Symptom Experiences and Coping Strategies in Turkish Patients with Implantable Cardioverter-Defibrillators: A Cross-Sectional Study Based on Interviews
Abstract
1. Introduction
Research Questions
2. Materials and Methods
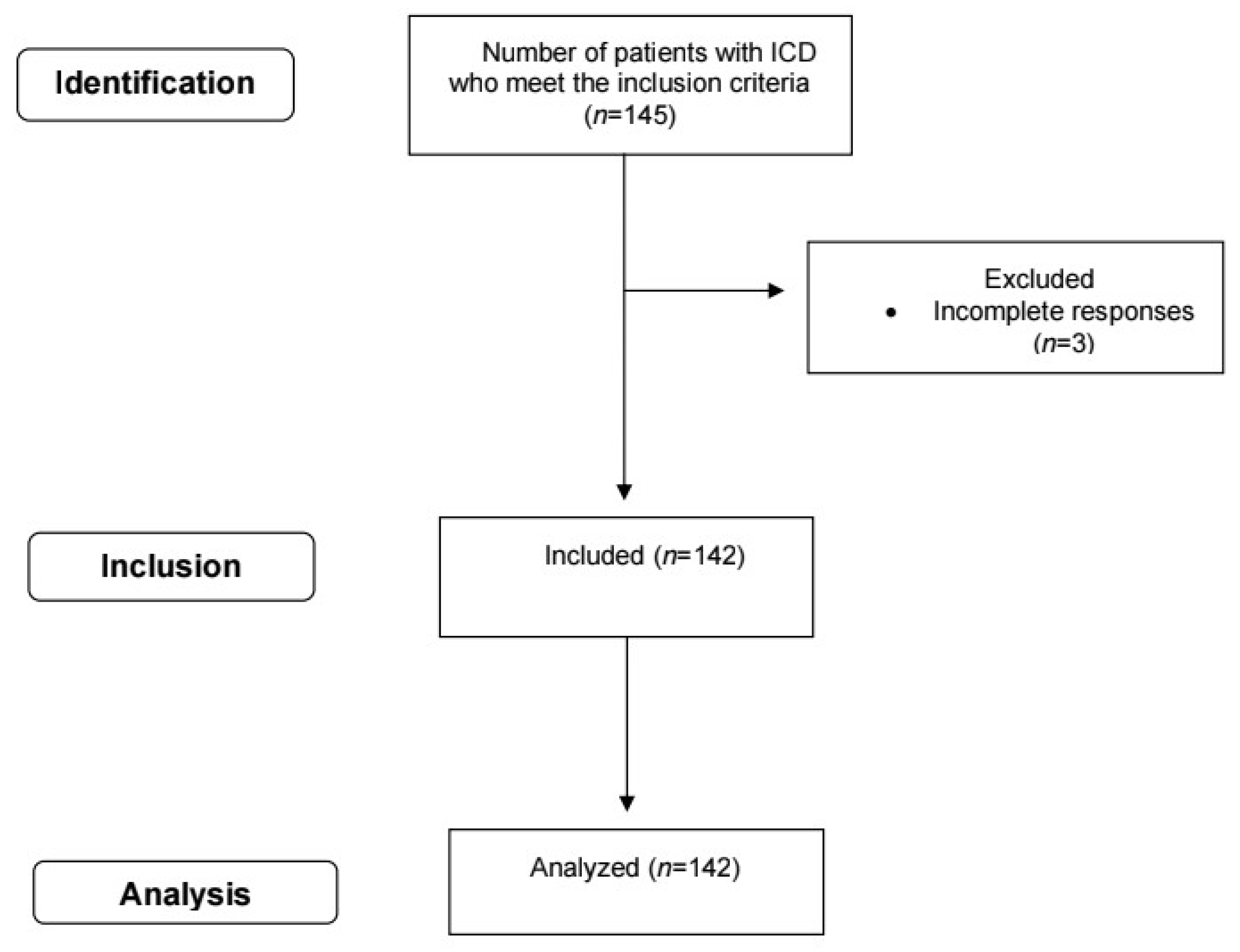
2.1. Type, Location, and Time of the Study
2.2. Population and Sample
2.2.1. Inclusion Criteria
- Patients who were informed about the study and voluntarily signed the informed consent form,
- Patients who had an ICD implanted at least three months prior to study enrollment,
- Patients without communication difficulties (able to speak Turkish and without cognitive impairment),
2.2.2. Exclusion Criteria
- Patients unable to sign the informed consent form,
- Patients who have undergone ICD implantation within the last three months,
- Patients with communication difficulties,
- Patients under 18 years of age,
2.3. Data Collection
2.3.1. Information Obtained from Medical Records
2.3.2. Sociodemographic Information
2.3.3. Symptom Experiences and Coping Methods
2.4. Data Analysis
3. Results
4. Discussion
5. Future Directions
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzeis, S. Sudden Cardiac Death: We Are Doing Well … but We Need to Do Better! Heart 2021, 107, 1276–1277. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report from the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Onat, A.; Can, G. Prevalence of Heart Disease, New Coronary Events, and Cardiac Mortality in Our Adults. In TEKHARF 2017 Leading the Medical World’s Approach to Chronic Diseases; Onat, A., Ed.; Logos Yayıncılık Tic. A.Ş.: Istanbul, Turkey, 2017; pp. 21–28. [Google Scholar]
- Onat, A.; Karakoyun, S.; Akbaş, T.; Özpamuk Karadeniz, F.; Çakır, H.; Şimşek, B.; Can, G. TEKHARF 2014 Screening and Incidence of Coronary Disease According to Geographic Regions in Türkiye. Turk. Soc. Cardiol. Arch. 2015, 43, 326–332. [Google Scholar] [CrossRef]
- John Camm, A. Indications for Implantable Cardioverter Defibrillators. In Manual of Cardiovascular Medicine; Oxford University Press: Oxford, UK, 2021; pp. 253–258. [Google Scholar]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- de Almeida Fernandes, D.; António, N.; Sousa, P.A.; Preto, L.; Madeira, M.; Elvas, L.; Gonçalves, L. “Real-World” Analysis of Battery Longevity of Implantable Cardioverter-Defibrillators: An in-Depth Analysis of a Prospective Defibrillator Database. BMC Cardiovasc. Disord. 2023, 23, 609. [Google Scholar] [CrossRef]
- Sampson, M. Ambulatory Electrocardiography: Indications and Devices. Br. J. Card. Nurs. 2019, 14, 114–121. [Google Scholar] [CrossRef]
- Ghezzi, E.S.; Sharman, R.L.S.; Selvanayagam, J.B.; Psaltis, P.J.; Sanders, P.; Astley, J.M.; Knayfati, S.; Batra, V.; Keage, H.A.D. Burden of Mood Symptoms and Disorders in Implantable Cardioverter Defibrillator Patients: A Systematic Review and Meta-Analysis of 39,954 Patients. Europace 2023, 25, euad130. [Google Scholar] [CrossRef]
- Ghezzi, E.; Sharman, R.; Selvanayagam, J.; Psaltis, P.; Sanders, P.; Astley, J.; Knayfati, S.; Batra, V.; Keage, H. Anxiety, Depression and Post-Traumatic Stress Disorder in Implantable Cardioverter Defibrillator Patients: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2023, 32, S336. [Google Scholar] [CrossRef]
