Under-Detection of Lyme Disease in Canada
Abstract
1. Introduction
2. Materials and Methods
2.1. Source Data
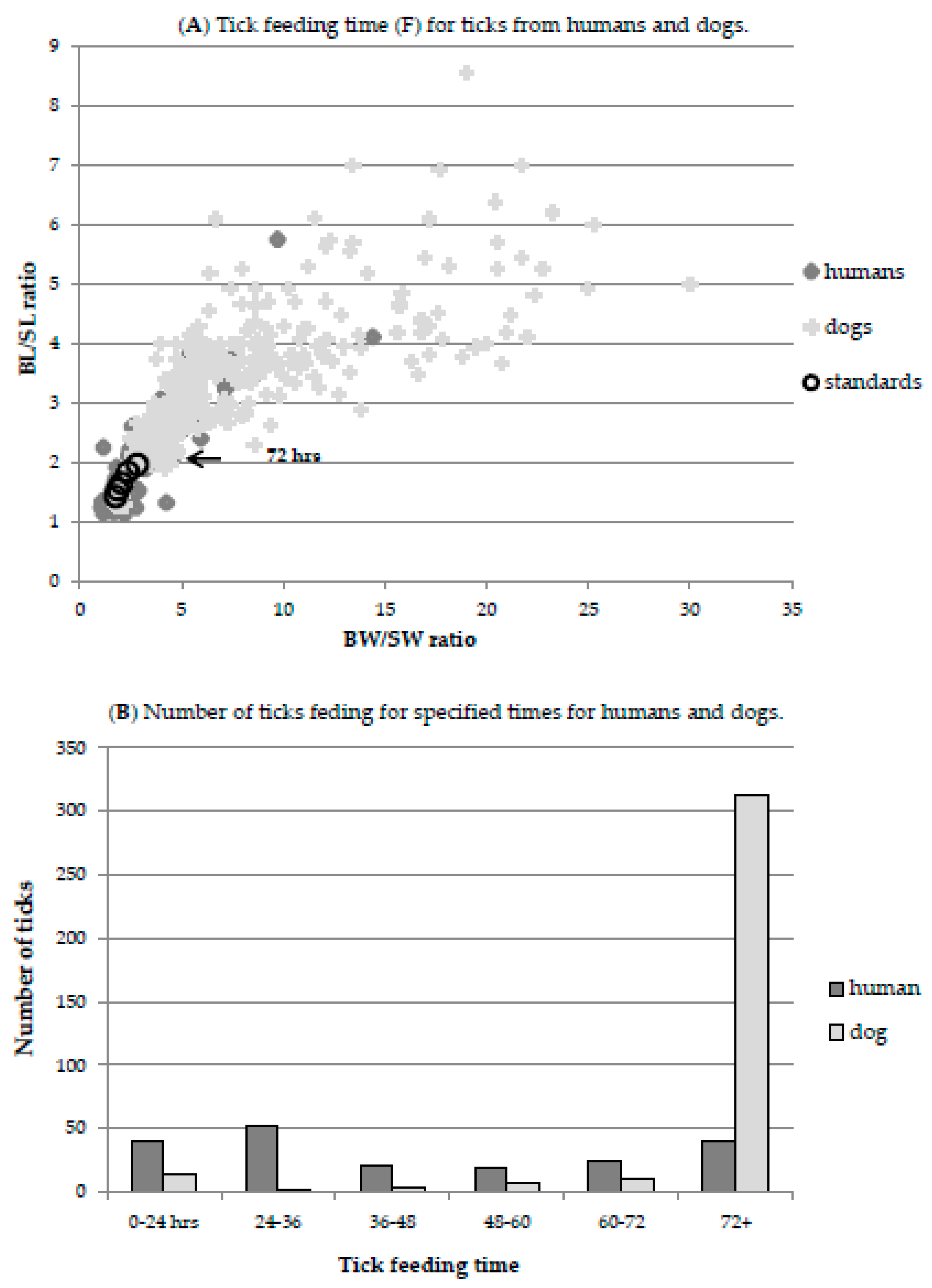
2.2. Estimation of Tick Feeding Time
2.3. Statistics
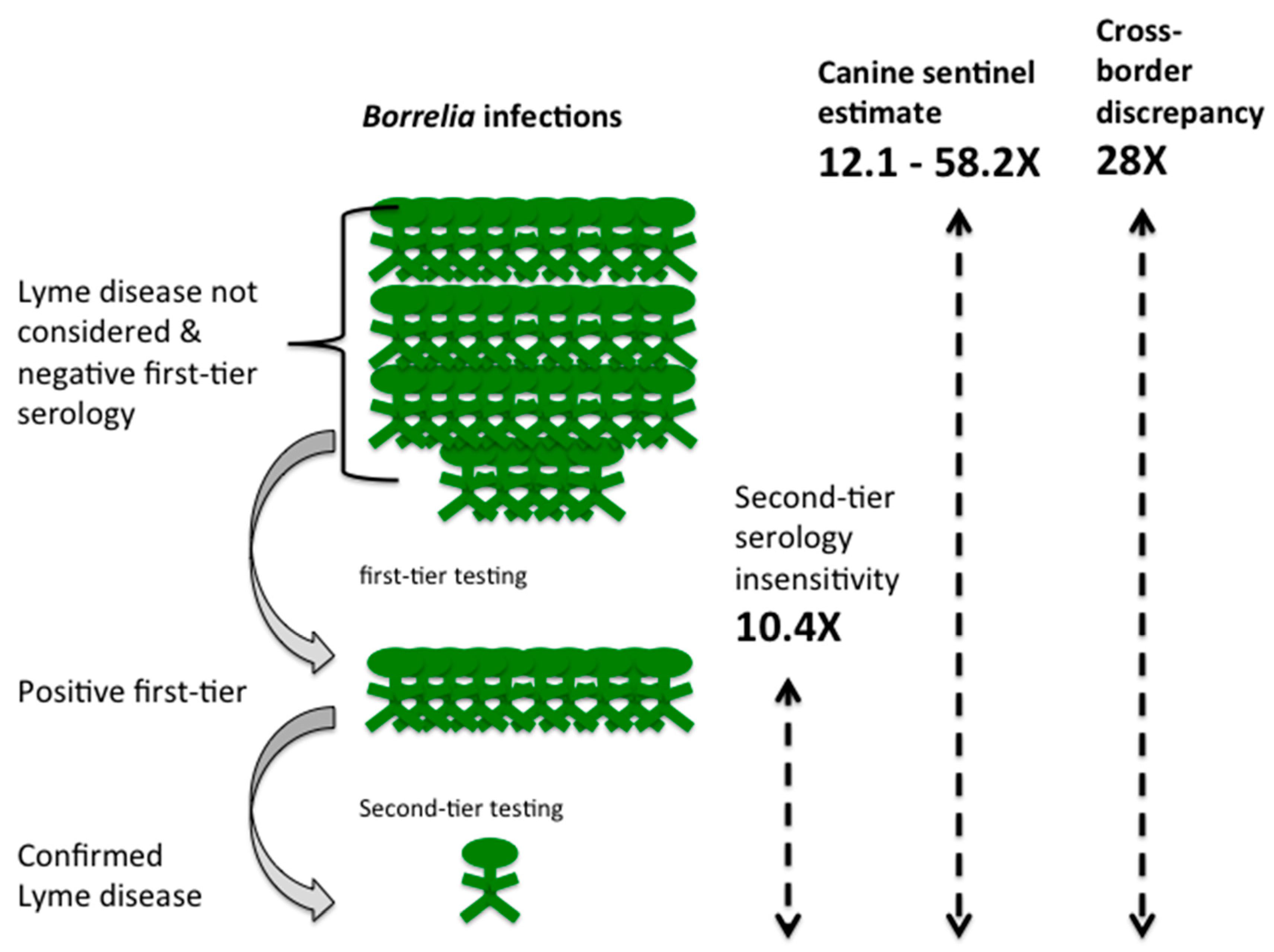
3. Results
3.1. Comparison of Lyme Disease Incidence in Canada and the United States
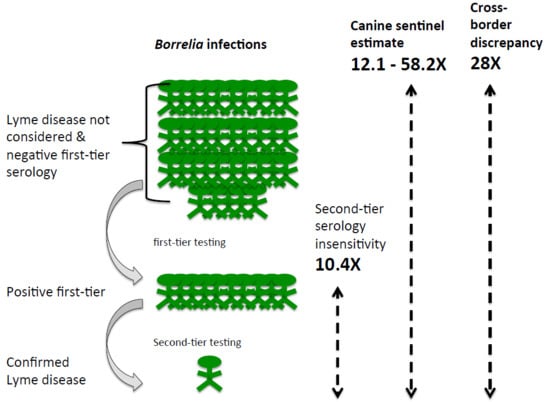
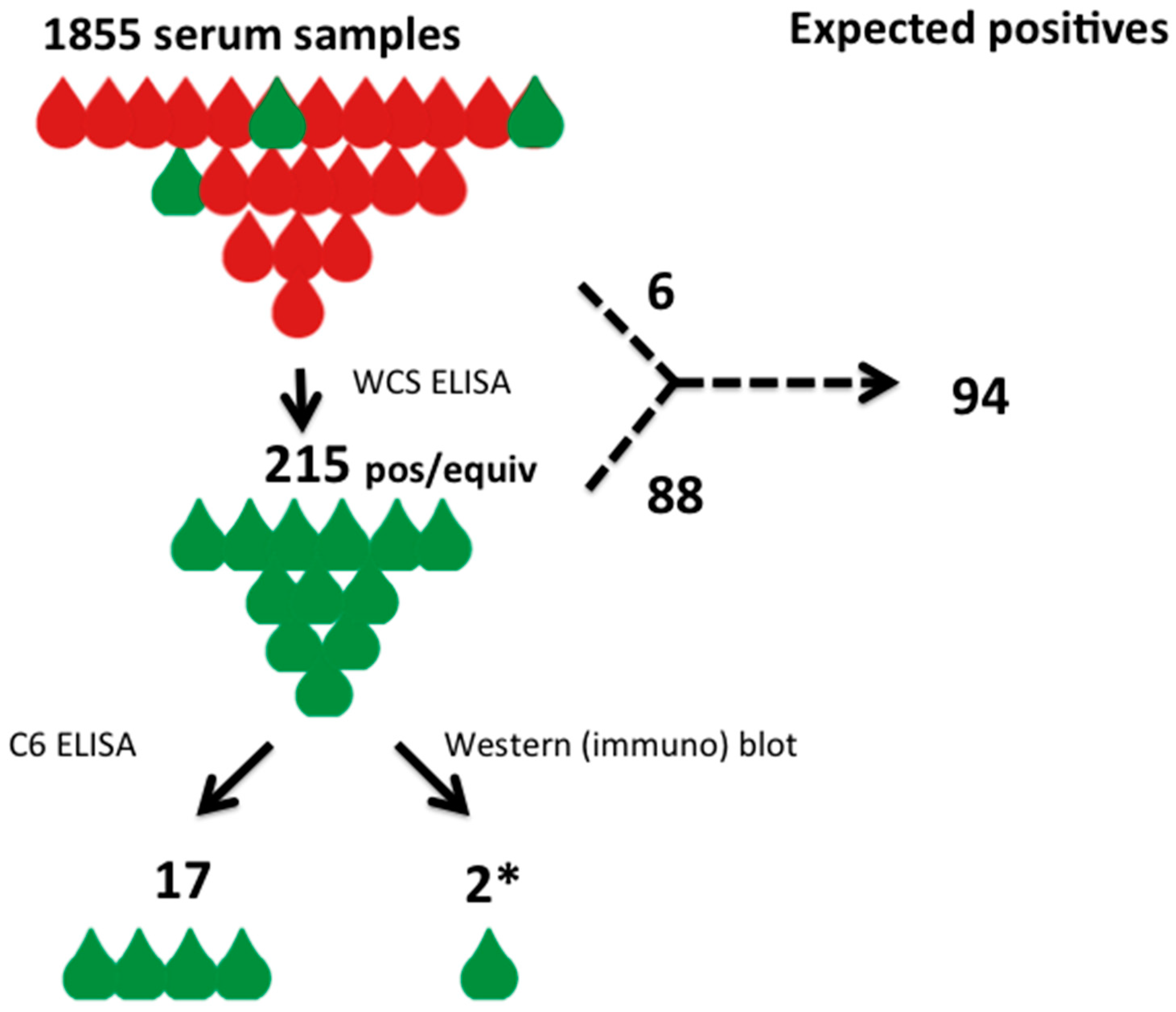
3.2. Assessment of Serological Diagnostics
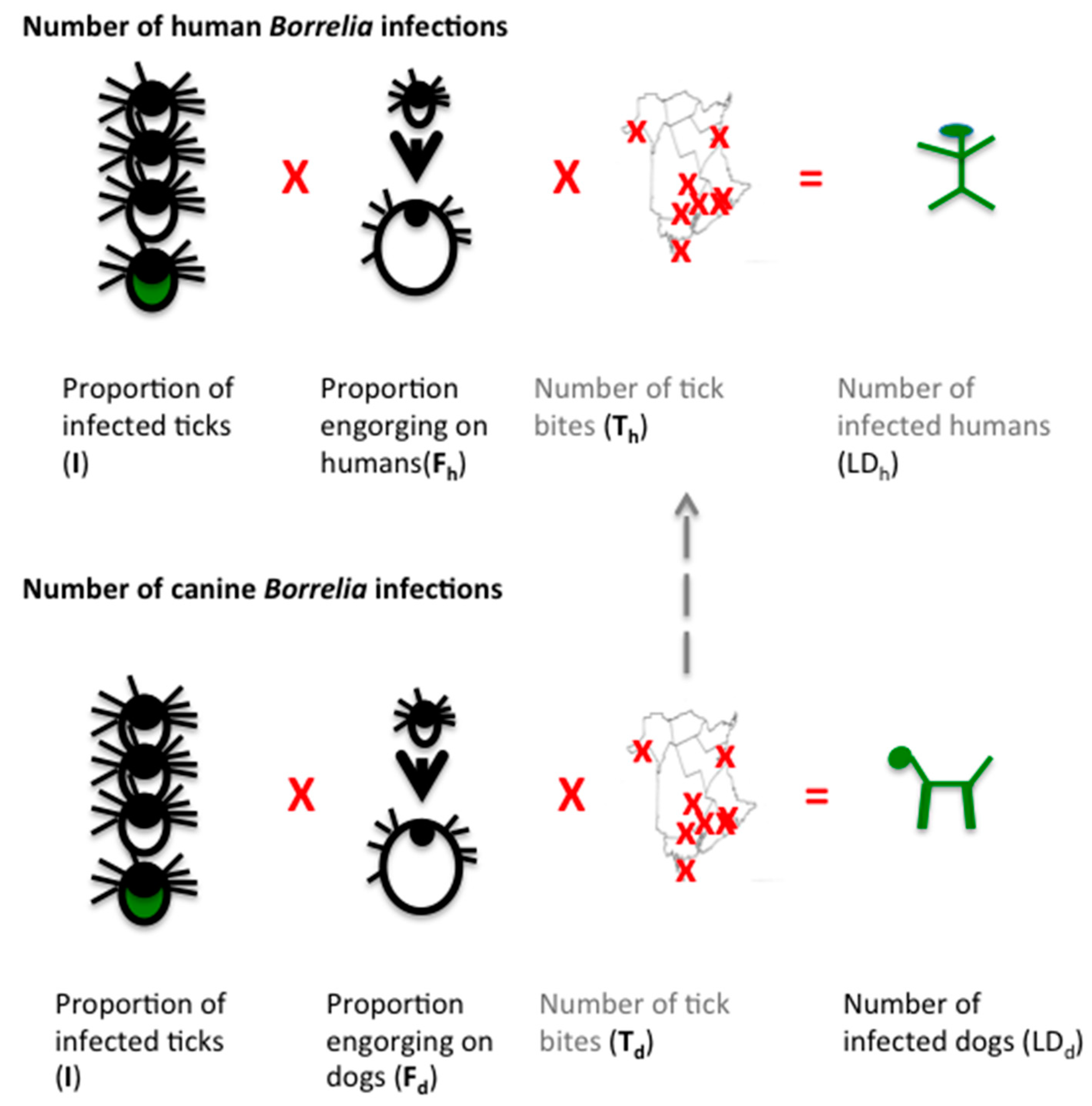
3.3. Estimation of Human Lyme Borreliosis Prevalence from Canine Sentinel Data
3.3.1. I—Proportion of Infected Ticks
3.3.2. F—Proportion of Tick Sufficiently Engorged to Transmit Infection
3.3.3. T—Number of Tick Bites
3.3.4. Extrapolating Dog Tick Bites to Human Tick Bites
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sperling, J.L.; Sperling, F.A. Lyme borreliosis in Canada: Biological diversity and diagnostic complexity from an entomological perspective. Can. Entomol. 2009, 141, 521–549. [Google Scholar] [CrossRef]
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oliver, J.H., Jr. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick-Borne Dis. 2011, 2, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.C.; Steere, A.C.; Duray, P.H.; Anderson, A.E.; Mahoney, E.B. Fatal pancarditis in a patient with coexistent Lyme disease and babesiosis. Demonstration of spirochetes in the myocardium. Ann. Intern. Med. 1985, 103, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Reimers, C.D.; de Koning, J.; Neubert, U.; Preac-Mursic, V.; Koster, J.G.; Müller-Felber, W.; Pongratz, D.E.; Duray, P.H. Borrelia burgdorferi myositis: Report of eight patients. J. Neurol. 1993, 240, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.J. Consequences of treatment delay in Lyme disease. J. Eval. Clin. Pract. 2007, 13, 470–472. [Google Scholar] [CrossRef]
