The Role of Gender in the Relationship Between Waist-to-Hip Ratio, Triglyceride–Glucose Index, and Insulin Resistance in Korean Children
Abstract
1. Introduction
2. Materials and Methods
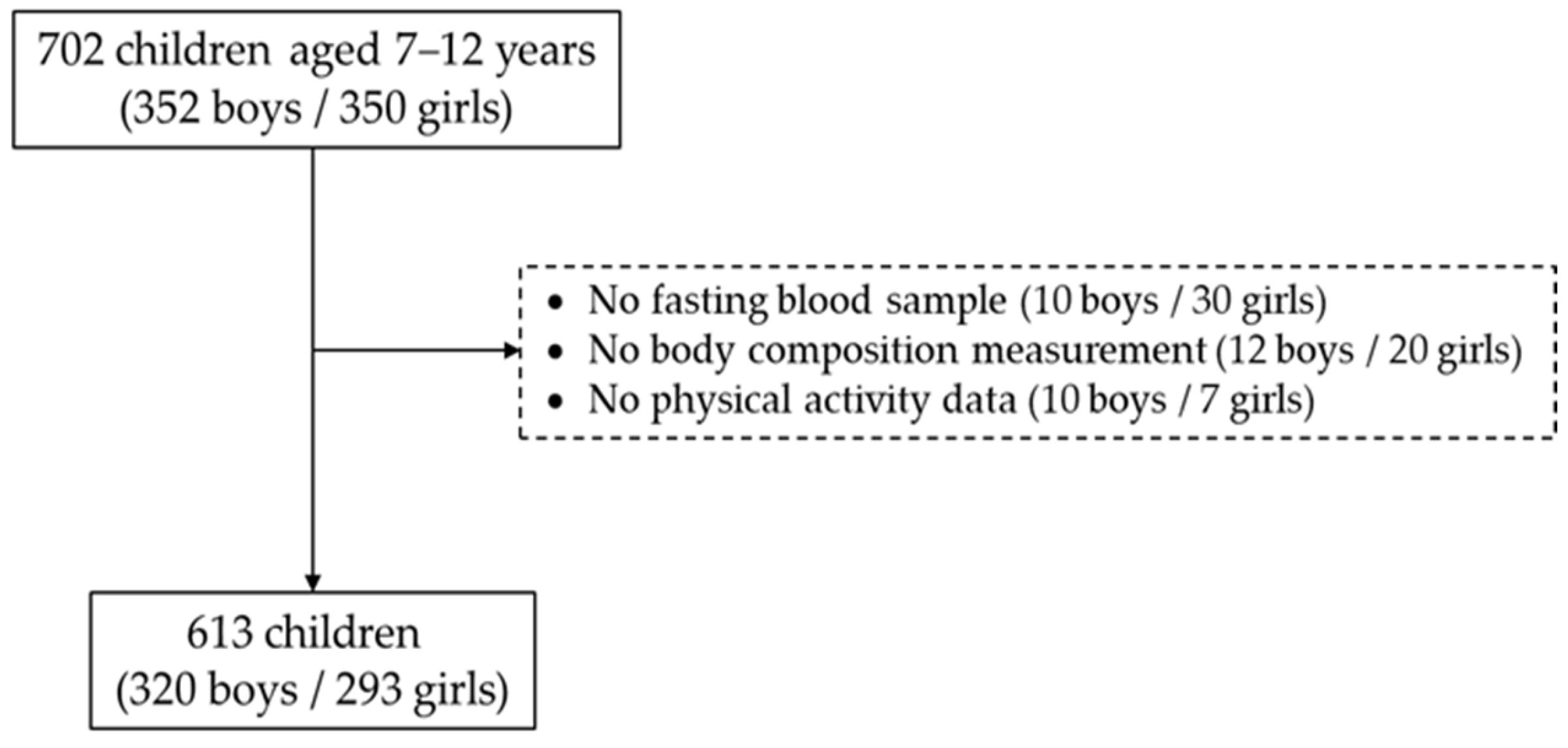
2.1. Study Participants
2.2. Measurement of Anthropometrics and Physical Activity
2.3. Definition of Whole-Body Insulin Resistance
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TyG | triglyceride–glucose |
| IR | insulin resistance |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| WHtR | wasit-to-hip ratio |
| CI | confidence interval |
| MetS | metabolic syndrome |
| T2D | type-2 diabetes |
| CVD | cardiovascular disease |
| BMI | body mass index |
| WC | waist circumference |
| FBG | fasting blood glucose |
| TC | total cholesterol |
| TG | triglycerides |
| HDLC | high-density lipoprotein cholesterol |
| BP | blood pressure |
References
- Tagi, V.M.; Chiarelli, F. Obesity and insulin resistance in children. Curr. Opin. Pediatr. 2020, 32, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [CrossRef]
- Guo, S. Insulin signaling, resistance, and the metabolic syndrome: Insights from mouse models into disease mechanisms. J. Endocrinol. 2014, 220, T1–T23. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Sourlas, A.; Oikonomakis, K.; Zoumi, E.-A.; Papadimitriou, A.; Kostara, C.E. Biomarkers of insulin sensitivity/resistance. J. Int. Med. Res. 2024, 52, 1–40. [Google Scholar] [CrossRef]
- Yan, W.; Wu, S.; Liu, Q.; Zheng, Q.; Gu, W.; Li, X. The link between obesity and insulin resistance among children: Effects of key metabolites. J. Diabetes 2023, 15, 1020–1028. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Savolainen, O.; Domínguez-Riscart, J.; Landberg, R.; Lechuga-Sancho, A.; González-Domínguez, R. Probing erythrocytes as sensitive and reliable sensors of metabolic disturbances in the crosstalk between childhood obesity and insulin resistance: Findings from an observational study, in vivo challenge tests, and ex vivo incubation assays. Cardiovasc. Diabetol. 