- Barisone, M.; Hayter, M.; Ghirotto, L.; Catania, G.; Zanini, M.; Dal Molin, A.; Sasso, L.; Bagnasco, A. The Experience of Patients with an Implantable Cardioverter-Defibrillator: A Systematic Review and Meta-Synthesis of Qualitative Studies. Eur. J. Cardiovasc. Nurs. 2022, 21, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Tuna, S. Problems Experienced by Patients with Implantable Cardioverter Defibrillators and Nursing Management. Gazi Health Sci. J. 2024, 9, 94–102. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary. Heart Rhythm 2018, 15, e190–e252. [Google Scholar] [CrossRef] [PubMed]
- Streur, M.M.; Auld, J.P.; Chang, W.; Choupani, F.; Zheng, T.; Frazier, E.; Liberato, A.C.S.; Pike, K.C.; Thompson, E.A.; Dougherty, C.M. Social Determinants of Health-Related Outcomes in an Intervention Following Initial Implantable Cardioverter-Defibrillator Implantation. J. Am. Heart Assoc. 2025, 14, e039238. [Google Scholar] [CrossRef]
- Retting, J.; Klapproth, C.P.; Rose, M.; Blaschke, F.; Parwani, A.; Baehr, F.; Boldt, L.H. Age- and Gender-Related Differences in Emotional Reactions of Patients Related to Their Implantable Cardioverter Defibrillator. Europace 2025, 27, euaf085.582. [Google Scholar] [CrossRef]
- van den Heuvel, L.M.; Sarina, T.; Sweeting, J.; Yeates, L.; Bates, K.; Spinks, C.; O’Donnell, C.; Sears, S.F.; McGeechan, K.; Semsarian, C.; et al. A Prospective Longitudinal Study of Health-Related Quality of Life and Psychological Wellbeing after an Implantable Cardioverter-Defibrillator in Patients with Genetic Heart Diseases. Heart Rhythm O2 2022, 3, 143–151. [Google Scholar] [CrossRef]
- Allemann, H.; Strömberg, A.; Thylén, I. Perceived Social Support in Persons with Heart Failure Living with an Implantable Cardioverter Defibrillator. J. Cardiovasc. Nurs. 2018, 33, E1–E8. [Google Scholar] [CrossRef]
- Mektebî, M. Emergency Department Presentations/Complaints/Complications in Patients with Pacemakers and ICDs. Medical Specialty Thesis, Gazi University Faculty of Medicine, Ankara, Turkey, 2015. [Google Scholar]
- Fidan Yolay, İ.; İşler, Y.; Kaya, H.; Yüksel, M.; Ay, M.O.; Ocak, U. Factors Influencing Mortality in Patients with Pacemaker/ICD Dysfunction Presenting to Emergency Departments. Eurasian J. Emerg. Med. 2024, 23, 270–277. [Google Scholar] [CrossRef]
- Gupta, K.; Kaminska, M.; Gupta, S.; Waraich, H.; Malik, S.; Meghdadi, A.; Marcotte, L.; Vazquez, G.H.; Baranchuk, A. The Psychological Impact of Implantable Cardioverter Defibrillators: A Narrative Review. Am. J. Cardiol. 2025, 255, 83–88. [Google Scholar] [CrossRef]
- Streur, M.M.; Thompson, E.A.; Dougherty, C.M. Multisymptom Profile Predicts Increased Risk of Poor Outcomes After Initial Placement of Implantable Cardioverter Defibrillator. J. Pain Symptom Manag. 2020, 59, 658–667. [Google Scholar] [CrossRef]
- Perini, A.P.; Kutyifa, V.; Veazie, P.; Daubert, J.P.; Schuger, C.; Zareba, W.; McNitt, S.; Rosero, S.; Tompkins, C.; Padeletti, L.; et al. Effects of Implantable Cardioverter/Defibrillator Shock and Antitachycardia Pacing on Anxiety and Quality of Life: A MADIT-RIT Substudy. Am. Heart J. 2017, 189, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Polikandrioti, M.; Tzirogiannis, K.; Zyga, S.; Koutelekos, I.; Vasilopoulos, G.; Theofilou, P.; Panoutsopoulos, G. Effect of Anxiety and Depression on the Fatigue of Patients with a Permanent Pacemaker. Arch. Med. Sci. Atheroscler. Dis. 2018, 3, 8–17. [Google Scholar] [CrossRef]