- Muehlenbachs, A.; Bollweg, B.C.; Schulz, T.J.; Forrester, J.D.; Carnes, M.D.; Molins, C.; Ray, G.S.; Cummings, P.M.; Ritter, J.M.; Blau, D.M.; et al. Cardiac tropism of Borrelia burgdorferi: An autopsy study of sudden cardiac death associated with Lyme carditis. Am. J. Pathol. 2016, 186, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Baranchuk, A. Lyme carditis and atrioventricular block. Can. Med. Assoc. J. 2018, 190, E622. [Google Scholar] [CrossRef] [PubMed]
- Aucott, J.N.; Seifter, A.; Rebman, A.W. Probable late lyme disease: A variant manifestation of untreated Borrelia burgdorferi infection. BMC Infect. Dis. 2012, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Aucott, J.N.; Crowder, L.A.; Kortte, K.B. Development of a foundation for a case definition of post-treatment Lyme disease syndrome. Int. J. Infect. Dis. 2013, 17, 443–449. [Google Scholar] [CrossRef]
- Dattwyler, R.J.; Luft, B.J.; Kunkel, M.J.; Finkel, M.F.; Wormser, G.P.; Rush, T.J.; Grunwaldt, E.; Agger, W.A.; Franklin, M.; Oswald, D.; et al. Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. N. Engl. J. Med. 1997, 337, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Shadick, N.A.; Phillips, C.B.; Logigian, E.L.; Steere, A.C.; Kaplan, R.F.; Berardi, V.P.; Duray, P.H.; Larson, M.G.; Wright, E.A.; Ginsburg, K.S.; et al. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann. Int. Med. 1994, 121, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Asch, E.S.; Bujak, D.I.; Weiss, M.; Peterson, M.G.; Weinstein, A. Lyme disease: An infectious and postinfectious syndrome. J. Rheumatol. 1994, 21, 454–461. [Google Scholar] [PubMed]
- Ogden, N.H.; Lindsay, L.R.; Hanincová, K.; Barker, I.K.; Bigras-Poulin, M.; Charron, D.F.; Heagy, A.; Francis, C.M.; O’Callaghan, C.J.; Schwartz, I.; Thompson, R.A. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008, 74, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Anderson, J.F.; Durden, L. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J. Parasitol. 