Have Studies that Measure Lumbar Kinematics and Muscle Activity Concurrently during Sagittal Bending Improved Understanding of Spinal Stability and Sub-System Interactions? A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
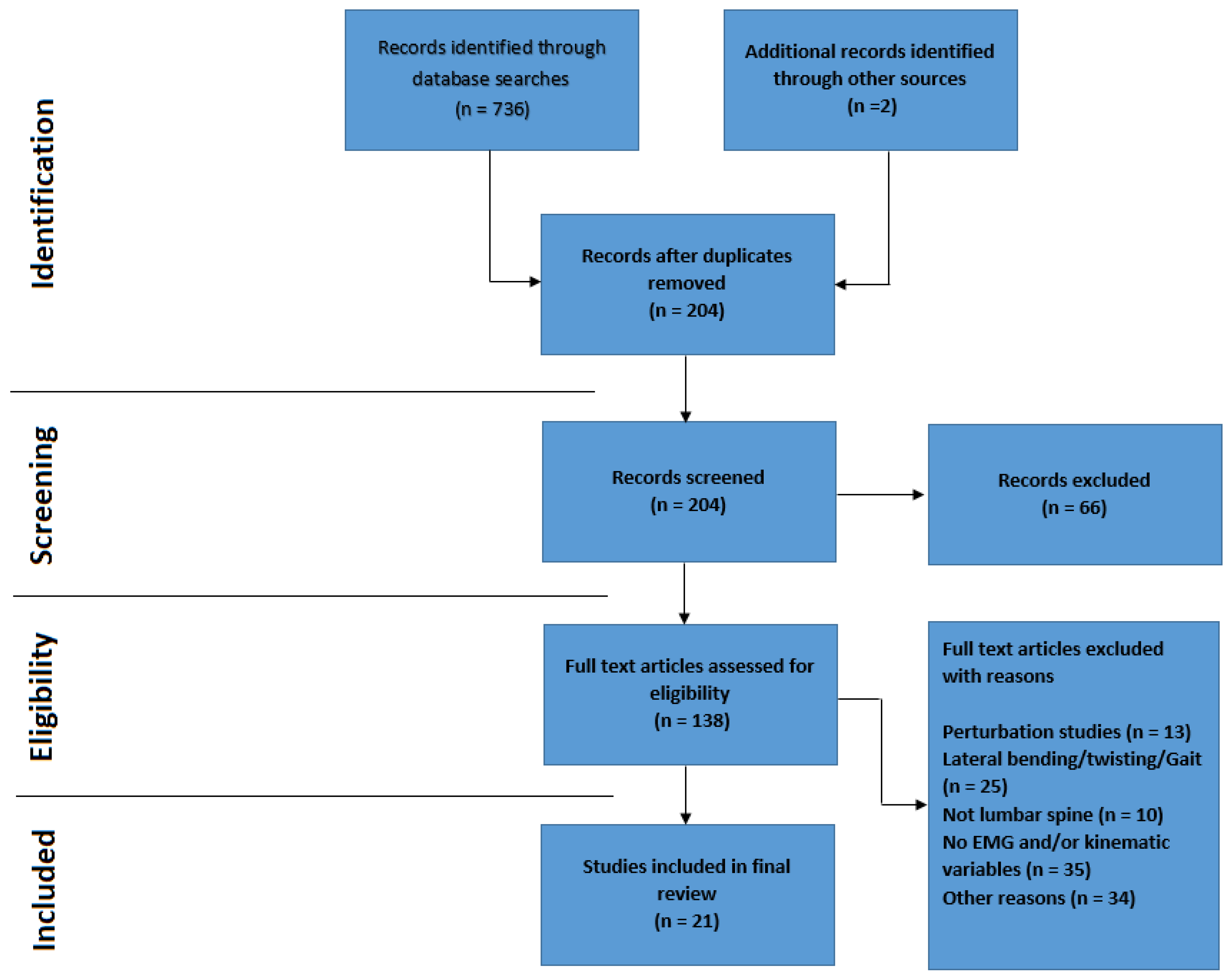
2.3. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)
2.4. Study Quality Assessment
2.4.1. Overall Quality Assessment
2.4.2. Specific EMG Quality Assessment
3. Results
3.1. Overall and EMG Quality Assessment
3.2. General Characteristics of the Reviewed Studies
3.3. Comparing Healthy Control and Low Back Pain Groups
3.4. Flexion Relaxation Studies
3.5. Models
4. Discussion
4.1. Quality Assessment
4.2. Spinal Stability and Sub-System Interaction
4.3. Can the Information Aquired by Combining Lumbar Kinematic and Muscle Activity Measurements during Functional Movements Assist in Distinguishing between Groups of Healthy Controls and Those with Low Back Pain?
4.4. Are There Opportunities to Improve Understanding of Sub-System Interactions and Low Back Pain Using Studies That Utilise Kinematic and EMG Measurements Concurrently?
4.5. Key findings and recommendations
- Increased muscle activity and co-contraction are strategies adopted to stabilise the lumbar spine.
- Whilst generalised conclusions regarding spinal stabilisation were seen throughout the literature, insights into the understanding of spinal sub-system interactions at the motion segment level were limited.