- Plakoutsi, S.; Florou, E.; Sfairopoulos, D.; Skapinakis, P.; Korantzopoulos, P. Depression and Anxiety in Patients Receiving an Implantable Cardioverter Defibrillator with or without Cardiac Resynchronization Therapy. J. Geriatr. Cardiol. 2025, 22, 255–264. [Google Scholar] [CrossRef]
- Andersen, C.M.; Theuns, D.A.M.J.; Johansen, J.B.; Pedersen, S.S. Anxiety, Depression, Ventricular Arrhythmias and Mortality in Patients with an Implantable Cardioverter Defibrillator: 7 Years’ Follow-up of the MIDAS Cohort. Gen. Hosp. Psychiatry 2020, 66, 154–160. [Google Scholar] [CrossRef]
- Zormpas, C.; Kahl, K.G.; Hohmann, S.; Oswald, H.; Stiel, C.; Veltmann, C.; Bauersachs, J.; Duncker, D. Depressive Symptoms and Quality of Life in Patients with Heart Failure and an Implantable Cardioverter-Defibrillator. Front. Psychiatry 2022, 13, 827967. [Google Scholar] [CrossRef]
- Flemme, I.; Johansson, I.; Strömberg, A. Living with Life-saving Technology—Coping Strategies in Implantable Cardioverter Defibrillators Recipients. J. Clin. Nurs. 2012, 21, 311–321. [Google Scholar] [CrossRef]
- Humphreys, N.K.; Lowe, R.; Rance, J.; Bennett, P.D. Living with an Implantable Cardioverter Defibrillator: The Patients’ Experience. Heart Lung 2016, 45, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.L.; He, H.-G.; Dong, Y.; Wang, W. Perceptions and Experiences of Patients Living with Implantable Cardioverter Defibrillators: A Systematic Review and Meta-Synthesis. Health Qual. Life Outcomes 2016, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Erbil, P.; Ahmadi, N.; Cetrez, Ö.A. Religion, Culture and Meaning-Making Coping: A Study Among Cancer Patients in Türkiye. J. Relig. Health 2019, 58, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Ar, Y.; Karanci, A.N. Turkish Adult Children as Caregivers of Parents with Alzheimer’s Disease: Perceptions and Caregiving Experiences. Dementia 2019, 18, 882–902. [Google Scholar] [CrossRef]
- Us, S.A.; Taşcı, S.; Demirtaş, A.O. The Effect of Lavender Essential Oil Aromatherapy on Anxiety and Fatigue in Patients with Implantable Cardioverter Defibrillator: Randomized Controlled Trial. EXPLORE 2025, 21, 103233. [Google Scholar] [CrossRef]
- Polikandrioti, M.; Tzirogiannis, K.; Zyga, S.; Gerogianni, G.; Stefanidou, S.; Tsami, A.; Panoutsopoulos, G. Assessment of Fatigue in Patients with a Permanent Cardiac Pacemaker: Prevalence and Associated Factors. Arch. Med. Sci. Atheroscler. Dis. 2018, 3, 166–173. [Google Scholar] [CrossRef]
- Sert, M. Death Anxiety and Sleep Quality in Patients with Implantable Cardioverter Defibrillators. J. Psychiatr. Nurs. 2024, 15, 399–409. [Google Scholar] [CrossRef]
- Squara, F.; Bateau, J.; Scarlatti, D.; Bun, S.-S.; Moceri, P.; Ferrari, E. Virtual Reality for the Management of Pain and Anxiety in Patients Undergoing Implantation of Pacemaker or Implantable Cardioverter Defibrillator: A Randomized Study. J. Med. Syst. 2024, 48, 28. [Google Scholar] [CrossRef]
