Abstract
In the contemporary context of health challenges, the focus on physical health has become a social and individual priority. Within this framework, body composition emerges as one of the key determinants of physical health, with deviations from normal body composition being associated with numerous pathological conditions that can lead to serious health issues. Consequently, there is an urgent need to synthesize the available knowledge and increase awareness regarding healthy body composition and the factors that shape its components. This narrative review aims to summarize the knowledge regarding the main components of body composition and the key factors that influence their development. The fundamental morphological characteristics and functions of the primary components of body composition—including adipose tissue, muscle mass, and bone tissue—are addressed. Furthermore, the available methods for assessing body composition are outlined. The role of three key factors that influence body composition is outlined, including, but not limited to, physical activity, sleep quality, and stress levels. Additionally, hormonal fluctuations that determine body composition in relation to the variability of these factors are discussed. The review provides evidence-based information that will be valuable both for disease prevention related to non-communicable diseases and for the promotion of health strategies aimed at long-term physical well-being.
1. Introduction
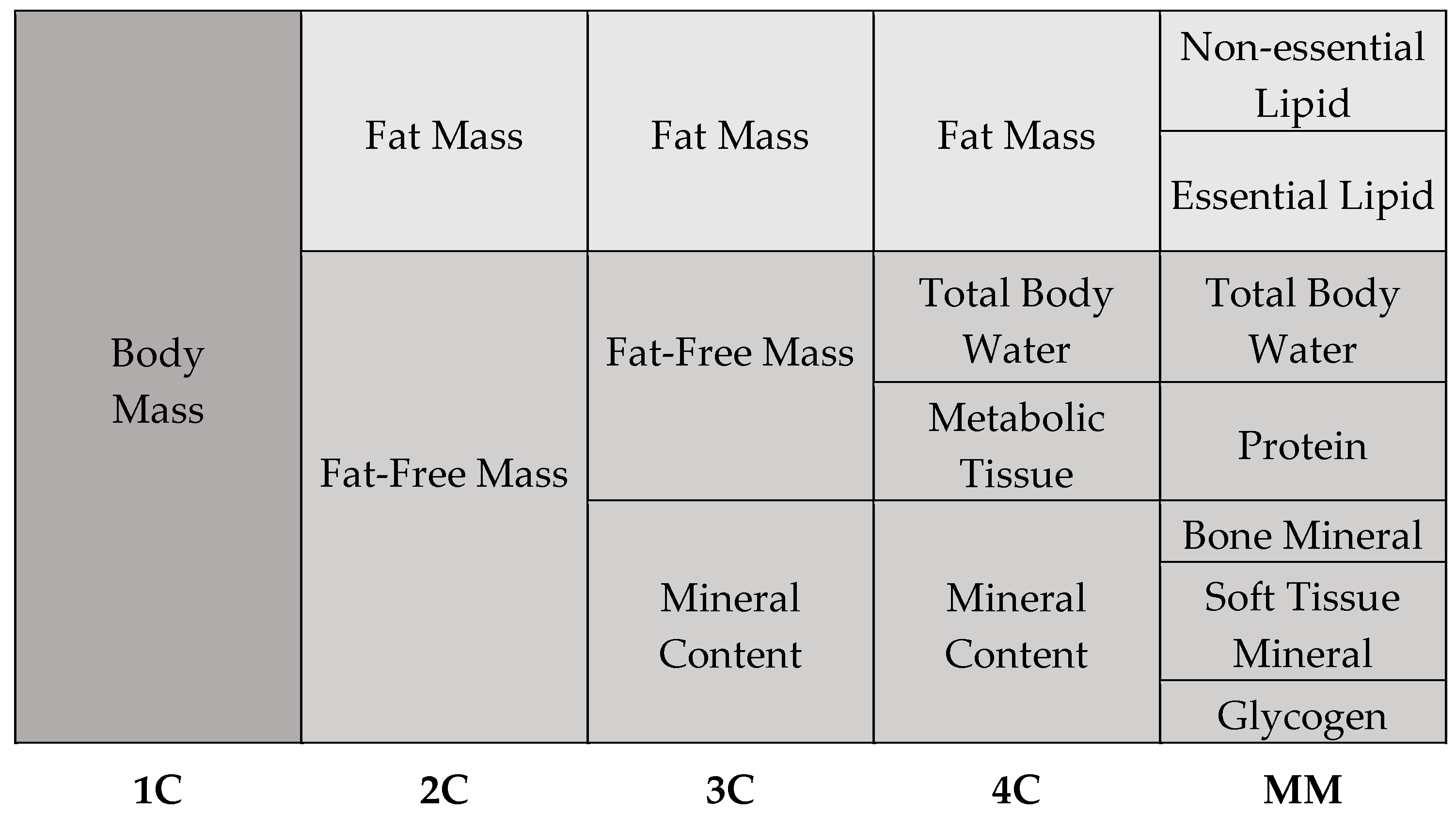
The human body is an extremely complex and yet highly organized structure. Its organization follows a hierarchical structure, starting with the atoms, through the cells, to the tissues and organs. The selected components of the human organism form a heterogeneous body composition. With regard to the chemical composition of the body, five models of body composition are distinguished, including one-compartment, two-compartment, three-compartment, four-compartment, and multi-compartment models (Figure 1) [1,2].
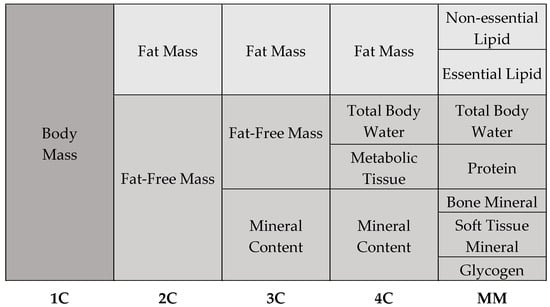
Figure 1.
Types of body composition models.
The one-compartment (1C) model provides the foundation for body composition analysis, as it considers body mass as a homogenous whole without discriminating between its many components. According to this idea, the human body is considered as a single mass that includes all tissues and chemicals, regardless of their individual characteristics [1,3]. To create a more accurate picture of body composition, more complex models are used, such as the two-compartment (2C) model. This model assumes that the human body consists of fat-free mass and adipose tissue. Fat-free mass includes water, muscle, bone tissue, and internal organs, while fat mass consists of total and visceral fat. The three-compartment model (3C) extends to the 2C model and is more complex and precise. This model divides body composition into fat mass, fat-free mass, and bone minerals. An even more detailed subdivision than the 3C model is the four-compartment model (4C). This model is very precise and enables detailed differentiation of body composition. It assumes that the human body is made up of fat mass, water, metabolic tissue, and bone minerals. According to current knowledge, the 4C model is considered the “gold standard” of body composition models [1,3]. The last and most complex model of body composition is the multicompartment model (MM). Despite the fact that MM models provide precise body composition measurements, their utilization is constrained by a number of factors. These include the absence of suitable devices, the high costs associated with their use, and the exposure to radiation that they entail [1].
Body composition plays a fundamental role in maintaining health, although its full significance often only becomes apparent in pathological conditions [4]. Each component of body mass has a specific morphological structure and fulfils different functions in the human body. Maintaining appropriate levels of each component and the correct proportions between them is crucial for health. Adipose tissue, for example, fulfils several important functions, including metabolic and endocrinological roles, such as the release of hormones that regulate energy expenditure [5]. However, when excessive amounts of adipose tissue accumulate, as is the case with obesity, it has a detrimental effect on health [6]. Obesity has been linked to numerous health conditions, including neurological deficits [7], insulin resistance, cardiovascular disease [8], and cancer [9], which can contribute to a significant reduction in life expectancy. In addition, reduced muscle mass resulting from an imbalance between catabolic and anabolic processes leads to muscle atrophy [10]. Later in life, this condition can contribute to reduced functional capacity, disability, reduced quality of life, and increased mortality [11,12]. Furthermore, reduced bone mass can lead to osteoporosis, a condition characterized by low bone mineral density and accelerated bone loss [13]. Such a condition is a major risk factor for fractures and falls [14]. Obesity, muscle wasting, and osteoporosis are all components of changes in body composition that lead to serious health consequences and place a significant burden on individual health and healthcare systems [15].
Synthesizing knowledge and increasing awareness within the community regarding body composition is therefore warranted. It is equally important to identify pathways that help maintain body composition within healthy ranges or improve pathological changes in body composition. The present article addresses the topic of body composition, its importance for health, and the essential factors that determine it, including physical activity, sleep, and stress. In-depth analysis is provided of the individual components of body composition, along with an overview of the methods commonly used for their assessment. The review also explores the key morphological characteristics and functions of each component, providing essential insights into their interactions and impact on overall health. The article is also notable for emphasizing not only the physiological aspects of body composition, but also modifiable factors such as physical activity, sleep, and stress that individuals can control and actively influence. While these factors are often discussed individually in existing reviews [16,17], this review goes further by examining how they collectively affect body composition. The manuscript is also enriched by analyzing the hormonal influences on body composition in relation to the variability of these factors, which is an intriguing but often overlooked aspect of the current literature.
