Accelerating the Delivery of Psychological Therapies After Stroke: A Feasibility Stepped-Wedge Cluster Randomised Controlled Trial
Abstract
1. Background
Objectives
2. Methods
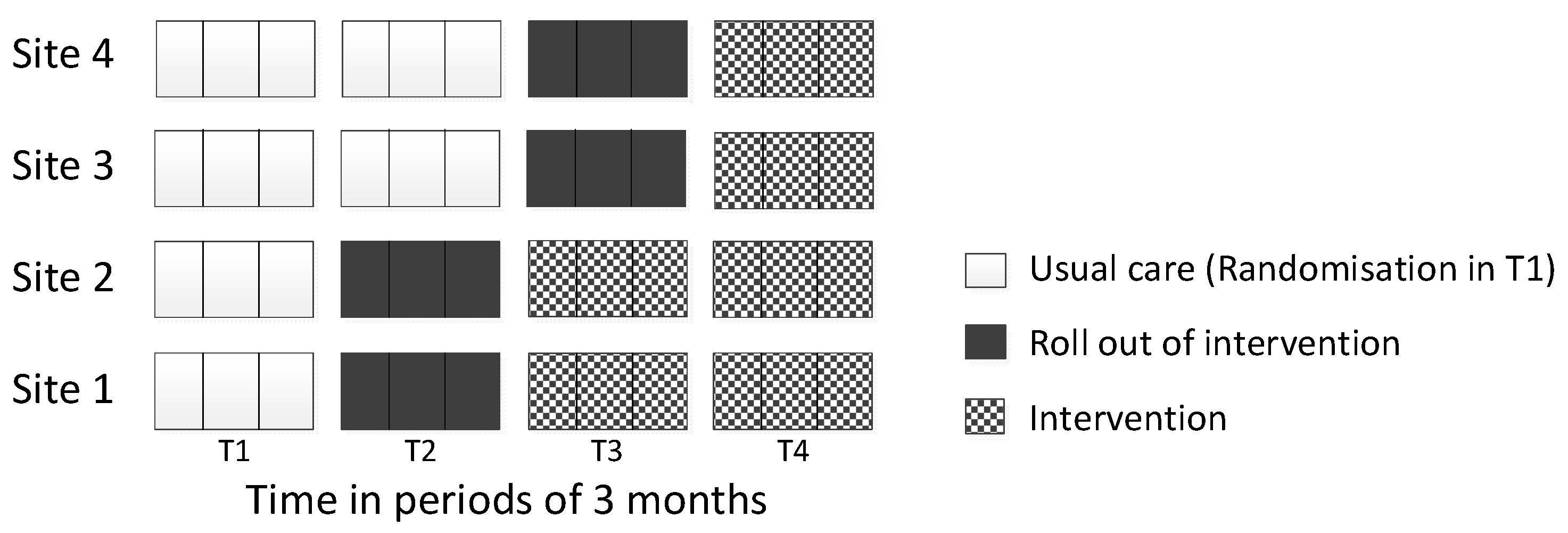
2.1. Study Design and Ethics
2.2. Setting
2.3. Patient Carer and Public Involvement (PCPI)
2.4. Randomisation
2.5. Phase 1
2.6. Phase 2: Feasibility Trial
- Number of patients suffering from psychological distress (anxiety or depression according to the Psychological Distress Algorithm (Appendix A)) and who received psychological support at each time point;
- Number of patients with anti-depressant use at each time point;
- Number of patients when psychological treatment was first received;
- Number of patients who required a letter to be sent to their GP to notify them of a potential issue concerning psychological distress;
- Number of patients with further stroke, TIA or other major health problems which required hospital admission (electronic health records were compared to participant reported problems at each follow-up using kappa statistics);
- Number of reminders sent to encourage participants to return the questionnaires.