2012, 98, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Barker, I.K.; Francis, C.A.; Heagy, A.; Lindsay, L.R.; Hobson, K.A. How far north are migrant birds transporting the tick Ixodes scapularis in Canada? Insights from stable hydrogen isotope analyses of feathers. Ticks Tick-Borne Dis. 2015, 6, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D. Studies abound on how far north Ixodes scapularis ticks are transported by birds. Ticks Tick-Borne Dis. 2016, 7, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Bouchard, C.; Kurtenbach, K.; Margos, G.; Lindsay, L.R.; Trudel, L.; Nguon, S.; Milord, F. Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of Lyme disease risk emergence in Canada. Environ. Health Perspect. 2010, 118, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Lieske, D.J.; Lloyd, V.K. Combining public participatory surveillance and occupancy modelling to predict the distributional response of Ixodes scapularis to climate change. Ticks Ticks Tick Borne-Dis. 2018, 9, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Lyme Disease 2016 Case Definition; Public Health Agency of Canada: Ottawa, ON, Canada, 2016. Available online: https://www.canada.ca/en/public-health/services/diseases/lyme-disease/surveillance-lyme-disease/case-definition.html (accessed on 18 June 2018).
- Gasmi, S.; Ogden, N.H.; Leighton, P.A.; Adam-Poupart, A.; Milord, F.; Lindsay, L.R.; Barkati, S.; Thivierge, K. Practices of Lyme disease diagnosis and treatment by general practitioners in Quebec, 2008–2015. BMC Fam. Pract. 2017, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Office of the Chief Medical Officer of Health, Government of New Brunswick. 2016 Survey of New Brunswick Family Physicians: Lyme Disease Awareness, Knowledge and Practice. Available online: https://www2.gnb.ca/content/dam/gnb/Departments/h-s/pdf/en/CDC/SurveyFamilyPhysicians.pdf (accessed on 1 September 2018).
