The Promises and Challenges of Ecological Momentary Assessment in Schizophrenia: Development of an Initial Experimental Protocol
Abstract
:1. Introduction and Background
1.1. Treatment Nonadherence Is a Major Public Health Problem in Schizophrenia
1.2. Our Knowledge of Adherence Predictors and Interventions Is Limited
1.3. Ecological Momentary Assessment (EMA) Can Improve Our Understanding of Nonadherence
1.4. Rationale and Hypotheses for Current Project
2. EMA Study Procedures
2.1. Sample Selection
2.2. Feasibility of EMA
2.3. Choice of EMA Variables
| Schedule | Dynamic Variables | Sample Items |
|---|---|---|
| Random Assessment | Context | Where are you right now? [42] |
| Substance Use | Have you used any of the following substances? | |
| Affect | Rate the following words based on how you feel right now [60]. | |
| Psychotic Symptoms | Was someone spying on you or plotting against you? [42] | |
| Psychological Coping | How did you deal with any difficulties you were having? [61] | |
| Psychological Acceptance | I simply noticed my feelings and continued with what I was doing [62]. | |
| Stressors | Has something stressful or bad happened in your life? [63] | |
| Social Support | I am getting the emotional help and support I need from my family or friends [64]. | |
| End of Day Assessment | Behavioral Adherence | Did you attend any treatment appointments today? |
| Medication Adherence | I’ve taken my psychiatric medications as prescribed today. | |
| Therapeutic Alliance | Do you and your treatment provider understand each other? [65] | |
| Reasons for Nonadherence | My medications make me feel strange or “doped up.” [66,67,68,69] | |
| Medication Side Effects | How bothered were you by these side effects? [70] |
2.4. EMA Procedures
2.5. Traditional (Non-EMA) Assessments
| Measure | Topic | Format | Time Point |
|---|---|---|---|
| Treatment History Interview-4 (THI-4) [81] | Treatment Utilization/ Behavioral Adherence | Interview | BL, 1, 2, 4 |
| Medication Event Monitoring System (MEMS) [77] | Medication Adherence | Electronic | BL, 1, 2, 4 |
| Pill Counts [82] | Medication Adherence | Behavioral | BL, 1, 2, 4 |
| Brief Adherence Rating Scale (BARS) [78] | Medication Adherence | Interview | BL, 1, 2, 4 |
| Ratings of Medication Influences Scale (ROMI) [69] | Reasons for Nonadherence | Interview | BL, 1, 2, 4 |
| Drug Attitude Inventory-10 (DAI-10) [68] | Medication Attitudes | Self-Report | BL, 1, 2, 4 |
| Antipsychotic Side Effect Checklist (ASC) [83] | Medication Side Effects | Interview | BL, 1, 2, 4 |
| Working Alliance Inventory-Short Version (WAI-S) [65] | Doctor-Patient Therapeutic Alliance | Self-Report | BL, 1, 2, 4 |
| Brief Psychiatric Rating Scale (BPRS) [84] | Psychiatric Symptoms | Interview | BL, 1, 2, 4 |
| Positive and Negative Affect Schedule-Expanded (State) (PANAS) [60] | Mood State | Self-Report | BL, 1, 2, 4 |
| Consumer Experiences of Stigma Questionnaire (CESQ) [67] | Stigma/Discrimination | Self-Report | BL, 1, 2, 4 |
| Life Events Assessment (LEA) [63] | Stressful Life Events | Interview | BL, 1, 2, 4 |
| Multidimensional Scale of Perceived Social Support (MSPSS) [64] | Perceived Social Support | Self-Report | BL, 1, 2, 4 |
| Emotional Regulation Questionnaire (ERQ) [85] | Psychological Coping | Self-Report | BL, 1, 2, 4 |
| Acceptance and Action Questionnaire-II (AAQ) [86] | Psychological Flexibility/ Experiential Avoidance | Self-Report | BL, 1, 2, 4 |
