Cytoreductive Surgery (CS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC): Postoperative Evolution, Adverse Outcomes and Perioperative Risk Factors
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Preoperative and Intraoperative Characteristics
3.2. Postoperative Evolution
3.2.1. Postoperative Complications
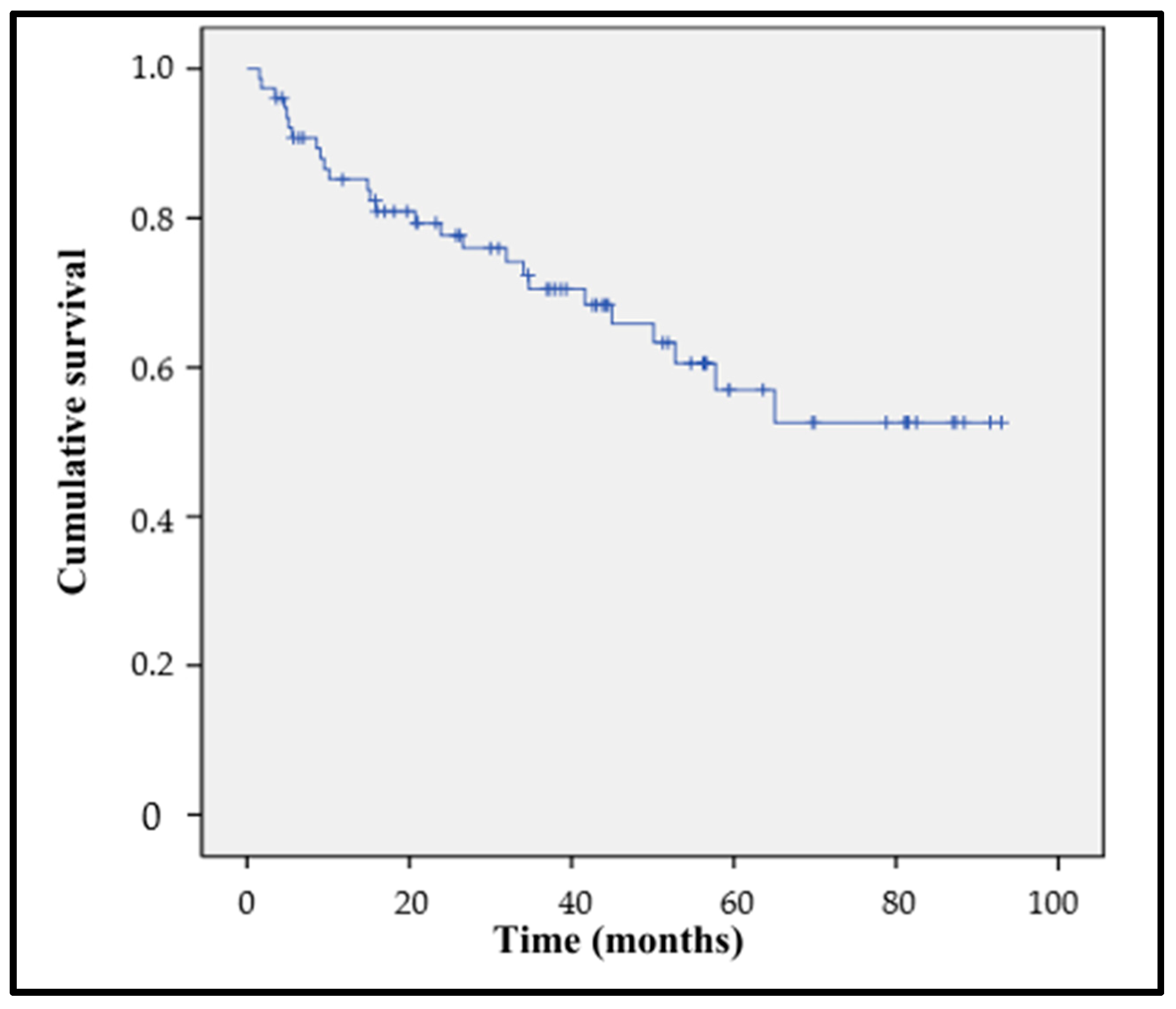
3.2.2. Postoperative Mortality
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- Alberto V, M.E.; Zuluaga, D.; Winter, A.; Pratschke, J.; Rau, B.; Gül, S. Complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy—Can we do better? J. Surg. Oncol. 2024, 130, 1403–1421. [Google Scholar] [CrossRef]
- Deng, H.; Li, B.; Qin, X. The short- and long-term survival of hyperthermic intraperitoneal chemotherapy (HIPEC) in the advanced gastric cancer with/without peritoneal carcinomatosis: A systematic review and meta-analysis of randomized controlled trials. Updates Surg. 2022, 74, 1805–1816. [Google Scholar] [CrossRef]
- Simkens, G.A.; van Oudheusden, T.R.; Luyer, M.D.; Nienhuijs, S.W.; Nieuwenhuijzen, G.A.; Rutten, H.J.; de Hingh, I.H. Serious postoperative complications affect eartly recurrence after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis. Ann. Surg. Oncol. 2015, 22, 2656–2662. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Ripollés-Melchor, J.; Abad-Motos, A.; Zorrilla-Vaca, A. Enhanced Recovery After Surgery (ERAS) in Surgical Oncology. Curr. Oncol. Rep. 2022, 24, 1177–1187. [Google Scholar] [CrossRef]
- Kepenekian V Bhatt, A.; Péron, J.; Alyami, M.; Benzerdjeb, N.; Bakrin, N.; Falandry, C.; Passot, G.; Rousset, P.; Glehen, O. Advances in the management of peritoneal malignancies. Nat. Rev. Clin. Oncol. 2022, 19, 698–718. [Google Scholar] [CrossRef]
- Becerra-Bolaños, A.; Hernández-Aguiar, Y.; Rodríguez-Pérez, A. Preoperative frailty and postoperative complications after non-cardiac surgery: A systematic review. J. Int. Med. Res. 2024, 52, 3000605241274553. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Chouliaras, K.; Levine, E.A.; Lee, B.; Votanopoulos, K.I. Frailty correlates with postoperative mortality and mayor morbidity after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2017, 24, 3825–3830. [Google Scholar] [CrossRef]
- Robella, M.; Tonello, M.; Berchialla, P.; Sciannameo, V.; Ilari Civit, A.M.; Sommariva, A.; Sassaroli, C.; Di Giorgio, A.; Gelmini, R.; Ghirardi, V.; et al. Enhaced recovery after surgery (ERAS) program for patients with peritoneal surface malignancies undergoing cytoreductive surgery with or without HIPEC: A systematic review and meta-analysis. Cancers 2023, 15, 570. [Google Scholar] [CrossRef]
- Hübner, M.; Kusamura, S.; Villeneuve, L.; Al-Niaimi, A.; Alyami, M.; Balonov, K.; Bell, J.; Bristow, R.; Guiral, D.C.; Fagotti, A.; et al. Guidelines for perioperative care in cytoreductive surgery (CRS) with or without hyperthermic intraperitoneal chemotherapy (HIPEC): Enhanced recovery after surgery (ERAS®) Society Recommendations—Part I: Preoperative and intraoperative management. Eur. J. Surg. Oncol. 2020, 46, 2292–2310. [Google Scholar] [PubMed]