2. Components of Body Composition
The most important and most abundant component of the human organism is water. The body consists of 50–60% water, with the proportion in men being around 60%, in women 50–55%, and in children 75% of the total body mass. Differences in water content can also be observed between the distinct types of tissue. In muscle tissue, the water content is around 76%, in fat-free mass 73%, and in adipose tissue around 10% [18]. The total water content in the human body is the sum of intracellular and extracellular water and can be expressed as a percentage of body mass or in kilograms. The considerable amount of water in the human body enables it to perform a variety of vital functions. The most important functions include transporting nutrients in the blood and interstitial fluid, maintaining a constant body temperature, lubricating the mouth, joints, and eyes, detoxification, facilitating the function of all organs, and supporting cell metabolism [19]. In addition, water is the main component of cells and is responsible for all processes in the body. Given the numerous functions of water in the human organism, it is crucial to maintain an adequate water balance to ensure an optimal environment for the proper functioning of the body’s organs. Importantly, neglecting the body’s water balance can lead to many negative health consequences, such as fatigue, weakness, dizziness, muscle cramps, rapid breathing, dry skin and mouth, thirst, and palpitations [20].
Alongside water, muscle mass makes up a considerable proportion of body composition. It refers to the total amount of muscle tissue in the body, including skeletal muscle, smooth muscle, and heart muscle. Skeletal muscles play a fundamental role in the human body and account for about 40% of total body mass. They consist mainly of water (75%), protein (20%), and other substances, including minerals, fats, carbohydrates, and inorganic salts (5%) [21]. The content of muscle mass varies according to age and sex. It is assumed that skeletal muscle mass accounts for around 38% of body mass in men and 30% in women [17]. Skeletal muscles fulfil a variety of essential functions in the human body. They are primarily involved in the generation of movement and strength and produce heat, which helps to maintain optimal body temperature. In addition, they play a role in maintaining posture and balance and support the efficient functioning of many processes, including respiration and digestion [22]. Notably, insufficient muscle mass or its loss is associated with slower and poorer wound healing, longer recovery time from illness, lower quality of life, lower basal metabolic rate, and higher healthcare costs [23].
Skeletal muscles are a fundamental component of body composition and fulfil many principal functions in the human body. However, in order for them to perform their basic locomotor function, the presence of bone is required, as bones serve as attachment points for skeletal muscles. Bone tissue makes up about 15% of the total body mass and is the most durable and rigid tissue in the human body. Its structure mainly comprises the cortical bone, which is denser; the trabecular bone, which is more porous; and the bone marrow cavity [24]. Beyond enabling movement, bones provide structural support, protect the internal organs, serve as a reservoir for minerals such as calcium and phosphate, and are responsible for the production of blood cells [24]. Additionally, scientific literature shows that males have larger and stronger bones compared to females [25].
So far, the focus of the discussion on body composition has been on the components of fat-free mass. However, it is important to know that fat mass is a significant part of overall body composition. In the human body, adipose tissue is a complex and heterogeneous structure consisting mainly of fat cells and a small proportion of water [26]. Two main types of adipose tissue are distinguished in the literature: brown and white [27]. Brown adipose tissue is characterized by rich vascularization, and its main function is thermogenesis. White adipose tissue, on the other hand, contains a small number of mitochondria and is found in many areas of the human body. There are two types of white adipose tissue: visceral adipose tissue and subcutaneous adipose tissue. Visceral fat surrounds the internal organs, while subcutaneous fat is found under the skin. In the human body, white adipose tissue functions as an energy reservoir, acts as a heat insulator, and plays an important endocrinological role [27]. This means that fat cells secrete certain hormonal factors in response to physiological signals, which regulate various processes such as appetite and glucose homeostasis. Notably, while a certain amount of adipose tissue is necessary for health, excessive accumulation contributes to the development of various health problems [28].
3. Methods of Body Composition Analysis and Assessment
There are currently numerous techniques for analyzing body composition, which can be divided into objective and subjective methods. Objective methods are characterized by high measurement accuracy and use advanced technologies that require special equipment. The most commonly used methods include dual-energy X-ray absorptiometry (DEXA), bioelectrical impedance analysis (BIA), magnetic resonance imaging (MRI), ultrasonography, air displacement plethysmography, and hydrostatic weighing [4,29]. DEXA is considered the gold standard for body composition assessment. This technique is a proven body composition analysis tool that provides precise and standardized measurements of tissue parameters [30]. Due to the excessive costs of DEXA, the BIA method is more commonly used in clinical practice. BIA is a modern, non-invasive, painless, and reliable method of body composition analysis that can serve as a substitute for DEXA [31]. The BIA method works based on the electrical properties of tissues and involves measuring the total electrical resistance of the body, which consists of resistance (passive opposition) and reactance (active opposition) [32]. Notably, tissues in the human body exhibit individual hydration levels and different conductive properties, which translate into varying electrical resistance. Adipose tissue is less hydrated and exhibits no capacitive resistance (reactance), whereas it possesses resistance, or active electrical opposition. In contrast, tissues that are well-hydrated, such as muscle tissue, exhibit reactance. Accurate estimation of total body water content, and consequently assessment of other body composition components, is enabled by understanding these differences in tissue characteristics.
Due to accessibility issues for the above methods, anthropometric measurements are increasingly being used to assess body composition. Anthropometry is a subjective technique for measuring body composition that focuses on the study of the physical dimensions and proportions of the human body. It is a non-invasive method that provides reliable and valuable information about nutritional status and body fat distribution [33]. Commonly used anthropometric measurements include height, body weight, waist circumference (WC), hip circumference (HC), body mass index (BMI), waist-to-hip ratio (WHR), waist-to-height ratio (WHtR), and skinfold thickness measurements. In addition, in response to the growing problem of obesity, derived anthropometric indices have been developed to serve as predictors of fat mass [34]. Among the most widely used are the relative body fat index (RFM), the body roundness index (BRI), the body shape index (ABSI) and the body adiposity index (BAI) [35]. A set of the most common anthropometric indicators along with their characteristics is presented in Table 1.

Table 1.
The most common anthropometric indicators.
4. Key Determinants of Body Composition
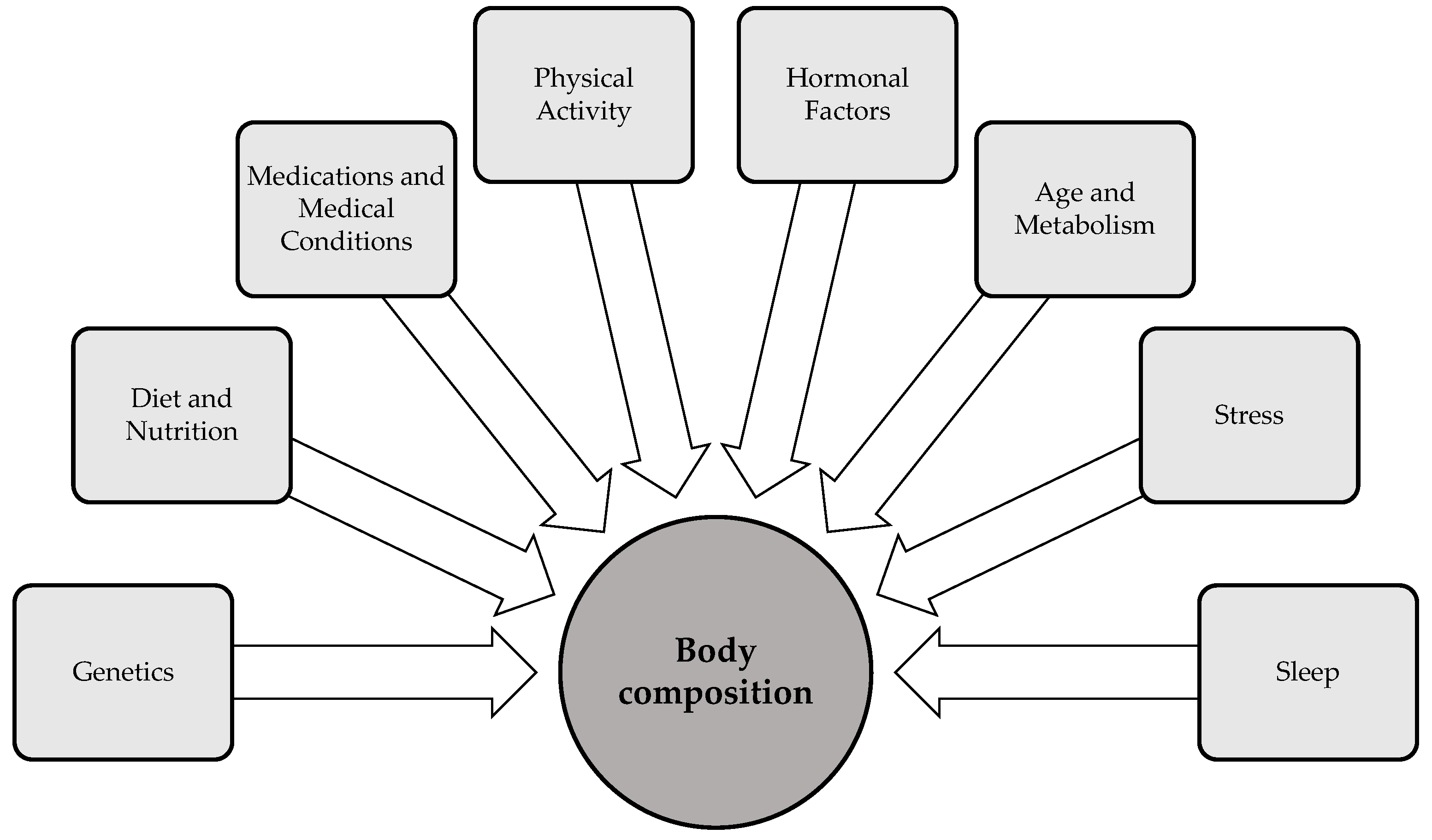
Body composition is influenced by a variety of factors that interact in complex ways to shape an individual’s health status. Key factors—including genetics, age, gender, physical activity, sleep, diet, and stress—significantly impact body fat distribution, muscle mass, and bone density [50] (Figure 2). Hormonal fluctuations, particularly those involved in metabolic regulation, also play a critical role in shaping body composition by modulating fat storage, muscle development, and energy expenditure. Additionally, environmental factors, such as exposure to endocrine-disrupting chemicals, have the capacity to modulate body composition by altering hormonal pathways and metabolic processes. The following section examines important factors that influence body composition, in particular physical activity, sleep, and stress. While these factors are not the primary determinants of body composition, they are modifiable variables that individuals can actively regulate to influence their body composition.