- Hinckley, A.F.; Connally, N.P.; Meek, J.I.; Johnson, B.J.; Kemperman, M.M.; Feldman, K.A.; White, J.L.; Mead, P.S. Lyme disease testing by large com- mercial laboratories in the United States. Clin. Infect. Dis. 2014, 59, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A.; Saha, S.; Kugeler, K.J.; Delorey, M.J.; Shankar, M.B.; Hinckley, A.F.; Mead, P.S. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg. Infect. Dis. 2015, 21, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Lyme Disease in Canada: A Federal Framework; Public Health Agency of Canada: Ottawa, ON, Canada, 2017. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/lyme-disease-canada-federal-framework.html (accessed on 26 June 2018).
- Payne, E. Sharp Rise in Lyme Disease Cases a Concern to Chief Medical Officer. 13 May 2016. Available online: http://ottawacitizen.com/news/local-news/sharp-rise-in-lyme-disease-cases-a-concern-to-chief-medical-officer (accessed on 24 June 2018).
- Henry, B.; Roth, D.; Reilly, R.; MacDougall, L.; Mak, S.; Li, M.; Muhamad, M. How big is the Lyme problem? Using novel methods to estimate the true number of Lyme disease cases in British Columbia residents from 1997 to 2008. Vector Borne Zoo Dis. 2011, 11, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Zubek, E. The Lyme law. Can. Med. Assoc. J. 2015, 187, 520. [Google Scholar] [CrossRef] [PubMed]
- Mosseler, A.; Lynds, J.A.; Major, J.E. Old-growth forests of the Acadian forest region. Environ. Rev. 2003, 11, S47–S77. [Google Scholar] [CrossRef]
- LoGiudice, K.; Ostfeld, R.S.; Schmidt, K.A.; Keesing, F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. USA 2003, 100, 567–571. [Google Scholar] [CrossRef] [PubMed]
- McPherson, M.; García-García, A.; Cuesta-Valero, F.J.; Beltrami, H.; Hansen-Ketchum, P.; MacDougall, D.; Ogden, N.H. Expansion of the Lyme disease vector Ixodes scapularis in Canada inferred from CMIP5 climate projections. Environ. Health Perspect. 2017, 125, 057008. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Lyme Disease Data and Statistics. Available online: https://www.cdc.gov/lyme/stats/index.html (accessed on 18 June 2018).
