Persistent Borrelia Infection in Patients with Ongoing Symptoms of Lyme Disease
Abstract
1. Introduction
2. Methods
2.1. Subject Selection
2.2. Control Selection
2.3. Informed Consent
2.4. Cultures
2.5. Dieterle and Anti-Bb Immunostaining
2.6. Molecular Testing
2.7. PCR—University of New Haven
2.8. PCR—Australian Biologics
2.9. PCR—University California Irvine
3. Results
3.1. Subject Histories
3.1.1. Case 1
3.1.2. Case 2
3.1.3. Case 3
3.1.4. Case 4
3.1.5. Case 5
3.1.6. Case 6
3.1.7. Case 7
3.1.8. Case 8
3.1.9. Case 9
3.1.10. Case 10
3.1.11. Case 11
3.1.12. Case 12
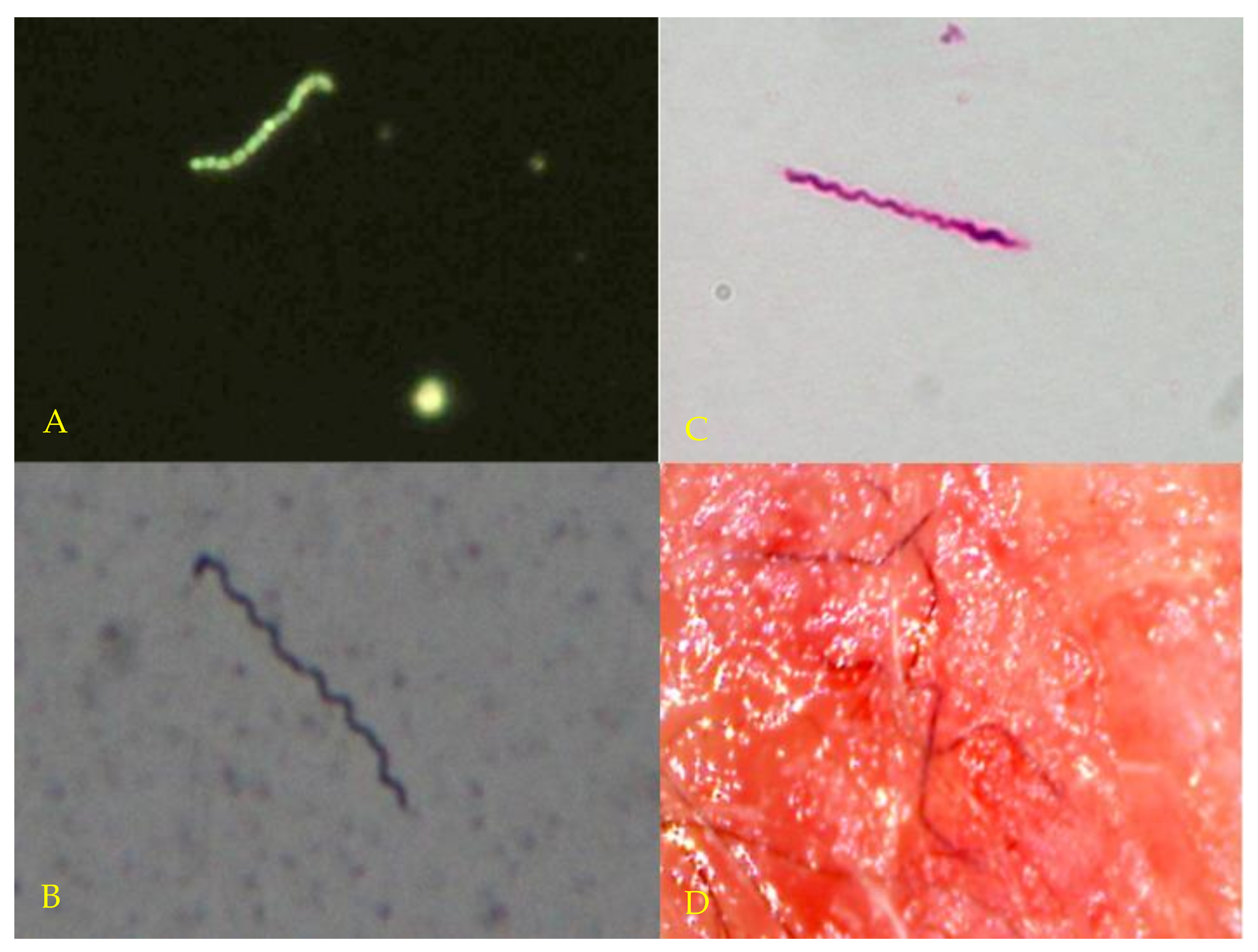
3.2. Microscopy and Histopathology
3.3. Molecular Testing
PCR Detection of Borrelia
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steere, A.C.; Malawista, S.E.; Snydman, D.R.; Shope, R.E.; Andiman, W.A.; Ross, M.R.; Steele, F.M. Lyme arthritis: An epidemic of oligoarticular arthritis in children and adults in three Connecticut Communities. Arthritis Rheum. 1977, 20, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex—Clinical significance of genomic species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J.; Ruzic-Sabljic, E.; Potkonjak, A. Emerging borreliae—Expanding beyond Lyme borreliosis. Mol. Cell. Probes 2017, 31, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.; Golovchenko, M.; Vancova, M.; Clark, K.; Grubhoffer, L.; Oliver, J.H., Jr. Isolation of live Borrelia burgdorferi sensu lato spirochaetes from patients with undefined disorders and symptoms not typical for Lyme borreliosis. Clin. Microbiol. Infect. 2016, 22, 267.e9–267.e15. [Google Scholar] [CrossRef] [PubMed]
- Dorward, D.W.; Fischer, E.R.; Brooks, D.M. Invasion and cytopathic killing of human lymphocytes by spirochetes causing Lyme disease. Clin. Infect. Dis. 1997, 25, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Johnson, L.B.; Maloney, E.L. Evidence assessments and guideline recommendations in Lyme disease: The clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev. Anti Infect. Ther. 2014, 12, 1103–1135. [Google Scholar] [CrossRef] [PubMed]
- Meriläinen, L.; Brander, H.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Pleomorphic forms of Borrelia burgdorferi induce distinct immune responses. Microbes Infect. 2016, 18, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Miklossy, J. Bacterial amyloid and DNA are important constituents of senile plaques: Further evidence of the spirochetal and biofilm nature of senile plaques. J. Alzheimers Dis. 2016, 53, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Johnson, L. Lyme disease diagnosis and treatment: Lessons from the AIDS epidemic. Minerva Med. 2010, 101, 419–425. [Google Scholar] [PubMed]
- Stricker, R.B.; Johnson, L. Lyme disease: The next decade. Infect. Drug Resist. 2011, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Caruso, V.G. Facial paralysis from Lyme disease. Otolaryngol. Head Neck Surg. 1985, 93, 550–553. [Google Scholar] [PubMed]
- Habicht, G.S.; Beck, G.; Benach, J.L. Lyme disease. Sci. Am. 1987, 257, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Middelveen, M.J. Sexual transmission of Lyme disease: Challenging the tickborne disease paradigm. Expert Rev. Anti Infect. Ther. 2015, 13, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Feder, H.M.; Johnson, B.J.B.; O’Connell, S.; Shapiro, E.D.; Steere, A.C.; Wormser, G.P. Ad Hoc International Lyme Disease Group. A critical appraisal of “chronic Lyme disease”. N. Engl. J. Med. 2007, 357, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Johnson, L. Spirochetal ‘debris’ versus persistent infection in chronic Lyme disease: From semantics to science. Fut. Microbiol. 