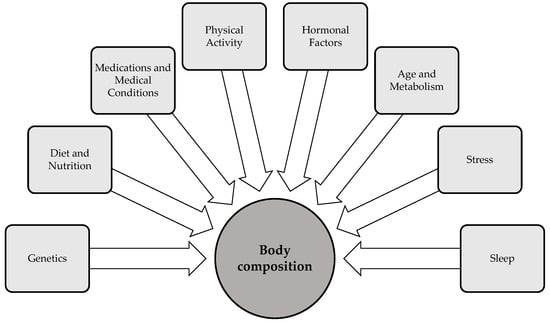
Figure 2.
Determinants of body composition [50,51,52].
4.1. The Role of Physical Activity in Determining Body Composition
Physical activity plays a key role in building muscle mass, and its effect is well documented in the literature. According to the available scientific data, regular physical activity contributes to an increase in the rate of muscle protein synthesis [53], an improvement in muscle strength and mass [54], and an improvement in cardiovascular and respiratory endurance and functional abilities such as walking speed [55]. Exercise also increases muscle cell sensitivity to insulin, improves glucose tolerance, and supports glycogen storage [56,57]. Moreover, an association has been demonstrated between physical activity and improved mitochondrial function, increased metabolic flexibility, and overall better muscle health. These factors collectively contribute to better metabolic regulation and overall physical health.
In addition to the well-known role that physical activity plays in modulating muscle mass, it is also fundamental in shaping the morphology and function of adipose tissue. Scientific reports indicate that regular physical activity promotes weight loss by reducing adipose tissue and adipocyte size [58]. It also enhances lipolysis and insulin sensitivity within adipose tissue [59], while decreasing lipid storage, lipogenesis, and inflammation. Moreover, consistent physical activity improves the profile of inflammatory markers [60], leading to a reduction in overall body mass and contributing to better metabolic health.
In the context of body composition, physical activity plays a key role not only in the regulation of muscle and adipose tissue content, but also in bone remodeling processes. The scientific literature shows that physical activity improves the mineralization of bones [61] and increases their mechanical strength [62]. This phenomenon results from the fact that the regulation of bone turnover is closely linked to the mechanical stress to which the bones are subjected during physical activity. This mechanical stress is a crucial factor that influences the activity of bone cells, which convert mechanical stimuli into intercellular signals and trigger a cascade of molecular events that lead to increased bone turnover. Regular engagement in physical activity also increases the proliferation of chondrocytes and enhances their differentiation, as well as reduces bone resorption [63]. In the context of bone health, it is noteworthy that physical activity has a positive influence on bone tissue at different ages. In youth, physical activity promotes the growth plates of long bones, while in old age it plays a crucial role in the prevention of osteoporosis [64,65]. Importantly, certain types of physical activity, such as cycling and swimming, do not have a positive impact on bone mineral density. These activities, although beneficial for overall fitness, do not provide the same bone-loading stimulus as weight-bearing exercises, which are essential for maintaining or increasing bone mineral density, particularly in females [66]. In Table 2, the effects of physical activity on the components of body composition are summarized.

Table 2.
Effects of physical activity on body composition components.
4.2. The Role of Sleep in Determining Body Composition
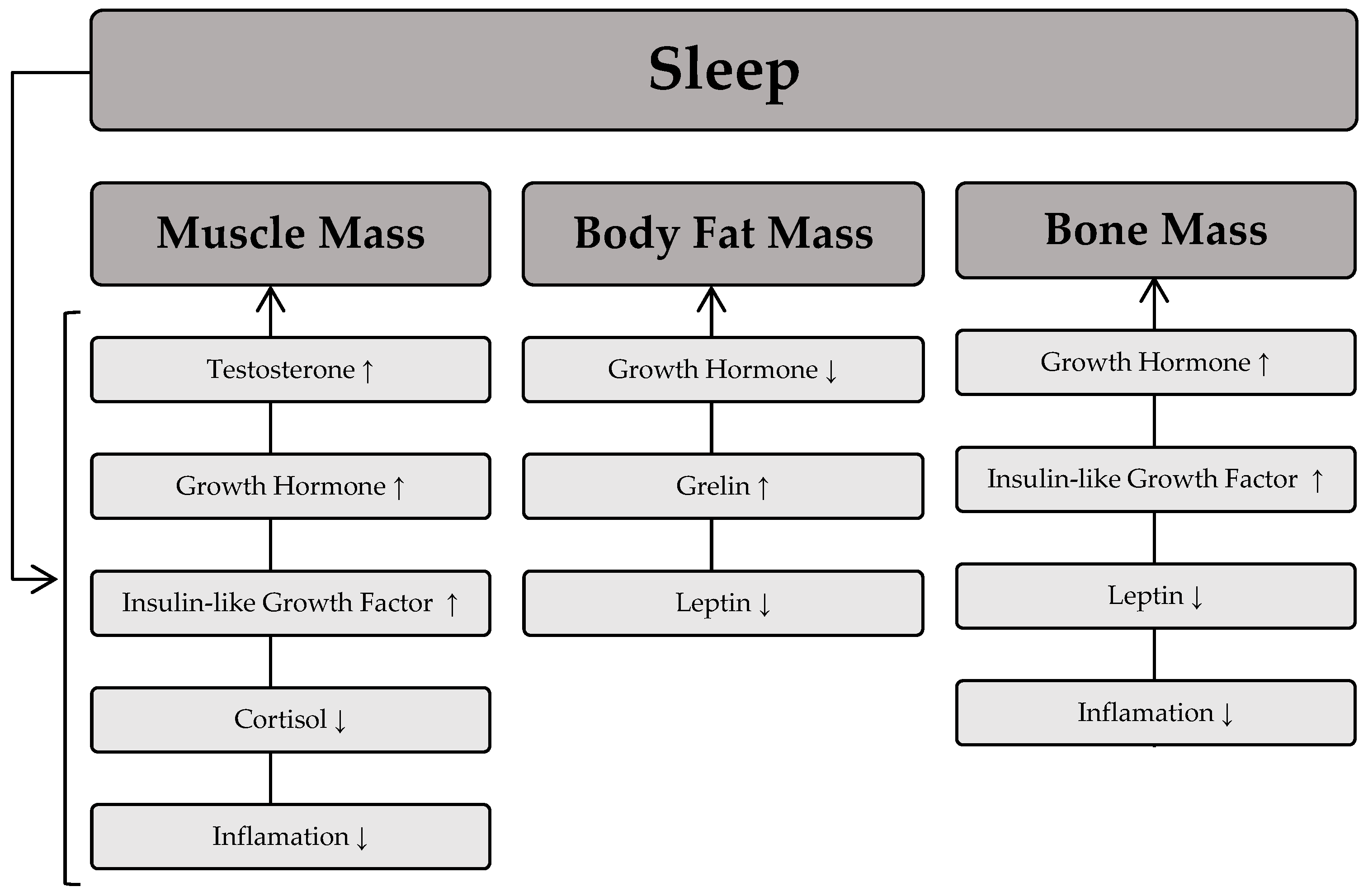
The sleep process is thought to play a significant role in the regulation of skeletal muscle mass [67]. This occurs through numerous pathways responsible for muscle protein synthesis and degradation. Remarkably, maintaining a stable level of muscle mass depends on the balance between anabolic and catabolic processes. This means that the equilibrium between protein synthesis and degradation processes will determine the maintenance of muscle mass; a dominance of protein synthesis processes will lead to muscle hypertrophy, whereas a predominance of protein degradation processes will cause muscle wasting. The scientific literature indicates that sleep deprivation is associated with lower muscle mass [68] and muscle strength [69]. Furthermore, shorter sleep duration is associated with greater muscle mass loss during weight loss [70]. The regulation of muscle mass during sleep is largely dependent on the secretion of hormones such as growth hormone, testosterone, and insulin-like growth factor (IGF-1), which have an anabolic effect and significantly influence muscle protein synthesis, ultimately leading to an increase in muscle mass. On the other hand, catabolic substances such as cortisol are released during sleep and contribute to a reduction in muscle mass. The available scientific evidence also suggests that poor sleep quality is associated with a slower decline in cortisol levels in the morning and later in the day, as well as elevated cortisol levels in the evening [71]. This phenomenon is related to the detrimental effect of cortisol on muscle mass due to its high activity in catabolic processes [72].