2.7. Phase 3: Process Evaluation Interviews
3. Results
3.1. Objective A: Evaluate the Feasibility of Collaboratively Developing and Implementing the Intervention Package
3.1.1. IP Component 1: Screening and Referral Pathway
3.1.2. IP Component 2: Training
3.1.3. IP Component 3: Manual
3.1.4. IP Component 4: Supervision
3.2. Objective B: Assess Whether the Development of the Intervention Package Impacted Psychological Service Provision
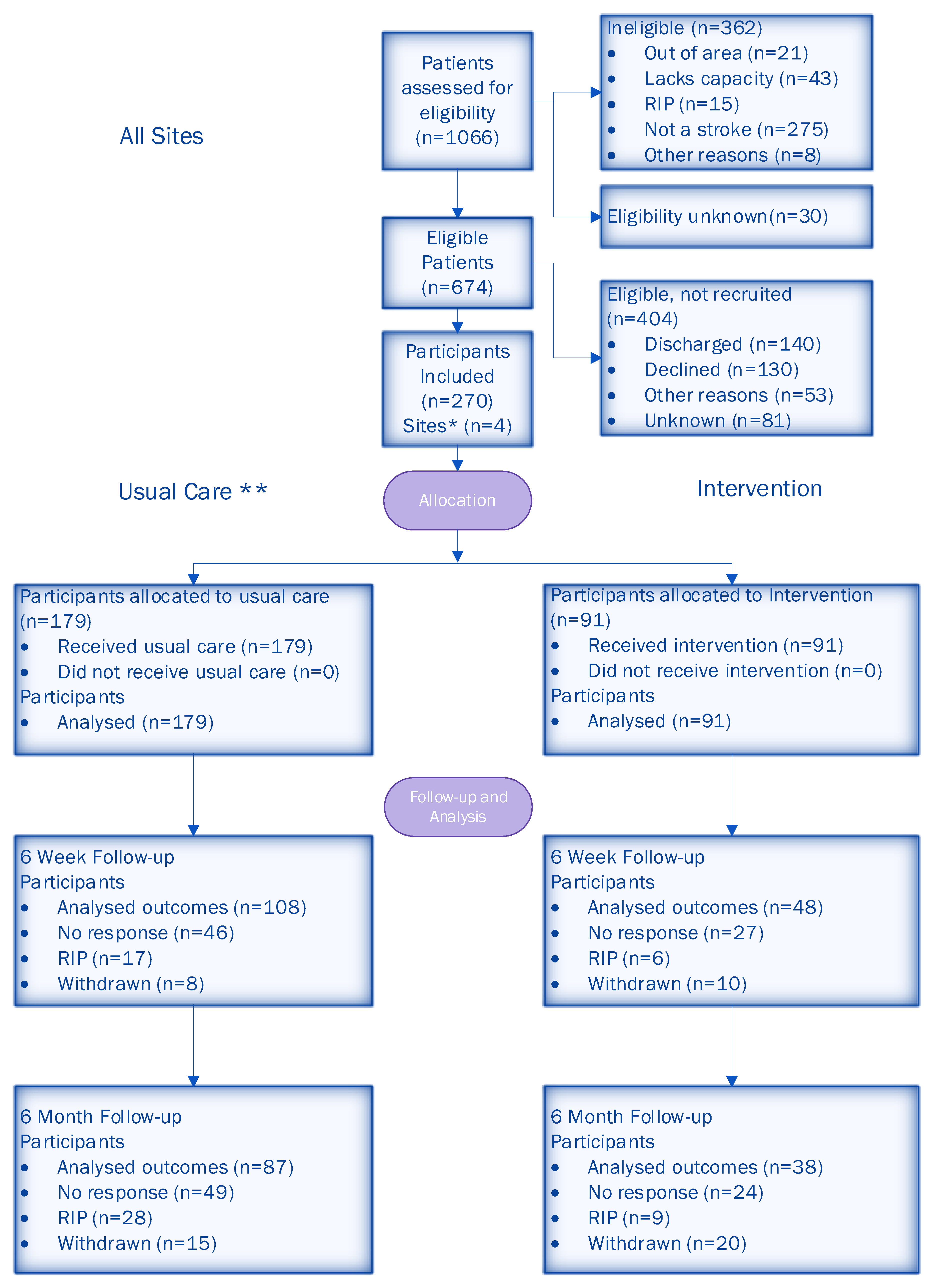
3.3. Objective C: Estimate the Eligibility, Recruitment and Attrition Rates for a Larger Trial
3.4. Objective D: Develop and Test Data Collection Systems, Outcome Measures and Follow-Up Protocols
3.4.1. Data Collection Systems: Questionnaire Type
3.4.2. Data Collection Systems: Outcome Measures
3.4.3. Follow-Up Protocol
3.5. Objective E: Estimate the Proportion of People with Psychological Distress, Time to First Referral and Time to Treatment
3.5.1. Estimating the Proportion of People with Psychological Distress
3.5.2. Time to First Referral for Psychological Support/Treatment and First Treatment for Psychological Distress
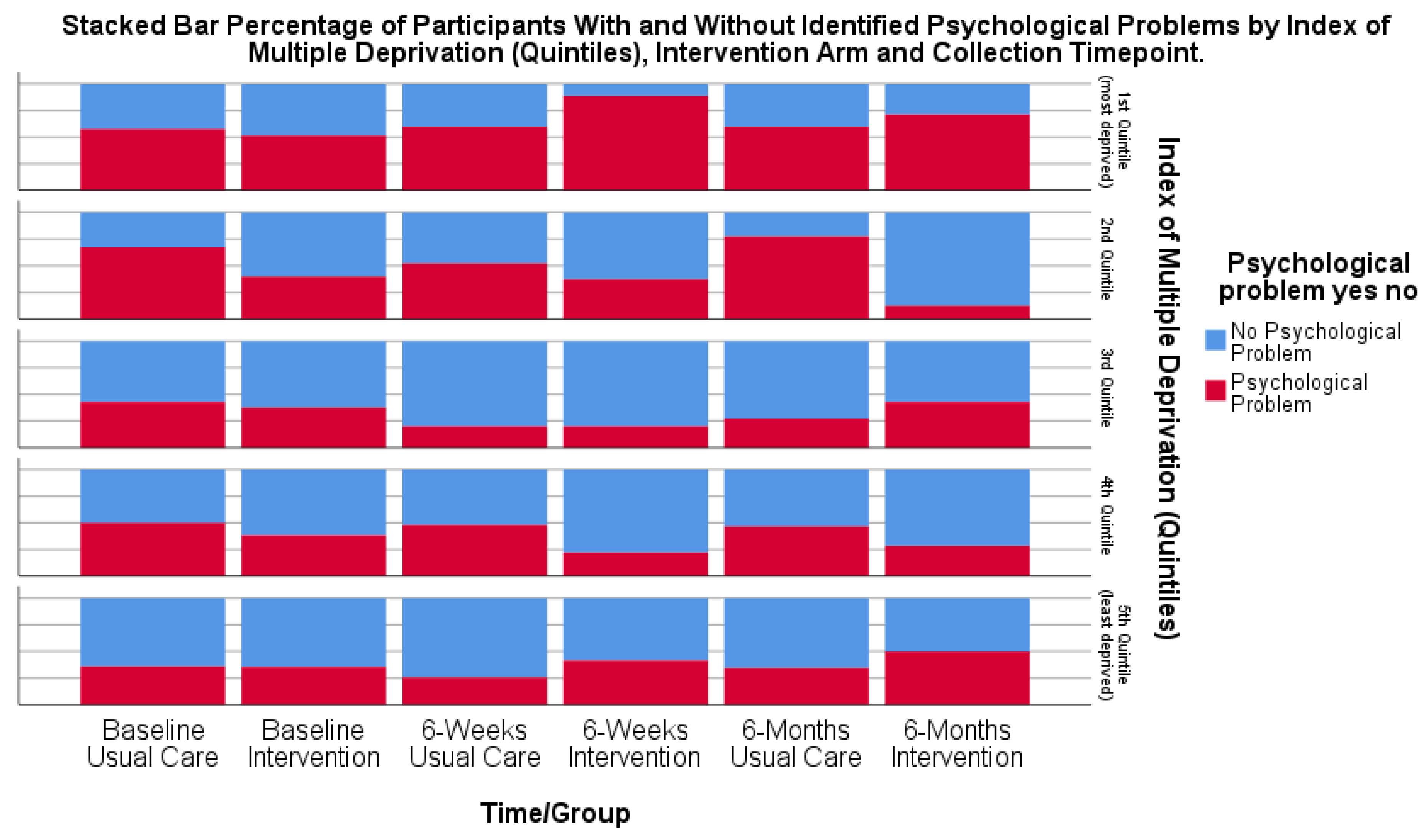
3.6. Objective F: Explore the Potential Benefits of the Intervention Package for Patients, Including for Different Stroke and Socio-Economic Subgroups
Potential Benefit of IPs for Patients and Subgroup Analysis of Socio-Economic Factors
3.7. Objective G: Investigate the Feasibility of the Stepped-Wedge Design to Evaluate the Delivery of the Intervention Package
4. Discussion
4.1. Feasibility of Stepped-Wedge Design
- A longer pre-implementation preparation period: Allocating a longer dedicated preparation phase (e.g., 6–9 months) prior to implementation may ensure readiness.
- Implementation support teams: Establishing local implementation leads or teams within each site could facilitate adaptation to service structures and improve engagement.
- Incentives and recognition: Providing professional development credits or recognition for engagement in implementation efforts.
- Tailored communication strategies: Regular, targeted communication (e.g., newsletters, briefing sessions) to maintain momentum.
- Alignment strategies with service development: Aligning intervention components with existing service priorities may enhance acceptability and integration.
- Clear accountability: Defining responsibilities in implementation plans to ensure engagement.