- Office of the Chief Medical Officer of Health (Public Health). Communicable Disease Control, 2016 NB CD Annual Report, Surveillance Annual Report 2014. Available online: http://www2.gnb.ca/content/gnb/en/departments/ocmoh/for_healthprofessionals/cdc.html (accessed on 26 June 2018).
- Ogden, N.H.; Arsenault, J.; Hatchette, T.F.; Mechai, S.; Lindsay, L.R. Antibody responses to Borrelia burgdorferi detected by western blot vary geographically in Canada. PLoS ONE 2017, 12, e0171731. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Trudel, L.; Artsob, H.; Barker, I.K.; Beauchamp, G.; Charron, D.F.; Drebot, M.A.; Galloway, T.D.; O’Handley, R.; Thompson, R.A.; et al. Ixodes scapularis ticks collected by passive surveillance in Canada: Analysis of geographic distribution and infection with Lyme borreliosis agent Borrelia burgdorferi. J. Med. Entomol. 2006, 43, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Dibernardo, A.; Cote, T.; Ogden, N.H.; Lindsay, L.R. The prevalence of Borrelia miyamotoi infection, and co-infections with other Borrelia spp. in Ixodes scapularis ticks collected in Canada. Parasites Vectors 2014, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J. Assessing the Risk of Lyme Disease in the Canadian Maritimes. Master’s Thesis, Mount Allison University, Sackville, NB, Canada, 2016. [Google Scholar]
- Bjurman, N.K.; Bradet, G.; Lloyd, V.K. Lyme disease risk in dogs in New Brunswick. Can. Vet. J. 2016, 57, 981–984. [Google Scholar] [PubMed]
- Herrin, B.H.; Peregrine, A.S.; Goring, J.; Beall, M.J.; Little, S.E. Canine infection with Borrelia burgdorferi, Dirofilaria immitis, Anaplasma spp. and Ehrlichia spp. in Canada, 2013–2014. Parasites Vectors 2017, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Evason, M.; Stull, J.; Buch, J.; Lailer, Z.; O’Connor, T.; Chandrashekar, R.; Jardine, C.; Pearl, D.; Peregrine, A.; Weese, J.S. Prevalence of Borrelia burgdorferi, Anaplasma spp., Ehrlichia spp. and Dirofilaria immitis in Canadian Dogs 2007–2016. J. Vet. Intern. Med. 2017, 31, 1591. [Google Scholar]
- Ogden, N.H.; Koffi, J.K.; Pelcat, Y.; Lindsay, L.R. Environmental risk from Lyme disease in central and eastern Canada: A summary of recent surveillance information. Can. Commun. Dis. Rep. 2014, 40, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.B.; Auckland, L.D.; Marra, P.P.; Hamer, S.A. Avian migrants facilitate invasions of neotropical ticks and tick-borne pathogens into the United States. Appl. Environ. Microbiol. 2015, 81, 8366–8378. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Stanek, G.; Kundi, M.; Kocianova, E. Dimensions of engorging Ixodes ricinus as a measure of feeding duration. Int. J. Med. Microbiol. 2005, 295, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Khatchikian, C.E.; Prusinski, M.A.; Stone, M.; Backenson, P.B.; Wang, I.N.; Foley, E.; Seifert, S.N.; Levy, M.Z.; Brisson, D. Recent and rapid population growth and range expansion of the Lyme disease tick vector, Ixodes scapularis, in North America. Evolution 2015, 69, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Hatchette, T.F.; Johnston, B.L.; Schleihauf, E.; Mask, A.; Haldane, D.; Drebot, M.; Baikie, M.; Cole, T.J.; Fleming, S.; Gould, R.; et al. Epidemiology of Lyme Disease, Nova Scotia, Canada, 2002–2013. Emerg. Infect. Dis. 2015, 21, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Branda, J.A.; Linskey, K. Two-Tiered Antibody Testing for Lyme Disease With Use of 2 Enzyme Immunoassays, a Whole-Cell Sonicate Enzyme Immunoassay Followed by a VlsE C6 Peptide Enzyme Immunoassay. Clin. Infect. Dis. 2011, 53, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Liveris, D.; Hanincová, K.; Brisson, D.; Ludin, S.; Stracuzzi, V.J.; Embers, M.E.; Philipp, M.T.; Levin, A.; Maria, A.R.; et al. Effect of Borrelia burgdorferi Genotype on the Sensitivity of C6 and 2-Tier Testing in North American Patients with Culture-Confirmed Lyme Disease. Clin. Infect. Dis. 2008, 47, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Waddell, L.A.; Greig, J.; Mascarenhas, M.; Harding, S.; Lindsay, R.; Ogden, N. The accuracy of diagnostic tests for Lyme disease in humans, a systematic review and meta-analysis of north American research. PLoS ONE 2016, 11, e0168613. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.H.; Beebee, H.; Lasserson, D.S. Philosophy of science and the diagnostic process. Fam. Pract. 2013, 30, 501–505. [Google Scholar] [CrossRef]
- Habegger, S. Lyme Disease in Canada: An Update on Case Definitions and Treatments; Purple Paper; National Collaborating Centre for Infectious Diseases (NCCID): Winnipeg, MB, Canada, 2014; Volume 44, pp. 1–10. [Google Scholar]
- Schulze, T.L.; Bosler, E.M.; Shisler, J.K.; Ware, I.C.; Lakat, M.F.; Parkin, W.E. Prevalence of canine Lyme disease from an endemic area as determined by serosurvey. Zentralbl. Bakteriol. Mikrobiol. Hyg. Ser. A 1986, 263, 427–434. [Google Scholar] [CrossRef]
- Magnarelli, L.A.; Anderson, J.F.; Schreier, A.B.; Ficke, C.M. Clinical and serologic studies of canine borreliosis. J. Am. Vet. Med. Assoc. 1987, 191, 1089–1094. [Google Scholar] [PubMed]