2024, 23, 336. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.H.; Kim, Y.; Im, M.; Han, H.S. The cutoff values of indirect indices for measuring insulin resistance for metabolic syndrome in Korean children and adolescents. Ann. Pediatr. Endocrinol. Metab. 2016, 21, 143–148. [Google Scholar] [CrossRef][Green Version]
- Yin, J.; Li, M.; Xu, L.; Wang, Y.; Cheng, H.; Zhao, X.; Jie, M. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol. Metab. Syndr. 2013, 5, 71. [Google Scholar] [CrossRef]
- Newbern, D.; Gumus Balikcioglu, P.; Balikcioglu, M.; Bain, J.; Muehlbauer, M.; Stevens, R.; Ilkayeva, O.; Dolinsky, D.; Armstrong, S.; Irizarry, K.; et al. Sex differences in biomarkers associated with insulin resistance in obese adolescents: Metabolomic profiling and principal components analysis. J. Clin. Endocrinol. Metab. 2014, 99, 4730–4739, Erratum in J. Clin. Endocrinol. Metab. 2015, 100, 1709.. [Google Scholar] [CrossRef]
- Adams-Huet, B.; Zubirán, R.; Remaley, A.T.; Jialal, I. The triglyceride-glucose index is superior to homeostasis model assessment of insulin resistance in predicting metabolic syndrome in an adult population in the United States. J. Clin. Lipidol. 2024, 18, e518–e524. [Google Scholar] [PubMed]
- Jiang, M.; Li, X.; Wu, H.; Su, F.; Cao, L.; Ren, X.; Hu, J.; Tatenda, G.; Cheng, M.; Wen, Y. Triglyceride-Glucose Index for the Diagnosis of Metabolic Syndrome: A Cross-Sectional Study of 298,652 Individuals Receiving a Health Check-Up in China. Int. J. Endocrinol. 2022, 2022, 3583603. [Google Scholar] [PubMed]
- Wei, X.; Min, Y.; Song, G.; Ye, X.; Liu, L. Association between triglyceride-glucose related indices with the all-cause and cause-specific mortality among the population with metabolic syndrome. Cardiovasc. Diabetol. 2024, 23, 134. [Google Scholar]
- Miao, Y.; Wang, Y.; Wan, Q. Association between TyG index with obesity indicators and coronary heart disease: A cohort study. Sci. Rep. 2025, 15, 8920. [Google Scholar]