- Flexible training delivery: Offering asynchronous online training modules with optional live question and answer sessions which may improve accessibility.
- Integrating training into service training programmes: Embedding training into existing professional development frameworks which may reduce disruption.
4.2. Feasibility of Data Collection Procedures/Systems
- Post-discharge recruitment pathways: Allowing recruitment after hospital discharge.
- Personalised follow-up strategies: Using reminder calls, SMS messages and flexible follow-up options (e.g., visits, virtual check-ins) may improve retention.
- Simplified data collection: Streamlining questionnaires to reduce participant burden may improve response rates.
- Targeted outreach: Proactive engagement in underserved areas to improve accessibility.
- Flexible service delivery models: Offering telephone or virtual psychological support to help to reduce barriers.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Psychological Distress Algorithm
- (1)
- A patient will be identified as being in psychological distress for anxiety using the following algorithm:
- (i)
- If a patient does not have a consultee and does not have aphasia, they will be identified as being in psychological distress due to anxiety if they scored 10 or more on the GAD-7.
- (ii)
- If the patient has not completed the patient self-completed questionnaire and does have a consultee or carer, then the corresponding measure (GAD-7 for consultee and BOA for carer questionnaire) will be used to indicate whether a patient is in psychological distress due to anxiety. If, for either of the corresponding measures, the patient has scored above the cut-off (10 or more on the GAD-7 and 14 or more on the BOA), then the patient be identified as being in psychological distress due to anxiety.
- (2)
- If the patient is identified as not being in psychological distress due to anxiety from either the GAD-7 or BOA, and is not identified as being in psychological distress due to anxiety from (1) above, then they will be identified as not being in psychological distress due to anxiety.
- (3)
- If, from (1) and (2) above, the patient cannot be classed as either being in psychological distress due to anxiety or as not being in psychological distress due to anxiety, their status for psychological distress due to anxiety will be set to ‘missing’.
