Clinical Trial Awareness, Perceptions, and Participation Among Cancer Patients in Saudi Arabia
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. Questionnaire
2.4. Statistical Analysis
2.4.1. Population and Sample Size
2.4.2. Data Collection Method
2.4.3. Variables and Their Measurement
3. Results
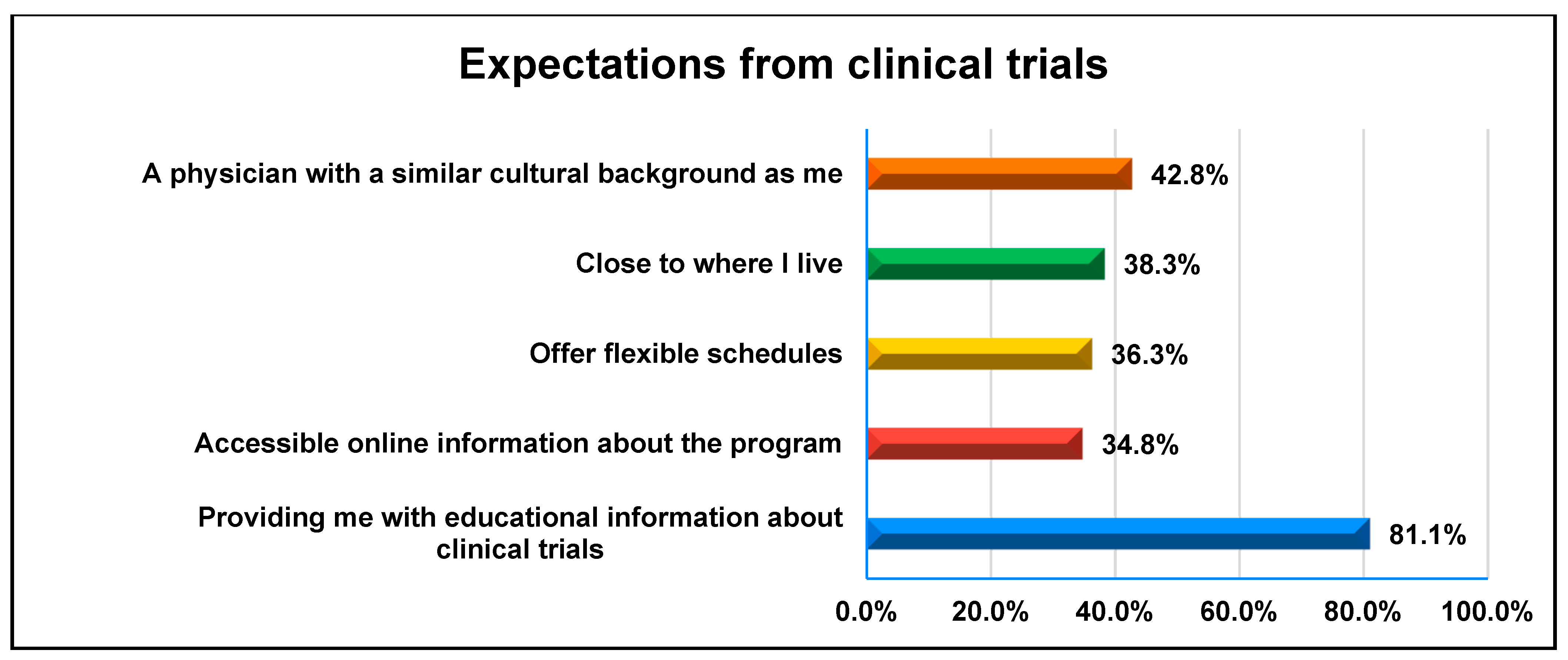
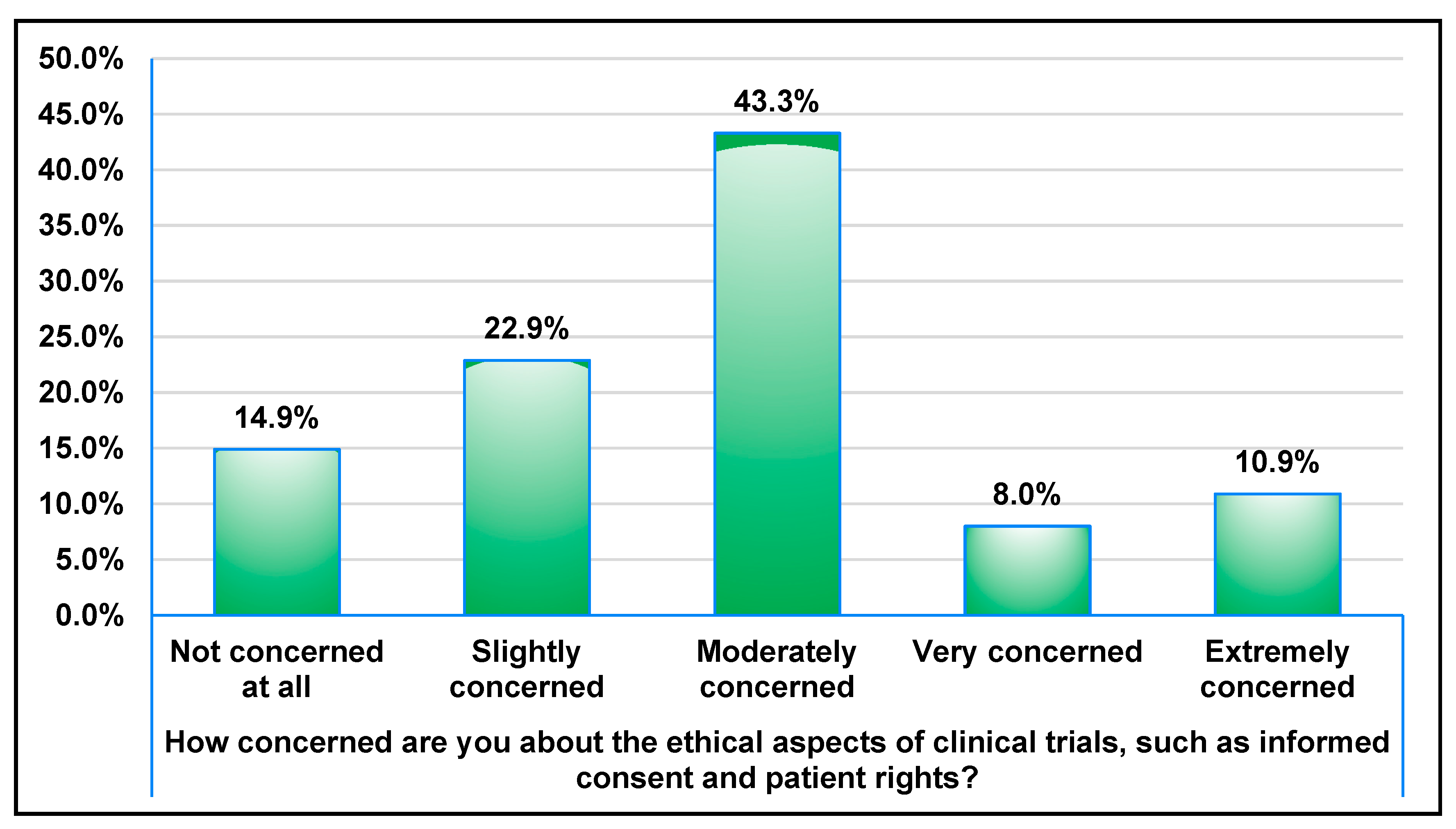
3.1. Awareness and Perceptions of Clinical Trials
3.2. Barriers to Participation in Clinical Trials
3.3. Factors Influencing Perception, Willingness to Participate, and Awareness of Clinical Trials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ranjan, R.; Agarwal, N.B.; Kapur, P.; Marwah, A.; Parveen, R. Study of Awareness and Practice of Informed Consent Process Among Clinical Trial Participants and Their Motives Behind Participation. Asia-Pac. J. Public Health 2019, 31, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Comis, R.L.; Miller, J.D.; Aldigé, C.R.; Krebs, L.; Stoval, E. Public attitudes toward participation in cancer clinical trials. J. Clin. Oncol. 2003, 21, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, S.J.; Chung, Y.H.; Kwon, J.H.; Lee, H.J.; Chung, Y.J.; Kim, Y.J.; Oh, D.Y.; Lee, S.H.; Kim, D.W.; et al. Cancer patients’ awareness of clinical trials, perceptions on the benefit and willingness to participate: Korean perspectives. Br. J. Cancer 2008, 99, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Bazarbashi, S.; Hassan, A.; Eldin, A.M.; Soudy, H.; Hussain, F. Awareness and Perceptions of Clinical Trials in Cancer Patients and Their Families in Saudi Arabia. J. Cancer Educ. 2015, 30, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, K.M.; Alonazi, W.B.; Alodhayani, A.A.; Vinluan, J.M.; Moussa, M.; Al-Ajlan, A.S.; Alsaleh, K.; Alruwaimi, D.; Alotaibi, N.E. Barriers to Cancer Clinical Trial Participation Among Saudi Nationals: A Cross-Sectional Study. J. Relig. Health 2017, 56, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Catania, C.; De Pas, T.; Goldhirsch, A.; Radice, D.; Adamoli, L.; Medici, M.; Verri, E.; Marenghi, C.; De Braud, F.; Nolè, F. Participation in clinical