- Eng, T.R.; Wilson, M.L.; Spielman, A.; Lastavica, C.C. Greater risk of Borrelia burgdorferi infection in dogs than in people. J. Infect. Dis. 1988, 158, 1410–1411. [Google Scholar] [CrossRef] [PubMed]
- Rand, P.W.; Smith, R.P., Jr.; Lacombe, E.H. Canine seroprevalence and the distribution of Ixodes dammini in an area of emerging Lyme disease. Am. J. Public Health 1991, 81, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.J.; Fish, D.; Levine, J.F.; Greco, M.A.; Eaton, A.T.; Padgett, P.J.; LaPointe, D.A. Canine exposure to Borrelia burgdorferi and prevalence of Ixodes dammini (Acari: Ixodidae) on deer as a measure of Lyme disease risk in the northeastern United States. J. Med. Entomol. 1993, 30, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Falco, R.C.; Smith, H.A.; Fish, D.; Mojica, B.A.; Bellinger, M.A.; Harris, H.L.; Hechemy, K.E. The distribution of canine exposure to Borrelia burgdorferi in a Lyme-Disease endemic area. Am. J. Public Health 1993, 83, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, J.M.; Marshall, D.; Onderdonk, A.B. Dogs as sentinels for Lyme disease in Massachusetts. Am. J. Public Health 1991, 81, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.D.; Stobierski, M.G.; Poplar, M.L.; Smith, T.W.; Murphy, A.J.; Smith, P.C.; Schmitt, S.M.; Cooley, T.M.; Kramer, C.M. Geographic distribution of ticks (Acari: Ixodidae) in Michigan, with emphasis on Ixodes scapularis and Borrelia burgdorferi. J. Med. Entomol. 1998, 35, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.A.; Walker, E.D.; Kitron, U. Canine surveillance system for Lyme borreliosis in Wisconsin and northern Illinois: Geographic distribution and risk factor analysis. Am. J. Trop. Med. Hyg. 2001, 65, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Ginsberg, H.S.; Zhioua, E.; Whitworth, U.G., Jr.; Markowski, D.; Hyland, K.E.; Hu, R. Passive tick surveillance, dog seropositivity, and incidence of human Lyme disease. Vector Borne Zoonotic Dis. 2004, 4, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.W.; Correa, M.T.; Levine, J.F.; Breitschwerdt, E.B. The dog as a sentinel for human infection: Prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic States. Vector Borne Zoonotic Dis. 2005, 5, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Hamer, S.A.; Tsao, J.I.; Walker, E.D.; Mansfield, L.S.; Foster, E.S.; Hickling, G.J. Use of tick surveys and serosurveys to evaluate pet dogs as a sentinel species for emerging Lyme disease. Am. J. Vet. Res. 2009, 70, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mead, P.; Goel, R.; Kugeler, K. Canine serology as adjunct to human Lyme disease surveillance. Emerg. Infect. Dis. 2011, 17, 1710–1712. [Google Scholar] [CrossRef] [PubMed]
- Doby, J.M.; Chevrier, S.; Couatarmanach, A. Tick spirochetosis by Borrelia burgdorferi in dogs in the west of France: Systematic serological examination of 806 hounds and 88 military dogs from 14 departments. Recl. Med. Vet. 1988, 164, 367–374. [Google Scholar]
- Delgado, S.; Cármenes, P. Seroepidemiological survey for Borrelia burgdorferi (Lyme disease) in dogs from northwestern of Spain. Eur. J. Epidemiol. 1995, 11, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Isogai, E.; Isogai, H.; Sato, N.; Yuzawa, M.; Kawakami, M. Antibodies to Borrelia burgdorferi in dogs in Hokkaido. Microbiol. Immunol. 1990, 34, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Artsob, H.; Barker, I.K.; Fister, R.; Sephton, G.; Dick, D.; Lynch, J.A.; Key, D. Serological studies on the infection of dogs in Ontario with Borrelia burgdorferi, the etiological agent of Lyme disease. Can. Vet. J. 1993, 34, 543–548. [Google Scholar] [PubMed]
- Banerjee, S.; Stephen, C.; Fernando, K.; Coffey, S.; Dong, M. Evaluation of dogs as sero-indicators of the geographic distribution of Lyme borreliosis in British Columbia. Can. Vet. J. 1996, 37, 168–169. [Google Scholar] [PubMed]
- Gary, A.T.; Webb, J.A.; Hegarty, B.C.; Breitschwerdt, E.B. The low seroprevalence of tick-transmitted agents of disease in dogs from southern Ontario and Quebec. Can. Vet. J. 2006, 47, 1194–1200. [Google Scholar] [PubMed]
- Villeneuve, A.; Goring, J.; Marcotte, L.; Overvelde, S. Seroprevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, Ehrlichia canis, and Dirofilaria immitis among dogs in Canada. Can. Vet. J. 2011, 52, 527–530. [Google Scholar] [PubMed]
- Schurer, J.M.; Ndao, M.; Quewezance, H.; Elmore, S.A.; Jenkins, E.J. People, pets, and parasites: One health surveillance in southeastern Saskatchewan. Am. J. Trop. Med. Hyg. 2014, 90, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.J. Lyme borreliosis: A review of data on transmission time after tick attachment. Int. J. Gen. Med. 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.M.; Spielman, A. Accelerated transmission of Lyme disease spirochetes by partially fed vector ticks. J. Clin. Microbiol. 1993, 31, 2878–2881. [Google Scholar] [PubMed]