2012, 7, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Duray, P.H.; Johnson, R.C. The histopathology of experimentally infected hamsters with the Lyme disease spirochete Borrelia burgdorferi. Proc. Soc. Exp. Biol. Med. 1986, 181, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Moody, K.D.; Barthold, S.W.; Terwilliger, G.A. Lyme borreliosis in laboratory animals: Effect of host species and in vitro passage Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 1990, 43, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Preac Mursic, V.; Patsouris, E.; Wilske, B.; Reinhardt, S.; Gross, B.; Mehraein, P. Persistence of Borrelia burgdorferi and histopathological alterations in experimentally infected animals. A comparison with histopathological findings in human Lyme disease. Infection 1990, 18, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.L.; Jurkovich, P.; Kodner, C.; Johnson, R.C. Persistent cardiac and urinary tract infections with Borrelia burgdorferi in experimentally infected Syrian hamsters. J. Clin. Microbiol. 1991, 29, 894–896. [Google Scholar] [PubMed]
- Schmitz, J.L.; Schell, R.F.; Lovrich, S.D.; Callister, S.M.; Coe, J.E. Characterization of the protective antibody response to Borrelia burgdorferi in experimentally infected LSH hamsters. Infect. Immun. 1991, 59, 1916–1921. [Google Scholar] [PubMed]
- Malawista, S.E.; Barthold, S.W.; Persing, D.H. Fate of Borrelia burgdorferi DNA in tissues of infected mice after antibiotic treatment. J. Infect. Dis. 1994, 170, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Moody, K.D.; Adams, R.L.; Barthold, S.W. Effectiveness of antimicrobial treatment against Borrelia burgdorferi infection in mice. Antimicrob. Agents Chemother. 1994, 38, 1567–1572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sonnesyn, S.W.; Manivel, J.C.; Johnson, R.C.; Goodman, J.L. A guinea pig model for Lyme disease. Infect. Immun. 1993, 61, 4777–4784. [Google Scholar] [PubMed]
- Roberts, E.D.; Bohm, R.P., Jr.; Cogswell, F.B.; Lanners, H.N.; Lowrie, R.C., Jr.; Povinelli, L.; Piesman, J.; Philipp, M.T. Chronic Lyme disease in the rhesus monkey. Lab. Invest. 1995, 72, 146–160. [Google Scholar] [PubMed]
- Straubinger, R.K.; Summers, B.A.; Chang, Y.F.; Appel, M.J. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 1997, 35, 111–116. [Google Scholar] [PubMed]
- Straubinger, R.K. PCR-Based quantification of Borrelia burgdorferi organisms in canine tissues over a 500-Day postinfection period. J. Clin. Microbiol. 2000, 38, 2191–2199. [Google Scholar] [PubMed]
- Pachner, A.R.; Cadavid, D.; Shu, G.; Dail, D.; Pachner, S.; Hodzic, E.; Barthold, S.W. Central and peripheral nervous system infection, immunity, and inflammation in the NHP model of Lyme borreliosis. Ann. Neurol. 2001, 50, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Cadavid, D.; Bai, Y.; Hodzic, E.; Narayan, K.; Barthold, S.W.; Pachner, A.R. Cardiac involvement in non-human primates infected with the Lyme disease spirochete Borrelia burgdorferi. Lab. Invest. 2004, 84, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Ku, Y.W.; Chang, C.F.; Chang, C.D.; McDonough, S.P.; Divers, T.; Pough, M.; Torres, A. Antibiotic treatment of experimentally Borrelia burgdorferi-infected ponies. Vet. Microbiol. 2005, 107, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.C.; Narayan, K.; Stevenson, B.; Pachner, A.R. Expression of Borrelia burgdorferi erp genes during infection of non-human primates. Microb. Pathog. 2005, 39, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bockenstedt, L.K.; Mao, J.; Hodzic, E.; Barthold, S.W.; Fish, D. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J. Infect. Dis. 2002, 186, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, E.; Feng, S.; Holden, K.; Freet, K.J.; Barthold, S.W. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 2008, 52, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Barthold, S.W.; Hodzic, E.; Imai, D.M.; Feng, S.; Yang, X.; Luft, B.J. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob. Agents Chemother. 