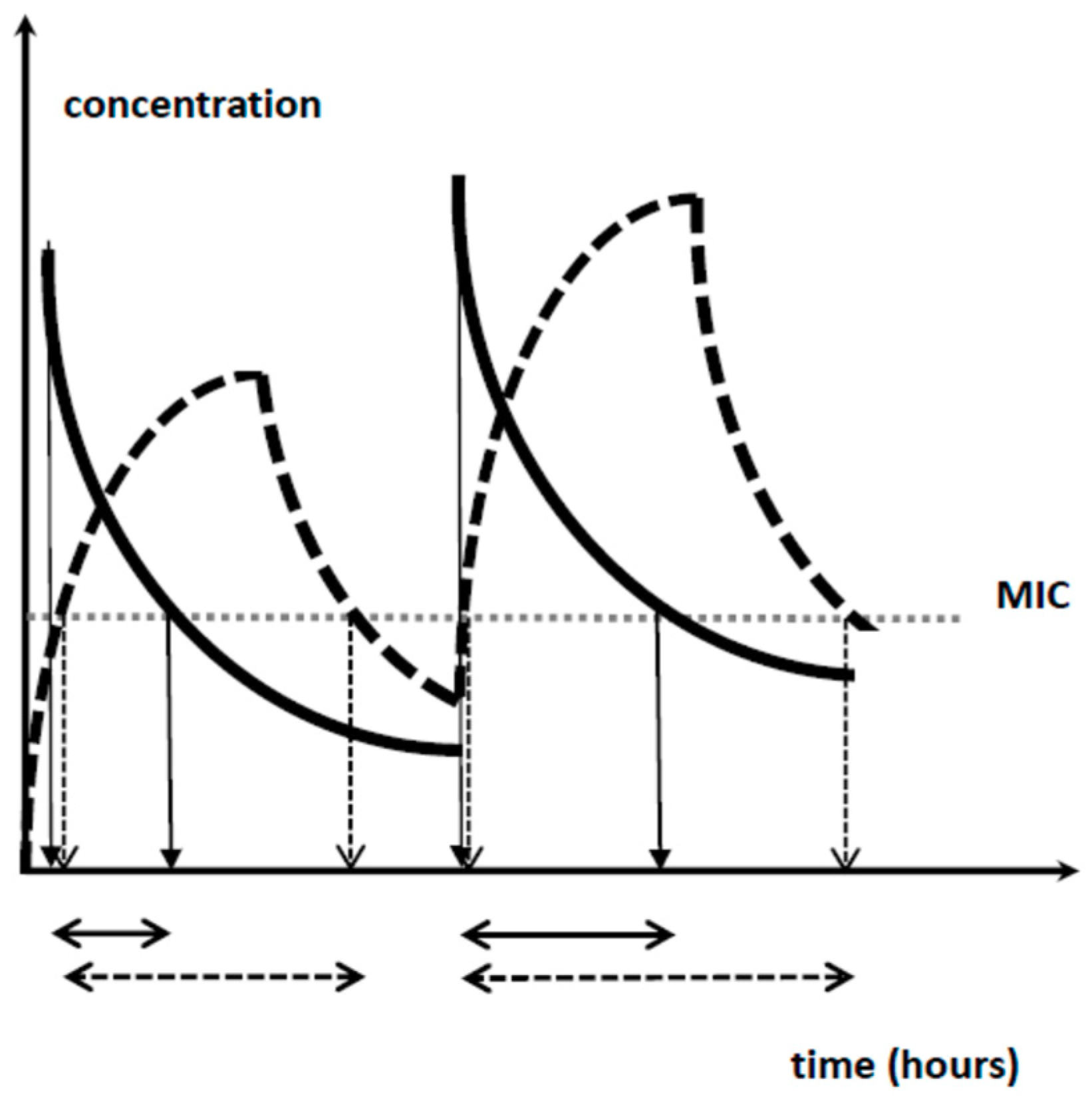
4.2. Aminoglycosides
4.3. Vancomycin
4.4. Other Routes of Administration
4.5. Duration of Treatment
5. Discussion: How to Improve the Current Setting
5.1. Understand Neonatal Pharmacology
5.2. Let Us Make Our Practices More Targeted and Smarter
5.3. We Should be Aware of Our Limitations
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Essential Medicines and Health Products. The Pursuit of Responsible Use of Medicines. Available online: htpps://www.who.int/medicines/areas/rational_use/en (accessed on 12 January 2019).
