The Many Faces of Intestinal Tumors in Adults, Including the Primary Role of CT Imaging in Emergencies and the Important Role of Cross-Sectional Imaging: A Pictorial Review
Abstract
1. Introduction
2. Updates on the Role of Imaging in the Evaluation of Small Bowel Tumors
2.1. Small Bowel Tumors: The Role of Imaging in Emergencies and the Importance of Cross-Sectional Imaging Studies
2.2. Small Bowel Tumors: The Emerging Role of Artificial Intelligence and Radiomics
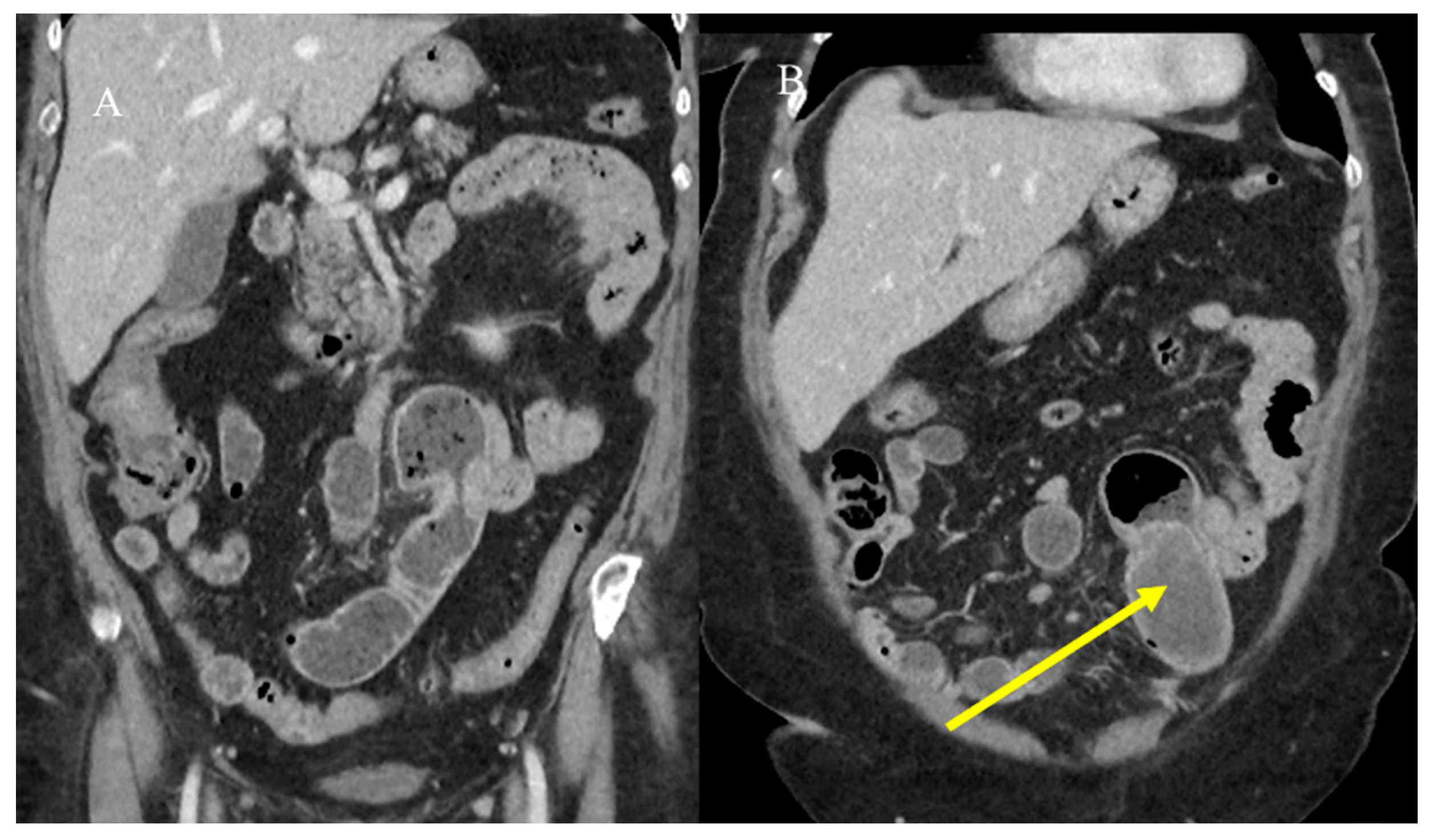
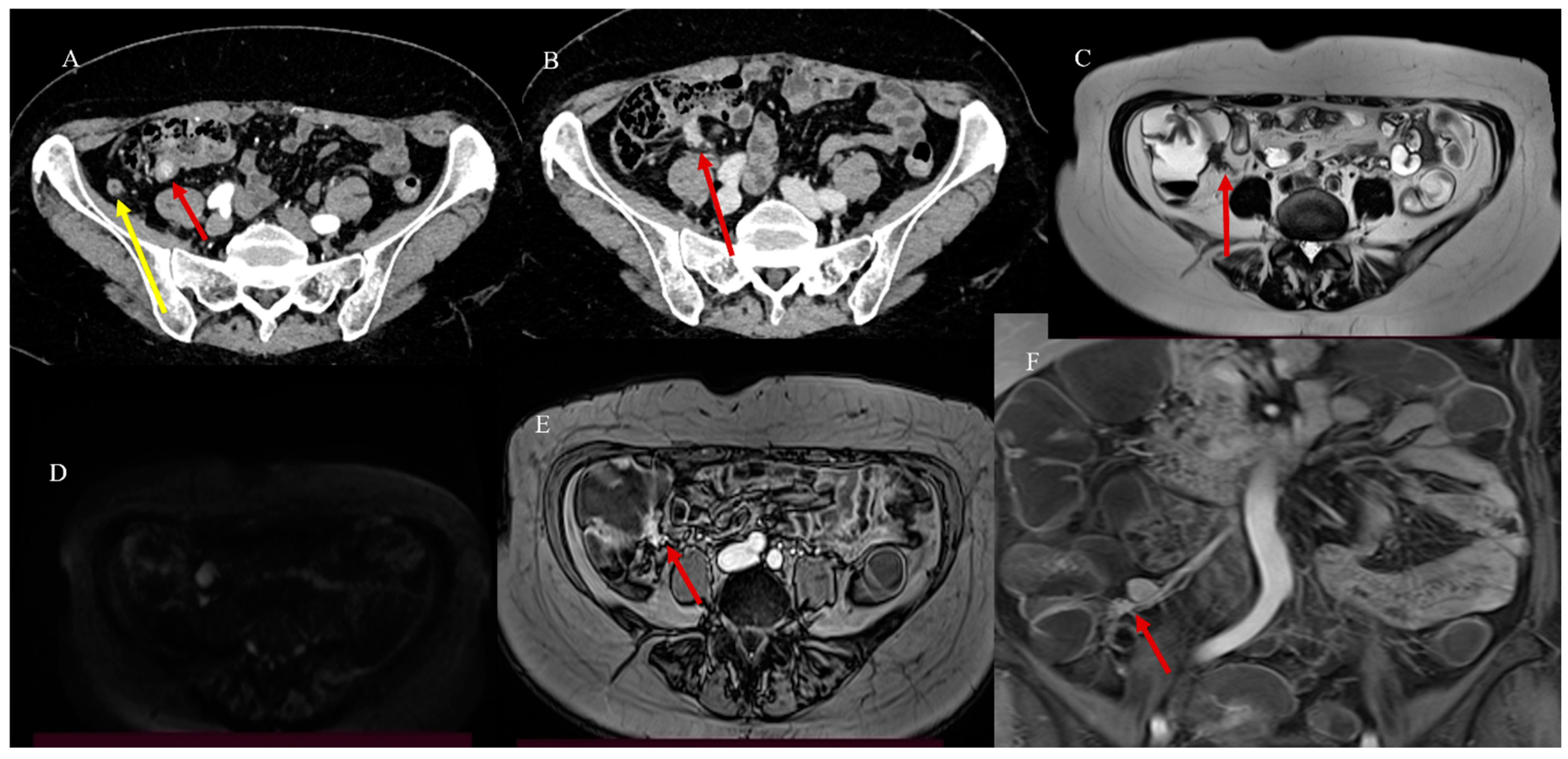
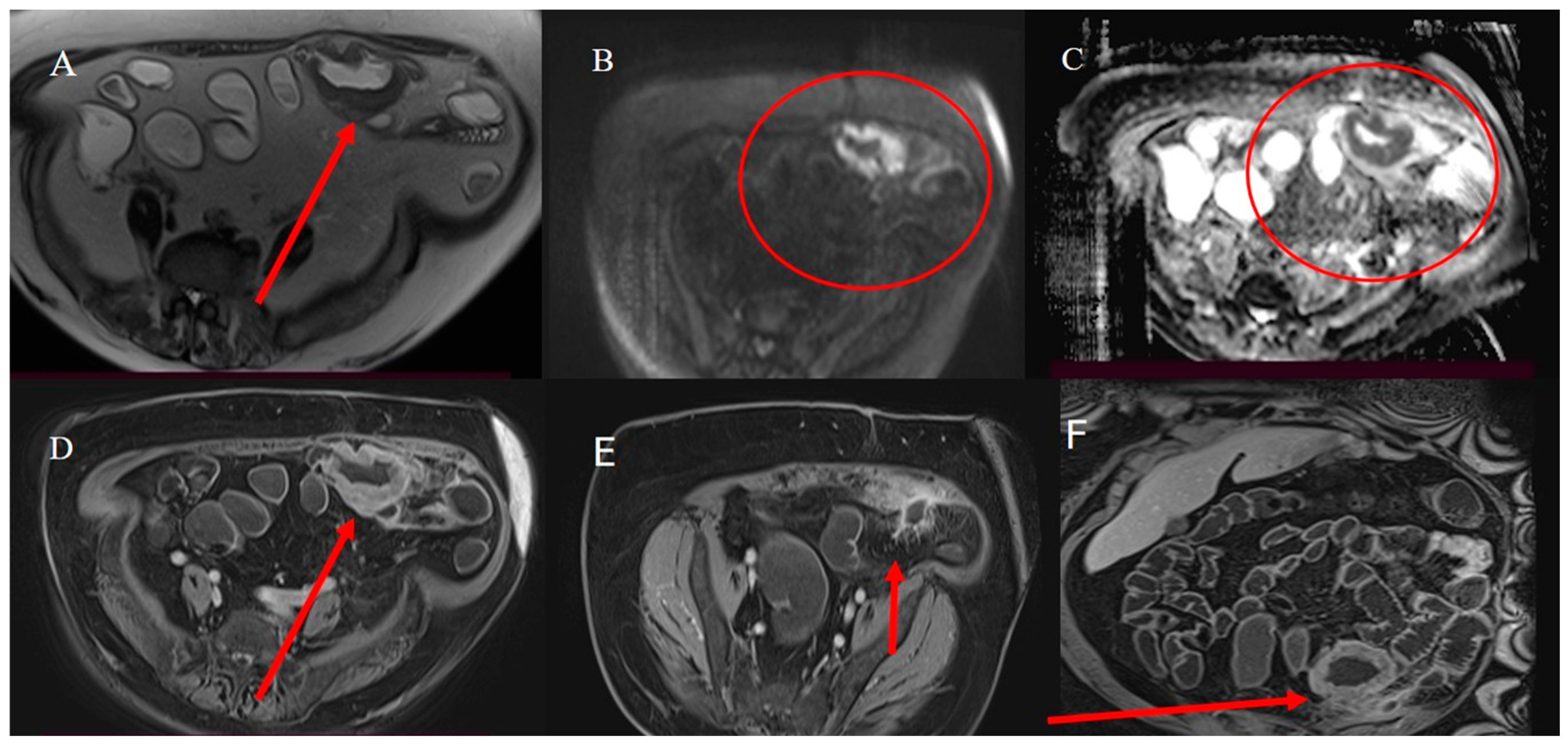
2.3. Small Bowel Tumor Presentation in Emergencies with Intestinal Intussusception
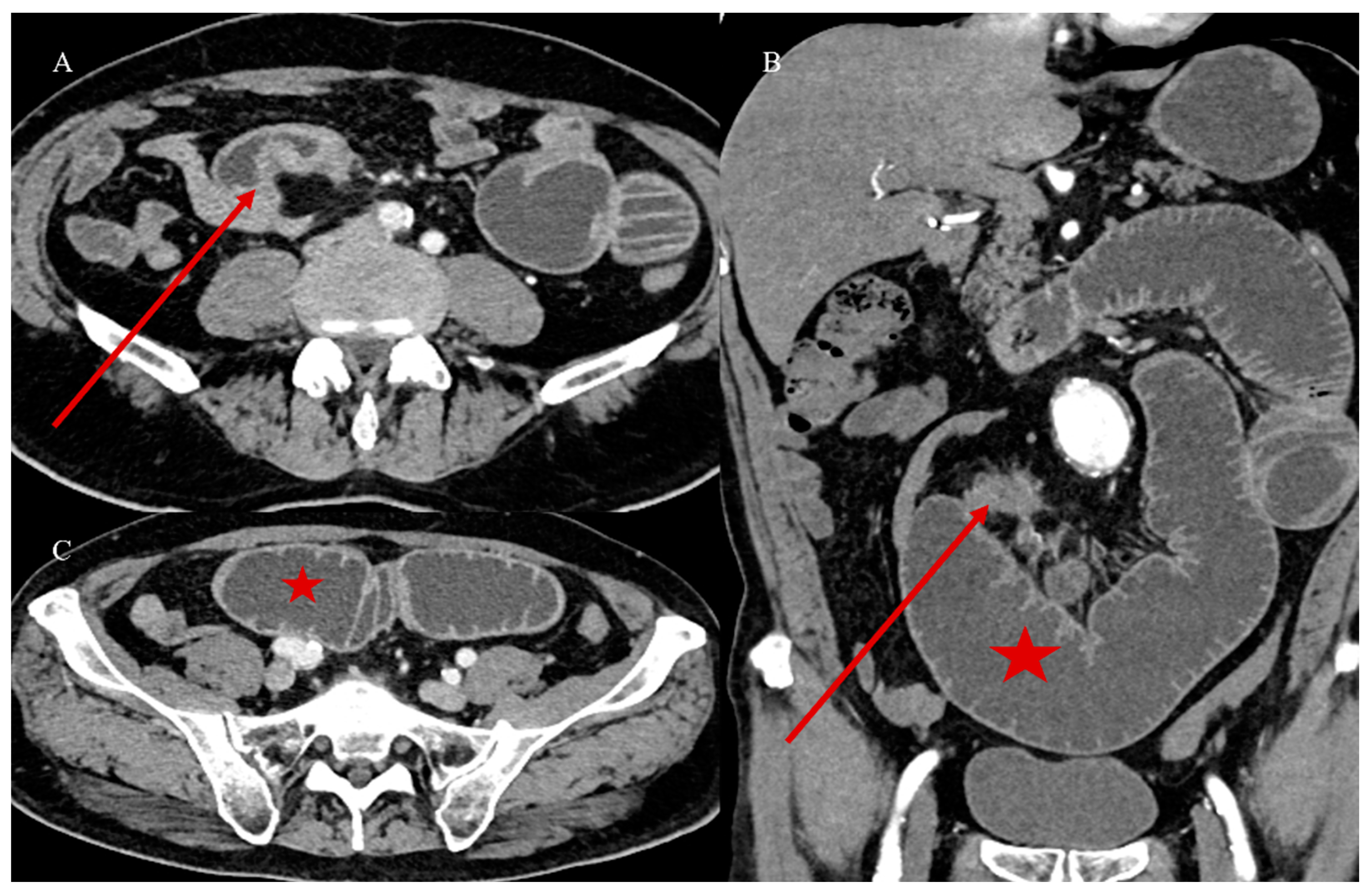
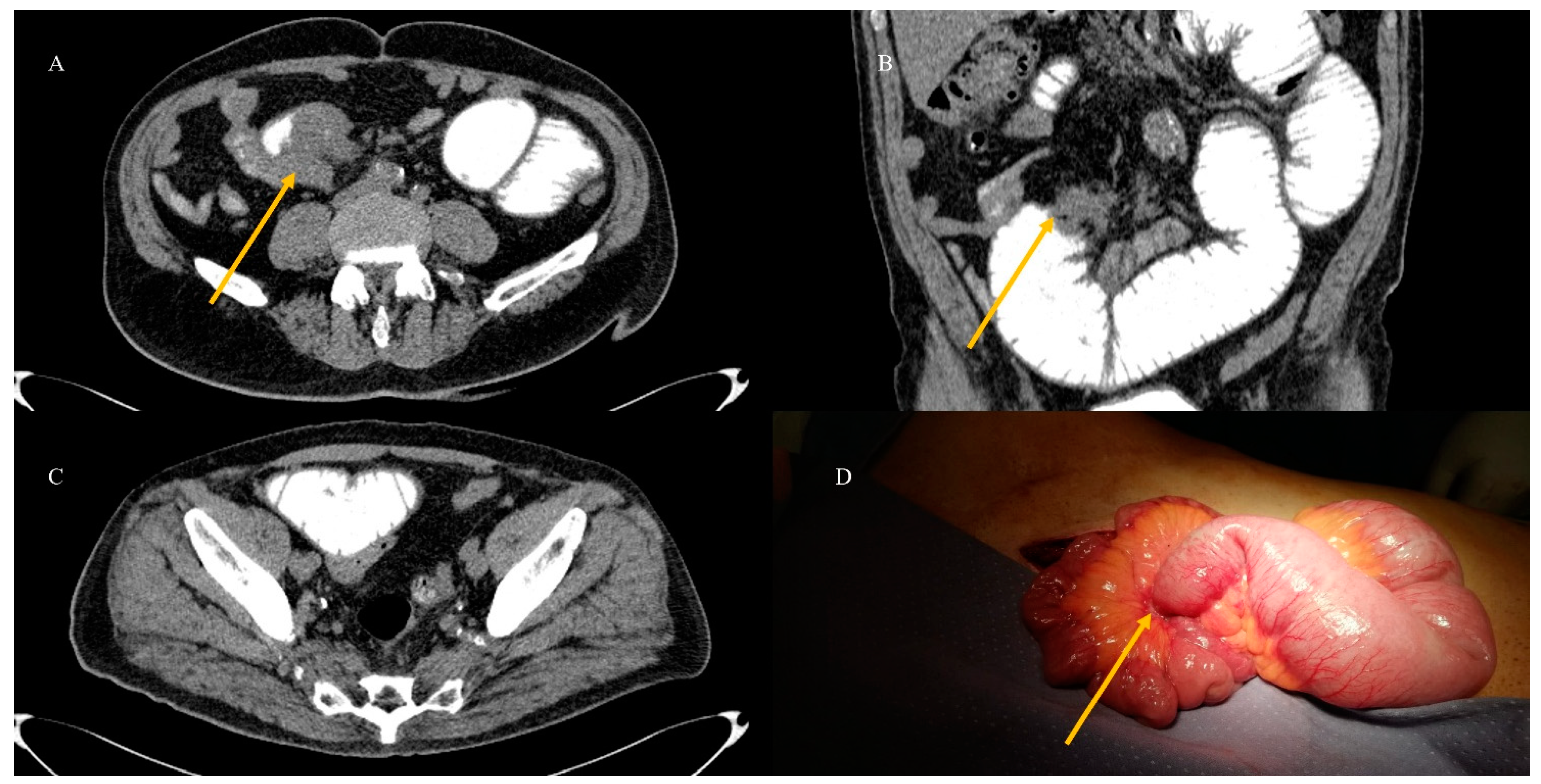
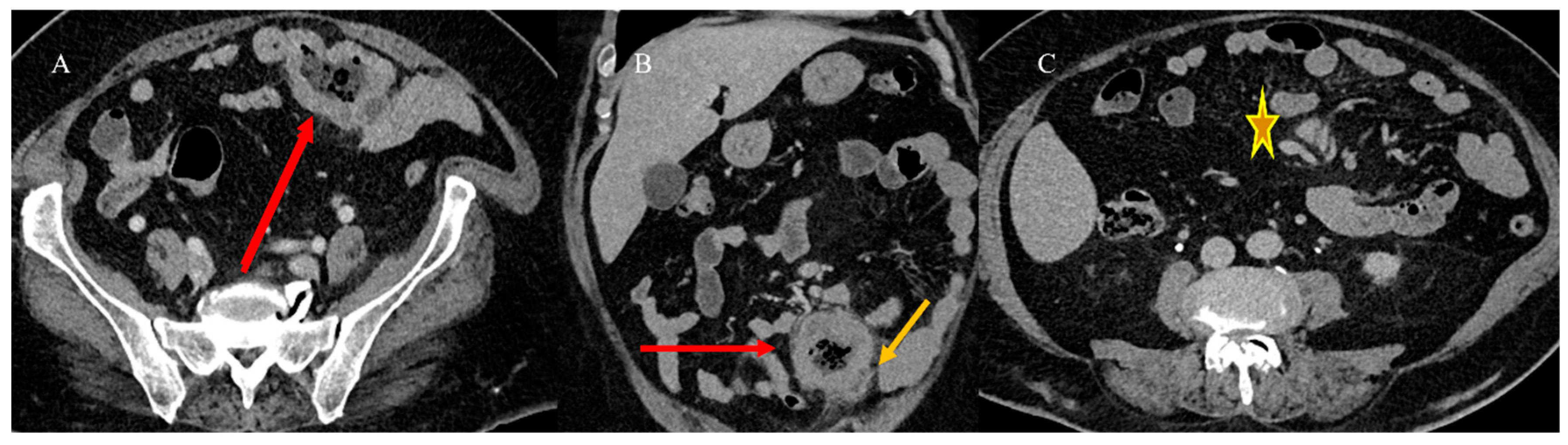
2.4. Small Bowel Tumors Causing Small Bowel Occlusion
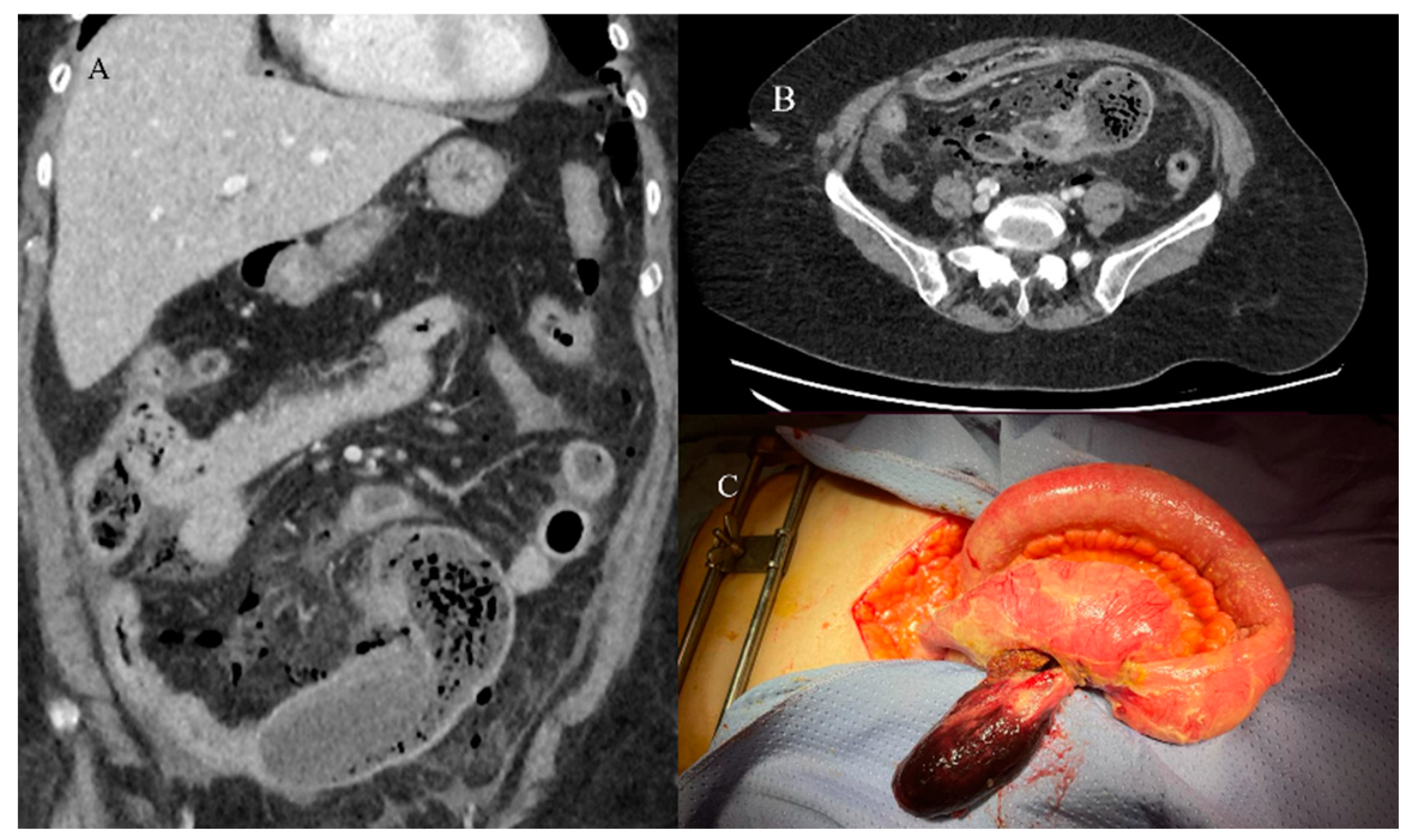
2.5. Small Bowel Tumors Presenting with Bleeding, Ischemia, and Perforation
2.6. Small Bowel Tumors Mimicking Inflammatory Disease
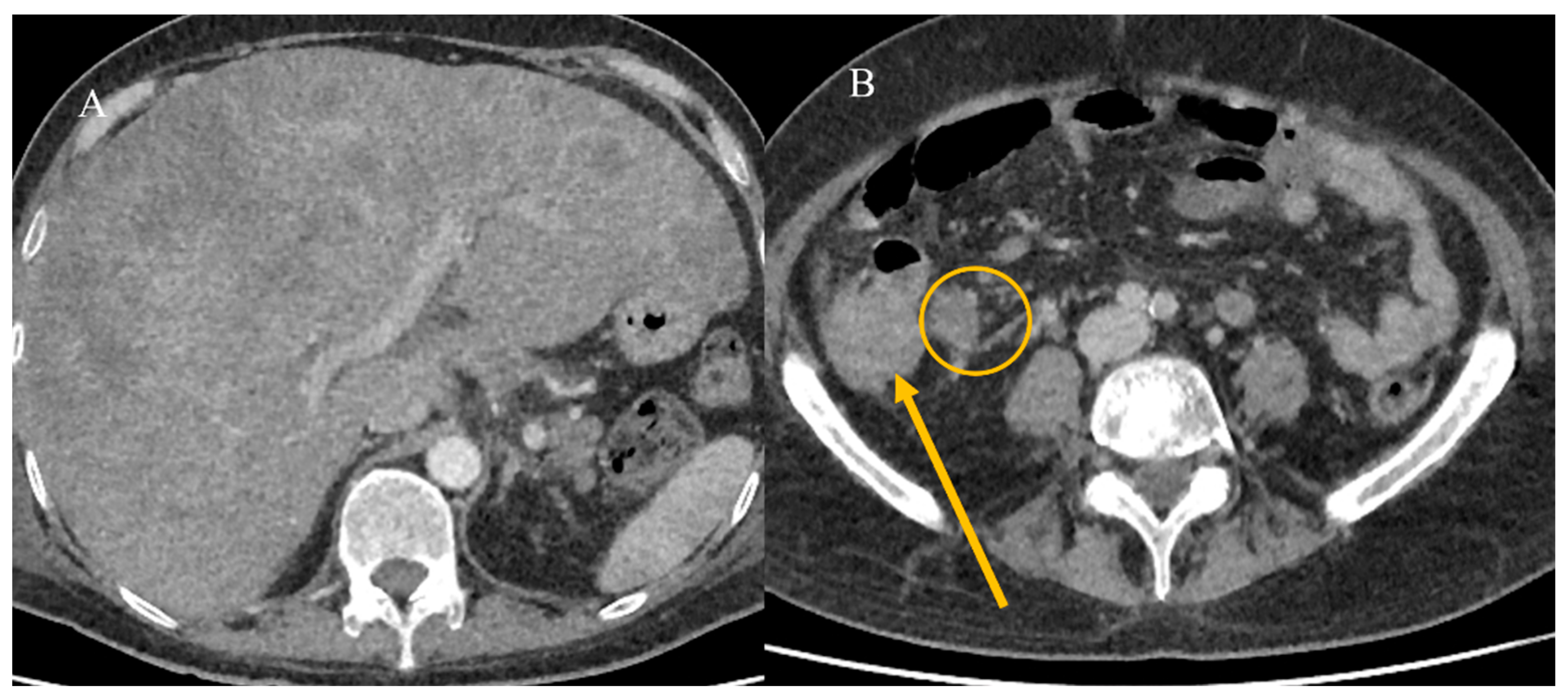
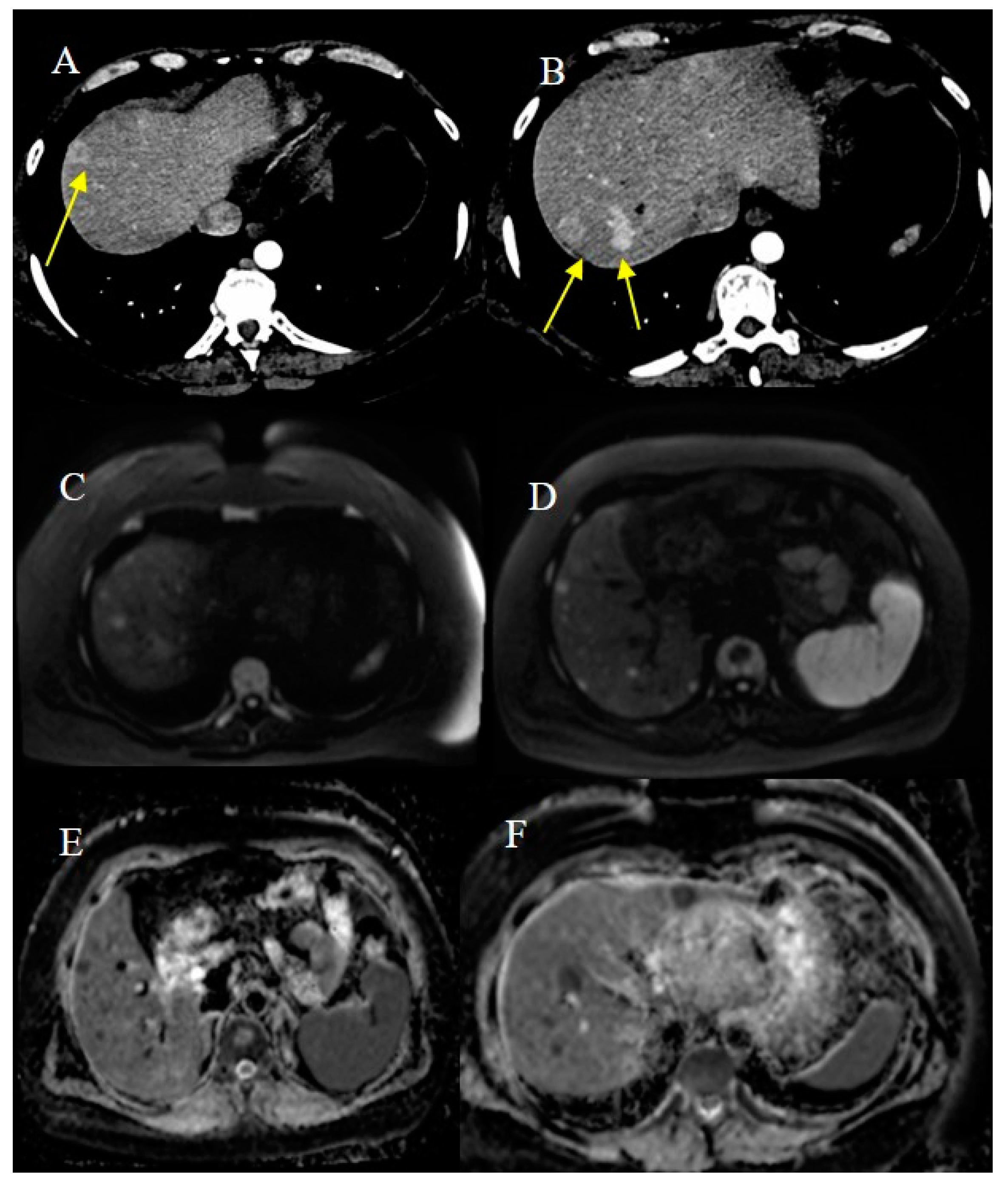
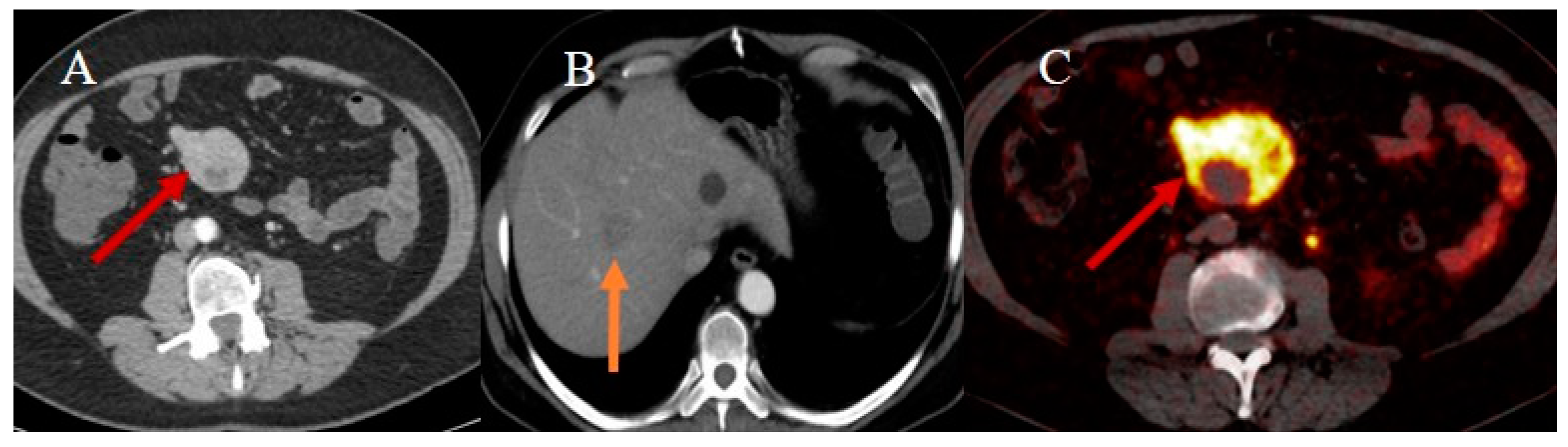
2.7. Metastatic Presentation of Small Bowel Tumors
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Chan, S.C.; Fung, Y.C.; Mak, F.Y.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; et al. Incidence, risk factors, and temporal trends of small intestinal cancer: A global analysis of cancer registries. Gastroenterology 2023, 165, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, A.M.; Robaszkiewicz, M.; Jooste, V.; Cariou, M.; Drouillard, A.; Bouvier, V.; Nousbaum, J.B. Trends in incidence of small bowel cancer according to histology: A population-based study. J. Gatroenterol. 2020, 55, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Wilson, B.E.; Chen, N.; Kong, W.; Patel, S.V.; Booth, C.M.; Merchant, S.J. Small bowel cancers: A population-based analysis of epidemiology, treatment and outcomes in Ontario, Canada from 2005–2020. Surg. Oncol. Ins. 2024, 1, 100096. [Google Scholar] [CrossRef]
- Wang, M.; Yu, M.; Kong, W.J.; Cui, M.; Gao, F. Association between intestinal neoplasms and celiac disease: A review. World J. Gastrointest. Oncol. 2021, 13, 1017. [Google Scholar] [CrossRef]
- Aparicio, T.; Zaanan, A.; Svrcek, M.; Laurent-Puig, P.; Carrere, N.; Manfredi, S.; Locher, C.; Afchain, P. Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis and treatment. Dig. Liver Dis. 2014, 46, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Michael, M.; Jürgen, R. Chronic inflammation in cancer development. Front. Immnunol. 2012, 2, 98. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Shahgoli, V.K.; Noorolyai, S.; Ahmadpour Youshanlui, M.; Saeidi, H.; Nasiri, H.; Mansoori, B.; Baradaran, B. Inflammatory bowel disease, colitis, and cancer: Unmasking the chronic inflammation link. Int. J. Color. Dis. 2024, 39, 173. [Google Scholar] [CrossRef]
- Faggiani, I.; D’amico, F.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Cicerone, C.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S.; Allocca, M. Small bowel cancer in Crohn’s disease. Cancers 2024, 16, 2901. [Google Scholar] [CrossRef]
- Bhatt, H.; Mathis, K.L. Small Bowel Carcinoma in the Setting of Inflammatory Bowel Disease. Clin. Colon. Rectal Surg. 2024, 37, 46–52. [Google Scholar] [CrossRef]
- Pavel, C.; Diculescu, M.M.; Ilie, M.; Plotogea, O.-M.; Sandru, V.; Enache, V.; Gheonea, D.-I.; Jichitu, A.; Constantinescu, A.; Serban, R.-E.; et al. Immunohistochemistry Analysis in Inflammatory Bowel Disease—Should We Bring to Light Interleukin-10? Biomedicines 2025, 13, 406. [Google Scholar] [CrossRef]