2010, 54, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Yrjänäinen, H.; Hytönen, J.; Hartiala, P.; Oski, J.; Vijanen, M.K. Persistence of borrelial DNA in the joints of Borrelia burgdorferi-infected mice after ceftriaxone treatment. APMIS 2010, 118, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Imai, D.M.; Barr, B.C.; Daft, B.; Bertone, J.J.; Feng, S.; Hodzic, E.; Johnston, J.M.; Olsen, K.J.; Barthold, S.W. Lyme neuroborreliosis in 2 horses. Vet. Pathol. 2011, 48, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Bockenstedt, L.K.; Gonzales, D.G.; Haberman, A.M.; Belperron, A.A. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J. Clin. Investig. 2012, 122, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Embers, M.E.; Hasenkampf, N.R.; Jacobs, M.B.; Tardo, A.C.; Doyle-Meyers, L.A.; Philipp, M.T.; Hodzic, E. Variable manifestations, diverse seroreactivity and post-treatment persistence in non-human primates exposed to Borrelia burgdorferi by tick feeding. PLoS ONE 2017, 12, e0189071. [Google Scholar] [CrossRef] [PubMed]
- Craft, J.E.; Fischer, D.K.; Shimamoto, G.T.; Steere, A.C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J. Clin. Investig. 1986, 78, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, M.A.; Azzolini, A.; Tobia, F.; Pesce, C.M. Spirochetes in the spleen of a patient with chronic Lyme disease. Am. J. Clin. Pathol. 1989, 91, 95–97. [Google Scholar] [CrossRef] [PubMed]
- De Koning, J.; Hoogkamp-Korstanje, J.A.; van der Linde, M.R.; Crijns, H.J. Demonstration of spirochetes in cardiac biopsies of patients with Lyme disease. J. Infect. Dis. 1989, 160, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Preac-Mursic, V.; Weber, K.; Pfister, H.W.; Wilske, B.; Gross, B.; Baumann, A.; Prokop, J. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection 1989, 17, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.D.; Kong, L.I.; Miller, F.W. Molecular detection of persistent Borrelia burgdorferi in a man with dermatomyositis. Clin. Exp. Rheumatol. 1992, 10, 387–390. [Google Scholar] [PubMed]
- Battafarano, D.F.; Combs, J.A.; Enzenauer, R.J.; Fitxpatrick, J.E. Chronic septic arthritis caused by Borrelia burgdorferi. Clin. Orthop. Relat. Res. 1993, 279, 238–241. [Google Scholar]
- Liegner, K.B.; Shapiro, J.R.; Ramsay, D.; Halperin, A.J.; Hogrefe, W.; Kong, L. Recurrent erythema migrans despite extended antibiotic treatment with minocycline in a patient with persisting Borrelia burgdorferi infection. J. Am. Acad. Dermatol. 1993, 28, 312–314. [Google Scholar] [CrossRef]
- Asch, E.S.; Bujak, D.I.; Weiss, M.; Peterson, M.G.; Weinstein, A. Lyme disease: An infectious and postinfectious syndrome. J. Rheumatol. 1994, 21, 454–461. [Google Scholar] [PubMed]
- Nocton, J.J.; Dressler, F.; Rutledge, B.J.; Rys, P.N.; Persing, D.H.; Steere, A.C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 1994, 330, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bayer, M.E.; Zhang, L.; Bayer, M.H. Borrelia burgdorferi DNA in the urine of treated patients with chronic Lyme disease symptoms. A PCR study of 97 cases. Infection 1996, 24, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Valesova, M.; Trnavský, K.; Hulínská, D.; Alusík, S.; Janousek, J.; Jirous, J. Detection of Borrelia in the synovial tissue from a patient with Lyme borreliosis by electron microscopy. J. Rheumatol. 1989, 16, 1502–1505. [Google Scholar] [PubMed]
- Donta, S.T. Tetracycline therapy for chronic Lyme disease. Clin. Infect. Dis. 1997, 25, S52–S56. [Google Scholar] [CrossRef] [PubMed]
- Priem, S.; Burmester, G.R.; Kamradt, T.; Wolbart, K.; Rittig, M.G.; Krause, A. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann. Rheum. Dis. 1998, 57, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.J.; Stewart, M.; Lennox, V.A.; Fukunaga, M.; Yabuki, M.; Macorison, H.; Kitchener-Smith, J. Culture-positive Lyme borreliosis. Med. J. Aust. 1998, 168, 500–502. [Google Scholar] [PubMed]