| World Health Organization Disability Assessment Schedule 2.0–12 Item Version (WHODAS 2.0) [87] | Psychosocial Functioning | Self-Report | BL, 1, 2, 4 |
| Addiction Severity Index (ASI) [88] | Illicit Drug Use | Interview | BL, 1, 2, 4 |
| Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) [89] | Hazardous Drinking | Self-Report | BL, 1, 2, 4 |
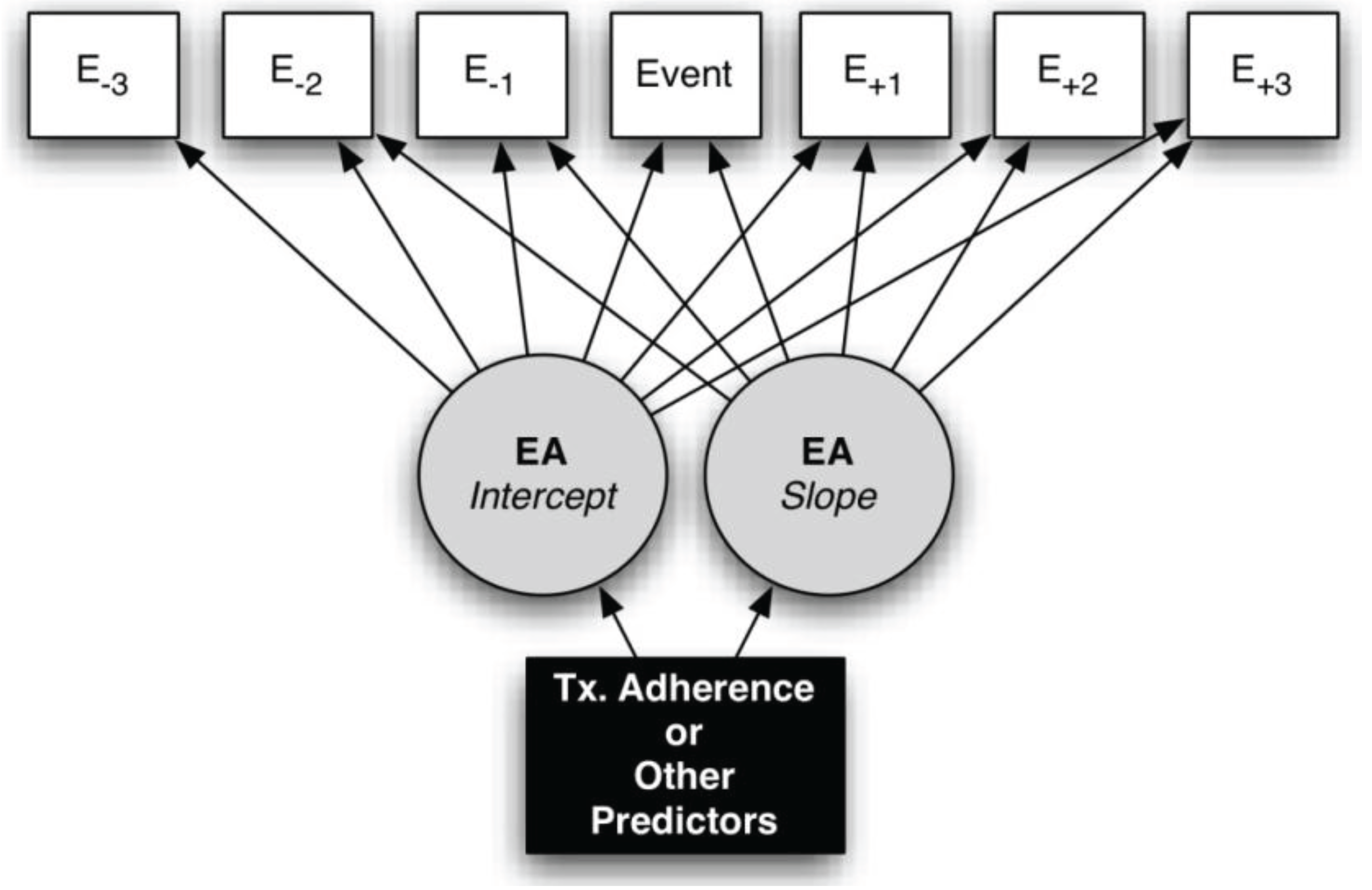
2.6. EMA Data Analysis Strategy
2.7. Future Ecological Momentary Intervention Development
3. Lessons Learned Thus Far
3.1. Choice of Mobile Devices and Programming
3.2. Recruitment Strategies
3.3. Retention Strategies
3.4. Technical Troubleshooting with Patients
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murray, C.; Lopez, A. The Global Burden of Disease; World Health Organization (WHO): Geneva, Swizerland, 1996. [Google Scholar]
- Wu, E.Q.; Birnbaum, H.G.; Shi, L.; Ball, D.E.; Kessler, R.C.; Moulis, M.; Aggarwal, J. The economic burden of schizophrenia in the United States in 2002. J. Clin. Psychiatry 2005, 66, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Staring, A.B.; van der Gaag, M.; Koopmans, G.T.; Selten, J.P.; van Beveren, J.M.; Hengeveld, M.W.; Loonen, A.J.; Mulder, C.L. Treatment adherence therapy in people with psychotic disorders: Randomised controlled trial. Br. J. Psychiatry 2010, 197, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Byerly, M.J.; Nakonezny, P.A.; Lescouflair, E. Antipsychotic medication adherence in schizophrenia. Psychiatry Clin. N. Am. 2007, 30, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.C.; Olfson, M. Outpatient antipsychotic treatment and inpatient costs of schizophrenia. Schizophr. Bull. 2008, 34, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ruscher, S.M.; de Wit, R.; Mazmanian, D. Psychiatric patients’ attitudes about medication and factors affecting noncompliance. Psychiatry Serv. 1997, 48, 82–85. [Google Scholar]
- Kreyenbuhl, J.; Slade, E.P.; Medoff, D.R.; Brown, C.H.; Ehrenreich, B.; Afful, J.; Dixon, L.B. Time to discontinuation of first- and second-generation antipsychotic medications in the treatment of schizophrenia. Schizophr. Res. 2011, 131, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.D.; Obeidat, N.A.; Cuffel, B.J.; Naradzay, J.; Loebel, A.D. Risk of discontinuation of atypical antipsychotic agents in the treatment of schizophrenia. Schizophr. Res. 2008, 98, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Novick, D.; Haro, J.M.; Suarez, D.; Perez, V.; Dittmann, R.W.; Haddad, P.M. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010, 176, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bergen, J.; Hunt, G.; Armitage, P.; Bashir, M. Six-month outcome following a relapse of schizophrenia. Aust. N. Z. J. Psychiatry 1998, 32, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Lacro, J.P.; Dunn, L.B.; Dolder, C.R.; Leckband, S.G.; Jeste, D.V. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: A comprehensive review of recent literature. J. Clin. Psychiatry 2002, 63, 892–909. [Google Scholar] [CrossRef] [PubMed]
- Bradburn, N.M.; Rips, L.J.; Shevell, S.K. Answering autobiographical questions: The impact of memory and inference on surveys. Science 1987, 236, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Csikszentmihalyi, M.; Larson, R. Validity and Reliability of the Experience-Sampling Method. J. Nerv. Ment. Dis. 1987, 175, 526–536. [Google Scholar] [CrossRef]
