Cognitive Interventions for the Treatment of Insomnia or Poor-Quality Sleep in Community-Dwelling Older People: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methodology
2.1. Sources of Information
2.2. Research Question and Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Selection Process
2.5. Quality Assessment
2.6. Data Extraction
- -
- Identification of the article using the title and summary.
- -
- Methodology: Type of intervention chosen and experimentality.
- -
- Participants: average age, control and intervention groups, number of participants, and inclusion criteria and selection.
- -
- Cognitive interventions, techniques, or therapies are applied to improve sleep quality.
- -
- The following variables were measured: quality and quantity of sleep, number of nocturnal awakenings, and time.
- -
- Results of the intervention: maintenance or improvement in the aspects measured with the variables.
2.7. Analysis of the Data Obtained
3. Results
3.1. Study Selection and Characteristics
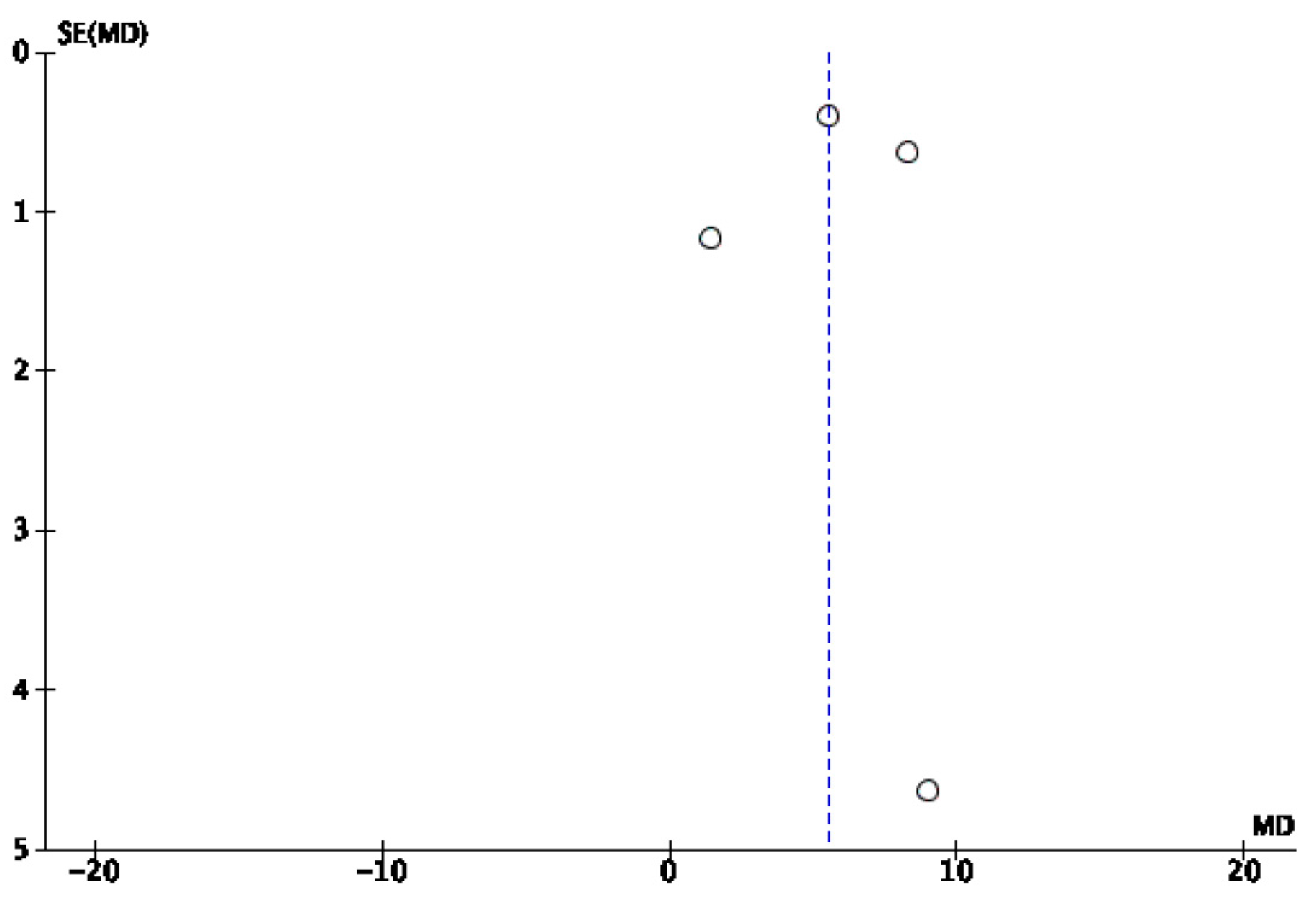
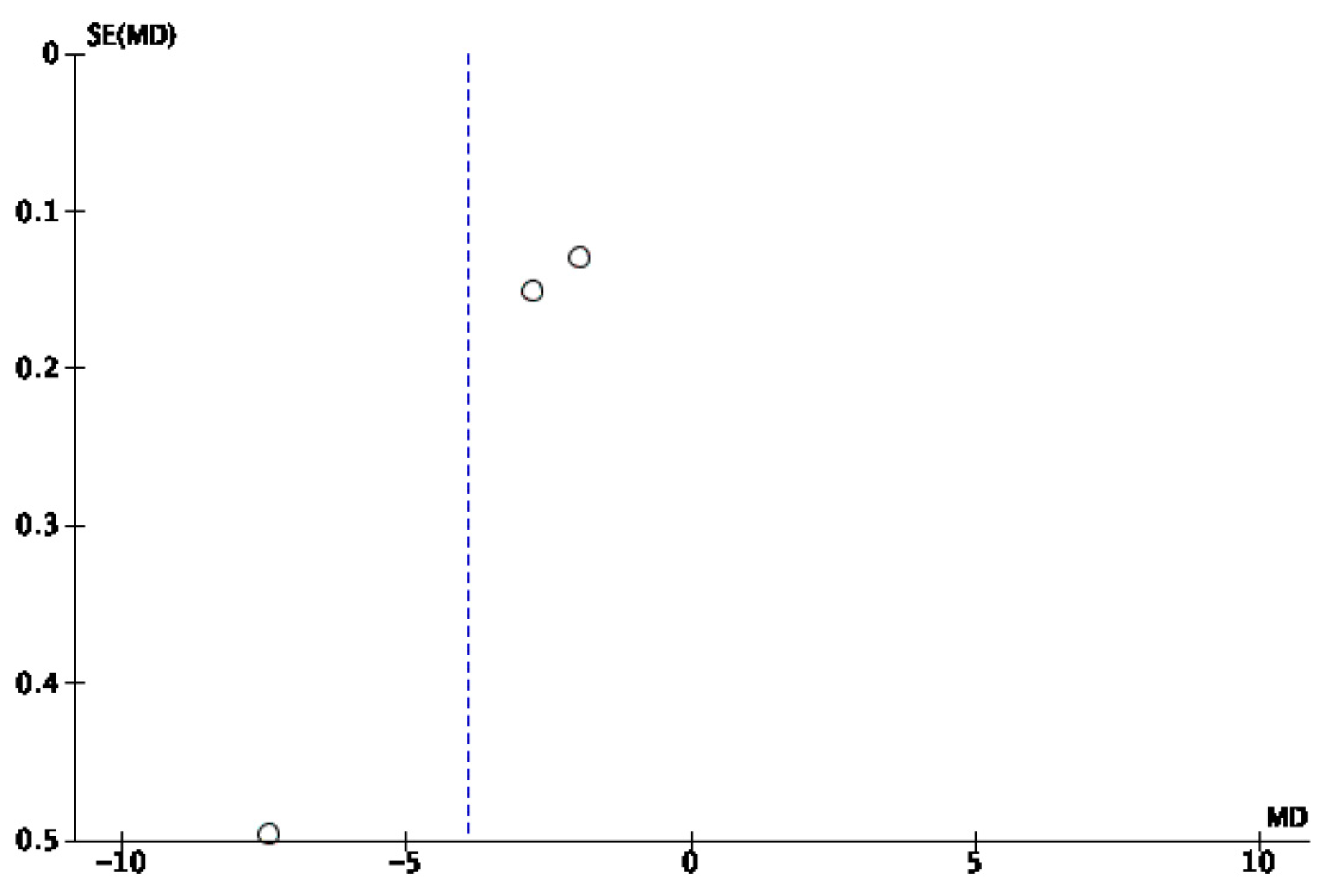
3.2. Risk of Bias
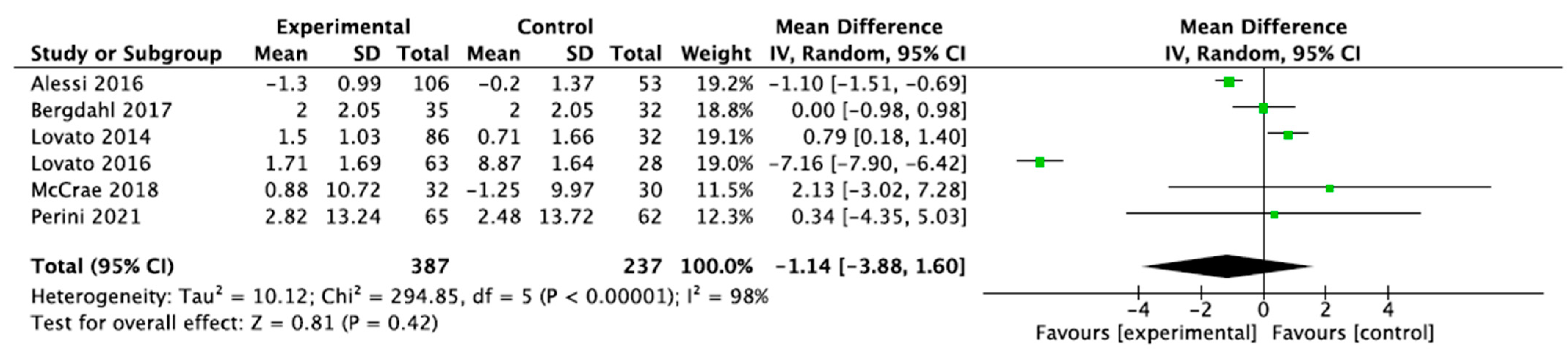
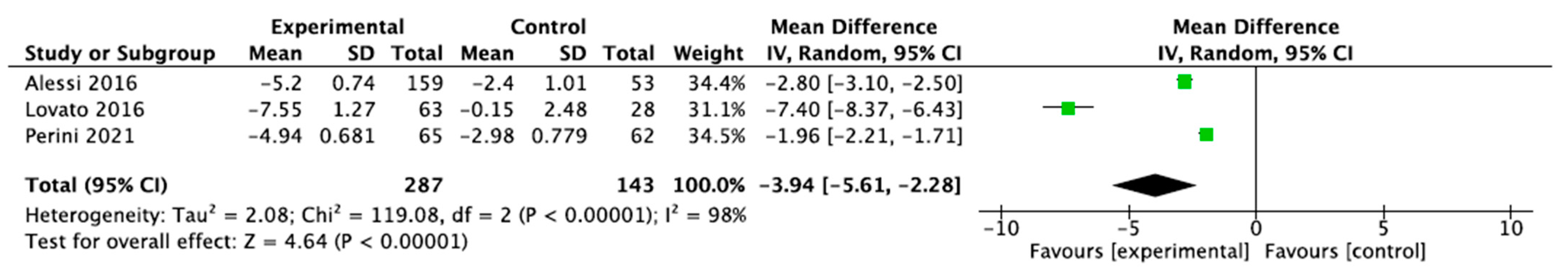
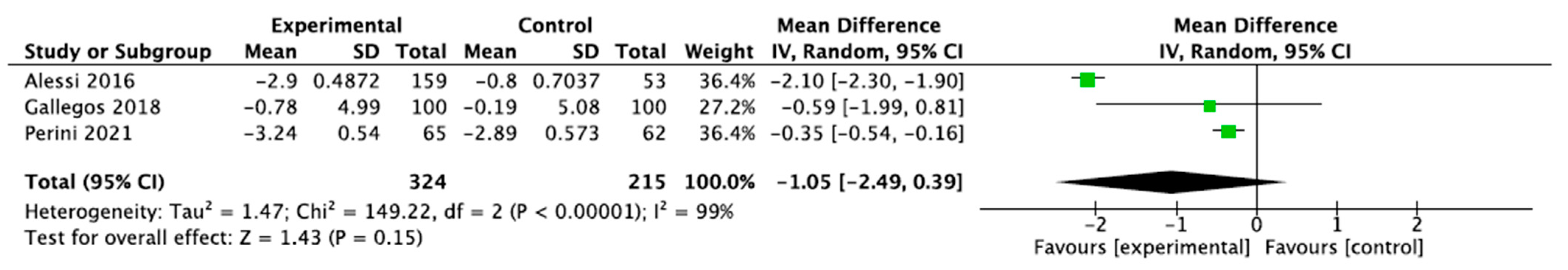
3.3. Sleep Measurements
3.3.1. Sleep Quality and Cycle
3.3.2. Sleep Diary
3.3.3. Scoring on the Insomnia Severity Index (ISI) Scale
3.3.4. Scoring on the Pittsburgh Sleep Quality Index (PSQI) Scale