- Oksi, J.; Nikoskelainen, J.; Vilajanen, M.K. Comparison of oral cefixime and intravenous ceftriaxone followed by oral amoxicillin in disseminated Lyme borreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Oksi, J.; Marjamäki, M.; Nikoskelainen, J.; Vilajanen, M.K. Borrelia burgdorferi detected by culture and PCR in clinical relapse of disseminated Lyme borreliosis. Ann. Med. 1999, 31, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Berglund, J.; Stjernberg, L.; Ornstein, K.; Tykesson-Joelsson, K.; Walter, H. Follow-up study of patients with neuroborreliosis. Scand. J. Infect. Dis. 2002, 34, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R. Clinical courses of acute and chronic neuroborreliosis following treatment with ceftriaxone. Nervenarzt 2004, 75, 553–557. [Google Scholar] [PubMed]
- Cameron, D. Severity of Lyme disease with persistent symptoms. Insights from a double-blind placebo-controlled clinical trial. Minerva Med. 2008, 99, 489–496. [Google Scholar] [PubMed]
- Fallon, B.A.; Keilp, J.G.; Corbera, K.M.; Petkova, E.; Britton, C.B.; Dwyer, E.; Slavov, I.; Cheng, J.; Dobkin, J.; Nelson, D.R.; et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008, 70, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Delong, A.K.; Green, C.L.; Savely, V.R.; Chamallas, S.N.; Johnson, L. Benefit of intravenous antibiotic therapy in patients referred for treatment of neurologic Lyme disease. Int. J. Gen. Med. 2011, 4, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Middelveen, M.J.; McClain, S.A.; Bandoski, C.; Israel, J.R.; Burke, J.; MacDonald, A.B.; Timmaraju, A.; Sapi, E.; Wang, Y.; Franco, A.; et al. Granulomatous hepatitis associated with chronic Borrelia burgdorferi infection: A case report. Res. Open Access. 2014, 1, 875. [Google Scholar] [CrossRef]
- Shah, J.S.; Du Cruz, I.; Narciso, W.; Lo, W.; Harris, N.S. Improved sensitivity of Lyme disease Western blots prepared with a mixture of Borrelia burgdorferi strains 297 and B31. Chronic Dis. Int. 2014, 1, 7. [Google Scholar]
- Middelveen, M.J.; Bandowski, C.; Burke, J.; Sapi, E.; Filush, K.R.; Wang, Y. Exploring the association between Morgellons disease and Lyme disease: Identification of Borrelia burgdorferi in Morgellons disease patients. BMC Dermatol. 2015, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, T.; Chaconas, G. The role of VlsE antigenic variation in the Lyme disease spirochete: Persistence through a mechanism that differs from other pathogens. Mol. Microbiol. 2007, 65, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.; Traweger, A.; Lusa, L.; Stupica, D.; Maraspin, V.; Barrett, P.N.; Strle, F.; Livey, I. Quantitative detection of Borrelia burgdorferi sensu lato in erythema migrans skin lesions using internally controlled duplex real time PCR. PLoS ONE 2013, 8, e63968. [Google Scholar] [CrossRef] [PubMed]
- Margos, G.; Hojgaard, A.; Lane, R.S.; Cornet, M.; Fingerle, V.; Rudenko, N.; Ogden, N.; Aanensen, D.M.; Fish, D.; Piesman, J. Multilocus sequence analysis of Borrelia bissettii strains from North America reveals a new Borrelia species, Borrelia kurtenbachii. Ticks Tick Borne Dis. 2010, 1, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.L.; Leydet, B.; Hartman, S. Lyme borreliosis in human patients in Florida and Georgia, USA. Int. J. Med. Sci. 2013, 10, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Pabbati, N.; Datar, A.; Davies, E.M.; Rattelle, A.; Kuo, B.A. Improved culture conditions for the growth and detection of Borrelia from human serum. Int. J. Med. Sci. 2013, 10, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Mayne, P.J. Investigation of Borrelia burgdorferi genotypes in Australia obtained from erythema migrans tissue. Clin. Cosmet. Investig. Dermatol. 