- Rambod, M.; Rohaninasab, S.; Pasyar, N.; Nikoo, M.H. The Effect of Virtual Interactive Nurse-Led Support Group Intervention on Fatigue, Shock Anxiety, and Acceptance of Implantable Cardioverter Defibrillator Patients: A Randomized Trial. BMC Cardiovasc. Disord. 2024, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Mamoureli, A.; Babatsikou, F.; Flevari, P.; Leftheriotis, D.; Fragiadakis, E.; Polikandrioti, M. Anxiety and Depression in Patients with Permanent Pacemaker. Perioper. Nurs. 2017, 6, 107–123. [Google Scholar] [CrossRef]
- Dunbar, S.B.; Jenkins, L.S.; Hawthorne, M.; Kimble, L.P.; Dudley, W.N.; Slemmons, M.; Purcell, J.A. Factors Associated with Outcomes 3 Months after Implantable Cardioverter Defibrillator Insertion. Heart Lung 1999, 28, 303–315. [Google Scholar] [CrossRef]
- Riley, J.L., III; Robinson, M.E.; Geisser, M.E. Empirical Subgroups of the Coping Strategies Questionnaire-Revised: A Multisample Study. Clin. J. Pain 1999, 15, 111–116. [Google Scholar] [CrossRef]
- Robinson, M.E.; Riley, J.L., III; Myers, C.D.; Sadler, I.J.; Kvaal, S.A.; Geisser, M.E.; Keefe, F.J. The Coping Strategies Questionnaire: A Large Sample, Item-Level Factor Analysis. Clin. J. Pain 1997, 13, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Januszkiewicz, Ł.; Barra, S.; Providencia, R.; Conte, G.; de Asmundis, C.; Chun, J.K.R.; Farkowski, M.M.; Guerra, J.M.; Marijon, E.; Boveda, S. Long-Term Quality of Life and Acceptance of Implantable Cardioverter-Defibrillator Therapy: Results of the European Heart Rhythm Association Survey. EP Eur. 2022, 24, 860–867. [Google Scholar] [CrossRef]
- Willy, K.; Ellermann, C.; Reinke, F.; Rath, B.; Wolfes, J.; Eckardt, L.; Doldi, F.; Wegner, F.K.; Köbe, J.; Morina, N. The Impact of Cardiac Devices on Patients’ Quality of Life—A Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2022, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Boşnak Güçlü, M.; Barği, G.; Katayifçi, N.; Şen, F. Comparison of Functional and Maximal Exercise Capacity, Respiratory and Peripheral Muscle Strength, Dyspnea, and Fatigue in Patients with Heart Failure with Pacemakers and Healthy Controls: A Cross-Sectional Study. Physiother. Theory Pract. 2021, 37, 295–306. [Google Scholar] [CrossRef]
- Pasyar, N.; Rambod, M.; Zare, A.; Nikoo, M.H. The Predictor Roles of Spiritual Well-Being, Healthcare Professionals’ Support and Shock Anxiety in Implantable Cardioverter-Defibrillator Device Acceptance. Malays. J. Med. Sci. 2022, 29, 80–89. [Google Scholar] [CrossRef]
- Moghtader, S.; Kanbar-Agha, F.; Sharafkhaneh, A. Treating Fatigue in Patients with Chronic Heart and Lung Disease. In Fatigue Management; Springer: New York, NY, USA, 2018; pp. 151–161. [Google Scholar]
- Razavi, M.; Khatiban, M.; Ahmadi, F.; Oshvandi, K. Adaptation Status and Related Factors in Patients Living with Implantable Cardioverter Defibrillators. J. Fam. Med. Prim. Care 2022, 11, 4467–4472. [Google Scholar] [CrossRef]
- Sandhu, U.; Nguyen, A.T.; Dornblaser, J.; Gray, A.; Paladino, K.; Henrikson, C.A.; Kovacs, A.H.; Nazer, B. Patient-Reported Outcomes in a Multidisciplinary Electrophysiology-Psychology Ventricular Arrhythmia Clinic. J. Am. Heart Assoc. 2022, 11, 4467–4472. [Google Scholar] [CrossRef]