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Aging and Health. October 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 10 November 2023).
- Duque-Fernández, L.M.; Ornelas-Contreras, M.; Benavides-Pando, E.V. Physical activity and its relationship with aging and functional capacity: A review of the research literature. Psychol. Health 2020, 30, 45–57. [Google Scholar]
- Piña Morán, M.; Olivo Viana, M.G.; Martínez Matamala, C.; Poblete Troncoso, M.; Guerra Guerrero, V. Aging, quality of life and health. Challenges for the social roles of older people. Rumbos TS 2022, 17, 7–27. [Google Scholar]
- Conde-Ruiz, J.I.; González, C.I. Studies on the Spanish Economy-2021/07. The aging process in Spain. Mediterráneo Económico, 2021; 34, 73–93. [Google Scholar]
- National Institute of Statistics. An Aging Population. 2021. Available online: https://www.ine.es/prodyser/demografia_UE/bloc-1c.html (accessed on 26 March 2025).
- Esmeraldas Vélez, E.E.; Falcones Centeno, M.R.; Vásquez Zevallos, M.G.; Solórzano Vélez, J.A. The aging of the elderly and its main characteristics. Recimundo 2019, 3, 58–74. [Google Scholar] [CrossRef]
- Patel, D.; Steinberg, J.; Patel, P. Insomnia in the Elderly: A Review. J. Clin. Sleep Med. 2018, 14, 1017–1024. [Google Scholar] [CrossRef]
- Cruz, T.; García, L.; Álvarez, M.A.; Manzanero, A.L. Sleep quality and memory deficits in healthy aging. Neurología 2022, 37, 31–37. [Google Scholar] [CrossRef]