- Nabipoorashrafi, S.A.; Seyedi, S.A.; Rabizadeh, S.; Ebrahimi, M.; Ranjbar, S.A.; Reyhan, S.K.; Meysamie, A.; Nakhjavani, M.; Esteghamati, A. The accuracy of triglyceride-glucose (TyG) index for the screening of metabolic syndrome in adults: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2677–2688. [Google Scholar]
- Sun, Y.; Ji, H.; Sun, W.; An, X.; Lian, F. Triglyceride glucose (TyG) index: A promising biomarker for diagnosis and treatment of different diseases. Eur. J. Intern. Med. 2025, 131, 3–14. [Google Scholar]
- Sanchez, B.N.; Volek, J.S.; Kraemer, W.J.; Saenz, C.; Maresh, C.M. Sex differences in energy metabolism: A female-oriented discussion. Sports Med. 2024, 54, 2033–2057. [Google Scholar]
- Mauvais-Jarvis, F. Sex differences in energy metabolism: Natural selection, mechanisms and consequences. Nat. Rev. Nephrol. 2024, 20, 56–69. [Google Scholar]
- Ciarambino, T.; Crispino, P.; Guarisco, G.; Giordano, M. Gender differences in insulin resistance: New knowledge and perspectives. Curr. Issues Mol. Biol. 2023, 45, 7845–7861. [Google Scholar] [CrossRef]
- Gado, M.; Tsaousidou, E.; Bornstein, S.R.; Perakakis, N. Sex-based differences in insulin resistance. J. Endocrinol. 2024, 261, e230245. [Google Scholar]
- Wang, C.; Liu, D.; Lu, J.; Huang, B.; Feng, B.; Yin, J.; Qiu, J.; Zhang, Z. Gender differences in the relationship between the triglyceride-glucose index and serum Klotho concentrations among the middle-aged and elderly: A cross-sectional analysis. BMC Endocr. Disord. 2024, 24, 185. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.W.; Chang, C.C.; Chou, R.H.; Tsai, Y.L.; Liu, L.K.; Chen, L.K.; Huang, P.H.; Lin, S.J. Gender difference in the association between TyG index and subclinical atherosclerosis: Results from the I-Lan Longitudinal Aging Study. Cardiovasc. Diabetol. 2021, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.W.; Tsai, C.T.; Chou, R.H.; Tsai, Y.L.; Kuo, C.S.; Huang, P.H.; Lin, S.J. Sex difference in the association of the triglyceride glucose index with obstructive coronary artery disease. Sci. Rep. 2023, 13, 9652. [Google Scholar] [CrossRef]