- Rosli, R.; Dali, A.F.; Abd Aziz, N.; Abdullah, A.H.; Ming, L.C.; Manan, M.M. Drug utilization on neonatal wards: A systematic review of observational studies. Front. Pharmacol. 2017, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Liem, T.B.; Heerdink, E.R.; Egberts, A.C.; Rademaker, C.M. Quantifying antibiotic use in paediatrics: A proposal for neonatal DDDs. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Liem, T.B.Y.; Slob, E.M.A.; Termote, J.U.M.; Wolfs, T.F.W.; Egberts, A.C.G.; Rademaker, C.M.A. Comparison of antibiotic dosing recommendations for neonatal sepsis from established reference sources. Int. J. Clin. Pharm. 2018, 40, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Laine, N.; Kaukonen, A.M.; Hoppu, K.; Airaksinen, M.; Saxen, H. Off-label use of antimicrobials in neonates in a tertiary children’s hospital. Eur. J. Clin. Pharmacol. 2017, 73, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Sequi, M.; Campi, R.; Clavenna, A.; Bonati, M. Methods in pharmacoepidemiology: A review of statistical analyses and data reporting in pediatric drug utilization studies. Eur. J. Clin. Pharmacol. 2013, 69, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, E.M.; Hornik, C.P.; Clark, R.H.; Laughon, M.M.; Benjamin, D.K., Jr.; Smith, P.B. Best Pharmaceuticals for Children Act-Pediatric Trials Network. Medication use in the neonatal intensive care unit. Am. J. Perinatol. 2014, 31, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Nellis, G.; Lutsar, I.; Varendi, H.; Toompere, K.; Turner, M.A.; Duncan, J.; Metsvaht, T. Comparison of two alternative study designs in assessment of medicines utilisation in neonates. BMC Med. Res. Methodol. 2014, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.A.; Duncan, J.; Shah, U.; Metsvaht, T.; Varendi, H.; Nellis, G.; Lutsar, I.; Vaconsin, P.; Storme, T.; Rieutord, A.; et al. European study of neonatal exposure to excipients: An update. Int. J. Pharm. 2013, 457, 357–358. [Google Scholar] [CrossRef]
- Versporten, A.; Sharland, M.; Bielicki, J.; Drapier, N.; Vankerckhoven, V.; Goossens, H.; ARPEC Project Group Members. The antibiotic resistance and prescribing in European Children project: A neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr. Infect. Dis. J. 2013, 32, e242–e253. [Google Scholar] [CrossRef]
- Allegaert, K. Rational use of medicines in neonates: Current observations, areas for research and perspectives. Healthcare 2018, 6, 115. [Google Scholar] [CrossRef]
- Ward, R.M.; Benjamin, D.; Barrett, J.S.; Allegaert, K.; Portman, R.; Davis, J.M.; Turner, M.A. Safety, dosing, and pharmaceutical quality for studies that evaluate medicinal products (including biological products) in neonates. Pediatr. Res. 2017, 81, 692–711. [Google Scholar] [CrossRef]
- Russell, A.B.; Sharland, M.; Heath, P.T. Improving antibiotic prescribing in neonatal units: Time to act. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F141–F146. [Google Scholar] [CrossRef]
- Klingenberg, C.; Kornelisse, R.F.; Buonocore, G.; Maier, R.F.; Stocker, M. Culture-negative early-onset neonatal sepsis—At the crossroad between efficient sepsis care and antimicrobial stewardship. Front. Pediatr. 2018, 6, 285. [Google Scholar] [CrossRef]
- Kuzniewicz, M.W.; Puopolo, K.M.; Fischer, A.; Walsh, E.M.; Li, S.; Newman, T.B.; Kipnis, P.; Escobar, G.J. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr. 2017, 171, 365–371. [Google Scholar] [CrossRef]