- Parameters shown to distinguish between non-LBP and LBP populations include spinal ROM and trunk muscle activation, including the FRP. Typically, LBP groups demonstrated comparatively reduced ROM, increased muscle activity and an absent FRP.
- Future studies should consider more frequent use of sub-divided regional or inter-vertebral kinematic measurements, and would benefit from methodological standardisation.
- More extensive exploration of thoracic kinematic and muscle activity parameters may be beneficial in order to enhance understanding of lumbar spinal stabilisation mechanisms.
4.6. Limitations
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Panjabi, M.M. The stabilising system of the spine—Part 1: Function, dysfunction, adaptation and enhancement. J. Spinal Disord. 1992, 5, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M. The stabilising system of the spine—Part 2: Neutral zone and instability hypothesis. J. Spinal Disord. 1992, 5, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.W.; Li, L.; Solomonow, M. Flexion-relaxation response to cyclic lumbar flexion. Clin. Biomech. 2004, 19, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Mirka, G.A. An in vivo assessment of the low back response to prolonged flexion: Interplay between active and passive tissues. Clin. Biomech. 2007, 22, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Van Dieen, J.H.; Cholewicki, J.; Radebold, A. Trunk Muscle Recruitment Patterns in Patients With Low Back Pain Enhance the Stability of the Lumbar Spine. Spine 2003, 28, 834–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lariviere, C.; Gagnon, D.; Loisel, P. The comparison of trunk muscles EMG activiation between subjects with and without chronic low back pain during flexion-extension and lateral bending tasks. J. Electromyogr. Kinesiol. 2000, 10, 79–91. [Google Scholar] [CrossRef]
- Dankaerts, W.; O’Sullivan, P.B.; Burnett, A.F.; Straker, L.M.; Davey, P.; Gupta, R. Discriminating Healthy Controls and Two Clinical Subgroups of Nonspecific Chronic Low Back Pain Patients Using Trunk Muscle Activation and Lumbosacral Kinematics of Postures and Movements. Spine 2009, 34, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Silfies, S.P.; Mehta, R.; Smith, S.S.; Karduna, A.R. Differences in Feedforward Trunk Muscle Activity in Subgroups of Patients With Mechanical Low Back Pain. Arch. Phys. Med. Rehabil. 2009, 90, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Granata, K.P.; Orishimo, K.F. Response of trunk muscle coactivation to changes in spinal stability. J. Biomech. 2001, 34, 1117–1123. [Google Scholar] [CrossRef]
- Sanchez-Zuriaga, D.; Adams, M.A.; Dolan, P. Is Activation of the Back Muscles Impaired by Creep or Muscle Fatigue? Spine 2010, 35, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Abboud, J.; Nougarou, F.; Descarreaux, M. Muscle Activity Adaptations to Spinal Tissue Creep in the Presence of Muscle Fatigue. PLoS ONE 2016, 11, e0149076. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, B.; Bazrgari, B.; Muslim, K.; Toosizadeh, N.; Nussbaum, M.A.; Madigan, M.L. Disturbance and recovery of trunk stiffness and reflexive muscle responses following prolonged trunk flexion: Influences of flexion angle and duration. Clin. Biomech. 2011, 26, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Abboud, J.; Lardon, A.; Boivin, F.; Duga, C.; Descarreaux, M. Effects of Muscle Fatigue, Creep, and Musculoskeletal Pain on Neuromuscular Responses to Unexpected Perturbation of the Trunk: A Systematic Review. Front. Hum. Neurosci. 2017, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Floyd, W.F.; Silver, P.H.S. The function of the erectores spinae muscles in certain movements and postures in man. J. Physiol. 1955, 129, 184–203. [Google Scholar] [CrossRef] [PubMed]
- McGill, S.M.; Kippers, V. Transfer of loads between lumbar tissues during the flexion-relaxation phenomenon. Spine 1994, 19, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Luhring, S.; Schinkel-Ivy, A.; Drake, J.D.M. Evaluation of the lumbar kinematic measures that most consistently characterize lumbar muscle activation patterns during trunk flexion: A cross-sectional study. J. Manip. Physiol. Ther. 2015, 38, 44–50. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, P.P.; Dankaerts, W.P.; Burnett, A.P.; Chen, D.M.; Booth, R.M.; Carlsen, C.M.; Schultz, A.M. Evaluation of the Flexion Relaxation Phenomenon of the Trunk Muscles in Sitting. Spine 2006, 31, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Sarti, M.A.; Lison, J.F.; Monfort, M.; Fuster, M.A. Response of the Flexion-Relaxation Phenomenon Relative to the Lumbar Motion to Load and Speed. Spine 2001, 26, E421–E426. [Google Scholar] [CrossRef] [PubMed]