- Wang, C.; Li, G.; Zheng, L.; Meng, X.; Meng, Q.; Wang, S.; Yin, H.; Chu, J.; Chen, L. Effects of music intervention on Sleep quality of older adults: A systematic review and meta-analysis. Complement. Ther. Med. 2021, 59, 102719. [Google Scholar] [CrossRef]
- Chaput, J.P.; Dutil, C.; Featherstone, R.; Ross, R.; Giangregorio, L.; Saundders, T.J.; Janssen, I.; Poitras, V.J.; Kho, M.E.; Ross-White, A.; et al. Sleep timing, sleep consistency, and health in adults: A systematic review. Appl. Physiol. Nutr. Metab. 2020, 45, S232–S247. [Google Scholar] [CrossRef]
- Kim, J.H.; Elkhadem, A.R.; Duffy, J.F. Circadian Rhythm Sleep–Wake Disorders in Older Adults. Sleep Med. Clin. 2022, 17, 241–252. [Google Scholar] [CrossRef]
- Paúl, C.; Ribeiro, O.; Teixeira, L. Active aging: An empirical approach to the WHO model. Curr. Gerontol. Geriatr. Res. 2012, 2012, 382972. [Google Scholar]
- Petrettoa, D.R.; Pilib, R.; Gavianoa, L.; Matos López, C.; Zuddas, C. Active and successful or healthy aging: A brief history of conceptual models. Rev. Esp. Geriatr. Gerontol. 2016, 51, 229–241. [Google Scholar]
- International Council on Active Aging. Active Aging and Well-Being. 2023. Available online: https://www.icaa.cc/activeagingandwellness/what-is-active-aging.htm (accessed on 10 November 2023).
- Institute of the Elderly and Social Services. White Paper on “Active Aging”. December 2022. [Google Scholar]
- National Institute on Aging. What Do We Know About Healthy Aging? 2021. Available online: https://www.nia.nih.gov/health/healthy-aging/what-do-we-know-about-healthy-aging (accessed on 9 April 2025).