- Ebner-Priemer, U.W.; Trull, T.J. Ecological momentary assessment of mood disorders and mood dysregulation. Psychol. Assess. 2009, 21, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, P.W.; Liberman, R.P.; Engel, J.D. From noncompliance to collaboration in the treatment of schizophrenia. Hosp. Community Psychiatry 1990, 41, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Kane, J.M.; Marder, S.R.; Brauzer, B.; Gierl, B.; Schooler, N.; Casey, D.E.; Hassan, M. Dose response of prophylactic antipsychotics. J. Clin Psychiatry 1993, 54, 24–30. [Google Scholar] [PubMed]
- Fenton, W.S.; Blyler, C.R.; Heinssen, R.K. Determinants of medication compliance in schizophrenia: Empirical and clinical findings. Schizophr. Bull. 1997, 23, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Olfson, M.; Mechanic, D.; Hansell, S.; Boyer, C.A.; Walkup, J.; Weiden, P.J. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatry Serv. 2000, 51, 216–222. [Google Scholar] [CrossRef]
- Meier, J.; Becker, T.; Patel, A.; Robson, D.; Schene, A.; Kikkert, M.; Barbui, C.; Burti, L.; Puschner, B. Effect of medication-related factors on adherence in people with schizophrenia: A European multi-centre study. Epidemiol. Psichiatry Soc. 2010, 19, 251–259. [Google Scholar] [CrossRef]
- Perkins, D.O.; Johnson, J.L.; Hamer, R.M.; Zipursky, R.B.; Keefe, R.S.; Centorrhino, F.; Green, A.I.; Glick, I.B.; Kahn, R.S.; Sharma, T.; et al. Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophr. Res. 2006, 83, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Mutsatsa, S.H.; Joyce, E.M.; Hutton, S.B.; Webb, E.; Gibbins, H.; Paul, S.; Barnes, T.R. Clinical correlates of early medication adherence: West London first episode schizophrenia study. Acta Psychiatry Scand. 2003, 108, 439–446. [Google Scholar] [CrossRef]
- Schennach-Wolff, R.; Jager, M.; Seemuller, F.; Obermeier, M.; Messer, T.; Laux, G.; Pfeiffer, H.; Naber, D.; Schmidt, L.G.; Gaebel, W.; et al. Attitude towards adherence in patients with schizophrenia at discharge. J. Psychiatry Res. 2009, 43, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.M.T.; Tsang, H.W.H.; Corrigan, P.W. Self-stigma of people with schizophrenia as predictor of their adherence to psychosocial treatment. Psychiatry Rehabil. J. 2008, 32, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hudson, T.J.; Owen, R.R.; Thrush, C.R.; Han, X.; Pyne, J.M.; Thapa, P.; Sullivan, G. A pilot study of barriers to medication adherence in schizophrenia. J. Clin. Psychiatry 2004, 65, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Granholm, E.; Ben-Zeev, D.; Link, P.C.; Bradshaw, K.R.; Holden, J.L. Mobile Assessment and Treatment for Schizophrenia (MATS): A pilot trial of an interactive text-messaging intervention for medication adherence, socialization, and auditory hallucinations. Schizophr. Bull. 2012, 38, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Acosta, F.J.; Bosch, E.; Sarmiento, G.; Juanes, N.; Caballero-Hidalgo, A.; Mayans, T. Evaluation of noncompliance in schizophrenia patients using electronic monitoring (MEMS (R)) and its relationship to sociodemographic, clinical and psychopathological variables. Schizophr. Res. 2009, 107, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Cuffel, B.J.; Alford, J.; Fischer, E.P.; Owen, R.R. Awareness of illness in schizophrenia and outpatient treatment adherence. J. Nerv. Ment. Dis. 1996, 184, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Rittmannsberger, H.; Pachinger, L.; Keppelmuller, P.; Wancata, J. Medication adherence among psychotic patients before admission to inpatient treatment. Psychiatry Serv. 2004, 55, 174–179. [Google Scholar] [CrossRef]