- Griffin, M.P.; O´Shea, T.M.; Bissonette, E.A.; Harrell, F.E., Jr.; Lake, D.E.; Moorman, J.R. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr. Res. 2003, 53, 920–926. [Google Scholar] [CrossRef]
- Coggins, S.A.; Weitkamp, J.H.; Grunwald, L.; Stark, A.R.; Reese, J.; Walsh, W.; Wynn, J.L. Heart rate characteristic index monitoring for bloodstream infection in a NICU: A 3-year experience. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F329–F332. [Google Scholar] [CrossRef]
- Litz, J.E.; Goedicke-Fritz, S.; Härtel, C.; Zemlin, M.; Simon, A. Management of early- and late-onset sepsis: Results from a survey in 80 German NICUs. Infection 2019. [Google Scholar] [CrossRef]
- Schelonka, R.L.; Chai, M.K.; Yoder, B.A.; Hensley, D.; Brockett, R.M.; Ascher, D.P. Volume of blood required to detect common neonatal pathogens. J. Pediatr. 1996, 129, 275–278. [Google Scholar] [CrossRef]
- Mukherjee, A.; Davidson, L.; Anguvaa, L.; Duffy, D.A.; Kennea, N. NICE neonatal early onset sepsis guidance: Greater consistency, but more investigations, and greater length of stay. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F248–F249. [Google Scholar] [CrossRef]
- Stocker, M.; van Herk, W.; El Helou, S.; Dutta, S.; Fontana, M.S.; Schuerman, F.A.B.A.; van den Tooren-de Groot, R.K.; Wieringa, J.W.; Janota, J.; van der Meer-Kappelle, L.H.; et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: A multicenter, randomised controlled trial (NeoPins). Lancet 2017, 390, 871–881. [Google Scholar] [CrossRef]
- Van Donge, T.; Bielicki, J.A.; van den Anker, J.; Pfister, M. Key components for antibiotic dose optimization of sepsis in neonates and infants. Front. Pediatr. 2018, 6, 325. [Google Scholar] [CrossRef]
- Tröger, B.; Göpel, W.; Faust, K.; Müller, T.; Jorch, G.; Felderhoff-Müser, U.; Gortner, L.; Heitmann, F.; Hoehn, T.; Kribs, A.; et al. Risk for late-onset blood-culture proven sepsis in very-low-birth weight infants born small for gestational age: A large multicenter study form the German Neonatal Network. Pediatr. Infect. Dis. J. 2014, 33, 238–243. [Google Scholar] [CrossRef]
- Alshaikh, B.; Yee, W.; Lodha, A.; Henderson, E.; Yusuf, K.; Sauve, R. Coagulase-negative staphylococcus sepsis in preterm infants and long-term neurodevelopmental outcome. J. Perinatol. 2014, 34, 125–129. [Google Scholar] [CrossRef]
- Pauwels, S.; Allegaert, K. Therapeutic drug monitoring in neonates. Arch. Dis. Child. 2016, 101, 377–381. [Google Scholar] [CrossRef]
- Esaiassen, E.; Fjalstad, J.W.; Juvet, L.K.; van den Anker, J.N.; Klingenberg, C. Antibiotic exposure in neonates and early adverse outcomes: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2017, 72, 1858–1870. [Google Scholar] [CrossRef]
- De Man, P.; Verhoeven, B.A.; Verbrugh, H.A.; Vos, M.C.; van den Anker, J.N. An antibiotic policy to prevent emergence of resistant bacilli. Lancet 2000, 355, 973–978. [Google Scholar] [CrossRef]
- Allegaert, K.; van den Anker, J. Neonates are not just little children and need more finesse in dosing of antibiotics. Acta. Clin. Belg. 2018. [Google Scholar] [CrossRef]
- Fuchs, A.; Li, G.; van den Anker, J.N.; Bielicki, J. Optimising β–lactam dosing in neonates: A review of pharmacokinetics, drug exposure and pathogens. Curr. Pharm. Des. 2017, 23, 5805–5838. [Google Scholar] [CrossRef]