- World Health Organization. Decade of Healthy Aging. May 2020. [Google Scholar]
- Pardo Crego, C.; Gonzalez Pena, C.M. Insomnia prevalence and environmental conditions in patients older than 65 years at Primary Care. Gerokomos 2017, 28, 121–126. [Google Scholar]
- Abad, V.C.; Guilleminault, C. Insomnia in Elderly Patients: Recommendations for Pharmacological Management. Drugs Aging 2018, 35, 791–817. [Google Scholar] [CrossRef]
- Kuula, L.; Halonen, R.; Kajanto, K.; Lipsanen, J.; Makkonen, T.; Peltonen, M.; Pesonen, A.K. The Effects of Presleep Slow Breathing and Music Listening on Polysomnographic Sleep Measures—A pilot trials. Sci. Rep. 2020, 10, 7427. [Google Scholar] [CrossRef] [PubMed]
- Redeker, N.S.; Yaggi, H.K.; Jacoby, D.; Hollenbeak, C.S.; Breazeale, S.; Conley, S.; Hwang, Y.; Iennaco, J.; Linsky, S.; Nwanaji-Enwerem, U.; et al. Cognitive behavioral therapy for insomnia has sustained effects on insomnia, fatigue, and function among people with chronic heart failure and insomnia: The HeartSleep Study. Sleep 2022, 45, zsab252. [Google Scholar] [CrossRef]
- Rusch, H.L.; Rosario, M.; Levison, L.M.; Olivera, A.; Livingston, W.S.; Wu, T.; Gill, J.M. The effect of mindfulness meditation on sleep quality: A systematic review and meta-analysis of randomized controlled trials. Ann. N. Y. Acad. Sci. 2019, 1445, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Chen, Y.C.; Tseng, Y.C.; Tsai, S.T.; Tseng, Y.H. Physical activity and successful aging among middle-aged and older adults: A systematic review and meta-analysis of cohort studies. Aging 2020, 12, 7704–7716. [Google Scholar] [CrossRef]
- Jiménez-Zazo, F.; Romero-Blanco, C.; Castro-Lemus, N.; Dorado-Suárez, A.; Aznar, S. Transtheoretical Model for Physical Activity in Older Adults: Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 9262. [Google Scholar] [CrossRef]
- Ramsey, K.A.; Rojer, A.G.M.; D’Andrea, L.; Otten, R.H.J.; Heymans, M.W.; Trappenburg, M.C.; Verlaan, S.; Whittaker, A.C.; Meskers, C.G.M.; Maier, A.B. The associations of objectively measured physical activity and sedentary behavior with Skeletal muscle strength and muscle power in older adults: A systematic review and meta-analysis. Aging Res. Rev. 2021, 67, 101266. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- National Institute for Care Health Research Prospero Centre for Reviews Dissemination University of York, U.K. Available online: https://www.crd.york.ac.uk/PROSPERO/login (accessed on 10 January 2024).
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision; United Nations: New York, NY, USA, 2017; Available online: https://www.un.org/en/desa/world-population-prospects-2017-revision (accessed on 26 March 2025).
- Health Sciences Descriptors, DeCS/MeSH. Virtual Health Library. Bireme, PAHO, WHO. 2023. Available online: https://decs.bvsalud.org/es/ths?filter=ths_termall&q=aged (accessed on 23 December 2023).
- Li, J.; Vitiello, M.V.; Gooneratne, N.S. Sleep in Normal Aging. J. Am. Geriatr. Soc. 2018, 66, 1130–1139. [Google Scholar] [CrossRef]
- Cabello, J.B. Template to help you understand a Clinical Trial. CASPe Read. Guides Med. Lit. Crit. 2005, 1, 5–8. [Google Scholar]
- Lovato, N.; Lack, L.; Wright, H.; Kennaway, D.J. Evaluation of a brief treatment program of Cognitive behavior therapy for insomnia in older adults. Sleep 2014, 37, 117–126. [Google Scholar] [CrossRef]
- Alessi, C.; Martin, J.L.; Fiorentino, L.; Fung, C.H.; Dzierzewski, J.M.; Rodriguez Tapia, J.C.; Song, Y.; Josephson, K.; Jouldjian, S.; Mitchell, M.N. Cognitive Behavioral Therapy for Insomnia in Older Veterans Using Nonclinician Sleep Coaches: Randomized Controlled Trial. J. Am. Geriatr. Soc. 2016, 64, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Lovato, N.; Lack, L.; Kennaway, D.J. Comparing and contrasting the therapeutic effects of cognitive-behavior therapy for older adults with short- and long-term insomnia objective sleep duration. Sleep Med. 2016, 22, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Bergdahl, L.; Broman, J.E.; Berman, A.H.; Haglund, K.; von Knorring, L.; Markström, A. Sleep patterns in a randomized controlled trial of auricular acupuncture and cognitive behavioral therapy for insomnia. Complement. Ther. Clin. Pract. 2017, 28, 220–226. [Google Scholar] [CrossRef]
- Gallegos, A.M.; Moynihan, J.; Pigeon, W.R. A Secondary Analysis of Sleep Quality Changes in Older Adults from a Randomized Trial of an MBSR Program. J. Appl. Gerontol. 2018, 37, 1327–1343. [Google Scholar] [CrossRef]
- McCrae, C.S.; Curtis, A.F.; Williams, J.M.; Dautovich, N.D.; McNamara, J.P.H.; Stripling, A.; Dzierzewski, J.M.; Chan, W.S.; Berry, R.B.; McCoy, K.J.M.; et al. Efficacy of brief behavioral treatment for insomnia in older adults: Examination of sleep, mood, and cognitive outcomes. Sleep Med. 2018, 51, 153–166. [Google Scholar] [CrossRef]
- Dzierzewski, J.M.; Martin, J.L.; Fung, C.H.; Song, Y.; Fiorentino, L.; Jouldjian, S.; Rodriguez, J.C.; Mitchell, M.; Josephson, K.; Alessi, C.A. CBT for late-life insomnia and the accuracy of sleep and wake perceptions: Results from a randomized controlled trial. J. Sleep. Res. 2019, 28, e12809. [Google Scholar] [CrossRef]
- Perini, F.; Wong, K.F.; Lin, J.; Hassirim, Z.; Ong, J.L.; Lo, J.; Ong, J.C.; Doshi, K.; Lim, J. Mindfulness-based therapy for insomnia for older adults with sleep difficulties: A randomized clinical trial. Psychol. Med. 2021, 53, 1038–1048. [Google Scholar] [CrossRef]