- Descarreaux, M.; Lafond, D.; Jeffrey-Gauthier, R.; Centomo, H.; Cantin, V. Changes in the flexion relaxation response induced by lumbar muscle fatigue. BMC Musculoskelet. Disord. 2008, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Kaigle, A.M.; Wesberg, P.; Hansson, T.H. Muscular and kinematic behavior of the lumbar spine during flexion-extension. J. Spinal Disord. 1998, 11, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Solomonow, M.; Zhou, B.H.; Harris, M.; Lu, Y.; Baratta, R.V. The ligamento-muscular stabilizing system of the spine. Spine 1998, 23, 2552–2562. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Macedo, L.G. Multifidus and Paraspinal Muscle Group Cross-Sectional Areas of Patients With Low Back Pain and Control Patients: A Systematic Review with a Focus on Blinding. Phys. Ther. 2013, 93, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Haq, I.; Lee, R.Y. An Investigation Into the Onset, Pattern, and Effects of Pain Relief on Lumbar Extensor Electromyography in People with Acute and Chronic Low Back Pain. J. Manip. Physiol. Ther. 2013, 36, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Nelson-Wong, E.; Alex, B.; Csepe, D.; Lancaster, D.; Callaghan, J.P. Altered muscle recruitment during extension from trunk flexion in low back pain developers. Clin. Biomech. 2012, 27, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Reeves, N.P.; Cholewicki, J.; Silfies, S.P. Muscle activation imbalance and low-back injury in varsity athletes. J. Electromyogr. Kinesiol. 2006, 16, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Zuriaga, D.; Lopez-Pascual, J.; Garrido-Jaen, D.; Garcia-Mas, M.A. A Comparison of Lumbopelvic Motion Patterns and Erector Spinae Behavior Between Asymtomatic Subjects and Patients with Recurrent Low Back Pain During Pain-Free Periods. J. Manip. Physiol. Ther. 2015, 38, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Ahern, D.K.; Follick, M.J.; Council, J.R.; Laserwolston, N.; Litchman, H. Comparison of lumbar paravertebral Emg patterns in chronic low back pain patients and non-patient controls. Pain 1988, 34, 153–160. [Google Scholar] [CrossRef]
- Kuriyama, N.; Ito, H. Electromyographic Functional Analysis of the Lumbar Spinal Muscles with Low Back Pain. J. Nippon. Med Sch. 2005, 72, 165–173. [Google Scholar] [CrossRef] [PubMed]
- McGregor, A.H.; McCarthy, I.D.; Dore, C.J.; Hughes, S.P. Quantitative assessment of the motion of the lumbar spine in the low back pain population and the effect of different spinal pathologies on this motion. Eur. Spine J. 1997, 6, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, J.; Fritz, J.; McCane, B.; Shultz, B.; Herbison, P.; Lyons, B.; Stefanko, G.; Walsh, R. Lumbar segmental mobility disorders: Comparison of two methods of defining abnormal displacement kinematics in a cohort of patients with non-specific mechanical low back pain. BMC Musculoskelet. Disord. 2006, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Kulig, K.; Powers, C.; Landel, R.; Chen, H.; Fredericson, M.; Guillet, M.; Butts, K. Segmental lumbar mobility in individuals with low back pain: In vivo assessment during manual and self-imposed motion using dynamic MRI. BMC Musculoskelet. Disord. 2007, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Teyhen, D.S.; Flynn, T.W.; Childs, J.D.; Kuklo, T.R.; Rosner, M.K.; Polly, D.W.; Abraham, L.D. Fluoroscopic Video to Identify Aberrant Lumbar Motion. Spine 2007, 32, E220–E229. [Google Scholar] [CrossRef] [PubMed]
- Mellor, F.E.; Thomas, P.; Thompson, P.; Breen, A.C. Proportional lumbar spine inter-vertebral motion patterns: A comparison of patients with chronic non-specific low back pain and healthy controls. Eur. Spine J. 2014, 23, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.A.; Amin, A.; Omeis, I.; Gokaslan, Z.L.; Gottfried, O.N. Implications of Spinopelvic Alignment for the Spine Surgeon. Neurosurgery 2012, 70, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Peach, J.P.; Sutarno, C.G.; McGill, S.M. Three-Dimensional Kinematics and Trunk Muscle Myoelectric Activity in the Young Lumbar Spine: A Database. Arch. Phys. Med. Rehabil. 1998, 79, 663–669. [Google Scholar] [CrossRef]
- Mitchell, T.; O’Sullivan, P.B.; Burnett, A.F.; Straker, L.; Smith, A. Regional differences in lumbar spinal posture and the influence of low back pain. BMC Musculoskelet. Disord. 2008, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Hemming, R.; Sheeran, L.; van Deursen, R.; Martin, R.W.; Sparkes, V. Regional spinal kinematics during static postures and functional tasks in people with non-specific chronic low back pain. Int. J. Ther. Rehabil. 2015, 22, S8. [Google Scholar] [CrossRef]
- Colloca, C.J.; Hinrichs, R.N. The biomechanical and clinical significance of the lumbar erector spinae flexion-relaxation phenomenon: A review of literature. J. Manip. Physiol. Ther. 2005, 28, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hagg, G. European Recommendations for Surface Electromyography: Results of the SENIAM Project; Roessingh Research and Development: Enschede, The Netherlands, 1999. [Google Scholar]
- Arjmand, N.; Gagnon, D.; Plamondon, A.; Shirazi-Adl, S.; Lariviere, C. A comparative study of two trunk biomechanical models under symmetric and asymmetric loadings. J. Biomech. 2010, 43, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.F.; Cornelius, M.W.; Dankaerts, W.; O’Sullivan, P.B. Spinal kinematics and trunk muscle activity in cyclists: A comparison between healthy controls and non-specific chronic low back pain subjects—A pilot investigation. Man. Ther. 2004, 9, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, J.C.; Dunk, N.M. Examination of the flexion relaxation phenomenon in erector spinae muscles during short duration slumped sitting. Clin. Biomech. 