- (4)
- If their status for psychological distress due to anxiety is missing and the unused measure from (1) above indicated psychological distress, then they will be indicated as being in psychological distress due to anxiety.
- (1)
- A patient will be identified as being in psychological distress due to depression using the following algorithm:
- (i)
- If a patient completes the patient self-completed questionnaire (that is, they do not have a consultee and do not have aphasia), they will be identified as being in psychological distress due to depression if they scored 10 or more on the PHQ-9.
- (ii)
- If a patient does not have a consultee but does have aphasia, they will be identified as being in psychological distress due to depression if they scored 3 or more on the DISCs.
- (iii)
- If a patient has responded ‘Yes’ to the Yale question (on either the patient self-completed or aphasia-friendly patient questionnaire).
- (iv)
- If the patient has not completed the patient self-completed questionnaire and does have a consultee or carer, the consultee and/or carer questionnaire will be used to indicate whether a patient is in psychological distress due to depression. If a carer scored their relative/friend as 14 or more on the SADQ-10, then the patient would be identified as being in psychological distress due to depression; likewise, if the relative/friend is screened positive for mood on the Yale question on either the consultee or carer questionnaire, the patient will be identified as being in psychological distress due to depression.
- (v)
- A patient will also be identified as being in psychological distress due to depression if a letter has been sent to their GP.
- (2)
- If the patient is identified as not being in psychological distress due to depression from any of the measures detailed in (1) (i)–(iv) above (PHQ-9; DISCs; patient, consultee or carer Yale question) and is not identified as being in psychological distress due to depression in (1) (v) above, then they will be identified as not being in psychological distress due to depression.
- (3)
- If, from (1) and (2) above, the patient cannot be classed as either being in psychological distress due to depression or as not being in psychological distress due to depression, their status for psychological distress due to depression will be set to ‘missing’.
- (4)
- If their status for psychological distress due to depression is missing and an unused measure from (1) above indicated psychological distress, then they will be indicated as being in psychological distress due to depression.
- (1)
- A patient will be identified as being in psychological distress if they are recorded as having psychological distress due to anxiety or recorded as having psychological distress due to depression (or both).
- (2)
- A patient will be identified as not being in psychological distress if they are recorded as not having psychological distress due to anxiety and recorded as not having psychological distress due to depression (or both).
- (3)
- If a patient is not recorded as being in psychological distress and is recorded as ‘missing’ on either (or both) psychological distress due to anxiety and psychological distress due to depression, then they will be recorded as ‘missing’ for psychological distress.
References
- King’s College London Sentinel Stroke National Audit Programme (SSNAP) on behalf of the Intercollegiate Stroke Working Party. State of the Nation Report 2024; Health Quality Improvement Partnership: London, UK, 2024. [Google Scholar]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [PubMed]
- Hackett, M.; Pickles, K. Part I: Frequency of depression after stroke: An updated systematic review and meta-analysis of observational studies. Int. J. Stroke 2014, 9, 1017–1025.6. [Google Scholar] [CrossRef] [PubMed]
- Knapp, P.; Dunn-Roberts, A.; Sahib, N.; Cook, L.; Astin, F.; Kontou, E.; Thomas, S.A. Frequency of anxiety after stroke: An updated systematic review and meta-analysis of observational studies. Int. J. Stroke 2020, 15, 244–255, Erratum in Int. J. Stroke 2021, 16, NP2. [Google Scholar] [CrossRef]
- Gillespie, D.C.; Cadden, A.P.; Lees, R.; West, R.M.; Broomfield, N.M. Prevalence of Pseudobulbar Affect following Stroke: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2016, 25, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.P.J.; Spauwen, P.J.J.; Bus, B.A.A.; Rijnen, S.J.M.; Ponds, R.W.H.M. Prevalence of posttraumatic stress disorder after stroke: A systematic literature review. J. Psychosom. Res. 2024, 187, 111914. [Google Scholar] [CrossRef]
- Ghose, S.S.; Williams, L.S.; Swindle, R.W. Depression and other mental health diagnoses after stroke increase inpatient and outpatient medical utilization three years poststroke. Med. Care 2005, 43, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Pohjasvaara, T.; Vataja, R.; Lepparuor, A.; Kaste, M.; Erkinjuntti, T. Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur. J. Neurol. 2001, 8, 315–319. [Google Scholar] [CrossRef]
- De Wit, L.; Theuns, P.; Dejaeger, E.; Devos, S.; Gantenbein, A.R.; Kerckhofs, E.; Schuback, B.; Schupp, W.; Putman, K. Long-term impact of stroke on patients’ health-related quality of life. Disabil. Rehabil. 2017, 39, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; Venturini, P.; Lamis, D.A.; Giordano, G.; Serafini, G.; Murri, M.B.; Amore, M.; Girardi, P. Suicide in Stroke Survivors: Epidemiology and Prevention. Drugs Aging 2015, 32, 21–29. [Google Scholar] [CrossRef]
- House, A.O.; Anderson, A.; Hackett, M. Effects of antidepressants and psychological therapies for reducing the emotional impact of stroke. J. R. Coll. Surg. Edinb. 2001, 31, 50–60. [Google Scholar]
- Kowalska, K.; Krzywoszański, Ł.; Droś, J.; Pasińska, P.; Wilk, A.; Klimkowicz-Mrowiec, A. Early Depression Independently of Other Neuropsychiatric Conditions, Influences Disability and Mortality after Stroke (Research Study-Part of PROPOLIS Study). Biomedicines 2020, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Husaini, B.; Levine, R.; Sharp, L.; Cain, V.; Novotny, M.; Hull, P.; Orum, G.; Samad, Z.; Sampson, U.; Moonis, M. Depression Increases Stroke Hospitalization Cost: An Analysis of 17,010 Stroke Patients in 2008 by Race and Gender. Stroke Res. Treat. 2013, 2013, 846732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hackett, M.L.; Yapa, C.; Parag, V.; Anderson, C.S. Frequency of Depression After Stroke. Stroke 2005, 36, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- King’s College London Sentinel Stroke National Audit Programme (SSNAP) on behalf of the Intercollegiate Stroke Working Party. Acute Organisational Audit 2021; Health Quality Improvement Partnership: London, UK, 2022. [Google Scholar]
- Royal College of Physicians Sentinel Stroke National Audit Programme (SSNAP). National Report 2015—Phase 2 - Post-acute stroke service provider audit. Prepared by Royal College of Physicians, Clinical Effectiveness and Evaluation Unit on behalf of the Intercollegiate Stroke Working Party. Available online: https://strokeaudit.org/Results2/PostAcute/National.aspx (accessed on 25 February 2025).