Beyond its influence on muscle mass, sleep also plays a crucial role in modulating body fat levels. It is generally believed that the development of excessive fat accumulation is primarily due to low levels of physical activity and poor dietary choices. The role of sleep in determining body fat is often underestimated, but numerous studies have confirmed an association between body fat indices and sleep deprivation [73,74]. In fact, sleep deprivation is associated with higher levels of body fat in different age groups [75,76]. It is hypothesized that the mechanism underlying the increased fat mass associated with sleep disturbance involves changes in endocrine function towards a hormonal pattern conducive to fat storage, such as elevated morning cortisol levels. Another mechanism linking sleep and excess fat accumulation is the regulation of food intake, which is modulated by two hormones: leptin and ghrelin [77]. Leptin is a hormone secreted by fat cells that signals the body’s energy status to the brain. In contrast, ghrelin, which is mainly secreted by the stomach, stimulates appetite and leads to increased food intake in individuals. The available scientific literature also suggests that sleep deprivation is associated with increased ghrelin levels [78]. Moreover, in contrast to the anabolic effect of growth hormone on most human tissues, its effect on adipose tissue promotes catabolic processes, thereby increasing lipolysis [79].
In addition to its effects on muscle mass and adipose tissue, sleep has been shown to be a crucial factor in the regulation of bone turnover. According to recent scientific reports, both long and short sleep duration are associated with low bone mineral density and osteoporosis [80,81]. One of the mechanisms underlying the effect of sleep on bone health is hyperactivity of the sympathetic nervous system. In addition, short sleep duration is associated with an inflammatory state that contributes to low bone mass. Chronic activation of the sympathetic nervous system is also thought to increase levels of leptin in the blood, leading to a reduction in bone mass, particularly cortical bone thickness [82]. On the other hand, good quality sleep promotes increased secretion of growth hormone, which increases the proliferation and differentiation of chondrocytes, thereby benefiting bone health. Figure 3 depicts the relationship between sleep and the influence of specific hormones on body composition components.
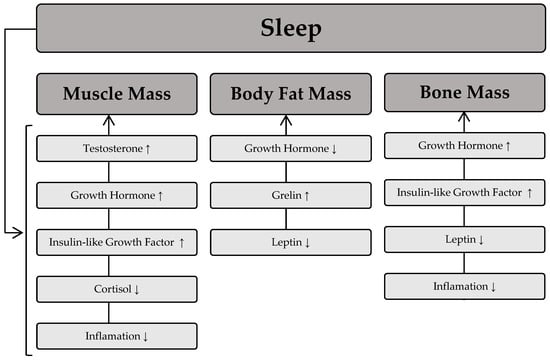
Figure 3.
Relationship between sleep and the influence of specific hormones on body composition components. Sleep process → induces fluctuations in specific hormones, which then influence body composition components. An upward arrow (↑) denotes an increase in the respective body composition components as a result of the hormone, while a downward arrow (↓) indicates a decrease in the respective body composition component due to the hormone.
4.3. The Role of Stress Level in Determining Body Composition
Stress is a physiological and psychological reaction to perceived challenges or threats that disrupt homeostasis [83]. It can be divided into eustress (positive) and distress (negative). Eustress motivates action and mobilizes resources, while distress depletes resources and is detrimental to health [83]. Excessive stress exceeds the body’s ability to adapt and leads to chronic tension, health problems and reduced quality of life. Its characteristic features include a long duration, high intensity, and the lack of effective coping mechanisms that prevent the body from restoring its balance [83].
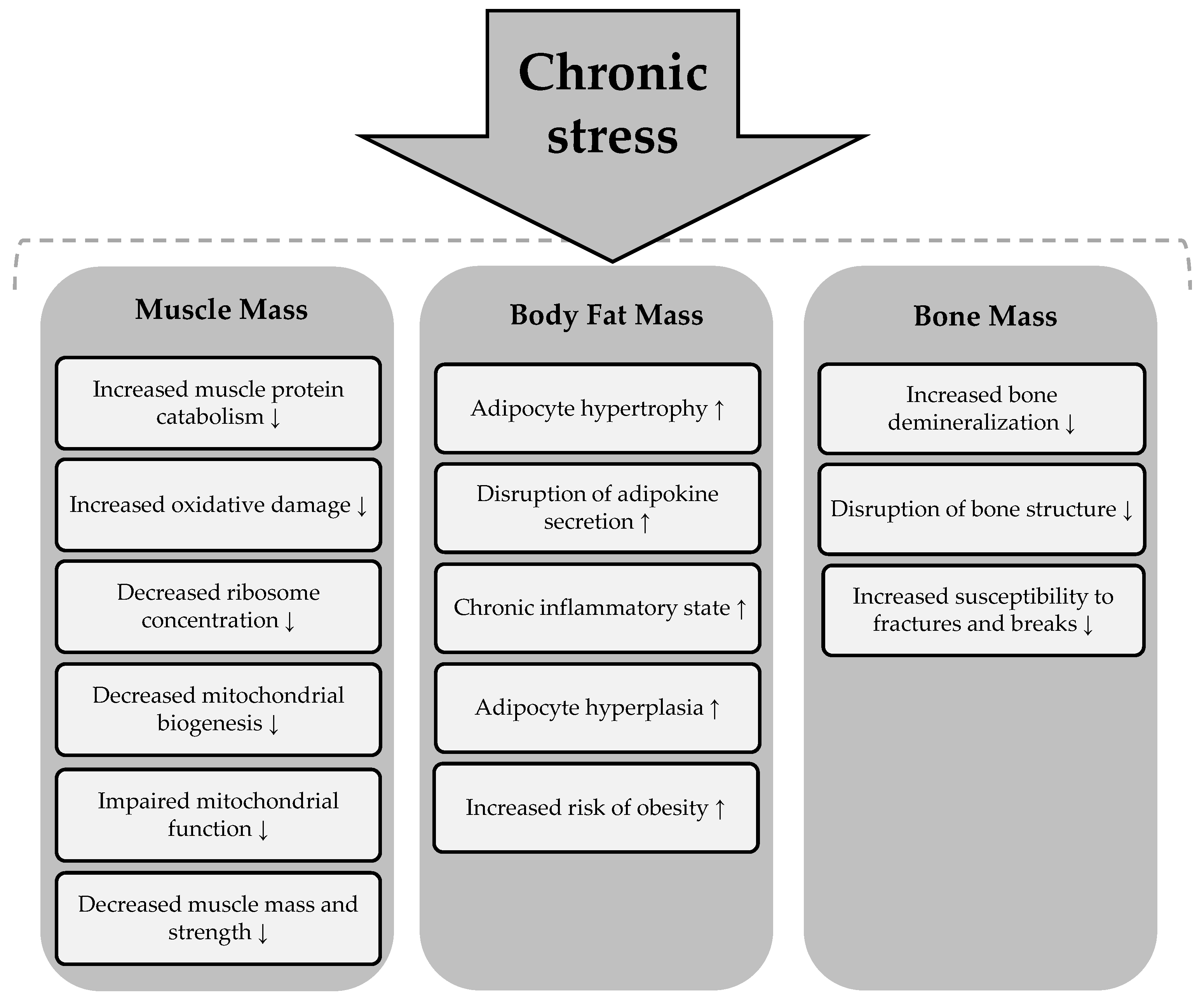
The literature suggests that prolonged exposure to high levels of stress induces a number of detrimental changes in skeletal muscle morphology and architecture. This occurs because when an individual experiences chronic stress, the hypothalamic–pituitary–adrenal (HPA) axis becomes overactive, leading to excessive cortisol secretion and a systemic inflammatory state. Such conditions promote catabolism muscle protein, [84] induce oxidative damage, reduce ribosome concentration, decrease mitochondrial biogenesis, and impair mitochondrial function [85]. As a result of these changes, a significant decrease in muscle mass and strength is observed, as well as the loss of normal movement patterns [84].
Chronic exposure to high levels of stress not only affects skeletal muscle but also has several adverse effects on adipose tissue. Prolonged stress is responsible for adipocyte hypertrophy and hyperplasia, disruption of adipokine secretion, and fat accumulation, particularly in the abdominal region [86]. Furthermore, long-term exposure to stressors is associated with a chronic inflammatory state characterized by elevated levels of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6. Scientific reports also suggest that the prolonged hypercortisolemia associated with chronic stress is associated with an increased risk of obesity [87].