- Somashekhar, S.P.; Deo, S.; Thammineedi, S.R.; Chaturvedi, H.; Mandakukutur Subramanya, G.; Joshi, R.; Kothari, J.; Srinivasan, A.; Rohit, K.C.; Ray, M.; et al. Enhanced recovery after surgery in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: National survey of peri-operative practice by Indian society of peritoneal surface malignancies. Pleura Peritoneum. 2023, 8, 91–99. [Google Scholar] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Q.; Min, Y.; Wang, S.Y.; Yang, X.J.; Liu, Y.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: A systematic review and meta-analysis of current evidence. Oncotarget 2017, 8, 55657–55683. [Google Scholar]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar]
- Lundy, M.E.; Moaven, O.; Perry, K.C.; Mangieri, C.W.; Valenzuela, C.D.; Russell, G.B.; Bordelon, R.; Shen, P.; Votanopoulos, K.I.; Levine, E.A. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Management of Colorectal Cancer with Peritoneal Dissemination: 30 Years of Experience at a Single Institution. J. Am. Coll. Surg. 2022, 234, 546–556. [Google Scholar] [CrossRef]
- Rovers, K.P.; Bakkers, C.; Nienhuijs, S.W.; Burger, J.W.A.; Creemers, G.M.; Thijs, A.M.J.; Brandt-Kerkhof, A.R.M.; Madsen, E.V.E.; van Meerten, E.; Tuynman, J.B.; et al. Perioperative Systemic Therapy vs Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Alone for Resectable Colorectal Peritoneal Metastases: A Phase 2 Randomized Clinical Trial. JAMA Surg. 2021, 156, 710–720. [Google Scholar]
- Rau, B.; Lang, H.; Koenigsrainer, A.; Gockel, I.; Rau, H.G.; Seeliger, H.; Lerchenmueller, C.; Reim, D.; Wahba, R.; Angele, M.; et al. Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer With Synchronous Peritoneal Metastases: The Phase III GASTRIPEC-I Trial. J. Clin. Oncol. 2024, 42, 146–156. [Google Scholar]
- Manzanedo, I.; Pereira, F.; Cascales-Campos, P.; Muñoz-Casares, C.; Asensio, E.; Torres-Melero, J.; Prada-Villaverde, A.; Caravaca-García, I.; Gutiérrez-Calvo, A.; Vaqué, J.; et al. Treatment of Peritoneal Surface Malignancies by Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Spain: Results of the National Registry of the Spanish Group of Peritoneal Oncologic Surgery (REGECOP). J. Clin. Med. 2023, 12, 3774. [Google Scholar] [CrossRef]
- Jafari, M.D.; Halabi, W.J.; Stamos, M.J.; Nguyen, V.Q.; Carmichael, J.C.; Mills, S.D.; Pigazzi, A. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: Analysis of the american college of surgeons national surgical quality improvement program. JAMA Surg. 2014, 149, 170–175. [Google Scholar] [CrossRef]
- Guchelaar, N.A.D.; Noordman, B.J.; Koolen, S.L.W.; Mostert, B.; Madsen, E.V.E.; Burger, J.W.A.; Brandt-Kerkhof, A.R.M.; Creemers, G.J.; de Hingh, I.H.J.T.; Luyer, M.; et al. Intraperitoneal Chemotherapy for Unresectable Peritoneal Surface Malignancies. Drugs 2023, 83, 159–180. [Google Scholar] [CrossRef] [PubMed]
- Cascales-Campos, P.A.; Sánchez-Fuentes, P.A.; Gil, J.; Gil, E.; López-López, V.; Rodriguez Gomez-Hidalgo, N.; Fuentes, D.; Parrilla, P. Effectiveness and failures of a fast track protocol after cytoreduction and hyperthermic intraoperative intraperitoneal chemotherapy in patients with peritoneal surface malignancies. Surg. Oncol. 2016, 25, 349–354. [Google Scholar] [CrossRef]