2012, 5, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Mayne, P. Clinical determinants of Lyme borreliosis, babesiosis, bartonellosis, anaplasmosis, and ehrlichiosis in an Australian cohort. Int. J. Gen. Med. 2014, 8, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bunikis, J.; Tsao, J.; Garpmo, U.; Berglund, J.; Fish, D.; Barbour, A.G. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology 2004, 150, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Travinsky, B.; Bunikis, J.; Barbour, A.G. Geographic differences in genetic locus linkages for Borrelia burgdorferi. Emerg. Infect. Dis. 2010, 16, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Snydman, D.R.; Schenkein, D.P.; Berardi, V.P.; Lastavica, C.C.; Pariser, K.M. Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann. Intern. Med. 1986, 104, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Klein, J.; Bittner, R.; Glogar, D. Isolation of Borrelia burgdorferi from the myocardium of a patient with longstanding cardiomyopathy. N. Engl. J. Med. 1990, 322, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sturrock, A.; Weis, J.J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect. Immun. 1991, 59, 671–678. [Google Scholar] [PubMed]
- Georgilis, K.; Peacocke, M.; Klempner, M.S. Fibroblasts protect the Lyme disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitro. J. Infect. Dis. 1992, 166, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Haupl, T.; Hahn, G.; Rittig, M.; Krause, A.; Schoerner, C.; Schönherr, U.; Kalden, J.R.; Burmester, G.R. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 1993, 36, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Klempner, M.S.; Noring, R.; Rogers, R.A. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 1993, 167, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Aberer, E.; Kersten, A.; Klade, H.; Poitschek, C.; Jurecka, W. Heterogeneity of Borrelia burgdorferi in the skin. Am. J. Dermatopathol. 1996, 18, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Girschick, H.J.; Huppertz, H.I.; Rüssmann, H.; Krenn, V.; Karch, H. Intracellular persistence of Borrelia burgdorferi in human synovial cells. Rheumatol. Int. 1996, 16, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Nanagara, R.; Duray, P.H.; Schumacher, H.R. Ultrastructural demonstration of spirochetal antigens in synovial uid and synovial membrane in chronic Lyme disease: Possible factors contributing to persistence of organisms. Hum. Pathol. 1996, 27, 1025–1034. [Google Scholar] [CrossRef]
- Hastey, C.J.; Elsner, R.A.; Barthold, S.W.; Baumgarth, N. Delays and diversions mark the development of B cell responses to Borrelia burgdorferi infection. J. Immunol. 2012, 188, 5612–5622. [Google Scholar] [CrossRef] [PubMed]
- Livengood, J.A.; Gilmore, R.D. Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes Infect. 2006, 8, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Johnson, L. Borrelia burgdorferi aggrecanase activity: More evidence for persistent infection in Lyme disease. Front. Cell Infect. Microbiol. 2013, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Weening, E.H.; Faske, J.B.; Höök, M.; Skare, J.T. Invasion of eukaryotic cells by Borrelia burgdorferi requires β(1) integrins and Src kinase activity. Infect. Immun. 2011, 79, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Balasubramanian, K.; Poruri, A.; Maghsoudlou, J.S.; Socarras, K.M.; Timmaraju, A.V.; Filush, K.R.; Gupta, K.; Shaikh, S.; Theophilus, P.A.; et al. Evidence of in vivo existence of Borrelia biofilm in Borrelial lymphocytomas. Eur. J. Microbiol. Immunol. 2016, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, S.; Shi, W.; Zhang, Y. Ceftriaxone pulse dosing fails to eradicate biofilm-like microcolony B. burgdorferi persisters which are sterilized by daptomycin/doxycycline/cefuroxime without pulse dosing. Front. Microbiol. 2016, 7, 1744. [Google Scholar] [CrossRef] [PubMed]