- Shenoy, S. Genetic risks and familial associations of small bowel carcinoma. World J. Gastrointest. Oncol. 2016, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Puccini, A.; Battaglin, F.; Lenz, H.J. Management of advanced small bowel cancer. Curr. Treat. Options Oncol. 2018, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.A.; Garrido-Laguna, I.; et al. Small bowel adenocarcinoma, version 1.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 2019, 17, 1109–1133. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, E.; Koffas, A.; Toumpanakis, C.; Keuchel, M. Updates in the diagnosis and management of small-bowel tumors. Best. Pract. Res. Clini Gastroenterol. 2023, 64, 101860. [Google Scholar] [CrossRef]
- Obleagă, C.V.; Streba, C.T.; Mirea, C.S.; Vîlcea, I.D.; Florescu, D.N.; Ciorbagiu, M.C.; Turcu, T.; Florescu, M.M.; Șerbănescu, M.S.; Mehedințeanu, A.-M.; et al. Primitive Resectable Small Bowel Cancer Clinical–Pathological Analysis: A 10-Year Retrospective Study in a General Surgery Unit. Cancers 2024, 16, 3713. [Google Scholar] [CrossRef]
- Farhat, M.H.; Shamseddine, A.I.; Barada, K.A. Small bowel tumors: Clinical presentation, prognosis, and outcome in 33 patients in a tertiary care center. J. Oncol. 2008, 2008, 212067. [Google Scholar] [CrossRef]
- Talamonti, M.S.; Goetz, L.H.; Rao, S.; Joehl, R.J. Primary cancers of the small bowel: Analysis of prognostic factors and results of surgical management. Arch. Surg. 2002, 137, 564–571. [Google Scholar] [CrossRef]
- Yano, T.; Yamamoto, H. Endoscopic Diagnosis of Small Bowel Tumor. Cancers 2024, 16, 1704. [Google Scholar] [CrossRef]
- Singeap, A.-M.; Sfarti, C.; Minea, H.; Chiriac, S.; Cuciureanu, T.; Nastasa, R.; Stanciu, C.; Trifan, A. Small Bowel Capsule Endoscopy and Enteroscopy: A Shoulder-to-Shoulder Race. J. Clin. Med. 2023, 12, 7328. [Google Scholar] [CrossRef] [PubMed]
- Jasti, R.; Carucci, L.R. Small bowel neoplasms: A pictorial review. Radiographics 2020, 40, 1020–1038. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, H.C.; Oh, J.; Won, K.Y.; Park, S.J.; Yang, D.M. Tumors of the jejunum and ileum: A pattern-based imaging approach on CT. Abdom. Radiol. 2019, 44, 2337–2345. [Google Scholar] [CrossRef]
- Gupta, P.; Lamichane, S.; Bhatia, H.; Singhal, M.; Sharma, V.; Singh, H.; Kumar, R.; Sandhu, M.S. Imaging of Small Bowel Tumors and Mimics. J. Gastrointest. Abdom. Radiol. 2024, 7, 55–64. [Google Scholar] [CrossRef]
- Tran, C.G.; Sherman, S.K.; Howe, J.R. Small bowel neuroendocrine tumors. Curr. Probl. Surg. 2020, 57, 100823. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, D.; Bhosale, P.; Yang, T.; Kundra, V. Imaging features of carcinoid tumors of the gastrointestinal tract. AJR Am. J. Roentgenol. 2013, 201, 773–786. [Google Scholar] [CrossRef]
- Gupta, A.; Lubner, M.G.; Menias, C.O.; Mellnick, V.M.; Elsayes, K.M.; Pickhardt, P.J. Multimodality imaging of ileal neuroendocrine (carcinoid) tumor. AJR Am. J. Roentgenol. 2019, 213, 45–53. [Google Scholar] [CrossRef]
- Gangi, A.; Siegel, E.; Barmparas, G.; Lo, S.; Jamil, L.H.; Hendifar, A.; Nissen, N.N.; Wolin, E.M.; Amersi, F. Multifocality in small bowel neuroendocrine tumors. J. Gastrointest. Surg. 2018, 22, 303–309. [Google Scholar] [CrossRef]
- Paparo, F.; Panvini, N.; Montale, A.; Pigati, M.; Marinaro, E.; Melani, E.F.; Piccardo, A.; Molini, L. Multimodality imaging features of small bowel cancers complicating Crohn’s disease: A pictorial review. Abdom. Radiol. 2024, 49, 2083–2097. [Google Scholar] [CrossRef]
- Khosla, D.; Dey, T.; Madan, R.; Gupta, R.; Goyal, S.; Kumar, N.; Kapoor, R. Small bowel adenocarcinoma: An overview. World J. Gastroint. Oncol. 2022, 14, 413. [Google Scholar] [CrossRef]
- Re, G.L.; Federica, V.; Midiri, F.; Picone, D.; La Tona, G.; Galia, M.; Casto, A.L.; Lagalla, R.; Midiri, M. Radiological features of gastrointestinal lymphoma. Gastroenterol. Res. Pract. 2016, 2016, 2498143. [Google Scholar] [CrossRef]
- Brogna, B.; Imbriani, G.C.; Forte, N.R.; Schettino, M.; Morelli, R.; Venditti, M.; Manganiello, C.; Biondo, F.G. Multifocal gastrointestinal stromal tumor: A case report with CT, surgical, and histopathologic correlation. Radiol. Case Rep. 2019, 14, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Ota, S.; Yamasaki, M.; Batsaikhan, B.; Furukawa, A.; Watanabe, Y. Gastrointestinal stromal tumors: A comprehensive radiological review. Jpn. J. Radiol. 2022, 40, 1105–1120. [Google Scholar] [CrossRef]