2002, 17, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Cholewicki, J.; Panjabi, M.M.; Khachatryan, A. Stabilizing function of trunk flexor-extensor muscles around a neutral spine posture. Spine 1997, 22, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Hashemirad, F.; Talebian, S.; Hatef, B.; Kahlaee, A.H. The relationship between flexibility and EMG activity pattern of the erector spinae muscles during trunk flexion-extension. J. Electromyogr. Kinesiol. 2009, 19, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.; Wachowiak, M.P.; Graham, R.B. Evaluating the relationship between muscle activation and spine kinematics through wavelet coherence. J. Appl. Biomech. 2016, 32, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Kienbacher, T.; Ferhmann, E.; Habenicht, R.; Koller, D.; Oeffel, C.; Kollmitzer, J.; Mair, P.; Ebenbichler, G.R. Age and gender related neuromuscular pattern during trunk flexion-extension in chronic low back pain patients. J. NeuroEng. Rehabil. 2016, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, J.; Hu, Y. Network modeling and analysis of lumbar muscle surface EMG signals during flexion–extension in individuals with and without low back pain. J. Electromyogr. Kinesiol. 2011, 21, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Mayer, T.; Neblett, R.; Brede, E.; Gatchel, R.J. The Quantified Lumbar Flexion-Relaxation Phenomenon Is a Useful Measurement of Improvement in a Functional Restoration Program. Spine 2009, 34, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Nairn, B.; Chisholm, S.; Drake, J.D.M. What is slumped sitting? A kinematic and electromyographical evaluation. Man. Ther. 2013, 18, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Neblett, R.; Mayer, T.G.; Gatchel, R.J.; Keeley, J.; Proctor, T.; Anagnostis, C. Quantifying the Lumbar Flexion-Relaxation Phenomenon: Theory, Normative Data, and Clinical Applications. Spine 2003, 28, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Jin, S.; Mirka, G.A. Describing the active region boundary of EMG-assisted biomechanical models of the low back. Clin. Biomech. 2012, 27, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquet, N.; Malouin, F.; Richards, C.L. Hip-Spine Movement Interaction and Muscle Activation Patterns During Sagittal Trunk Movements in Low Back Pain Patients. Spine 1994, 19, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Bergmark, A. Stability of the lumbar spine: A study in mechanical engineering. Acta Orthop. Scand. 1989, 60, 1–54. [Google Scholar] [CrossRef]
- Cerveri, P.; Pedotti, A.; Ferrigno, G. Kinematical models to reduce the effect of skin artifacts on marker-based human motion estimation. J. Biomech. 2005, 38, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, J. Model-guided derivation of lumbar vertebral kinematics in vivo reveals the difference between external marker-defined and internal segmental rotations. J. Biomech. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Ogston, N.G.; King, G.J.; Gertzbein, S.D.; Tile, M.M.D.; Kapasouri, A.; Rubenstein, J.D. Centrode Patterns in the Lumbar Spine: Baseline Studies in Normal Subjects. Spine 1986, 11, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Pearcy, M.J.; Portek, I.; Shepherd, J. Three dimensional x-ray analysis of normal movement in the lumbar spine. Spine 1984, 9, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Maroufi, N.; Behtash, H.; Zekavat, H.; Parnianpour, M. Kinematic analysis of dynamic lumbar motion in patients with lumbar segmental instability using digital videofluoroscopy. Eur. Spine J. 2009, 18, 1677–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breen, A.C.; Teyhen, D.S.; Mellor, F.E.; Breen, A.C.; Wong, K.W.N.; Deitz, A. Measurement of inter-vertebral motion using quantitative fluoroscopy: Report of an international forum and proposal for use in the assessment of degenerative disc disease in the lumbar spine. Adv. Orthop. 2012, 2012, 802350. [Google Scholar] [CrossRef] [PubMed]
- Du Rose, A.; Breen, A. Relationships between lumbar inter-vertebral motion and lordosis in healthy adult males: A cross sectional cohort study. BMC Musculoskelet. Disord. 2016, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Du Rose, A.; Breen, A. Relationships between Paraspinal Muscle Activity and Lumbar Inter-Vertebral Range of Motion. Healthcare 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Luk, K.; Leong, J.; Wong, S.; Wong, K. Continuous dynamic spinal motion analysis. Spine 2006, 31, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Breen, A.; Muggleton, J.; Mellor, F. An objective spinal motion imaging assessment (OSMIA): Reliability, accuracy and exposure data. BMC Musculoskelet. Disord. 2006, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Teyhen, D.S.; Flynn, T.W.; Bovik, A.C.; Abraham, L.D. A new technique for digital fluoroscopic video assessment of sagittal plane lumbar spine motion. Spine 2005, 30, E406–E413. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.S.; Cook, D.J.; Cheng, B.C. Reliability of computer-assisted lumbar intervertebral measurement using a novel vertebral motion analysis system. Spine J. 2014, 14, 274–281. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, P. Diagnosis and classificiation of chronic low back pain disorders: Maladaptive movement and motor control impairments as underlying mechanism. Man. Ther. 2005, 10, 242–255. [Google Scholar] [CrossRef] [PubMed]