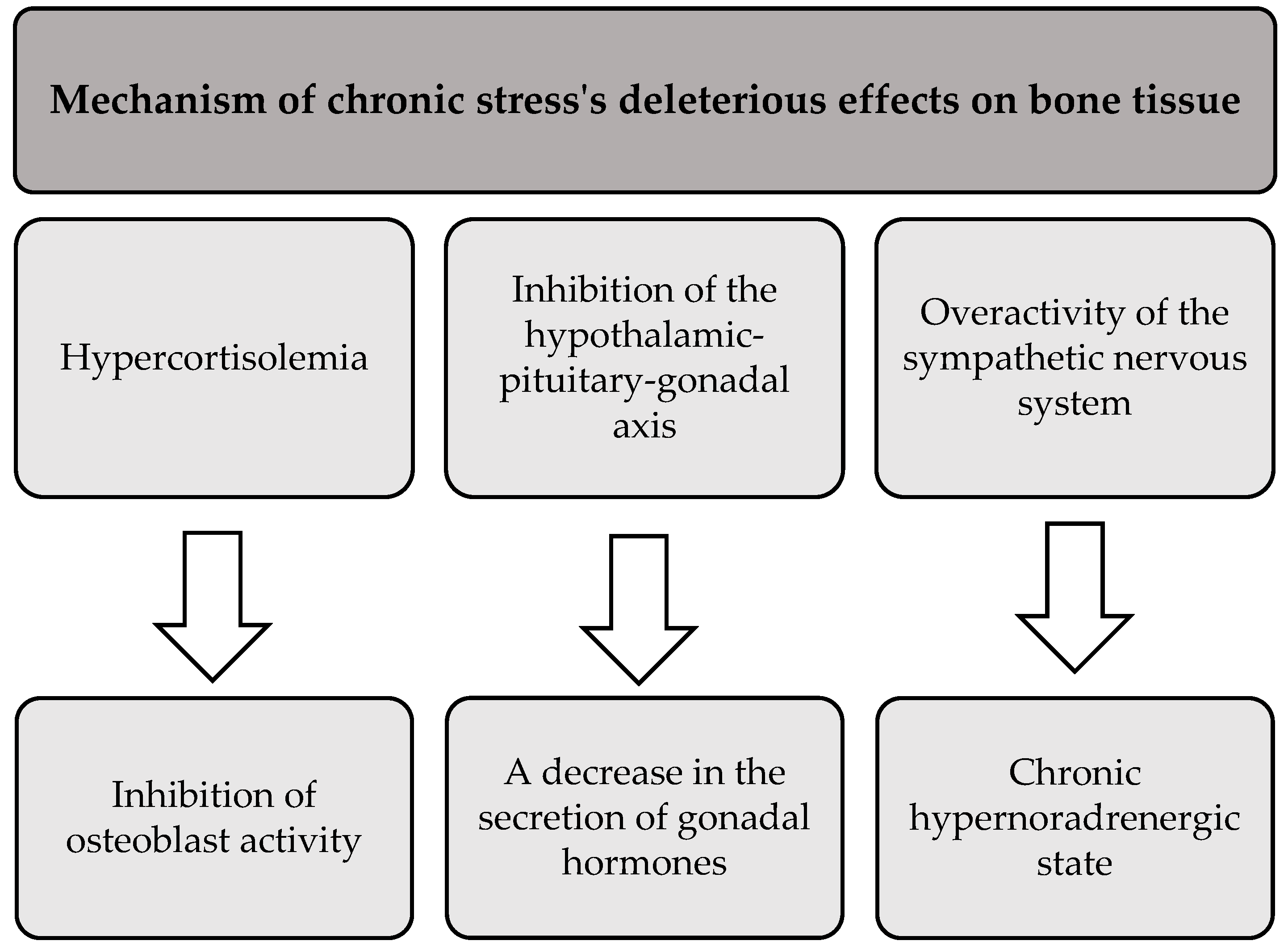
In addition to its negative effects on skeletal muscle and adipose tissue, prolonged stress also adversely affects bone health. The literature suggests that chronic exposure to stress leads to bone demineralization and disruption of bone architecture. This results in reduced bone mineral density and increased susceptibility to fractures and breaks [88]. Three main mechanisms that mediate the deleterious effects of chronic stress on bone tissue are presented in Figure 4 [88,89].
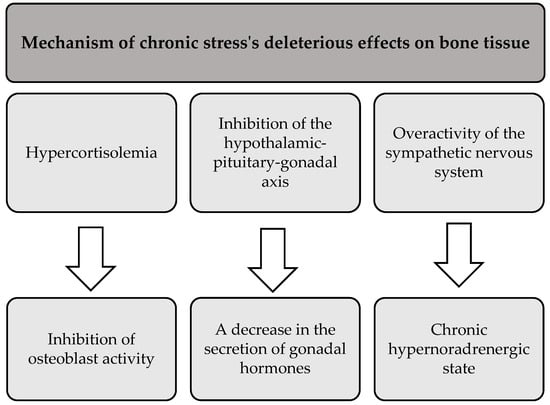
Figure 4.
The processes underlying the detrimental effects of chronic stress on bone tissue.
Chronic stress can lead to disturbances in all body composition parameters, including adipose, muscle, and bone tissue. Its detrimental effects are reflected in overall health, contributing to an increased risk of various health conditions [90]. The summary of the effects of chronic stress on body composition parameters is presented in Figure 5.
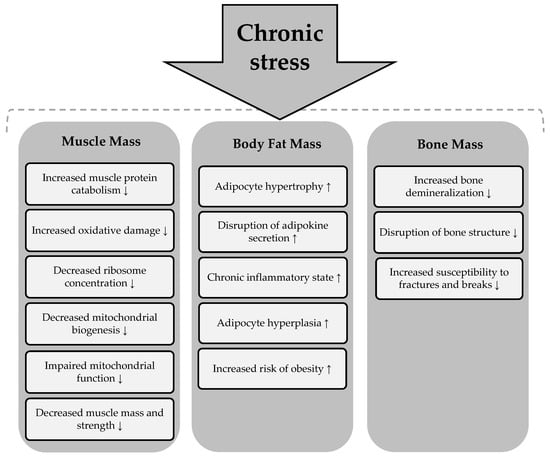
Figure 5.
Effects of chronic stress on body composition components. Chronic stress → induces a process or condition that subsequently affects the components of body composition. An upward arrow (↑) indicates an increase in the corresponding body composition component as a result of the process, while a downward arrow (↓) signifies a decrease in the respective body composition component due to the process.
5. The Interplay Between Physical Activity, Sleep, and Stress
The interactions between physical activity, sleep, and stress have attracted increasing scientific interest due to their joint influence on physiological homeostasis and general health. All three factors that contribute to the regulation of body composition exhibit complex mutual interactions.
Physical activity extends beyond its impact on overall health and body composition to encompass a broad range of physiological processes, including the regulation of sleep. There is empirical evidence that regular physical activity contributes to improvements in sleep quality and duration [91,92] and is an effective non-pharmacological intervention for sleep disorders such as insomnia [93]. The underlying mechanisms involve modulation of neuroendocrine pathways, including upregulation of melatonin secretion, attenuation of stress-related physiological responses and improvement of mood. Together, these factors form a positive feedback loop in which improved sleep further facilitates adherence to physical activity and reinforces its beneficial effects [94].
Chronic stress, on the other hand, disrupts this balance by inducing widespread physiological dysregulation, particularly in sleep architecture. There is evidence of a strong inverse relationship between stress intensity and sleep parameters, with increased stress correlating with shortened sleep duration [95], impaired sleep quality [96], and increased incidence of insomnia [97]. The pathophysiological mechanisms underlying this association are multifactorial and include dysregulated activity of the hypothalamic–pituitary–adrenal axis, excessive cortisol secretion, and subsequent suppression of melatonin secretion [98]. Persistently elevated cortisol levels impair melatonin synthesis and disrupt the onset and consolidation of sleep [99]. Stress also increases sympathetic nervous system activity, marked by heightened noradrenaline and adrenaline release, which sustains physiological arousal and further impairs sleep regulation [98]. Together, these neurobiological changes contribute to sleep disorders and reinforce the harmful interplay between stress and sleep homeostasis.
Recent findings suggest that the interaction of physical activity and sleep could have a synergistic effect on body composition. While both factors contribute independently to the regulation of body weight and metabolic health, new studies have begun to investigate how their interaction may improve these outcomes. For instance, a randomized controlled trial by Jåbekk et al. examined the effects of a 10-week resistance training program combined with sleep education [100]. Participants who took part in both interventions showed a significantly greater reduction in fat mass than those who exercised alone, highlighting the added benefit of optimizing sleep alongside physical training. Similarly, Moradell et al. conducted an eight-month multicomponent intervention in older adults combining structured exercise with sleep quality monitoring [101]. The results demonstrated a 10% reduction in body fat percentage and a 47% improvement in sleep quality in the intervention group, suggesting that improvements in sleep may enhance the effects of physical activity on body composition. The results support the hypothesis that combining sleep behavior modification with physical activity may yield more positive changes in body composition than using either factor in isolation.