- Hofer, A.; Kemmler, G.; Eder, U.; Honeder, M.; Hummer, M.; Fleischhacker, W.W. Attitudes toward antipsychotics among outpatient clinic attendees with schizophrenia. J. Clin. Psychiatry 2002, 63, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Dolder, C.R.; Lacro, J.P.; Leckband, S.; Jeste, D.V. Interventions to improve antipsychotic medication adherence: Review of recent literature. J. Clin. Psychopharm. 2003, 23, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, A.; Olfson, M.; Boyer, C.A.; Mechanic, D. Interventions to improve medication adherence in schizophrenia. Am. J. Psychiatry 2002, 159, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Kemp, R.; Hayward, P.; Applewhaite, G.; Everitt, B.; David, A. Compliance therapy in psychotic patients: Randomised controlled trial. Br. Med. J. 1996, 312, 345–349. [Google Scholar] [CrossRef]
- O’Donnell, C.; Donohoe, G.; Sharkey, L.; Owens, N.; Migone, M.; Harries, R.; Kinsella, A.; Larkin, C.; O’Callaghan, E. Compliance therapy: A randomised controlled trial in schizophrenia. Br. Med. J. 2003. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.B.; Dickerson, F.; Bellack, A.S.; Bennett, M.; Dickinson, D.; Goldberg, R.W.; Lehman, A.; Tenhula, W.N.; Calmes, C.; Pasillas, R.M.; et al. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr. Bull. 2010, 36, 48–70. [Google Scholar] [CrossRef] [PubMed]
- Gaudiano, B.A.; Weinstock, L.M.; Miller, I.W. Improving treatment adherence in bipolar disorder: A review of current psychosocial treatment efficacy and recommendations for future treatment development. Behav. Modif. 2008, 32, 267–301. [Google Scholar] [CrossRef] [PubMed]
- Colom, F.; Vieta, E.; Tacchi, M.J.; Sanchez-Moreno, J.; Scott, J. Identifying and improving non-adherence in bipolar disorders. Bipolar Disord. 2005, 7, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.S.; Swanson, J.W.; Wagner, H.R.; Burns, B.J.; Hiday, V.A. Effects of involuntary outpatient commitment and depot antipsychotics on treatment adherence in persons with severe mental illness. J. Nerv. Ment. Dis 2001, 189, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Bolger, N.; Davis, A.; Rafaeli, E. Diary methods: Capturing life as it is lived. Ann. Rev. Psychol. 2003, 54, 579–616. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.C.; Lim, C.S.; Yu, N.; Geller, D.; Wagner, M.H.; Quittner, A.L. A multi-method assessment of treatment adherence for children with cystic fibrosis. J. Cystic Fibrosis 2006, 5, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Quittner, A.L.; Modi, A.C.; Lemanek, K.L.; Ievers-Landis, C.E.; Rapoff, M.A. Evidence-based assessment of adherence to medical treatments in pediatric psychology. J. Pediatry Psychol. 2008, 33, 916–936. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zeev, D.; Ellington, K.; Swendsen, J.; Granholm, E. Examining a cognitive model of persecutory ideation in the daily life of people with schizophrenia: A computerized experience sampling study. Schizophr. Bull. 2011, 37, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Granholm, E.; Loh, C.; Swendsen, J. Feasibility and validity of computerized ecological momentary assessment in schizophrenia. Schizophr. Bull. 2008, 34, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zeev, D.; McHugo, G.J.; Xie, H.; Dobbins, K.; Young, M.A. Comparing retrospective reports to real-time/real-place mobile assessments in individuals with schizophrenia and a nonclinical comparison group. Schizophr. Bull. 2012. [Google Scholar] [CrossRef] [PubMed]