| Quality Check | Category | Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors (year) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Score (/9 * or /10) | Score (%) |
| Arjmand et al. (2010) [41] | 0 | 1 | 0 | 1 | 1 | 0 | 0 | N/A | 1 | 0 | 4 * | 44 |
| Burnett et al. (2004) [42] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 90 |
| Callaghan and Dunk 2002 [43] | 1 | 0 | 0 | 1 | 1 | 0 | 1 | N/A | 1 | 1 | 6* | 67 |
| Cholewicki et al. (1997) [44] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | N/A | 1 | 1 | 7 * | 78 |
| Dankaerts et al. (2009) [7] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | 100 |
| Hashemirad et al. (2009) [45] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | N/A | 1 | 1 | 7 * | 78 |
| Hay et al. (2016) [46] | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | 60 |
| Kaigle et al. (1998) [20] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 8 | 80 |
| Kienbacher et al. (2016) [47] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | 100 |
| Lariviere et al. (2000) [6] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 | 90 |
| Liu et al. (2011) [48] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 7 | 70 |
| Luhring et al. (2015) [16] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | N/A | 1 | 1 | 8 * | 89 |
| Mayer et al. (2009) [49] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 7 | 70 |
| McGill and Kippers 1994 [15] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | N/A | 1 | 1 | 7 * | 78 |
| Nairn et al. (2013) [50] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | N/A | 1 | 1 | 7 * | 78 |
| Neblett et al. (2003) [51] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | N/A | 1 | 1 | 8 * | 89 |
| Ning et al. (2012) [52] | 1 | 1 | 0 | 1 | 1 | 0 | 0 | N/A | 1 | 1 | 6 * | 67 |
| O’Sullivan et al. (2006) [17] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | N/A | 1 | 1 | 9 * | 100 |
| Paquet et al. (1994) [53] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 9 | 90 |
| Peach et al. (1998) [35] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | N/A | 1 | 1 | 7 * | 78 |
| Sanchez-Zuriaga et al. (2015) [26] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 9 | 90 |
| EMG Quality Check | Category | Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors (year) | 1.1 | 1.2 | 1.3 | 2.1 | 2.2 | 2.3 | 2.4 | 3.1 | 3.2 | 3.3 | 3.4 | 4 | score (/3 * or /4) | score (%) |
| Arjmand et al. (2010) [41] | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 50 |
| Burnett et al. (2004) [42] | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 4 | 100 |
| Callaghan and Dunk 2002 [43] | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 50 |
| Cholewicki et al. (1997) [44] | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 50 |
| Dankaerts et al. (2009) [7] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 100 |
| Hashemerad et al. (2009) [45] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 100 |
| Hay et al. (2016) [46] | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | N/A | 2 * | 67 |
| Kaigle et al. (1998) [20] | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | N/A | 2 * | 67 |
| Kienbacher et al. (2016) [47] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 100 |
| Lariviere et al. (2000) [6] | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | N/A | 2 * | 67 |
| Liu et al. (2011) [48] | 0 | 0 | N/A | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 3 * | 100 |
| Luhring et al. (2015) [16] | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 3 | 75 |
| Mayer et al. (2009) [49] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 25 |
| McGill and Kippers 1994 [15] | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 3 | 75 |
| Nairn et al. (2013) [50] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 100 |
| Neblett et al. (2003) [51] | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | N/A | 2 * | 67 |
| Ning et al. (2012) [52] | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 50 |
| O’Sullivan et al. (2006) [17] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 100 |
| Paquet et al. (1994) [53] | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 25 |
| Peach et al. (1998) [35] | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 3 | 75 |
| Sanchez-Zuriaga et al. (2015) [26] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 100 |
| Authors (Year) | Quality Index Score (%) | EMG Quality Score (%) | Combined Score (%) |
|---|---|---|---|
| Arjmand et al. (2010) [41] | 44 | 50 | 47 |
| Burnett et al. (2004) [42] | 90 | 100 | 95 |
| Callaghan and Dunk 2002 [43] | 67 | 50 | 58.5 |
| Cholewicki et al. (1997) [44] | 78 | 50 | 64 |
| Dankaerts et al. (2009) [7] | 100 | 100 | 100 |
| Hashemerad et al. (2009) [45] | 78 | 100 | 89 |
| Hay et al. (2016) [46] | 60 | 67 | 63.5 |
| Kaigle et al. (1998) [20] | 80 | 67 | 73.5 |
| Kienbacher et al. (2016) [47] | 100 | 100 | 100 |
| Lariviere et al. (2000) [6] | 90 | 67 | 78.5 |
| Liu et al. (2011) [48] | 70 | 100 | 85 |
| Luhring et al. (2015) [16] | 89 | 75 | 82 |
| Mayer et al. (2009) [49] | 70 | 25 | 47.5 |
| McGill and Kippers 1994 [15] | 78 | 75 | 76.5 |
| Nairn et al. (2013) [50] | 78 | 100 | 89 |
| Neblett et al. (2003) [51] | 89 | 67 | 78 |
| Ning et al. (2012) [52] | 67 | 50 | 58.5 |
| O’Sullivan et al. (2006) [17] | 100 | 100 | 100 |