trials as viewed by the patient: Understanding cultural and emotional aspects which influence choice. Oncology 2008, 74, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Klabunde, C.N.; Springer, B.C.; Butler, B.; White, M.S.; Atkins, J. Factors Influencing Enrollment in Clinical Trials for Cancer Treatment. South. Med. J. 1999, 92, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Al-Tannir, M.A.; El-Bakri, N.; Abu-Shaheen, A.K. Knowledge, attitudes and perceptions of saudis towards participating in clinical trials. PLoS ONE 2016, 11, e0143893. [Google Scholar] [CrossRef] [PubMed]
- Al-Dakhil, L.O.; Alanazy, R.; Al-Hamed, R.E.; Al-Mandeel, H.; Alobaid, A. Attitudes of patients in developing countries toward participating in clinical trials: A survey of saudi patients attending primary health care services. Oman Med. J. 2016, 31, 284. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, Z.E.; Berthelsen, C.B. Cancer patients’ perceptions of factors influencing their decisions on participation in clinical drug trials: A qualitative meta-synthesis. J. Clin. Nurs. 2019, 28, 2443–2461. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Park, L.C.; Lee, J.; Kim, S.; Choi, M.K.; Hong, J.Y.; Park, S.; Maeng, C.H.; Chang, W.; Kim, Y.S.; et al. Unique perception of clinical trials by Korean cancer patients. BMC Cancer 2012, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.K.; Tsark, J.U.; Braun, K.L. Increasing primary care physician support for and promotion of cancer clinical trials. Hawai’i J. Med. Public Health 2014, 73, 84. [Google Scholar]
- Bartoszkiewicz, M.; Kufel-Grabowska, J.; Litwiniuk, M. Awareness of breast cancer patients in Poland about clinical trials as available treatment options. Breast Dis. 2021, 40, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 12 November 2024).