- Camino, M.; Satorres, E.; Delhom, I.; Real, E.; Abella, M.; Meléndez, J.C. Mindfulness-based Use of cognitive therapy to improve sleep quality in older adults with insomnia. Psychosoc. Interv. 2022, 31, 159–167. [Google Scholar] [CrossRef]
- Bellone, L.G.; Plano, S.A.; Cardinali, D.P.; Pérez, D.; Vigo, D.E.; Golombek, D.A. Actigraphy as diagnostic tool. Pren. Med. Agent. 2018, 104, 51–58. [Google Scholar]
- Brewster, G.S.; Riegel, B.; Gehrman, P.R. Insomnia in elderly individuals. Sleep Med. Clin. 2018, 13, 13–19. [Google Scholar] [CrossRef]
- Pfeiffer, P.N.; Ganoczy, D.; Zivin, K.; Gerlach, L.; Damschroder, L.; Ulmer, C.S. Guidelines concordant use of cognitive behavioral therapy for insomnia in the Veterans’ Health Administration. Sleep. Health 2023, 9, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.D.; Siriwardena, A.N.; Espie, C.A.; Yang, Y.; Petrou, S.; Ogburn, E.; Begum, N.; Maurer, L.F.; Robinson, B.; Gardner, C.; et al. Clinical and cost effectiveness of nurse-delivered sleep restriction therapy for insomnia in primary care (HABIT): A pragmatic, superiority, open-label, randomized controlled trial. Lancet 2023, 402, 975–987. [Google Scholar] [CrossRef] [PubMed]
- López-Trigo, J.A.; Álamo-González, C.; Gil-Gregorio, P.; González-Gil, P.; Merino-Andreu, M.; García-García, P. Good Clinical Practice Guideline in Geriatrics: Insomnia, 3rd ed.; SEGG: Madrid, Spain, 2015; Available online: https://www.segg.es/media/descargas/GBPCG_Insomnio.pdf (accessed on 8 May 2024).
- Álamo González, C.; Alonso Álvarez, M.L.; Cañellas Dols, F.; Martín Águeda, B.; Pérez Díaz, H.; Romero Santo-Tomás, O.; Santos, J.T. Guidelines for Action and Follow-Up: Insomnia. From Disease-Centered Practice to People-Centered Care. Commission for Continuing Education of Health Professions in the Community of Madrid. 1st Edition. IMC. FFOMC. Madrid. 2016. Available online: https://www.ses.org.es/docs/guia-de-insomnio-2016.pdf (accessed on 8 May 2024).
- Hertenstein, E.; Trinca, E.; Wunderlin, M.; Schneider, C.L.; Züst, M.A.; Fehér, K.D.; Su, T.; Straten, A.V.; Berger, T.; Baglioni, C. Cognitive behavioral therapy for insomnia in patients with mental disorders and comorbidities insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2022, 62, 101597. [Google Scholar] [CrossRef]
- Bergeyck, R.; Geoffroy, P.A. Insomnia in neurological disorders: Prevalence, mechanisms, impact and treatment approaches. Rev. Neurol. 2023, 179, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Solis-Navarro, L.; Masot, O.; Torres-Castro, R.; Otto-Yáñez, M.; Fernández-Jané, C.; Solà-Madurell, M.; Coda, A.; Cyrus-Barker, E.; Sitjà-Rabert, M.; Pérez, L.M. Effects on Sleep Quality of Physical Exercise Programs in Older Adults: A Systematic Review and Meta-Analysis. Clocks Sleep. 2023, 5, 152–166. [Google Scholar] [CrossRef]
- Chen, C.; Tung, H.; Fang, C.; Wang, J.; Ko, N.; Chang, Y.; Chen, Y. Effect of music therapy on improving sleep quality in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2021, 69, 1925–1932. [Google Scholar] [CrossRef]
- Chitra, J.; Eremita, M.S. Effect of Virtual Reality on Sleep-Deprived Individuals. Indian J. Psychol. Med. 2023, 45, 610–613. [Google Scholar] [CrossRef]
- Cinalioglu, K.; Lavín, P.; Bein, M.; Lesage, M.; Gruber, J.; Se, J.; Bukhari, S.; Sasi, N.; Noble, H.; Andree-Bruneau, M.; et al. Effects of virtual reality guided meditation in older adults: The protocol of a pilot randomized controlled trial. Front. Psychol. 2023, 14, 1083219. [Google Scholar] [CrossRef]
- Kline, C.; Kubala, A.; Egeler, M.; Buysse, D.; Hall, M.; Barinas-Mitchell, E. Combining cognitive behavioral therapy for insomnia with physical training in adults with insomnia and short sleep duration: Impact on sleep outcomes. Med. Sci. Sports Exerc. 2022, 54, 414–415. [Google Scholar] [CrossRef]
- Ferreira, W.S.; Santana, M.G.; Youngstedt, S.D.; de Assis, D.E.; de Assis, B.P.; de Cerqueira, D.P.; Mazzaro, M.C.; Passo, G.S. Effects of exercise training and exercise plus acupuncture on chronic insomnia: A feasibility study. Sleep. Sci. 2022, 15, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Estrella González, I.M.; Torres Prados, M.T. Sleep hygiene in elderly individuals, a task close to nursing. Gerokomos 2015, 26, 123–126. [Google Scholar]
- Gras, C.B.; Hidalgo, J.L.; García, Y.D.; Lapeira, J.T.; Ferrer, A.V.; Martínez, I.P. Sleep disorders and environmental conditions in people over 65 years of age. Aten Primaria 2019, 41, 564–569. [Google Scholar]
- Pajėdienė, E.; Urbonavičiūtė, V.; Ramanauskaitė, V.; Strazdauskas, L.; Stefani, A. Sex Differences in Insomnia and Circadian Rhythm Disorders: A Systematic Review. Medicina 2024, 60, 474. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-N.; Zong, Q.-Q.; Yang, Y.; Zhang, L.; Xiang, Y.-F.; Ng, C.H.; Chen, L.-G.; Xiang, Y.-T. Gender Difference in the Prevalence of Insomnia: A Meta-Analysis of Observational Studies. Front. Psychiatry 2020, 11, 577429. [Google Scholar] [CrossRef]
- Scott, H.; Muench, A.; Appleton, S.; Reynolds, A.C.; Loffler, K.A.; Bickley, K.; Haycock, J.; Lovato, N.; Micic, G.; Lack, L.; et al. Sex differences in response to cognitive behavioral therapy for insomnia: A chart review of 455 patients with chronic insomnia. Sleep Med. 2024, 116, 123–128. [Google Scholar] [CrossRef]




| Do Cognitive Therapies, Interventions and Techniques Improve Sleep Quality in Older People? | |||
|---|---|---|---|