- Fernandes, D.D.; Galwa, R.P.; Fasih, N.; Fraser-Hill, M. Cross-sectional imaging of small bowel malignancies. Can. Assoc. Radiol. J. 2012, 63, 215–221. [Google Scholar] [CrossRef]
- Williams, E.A.; Bowman, A.W. Multimodality imaging of small bowel neoplasms. Abdom. Radiol. 2019, 44, 2089–2103. [Google Scholar] [CrossRef]
- Brogna, B.; Frieri, C.; Risitiano, A.M.; Urciuoli, L.; Storti, G.; Santoro, L.; Urciuoli, E.; De Chiara, G.; Cretella, P.; Sementa, C.; et al. Intestinal and Extraintestinal Findings of Graft-versus-Host Disease on CT: A Case Series with Radiological and Histopathological Correlations. Biomedicines 2024, 12, 1516. [Google Scholar] [CrossRef] [PubMed]
- Megally, H.I.; Seifeldein, G.S.; Abbas, N.A.; Elamin, H.A. The diagnostic role of MDCT enterography in small bowel lesions. Egypt. J. Radiol. Nucl. Med. 2015, 46, 1–8. [Google Scholar] [CrossRef]
- Ilangovan, R.; Burling, D.; George, A.; Gupta, A.; Marshall, M.; Taylor, S.A. CT enterography: Review of technique and practical tips. Br. J. Radiol. 2012, 85, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, R.N.; Dolan, S.G.; Barlow, J.M.; Wells, M.L.; Sheedy, S.P.; Fidler, J.L.; Hansel, S.; Harmsen, S.; Fletcher, J.G. Impact of CT enterography on the diagnosis of small bowel gastrointestinal stromal tumors. Abdom. Radiol. 2017, 42, 1365–1373. [Google Scholar] [CrossRef]
- Patel, A.; Lalwani, N.; Kielar, A. Use of oral contrast in 2024: Primer for radiologists. Abdom. Radiol. 2024, 49, 2953–2959. [Google Scholar] [CrossRef]
- Almafreji, I.; Chinaka, U.; Hussain, A.; Lynch, M.; Cottrell, R. Role of Gastrografin in patients with small bowel obstruction. Cureus 2020, 12, e9695. [Google Scholar] [CrossRef]
- Mileto, A.; Ananthakrishnan, L.; Morgan, D.E.; Yeh, B.M.; Marin, D.; Kambadakone, A.R. Clinical implementation of dual-energy CT for gastrointestinal imaging. AJR Am. J. Roentgenol. 2021, 217, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-B.; Yu, N.; Jian, Y.-J.; Yu, Y.; Duan, H.-F.; Zhang, X.-R.; Ma, G.-M.; Guo, Y.; Duan, X. Spectral CT imaging in the differential diagnosis of small bowel adenocarcinoma from primary small intestinal lymphoma. Acad. Radiol. 2019, 26, 878–884. [Google Scholar] [CrossRef]
- Trabzonlu, T.A.; Mozaffary, A.; Kim, D.; Yaghmai, V. Dual-energy CT evaluation of gastrointestinal bleeding. Abdom. Radiol. 2020, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dane, B.; Gupta, A.; Wells, M.L.; Anderson, M.A.; Fidler, J.L.; Naringrekar, H.V.; Allen, B.C.; Brook, O.R.; Bruining, D.H.; Gee, M.S.; et al. Dual-Energy CT Evaluation of Gastrointestinal Bleeding. RadioGraphics 2023, 43, e220192. [Google Scholar] [CrossRef] [PubMed]
- Hoerning, A.; Jüngert, J.; Siebenlist, G.; Knieling, F.; Regensburger, A.P. Ultrasound in Pediatric Inflammatory Bowel Disease—A Review of the State of the Art and Future Perspectives. Children 2024, 11, 156. [Google Scholar] [CrossRef]
- Dell’era, A.; Cannatelli, R.; Ferretti, F.; Manzotti, C.; Dilillo, D.; Zuccotti, G.; Meneghin, F.; Ardizzone, S.; Maconi, G. Relevance of sonographic parameters for inflammatory bowel disease in children. J. Ultrasound 2023, 26, 815–822. [Google Scholar] [CrossRef]
- Fujita, M.; Manabe, N.; Honda, K.; Murao, T.; Osawa, M.; Kawai, R.; Akiyama, T.; Shiotani, A.; Haruma, K.; Hata, J. Usefulness of Ultrasonography for Diagnosis of Small Bowel Tumors. Medicine 2015, 94, e1464. [Google Scholar] [CrossRef]
- Yu, X.; Yang, Y.; Li, J. Application of ultrasound in the diagnosis of gastrointestinal tumors. Eur. J. Inflam. 2020, 18, 2058739220961194. [Google Scholar] [CrossRef]
- Pierro, A.; Minordi, L.M.; Larosa, L.; Cipri, C.; Guerri, G.; Quinto, F.; Rotondi, F.; Marcellino, A.; Basilico, R.; Iezzi, R.; et al. Small Bowel Imaging from Stepchild of Roentgenology to MR Enterography, Part II: The Reliable Disclosure of Crohn’s Disease and Non-Inflammatory Small Bowel Disorder Plot through MRI Findings. Life 2023, 13, 1836. [Google Scholar] [CrossRef]