6. Future Directions
The necessity for additional longitudinal studies to enhance comprehension of the long-term effects of physical activity, sleep quality, and stress on body composition is the primary area requiring further research. While extant research has furnished valuable insights into the immediate effects of these factors on body composition, the long-term impacts remain less well understood. Longitudinal studies can assist in identifying patterns of change in body composition over extended periods, thereby revealing how lifestyle factors contribute to the maintenance or deterioration of health across various stages of life. The exploration of potential cumulative effects—such as prolonged periods of physical inactivity, poor sleep, or chronic stress potentially leading to more significant shifts in body composition—is a future avenue for research in this area.
The role of hormonal fluctuations and their complex interactions with lifestyle factors requires further exploration, for example through advanced biomarker profiling and omics technologies. Hormones such as cortisol, insulin, and leptin significantly impact body composition [102]; however, the mechanisms underlying these interactions could be explored in greater depth. The application of advanced omics approaches has the potential to facilitate the identification of biomarkers that reflect these hormonal changes and their effects on fat, muscle, and bone tissue.
Moreover, it is becoming increasingly evident that environmental influences and gut microbiota are significant determinants of body composition [103]. Environmental exposures, such as endocrine disruptors, may alter hormonal regulation, while gut microbiota influences metabolism and fat storage. Investigating these complex interactions in future studies will provide valuable information in the field of body composition determinants. This will facilitate the optimization of body composition and the prevention of metabolic diseases.
Finally, it is also recommended that future research endeavors concentrate on the exploration of the dynamic interplay between body composition parameters, and the way their alterations contribute to the onset and progression of conditions such as cardiovascular disease, diabetes, and metabolic syndrome. Specifically, it would be valuable to investigate the role of sarcopenic obesity, where a loss of muscle mass occurs alongside an increase in fat mass, and how this condition influences metabolic dysfunction. This area represents an interesting direction for future exploration.
7. Conclusions
Body composition is inherently influenced by various factors, with physical activity, sleep, and stress being among those most amenable to individual regulation. These factors represent modifiable determinants that individuals can actively manage. Regular physical activity enhances muscle mass and strength, reduces adiposity, and improves bone mineral density and overall skeletal health. Adequate sleep is essential for maintaining optimal body composition, as sleep deprivation disrupts metabolic processes, increases fat accumulation (particularly in the abdominal region) and impairs muscle recovery. Chronic stress induces prolonged elevations in cortisol, contributing to increased visceral fat storage and muscle catabolism, while also influencing eating behavior and promoting weight gain. It is evident that these three modifiable factors play an important role in determining body composition, with modifying them being instrumental in achieving improvements in body composition, consequently enhancing overall health and preventing various diseases, notably non-communicable diseases.
All things considered, addressing these factors is essential, as interventions aimed at increasing physical activity, enhancing sleep quality, and mitigating stress can lead to significant improvements in body composition, thereby contributing to overall health optimization. These interventions also can help prevent or manage chronic conditions, improve mental well-being, and enhance quality of life, making them critical components of a holistic approach to health.
Author Contributions
Conceptualization, K.M., W.K. and M.K.; methodology, K.M., K.K. and W.K.; software, M.K. and J.B.; validation, K.M., J.B. and K.K.; resources, K.M. and W.K.; data curation, K.M. and J.B.; writing-original draft preparation, K.M., M.K. and K.K.; writing-review and editing, K.M. and M.K.; visualization, K.M. and W.K.; supervision, M.K., J.B. and K.K.; project administration, K.M. and M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kuriyan, R. Body composition techniques. Indian J. Med. Res. 2018, 148, 648–658. [Google Scholar] [CrossRef]
- Ellis, K.J. Human body composition: In vivo methods. Physiol. Rev. 2000, 80, 649–680. [Google Scholar] [CrossRef] [PubMed]
- Toomey, C.M.; Cremona, A.; Hughes, K.; Norton, C.; Jakeman, P. A Review of Body Composition Measurement in the Assessment of Health. Top. Clin. Nutr. 2015, 30, 16–32. [Google Scholar] [CrossRef]
- Andreoli, A.; Garaci, F.; Cafarelli, F.P.; Guglielmi, G. Body composition in clinical practice. Eur. J. Radiol. 2016, 85, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, S.; Zhang, C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare 2022, 10, 1616. [Google Scholar] [CrossRef]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef]
- Welsh, A.; Hammad, M.; Piña, I.L.; Kulinski, J. Obesity and cardiovascular health. Eur. J. Prev. Cardiol. 2024, 31, 1026–1035. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol 2013, 45, 2121–2129. [Google Scholar] [CrossRef]
- Arango-Lopera, V.E.; Arroyo, P.; Gutiérrez-Robledo, L.M.; Pérez-Zepeda, M.U.; Cesari, M. Mortality as an adverse outcome of sarcopenia. J. Nutr. Health Aging 2013, 17, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Purcell, S.A.; Alish, C.; Pereira, S.L.; Deutz, N.E.; Heyland, D.K.; Goodpaster, B.H.; Tappenden, K.A.; Heymsfield, S.B. Implications of low muscle mass across the continuum of care: A narrative review. Ann. Med. 2018, 50, 675–693. [Google Scholar] [CrossRef]
- Colón, C.J.P.; Molina-Vicenty, I.L.; Frontera-Rodríguez, M.; García-Ferré, A.; Rivera, B.P.; Cintrón-Vélez, G.; Frontera-Rodríguez, S. Muscle and Bone Mass Loss in the Elderly Population: Advances in diagnosis and treatment. J. Biomed. 2018, 3, 40–49. [Google Scholar] [CrossRef]
- Möckel, L. Risk of falls in patients with low bone mineral density: Analysis of placebo arms from clinical trials. Z. für Gerontol. Und Geriatr. 2021, 54, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Goates, S.; Du, K.; Arensberg, M.B.; Gaillard, T.; Guralnik, J.; Pereira, S.L. Economic Impact of Hospitalizations in US Adults with Sarcopenia. J. Frailty Aging 2019, 8, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.; Brasil, I.; Monteiro, W.; Farinatti, P. Effects of physical activity on body mass and composition of school-age children and adolescents with overweight or obesity: Systematic review focusing on intervention characteristics. J. Bodyw. Mov. Ther. 2023, 33, 154–163. [Google Scholar] [CrossRef]
- Stich, F.M.; Huwiler, S.; D’Hulst, G.; Lustenberger, C. The Potential Role of Sleep in Promoting a Healthy Body Composition: Underlying Mechanisms Determining Muscle, Fat, and Bone Mass and Their Association with Sleep. Neuroendocrinology 2022, 112, 673–701. [Google Scholar] [CrossRef]
- Serra-Prat, M.; Lorenzo, I.; Palomera, E.; Ramírez, S.; Yébenes, J.C. Total Body Water and Intracellular Water Relationships with Muscle Strength, Frailty and Functional Performance in an Elderly Population. J. Nutr. Health Aging 2019, 23, 96–101. [Google Scholar] [CrossRef]
- Lorenzo, I.; Serra-Prat, M.; Yébenes, J.C. The Role of Water Homeostasis in Muscle Function and Frailty: A Review. Nutrients 2019, 11, 1857. [Google Scholar] [CrossRef]
- Shaheen, N.A.; Alqahtani, A.A.; Assiri, H.; Alkhodair, R.; Hussein, M.A. Public knowledge of dehydration and fluid intake practices: Variation by participants’ characteristics. BMC Public Health 2018, 18, 1346. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- McCuller, C.; Jessu, R.; Callahan, A.L. Physiology, Skeletal Muscle; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef]
- Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Zhou, H.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int. J. Biol. Sci. 2019, 15, 776–787. [Google Scholar] [CrossRef]
- Vilaca, T.; Eastell, R.; Schini, M. Osteoporosis in men. Lancet Diabetes Endocrinol. 2022, 10, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Matafome, P.; Seiça, R. Function and Dysfunction of Adipose Tissue. Adv. Neurobiol. 2017, 19, 3–31. [Google Scholar] [PubMed]