- Gittins, M.; Vail, A.; Bowen, A.; Lugo-Palacios, D.; Paley, L.; Bray, B.; Gannon, B.; Tyson, S. Factors influencing the amount of therapy received during inpatient stroke care: An analysis of data from the UK Sentinel Stroke National Audit Programme. Clin. Rehabil. 2020, 34, 981–991. [Google Scholar] [CrossRef]
- National Clinical Guideline for Stroke for the UK and Ireland. London: Intercollegiate Stroke Working Party. Available online: www.strokeguideline.org (accessed on 4 May 2023).
- The British Psychological Society. Recommendations for Integrated Community Stroke Services 2023. Available online: https://www.bps.org.uk/guideline/recommendations-integrated-community-stroke-services (accessed on 25 February 2025).
- Harriman, E.; Poh, J.; Steverson, T. A clinical psychology service in stroke rehabilitation: A review of five years of referrals and an evaluation of a matched care model. Neuropsychol. 2021, 11, 38–46. [Google Scholar] [CrossRef]
- Wagenaar, B.H.; Petersen, I.; Rao, D.; Chwastiak, L. 7—Collaborative care models: A global perspective. In Global Mental Health in Practice, Global Mental Health and Psychotherapy; Stein, D.J., Bass, J.K., Hofmann, S.G., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 153–170. ISBN 9780128149324. [Google Scholar] [CrossRef]
- Rugkåsa, J.; Tveit, O.G.; Berteig, J.; Hussain, A.; Ruud, T. Collaborative care for mental health: A qualitative study of the experiences of patients and health professionals. BMC Health Serv. Res. 2020, 20, 844. [Google Scholar] [CrossRef] [PubMed]
- Marshall, I.J.; Wang, Y.; Crichton, S.; McKevitt, C.; Rudd, A.G.; Wolfe, C.D.A. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol. 2015, 14, 1206–1218. [Google Scholar] [CrossRef]
- Fellow-Smith, E.; Moss-Morris, R.; Tylee, A.; Fossey, M.; Cohen, A.; Nixon, T. Investing in Emotional and Psychological Wellbeing for Patients with Long-Term Conditions. Available online: https://www.choiceforum.org/docs/iep.pdf (accessed on 25 February 2025).
- Department of Health and Social Care. Long-Term Conditions Positive Practice Guide. London: Department of Health and Social Care. 2008. Available online: http://www.uea.ac.uk/documents/246046/11991919/longterm-conditions-positive-practice-guide.pdf/f9e2b540-2061-4950-a428-b2c9c3ddb35d (accessed on 25 February 2025).