- Guo, R.; Tong, J.; Wang, R.; Ma, S.; Wei, L.; Zhao, W. Gender differences in triglyceride glucose index predictive power for type 2 diabetes mellitus: A Chinese cohort study. Int. J. Diabetes Dev. Ctries. 2024. [Google Scholar] [CrossRef]
- Owen, K.B.; Corbett, L.; Ding, D.; Eime, R.; Bauman, A. Gender differences in physical activity and sport participation in adults across 28 European countries between 2005 and 2022. Ann. Epidemiol. 2025, 101, 52–57. [Google Scholar] [CrossRef]
- Choi, J.; Yoon, T.W.; Yu, M.H.; Kang, D.R.; Choi, S. Gender and age differences in the prevalence and associated factors of metabolic syndrome among children and adolescents in South Korea. Child. Health Nurs. Res. 2021, 27, 160–170. [Google Scholar] [CrossRef]
- Kumahara, H.; Schutz, Y.; Ayabe, M.; Yoshioka, M.; Yoshitake, Y.; Shindo, M.; Ishii, K.; Tanaka, H. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: A validation study against whole-body indirect calorimetry. Br. J. Nutr. 2004, 91, 235–243. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Yoon, J.S.; Shim, Y.S.; Lee, H.S.; Hwang, I.T.; Hwang, J.S. A population-based study of TyG index distribution and its relationship to cardiometabolic risk factors in children and adolescents. Sci. Rep. 2021, 11, 23660. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, H.J.; Jeong, H.R.; Shim, Y.S.; Kang, M.J.; Hwang, I.T. Triglyceride glucose index is superior biomarker for predicting type 2 diabetes mellitus in children and adolescents. Endocr. J. 2022, 69, 559–565. [Google Scholar] [CrossRef]
- Kim, B.; Jin, H.Y.; Yoon, J.S.; Noh, E.S.; Hwang, I.T. Triglyceride glucose index is associated with ultrasonographic fatty liver indicator in children and adolescents with non-alcoholic fatty liver disease. J. Clin. Res. Pediatr. Endocrinol. 2024, 16, 306–313. [Google Scholar] [PubMed]