- Klempner, M.S.; Hu, L.T.; Evans, J.; Schmid, C.H.; Johnson, G.M.; Trevino, R.P.; Trevino, B.S.; DeLona Norton, M.P.H.; Lois Levy, M.S.W.; Diane Wall, R.N.; et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N. Engl. J. Med. 2001, 345, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Nadelman, R.B.; Schwartz, I. The amber theory of Lyme arthritis: Initial description and clinical implications. Clin. Rheumatol. 2012, 31, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Aucott, J.N. Post-treatment Lyme disease syndrome. Infect. Dis. Clin. N. Am. 2015, 29, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.J.; McCarter, A.L.; Neifer-Sadhwani, K.; Wooten, R.M. ELISA-based measurement of antibody responses and PCR-based detection profiles can distinguish between active infection and early clearance of Borrelia burgdorferi. Clin. Dev. Immunol. 2012, 2012, 138069. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shi, W.; Zhang, S.; Zhang, Y. Persister mechanisms in Borrelia burgdorferi: Implications for improved intervention. Emerg. Microbes Infect. 2015, 4, e51. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Brown, A.V.; Matluck, N.E.; Hu, L.T.; Lewis, K. Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrob. Agents Chemother. 2015, 59, 4616–4624. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C.; Godfrey, H.P.; Bugrysheva, J.; Newman, S.A. Sleeper cells: The stringent response and persistence in the Borreliella (Borrelia) burgdorferi enzootic cycle. Environ. Microbiol. 2017, 19, 3846–3862. [Google Scholar] [CrossRef] [PubMed]
- Middelveen, M.J.; Stricker, R.B. Morgellons disease: A filamentous borrelial dermatitis. Int. J. Gen. Med. 2016, 9, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Middelveen, M.J.; Fesler, M.C.; Stricker, R.B. History of Morgellons disease: From delusion to definition. Clin. Cosmet. Investig. Dermatol. 2018, 11, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Savely, V.R.; Stricker, R.B. Morgellons disease: The mystery unfolds. Expert Rev. Dermatol. 2007, 2, 585–591. [Google Scholar] [CrossRef]
- Savely, V.R.; Stricker, R.B. Morgellons disease: Analysis of a population with clinically confirmed microscopic subcutaneous fibers of unknown etiology. Clin. Cosmet. Investig. Dermatol. 2010, 3, 67–78. [Google Scholar] [PubMed]
- Middelveen, M.J.; Stricker, R.B. Filament formation associated with spirochetal infection: A comparative approach to Morgellons disease. Clin. Cosmet. Investig. Dermatol. 2011, 4, 167–177. [Google Scholar] [PubMed]
- Middelveen, M.J.; Mayne, P.J.; Kahn, D.G.; Stricker, R.B. Characterization and evolution of dermal filaments from patients with Morgellons disease. Clin. Cosmet. Investig. Dermatol. 2013, 6, 1–21. [Google Scholar] [PubMed]
- Middelveen, M.J.; Burugu, D.; Poruri, A.; Burke, J.; Mayne, P.J.; Sapi, E.; Kahn, D.G.; Stricker, R.B. Association of spirochetal infection with Morgellons disease. F1000Res 2013, 2, 25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chmielewski, T.; Tylewska-Wierzbanowska, S. Interactions between Borrelia burgdorferi and mouse fibroblasts. Pol. J. Microbiol. 2010, 59, 157–160. [Google Scholar] [PubMed]
- Radolf, J.D.; Deka, R.K.; Anand, A.; Šmajs, D.; Norgard, M.V.; Yang, X.F. Treponema pallidum, the syphilis spirochete: Making a living as a stealth pathogen. Nat. Rev. Microbiol. 2016, 14, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Brorson, Ø.; Brorson, S.H. Transformation of cystic forms of Borrelia burgdorferi to normal mobile spirochetes. Infection 1997, 25, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Murgia, R.; Cinco, M. Induction of cystic forms by different stress conditions in Borrelia burgdorferi. APMIS 2004, 112, 57–62. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.B. Borrelia burgdorferi tissue morphologies and imaging methodologies. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Mestecky, J.; Moldoveanu, Z.; Russell, M.W. Immunologic uniqueness of the genital tract: Challenge for vaccine development. Am. J. Reprod. Immunol. 2005, 53, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Cu-Uvin, S.; DeLong, A.K.; Venkatesh, K.K.; Hogan, J.W.; Ingersoll, J.; Kurpewski, J.; De Pasquale, M.P.; D’Aquila, R.; Caliendo, A.M. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS 2010, 24, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Mzingwane, M.L.; Tiemessen, C.T. Mechanisms of HIV persistence in HIV reservoirs. Rev. Med. Virol. 2017, 27, e1924. [Google Scholar] [CrossRef] [PubMed]
- Vetter, P.; Fischer, W.A., II; Schibler, M.; Jacobs, M.; Bausch, D.G.; Kaiser, L. Ebola virus shedding and transmission: Review of current evidence. J. Infect. Dis. 2016, 214, S177–S184. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Peixoto, T.M.; Siqueira, A.M.; Lamas, C.C. Sexually acquired Zika virus: A systematic review. Clin. Microbiol. Infect. 2017, 23, 296–305. [Google Scholar] [CrossRef] [PubMed]
| Case # | Age/Gender | EM Rash | Sx | MD Lesions | LD Seroreactivity | Co-Infections | Abx |
|---|---|---|---|---|---|---|---|
| Case 1 | 50F | No | MS, F | Yes | Negative | Unknown | Yes |
| Case 2 | 54F | Yes | MS, F | Yes | Negative | Bab, Bart | No |
| Case 3 | 63M | No | MS, F | No | Positive | Bab, Ana | Yes |
| Case 4 | 53F | Yes | MS, F | No | Negative, seroconverting to positive | Bab, Ana | Yes |
| Case 5 | 40F | No | MS, N | No | Positive | Bab | Yes |
| Case 6 | 42M | No | MS, N | No | Positive | None | Yes |
| Case 7 | 36F | No | MS, N | No | Positive | None | Yes |
| Case 8 | 39M | No | MS, N | No | Positive | Unknown | No |
| Case 9 | 71F | No | MS, F, N | No | Positive | None | No |
| Case 10 | 72M | No | MS, F, N | No | Positive | None | No |
| Case 11 | 57F | No | MS | No | Positive | Bab, Ehr, Bart | No |
| Case 12 | 46F | No | MS | Yes | Positive | Bab | No |
| Patient Number | Western Blot IgM | Western Blot IgG |
|---|---|---|
| 1 | Negative | Negative |
| 2 | Negative | Negative |
| 3 | Negative | Positive |
| 4 | Positive | Negative |
| 5 | Positive | Negative |
| 6 | Positive | Negative |
| 7 | Positive | Negative |
| 8 | Positive | Negative |
| 9 | Positive | Positive |
| 10 | Positive | Negative |
| 11 | Positive | Positive |
| 12 | Negative | Positive |
| Case # | Sample Type | Darkfield | Dieterle | Bb Immunostain |
|---|---|---|---|---|
| Case 1 | whole callus | N/A | spirochetes | positive, spirochetes |
| blood culture | spirochetes | N/A | N/A | |
| Case 2 | blood culture | spherules | spherules | positive, spherules |
| vaginal culture | spirochetes | spirochetes | positive, spirochetes, biofilm | |
| Case 3 | blood culture | spirochetes/spherules | spirochetes/spherules | positive spirochetes/spherules |
| seminal culture | spirochetes | spirochetes | positive, spirochetes | |
| Case 4 | blood culture | spirochetes/spherules | spirochetes/spherules | positive spirochetes/spherules |
| vaginal culture | spirochetes | spirochetes | positive, spirochetes | |
| Case 5 | blood culture | spherules | spherules | positive, spherules |
| vaginal culture | spirochetes | spirochetes | positive, spirochetes | |
| Case 6 | blood culture | spherules | spherules | positive, spherules |
| seminal culture | spirochetes | spirochetes | positive, spirochetes | |
| Case 7 | vaginal culture | spirochetes | spirochetes | positive, spirochetes |
| Case 8 | seminal culture | spirochetes | spirochetes | positive, spirochetes |
| Case 9 | vaginal culture | spirochetes | spirochetes | positive, spirochetes |
| Case 10 | seminal culture | spirochetes | spirochetes | positive, spirochetes |
| Case 11 | vaginal culture | spirochetes | spirochetes | positive, spirochetes |
| Case 12 | blood culture | spherules | spherules | positive, spherules |