- Allegaert, K.; Verbesselt, R.; Naulaers, G.; van den Anker, J.N.; Rayyan, M.; Debeer, A.; de Hoon, J. Developmental pharmacology: Neonates are not just small adults…. Acta Clin. Belg. 2008, 63, 16–24. [Google Scholar]
- Sherwin, C.M.; Medlicott, N.J.; Reith, D.M.; Broadbent, R.S. Intravenous drug delivery in neonates: Lessons learnt. Arch. Dis. Child. 2014, 99, 590–594. [Google Scholar] [CrossRef]
- Leroux, S.; Zhao, W.; Betremieux, P.; Pladys, P.; Saliba, E.; Jacqz-Aigrain, E.; French Society of Neonatology. Therapeutic guidelines for prescribing antibiotics in neonates should be evidence-based: A French national survey. Arch. Dis. Child. 2015, 100, 394–398. [Google Scholar] [CrossRef]
- Metsvaht, T.; Nellis, G.; Varendi, H.; Nunn, A.J.; Graham, S.; Rieutord, A.; Storme, T.; McElnay, J.; Mulla, H.; Turner, M.A.; Lutsar, I. High variability in the dosing of commonly used antibiotics revealed by a Europe-wide point prevalence study: Implications for research and dissemination. BMC Pediatr. 2015, 15, 41. [Google Scholar] [CrossRef]
- Van der Zanden, T.M.; de Wildt, S.N.; Liem, Y.; Offringa, M.; de Hoog, M.; Dutch Paediatric Pharmacotherapy Expertise Network NKFK (Nederlands Kenniscentrum voor Farmacotherapie bij Kinderen). Developing a paediatric drug formulary for the Netherlands. Arch. Dis. Child. 2017, 102, 357–361. [Google Scholar] [CrossRef]
- Dalhoff, A. Seventy-five years of research on protein binding. Antimicrob. Agents Chemother. 2018, 62, e01663-17. [Google Scholar] [CrossRef]
- Wilbaux, M.; Fuchs, A.; Samardzic, J.; Rodieux, F.; Csajka, C.; Allegaert, K.; van den Anker, J.N.; Pfister, M. Pharmacometric approaches to personalize use of primarily renally eliminated antibiotics in preterm and term neonates. J. Clin. Pharmacol. 2016, 56, 909–935. [Google Scholar] [CrossRef]
- De Cock, R.F.; Smits, A.; Allegaert, K.; de Hoon, J.; Saegeman, V.; Danhof, M.; Knibbe, C.A. Population pharmacokinetic modelling of total and unbound cefazolin plasma concentrations as a guide for dosing in preterm and term neonates. J. Antimicrob. Chemother. 2014, 69, 1330–1338. [Google Scholar] [CrossRef]
- Van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental changes in pharmacokinetics and pharmacodynamics. J. Clin. Pharmacol. 2018, 58 (Suppl. 10), S10–S25. [Google Scholar] [CrossRef]
- Allegaert, K.; van den Anker, J. Neonatal drug therapy: The first frontier of therapeutics for children. Clin. Pharmacol. Ther. 2015, 98, 288–297. [Google Scholar] [CrossRef]
- Padari, H.; Metsvaht, T.; Germovsek, E.; Barker, C.I.; Kipper, K.; Herodes, K.; Standing, J.F.; Oselin, K.; Tasa, T.; Soeorg, H.; Lutsar, I. Pharmacokinetics of penicillin G in preterm and term neonates. Antimicrob. Agents Chemother. 2018, 62, e02238-17. [Google Scholar] [CrossRef]
- Leroux, S.; Roué, J.M.; Gouyon, J.B.; Biran, V.; Zheng, H.; Zhao, W.; Jacqz-Aigrain, E. A population and developmental pharmacokinetic analysis to evaluate and optimize cefotaxime dosing regimen in neonates and young infants. Antimicrob. Agents Chemother. 2016, 60, 6626–6634. [Google Scholar] [CrossRef]
- Van den Anker, J.N.; Pokorna, P.; Kinzig-Schippers, M.; Martinkova, J.; de Groot, R.; Drusano, G.L.; Sorgel, F. Meropenem pharmacokinetics in the newborn. Antimicrob. Agents Chemother. 2009, 53, 3871–3879. [Google Scholar] [CrossRef]
- Shabaan, A.E.; Nour, I.; Elsayed Eldegla, H.; Nasef, N.; Shouman, B.; Abdel-Hady, H. Conventional versus prolonged infusion of meropenem in neonates with Gram-negative late-onset sepsis: A randomized controlled trial. Pediatr. Infect. Dis. J. 2017, 36, 358–363. [Google Scholar] [CrossRef]