- Masselli, G.; Casciani, E.; Polettini, E.; Laghi, F.; Gualdi, G. Magnetic resonance imaging of small bowel neoplasms. Cancer Imaging 2013, 13, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Faggian, A.; Fracella, M.R.; D’alesio, G.; Alabiso, M.E.; Berritto, D.; Feragalli, B.; Miele, V.; Iasiello, F.; Grassi, R. Small-Bowel Neoplasms: Role of MRI Enteroclysis. Gastroenterol. Res. Pract. 2016, 2016, 9686815. [Google Scholar] [CrossRef]
- Boone, D.; Taylor, S.A. Magnetic Resonance of the Small Bowel: How to Do It. Magn. Reson. Imaging Clin. N. Am. 2019, 28, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, F.; Busato, L.; Valenti, A.; Cardaccio, S.; Longhi, A.; Catalano, C. Magnetic resonance imaging of the gastrointestinal tract: Current role, recent advancements and future prospectives. Diagnostics 2023, 13, 2410. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.; Bicci, E.; Danti, G.; Flammia, F.; Chiti, G.; Palumbo, P.; Bruno, F.; Borgheresi, A.; Grassi, R.; Grassi, F.; et al. The role of magnetic resonance enterography in crohn’s disease: A review of recent literature. Diagnostics 2022, 12, 1236. [Google Scholar] [CrossRef]
- Reda, A.M.; Saleh, R.A.; Elgawish, M.A.; Elsharkawy, A. MR enterography in small bowel diseases, adding multipoint Dixon sequence, is it worth? Egypt. J. Radiol. Nucl. Med. 2023, 54, 87. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Z.; Morelli, J.N.; Yu, H.; Luo, Y.; Hu, X.; Li, Z.; Hu, D.; Shen, Y. A systematic review of technical parameters for MR of the small bowel in non-IBD conditions over the last ten years. Sci. Rep. 2019, 9, 14100. [Google Scholar] [CrossRef]
- Maccioni, F.; Alfieri, G.; Assanto, G.M.; Mattone, M.; Silveri, G.G.; Viola, F.; De Maio, A.; Frantellizzi, V.; Di Rocco, A.; De Vincentis, G.; et al. Whole body MRI with Diffusion Weighted Imaging versus 18F-fluorodeoxyglucose-PET/CT in the staging of lymphomas. Radiol. Med. 2023, 128, 556–564. [Google Scholar] [CrossRef]
- Pezzella, M.; Brogna, B.; Romano, A.; Torelli, F.; Esposito, G.; Petrillo, M.; Romano, F.; Di Martino, N.; Reginelli, A.; Grassi, R. Detecting a rare composite small bowel lymphoma by Magnetic Resonance Imaging coincidentally: A case report with radiological, surgical and histopathological features. Int. J. Surg. Case Rep. 2018, 46, 50–55. [Google Scholar] [CrossRef]
- Maccioni, F.; Al Ansari, N.; Mazzamurro, F.; Barchetti, F.; Marini, M. Surveillance of patients affected by Peutz-Jeghers syndrome: Diagnostic value of MR enterography in prone and supine position. Abdom. Imaging 2012, 37, 279–287. [Google Scholar] [CrossRef]
- Jayaprakasam, V.S.; Paroder, V.; Schöder, H. Variants and pitfalls in PET/CT imaging of gastrointestinal cancers. Semin. Nucl. Med. 2021, 51, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Koppula, B.R.; Fine, G.C.; Salem, A.E.; Covington, M.F.; Wiggins, R.H.; Hoffman, J.M.; Morton, K.A. PET-CT in clinical adult oncology: III. Gastrointestinal malignancies. Cancers 2022, 14, 2668. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.T.; Howe, J.R. Management of small bowel neuroendocrine tumors. J. Oncol. Pract. 2018, 14, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Kandathil, A.; Subramaniam, R.M. Gastroenteropancreatic neuroendocrine tumor diagnosis: DOTATATE PET/CT. PET Clin. 2023, 18, 189–200. [Google Scholar] [CrossRef]
- Shell, J.; Keutgen, X.M.; Millo, C.; Nilubol, N.; Patel, D.; Sadowski, S.; Boufraqech, M.; Yang, L.; Merkel, R.; Atallah, C.; et al. 68-Gallium DOTATATE scanning in symptomatic patients with negative anatomic imaging but suspected neuroendocrine tumor. Int. J. Endocr. Oncol. 2018, 5, IJE04. [Google Scholar] [CrossRef]
- Bonomi, A.; Romario, U.F.; Funicelli, L.; Conti, G.; Luc, M.R.; Ceci, F.; Pozzi, S.; Radice, D.; Fazio, N.; Bertani, E. Diagnosis and staging of small intestinal neuroendocrine tumors with CT enterography and PET with Gallium-68: Preoperative risk stratification protocol. Langenbecks Arch. Surg. 2024, 409, 63. [Google Scholar] [CrossRef]