- Kimhy, D.; Delespaul, P.; Ahn, H.; Cai, S.; Shikhman, M.; Lieberman, J.A.; Malaspina, D.; Sloan, R.P. Concurrent measurement of “real-world” stress and arousal in individuals with psychosis: Assessing the feasibility and validity of a novel methodology. Schizophr. Bull. 2010, 36, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Swendsen, J.; Ben-Zeev, D.; Granholm, E. Real-time electronic ambulatory monitoring of substance use and symptom expression in schizophrenia. Am. J. Psychiatry 2011, 168, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Oorschot, M.; Lataster, T.; Thewissen, V.; Wichers, M.; Myin-Germeys, I. Mobile assessment in schizophrenia: A data-driven momentary approach. Schizophr. Bull. 2012. [Google Scholar] [CrossRef] [PubMed]
- So, S.H.; Peters, E.R.; Swendsen, J.; Garety, P.A.; Kapur, S. Detecting improvements in acute psychotic symptoms using experience sampling methodology. Psychiatry Res. 2013, 210, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Depp, C.A.; Mausbach, B.; Granholm, E.; Cardenas, V.; Ben-Zeev, D.; Patterson, T.L.; Lebowitz, B.D.; Jeste, D.V. Mobile interventions for severe mental illness: Design and preliminary data from three approaches. J. Nerv. Ment. Dis. 2010, 198, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.M.; Medina, E.; Gomez-Beneyto, M.; Maurino, J. A short message service (SMS)-based strategy for enhancing adherence to antipsychotic medication in schizophrenia. Psychiatry Res. 2012, 200, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Wang, L.J. Statistical power analysis for growth curve models using SAS. Behav. Res. Methods 2009, 41, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Armey, M.F.; Schatten, H.T.; Haradhvala, N.; Miller, I.W. Ecological momentary assessment (EMA) of depression-related phenomena. Curr. Opin. Psychol. 2015, 4, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, J.R.; Wilkinson, I.B.; Yki-Jarvinen, H. Multiple risk factor intervention in type 2 diabetes: An opportunity not to be missed. Diabetes Obes. Metab. 2001, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hjermann, I. Multiple risk factor intervention in coronary heart disease. Ciba Found. Symp. 1985, 110, 110–125. [Google Scholar] [PubMed]
- Kuller, L.; Neaton, L.; Caggiula, A.; Falvo-Gerard, L. Primary prevention of heart attacks: The multiple risk factor intervention trial. Am. J. Epideiol. 1980, 112, 185–199. [Google Scholar]
- Stamler, J.; Vaccaro, O.; Neaton, J.D.; Wentworth, D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993, 16, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Loffler, W.; Kilian, R.; Toumi, M.; Angermeyer, M.C. Schizophrenic patients’ subjective reasons for compliance and noncompliance with neuroleptic treatment. Pharmacopsychiatry 2003, 36, 105–112. [Google Scholar] [PubMed]
- Moitra, E.; Herbert, J.D.; Forman, E.M. Acceptance-based behavior therapy to promote HIV medication adherence. AIDS Care 2011, 23, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Vilardaga, R.; McDonell, M.; Leickly, E.; Ries, R. Ecological momentary assessments in psychosis: A contextual behavioral approach to studying mindfulness and acceptance. In Incorporating Mindfulness and Acceptance into the Treatment of Psychosis: Current Trends and Future Directions; Gaudiano, B.A., Ed.; Oxford: New York, NY, USA, 2015; pp. 25–56. [Google Scholar]