- Ahram, M.; Farkouh, A.; Haddad, M.; Kalaji, Z.; Yanis, A. Knowledge of, attitudes to and participation in clinical trials in Jordan: A population-based survey. East. Mediterr. Health J. 2020, 26, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Occa, A.; Merritt, A.S.; Leip, A.; Stapleton, J.L. What influences trust in and understanding of clinical trials? An analysis of information and communication technology use and online health behavior from the Health Information National Trends Survey. Clin. Trials 2024, 21, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Sumayyia, M.D.; Al-Madaney, M.M.; Almousawi, F.H. Health information on social media. Perceptions, attitudes, and practices of patients and their companions. Saudi Med. J. 2019, 40, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.; Chao, J.H.; Lee, D.; Wu, Y.; Kloecker, G.H. Patient perceptions concerning clinical trials in oncology patients. Contemp. Clin. Trials Commun. 2016, 4, 179–185. [Google Scholar] [CrossRef] [PubMed]

| Items | Studied Participants (N = 201) | |
|---|---|---|
| N | % | |
| Age | ||
| Mean± SD | 39.51± 14.86 | |
| Median (Range) | 38.5 (14–83) | |
| ≤18 years | 11 | 5.5% |
| 19–40 years | 100 | 49.8% |
| 41–60 years | 80 | 39.8% |
| >60 years | 10 | 5.0% |
| Gender | ||
| Female | 139 | 69.2% |
| Male | 62 | 30.8% |
| Marital status | ||
| Married | 108 | 53.7% |
| Single | 65 | 32.3% |
| Divorced | 16 | 8.0% |
| Widowed | 9 | 4.5% |
| Prefer not to say | 3 | 1.5% |
| Educational level | ||
| Illiterate | 8 | 4.0% |
| Primary School | 14 | 7.0% |
| Secondary School | 57 | 28.4% |
| Bachelor’s Degree | 94 | 46.8% |
| Diploma | 3 | 1.5% |
| Master’s Degree | 14 | 7.0% |
| Doctorate | 10 | 5.0% |
| Others | 1 | 0.5% |
| Employment Status | ||
| Employed full-time | 64 | 31.8% |
| Employed part-time | 10 | 5.0% |
| Freelancer | 4 | 2.0% |
| Housewife | 4 | 2.0% |
| Not working | 4 | 2.0% |
| Retired | 22 | 10.9% |
| Student | 33 | 16.4% |
| Unemployed | 60 | 29.9% |
| Monthly family income | ||
| Less than 6000 SAR | 63 | 31.3% |
| 6000–15,000 SAR | 68 | 33.8% |
| 15,001–30,000 SAR | 30 | 14.9% |
| 30,001–50,000 SAR | 6 | 3.0% |
| More than 50,000 SAR | 1 | 0.5% |
| Prefer not to say | 33 | 16.4% |
| Province | ||
| Makkah province | 83 | 41.3% |
| Riyadh province | 56 | 27.9% |
| Northern province | 33 | 16.4% |
| Eastern province | 15 | 7.5% |
| Madinah province | 7 | 3.5% |
| Southern province | 7 | 3.5% |