| Population (P) | Intervention (I) | Comparison (C) | Outcome (O) |
| People over 60 years of age with poor sleep quality or insomnia. | Cognitive interventions, therapies, and techniques to improve sleep quality. | People over 60 years of age in whom interventions to improve sleep quality are not applied. | Increase and improvement in sleep quality in older people after the intervention. |
| Database | Search Strategy |
|---|---|
| Pubmed | ((cognitive[Title/Abstract]) AND (Aged[Title/Abstract]) OR (“older people”[Title/Abstract]) OR (elderly[Title/Abstract])) AND ((sleep[Title/Abstract]) OR (insomnia[Title/Abstract])) |
| Web of Science | (cognitive) AND (Aged OR elderly) AND (sleep OR insomnia) |
| Clinicaltrials.gov | “cognitive AND aged AND sleep OR insomnia” |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Type of studies: randomized experimental studies with a control group. | Studies with more than 10 years of experience. |
| Population over 60 years of age with worsening sleep quality or insomnia who do not suffer from other serious pathologies | Elderly population living in nursing homes. |
| Studies written in English. | Studies that analyze the effectiveness of physical or pharmacological interventions on sleep quality and that do not use cognitive interventions, therapies, or techniques to improve sleep quality. |
| First Author, Year, Country | Design | Participants and Characteristics | Interventions and Follow-Up | Variables and Measures | Evaluation and Results | Losses to Follow-up, Adverse Effects and Limitations |
|---|---|---|---|---|---|---|
| Lovato et al. [32], 2014. Adelaide. | Randomized con-trolled clinical trial. | N = 118 (55 M, 66 W) IG: 86 CG: 32 A: 63.7 C: (1) AHI less than 15. (2) They do not suffer from mental disorders. (3) They have not consumed hypnotics or large amounts of caffeine for at least 1 month. (4) They have nocturnal awakenings of +30 min at least 3 nights per week for 6 months. (5) They suffer from fatigue or memory problems during the day. | IG: Brief CBT-I applied in 4 sessions of 60 min during 4 weeks in groups of 4 or 5 people. The first session dealt with the behavioral component, the 2nd and 3rd sessions dealt with sleep hygiene and education, and the 4th session summarized and reviewed what had been worked on. CG: The same follow-up as GI was carried out, but the intervention was not applied until after the study had finished. | Sleep diary: recorded hours of going to bed, lights out, getting out of bed, number and duration of awakenings, and estimated sleep latency. 7 days per assessment. Wrist actigraphy: sleep latency and number of awakenings. 7 days per assessment. ISI: changes in insomnia. Flinders Fatigue Scale, Epworth Sleepiness Scale, and Daytime Feeling and Functioning Scale: daytime performance. | Evaluation T1: during the intervention. T2: after the intervention. T3: 3 months post-intervention. Results Significant reduction in night awakenings and improvement in sleep efficiency after the application of the intervention in IG compared to CG. | IG: 2 did not receive interv, 14 lost in phone tracking. CG: 2 do not receive interv, 5 lost in phone tracking. |
| Alessi et al. [33], 2016. Los Angeles. | Randomized con-trolled clinical trial. | N = 159 IG: 106 (102 M, 4 W) CG: 53 (52 M, 1 W) A: 64–80 C: (1) +60 years. (2) Results higher than 24 in Mini Mental Test. (3) Do not present severe mental disorder. (4) AHI less than 20. | IG: Cognitive behavioral therapy was applied in 5 sessions of 1 h over 6 weeks + telephone call for control in week 5. Stimulus control, sleep restriction, cognitive therapy, sleep hygiene, and relapse prevention were treated. CG: General sleep education pro-gram at the same frequency and intervals as GI. | Sleep efficiency (sleep onset latency, total wake time, and sleep efficiency): diary recording. Sleep parameters: wrist actigraphy. Insomnia: ISI. Sleep quality: PSQI. | Evaluation T0: prior to application of the intervention. T1: one-week post-intervention. T2: 6 months post-randomization. T3: 12 months post-randomization. Results Statistically significant improvement in IG compared to CG in sleep quality and insomnia after application of the intervention. Effects lasting + 12 months. | Almost all the sample were men. IG: 9 refused the interview, 3 died, 2 impossible to contact, 3 withdrew from the study. CG: 1 withdrew, 1 impossible to contact. |
| Lovato et al. [34], 2016. Adelaide | Randomized con-trolled clinical trial. | N = 91 (43 M, 48 W) IG: 63 CG: 28 A: 63.34 C: (1) Waking up during the night for more than 30 min. at least 3 days a week for 6 months. (2) Fatigue, poor performance, and even memory problems during the day. (3) Not taking any drug treatment for at least 1 month prior to the intervention. (4) AHI less than 15. (5) Not having severe mental problems or consuming excessive caffeine. | IG: Divided into short sleepers (−6 h) and long sleepers (+6 h). A 60 min weekly session of CBT-I consisting of sleep restriction, cognitive and educational therapy aimed at addressing sleep misperception and cognitive-emotional aspects was administered for 4 weeks. CG: Received the intervention after completing the 3-month follow-up. The same divisions were made into short and long sleepers as in IG. | Polysomnography: classification into short or long sleep. Sleep diary and actigraphy: for 7 days per measurement to calculate quality, awakenings, duration of sleep. ISI: insomnia. Flinders Fatigue Scale, Epworth Sleepiness Scale and Daytime Feeling and Functioning Scale: performance during the day. | Evaluation T0: pre-interv. T1: post-interv. T2: 3 months post-interv. Results The intervention produced lasting improvements in subjective sleep quality, perceived insomnia severity, daily performance, and thoughts about sleep in IG compared to CG. The improvements were more significant in the short-sleeper group. | IG: 1 does not receive interv, 9 are not followed up. CG: 1 does not receive interv, 9 are not followed up |