- Cohen, P.; Spiegelman, B.M. Cell biology of fat storage. Mol. Biol. Cell 2016, 27, 2523–2527. [Google Scholar] [CrossRef]
- Lemieux, I.; Després, J.P. Metabolic Syndrome: Past, Present and Future. Nutrients 2020, 12, 3501. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef]
- Imboden, M.T.; Welch, W.A.; Swartz, A.M.; Montoye, A.H.; Finch, H.W.; Harber, M.P.; Kaminsky, L.A. Reference standards for body fat measures using GE dual energy x-ray absorptiometry in Caucasian adults. PLoS ONE 2017, 12, e0175110. [Google Scholar] [CrossRef]
- McLester, C.N.; Nickerson, B.S.; Kliszczewicz, B.M.; McLester, J.R. Reliability and Agreement of Various InBody Body Composition Analyzers as Compared to Dual-Energy X-Ray Absorptiometry in Healthy Men and Women. J. Clin. Densitom. 2020, 23, 443–450. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, A.; Fernández-Lucas, M.; Teruel, J.L. The electrical basis of bioimpedance. Nefrologia 2012, 32, 133–135. [Google Scholar]
- Piqueras, P.; Ballester, A.; Durá-Gil, J.V.; Martinez-Hervas, S.; Redón, J.; Real, J.T. Anthropometric Indicators as a Tool for Diagnosis of Obesity and Other Health Risk Factors: A Literature Review. Front. Psychol. 2021, 12, 631179. [Google Scholar] [CrossRef]
- Adegoke, O.; Ozoh, O.B.; Odeniyi, I.A.; Bello, B.T.; Akinkugbe, A.O.; Ojo, O.O.; Agabi, O.P.; Okubadejo, N.U. Prevalence of obesity and an interrogation of the correlation between anthropometric indices and blood pressures in urban Lagos, Nigeria. Sci. Rep. 2021, 11, 3522. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, T. Anthropometric Indicators of Obesity. Are the New Indicators a Better Predictor of Body Fat Content Than BMI? J. Educ. Health Sport 2021, 11, 11–23. [Google Scholar] [CrossRef]
- Sweatt, K.; Garvey, W.T.; Martins, C. Strengths and Limitations of BMI in the Diagnosis of Obesity: What is the Path Forward? Curr. Obes. Rep. 2024, 13, 584–595. [Google Scholar] [CrossRef]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. Study: Waist-to-Hip Ratio Might Predict Mortality Better Than BMI. JAMA 2023, 330, 1515–1516. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, X.; Dahl, A.K.; Xu, W. Waist-to-height ratio is the best indicator for undiagnosed type 2 diabetes. Diabet. Med. 2013, 30, e201–e207. [Google Scholar] [CrossRef]
- Yoo, E.G. Waist-to-height ratio as a screening tool for obesity and cardiometabolic risk. Korean J. Pediatr. 2016, 59, 425–431. [Google Scholar] [CrossRef]
- Abdi Dezfouli, R.; Mohammadian Khonsari, N.; Hosseinpour, A.; Asadi, S.; Ejtahed, H.S.; Qorbani, M. Waist to height ratio as a simple tool for predicting mortality: A systematic review and meta-analysis. Int. J. Obes. 2023, 47, 1286–1301. [Google Scholar] [CrossRef]
- Woolcott, O.O.; Bergman, R.N. Relative fat mass (RFM) as a new estimator of whole-body fat percentage—A cross-sectional study in American adult individuals. Sci. Rep. 2018, 8, 10980. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-León, A.E.; Velarde, A.G.; Vidal-Salas, M.; Urquijo-Ruiz, L.G.; Caraveo-Gutiérrez, L.A.; Valencia, M.E. External validation of the relative fat mass (RFM) index in adults from north-west Mexico using different reference methods. PLoS ONE 2019, 14, e0226767. [Google Scholar] [CrossRef]
- Bozorgmanesh, M.; Sardarinia, M.; Hajsheikholeslami, F.; Azizi, F.; Hadaegh, F. CVD-predictive performances of “a body shape index” versus simple anthropometric measures: Tehran lipid and glucose study. Eur. J. Nutr. 2016, 55, 147–157. [Google Scholar] [CrossRef]
- Christakoudi, S.; Tsilidis, K.K.; Muller, D.C.; Freisling, H.; Weiderpass, E.; Overvad, K.; Söderberg, S.; Häggström, C.; Pischon, T.; Dahm, C.C.; et al. A Body Shape Index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: Results from a large European cohort. Sci. Rep. 2020, 10, 14541. [Google Scholar] [CrossRef] [PubMed]
- Gawrys, W.; Zyska, A.; Ślęzak, A. Anthropometric indicators and their applications for assessing population’s health condition. Hygeia Public Health 2017, 52, 41–47. [Google Scholar]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A better index of body adiposity. Obesity 2011, 19, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; Maeda, Y.; McDougall, A.; Peterson, C.M.; Ravussin, E.; et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity 2013, 21, 2264–2271. [Google Scholar] [CrossRef]
- Liu, B.; Liu, B.; Wu, G.; Yin, F. Relationship between body-roundness index and metabolic syndrome in type 2 diabetes. Diabetes Metab. Syndr. Obes. 2019, 12, 931–935. [Google Scholar] [CrossRef]
- Holmes, C.J.; Racette, S.B. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Ilich, J.Z. Nutritional and Behavioral Approaches to Body Composition and Low-Grade Chronic Inflammation Management for Older Adults in the Ordinary and COVID-19 Times. Nutrients 2020, 12, 3898. [Google Scholar] [CrossRef]
- Ghosh, S. A comparative analysis of dietary intake and body composition among two ethnically distinct tribal populations from India. Eur. J. Clin. Nutr. 2022, 76, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Phillips, S.M.; Libardi, C.A.; Vechin, F.C.; Lixandrão, M.E.; Jannig, P.R.; Costa, L.A.; Bacurau, A.V.; Snijders, T.; Parise, G.; et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J. Physiol. 2016, 594, 5209–5222. [Google Scholar] [CrossRef] [PubMed]
- Benito, P.J.; Cupeiro, R.; Ramos-Campo, D.J.; Alcaraz, P.E.; Rubio-Arias, J.Á. A Systematic Review with Meta-Analysis of the Effect of Resistance Training on Whole-Body Muscle Growth in Healthy Adult Males. Int. J. Environ. Res. Public Health 2020, 17, 1285. [Google Scholar] [CrossRef]
- Guadalupe-Grau, A.; Aznar-Laín, S.; Mañas, A.; Castellanos, J.; Alcázar, J.; Ara, I.; Mata, E.; Daimiel, R.; García-García, F.J. Short- and Long-Term Effects of Concurrent Strength and HIIT Training in Octogenarians with COPD. J. Aging Phys. Act. 2017, 25, 105–115. [Google Scholar] [CrossRef]
- Langleite, T.M.; Jensen, J.; Norheim, F.; Gulseth, H.L.; Tangen, D.S.; Kolnes, K.J.; Heck, A.; Storås, T.; Grøthe, G.; Dahl, M.A.; et al. Insulin sensitivity, body composition and adipose depots following 12 w combined endurance and strength training in dysglycemic and normoglycemic sedentary men. Arch. Physiol. Biochem. 2016, 122, 167–179. [Google Scholar] [CrossRef]
- Jelstad, S.; Ditta Valsdottir, T.; Johansen, E.I.; Jensen, J.R. Eight sessions of endurance training decrease fasting glucose and improve glucose tolerance in middle-aged overweight males. Arch. Physiol. Biochem. 2021, 127, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Swift, D.L.; Johannsen, N.M.; Lavie, C.J.; Earnest, C.P.; Church, T.S. The role of exercise and physical activity in weight loss and maintenance. Prog. Cardiovasc. Dis. 2014, 56, 441–447. [Google Scholar] [CrossRef]
- Riis, S.; Christensen, B.; Nellemann, B.; Møller, A.B.; Husted, A.S.; Pedersen, S.B.; Schwartz, T.W.; Jørgensen, J.O.L.; Jessen, N. Molecular adaptations in human subcutaneous adipose tissue after ten weeks of endurance exercise training in healthy males. J. Appl. Physiol. 2019, 126, 569–577. [Google Scholar] [CrossRef]
- Vella, C.A.; Allison, M.A.; Cushman, M.; Jenny, N.S.; Miles, M.P.; Larsen, B.; Lakoski, S.G.; Michos, E.D.; Blaha, M.J. Physical Activity and Adiposity-related Inflammation: The MESA. Med. Sci. Sports Exerc. 2017, 49, 915–921. [Google Scholar] [CrossRef]
- Moreira, L.D.F.; Oliveira, M.L.D.; Lirani-Galvão, A.P.; Marin-Mio, R.V.; Santos, R.N.D.; Lazaretti-Castro, M. Physical exercise and osteoporosis: Effects of different types of exercises on bone and physical function of postmenopausal women. Arq. Bras. Endocrinol. Metabol. 2014, 58, 514–522. [Google Scholar] [CrossRef]
- Gabel, L.; Macdonald, H.M.; Nettlefold, L.; McKay, H.A. Physical Activity, Sedentary Time, and Bone Strength From Childhood to Early Adulthood: A Mixed Longitudinal HR-pQCT study. J. Bone Miner. Res. 2017, 32, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Lassandro, G.; Chiarito, M.; Valente, F.; Ciaccia, L.; Giordano, P. How Physical Activity across the Lifespan Can Reduce the Impact of Bone Ageing: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 1862. [Google Scholar] [CrossRef] [PubMed]
- Mirtz, T.A.; Chandler, J.P.; Eyers, C.M. The effects of physical activity on the epiphyseal growth plates: A review of the literature on normal physiology and clinical implications. J. Clin. Med. Res. 2011, 12, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinheiro, M.B.; Oliveira, J.; Bauman, A.; Fairhall, N.; Kwok, W.; Sherrington, C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: A systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 150. [Google Scholar] [CrossRef]