| Type of cancer | ||
| Breast cancer | 65 | 32.3% |
| Lymphoma | 36 | 17.9% |
| Colorectal cancer | 15 | 7.5% |
| Renal carcinoma | 6 | 3.0% |
| Prostate cancer | 5 | 2.5% |
| Lung cancer | 5 | 2.5% |
| Other cancer (Not specified) | 69 | 34.3% |
| Studied Participants (N = 201) | ||
|---|---|---|
| No | % | |
| What potential barriers would prevent you from participating in a clinical trial? | ||
| Fear of side effects | 166 | 82.6% |
| Fear of experimental treatment | 115 | 57.2% |
| Lack of information | 86 | 42.8% |
| Fear of receiving a placebo | 83 | 41.3% |
| Lack of trust in healthcare providers | 49 | 24.4% |
| Concerns about privacy | 48 | 23.9% |
| Financial constraints | 38 | 18.9% |
| Time constraints | 21 | 10.4% |
| Family or cultural beliefs | 18 | 9.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, M.; Alsolami, L.; AlHarbi, S.; Alagha, A.; Alqurashi, L.; Badr, R.; Almatrafi, N.; Aladwani, G.; Alotaibi, A.; Shahbar, A.; et al. Clinical Trial Awareness, Perceptions, and Participation Among Cancer Patients in Saudi Arabia. Healthcare 2025, 13, 1044. https://doi.org/10.3390/healthcare13091044
Alotaibi M, Alsolami L, AlHarbi S, Alagha A, Alqurashi L, Badr R, Almatrafi N, Aladwani G, Alotaibi A, Shahbar A, et al. Clinical Trial Awareness, Perceptions, and Participation Among Cancer Patients in Saudi Arabia. Healthcare. 2025; 13(9):1044. https://doi.org/10.3390/healthcare13091044
Chicago/Turabian StyleAlotaibi, Maryam, Laila Alsolami, Sarah AlHarbi, Amal Alagha, Lina Alqurashi, Rahaf Badr, Nouf Almatrafi, Ghada Aladwani, Amal Alotaibi, Alaa Shahbar, and et al. 2025. "Clinical Trial Awareness, Perceptions, and Participation Among Cancer Patients in Saudi Arabia" Healthcare 13, no. 9: 1044. https://doi.org/10.3390/healthcare13091044
APA StyleAlotaibi, M., Alsolami, L., AlHarbi, S., Alagha, A., Alqurashi, L., Badr, R., Almatrafi, N., Aladwani, G., Alotaibi, A., Shahbar, A., & Alnuhait, M. (2025). Clinical Trial Awareness, Perceptions, and Participation Among Cancer Patients in Saudi Arabia. Healthcare, 13(9), 1044. https://doi.org/10.3390/healthcare13091044