| Bergdahl et al. [35], 2017. Uppsala. | Randomized con-trolled clinical trial. | N = 67 (9 M, 58 W) IG: 35 (5 M, 30 W) CG: 32 (4 M, 28 W) A = 60.5 C: (1) Having been using nonbenzodiazepine hypnotics at least 3 times a week for 6 months, but with persistent symptoms of insomnia, wanting to stop taking them and stopping them 5 days before the intervention. (2) Speak Swedish. (3) Have no mental pathologies. | IG: CBT-I + a group manual for the intervention. Weekly 90 min sessions for 6 weeks by clinical psychologists. CG: 2 weekly sessions for 4 weeks of auricular acupuncture. | Actigraphy: daily patterns, specifically sleep periods. Short Form-12: physical and mental health, quality of life. | Evaluation T0: pre-intervention T1: post-intervention T2: 6 months post-intervention. Results Insomnia symptoms were significantly reduced in IG after the intervention. In CG, the intervention was not effective in reducing insomnia. | Sample majority of women. IG: 6 declined the interview, 6 dropped the inteview. CG: 4 declined to participate. |
| Gallegos et al. [36], 2018. New York. | Randomized con-trolled clinical trial. | N = 200 (76 M, 124 W) GI: 100 (38 M, 62 W) GC: 100 (38 M, 62 W) A: 72.5 IG: 72 CG: 73 C: (1) E: +65 (2) Speak English (3) Not have serious mental pathologies. (4) Have a stable pharmacological treatment of antidepressants or anxiolytics for at least 8 weeks prior to the interval and stop taking them during the treatment. | IG: Weekly 120 min sessions for 8 weeks and one intensive 7 h session of mindfulness-based stress reduction therapy in groups of 15 to 20 participants. Sessions included breathing exercises, sitting meditation or body scanning followed by informal practice at home. CG: Placebo, no intervention was applied, only assessments were performed. | PSQI: sleep quality. | Evaluation T0: pre-interv. T1: 8 weeks post-interv. T2: 6 months post-interv. Results This study was not directly designed to improve sleep quality, but significant improvements in sleep quality were observed following implementation of the intervention. | Unknown. |
| McCrae, et al. [37], 2018. Gainesville. | Randomized con-trolled clinical trial | N = 62 (20 M, 42 W) GI: 32 (10 M, 22 W) GC: 30 (8 M, 22 W) A = 62.45 C: (1) +65 years. (2) Agreed to randomization and speak and write English. (3) Suffer from insomnia. (4) Have no serious mental illness. (5) Have not taken prescribed sleeping medication in the last month. | IG: 1 weekly 1 h session for 4 weeks of an individually delivered behavioral treatment pro-gram on sleep hygiene, stimulus control, sleep restriction, and relaxation. CG: 1 weekly 1 h session for 4 weeks where you discuss topics unrelated to sleep or the IG with a therapist. | Sleep diary and actigraphy: for 14 days per assessment to measure quality, duration of sleep, night awakenings, etc. Geriatric Depression Scale, Beck Depression Inventory, Second Edition, State-Trait Anxiety Inventory-Formula, and Neuropsychological Battery: Mood and Depression. | Evaluation: T0: pre-interv. T1: during interv. T2: 3 months post-interv. Results: The IG experienced significant improvements in sleep measures that continued 3 months post-intervention. The CG did not experience significant improvements. | Unusually, 60% of older adults had higher education. IG: 5 did not receive interv, 8 lost to follow-up. CG: 7 did not receive interv, 2 lost to follow-up. |
| Dzierzewski et al. [38], 2019. Los Angeles. | Randomized con-trolled clinical trial. | N = 159 (154 M, 5 W) IG: 106 (102 M, 4 W) -52: CBTI group -54: CBTI individual CG: 53 (52 M, 1 W) A = 72.2 IG: 72.1 CG: 72.4 C: (1) +60 years. (2) Diagnosis of insomnia. (3) Not having sleep apnea, or scores below 20 on the apnea hypopnea index. (4) Results higher than 24 on the Mini Mental Test. (5) Not having serious physical or mental pathologies. | IG: CBTI was applied in 5 sessions spread over 6 weeks including psychoeducation, sleep restriction, stimulus control, and cognitive therapy. CG: A general sleep education pro-gram structured in the same way as IG was applied. | Sleep diary and actigraphy: for 7 days per assessment. Sleep quality and quantity. PSQI: sleep quality. | Evaluation T0: pre-interv. T1: 6 months post-interv. T2: 12 months post-interv. Results Improved sleep quality in IG compared to CG. There are no significant differences between perceived and measured sleep time. | IG: 9 did not complete the interv, 8 were lost to follow-up. CG: 2 were lost to follow-up. |