- Sertour, N.; Cotté, V.; Garnier, M.; Malandrin, L.; Ferquel, E.; Choumet, V. Infection kinetics and tropism of Borrelia burgdorferi sensu lato in mouse after natural (via ticks) or artificial (needle) infection depends on the bacterial strain. Front. Microbiol. 2018, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, R.; Mainville, C.A.; Beall, M.J.; O’Connor, T.; Eberts, M.D.; Alleman, A.R.; Gaunt, S.D.; Breitschwerdt, E.B. Performance of a commercially available in-clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrelia burgdorferi and Dirofilaria immitis antigen in dogs. Am. J. Vet. Res. 2010, 71, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Mather, T.N.; Fish, D.; Coughlin, R.T. Competence of dogs as reservoirs for Lyme disease spirochetes (Borrelia burgdorferi). J. Am. Vet. Med. Assoc. 1994, 205, 186–188. [Google Scholar] [PubMed]
- Philipp, M.T.; Bowers, L.C.; Fawcett, P.T.; Jacobs, M.B.; Liang, F.T.; Marques, A.R.; Mitchell, P.D.; Purcell, J.E.; Ratterree, M.S.; Straubinger, R.K. Antibody response to IR6, a conserved immunodominant region of the VlsE lipoprotein, wanes rapidly after antibiotic treatment of Borrelia burgdorferi infection in experimental animals and in humans. J. Infect. Dis. 2001, 184, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Szkotnicki, J. Canadian Animal Health Sector—An Overview of the Challenges and Opportunities. Int. Anim. Health J. 2014, 1, 15. [Google Scholar]
- Annual Estimates of Population for Canada, Provinces and Territories…Statistics Canada. Available online: www.stats.gov.nl.ca/statistics/population/pdf/annual_pop_prov.pdf (accessed on 27 June 2018).
- Focus on Geography Series, 2011 Census, Province of New Brunswick. Available online: http://www12.statcan.gc.ca/census-recensement/2011/as-sa/fogs-spg/Facts-pr-eng.cfm?Lang=Eng&GC=13 (accessed on 27 June 2018).
- Krupka, I.; Knauer, J.; Lorentzen, L.; O’Connor, T.P.; Saucier, J.; Straubinger, R.K. Borrelia burgdorferi sensu lato species in Europe induce diverse immune responses against C6 peptides in infected mice. Clin. Vaccine Immunol. 2009, 16, 1546–1562. [Google Scholar] [CrossRef]
- Pachner, A.R.; Delaney, E.; O’Neill, T.; Major, E. Inoculation of non-human primates with the N40 strain of Borrelia burgdorferi leads to a model of Lyme neuroborreliosis faithful to the human disease. Neurology 1995, 45, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Du Cruz, I.; Narciso, W.; Lo, W.; Harris, N.S. Improved sensitivity of Lyme disease western blots prepared with a mixture of Borrelia Burgdorferi strains 297 and B31. Chronic Dis. Int. 2014, 1, 1–7. [Google Scholar]
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Barbour, A.G.; Platonov, A.E.; Brancato, J.; Lepore, T.; Dardick, K.; Mamula, M.; Rollend, L.; et al. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the Northeastern United States. Emerg. Infect. Dis. 2014, 20, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Makhani, N.; Morris, S.K.; Page, A.V.; Brophy, J.; Lindsay, L.R.; Banwell, B.L.; Richardson, S.E. A twist on Lyme: The challenge of diagnosing European Lyme neuroborreliosis. J. Clin. Microbiol. 2011, 49, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Sudhindra, P.; Wang, G.; Schriefer, M.E.; McKenna, D.; Zhuge, J.; Krause, P.J.; Marques, A.R.; Wormser, G.P. Insights into Borrelia miyamotoi infection from an untreated case demonstrating relapsing fever, monocytosis and a positive C6 Lyme serology. Diagn. Microbiol. Infect. Dis. 2016, 86, 93–96. [Google Scholar] [CrossRef]
- Ogden, N.H.; Margos, G.; Aanensen, D.M.; Drebot, M.A.; Feil, E.J.; Hanincová, K.; Schwartz, I.; Tyler, S.; Lindsay, L.R. Investigation of genotypes of Borrelia burgdorferi in Ixodes scapularis ticks collected during surveillance in Canada. BMC Public Health 2014, 14, 1298. [Google Scholar] [CrossRef] [PubMed]
- Tyler, S.; Tyson, S.; Dibernardo, A.; Drebot, M.; Feil, E.J.; Graham, M.; Knox, N.C.; Lindsay, L.R.; Margos, G.; Mechai, S.; et al. Whole genome sequencing and phylogenetic analysis of strains of the agent of Lyme disease Borrelia burgdorferi from Canadian emergence zones. Sci. Rep. 2018, 8, 10552. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, K.; Gretchen, A. Higher sensitivity of recomLine Borrelia IgG Immunoblot Kit compared with Standard Lyme IgG Immunoblot Following CDC Testing Criteria. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Schriefer, M.; Aguero-Rosenfeld, M.E.; Levin, A.; Steere, A.C.; Nadelman, R.B.; Nowakowski, J.; Marques, A.; Johnson, B.J.; Dumler, J.S. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn. Microbiol. Infect. Dis. 2013, 75, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Meek, J.I.; Roberts, C.L.; Smith, E.V., Jr.; Cartter, M.L. Underreporting of Lyme disease by Connecticut physicians, 1992. J. Public Health Manag. Pract. 1996, 2, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Surveillance of Lyme Disease; Government of Canada: Ottawa, ON, Canada. Available online: https://www.canada.ca/en/public-health/services/diseases/lyme-disease/surveillance-lyme-disease.html#a3 (accessed on 27 June 2018).