- Huang, H. The application of radiomics and artificial intelligence in cancer imaging. Front. Oncol. 2022, 12, 864940. [Google Scholar] [CrossRef]
- Zheng, S.; Cui, X.; Ye, Z. Integrating artificial intelligence into radiological cancer imaging: From diagnosis and treatment response to prognosis. Cancer Biol. Med. 2025, 22, 6–13. [Google Scholar] [CrossRef]
- Limkin, E.J.; Sun, R.; Dercle, L.; Zacharaki, E.I.; Robert, C.; Reuzé, S.; Schernberg, A.; Paragios, N.; Deutsch, E.; Ferté, C. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann. Oncol. 2017, 28, 1191–1206. [Google Scholar] [CrossRef]
- Al-Bayati, K.; Stone, J.K.; Berzin, T.M. The Use of Artificial Intelligence for Endoscopic Evaluation of the Small Bowel. Gastrointest. Endosc. Clin. N. Am. 2025, 35, 355–366. [Google Scholar] [CrossRef]
- Mota, J.; Almeida, M.J.; Mendes, F.; Martins, M.; Ribeiro, T.; Afonso, J.; Cardoso, P.; Cardoso, H.; Andrade, P.; Ferreira, J.; et al. From data to insights: How is AI revolutionizing small-bowel endoscopy? Diagnostics 2024, 14, 291. [Google Scholar] [CrossRef]
- He, Y.S.; Su, J.R.; Li, Z.; Zuo, X.L.; Li, Y.Q. Application of artificial intelligence in gastrointestinal endoscopy. J. Dig. Dis. 2019, 20, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Berbís, M.A.; Aneiros-Fernández, J.; Olivares, F.J.M.; Nava, E.; Luna, A. Role of artificial intelligence in multidisciplinary imaging diagnosis of gastrointestinal diseases. World J. Gastroenterol. 2021, 27, 4395. [Google Scholar] [CrossRef]
- Goyal, H.; Sherazi, S.A.A.; Mann, R.; Gandhi, Z.; Perisetti, A.; Aziz, M.; Chandan, S.; Kopel, J.; Tharian, B.; Sharma, N.; et al. Scope of artificial intelligence in gastrointestinal oncology. Cancers 2021, 13, 5494. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Sandhya, T.; Srilatha, M.; Ganji, S.P.; Saddala, M.S.; El-Rayes, B.F. Artificial intelligence in gastrointestinal cancers: Diagnostic, prognostic, and surgical strategies. Cancer Lett. 2025, 612, 217461. [Google Scholar] [CrossRef] [PubMed]
- Mervak, B.M.; Fried, J.G.; Wasnik, A.P. A review of the clinical applications of artificial intelligence in abdominal imaging. Diagnostics 2023, 13, 2889. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, L.; Zhang, C.; Huang, C.; Wang, M.; Feng, Z.; Xiong, Y. CT radiomics model for discriminating the risk stratification of gastrointestinal stromal tumors: A multi-class classification and multi-center study. Front. Oncol. 2021, 11, 654114. [Google Scholar] [CrossRef]
- Rengo, M.; Onori, A.; Caruso, D.; Bellini, D.; Carbonetti, F.; De Santis, D.; Vicini, S.; Zerunian, M.; Iannicelli, E.; Carbone, I.; et al. Development and validation of artificial-intelligence-based radiomics model using computed tomography features for preoperative risk stratification of gastrointestinal stromal tumors. J. Pers. Med. 2023, 13, 717. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Ren, J.; Jia, L.; Ma, L.; Yin, X.; Yang, F.; Gao, B.-L. Malignancy risk of gastrointestinal stromal tumors evaluated with noninvasive radiomics: A multi-center study. Front. Oncol. 2022, 12, 966743. [Google Scholar] [CrossRef]
- Zhuo, M.; Tang, Y.; Guo, J.; Qian, Q.; Xue, E.; Chen, Z. Predicting the risk stratification of gastrointestinal stromal tumors using machine learning-based ultrasound radiomics. J. Med. Ultrason. 2024, 51, 71–82. [Google Scholar] [CrossRef]
- Marsicovetere, P.; Ivatury, S.J.; White, B.; Holubar, S.D. Intestinal intussusception: Etiology, diagnosis, and treatment. Clin. Colon. Rectal Surg. 2017, 30, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Blake, M.A.; Harisinghani, M.G.; Archer-Arroyo, K.; Hahn, P.F.; Pitman, M.B.; Mueller, P.R. Adult intestinal intussusception: CT appearances and identification of a causative lead point. Radiographics 2006, 26, 733–744. [Google Scholar] [CrossRef]
- Choi, S.H.; Han, J.K.; Kim, S.H.; Lee, J.M.; Lee, K.H. Intussusception in adults: From stomach to rectum. AJR Am. J. Roentgenol. 2004, 183, 691–698. [Google Scholar] [CrossRef]