| Perini et al. [39], 2021. Singapore | Randomized con-trolled clinical trial. | N = 127 (53 M, 74 W) IG: 65 (29 M, 36 W) CG: 62 (24 M, 38 W) A: 60.9 IG: 61.2 CG: 60.7 C: (1) Be fluent in English. (2) Have no cognitive deficiencies or score greater than or equal to 26 on the Mini Mental State Test or greater than or equal to 23 on the Montreal Cognitive Assessment. (3) Have reported sleep problems in the last month | IG: Weekly 2 h intervention for 8 weeks in which mindfulness exercises, meditation, and body scans were carried out. Afterwards, participants shared their experiences with these practices and how it had affected their sleep quality. Most of the classes contained educational content on sleep hygiene and behavioral strategies. They were taught by certified mindfulness teachers. Each participant was provided with a booklet and audios to practice mindfulness at home. CG: They received a different intervention of the same duration in which a clinical psychologist provided information on sleep biology, self-monitoring of sleep behavior, and were taught changes in habits and environment that could improve sleep quality. They also learned exercises to promote sleep and were provided with booklets and audios to practice at home. | PSQI, ISI: Sleep quality. Actigraphy and polysomnography: sleep latency and awakenings. Five-facet Mind-fulness Questionnaire, Pre-Sleep Arousal Scale, and Dysfunctional Beliefs and Atti-tudes about Sleep Questionnaire: time in bed, total time sleeping, and sleep efficiency. | Evaluation T0: pre-interv. T1: week 4 of interv. T2: post-interv. T3: 6 months post-interv. Results Both CG and IG achieved significant improvement in sleep quality. IG: achieved better results in reducing insomnia. | The sessions could not be recorded to ensure that the protocols were followed correctly and to preserve the privacy of the participants. IG: 2 did not attend the interview, 6 did not complete the sessions, 5 were lost to follow-up, 7 did not take the correct measurements. CG: 1 did not receive the interview, 3 did not complete the sessions, 26 did not take the correct measurements, 6 were lost to follow-up. |
| Camino et al. [40], 2022. Valencia. | Randomized con-trolled clinical trial. | N = 96 (20 M, 76 W) IG: 48 (10 M, 38 W) IGsubclinic: 23 IGmoderate: 25 CG: 48 (10 M, 38 W) CGsubclinic: 24 CGmoderate: 24 A: 72.9 C: (1) +60 years. (2) Be able to read and have no vision problems. (3) Have an ISI score be-tween 8 and 21 and at least 6 on the Pittsburgh index. (4) Not have serious physical or mental health problems. Participants were divided into 2 groups: with subclinical insomnia and with moderate insomnia. They were then randomized into CG and IG. | IG: 1 weekly session for 8 weeks lasting 1.15 h using cognitive mindfulness therapy to improve sleep quality. The first 15 min were meditation, followed by a discussion and sharing of participants’ emotions and progress during the week, then moving on to the different mindfulness sessions of autopilot, facing the challenge, conscious breathing, living in the present, letting go, thoughts are not facts, ways to take better care of myself and use what I have learned. CG: 1 weekly session for 8 weeks of films about active aging. | ISI: Participants’ perception of insomnia. PSQI: Qualitative and quantitative aspects of sleep. | Evaluation T0: pre-interv. T1: post interv. Results The intervention significantly improved sleep quality in older adults with moderate and subclinical insomnia. It also significantly improved the severity of insomnia in subjects with moderate insomnia. After the intervention, treatment was more effective in participants with moderate insomnia than in those with subclinical insomnia. | IG: 4 excluded for medical reasons, 1 did not attend all sessions. CG: 5 did not complete the second evaluation. |
| Lovato et al. [32], 2014. Adelaide. | Alessi et al. [33], 2016. Los Angeles | Lovato et al. [34], 2016. Adelaide. | Bergdahl et al. [35], 2017. Uppsala. | Gallegos et al. [36], 2018. New York | McCrae et al. [37], 2018. Gainesville. | Dzierzewski et al. [38], 2019. Los Angeles. | Perini et al. [39], 2021. Singapore. | Camino et al. [40], 2022. Valencia. | |
|---|---|---|---|---|---|---|---|---|---|
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| No | No | No | No | No | No | No | No | No |
| Yes | Yes | I don’t know | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total | 10/11 | 10/11 | 9/11 | 9/11 | 10/11 | 10/11 | 10/11 | 10/11 | 10/11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Paz-Montón, L.P.; Laredo-Aguilera, J.A.; Carmona-Torres, J.M. Cognitive Interventions for the Treatment of Insomnia or Poor-Quality Sleep in Community-Dwelling Older People: A Systematic Review and Meta-Analysis. Healthcare 2025, 13, 1078. https://doi.org/10.3390/healthcare13091078
de Paz-Montón LP, Laredo-Aguilera JA, Carmona-Torres JM. Cognitive Interventions for the Treatment of Insomnia or Poor-Quality Sleep in Community-Dwelling Older People: A Systematic Review and Meta-Analysis. Healthcare. 2025; 13(9):1078. https://doi.org/10.3390/healthcare13091078
Chicago/Turabian Stylede Paz-Montón, Laura Pilar, José Alberto Laredo-Aguilera, and Juan Manuel Carmona-Torres. 2025. "Cognitive Interventions for the Treatment of Insomnia or Poor-Quality Sleep in Community-Dwelling Older People: A Systematic Review and Meta-Analysis" Healthcare 13, no. 9: 1078. https://doi.org/10.3390/healthcare13091078
APA Stylede Paz-Montón, L. P., Laredo-Aguilera, J. A., & Carmona-Torres, J. M. (2025). Cognitive Interventions for the Treatment of Insomnia or Poor-Quality Sleep in Community-Dwelling Older People: A Systematic Review and Meta-Analysis. Healthcare, 13(9), 1078. https://doi.org/10.3390/healthcare13091078