- Wajekar, A.S.; Solanki, S.L.; Patil, V.P. Postoperative complications and critical care management after cytoreduction surgery and hyperthermic intraperitoneal chemotherapy: A systematic review of the literature. World J. Crit. Care Med. 2022, 11, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Şen, Ö.; Aslan, E.; Kalaycı, D.; Küçük, A.; Başkan, S.; Sezen, Ş.C.; Arslan, M.; Ünal, Y.; Tosun, M. Evaluation of the Effect of Different Inhalation Agents on Ovaries with Hyperthermic Intraperitoneal Chemotherapy: An Experimental Study. Medicina 2024, 60, 1403. [Google Scholar] [CrossRef] [PubMed]
- Navarro Santana, B.; Garcia-Torralba, E.; Viveros-Carreño, D.; Rodriguez, J.; Pareja, R.; Martin, A.; Forte, S.; Krause, K.J.; González-Martín, J.M.; Ramirez, P.T. Complications of HIPEC for ovarian cancer surgery: Evaluation over two time periods. Int. J. Gynecol. Cancer 2024, 34, 1–9. [Google Scholar] [CrossRef]
- Patel, M.; Arora, A.; Mukherjee, D.; Mukherjee, S. Effect of hyperthermic intraperitoneal chemotherapy on survival and recurrence rates in advanced gastric cancer: A systematic review and meta-analysis. Int. J. Surg. 2023, 109, 2435–2450. [Google Scholar] [CrossRef]
- Lundbech, M.; Krag, A.E.; Iversen, L.H.; Hvas, A.M. Postoperative bleeding and venous thromboembolism in colorectal cancer patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: A systematic review and meta-analysis. Int. J. Color. Dis. 2022, 37, 17–33. [Google Scholar] [CrossRef]
- Khan, S.; Kelly, K.J.; Veerapong, J.; Lowy, A.M.; Baumgartner, J.M. Incidence, risk factors, and prevention strategies for venous thromboembolism after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2019, 26, 2276–2284. [Google Scholar] [CrossRef]
- Tao, J.; Ji, P.T.; Shen, J.J.; Lu, Y. Survival and complications of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in elderly patients: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5330–5348. [Google Scholar]
- Chen, D.; Ma, Y.; Li, J.; Wen, L.; Zhang, G.; Huang, C.; Yao, X. Risk factors for postoperative complications in patients undergoing cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: A meta-analysis and systematic review. Int. J. Color. Dis. 2024, 39, 167. [Google Scholar] [CrossRef]
- Tejedor, A.; Vendrell, M.; Bijelic, L.; Tur, J.; Bosch, M.; Martínez-Pallí, G. Predictors of major postoperative complications in cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy. Clin. Transl. Oncol. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Rubio-López, J.D.; Durán-Martínez, M.; Moreno-Blázquez, A.; Rodríguez-Ortiz, L.; Rufián-Andújar, B.; Valenzuela-Molina, F.; Adam, Á.C.; Sánchez-Hidalgo, J.M.; Rufián-Peña, S.; Romero-Ruiz, A.; et al. Intraoperative metabolic changes associated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Langenbeck’s Arch. Surg. 2023, 408, 34. [Google Scholar]
- Paulo, J.; Oliveira, J.; Silva, M.; Silva, P.; Leite, F.; Valente, R.; Sousa, A.; Lobo, M. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: Analysis of perioperative risk factors and impact on outcome. Cureus 2022, 14, e22937. [Google Scholar] [PubMed]