- Panzera, F.; Di Venere, B.; Rizzi, M.; Biscaglia, A.; Praticò, C.A.; Nasti, G.; Mardighian, A.; Nunes, T.F.; Inchingolo, R. Bowel intussusception in adult: Prevalence, diagnostic tools and therapy. World J. Methodol. 2021, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Honjo, H.; Mike, M.; Kusanagi, H.; Kano, N. Adult intussusception: A retrospective review. World J. Surg. 2015, 39, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Cui, X.Y.; Liu, Y.; Long, J.; Xu, Y.H.; Guo, R.X.; Guo, K.J. Adult intussusception: A retrospective review of 41 cases. World J. Gastroenterol. World J. Gastroenterol. 2009, 15, 3303. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Yeh, Y.T. Insidious presentation of intussusception with appendicitis. J. Surg. Case Rep. 2021, 2021, rjaa578. [Google Scholar] [CrossRef]
- Kee, H.M.; Park, J.Y.; Yi, D.Y.; Lim, I.S. A case of intussusception with acute appendicitis. Pediatr. Gastroenterol. Hepatol. Nutr. 2015, 18, 134–137. [Google Scholar] [CrossRef]
- Tuca, A.; Guell, E.; Martinez-Losada, E.; Codorniu, N. Malignant bowel obstruction in advanced cancer patients: Epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag. Res. 2012, 4, 159–169. [Google Scholar] [CrossRef]
- Alshamsan, B.; Aseafan, M.; Badran, A.; Shaheen, A.; Elshenawy, M.A.; Bazarbashi, S.; Aljubran, A.H. Characteristics and outcomes of small bowel adenocarcinoma: 14 years of experience at a single tertiary hospital in Saudi Arabia. Mol. Clin. Oncol. 2023, 18, 17. [Google Scholar] [CrossRef]
- Kroepfl, V.; Bellotti, R.; Gasser, E.; Esswein, K.; Esser, H.; Kafka-Ritsch, R.; Öfner, D.; Perathoner, A. Small bowel neuroendocrine tumors: An analysis of clinical presentation, diagnostic workup and surgical approach—A single center retrospective study. Front. Surg. 2023, 10, 1072435. [Google Scholar] [CrossRef]
- Basendowah, M.H.; Ashour, M.A.; Hassan, A.Y.; Alshaynawi, S.; Alyazidi, L.K. Multiple small intestinal neuroendocrine tumors with findings of intestinal obstruction. Cureus 2021, 13, e17629. [Google Scholar] [CrossRef] [PubMed]
- Behi, H.; Omry, A.; Dallagi, R.; Changuel, A.; Troudi, D.; Khalifa, M.B. Diagnosing and managing small bowel neuroendocrine tumors presenting as acute obstruction in an elderly patient: A case report and comprehensive management overview. Int. J. Surg. Case Rep. 2024, 122, 110126. [Google Scholar] [CrossRef]
- Song, Y.; Li, M.; Shan, J.; Ye, X.; Tang, S.; Fang, X.; Ding, K.; Yuan, Y. Acute small bowel obstruction: A rare initial presentation for the metastasis of the large-cell carcinoma of the lung. World J. Surg. Oncol. 2012, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Alsobahi, N.A.; Mohammed, T.A. Small bowel obstruction as first presentation of metastatic lobular breast cancer for pilgrim patient. Int. J. Surg. Case Rep. 2024, 120, 109855. [Google Scholar] [CrossRef]
- Naoshima, K.; Abe, K.; Murakami, K.; Takaya, K.; Nakano, T. Small Bowel Obstruction Caused by Small Intestinal Metastasis Secondary to Esophageal Carcinoma. Case Rep. Surg. 2021, 2021, 9728424. [Google Scholar] [CrossRef] [PubMed]
- Sparkman, B.K.; Pearce, J.; Klein, K.; Idowu, M.; Askari, K.; Fernandez, L.J.; Trevino, J.G.; Sullivan, S.A.; Miller, D.T.; Randall, L.M. Metastatic Uterine Leiomyosarcoma presenting as small bowel intussusception at two independent visits. Gynecol. Oncol. Rep. 2023, 51, 101306. [Google Scholar] [CrossRef]
- Rais, M.; Chahdi, H.; Elfahssi, M.; Albouzidi, A.; Oukabli, M. An unusual cause of intestinal obstruction in a young adult patient: Inflammatory fibroid polyp. Case Rep. Surg. 2017, 2017, 3675848. [Google Scholar] [CrossRef]
- Mohamud, S.O.; Motorwala, S.A.; Daniel, A.R.; Tworek, J.A.; Shehab, T.M. Giant ileal inflammatory fibroid polyp causing small bowel obstruction: A case report and review of the literature. Cases J. 2008, 1, 341. [Google Scholar] [CrossRef]
- Nelms, D.W.; Kann, B.R. Imaging modalities for evaluation of intestinal obstruction. Clin. Colon. Rectal Surg. 2021, 34, 205–218. [Google Scholar] [CrossRef]