- Nicmanis, M.; Chur-Hansen, A.; Oxlad, M. The Psychological, Social, and Quality of Life Outcomes of People with a Cardiac Implantable Electronic Device: An Umbrella Review. Eur. J. Cardiovasc. Nurs. 2024, 23, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Pasyar, N.; Rambod, M.; Nikoo, M.H.; Mansouri, P. An Evaluation of the Association between Quality of Life and Psychological Issues in Patients with Automated Implantable Cardioverter Defibrillator. J. Caring Sci. 2021, 11, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, Y.; Watabe, T.; Nakahara, D.; Taniguchi, K.; Oginosawa, Y.; Kataoka, M. Characteristics of Anxiety about Returning to Work after Implantable Cardioverter Defibrillator Implantation in Japan. J. Cardiol. 2025; Epub ahead of printing. [Google Scholar] [CrossRef]
- Rottmann, N.; Skov, O.; Andersen, C.M.; Theuns, D.A.M.J.; Pedersen, S.S. Psychological Distress in Patients with an Implantable Cardioverter Defibrillator and Their Partners. J. Psychosom. Res. 2018, 113, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.S.; van Domburg, R.T.; Theuns, D.A.; Jordaens, L.; Erdman, R.A. Concerns about the Implantable Cardioverter Defibrillator: A Determinant of Anxiety and Depressive Symptoms Independent of Experienced Shocks. Am. Heart J. 2005, 149, 664–669. [Google Scholar] [CrossRef]
- Rezaeipandari, H.; Morowatisharifabad, M.A.; Shaghaghi, A. Religious and Spiritual Coping Elements in Dealing with Chronic Diseases: A Qualitative Exploration of the Perspectives of Older Iranian Zoroastrians. J. Relig. Health 2023, 62, 3017–3041. [Google Scholar] [CrossRef]
- Reynolds, N.; Mrug, S.; Wolfe, K.; Schwebel, D.; Wallander, J. Spiritual Coping, Psychosocial Adjustment, and Physical Health in Youth with Chronic Illness: A Meta-Analytic Review. Health Psychol. Rev. 2016, 10, 226–243. [Google Scholar] [CrossRef]
- Hajian-Tilaki, E.; Hajian-Tilaki, K.; Moslemi, D.; Godazandeh, G.; Firouzbakht, M. Association of Social Support, Spirituality with Psychological Factors in Iranian Breast Cancer Survivors: An Evidence from a Cross-sectional Study. Nurs. Open 2022, 9, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.M.; Mahmood, Q.K.; Sher, F.; Saud, M.; Mas’udah, S.; Ida, R. Coping Through Religiosity, Spirituality and Social Support Among Muslim Chronic Hepatitis Patients. J. Relig. Health 2020, 59, 3126–3140. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n (%) | |
|---|---|---|
| Biological sex | Male | 103 (72.5) |
| Female | 39 (27.5) | |
| Educational level | Illiterate | 23 (16.2) |
| Literate | 5 (3.5) | |
| Elementary school graduate | 74 (52.1) | |
| Middle school graduate | 14 (9.9) | |
| High school graduate | 19 (13.4) | |
| University graduate | 6 (4.2) | |
| Master’s/Doctorate | 1 (0.7) | |
| Marital status | Married | 118 (83.1) |
| Single | 4 (2.8) | |
| Widowed | 20 (14.1) | |
| Family type | Nuclear family | 121 (85.2) |
| Extended family | 18 (12.7) | |
| Single-person household | 3 (2.1) | |
| Employment status | Employed | 21 (14.8) |
| Unemployed | 121 (85.2) | |
| Occupation | Civil servant | 3 (2.1%) |
| Worker | 15 (10.6%) | |
| Self-employed | 12 (8.5%) | |
| Retired | 90 (63.4%) | |