- Harrison, M.; Ryan, T.; Gardiner, C.; Jones, A. Psychological and emotional needs, assessment, and support post-stroke: A multi-perspective qualitative study. Top. Stroke Rehabil. 2017, 24, 119–125. [Google Scholar] [CrossRef]
- Hemming, K.; Haines, T.P.; Chilton, P.J.; Girling, A.J.; Lilford, R.J. The stepped wedge cluster randomised trial: Rationale, design, analysis, and reporting. BMJ 2015, 350, 391. [Google Scholar] [CrossRef]
- Enderby, P.M.; Wood, V.A.; Wade, D.T.; Hewer, R.L. The Frenchay Aphasia Screening Test: A short, simple test for aphasia appropriate for non-specialists. Int. Rehabil. Med. 1986, 8, 166–170. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.; Williams, W. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–616. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Kalmus, M.; Hirani, D.; Clegg, F. The Depression Intensity Scale Circles (DISCs): A first evaluation of a simple assessment tool for depression in the context of brain injury. J. Neurol Neurosurg. Psychiatry 2005, 76, 1273–1278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Lowe, B. A brief measure for assessing generalized anxiety disorder. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Hosey, M.M.; Leoutsakos, J.S.; Li, X.; Dinglas, V.D.; Bienvenu, O.J.; Parker, A.M.; Hopkins, R.O.; Needham, D.M.; Neufeld, K.J. Screening for posttraumatic stress disorder in ARDS survivors: Validation of the Impact of Event Scale-6 (IES-6). Crit Care 2019, 23, 276, Erratum in Crit Care 2020, 24, 37. https://doi.org/10.1186/s13054-020-2759-0. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]
- Ellul, J.; Watkins, C.; Barer, D. Estimating total Barthel scores from just three items: The European Stroke Database ‘minimum dataset’ for assessing functional status at discharge from hospital. Age Ageing 1998, 27, 115–122. [Google Scholar] [CrossRef][Green Version]
- Jenkinson, C.; Fitzpatrick, R.; Crocker, H.; Peters, M. The Stroke Impact Scale: Validation in a UK setting and development of a SIS short form and SIS index. Stroke 2013, 44, 2532–2535. [Google Scholar] [CrossRef] [PubMed]
- Mundt, J.C.; Marks, I.M.; Shear, M.K.; Greist, J.H. The Work and Social Adjustment Scale: A simple measure of impairment in functioning. Br. J. Psychiatry 2002, 180, 461–464. [Google Scholar] [CrossRef] [PubMed]
- The EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Sutcliffe, L.; Lincoln, N. The assessment of depression in aphasic stroke patients: The development of the stroke aphasic depression questionnaire. Clin. Rehabil. 1998, 12, 506–513. [Google Scholar] [CrossRef]
- Mahoney, J.; Drinka, T.J.K.; Abler, R.; Gunter-Hunt, G.; Matthews, C.; Gravenstein, S.; Carnes, M. Screening for depression: Single question versus GDS. J. Am. Geriatr. Soc. 1994, 9, 1006–1008. [Google Scholar] [CrossRef]
- Linley-Adams, B.; Morris, R.; Kneebone, I. The Behavioural Outcomes of Anxiety scale (BOA): A preliminary validation in stroke survivors. Br. J. Clin. Psychol. 2014, 53, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Hemming, K.; Taljaard, M.; McKenzie, J.E.; Hooper, R.; Copas, A.; Thompson, J.A.; Dixon-Woods, M.; Aldcroft, A.; Doussau, A.; Grayling, M.; et al. Reporting of stepped wedge cluster randomised trials: Extension of the CONSORT 2010 statement with explanation and elaboration. BMJ 2018, 363, k1614. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 24.0; IBM Corp: Armonk, NY, USA, 2016. [Google Scholar]
- StataCorp. Stata Statistical Software; Release 15; StataCorp LLC.: College Station, TX, USA, 2017. [Google Scholar]
- Kagan, A. Supported Conversation for Adults with Aphasia (SCA). In The SAGE Encyclopedia of Human Communication Sciences and Disorders; Damico, J.S., Ball, M.J., Eds.; SAGE Publishing: Los Angeles, CA, USA, 2019. [Google Scholar]
- Michie, S.; Johnston, M.; Abraham, C.; Lawton, R.; Parker, D.; Walker, A. on behalf of the ‘Psychological Theory’ Group: Making psychological theory useful for implementing evidence based practice: A consensus approach. Qual. Saf. Health Care 2005, 14, 26–33. [Google Scholar] [PubMed]
- Green, A.; Aarons, G.A. A comparison of policy and direct practice stakeholder perceptions of factors affecting evidence-based practice implementation using concept mapping. Implement Sci. 2011, 7, 104. [Google Scholar] [CrossRef]
- Palinkas, L.A.; Aarons, G.A.; Chorpita, B.F.; Hoagwood, K.; Landsverk, J.; Weisz, J.R. Cultural exchange and the implementation of evidence-based practices. Res. Soc. Work. Pract. 2009, 19, 602–612. [Google Scholar] [CrossRef]
- Pepin, R.; Hoyt, J.; Seifert, B.; Bartels, S.J. A multi-stakeholder process to transform a community-based screening and referral program to implement evidence-based depression care. J. Evid. Inf. Soc. Work 2016, 13, 362–372. [Google Scholar] [CrossRef]
- Bragstad, L.K.; Bronken, B.A.; Sveen, U.; Hjelle, E.G.; Kitzmuller, G.; Martinsen, R.; Kvigne, K.J.; Mangset, M.; Kirkevold, M. Implementation fidelity in a complex intervention promoting psychosocial well-being following stroke: An explanatory sequential mixed methods study. BMC Med. Res. Methodol. 2019, 19, 59. [Google Scholar] [CrossRef]
- Coventry, P.; Lovell, K.; Dickens, C.; Bower, P.; Chew-Graham, C.; McElvenny, D.; Hann, M.; Cherrington, A.; Garrett, C.; Gibbons, C.J.; et al. Integrated primary care for patients with mental and physical multimorbidity: Cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ 2015, 350, h638. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, M.; Marshall, I.J.; Wolfe, C.D.; Wang, Y.; O’Connell, M.D. Prevalence and natural history of depression after stroke: A systematic review and meta-analysis of observational studies. PLoS Med. 2023, 28, e1004200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tjokrowijoto, P.; Stolwyk, R.J.; Ung, D.; Kneebone, I.; Kilkenny, M.F.; Kim, J.; Olaiya, M.T.; Dalli, L.L.; Cadilhac, D.A.; Nelson, M.R.; et al. Receipt of Mental Health Treatment in People Living With Stroke: Associated Factors and Long-Term Outcomes. Stroke 2023, 54, 1519–1527, Erratum in Stroke 2023, 54, e230. [Google Scholar] [CrossRef] [PubMed]
- Office for National Statistics (ONS), Released 6 December 2022, ONS Website, Article, Cost of Living and Depression in Adults, Great Britain: 29 September to 23 October 2022. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/mentalhealth/articles/costoflivinganddepressioninadultsgreatbritain/29septemberto23october2022 (accessed on 25 February 2025).