- Paulson, E.K.; Thompson, W.M. Review of small-bowel obstruction: The diagnosis and when to worry. Radiology 2015, 275, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.C.; Chao, H.H.; Jan, Y.Y.; Chen, M.F. Perforation through small bowel malignant tumors. J. Gastrointest. Surg. 2005, 9, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Mooghal, M.; Ahmad, A.; Safi, A.; Khan, W.; Ahmad, N. Impending perforation near ileocecal junction due to phytobezoar impaction and intraluminal polyp: A case report. J. Med. Case Rep. 2022, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Carney, B.W.; Khatri, G.; Shenoy-Bhangle, A.S. The role of imaging in gastrointestinal bleed. Cardiovasc. Diagn. Ther. 2019, 9 (Suppl. S1), S88. [Google Scholar] [CrossRef]
- Pouli, S.; Kozana, A.; Papakitsou, I.; Daskalogiannaki, M.; Raissaki, M. Gastrointestinal perforation: Clinical and MDCT clues for identification of aetiology. Insights Imaging 2020, 11, 31. [Google Scholar] [CrossRef]
- Yavuz, A.; Ay, G.; Akan, K.; Ulaşoğlu, C.; Tuncer, İ. Small Bowel Adenocarcinoma Mimicking a Crohn’s Attack. Cureus 2021, 13, e15743. [Google Scholar] [CrossRef]
- Thomas, A.S.; Schwartz, M.; Quigley, E. Gastrointestinal lymphoma: The new mimic. BMJ Open Gastroenterol. 2019, 6, e000320. [Google Scholar] [CrossRef]
- Attiyeh, M.A.; Malhotra, G.K.; Li, D.; Manoukian, S.B.; Motarjem, P.M.; Singh, G. Defining MRI Superiority over CT for Colorectal and Neuroendocrine Liver Metastases. Cancers 2023, 15, 5109. [Google Scholar] [CrossRef]
- Shenoy-Bhangle, A.; Baliyan, V.; Kordbacheh, H.; Guimaraes, A.R.; Kambadakone, A. Diffusion weighted magnetic resonance imaging of liver: Principles, clinical applications and recent updates. World J. Hepatol. 2017, 9, 1081. [Google Scholar] [CrossRef]
- Naswa, N.; Sharma, P.; Kumar, A.; Nazar, A.H.; Kumar, R.; Chumber, S.; Bal, C. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: A prospective single-center study. AJR Am. J. Roentgenol. 2011, 197, 1221–1228. [Google Scholar] [CrossRef]
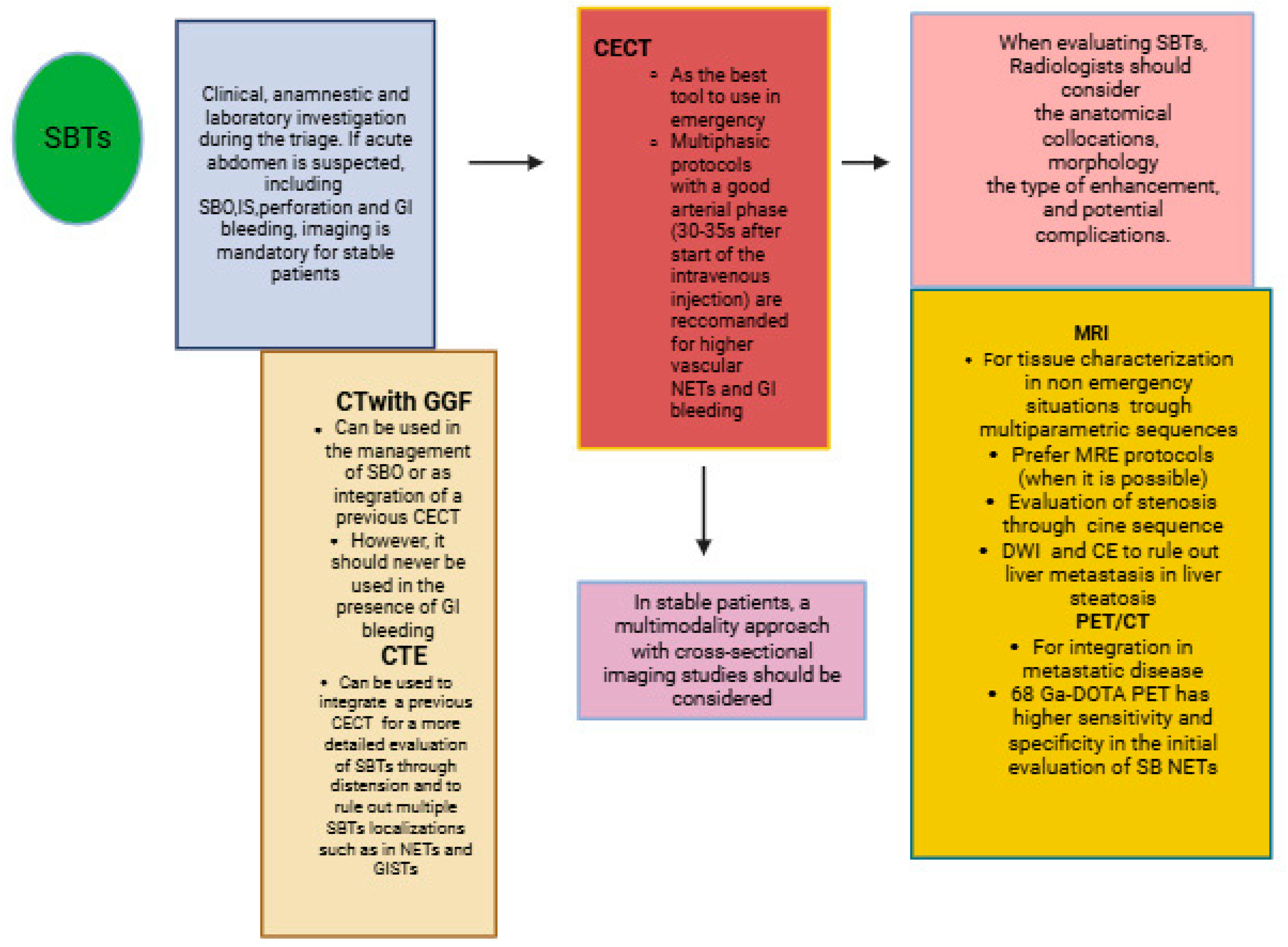
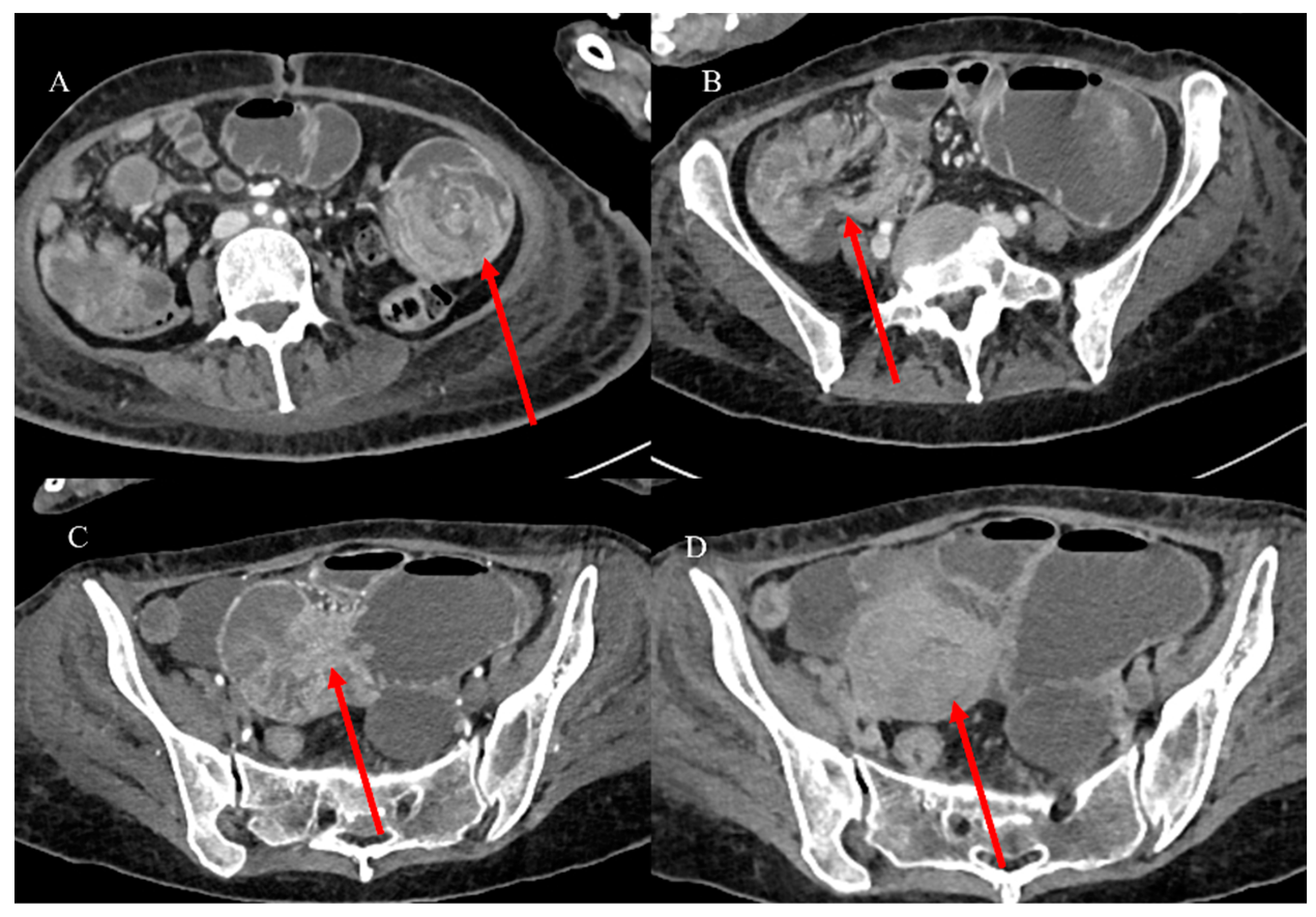
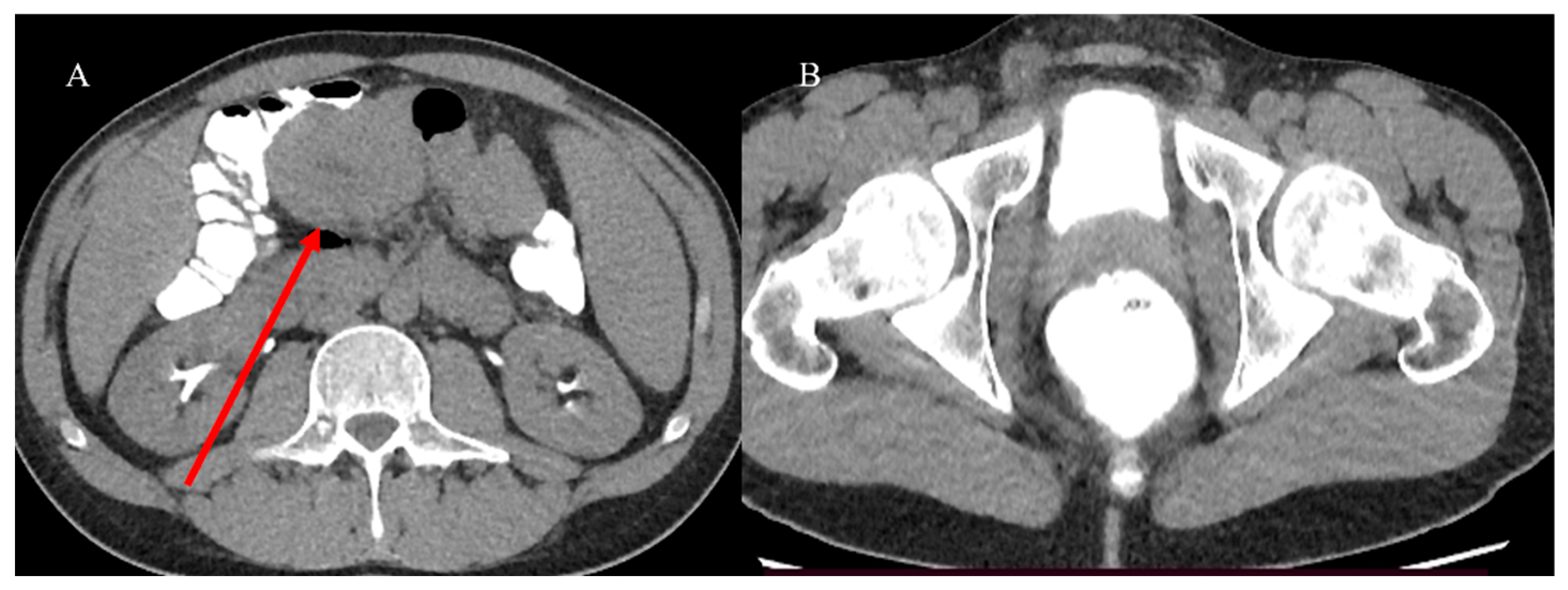

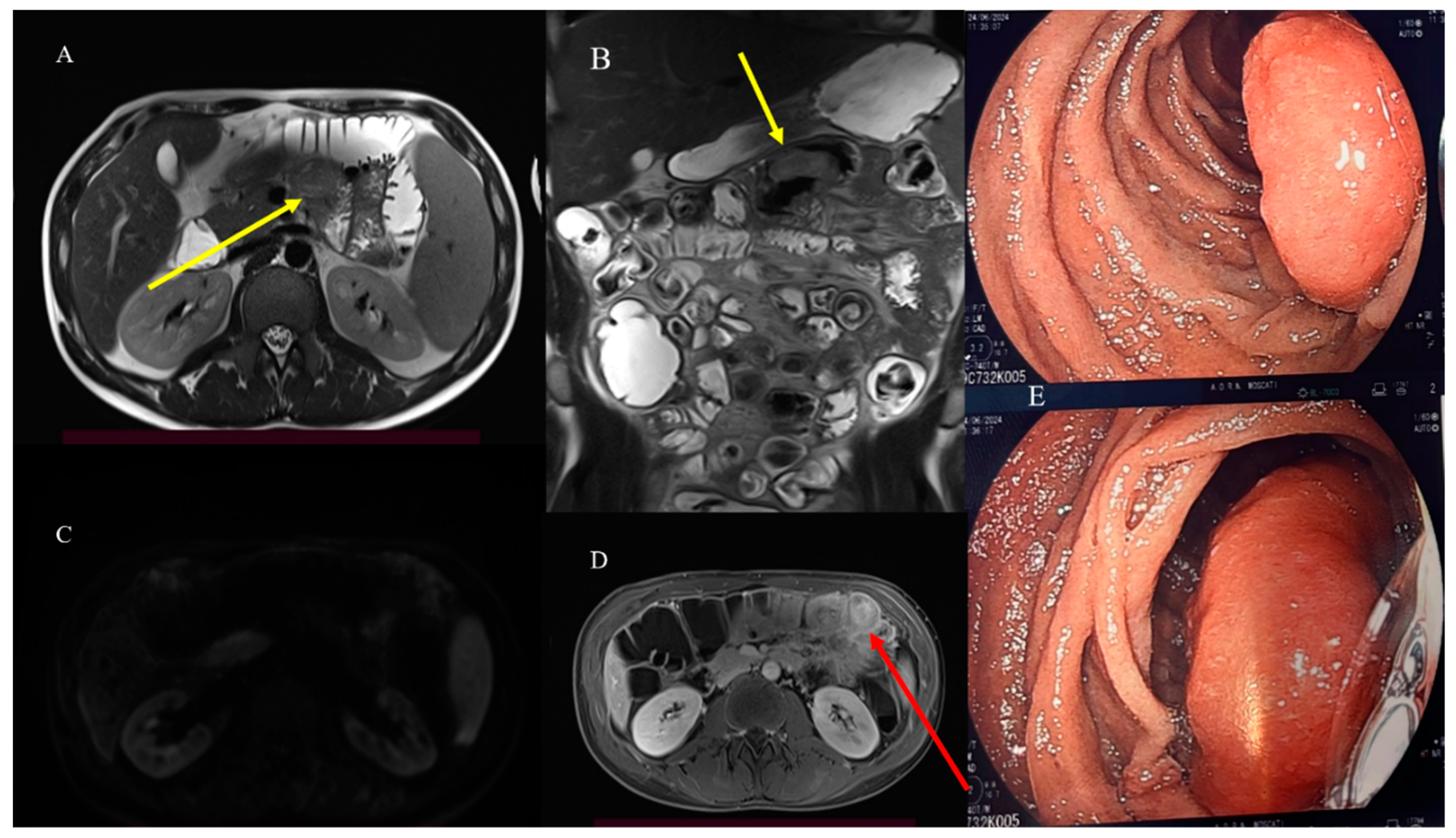
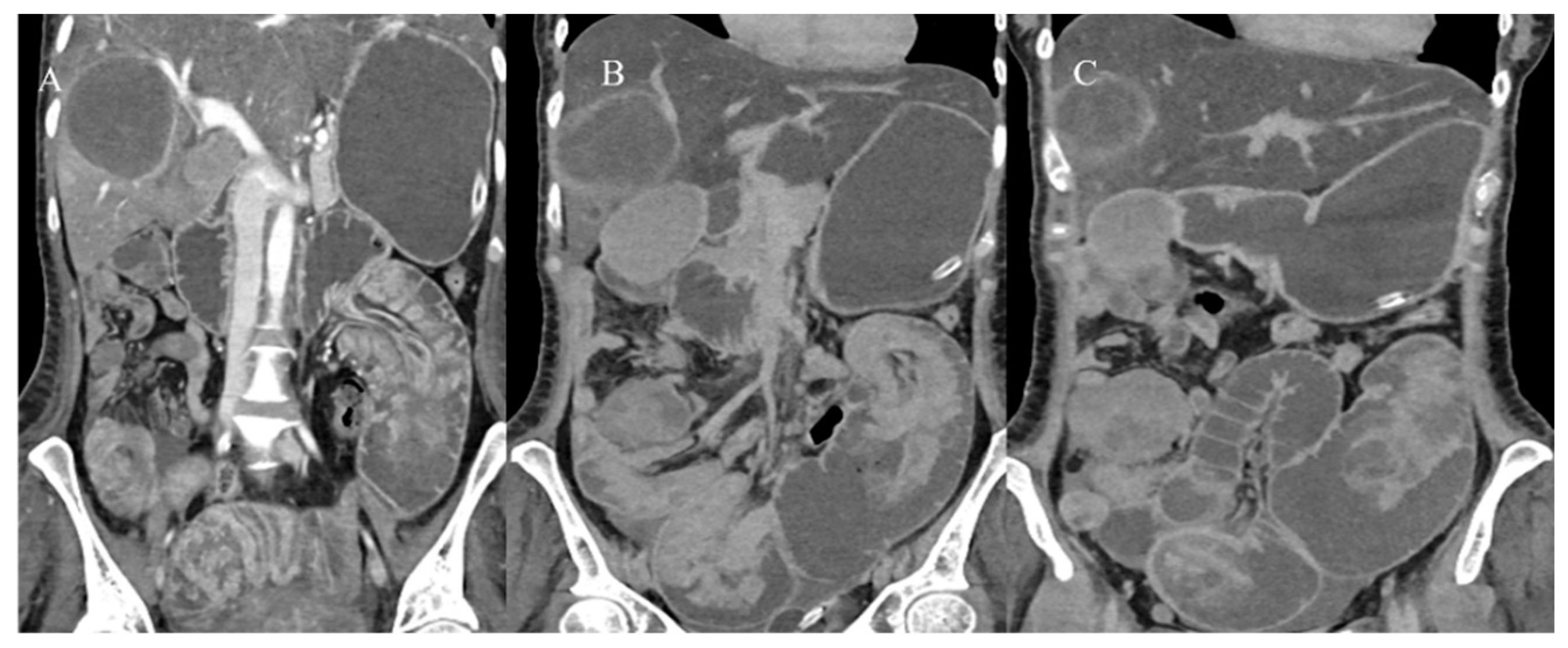

| Indications | Protocols | Advantages | Limitations | |
|---|---|---|---|---|
| Abdominal X-Ray | In the emergency department for acute abdomen with SBO or small bowel perforations | Anterior–posterior (AP) supine projection or PA prone, lateral decubitus, upright AP, and lateral cross-table (with the patient supine) | Low dose; easier availability; cost-effective | Poor sensitivity for SBO causes and for SB parietal evaluation |
| IUS | In pediatric patients; in patients with GI diseases, including IBD and GI obstruction | The small bowel is explored with a medium- or high-frequency convex or lineal transducer (5–15 MHz) | Radiation-free; non-invasive; cost-effective | The experience of the operator, artifacts produced by intestinal gas, and the patient’s body habitus, including BMI and the thickness of the fatty layer of the anterior abdominal wall |
| CT | ||||
| CECT | In an emergency and initial staging of SBTs | A good arterial phase (30–35 s) for higher-vascular SBT tumors as NETs and GI bleeding | Speed; easier availability; MPR reconstruction | Small intestinal tumors may be not visualized without distension; allergy to contrast agents |
| CT with GGF | In an emergency; in cases of SBO that do not require emergency surgery; for transit evaluation in SBTs with SBO; in cases of small bowel perforations | 100 mL of GGF diluted into 50 mL water via NG tube or taken orally | GGF can aid in the management of SBO, has a therapeutic effect in the conservative management of adhesive SBO, and can depict the presence of intestinal fistulas | Can mask GI bleeding in the intestinal lumen |