- Abrahin, O.; Rodrigues, R.P.; Marçal, A.C.; Alves, E.A.; Figueiredo, R.C.; de Sousa, E.C. Swimming and cycling do not cause positive effects on bone mineral density: A systematic review. Rev. Bras. Reumatol. Engl. Ed. 2016, 56, 345–351. [Google Scholar] [CrossRef]
- Song, J.; Park, S.J.; Choi, S.; Han, M.; Cho, Y.; Oh, Y.H.; Park, S.M. Effect of changes in sleeping behavior on skeletal muscle and fat mass: A retrospective cohort study. BMC Public Health 2023, 23, 1879. [Google Scholar] [CrossRef]
- Nam, G.E.; Han, K.; Kim, D.H.; Lee, J.H.; Seo, W.H. Sleep duration is associated with body fat and muscle mass and waist-to-height ratio beyond conventional obesity parameters in Korean adolescent boys. J. Sleep Res. 2017, 26, 444–452. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Y.; Chen, S.; Wu, Z. Relationship between sleep and muscle strength among Chinese university students: A cross-sectional study. J. Musculoskelet. Neuronal Interact. 2017, 17, 327–333. [Google Scholar]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Schoeller, D.A.; Penev, P.D. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann. Intern. Med. 2010, 153, 435–441. [Google Scholar] [CrossRef]
- Castro-Diehl, C.; Diez Roux, A.V.; Redline, S.; Seeman, T.; Shrager, S.E.; Shea, S. Association of Sleep Duration and Quality With Alterations in the Hypothalamic-Pituitary Adrenocortical Axis: The Multi-Ethnic Study of Atherosclerosis (MESA). J. Clin. Endocrinol. Metab. 2015, 100, 3149–3158. [Google Scholar] [CrossRef]
- Katsuhara, S.; Yokomoto-Umakoshi, M.; Umakoshi, H.; Matsuda, Y.; Iwahashi, N.; Kaneko, H.; Ogata, M.; Fukumoto, T.; Terada, E.; Sakamoto, R.; et al. Impact of Cortisol on Reduction in Muscle Strength and Mass: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2022, 107, e1477–e1487. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhai, L.; Zhang, D. Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med. 2014, 15, 1456–1462. [Google Scholar] [CrossRef]
- Sperry, S.D.; Scully, I.D.; Gramzow, R.H.; Jorgensen, R.S. Sleep Duration and Waist Circumference in Adults: A Meta-Analysis. Sleep 2015, 38, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Rahe, C.; Czira, M.E.; Teismann, H.; Berger, K. Associations between poor sleep quality and different measures of obesity. Sleep Med. 2015, 16, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Ferranti, R.; Marventano, S.; Castellano, S.; Giogianni, G.; Nolfo, F.; Rametta, S.; Matalone, M.; Mistretta, A. Sleep quality and duration is related with diet and obesity in young adolescent living in Sicily, Southern Italy. Sleep Sci. 2016, 9, 117–122. [Google Scholar] [CrossRef]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef]
- Benedict, C.; Hallschmid, M.; Lassen, A.; Mahnke, C.; Schultes, B.; Schiöth, H.B.; Born, J.; Lange, T. Acute sleep deprivation reduces energy expenditure in healthy men. Am. J. Clin. Nutr. 2011, 93, 1229–1236. [Google Scholar] [CrossRef]
- Bergan-Roller, H.E.; Sheridan, M.A. The growth hormone signaling system: Insights into coordinating the anabolic and catabolic actions of growth hormone. Gen. Comp. Endocrinol. 2018, 258, 119–133. [Google Scholar] [CrossRef]
- Niu, J.; Sahni, S.; Liao, S.; Tucker, K.L.; Dawson-Hughes, B.; Gao, X. Association between Sleep Duration, Insomnia Symptoms and Bone Mineral Density in Older Boston Puerto Rican Adults. PLoS ONE 2015, 10, e0132342. [Google Scholar] [CrossRef]
- Chen, G.; Chen, L.; Wen, J.; Yao, J.; Li, L.; Lin, L.; Tang, K.; Huang, H.; Liang, J.; Lin, W.; et al. Associations between sleep duration, daytime nap duration, and osteoporosis vary by sex, menopause, and sleep quality. J. Clin. Endocrinol. Metab. 2014, 99, 2869–2877. [Google Scholar] [CrossRef]
- Kuriyama, N.; Inaba, M.; Ozaki, E.; Yoneda, Y.; Matsui, D.; Hashiguchi, K.; Koyama, T.; Iwai, K.; Watanabe, I.; Tanaka, R.; et al. Association between loss of bone mass due to short sleep and leptin-sympathetic nervous system activity. Arch. Gerontol. Geriatr. 2017, 70, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Marwaha, K.; Sanvictores, T.; Awosika, A.O.; Ayers, D. Physiology, Stress Reaction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Stefanaki, C.; Pervanidou, P.; Boschiero, D.; Chrousos, G.P. Chronic stress and body composition disorders: Implications for health and disease. Hormones 2018, 17, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2016, 6, 422. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A.J. Stress and Obesity. Annu. Rev. Psychol. 2019, 70, 703–718. [Google Scholar] [CrossRef]
- Gianotti, L.; Belcastro, S.; D’Agnano, S.; Tassone, F. The Stress Axis in Obesity and Diabetes Mellitus: An Update. Endocrines 2021, 2, 334–347. [Google Scholar] [CrossRef]
- Azuma, K.; Adachi, Y.; Hayashi, H.; Kubo, K.Y. Chronic Psychological Stress as a Risk Factor of Osteoporosis. J. UOEH 2015, 37, 245–253. [Google Scholar] [CrossRef]
- Seibel, M.J.; Cooper, M.S.; Zhou, H. Glucocorticoid-induced osteoporosis: Mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol. 2013, 1, 59–70. [Google Scholar] [CrossRef]
- Helman, T.J.; Headrick, J.P.; Stapelberg, N.J.C.; Braidy, N. The sex-dependent response to psychosocial stress and ischaemic heart disease. Front. Cardiovasc. Med. 2023, 10, 1072042. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, S.; Chen, X.J.; Yu, H.H.; Yang, Y.; Wang, W. Effects of Exercise on Sleep Quality and Insomnia in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Psychiatry 2021, 12, 664499. [Google Scholar] [CrossRef]
- Rosa, C.C.; Tebar, W.R.; Oliveira, C.B.S.; Farah, B.Q.; Casonatto, J.; Saraiva, B.T.C.; Christofaro, D.G.D. Effect of Different Sports Practice on Sleep Quality and Quality of Life in Children and Adolescents: Randomized Clinical Trial. Sports Med. Open 2021, 7, 83. [Google Scholar] [CrossRef]
- Alnawwar, M.A.; Alraddadi, M.I.; Algethmi, R.A.; Salem, G.A.; Salem, M.A.; Alharbi, A.A. The Effect of Physical Activity on Sleep Quality and Sleep Disorder: A Systematic Review. Cureus 2023, 15, e43595. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E. Exercise-induced oxidative stress and melatonin supplementation: Current evidence. J. Physiol. Sci. 2021, 71, 27. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, S.Y.; Joo, J.H.; Choi, D.W.; Park, E.C. The Relationship between Sleep Duration and Perceived Stress: Findings from the 2017 Community Health Survey in Korea. Int. J. Environ. Res. Public Health 2019, 16, 3208. [Google Scholar] [CrossRef]
- Deng, X.; Liu, X.; Fang, R. Evaluation of the correlation between job stress and sleep quality in community nurses. Medicine 2020, 99, e18822. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.H.; Casement, M.D.; Troxel, W.M.; Matthews, K.A.; Bromberger, J.T.; Kravitz, H.M.; Krafty, R.T.; Buysse, D.J. Chronic Stress is Prospectively Associated with Sleep in Midlife Women: The SWAN Sleep Study. Sleep 2015, 38, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, C.; Tufik, S.; Andersen, M.L. Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 2015, 8, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, Y.; Aluru, N.; McGuire, A.; Park, Y.J.; Vijayan, M.M.; Takemura, A. Effect of cortisol on melatonin production by the pineal organ of tilapia, Oreochromis mossambicus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 155, 84–90. [Google Scholar] [CrossRef]
- Jåbekk, P.; Jensen, R.M.; Sandell, M.B.; Haugen, E.; Katralen, L.M.; Bjorvatn, B. A randomized controlled pilot trial of sleep health education on body composition changes following 10 weeks’ resistance exercise. J. Sports Med. Phys. Fit. 2020, 60, 743–748. [Google Scholar] [CrossRef]
- Forte, P.; Encarnação, S.G.; Branquinho, L.; Barbosa, T.M.; Monteiro, A.M.; Pecos-Martín, D. The Effects of an 8-Month Multicomponent Training Program in Body Composition, Functional Fitness, and Sleep Quality in Aged People: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 6603. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Severo, J.S.; Beserra, J.B.; de Oiveira, A.R.S.; Cruz, K.J.C.; de Sousa Melo, S.R.; do Nascimento, G.V.R.; de Macedo, G.F.S.; do Nascimento Marreiro, D. Association Between Cortisol, Insulin Resistance and Zinc in Obesity: A Mini-Review. Biol. Trace Elem. Res. 2019, 191, 323–330. [Google Scholar] [CrossRef]
- Remely, M.; Tesar, I.; Hippe, B.; Gnauer, S.; Rust, P.; Haslberger, A.G. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef. Microbes 2015, 6, 431–439. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).