- Smits, A.; Kulo, A.; van den Anker, J.; Allegaert, K. The amikacin research program: A stepwise approach to validate dosing regimens in neonates. Expert Opin. Drug Metab. Toxicol. 2017, 13, 157–166. [Google Scholar] [CrossRef]
- Cristea, S.; Smits, A.; Kulo, A.; Knibbe, C.A.J.; van Weissenbruch, M.; Krekels, E.H.J.; Allegaert, K. Amikacin pharmacokinetics to optimize dosing in neonates with perinatal asphyxia treated with hypothermia. Antimicrob. Agents Chemother. 2017, 61, e01282-17. [Google Scholar] [CrossRef]
- Allegaert, K.; Cossey, V.; Langhendries, J.P.; Naulaers, G.; Vanhole, C.; Devlieger, H.; Van Overmeire, B. Effects of co-administration of ibuprofen-lysine on the pharmacokinetics of amikacin in preterm infants during the first days of life. Biol. Neonate 2004, 86, 207–211. [Google Scholar] [CrossRef]
- Van Donge, T.; Pfister, M.; Bielicki, J.; Csajka, C.; Rodieux, F.; van den Anker, J.; Fuchs, A. Quantitative analysis of gentamicin exposure in neonates and infants calls into question its current dosing recommendations. Antimicrob. Agents Chemother. 2018, 62, e02004-17. [Google Scholar] [CrossRef]
- Valitalo, P.A.; van den Anker, J.N.; Allegaert, K.; de Cock, R.F.; de Hoog, M.; Simons, S.H.; Mouton, J.W.; Knibbe, C.A. Novel model-based dosing guidelines for gentamicin and tobramycin in preterm and term neonates. J. Antimicrob. Chemother. 2015, 70, 2074–2077. [Google Scholar] [CrossRef]
- De Cock, R.F.; Allegaert, K.; Sherwin, C.M.; Nielsen, E.I.; de Hoog, M.; van den Anker, J.N.; Danhof, M.; Knibbe, C.A. A neonatal amikacin covariate model can be used to predict ontogeny of other drugs eliminated through glomerular filtration in neonates. Pharm. Res. 2014, 31, 754–767. [Google Scholar] [CrossRef]
- Drennan, P.G.; Begg, E.J.; Gardiner, S.J.; Kirkpatrick, C.M.J.; Chambers, S.T. The dosing and monitoring of vancomycin—What it the best way forward? Int. J. Antimicrob. Agents 2018. [Google Scholar] [CrossRef]
- Tasa, T.; Metsvaht, T.; Kalamees, R.; Vilo, J.; Lutsar, I. DosOpt: a tool for personalized Bayesian dose adjustment of vancomycin in neonates. Ther. Drug Monit. 2017, 39, 604–613. [Google Scholar] [CrossRef]
- Zhao, W.; Kaguelidou, F.; Biran, V.; Zhang, D.; Allegaert, K.; Capparelli, E.V.; Holford, N.; Kimura, T.; Lo, Y.L.; Peris, J.E.; et al. External evaluation of population pharmacokinetic models of vancomycin in neonates: The transferability of published models to different clinical settings. Br. J. Clin. Pharmacol. 2013, 75, 1068–1080. [Google Scholar] [CrossRef]
- Smits, A.; Kulo, A.; Verbesselt, R.; Naulaers, G.; de Hoon, J.; Vermeersch, P.; Allegaert, K. Cefazolin plasma protein binding and its covariates in neonates. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3359–3365. [Google Scholar] [CrossRef]
- Fuchs, A.; Bielicki, J.; Mathur, S.; Sharland, M.; Van Den Anker, J.N. Reviewing the WHO guidelines of antibiotic use for sepsis in neonates and children. Paediatr. Int. Child Health 2018, 38 (Suppl. 1), S3–S15. [Google Scholar] [CrossRef]
- McMullan, B.J.; Andresen, D.; Blyth, C.C.; Avent, M.L.; Bowen, A.C.; Britton, P.N.; Clark, J.E.; Cooper, C.M.; Curtis, N.; Goeman, E.; et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect. Dis. 2016, 16, e139–e152. [Google Scholar] [CrossRef]