| Housewife | 22 (15.5%) | |
| Place of residence | Metropolitan | 18 (12.7) |
| Province | 19 (13.4) | |
| District | 84 (59.2) | |
| Town | 2 (1.4) | |
| Village | 19 (13.4) | |
| Access to healthcare services | Easy | 74 (52.1) |
| Difficult | 68 (47.9) | |
| Income status | Very good | 0 (0) |
| Good | 11 (7.7) | |
| Average | 80 (56.3) | |
| Poor | 39 (27.5) | |
| Very poor | 12 (8.5) | |
| Smoking status | Smoker | 31 (21.8) |
| Non-smoker | 42 (29.6) | |
| Ex-smoker | 69 (48.6) | |
| Regular medication use status | User | 128 (90.1) |
| Partial user | 10 (7) | |
| Non-user | 4 (2.8) | |
| Comorbidity status | Present | 130 (91.5) |
| None | 12 (8.5) | |
| NYHA classification | Class II | 82 (57.7) |
| Class III | 59 (41.5) | |
| Class IV | 1 (0.7) | |
| Shock status | Shocked | 50 (35.2%) |
| Non-shocked | 92(64.8%) | |
| ± SD | ||
| Age | 61.96 ± 11.93 | |
| ICD Implantation Duration | 4.68 ± 5.45 | |
| Ejection fraction | 32.89 ± 3.49 | |
| Symptoms | None | Sometimes | Frequently | Always | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Chest pain | Pre-implantation | 59 (41.5) | 51 (35.9) | 25 (17.6) | 7 (4.9) |
| Post-implantation | 95 (66.9) | 44 (31) | 2 (1.4%) | 1 (0.7) | |
| Palpitations | Pre-implantation | 33 (23.2) | 39 (27.5) | 45 (31.7%) | 25 (17.6) |
| Post-implantation | 86 (60.6) | 53 (37.3) | 3 (2.1%) | 0 (0) | |
| Dizziness | Pre-implantation | 88 (62%) | 41 (28.9) | 10 (7%) | 3 (2.1) |
| Post-implantation | 108 (76.1) | 33 (23.2) | 1 (0.7%) | 0 | |
| Syncope | Pre-implantation | 100 (70.4%) | 34 (23.9) | 8 (5.6) | 0 (0%) |
| Post-implantation | 126 (88.7%) | 15 (10.6) | 1 (0.7%) | 0 (0) | |
| Dyspnea | Pre-implantation | 30 (21.1%) | 47 (33.1) | 41 (28.9) | 24 (16.9) |
| Post-implantation | 60 (42.3) | 63 (44.4) | 16 (11.3) | 3 (2.1) | |
| Fatigue | Pre-implantation | 13 (9.2%) | 55 (38.7%) | 51 (35.9%) | 23 (16.2) |
| Post-implantation | 1 (0.7%) | 30 (21.1%) | 83 (58.5%) | 28 (19.7) | |
| Insomnia | Pre-implantation | 101 (71.1%) | 31 (21.8) | 7 (4.9%) | 3 (2.1) |
| Post-implantation | 97 (68.3) | 34 (23.9) | 8 (5.6%) | 3 (2.1) | |
| Fear | Pre-implantation | 98 (69%) | 36 (25.4) | 5 (3.5%) | 3 (2.1) |
| Post-implantation | 103 (72.5) | 35 (24.6) | 3 (2.1%) | 1 (0.7) | |
| Anxiety | Pre-implantation | 84 (59.2%) | 43 (30.3%) | 12 (8.5%) | 3 (2.1) |
| Post-implantation | 0 (0%) | 43 (30.3%) | 84 (59.2%) | 15 (10.6) |
| Symptoms | Pre-Implantation ± SD | Post-Implantation ± SD | Mean Difference %95 CI | p |
|---|---|---|---|---|
| Chest pain | 0.86 ± 0.88 | 0.36 ± 0.55 | 0.50 (0.36; 0.64) | <0.001 |
| Palpitations | 1.44 ± 1.03 | 0.42 ± 0.54 | 1.02 (0.84; 1.20) | <0.001 |
| Dizziness | 0.49 ± 0.72 | 0.25 ± 0.45 | 0.25 (0.12; 0.37) | <0.001 |
| Syncope | 0.35 ± 0.59 | 0.12 ± 0.35 | 0.23 (0.13; 0.33) | <0.001 |
| Dyspnea | 1.42 ± 1.01 | 0.73 ± 0.74 | 0.68 (0.51; 0.85) | <0.001 |
| Fatigue | 1.59 ± 0.87 | 1.97 ± 0.66 | −0.38 (−0.52; −0.24) | <0.001 |
| Insomnia | 0.38 ± 0.68 | 0.42 ± 0.7 | −0.04 (−0.12; 0.05) | 0.473 |
| Fear | 0.39 ± 0.66 | 0.31 ± 0.55 | 0.08 (−0.01; 0.16) | 0.082 |
| Anxiety | 0.54 ± 0.74 | 1.8 ± 0.61 | −1.27 (−1.39; −1.14) | <0.001 |
| Symptoms | Shock Status | ||
|---|---|---|---|
| Shocked | Non-Shocked | ||
| ± SD | ± SD | ||