| Paquet et al. (1994) [53] | 90 | 25 | 57.5 |
| Peach et al. (1998) [35] | 78 | 75 | 76.5 |
| Sanchez-Zuriaga et al. (2015) [26] | 90 | 100 | 95 |
| Authors | Study Aim | EMG Variable and Lumbar Paraspinal Muscles Recorded (LMU = Lumbar Multifidus, LES = Lumbar Erector Spinae, TES = Thoracic Erector Spinae) | Lumbar Kinematic Measurements | Study Findings | Participants | Analysis |
|---|---|---|---|---|---|---|
| Arjmand et al. (2010) [41] | To compare a single joint model to kinematic driven model during trunk flexion. | Normalised EMG activity. Muscles Longissimus (3 cm lateral to L1) Iliocostalis (3 cm lateral to L3) Multifidus (2 cm lateral to L5). | Optotrak 4 camera system (regional) Lumbar region LED’s placed on pelvis and T12. | In both models, global extensor activity peaked around 30° of flexion, due to the increase in contribution of passive structures at this point. Extensors became silent between 50–70°. | N = 1 A male participant with no recent history of LBP. | Quantitative comparison was not performed. |
| Burnett et al. (2004) [42] | To determine whether differences exist in spinal kinematics and trunk muscle activity in cyclists with and without NSCLBP. | EMG activity was quantified by obtaining the mean activation, during a 5 crank revolution period. Muscles TES (5 cm lateral to T9) LMU (2–3 cm lateral to L4–L5). | 3-Space Fastrak (regional) Lower lumbar L3 relative to S2 Upper lumbar T12 relative to L3. | The LBP group demonstrated greater lower lumbar flexion than controls associated with a loss of multifidus co-contraction. | N = 18 mean age 37.6 years 9 non low back pain 9 NSCLBP. | Independent sample t-tests. |
| Callaghan and Dunk 2002 [43] | To determine if FRP occurs in seated and slumped postures. | Ensemble average normalised EMG activity. Muscles TES (5 cm lateral to T9) LES (3 cm lateral to L3). | 3-Space ISOTRAK (regional) Lumbar region Sacrum relative to L1. | FRP was shown in the TES, but not the LES during Slumped sitting. TES silence during sitting also happened at earlier angle of lumbar flexion than during standing. | N = 22 low back pain free participants 11 males mean age 21.3 years 11 females mean age 21.9 years. | Three way ANOVA, and Tukey’s post hoc multiple comparisons. |
| Cholewicki et al. (1997) [44] | To test the hypothesis that the flexors and extensors of the trunk are co-activated around a neutral spine posture. | Normalised EMG activity. Muscles TES (5 cm lateral to T9) LES (3 cm lateral to L3) LMU (2 cm lateral to L5–L5). | The use of 2 pieces of string attached to a chest harness and two potentiometers (regional). | Co-activation of trunk flexors and extensors was shown in healthy participants around a neutral posture. | N = 10 low back pain free participants 8 males and 2 females mean age 27 years. | A two factor repeated measures ANOVA. |
| Dankaerts et al. (2009) [7] | To test the ability of a model to distinguish between flexion pattern (FP) and active extension pattern (AEP) subgroups and healthy controls using lumbar kinematics and trunk muscle activity. | Normalised EMG activity. Superficial LMU (at the level of L5 orientated by a line between the PSIS and the L1–L2 interspace. Iliocostalis lumborum pars thoracis (lateral to L1). | 3-Space Fastrak (regional) Upper lumbars T12 relative to L3 Lower lumbars L3 relative to S2. | Differences in muscle activity and spinal kinematics during flexion suggest that 2 distinct motor control patterns can exist in CNSLBP patients. | N = 67 participants 34 low back pain free controls, mean age 32 20 Flexion pattern NSLBP patients, mean age 36 13 Extension pattern NSLBP patients, mean age 40. | ANOVA and post hoc Bonferroni. |
| Hashemirad et al. (2009) [45] | To investigate the relationship between lumbar spine flexibility and LES activity during sagittal flexion and return. | Normalised EMG amplitude and signal onset/offset. Muscle LES (4 cm lateral to L3–L4). | Estimated using a camera and markers placed at the spinous processes of T12, L3 and S2 (regional). | During bending the ES of participants with high toe touch score deactivated at greater trunk and hip angles. Those with high modified Schober scores deactivated later and reactivated sooner in accordance with lumbar angle. | N = 30 low back pain free participants. | Pearson correlations and multiple linear regression analysis. |
| Hay et al. (2016) [46] | To show that wavelet coherence and phase plots can be used to provide insight into how muscle activation relates to kinematics. | EMG amplitude (linear envelope). Muscle Lumbar erector spinae (no details of positioning). | Oqus 400 motion capture system (regional) Reflective markers placed over T12 and S1. | The study showed good agreement between lumbar kinematics and linear enveloped sEMG. Validating the use of the wavelet coherence technique. | N = 14 low back pain free male participants. | The coefficient of determination (R²). |
| Kaigle et al. (1998) [20] | To concurrently quantify muscle activation of LES with the kinematics of lumbar motion segments, in low back patients and controls. | Root mean square (RMS) sEMG amplitude. Muscle LES (3 cm lateral to L3–L4). | A linkage transducer system secured by interosseous pins to L2-L3, L3-4 and L4-L5 motion segments (inter-vertebral). | ROM was less in low back pain patients and FRP occurred in participants when IV-ROM was complete before full trunk flexion | N = 13 6 low back pain free participants, mean age 40. 7 low back pain patients with suspected lumbar instability, mean age 51. | Wilcoxon rank-sum test and Wilcoxon matched-pairs signed rank test. |