- Gathwala, G.; Sindwani, A.; Singh, J.; Choudhry, O.; Chaudhary, U. Ten days vs. 14 days antibiotic therapy in culture-proven neonatal sepsis. J. Trop. Pediatr. 2010, 56, 433–435. [Google Scholar] [CrossRef]
- Rohatgi, S.; Dewan, P.; Faridi, M.M.A.; Kumar, A.; Malhotra, R.K.; Batra, P. Seven versus 10 days antibiotic therapy for culture-proven neonatal sepsis: A randomised controlled trial. J. Paediatr. Child Health 2017, 53, 556–562. [Google Scholar] [CrossRef]
- Linder, N.; Lubin, D.; Hernandez, A.; Amit, L.; Ashkenazi, S. Duration of vancomycin treatment for coagulase-negative Staphylococcus sepsis in very low birth weight infants. Br. J. Clin. Pharmacol. 2013, 76, 58–64. [Google Scholar] [CrossRef]
- Chowdhary, G.; Dutta, S.; Narang, A. Randomized controlled trial of 7-day vs. 14-day antibiotics for neonatal sepsis. J. Trop. Pediatr. 2006, 52, 427–432. [Google Scholar] [CrossRef]
- Turta, O.; Rautava, S. Antibiotics, obesity and the link to microbes—What are we doing to our children? BMC Med. 2016, 14, 57. [Google Scholar] [CrossRef]
- Avorn, J. The psychology of clinical decision making—Implications for medication use. N. Engl. J. Med. 2018, 378, 689–691. [Google Scholar] [CrossRef]
| Rank | Medicine | Exposure | Courses | Days of use |
|---|---|---|---|---|
| 1 | ampicillin | 681 | 709 | 3069 |
| 2 | gentamicin | 676 | 785 | 3521 |
| 5 | vancomycin | 91 | 150 | 987 |
| 15 | cefotaxime | 43 | 53 | 316 |
| 23 | tobramycin | 24 | 34 | 189 |
| 24 | erythromycin | 24 | 25 | 103 |
| 28 | clindamycin | 17 | 19 | 128 |
| 38 | ceftazidime | 12 | 15 | 99 |
| 41 | piperacillin/tazobactam | 11 | 15 | 115 |
| 43 | amoxicillin | 11 | 12 | 72 |
| 44 | metronidazole | 11 | 13 | 97 |
| 45 | oxacillin | 10 | 13 | 97 |
| 46 | nafcillin | 9 | 14 | 97 |
| 48 | amikacin | 8.8 | 12 | 77 |
| 51 | cefazolin | 7.5 | 8.1 | 27 |
| 52 | meropenem | 7 | 8.9 | 82 |
| 60 | cefipime | 6.1 | 7.7 | 58 |
| 66 | penicillin G | 4.7 | 4.9 | 38 |
| 74 | rifampin | 3.6 | 3.8 | 36 |
| 77 | imipenem + cilastatin | 3.0 | 3.3 | 29 |
| 90 | cephalexin | 1.9 | 2.0 | 9.5 |
| 91 | ceftriaxone | 1.8 | 1.8 | 5.7 |
| 94 | sulfamethoxazole + trimethoprim | 1.6 | 1.8 | 16 |
| 96 | cefoxitin | 1.5 | 1.6 | 4.9 |
| 98 | fosphenytoin | 1.4 | 1.6 | 10 |
| 100 | linezolid | 1.3 | 1.6 | 14 |
| Weight (gram) | Postnatal Age < 14 Days *,** | Postnatal Age ≥ 14 Days |
|---|---|---|
| <800 g | 16 mg/kg/48 h | 20 mg/kg/42 h |
| 800–1200 g | 16 mg/kg/42 h | 20 mg/kg/36 h |
| 1200–2000 g | 15 mg/kg/36 h | 18 mg/kg/30 h |
| 2000–2800 g | 15 mg/kg/36 h | 18 mg/kg/24 h |
| ≥2800 g | 15 mg/kg/30 h | 18 mg/kg/20 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Anker, J.; Allegaert, K. Rational Use of Antibiotics in Neonates: Still in Search of Tailored Tools. Healthcare 2019, 7, 28. https://doi.org/10.3390/healthcare7010028
van den Anker J, Allegaert K. Rational Use of Antibiotics in Neonates: Still in Search of Tailored Tools. Healthcare. 2019; 7(1):28. https://doi.org/10.3390/healthcare7010028
Chicago/Turabian Stylevan den Anker, John, and Karel Allegaert. 2019. "Rational Use of Antibiotics in Neonates: Still in Search of Tailored Tools" Healthcare 7, no. 1: 28. https://doi.org/10.3390/healthcare7010028
APA Stylevan den Anker, J., & Allegaert, K. (2019). Rational Use of Antibiotics in Neonates: Still in Search of Tailored Tools. Healthcare, 7(1), 28. https://doi.org/10.3390/healthcare7010028