| Chest pain | Pre-implantation | 1.04 ± 0.92 | 0.76 ± 0.84 |
| Post-implantation | 0.4 ± 0.64 | 0.34 ± 0.5 | |
| Mean Difference (%95 CI) | 0.64 (0.40; 0.88) | 0.42 (0.25; 0.60) | |
| p | <0.001 | <0.001 | |
| Palpitations | Pre-implantation | 1.62 ± 1.03 | 1.34 ± 1.03 |
| Post-implantation | 0.52 ± 0.58 | 0.36 ± 0.5 | |
| Mean Difference (%95 CI) | 1.10 (0.75; 1.45) | 0.98 (0.78; 1.18) | |
| p | <0.001 | <0.001 | |
| Dizziness | Pre-implantation | 0.44 ± 0.67 | 00.52 ± 0.75 |
| Post-implantation | 0.18 ± 0.39 | 0.28 ± 0.48 | |
| Mean Difference (%95 CI) | 0.26 (0.05; 0.47) | 0.24 (0.08; 0.40) | |
| p | 0.014 | 0.004 | |
| Syncope | Pre-implantation | 0.34 ± 0.56 | 0.36 ± 0.6 |
| Post-implantation | 0.22 ± 0.46 | 0.07 ± 0.25 | |
| Mean Difference (%95 CI) | 0.12 (−0.07; 0.31) | 0.29 (0.18; 0.41) | |
| p | 0.201 | <0.001 | |
| Dyspnea | Pre-implantation | 1.54 ± 1.03 | 1.35 ± 0.99 |
| Post-implantation | 0.8 ± 0.78 | 0.7 ± 0.72 | |
| Mean Difference (%95 CI) | 0.74 (0.47; 1.01) | 0.65 (0.43; 0.87) | |
| p | <0.001 | <0.001 | |
| Fatigue | Pre-implantation | 1.84 ± 0.87 | 1.46 ± 0.84 |
| Post-implantation | 2.1 ± 0.71 | 1.9 ± 0.63 | |
| Mean Difference (%95 CI) | −0.26 (−0.50; −0.02) | −0.45 (−0.62; −0.27) | |
| p | 0.047 | <0.001 | |
| Insomnia | Pre-implantation | 0.42 ± 0.64 | 0.36 ± 0.7 |
| Post-implantation | 0.36 ± 0.63 | 0.45 ± 0.73 | |
| Mean Difference (%95 CI) | 0.06 (−0.09; 0.21) | −0.09 (−0.19; 0.02) | |
| p | 0.405 | 0.108 | |
| Fear | Pre-implantation | 0.52 ± 0.76 | 0.32 ± 0.59 |
| Post-implantation | 0.44 ± 0.64 | 0.24 ± 0.48 | |
| Mean Difference (%95 CI) | 0.08 (−0.07; 0.23) | 0.08 (−0.03; 0.18) | |
| p | 0.317 | 0.157 | |
| Anxiety | Pre-implantation | 0.74 ± 0.88 | 0.42 ± 0.63 |
| Post-implantation | 1.8 ± 0.64 | 1.8 ± 0.6 | |
| Mean Difference (%95 CI) | −1.06 (−1.30; −0.82) | −1.38 (−1.52; −1.24) | |
| p | <0.001 | <0.001 | |
| Coping Category | Coping Method | Number of Uses n (%) | Effectiveness n (%) |
|---|---|---|---|
| Religious/Spiritual | Prayer/Remembrance | 95 (66.9) | Effective: 70 (73.7) Partially: 20 (21.1) Not effective: 5 (5.2) |
| Physical Activity | Walking | 34 (23.9) | Effective: 25 (73.5) Partially: 7 (20.6) Not effective: 2 (5.9) |
| Complementary/Traditional | Drinking herbal tea | 13 (9.2) | Effective: 5 (38.5) Partially: 8 (61.5) Not effective: 0 (0) |
| Characteristics | Prayer/Zikr n (%) | Herbal Tea Consumption n (%) | Walking n (%) | p |
|---|---|---|---|---|
| Biological sex | 0.062 * | |||
| Male | 63 (61.2) | 11 (10.7) | 29 (28.2) | |
| Female | 32 (82.1) | 2 (5.1) | 5 (12.8) | |
| Education Level | 0.554 * | |||
| Illiterate | 17 (73.9) | 2 (8.7) | 4 (17.4) | |
| Literate | 5 (100) | 0 (0) | 0 (0) | |
| Elementary school graduate | 52 (70.3) | 6 (8.1) | 16 (21.6) | |
| Middle school graduate | 8 (57.1) | 2 (14.3) | 4 (28.6) | |
| High school graduate | 8 (42.1) | 3 (15.8) | 8 (42.1) | |
| University graduate | 4 (66.7) | 0 (0) | 2 (33.3) | |
| Master’s/Doctorate | 1 (100) | 0 (0) | 0 (0) | |
| Marital Status | 0.042 * | |||
| Married | 77 (65.3) | 11 (9.3) | 30 (25.4) | |
| Single | 1 (25) | 0 (0) | 3 (75) | |
| Widowed | 17 (85) | 2 (10) | 1 (5) | |
| Family Type | 0.472 * | |||
| Nuclear family | 78 (64.5) | 11 (9.1) | 32 (26.4) | |