| Kienbacher et al. (2016) [47] | To determine whether lumbar extensor activity and flexion relaxation ratios could differentiate low back pain patients (of various age groups) during flexion-extension task. | Normalised RMS sEMG amplitudes. Muscle LMU (lateral to L5) a line joining the iliac crests, and 2–3 cm bilateral and distal from their middle). | 3-D accelerometers placed at the levels of T4 and L5. Used to calculate hip, lumbothoracic and gross trunk regions. (regional). | The sEMG activation was highest in over 60′s and female groups during standing. This possibly relates to why this group showed minimal changes during flexion. This group also demonstrated the highest hip, and lowest lumbothoracic angle changes. | N = 216 low back pain patients. 62 (60–90 year olds) 84 (40–59 year olds) 70 (18–39 year olds). | ANOVA and bootstrap confidence intervals. |
| Lariviere et al. (2000) [6] | To evaluate the sensitivity of trunk muscle EMG waveforms to trunk ROM and low back pain status during flexion-extension tasks. | Mean normalised EMG activity. Muscles LES and TES (exact locations not specified). | Video cameras and reflective markers. Trunk angles relative to the vertical plane were used to determine trunk flexion (A line between the hips and the centre of C7-T1) (regional). | Principal component analysis (PCA) distance measures were sensitive to trunk ROM but not low back status. The usefulness of PCA as an effective clinical tool was not established. | N = 33 15 low back pain patients, mean age 40 18 low back pain free participants, mean age 39. | ANOVA and ICC’s. |
| Liu et al. (2011) [48] | To develop a new test based on lumbar sEMG activity (the sEMG coordination network analysis approach) during flexion-extension, to distinguish between healthy control and low back pain groups. | Normalised RMS sEMG activity. Muscles An sEMG electrode array placed over the lumbar region (16 electrodes, target muscles not specified). | 30° of trunk flexion, measured by a protractor (no further details) (regional). | Group network analysis shows a loss of global symmetric patterns in the low back pain group. | N = 21 11 low back pain patients, mean age 40. 10 low back pain free participants, mean age 28. | Did not specify. (However, groups comparison statistics and symmetry scores were used). |
| Luhring et al. (2015) [16] | To determine a kinematic measurement that best determines the onset and offset of the FRP. | Normalised sEMG onset and cessation. Muscle LES (4 cm lateral to L3). | Vicon MX motion capture camera system. Reflective markers placed at various locations throughout the spine including T12, L5 and pelvis (regional). | Lumbar kinematic measurements are preferential when the FRP is considered clinically. | N = 20 low back pain free participants, mean age 24. | Coefficients of Variation (CV) and ICC’s. |
| Mayer et al. (2009) [49] | To determine when FRP occurs in patients and to correlate the findings with lumbar ROM. | Mean RMS sEMG with pre-determined cut-off values. Muscles Not identified within paper. | Gross lumbar, hip/pelvic ROM using an inclinometer (no further details provided) (regional). | After a functional restoration program, both normal FRP and normal lumbar ROM were restored in the majority of patients. | N = 134 30 low back pain free participants, mean age 38. 104 low back pain patients (mean age not provided). | Descriptive statistics including mean and SD. Sensitivity and specificity. P-values and Odds ratios (not specified). |
| McGill and Kippers 1994 [15] | To examine the tissue loading during the period of transition between active and passive tissues during flexion. | Normalised sEMG activity. Muscles TES (5 cm lateral to T9) LES (3 cm lateral to L3). | 3-Space Isotrak (regional) with sensors placed over the sacrum and T10. | The deactivation of lumbar extensor muscles during FRP occurs only in an electrical sense as they still provide force elastically. | N = 8 low back pain free participants, mean age 26. | Dynamic modelling. |
| Nairn et al. (2013) [50] | To quantify slumped sitting both in terms of spinal kinematics and sEMG. | Mean normalised sEMG activity. Muscles Lower TES (5 cm lateral to T9) LES (4 cm lateral to L3) LMU (Adjacent to L5 orientated along a line between the PSIS and the L1–L2 interspinous space. | Vicon motion capture camera system. Reflective markers placed at various locations throughout the spine including T12, L1 and bilateral PSIS’s (regional). | During slumped sitting lower sEMG activity was found in the thoracic and lumbar erector spinae compared to upright sitting. Patterns varied depending on the degree of bending at each area of the spine. Thoracic kinematic and EMG information is therefore useful in these type of studies | N = 12 low back pain free participants, mean age 23. | ANOVA and Bonferroni correction. |