- Hektner, J.M.; Schmidt, J.A.; Csíkszentmihályi, M. Experience Sampling Method: Measuring the Quality of Everyday Life; SAGE: London, UK, 2007. [Google Scholar]
- Watson, D.; Clark, L. The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form; The University of Iowa: Iowa City, IA, USA, 1994. [Google Scholar]
- Vilardaga, R.; Hayes, S.C.; Atkins, D.C.; Bresee, C.; Kambiz, A. Comparing experiential acceptance and cognitive reappraisal as predictors of functional outcome in individuals with serious mental illness. Behav. Res. Ther. 2013, 51, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Shawyer, F.; Ratcliff, K.; Mackinnon, A.; Farhall, J.; Hayes, S.C.; Copolov, D. The voices acceptance and action scale (VAAS): Pilot data. J. Clin. Psychol. 2007, 63, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, L.; Breznitz, S. Handbook of Stress: Theoretical and Clinical Aspects; The Free Press: New York, NY, USA, 1993; Volume 2. [Google Scholar]
- Zimet, G.D.; Dahlem, N.W.; Zimet, S.G.; Farley, G.K. The multidimensional scale of perceived social support. J. Pers. Assess. 1988, 52, 30–41. [Google Scholar] [CrossRef]
- Munder, T.; Wilmers, F.; Leonhart, R.; Linster, H.W.; Barth, J. Working Alliance Inventory-Short Revised (WAI-SR): Psychometric properties in outpatients and inpatients. Clin. Psychol. Psychother. 2010, 17, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Birchwood, M.; Smith, J.; Drury, V.; Healy, J.; Macmillan, F.; Slade, M. A self-report insight scale for psychosis: Reliability, validity and sensitivity to change. Acta Psychiatry Scand. 1994, 89, 62–67. [Google Scholar] [CrossRef]
- Dickerson, F.B.; Sommerville, J.; Origoni, A.E.; Ringel, N.B.; Parente, F. Experiences of stigma among outpatients with schizophrenia. Schizophr. Bull. 2002, 28, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.E.; Lindstrom, E.; Nielsen, J.; Levander, S. DAI-10 is as good as DAI-30 in schizophrenia. Eur. Neuropsychopharmacol. 2012, 22, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Weiden, P.; Rapkin, B.; Mott, T.; Zygmunt, A.; Goldman, D.; Horvitz-Lennon, M.; Frances, A. Rating of medication influences (ROMI) scale in schizophrenia. Schizophr. Bull. 1994, 20, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Dibonaventura, M.; Gabriel, S.; Dupclay, L.; Gupta, S.; Kim, E. A patient perspective of the impact of medication side effects on adherence: Results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry 2012. [Google Scholar] [CrossRef] [PubMed]
- Gaudiano, B.A.; Herbert, J.D. Acute treatment of inpatients with psychotic symptoms using Acceptance and Commitment Therapy: Pilot results. Behav. Res. Ther. 2006, 44, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Strosahl, K.D.; Wilson, K.G. Acceptance and Commitment Therapy: The Process and Practice of Mindful Change, 2nd ed.; Guilford: New York, NY, USA, 2012. [Google Scholar]
- Hayes, S.; Villatte, M.; Levin, M.; Hildebrandt, M. Open, aware, and active: Contextual approaches as an emerging trend in the behavioral and cognitive therapies. Ann. Rev. Clin. Psychol. 2011, 7, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Luoma, J.B.; Bond, F.W.; Masuda, A.; Lillis, J. Acceptance and commitment therapy: Model, processes and outcomes. Behav. Res. Ther. 2006, 44, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological momentary assessment. Ann. Rev. Clin. Psychol. 2008, 4, 1–32. [Google Scholar] [CrossRef]
- Froehlich, J. The Myexperience Tool. Avaliable online: http://myexperience.sourceforge.net/ (accessed on 20 June 2015).