- Furdela, V.; Pavlyshyn, H.; Shulhai, A.M.; Kozak, K.; Furdela, M. Triglyceride glucose index, pediatric NAFLD fibrosis index, and triglyceride-to-high-density lipoprotein cholesterol ratio are the most predictive markers of the metabolically unhealthy phenotype in overweight/obese adolescent boys. Front. Endocrinol. 2023, 14, 1124019. [Google Scholar]
- Tian, X.; Chen, S.; Wang, P.; Xu, Q.; Zhang, Y.; Luo, Y.; Wu, S.; Wang, A. Insulin resistance mediates obesity-related risk of cardiovascular disease: A prospective cohort study. Cardiovasc. Diabetol. 2022, 21, 289. [Google Scholar]
- Chen, W.; Ding, S.; Tu, J.; Xiao, G.; Chen, K.; Zhang, Y.; Huang, R.; Liao, Y. Association between the insulin resistance marker TyG index and subsequent adverse long-term cardiovascular events in young and middle-aged US adults based on obesity status. Lipids Health Dis. 2023, 22, 65. [Google Scholar]
- Xie, Y.; Guo, R.; Li, Z.; Guo, X.; Sun, G.; Sun, Z.; Zheng, J.; Sun, Y.; Zheng, L. Temporal relationship between body mass index and triglyceride-glucose index and its impact on the incident of hypertension. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1220–1229. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Wu, Y.; Huang, H.; Hu, F.; Zhang, M.; Sun, L.; Hu, D. Association of triglyceride-glucose index and its 6-year change with risk of hypertension: A prospective cohort study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 568–576. [Google Scholar] [CrossRef]
- Fritz, J.; Brozek, W.; Concin, H.; Nagel, G.; Kerschbaum, J.; Lhotta, K.; Ulmer, H.; Zitt, E. The triglyceride-glucose index and obesity-related risk of end-stage kidney disease in Austrian adults. JAMA Netw. Open 2021, 4, e212612. [Google Scholar] [CrossRef]
- Kretschmer, L.; Salali, G.D.; Andersen, L.B.; Hallal, P.C.; Northstone, K.; Sardinha, L.B.; Dyble, M.; Bann, D.; International Children’s Accelerometry Database (ICAD) Collaborators. Gender differences in the distribution of children’s physical activity: Evidence from nine countries. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 103. [Google Scholar]