- Nadelman, R.B.; Nowakowski, J.; Fish, D.; Falco, R.C.; Freeman, K.; McKenna, D.; Welch, P.; Marcus, R.; Agüero-Rosenfeld, M.E.; Dennis, D.T.; et al. Tick Bite Study Group. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N. Engl. J. Med. 2001, 345, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R. False-negative serology in patients with neuroborreliosis and the value of employing of different borrelial strains in serological assays. J. Med. Microbiol. 2000, 49, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; McHugh, G.; Damle, N.; Sikand, V.K. Prospective study of serologic tests for Lyme disease. Clin. Infect. Dis. 2008, 47, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Donta, S.T. Issues in the diagnosis and treatment of Lyme disease. Open Neurol. J. 2012, 6, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; HasenKampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in Rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE 2012, 7, e29914. [Google Scholar] [CrossRef]
- Wormser, G.P.; Tang, A.T.; Schimmoeller, N.R.; Bittker, S.; Cooper, D.; Visintainer, P.; Aguero-Rosenfeld, M.E.; Ogrinc, K.; Strle, F.; Stanek, G. Utility of serodiagnostics designed for use in the United States for detection of Lyme borreliosis acquired in Europe and vice versa. Med. Microbiol. Immunol. 2014, 203, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Elsner, R.A.; Hastey, C.J.; Olsen, K.J.; Baumgarth, N. Suppression of long-lived humoral immunity following Borrelia burgdorferi infection. PLoS Pathog. 2015, 11, e1004976. [Google Scholar] [CrossRef] [PubMed]
- Wagemakers, A.; Visser, M.C.; de Wever, B.; Hovius, J.W.; van de Donk, N.W.C.J.; Hendriks, E.J.; Peferoen, L.; Muller, F.F.; Ang, C.W. Case report: Persistently seronegative neuroborreliosis in an immunocompromised patient. BMC Infect. Dis. 2018, 18, 362. [Google Scholar] [CrossRef] [PubMed]
- Aenishaenslin, C.; Ravel, A.; Michel, P.; Gern, L.; Milord, F.; Waaub, J.P.; Bélanger, D. From Lyme disease emergence to endemicity: A cross sectional comparative study of risk perceptions in different populations. Appl. Environ. Microbiol. 2011, 77, 3244–3254. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Christen, B.; Leung, D.; Vigo-Pelfrey, C. Serodiagnosis of Lyme borreliosis by western immunoblot: Reactivity of various significant antibodies against Borrelia burgdorferi. J. Clin. Microbiol. 1992, 30, 370–376. [Google Scholar] [PubMed]
- Boudreau, C.R.; Lloyd, V.K.; Gould, O.N. Motivations and experiences of Canadians seeking treatment for Lyme disease outside of the conventional Canadian health-care system. J. Patient Exp. 2018, 5, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Meltzer, M.I.; Pena, C.A.; Hopkins, A.B.; Wroth, L.; Fix, A.D. Economic impact of Lyme disease. Emerg. Infect. Dis. 2006, 12, 653–660. [Google Scholar] [CrossRef] [PubMed]
| Province | Canada Incidence | US Incidence | Fold Difference | |||
|---|---|---|---|---|---|---|
| 2014 | 2016 | 2014 | 2016 | 2014 | 2016 | |
| AB | 0.05 | 0.2 | 0.5 (MT) | 1.2 (MT) | 10.0 | 6.0 |
| MB | 0.64 | 3.9 | 43.43 (ND, MN) | 103.47 (ND, MN) | 67.9 | 26.5 |
| ON | 0.72 | 2.7 | 11.82 (MN, WI, MI, NY) | 20.75 (MN, WI, MI, NY) | 16.4 | 7.7 |
| QC | 0.21 | 2.1 | 20.55 (NY, VT, ME) | 31.14 (NY, VT, ME) | 97.9 | 14.8 |
| NB | 0.66 | 1.5 | 87.9 (ME) | 86.4 (ME) | 133.2 | 57.6 |
| NS | 5.8 | 34.4 | 87.9 (ME) | 86.4 (ME) | 15 | 2.5 |
| ELISA outcome | Positive (Predicted) | Negative (Predicted) | Total (Observed) |
|---|---|---|---|
| WCS ELISA + | 88 | 127 | 215 |
| WCS ELISA − | 6 | 1634 | 1640 |
| Total | 94 | 1761 | 1855 |
| Parameter | Value | ||
| Prevalence | 5.1% | ||
| Sensitivity | 93.6% | ||
| Specificity | 92.8% | ||
| Positive Predictive Value | 40.9% | ||
| Negative Predictive Value | 99.6% | ||
| Likelihood ratio (positive) | 12.98 | ||
| Likelihood ratio (negative) | 0.07 | ||
| ELISA outcome | Lyme Disease Present | Lyme Disease Absent | Total |
|---|---|---|---|
| C6 ELISA test positive | 39 | 22 | 61 |
| C6 ELISA test negative | 13 | 1412 | 1425 |
| Total | 52 | 1434 | 1486 |
| Parameter | Value | ||
| Prevalence | 3.5% | ||
| Sensitivity | 75.0% | ||
| Specificity | 98.5% | ||
| Positive Predicted Value | 63.9% | ||
| Negative Predicted Value | 99.1% | ||
| Likelihood Ratio (positive) | 48.89 | ||
| Likelihood Ratio (negative) | 0.25 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lloyd, V.K.; Hawkins, R.G. Under-Detection of Lyme Disease in Canada. Healthcare 2018, 6, 125. https://doi.org/10.3390/healthcare6040125
Lloyd VK, Hawkins RG. Under-Detection of Lyme Disease in Canada. Healthcare. 2018; 6(4):125. https://doi.org/10.3390/healthcare6040125
Chicago/Turabian StyleLloyd, Vett K., and Ralph G. Hawkins. 2018. "Under-Detection of Lyme Disease in Canada" Healthcare 6, no. 4: 125. https://doi.org/10.3390/healthcare6040125
APA StyleLloyd, V. K., & Hawkins, R. G. (2018). Under-Detection of Lyme Disease in Canada. Healthcare, 6(4), 125. https://doi.org/10.3390/healthcare6040125