| Extended family | 14 (77.8) | 2 (11.1) | 2 (11.1) | |
| Living alone | 3 (100) | 0 (0) | 0 (0) | |
| Employment Status | 0.854 * | |||
| Working | 13 (61.9) | 2 (9.5) | 6 (28.6) | |
| Not working | 82 (67.8) | 11 (9.1) | 28 (23.1) | |
| Occupation | ||||
| Civil servant | 0 | 0 (0%) | 3 (100%) | 0.083 * |
| Worker | 10 (66.7%) | 1 (6.7%) | 4 (26.7%) | |
| Self-employed | 8 (66.7) | 1 (8.3%) | 3 (25%) | |
| Retired | 58 (64.4) | 10 (11.1%) | 22 (24.4%) | |
| Housewife | 19 (86.4) | 1 (4.5%) | 2 (9.1%) | |
| Place of Residence | 0.909 * | |||
| Metropolitan | 10 (55.6) | 3 (16.7) | 5 (27.8) | |
| Province | 14 (73.7) | 1 (5.3) | 4 (21.1) | |
| District | 57 (67.9) | 8 (9.5) | 19 (22.6) | |
| Town | 1 (50) | 0 (0) | 1 (50) | |
| Village | 13 (68.4) | 1 (5.3) | 5 (26.3) | |
| Access to Health Services | 0.823 * | |||
| Easy | 49 (66.2) | 6 (8.1) | 19 (25.7) | |
| Difficult | 46 (67.6) | 7 (10.3) | 15 (22.1) | |
| Income Status | 0.619 * | |||
| Good | 6 (54.5) | 1 (9.1) | 4 (36.4) | |
| Average | 56 (70) | 7 (8.8) | 17 (21.3) | |
| Poor | 24 (61.5) | 3 (7.7) | 12 (30.8) | |
| Very weak | 9 (75) | 2 (16.7) | 1 (8.3) | |
| Smoking Status | 0.523 * | |||
| Smoker | 23 (74.2) | 1 (3.2) | 7 (22.6) | |
| Non-smoker | 30 (71.4) | 4 (9.5) | 8 (19) | |
| Ex-smoker | 42 (60.9) | 8 (11.6) | 19 (27.5) | |
| Regular Medication Use | 0.090 | |||
| User | 85 (66.4) | 10 (7.8) | 33 (25.8) | |
| Partially user | 6 (60) | 3 (30) | 1 (10) | |
| Not a user | 4 (100) | 0 (0) | 0 (0) | |
| Comorbidity Status | 0.513 * | |||
| Current | 86 (66.2) | 13 (10) | 31 (23.8) | |
| NHYA Classification | 0.183 * | |||
| Class II | 52 (63.4) | 5 (6.1) | 25 (30.5) | |
| Class III Shock status Shocked Non-shocked | 42 (71.2) 37 (74) 58 (63) | 8 (13.6) 3 (6) 10 (10.9) | 9 (15.3) 10 (20) 24 (26.1) | 0.383 * |
| Age | ||||
| 60 years and under | 42 (67.7) | 4 (6.5) | 16 (25.8) | 0.592 * |
| Over 60 | 53 (66.3) | 9 (11.3) | 18 (22.5) | |
| ± SD | ± SD | ± SD | ||
| ICD Implantation Duration | 4.95 ± 6.28 | 4.77 ± 3.65 | 3.91 ± 3.01 | 0.638 ** |
| Ejection Fraction | 32.45 ± 4.74 | 33.08 ± 3.25 | 33.24 ± 3.23 | 0.627 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Us, S.A.; Taşcı, S. Symptom Experiences and Coping Strategies in Turkish Patients with Implantable Cardioverter-Defibrillators: A Cross-Sectional Study Based on Interviews. Healthcare 2026, 14, 59. https://doi.org/10.3390/healthcare14010059
Us SA, Taşcı S. Symptom Experiences and Coping Strategies in Turkish Patients with Implantable Cardioverter-Defibrillators: A Cross-Sectional Study Based on Interviews. Healthcare. 2026; 14(1):59. https://doi.org/10.3390/healthcare14010059
Chicago/Turabian StyleUs, Sebiha Aktaş, and Sultan Taşcı. 2026. "Symptom Experiences and Coping Strategies in Turkish Patients with Implantable Cardioverter-Defibrillators: A Cross-Sectional Study Based on Interviews" Healthcare 14, no. 1: 59. https://doi.org/10.3390/healthcare14010059
APA StyleUs, S. A., & Taşcı, S. (2026). Symptom Experiences and Coping Strategies in Turkish Patients with Implantable Cardioverter-Defibrillators: A Cross-Sectional Study Based on Interviews. Healthcare, 14(1), 59. https://doi.org/10.3390/healthcare14010059