| CTE | To diagnose and stage SBTs; integration with previous CECT in non-emergency cases | Small bowel distension through negative or positive oral contrast agents; split bolus technique: contrast agent injected twice, with the first injection consisting of 60% of the total dose, followed by injection of the remaining 40% | Improved visualization of the small bowel wall and lumen; enhanced detection of small intestine lesions and multiple localizations | Inadequate small bowel distention; less sensitive than capsule study to detect small bowel distension; cannot be tolerated by some patients |
| DECT | To diagnose and stage SBTs; in emergencies when available | Use of two different X-ray tube potentials to acquire images simultaneously or sequentially | A combination of low-energy monochromatic images, iodine maps, and virtual unenhanced images improves lesion detection and characterization; reduction of radiation dose and number of CT scans | Higher costs; lesser availability than conventional MDCT |
| MRI | ||||
| MRE | For SBT tissue characterization in non-emergency cases or in stable patients | Intestinal distension with oral biphasic contrast agents, with an optimal volume of 1000–1500 mL that can be ingested over 45–60 min before the examination; T2 HASTE with and without FS on axial and coronal plane; Trufi T2 on axial and coronal plane; DWI; VIBE T1 FS pre-contrast on coronal plane; if stenosis is present, it may be helpful to use the cine-balanced sequence | Tissue characterization through multiparametric sequence; DWI can be used to predict and monitor SBTs; DWI is more sensitive to detecting LI metastasis in liver steatosis | Claustrophobia; PMK not MRI-compatible |
| PET/CT | Staging and restaging of SBTs | 2-deoxy-2-18F-D-glucose (FDG) is the most commonly used tracer; 68-Gallium (68Ga)-DOTA peptide (DOTATATE, DOTATOC, and DOTANOC) PET/CT is used in small bowel NETs; full body scan acquisition | Ability to detect local and distant metastatic disease; 68-Gallium (68Ga)-DOTA peptide has higher sensitivity and specificity for small bowel; NETs tumor localization, staging, and receptor status assessment; 68-Gallium DOTATATE PET/CT is accurate for detecting initial or recurrent NETs in patients with carcinoid-like symptoms and negative anatomical imaging | PET/CT can produce false positives in patients with inflammation or infection; PET/CT has less spatial resolution than CT and MRI; PET and CT images can be misregistered due to the small bowel’s mobility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brogna, B.; Maccioni, F.; Sgambato, D.; Capuano, F.; Iovine, L.; Guarino, S.; Di Libero, L.; Amendola, A.; Faggioni, L.; Cioni, D. The Many Faces of Intestinal Tumors in Adults, Including the Primary Role of CT Imaging in Emergencies and the Important Role of Cross-Sectional Imaging: A Pictorial Review. Healthcare 2025, 13, 1071. https://doi.org/10.3390/healthcare13091071
Brogna B, Maccioni F, Sgambato D, Capuano F, Iovine L, Guarino S, Di Libero L, Amendola A, Faggioni L, Cioni D. The Many Faces of Intestinal Tumors in Adults, Including the Primary Role of CT Imaging in Emergencies and the Important Role of Cross-Sectional Imaging: A Pictorial Review. Healthcare. 2025; 13(9):1071. https://doi.org/10.3390/healthcare13091071
Chicago/Turabian StyleBrogna, Barbara, Francesca Maccioni, Dolores Sgambato, Fabiana Capuano, Lorenzo Iovine, Salvatore Guarino, Lorenzo Di Libero, Alfonso Amendola, Lorenzo Faggioni, and Dania Cioni. 2025. "The Many Faces of Intestinal Tumors in Adults, Including the Primary Role of CT Imaging in Emergencies and the Important Role of Cross-Sectional Imaging: A Pictorial Review" Healthcare 13, no. 9: 1071. https://doi.org/10.3390/healthcare13091071
APA StyleBrogna, B., Maccioni, F., Sgambato, D., Capuano, F., Iovine, L., Guarino, S., Di Libero, L., Amendola, A., Faggioni, L., & Cioni, D. (2025). The Many Faces of Intestinal Tumors in Adults, Including the Primary Role of CT Imaging in Emergencies and the Important Role of Cross-Sectional Imaging: A Pictorial Review. Healthcare, 13(9), 1071. https://doi.org/10.3390/healthcare13091071