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Pathophysiology of Physical Inactivity-Dependent Insulin Resistance: A Theoretical Mechanistic Review Emphasizing Clinical Evidence. J. Diabetes Res. 2021, 2021, 7796727. [Google Scholar]
- Lombardo, M.; Feraco, A.; Armani, A.; Camajani, E.; Gorini, S.; Strollo, R.; Padua, E.; Caprio, M.; Bellia, A. Gender differences in body composition, dietary patterns, and physical activity: Insights from a cross-sectional study. Front. Nutr. 2024, 11, 1414217. [Google Scholar]
- Hannon, T.S.; Janosky, J.; Arslanian, S.A. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr. Res. 2006, 60, 759–763. [Google Scholar] [CrossRef] [PubMed]
| Variables | IS (n = 533) | IR (n = 80) | Total (n = 613) | p for Group |
|---|---|---|---|---|
| Age (years) | 9.7 ± 1.3 | 10.7 ± 0.9 | 9.8 ± 1.3 | <0.001 |
| Gender | 0.001 | |||
| Boys, n (%) | 292 (91.3) | 28 (8.8) | 320 (52.2) | |
| Girls, n (%) | 241 (82.3) | 52 (17.7) | 293 (47.8) | |
| BMI (kg/m2) | 18.7 ± 3.1 | 22.2 ± 3.7 | 19.2 ± 3.4 | <0.001 |
| WC (cm) | 65.7 ± 11.3 | 76.1 ± 10.3 | 67.1 ± 11.7 | <0.001 |
| WHtR | 0.83 ± 0.06 | 0.85 ± 0.06 | 0.83 ± 0.06 | 0.041 |
| Tanner scale scores | 1.7 ± 0.9 | 2.3 ± 1.1 | 1.8 ± 1.0 | <0.001 |
| Systolic BP (mmHg) | 108.1 ± 16.0 | 114.8 ± 16.5 | 109.0 ± 16.2 | 0.001 |
| Diastolic BP (mmHg) | 65.8 ± 6.6 | 69.7 ± 13.1 | 66.3 ± 12.1 | 0.007 |
| FBG (mg/dL) | 90.9 ± 6.6 | 96.6 ± 7.7 | 91.7 ± 7.0 | <0.001 |
| TC (mg/dL) | 172.5 ± 26.7 | 171.6 ± 32.0 | 172.4 ± 27.4 | <0.001 |
| TGs (mg/dL) | 81.2 ± 44.4 | 106.1 ± 66.2 | 84.5 ± 48.5 | <0.001 |
| HDLC (mg/dL) | 59.4 ± 13.2 | 49.6 ± 10.6 | 58.1 ± 13.3 | <0.001 |
| TyG index | 8.10 ± 0.45 | 8.39 ± 0.52 | 8.14 ± 0.47 | <0.001 |
| Insulin (µU/mL) | 7.0 ± 2.9 | 18.7 ± 6.3 | 8.5 ± 5.3 | <0.001 |
| HOMA-IR | 1.6 ± 0.7 | 4.5 ± 1.5 | 2.0 ± 1.3 | <0.001 |
| Physical activity | ||||