| Usual Care (N = 179) | Intervention (N = 91) | All (N = 270) | |
|---|---|---|---|
| Age, median (IQR) n = 269 | 72 (62, 81) | 76 (61, 83) | 73 (62, 82) |
| Gender ^, n (%) | |||
| Female | 85 (47.8) | 44 (48.4) | 129 (48.0) |
| Ethnicity ^^, n (%) | |||
| White | 172 (97.2) | 87 (95.6) | 259 (96.6) |
| Employment status ^^, n (%) | |||
| Paid | 37 (20.9) | 16 (17.6) | 53 (19.8) |
| Living situation ^^, n (%) | |||
| At Home | 149 (84.2) | 78 (85.7) | 227 (84.7) |
| Index of Multiple Deprivation (quintiles), n (%) | |||
| 1st (most deprived) | 45 (25.1) | 32 (35.2) | 77 (28.5) |
| 2nd | 37 (20.7) | 16 (17.6) | 53 (19.6) |
| 3rd | 21 (11.7) | 16 (17.6) | 37 (13.7) |
| 4th | 38 (21.2) | 13 (14.3) | 51 (18.9) |
| 5th (least deprived) | 38 (21.2) | 14 (15.4) | 52 (19.3) |
| Type of stroke ^^^, n (%) | |||
| Ischaemic | 145 (81.9) | 86 (95.6) | 231 (86.5) |
| Intra-Cerebral Haemorrhage | 32 (18.1) | 4 (4.4) | 36 (13.5) |
| Side of body affected by stroke ^^^, n (%) | |||
| Left | 76 (43.2) | 38 (41.8) | 114 (42.7) |
| Right | 83 (47.2) | 43 (47.3) | 126 (47.2) |
| Bilateral | 2 (1.1) | 2 (2.2) | 4 (1.5) |
| Neither | 15 (8.5) | 8 (8.8) | 23 (8.6) |
| NIHSS score, median (IQR) n = 210 | 4 (2.5, 8.5) | 5 (2, 11) | 5 (2, 10) |
| Estimated Barthel Index, median (IQR) n = 265 | 16.3 (10, 20) | 17.5 (10, 20) | 17.5 (10, 20) |
| Modified Rankin ^^, n (%) | |||
| Moderate to Severe | 89 (49.7) | 42 (46.2) | 131 (48.9) |
| EQ5—VAS, median (IQR) n = 188 | 55 (40, 75) | 70(50, 80) | 60 (50, 80) |
| Sensory impairment (sight or hearing), n (%) | 61 (34.1) | 31 (34.1) | 92 (34.1) |
| Cognitive score (MOCA), median (IQR) *, n = 181 | 23 (18, 26) | 24 (19, 27) | 23 (18, 26) |
| Cognitive impairment, n (%) *, n = 181 | 90 (73.8) | 37 (62.7) | 127 (70.2) |
| Communication score (FAST), median (IQR) *, n = 148 | 29 (26, 30) | 29 (25, 30) | 29 (26, 30) |
| Communication problems, n (%) *, n = 148 | 19 (18.6) | 11 (23.9) | 30 (20.3) |
| Current/past use of anti-depressants ^^, n (%) | 36 (20.3) | 7 (7.7) | 43 (16.0) |
| Current/past use of psychological support ^^^, n (%) | 31 (17.6) | 10 (11.0) | 41 (15.4) |
| Self-reported psychological difficulties, n (%) | 80 (44.7) | 39 (42.9) | 119 (44.1) |
| Questionnaire | Problem *1 | Usual Care n (%) | Intervention n (%) | All Participants n (%) |
|---|---|---|---|---|
| GAD-7 *2 | Anxiety | 26 (21.3) | 11 (18.0) | 37 (20.2) |
| BOA | Anxiety | 50 (29.4) | 22 (25.9) | 72 (28.2) |
| PHQ-9 *2 | Depression | 29 (24.4) | 10 (16.4) | 39 (21.7) |
| SADQ-10 | Depression | 52 (30.1) | 21 (24.4) | 73 (28.5) |
| Yale *3 | Depression | 42 (33.6) | 23 (35.9) | 65 (34.4) |
| Carer Yale | Depression | 52 (30.6) | 20 (23.3) | 72 (28.1) |
| DISCs | Depression | 22 (17.6) | 10 (15.6) | 32 (16.9) |
| Usual Care (n = 108) | Intervention (n = 48) | All (n = 156) | |
|---|---|---|---|
| Estimated Barthel Index, median (IQR) n = 155 | 20 (16.3, 20) | 20 (15, 20) | 20 (15, 20) |
| Modified Rankin, n(%) | |||
| Moderate to severe | 48 (44.4) | 22 (47.8) | 70 (45.5) |
| EQ5—VAS, median (IQR) n = 119 | 70 (50, 87) | 70 (55, 90) | 70 (50, 90) |
| SF—SIS, median (IQR) n = 99 | 31 (23, 37) | 30 (20, 37) | 30.5 (23, 37) |