| Neblett et al. (2003) [51] | To assess EMG activity in terms of the FRP during dynamic flexion and to determine whether abnormal FRP patterns in NSLBP patients can be normalised. | RMS sEMG cut-off values. Muscles LES (2 cm lateral to L3). | Inclinometers at T12 and the sacrum (regional). | In asymptomatic participants, the flexion relaxation (FR) angle was always less than the maximal voluntary flexion (MVF) angle. Of the patients that completed a functional restoration program, 94% achieved FR compared to 30% pre-treatment. | N = 66 12 low back pain free participants, mean age 34. 54 chronically disabled work-related spinal disorder (CDWRSD) patients | Descriptive statistics for ROM and FRP |
| Ning et al. (2012) [52] | To determine a boundary at which the passive tissues begin to take a significant role in trunk extensor moment (and therefore at what point EMG assisted modelling is no longer valid). | Normalised EMG activity. Muscles LES at two levels (3 cm lateral to L3 and 4 cm lateral to L4). | A magnetic-field based motion tracking system with sensors placed at T12 and S1. Lumbar flexion calculated as the pitch of T12 relative to S1 (regional). | EMG-assisted models should consider the action of the passive tissues at lower flexion angles than previously thought. | N = 11 low back pain free participants, mean age 26. | ANOVA and Tukey–Kramer post-hoc testing |
| O’Sullivan et al. (2006) [17] | To investigate the FRP of spinal muscles in healthy participants during slumped sitting from an upright position. | Normalised EMG activity offset. Muscles TES (5 cm lateral to T9) LMU (Adjacent to L5 orientated along a line between the PSIS and the L1–L2 interspinous space. | 3- Space Fastrak with sensors placed over T6, T12 and S2. (regional). | LMU is active during neutral sitting and demonstrates FRP when moving from upright to slumped sitting. FRP of these muscles is also different to when standing. More variation was found in EMG patterns of the TES. | N = 24 low back pain free participants, mean age 32. | ANOVA and ICC’s |
| Paquet et al. (1994) [53] | To compare healthy controls and low back pain patients in terms of hip-spine movement interaction and EMG, and to verify the relationships between kinematics and EMG in these groups. | Raw EMG envelope. Area under the curve and ratio of activity at different parts of the flexion-extension cycle (not-specified). Muscles LES (at the level of L3, distance not-specified). | Electro goniometers measured angular displacements at the hip and lumbar spine using landmarks of T8 and S1 (regional). | LES activation patterns were found to be significantly different between groups when flexion was performed at the same rate and range. Abnormal hip-spine movement related to an absence of the FRP at full flexion. | N = 20 10 low back pain free participants, mean age 34. 10 low back pain patients, mean age 38. | Mann-Whitney U test and Kruskal-Wallis test |
| Peach et al. (1998) [35] | To document the lumbar kinematics and trunk EMG activation patterns of healthy controls during tasks including sagittal flexion | Mean normalised EMG. Muscles TES (5 cm lateral to T9) LES (3 cm lateral to L3) LMU (1–2 cm lateral to L5). | 3-Space Isotrak with sensors placed over T12 and Sacrum. (regional). | A database of normal lumbar spinal kinematics and EMG patterns was created for future reference against LBP groups. | N = 24 low back pain free participants, mean age 22. | Descriptive statistics, ANOVA and Tukey’s honestly significant difference (HSD) post-hoc testing |
| Sanchez-Zuriaga et al. (2015) [26] | To compare healthy controls and LBP patients in terms of lumbopelvic kinematics and erector spinae activity | Mean normalised EMG activity, and start and end of FRP. Muscle LES (3 cm lateral to L3). | A 3-dimensional videophotogrammetric system, with markers placed at T12, L3, L5 and the sacrum (regional). | During pain free periods, recurrent LBP patients showed significantly greater LES activity during flexion and extension. Lumbar ROM and FRP were not found to be useful to distinguish between groups. | N = 30 15 low back pain free participants, mean age 41. 15 patients with recurring low back pain (currently in a pain free stage), mean age 45. | Mann-Whitney U test |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du Rose, A. Have Studies that Measure Lumbar Kinematics and Muscle Activity Concurrently during Sagittal Bending Improved Understanding of Spinal Stability and Sub-System Interactions? A Systematic Review. Healthcare 2018, 6, 112. https://doi.org/10.3390/healthcare6030112
Du Rose A. Have Studies that Measure Lumbar Kinematics and Muscle Activity Concurrently during Sagittal Bending Improved Understanding of Spinal Stability and Sub-System Interactions? A Systematic Review. Healthcare. 2018; 6(3):112. https://doi.org/10.3390/healthcare6030112
Chicago/Turabian StyleDu Rose, Alister. 2018. "Have Studies that Measure Lumbar Kinematics and Muscle Activity Concurrently during Sagittal Bending Improved Understanding of Spinal Stability and Sub-System Interactions? A Systematic Review" Healthcare 6, no. 3: 112. https://doi.org/10.3390/healthcare6030112
APA StyleDu Rose, A. (2018). Have Studies that Measure Lumbar Kinematics and Muscle Activity Concurrently during Sagittal Bending Improved Understanding of Spinal Stability and Sub-System Interactions? A Systematic Review. Healthcare, 6(3), 112. https://doi.org/10.3390/healthcare6030112