| LPA (min/day) | 171 ± 41 | 164 ± 46 | 170 ± 42 | 0.151 |
| MPA (min/day) | 72 ± 24 | 76 ± 24 | 72 ± 24 | 1.58 |
| VPA (min/day) | 33 ± 16 | 28 ± 18 | 32 ± 17 | 0.011 |
| Variables | Mediation Analysis | |||
|---|---|---|---|---|
| β | SE | t | p-Value | |
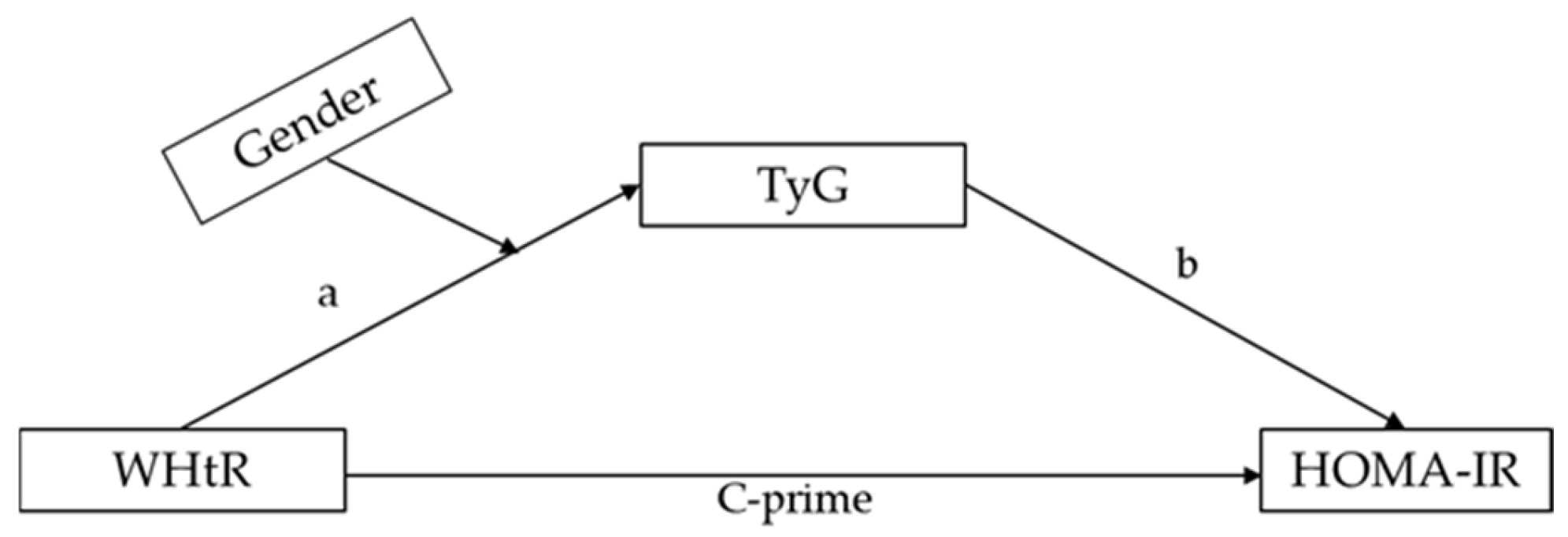
| WHtR (X) → TyG (M) | 1.8154 | 0.3155 | 5.7534 | <0.001 |
| WHtR (X) → HOMA-IR (Y) | 0.7284 | 0.1480 | 4.9189 | <0.001 |
| TyG (M) → HOMA-IR (Y) | 0.1924 | 0.0197 | 9.7718 | <0.001 |
| Variable | Bootstrap results for indirect effect (X → M → Y) | |||
| Effect | SE | LL 95% CI | UL 95% CI | |
| TyG | 0.3493 | 0.0748 | 0.2100 | 0.5037 |
| Variables | Dependent Variable: TyG | |||
|---|---|---|---|---|
| B | SE | t | p-Values | |
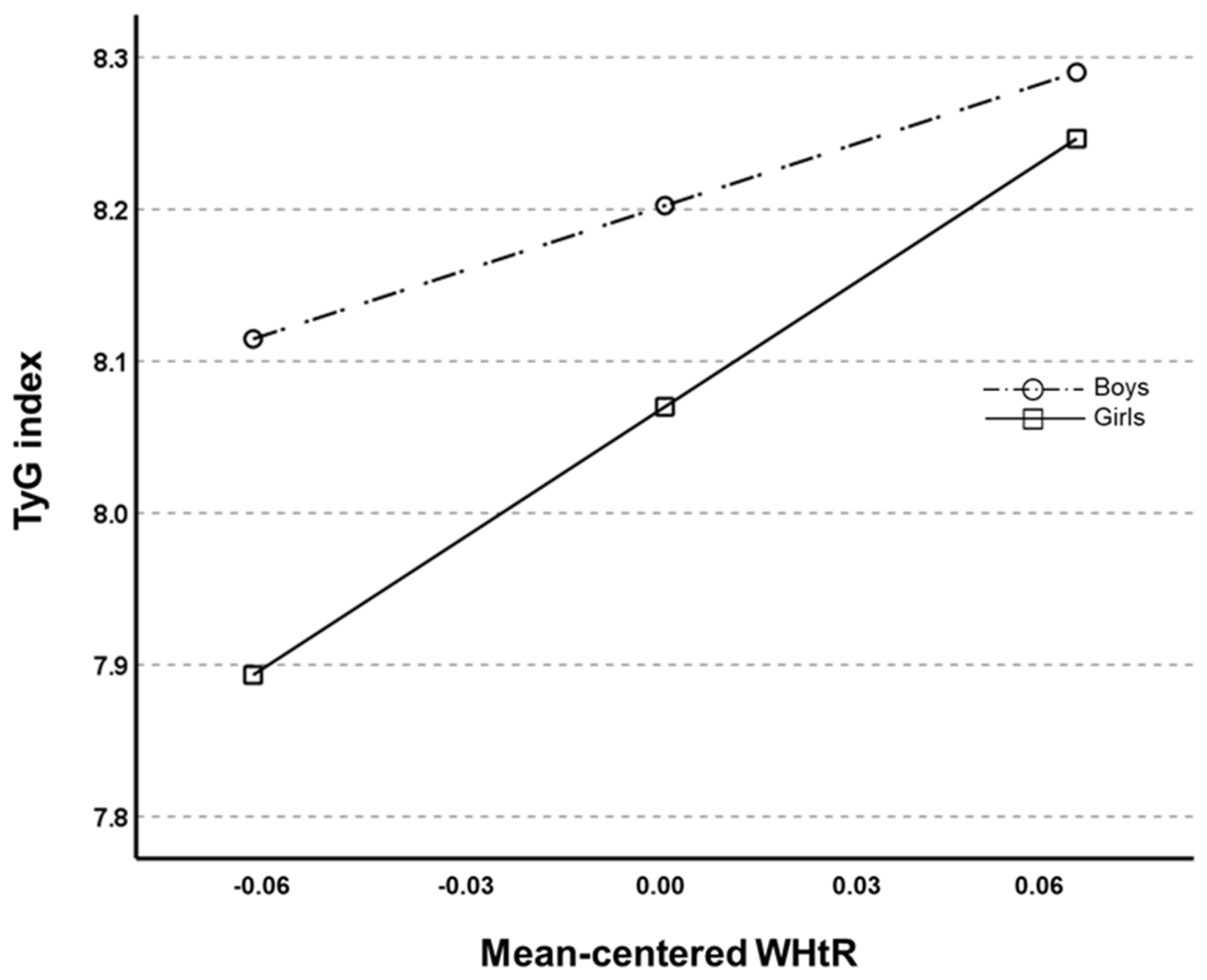
| WHtR (X) | 4.091 | 1.003 | 4.077 | 0.001 |
| Gender (W) | 0.133 | 0.048 | 2.784 | 0.006 |
| Interaction (X × W) | −1.369 | 0.631 | −2.168 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Qiu, X.; Kim, S.; Kang, H. The Role of Gender in the Relationship Between Waist-to-Hip Ratio, Triglyceride–Glucose Index, and Insulin Resistance in Korean Children. Healthcare 2025, 13, 823. https://doi.org/10.3390/healthcare13070823
Kang S, Qiu X, Kim S, Kang H. The Role of Gender in the Relationship Between Waist-to-Hip Ratio, Triglyceride–Glucose Index, and Insulin Resistance in Korean Children. Healthcare. 2025; 13(7):823. https://doi.org/10.3390/healthcare13070823
Chicago/Turabian StyleKang, Seamon, Xiaoming Qiu, Simon Kim, and Hyunsik Kang. 2025. "The Role of Gender in the Relationship Between Waist-to-Hip Ratio, Triglyceride–Glucose Index, and Insulin Resistance in Korean Children" Healthcare 13, no. 7: 823. https://doi.org/10.3390/healthcare13070823
APA StyleKang, S., Qiu, X., Kim, S., & Kang, H. (2025). The Role of Gender in the Relationship Between Waist-to-Hip Ratio, Triglyceride–Glucose Index, and Insulin Resistance in Korean Children. Healthcare, 13(7), 823. https://doi.org/10.3390/healthcare13070823