| WSAS, median (IQR) n = 123 | 12 (4, 28) | 10.5 (2, 26) | 12 (2, 28) |
| IES-6, median (IQR) n = 143 | 1 (0.5, 2) | 1 (0.2, 2.2) | 1 (0.3, 2) |
| Usual Care (n = 87) | Intervention (n = 38) | All (n = 125) | |
|---|---|---|---|
| Estimated Barthel Index, median (IQR) n = 122 | 20 (17.5, 20) | 20 (15, 20) | 20 (17.5, 20) |
| Modified Rankin, n(%) | |||
| Moderate to severe | 35 (40.2) | 15 (39.5) | 50 (40.0) |
| EQ5—VAS, median (IQR) n = 103 | 70 (50, 80) | 70 (65, 90) | 70 (50, 85) |
| SF—SIS, median (IQR) n = 115 | 32 (24, 37) | 30 (21, 38) | 32 (22, 37) |
| WSAS, median (IQR) n = 103 | 8 (1, 25.5) | 7 (0, 28) | 8 (0, 26) |
| IES-6, median (IQR) n = 114 | 0.8 (0.3, 1.7) | 0.8 (0.3, 2) | 0.8 (0.3, 1.8) |
| Usual Care | Intervention | Total | Missing | Adjusted OR * (95% CI) | |
|---|---|---|---|---|---|
| Baseline | n= 179 | n= 91 | n= 270 | ||
| Anxiety | 52 (29.1) | 22 (24.2) | 74 (27.4) | 5 (1.9) | N/A |
| Depression | 84 (46.9) | 36 (39.6) | 120 (44.4) | 2 (0.7) | N/A |
| Either | 92 (51.4) | 38 (41.8) | 130 (48.2) | 4 (1.5) | N/A |
| 6 Weeks | n= 108 | n= 48 | n= 156 | ||
| Anxiety | 27 (25.0) | 10 (20.8) | 37 (23.7) | 4 (2.6) | 0.74 (0.28, 1.93) |
| Depression | 42 (38.9) | 19 (39.6) | 61 (39.1) | 0 (0.0) | 1.18 (0.55, 2.50) |
| Either | 45 (41.7) | 20 (41.7) | 65 (41.7) | 1 (0.6) | 1.06 (0.50, 2.26) |
| 6 Months ** | n= 87 | n= 38 | n= 125 | ||
| Anxiety | 16 (18.4) | 7 (18.4) | 23 (18.4) | 4 (3.2) | 1.02 (0.35, 2.98) |
| Depression | 42 (48.3) | 15 (39.5) | 57 (45.6) | 0 (0.0) | 0.75 (0.31, 1.79) |
| Either | 42 (48.3) | 15 (39.5) | 57 (45.6) | 3 (2.4) | 0.72 (0.30, 1.77) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lightbody, C.E.; Patel, K.; Holland, E.-J.; Sutton, C.J.; Brown, C.; Tishkovskaya, S.V.; Bowen, A.; Read, J.; Thomas, S.; Roberts, T.; et al. Accelerating the Delivery of Psychological Therapies After Stroke: A Feasibility Stepped-Wedge Cluster Randomised Controlled Trial. Healthcare 2025, 13, 824. https://doi.org/10.3390/healthcare13070824
Lightbody CE, Patel K, Holland E-J, Sutton CJ, Brown C, Tishkovskaya SV, Bowen A, Read J, Thomas S, Roberts T, et al. Accelerating the Delivery of Psychological Therapies After Stroke: A Feasibility Stepped-Wedge Cluster Randomised Controlled Trial. Healthcare. 2025; 13(7):824. https://doi.org/10.3390/healthcare13070824
Chicago/Turabian StyleLightbody, C. Elizabeth, Kulsum Patel, Emma-Joy Holland, Chris J. Sutton, Christopher Brown, Svetlana V. Tishkovskaya, Audrey Bowen, Jessica Read, Shirley Thomas, Temitayo Roberts, and et al. 2025. "Accelerating the Delivery of Psychological Therapies After Stroke: A Feasibility Stepped-Wedge Cluster Randomised Controlled Trial" Healthcare 13, no. 7: 824. https://doi.org/10.3390/healthcare13070824
APA StyleLightbody, C. E., Patel, K., Holland, E.-J., Sutton, C. J., Brown, C., Tishkovskaya, S. V., Bowen, A., Read, J., Thomas, S., Roberts, T., & Watkins, C. L. (2025). Accelerating the Delivery of Psychological Therapies After Stroke: A Feasibility Stepped-Wedge Cluster Randomised Controlled Trial. Healthcare, 13(7), 824. https://doi.org/10.3390/healthcare13070824