| skin culture | spirochetes | spirochetes | positive, spirochetes |
| Case # | Sample Type | University of New Haven | Australian Biologics | UC-Irvine |
|---|---|---|---|---|
| 1 | whole callus | 16S rRNA (N), pyrG (N) *, fla (N) * | N/A | N/A |
| blood culture | pyrG (N), fla (N) * | N/A | N/A | |
| 2 | blood culture | 16S rRNA (N) | 16S rRNA (RT), rpoC (E) * | N/A |
| vaginal culture | pyrG (N) *, fla (N) | 16S rRNA (RT) * | qPCR 16S-23S intergenic spacer | |
| 3 | blood culture | 16S rRNA (N) * | 16S rRNA (RT) | N/A |
| seminal culture | 16S rRNA (RT), 16S rRNA (N) *, fla (N) | 16S rRNA (RT), rpoC (E) * | N/A | |
| 4 | blood culture | 16S rRNA (N), pyrG (N) | 16S rRNA (RT), rpoC (E) * | N/A |
| vaginal culture | 16S rRNA (RT), 16S rRNA (N), pyrG (N), fla (N) | 16S rRNA (RT), rpoC (E) * | N/A | |
| 5 | blood culture | 16S rRNA (RT), 16S rRNA (N), pyrG (N) | 16S rRNA (RT) | N/A |
| vaginal culture | 16S rRNA (N) | 16S rRNA (RT), rpoC (E) * | N/A | |
| 6 | blood culture | 16S rRNA (RT), 16S rRNA (N), pyrG (N) | 16S rRNA (RT) | N/A |
| seminal culture | 16S rRNA (RT), 16S rRNA (N) | 16S rRNA (RT) | N/A | |
| 7 | vaginal culture | 16S rRNA (N) * | N/A | N/A |
| 8 | seminal culture | 16S rRNA (N) * | N/A | N/A |
| 9 | vaginal culture | pyrG (N) | 16S rRNA (RT) | N/A |
| 10 | seminal culture | pyrG (N) | 16S rRNA (RT) | (+/−) qPCR16S-23S intergenic spacer |
| 11 | vaginal culture | 16S rRNA (N) | 16S rRNA (RT), rpoC (E) * | N/A |
| 12 | whole callus | uvrA (N) * | N/A | N/A |
| blood culture | pyrG | 16S rRNA (RT) | N/A |
| Case # | Culture Specimen | Sequence | Length | E-Value | BLAST Match Bbss | LAB |
|---|---|---|---|---|---|---|
| 1 | callus | pyrG | 680 | 0.0 | 100% | UNH |
| callus | fla | 367 | 2e-172 | 100% | UNH | |
| blood | fla F | 364 | 2e-176 | 99% | UNH | |
| blood | fla R | 367 | 2e-172 | 99% | UNH | |
| 2 | vaginal | pyrG F | 656 | 0.0 | 99% | UNH |
| vaginal | pyrG R | 659 | 0.0 | 99% | UNH | |
| vaginal | rpoC | 79 | 4e-32 | 100% | AB | |
| vaginal | 16S-23S Intergenic spacer | 474 | 0.0 | 100% | UCI | |
| 3 | blood | 16S rRNA F | 415 | 0.0 | 99% | UNH |
| blood | 16S rRNA R | 415 | 0.0 | 99% | UNH | |
| seminal | 16S rRNA | 388 | 0.0 | 99% | UNH | |
| blood 1 month Abx | rpoC | 103 | 0.11 | 96% | AB | |
| seminal 1 month Abx | rpoC | 146 | 9e-57 | 100% | AB | |
| seminal 4 months Abx | rpoC | 158 | 3e-52 | 98% | AB | |
| 4 | vaginal | rpoC | 118 | 1e-51 | 99% | AB |
| 5 | vaginal | rpoC | 109 | 6e-47 | 99% | AB |
| 7 | vaginal | 16S rRNA | 396 | 0.0 | 99% | UNH |
| 8 | seminal | 16S rRNA | 221 | 7e-10 | 100% | UNH |
| 10 | seminal | 16S-23S Intergenic spacer | 474 | 0.0 | 99% | UCI |
| 11 | vaginal | rpoC | 156 | 1e-25 | 100% | AB |
| 12 | callus | uvrA F | 653 | 0.0 | 99% | UNH |
| callus | uvrA R | 651 | 0.0 | 99% | UNH |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Middelveen, M.J.; Sapi, E.; Burke, J.; Filush, K.R.; Franco, A.; Fesler, M.C.; Stricker, R.B. Persistent Borrelia Infection in Patients with Ongoing Symptoms of Lyme Disease. Healthcare 2018, 6, 33. https://doi.org/10.3390/healthcare6020033
Middelveen MJ, Sapi E, Burke J, Filush KR, Franco A, Fesler MC, Stricker RB. Persistent Borrelia Infection in Patients with Ongoing Symptoms of Lyme Disease. Healthcare. 2018; 6(2):33. https://doi.org/10.3390/healthcare6020033
Chicago/Turabian StyleMiddelveen, Marianne J., Eva Sapi, Jennie Burke, Katherine R. Filush, Agustin Franco, Melissa C. Fesler, and Raphael B. Stricker. 2018. "Persistent Borrelia Infection in Patients with Ongoing Symptoms of Lyme Disease" Healthcare 6, no. 2: 33. https://doi.org/10.3390/healthcare6020033
APA StyleMiddelveen, M. J., Sapi, E., Burke, J., Filush, K. R., Franco, A., Fesler, M. C., & Stricker, R. B. (2018). Persistent Borrelia Infection in Patients with Ongoing Symptoms of Lyme Disease. Healthcare, 6(2), 33. https://doi.org/10.3390/healthcare6020033