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Byerly, M.J.; Nakonezny, P.A.; Rush, A.J. The brief adherence rating scale (bars) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophrenia Res. 2008, 100, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Ailinger, R.L.; Black, P.L.; Lima-Garcia, N. Use of electronic monitoring in clinical nursing research. Clin. Nurs. Res. 2008, 17, 89–97. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, R.; Dennis, M.; Johnston, M.; Sudlow, C. Improving adherence to medication in stroke survivors (IAMSS): A randomised controlled trial: Study protocol. BMC neurology 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linehan, M.M.; Heard, H.L. Treatment History Interview (THI); University of Washington: Seattle, WA, USA, 1987. [Google Scholar]
- Kalichman, S.C.; Amaral, C.M.; Cherry, C.; Flanagan, J.; Pope, H.; Eaton, L.; Kalichman, M.O.; Cain, D.; Detorio, M.; Caliendo, A.; et al. Monitoring medication adherence by unannounced pill counts conducted by telephone: Reliability and criterion-related validity. HIV Clin. Trials 2008, 9, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Weiden, P.J.; Miller, A.L. Which side effects really matter? Screening for common and distressing side effects of antipsychotic medications. J. Psychiatry Pract. 2001, 7, 41–47. [Google Scholar] [CrossRef]
- Overall, J.; Gorham, D. The brief psychiatric rating scale. Psychol. Rep. 1962, 10, 799–812. [Google Scholar] [CrossRef]
- Gross, J.J.; John, O.P. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 2003, 85, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Bond, F.W.; Hayes, S.C.; Baer, R.A.; Carpenter, K.M.; Guenole, N.; Orcutt, H.K.; Waltz, T.; Zettle, R.D. Preliminary psychometric properties of the Acceptance and Action Questionnaire-II: A revised measure of psychological inflexibility and experiential avoidance. Behav. Ther. 2011, 42, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Federici, S.; Meloni, F.; Mancini, A.; Lauriola, M.; Olivetti Belardinelli, M. World Health Organisation disability assessment schedule II: Contribution to the Italian validation. Disabil. Rehabil. 2009, 31, 553–564. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.T.; Luborsky, L.; Woody, G.E.; O’Brien, C.P. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J. Nerv. Ment. Dis. 1980, 168, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Heron, K.E.; Smyth, J.M. Ecological momentary interventions: Incorporating mobile technology into psychosocial and health behaviour treatments. Br. J. Health Psychol. 2010, 15, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zeev, D.; Kaiser, S.M.; Brenner, C.J.; Begale, M.; Duffecy, J.; Mohr, D.C. Development and usability testing of FOCUS: A smartphone system for self-management of schizophrenia. Psychiatry Rehabil. J. 2013, 36, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Zickuhr, K.; Smith, A. Digital Differences. 2012. Avilable online: http://www.pewinternet.org/2012/04/13/digital-differences/ (accessed on 20 June 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaudiano, B.A.; Moitra, E.; Ellenberg, S.; Armey, M.F. The Promises and Challenges of Ecological Momentary Assessment in Schizophrenia: Development of an Initial Experimental Protocol. Healthcare 2015, 3, 556-573. https://doi.org/10.3390/healthcare3030556
Gaudiano BA, Moitra E, Ellenberg S, Armey MF. The Promises and Challenges of Ecological Momentary Assessment in Schizophrenia: Development of an Initial Experimental Protocol. Healthcare. 2015; 3(3):556-573. https://doi.org/10.3390/healthcare3030556
Chicago/Turabian StyleGaudiano, Brandon A., Ethan Moitra, Stacy Ellenberg, and Michael F. Armey. 2015. "The Promises and Challenges of Ecological Momentary Assessment in Schizophrenia: Development of an Initial Experimental Protocol" Healthcare 3, no. 3: 556-573. https://doi.org/10.3390/healthcare3030556
APA StyleGaudiano, B. A., Moitra, E., Ellenberg, S., & Armey, M. F. (2015). The Promises and Challenges of Ecological Momentary Assessment in Schizophrenia: Development of an Initial Experimental Protocol. Healthcare, 3(3), 556-573. https://doi.org/10.3390/healthcare3030556