- Mazo, V.; Sabaté, S.; Canet, J.; Gallart, L.; de Abreu, M.G.; Belda, J.; Langeron, O.; Hoeft, A.; Pelosi, P. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014, 121, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Simkens, G.A.; van Oudheusden, T.R.; Braam, H.J.; Luyer, M.D.; Wiezer, M.J.; van Ramshorst, B.; Nienhuijs, S.W.; de Hingh, I.H. Treatment-Related Mortality After Cytoreductive Surgery and HIPEC in Patients with Colorectal Peritoneal Carcinomatosis is Underestimated by Conventional Parameters. Ann. Surg. Oncol. 2016, 23, 99–105. [Google Scholar] [CrossRef]
- Della Corte, L.; Conte, C.; Palumbo, M.; Guerra, S.; Colacurci, D.; Riemma, G.; De Franciscis, P.; Giampaolino, P.; Fagotti, A.; Bifulco, G.; et al. Hyperthermic Intraperitoneal Chemotherapy (HIPEC): New Approaches and Controversies on the Treatment of Advanced Epithelial Ovarian Cancer-Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7012. [Google Scholar] [CrossRef]
- Wang, X.; Li, T. Postoperative pain pathophysiology and treatment strategies after CRS + HIPEC for peritoneal cancer. World J. Surg. Oncol. 2020, 18, 62. [Google Scholar]
- Becerra-Bolaños, Á.; Armas-Domínguez, A.; Valencia, L.; Jiménez-Marrero, P.; López-Ruiz, S.; Rodríguez-Pérez, A. Pain prevalence and satisfaction with pain management in inpatients: A cross-sectional study. Healthcare 2023, 11, 3191. [Google Scholar] [CrossRef]
- Chua, T.C.; Yan, T.D.; Saxena, A.; Morris, D.L. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure? A systematic review of morbidity and mortality. Ann. Surg. 2009, 249, 900–907. [Google Scholar]
- Said, E.T.; Sztain, J.F.; Martin, E.I.; Abramson, W.B.; Meineke, M.N.; Furnish, T.J.; Swisher, M.W.; Gabriel, R.A. Association of trainee involvement in an acute pain service with postoperative opioid use in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Korean J. Anesthesiol. 2020, 73, 219–223. [Google Scholar] [CrossRef]


| n = 77 | ||
|---|---|---|
| Female gender, n (%) | 46 (59.7) | |
| Age, years | 61.6 ± 8.7 | |
| Height, m | 1.66 ± 0.09 | |
| Weight, kg | 73.2 ± 14.3 | |
| Body mass index, kg·m−2 | 26.37 ± 4.25 | |
| ASA | I, n (%) | 1 (1.3) |
| II, n (%) | 10 (12.9) | |
| III, n (%) | 38 (49.4) | |
| IV, n (%) | 28 (36.4) | |
| Comorbidities | Arterial hypertension, n (%) | 31 (40.3) |
| Diabetes mellitus, n (%) | 14 (18.2) | |
| Lung disease, n (%) | 7 (9.1) | |
| Heart diseases, n (%) | 6 (7.8) | |
| Chronic kidney disease, n (%) | 2 (2.6) | |
| Preoperative laboratory tests | Hemoglobin, g·dL−1 | 12.5 ± 1.6 |
| Creatinine, mg·dL−1 | 0.79 ± 0.19 | |
| Intraoperative management | Epidural analgesia, n (%) | 58 (75.3) |
| Transfusions, n (%) | 13 (16.9) | |
| Vasoactive support, n (%) | 33 (42.9) | |
| Duration of surgery, min | 477 ± 177 | |
| Immediate postoperative extubation in the operating room, n (%) | 68 (88.3) | |
| n = 77 | ||
|---|---|---|
| Postoperative therapies | Vasoactive support, n (%) | 22 (28.6) |
| Non-invasive mechanical ventilation, n (%) | 12 (15.6) | |
| Renal replacement therapies, n (%) | 4 (5.2) | |
| Immediate postoperative laboratory tests | Hemoglobin, g·dL−1 | 10.9 ± 1.9 |
| Creatinine, mg·dL−1 | 0.72 ± 0.31 | |
| Lactate, mmol·L−1 | 1.99 ± 1.69 | |
| C-reactive protein, mcg·L−1 | 81.61 ± 60.69 | |
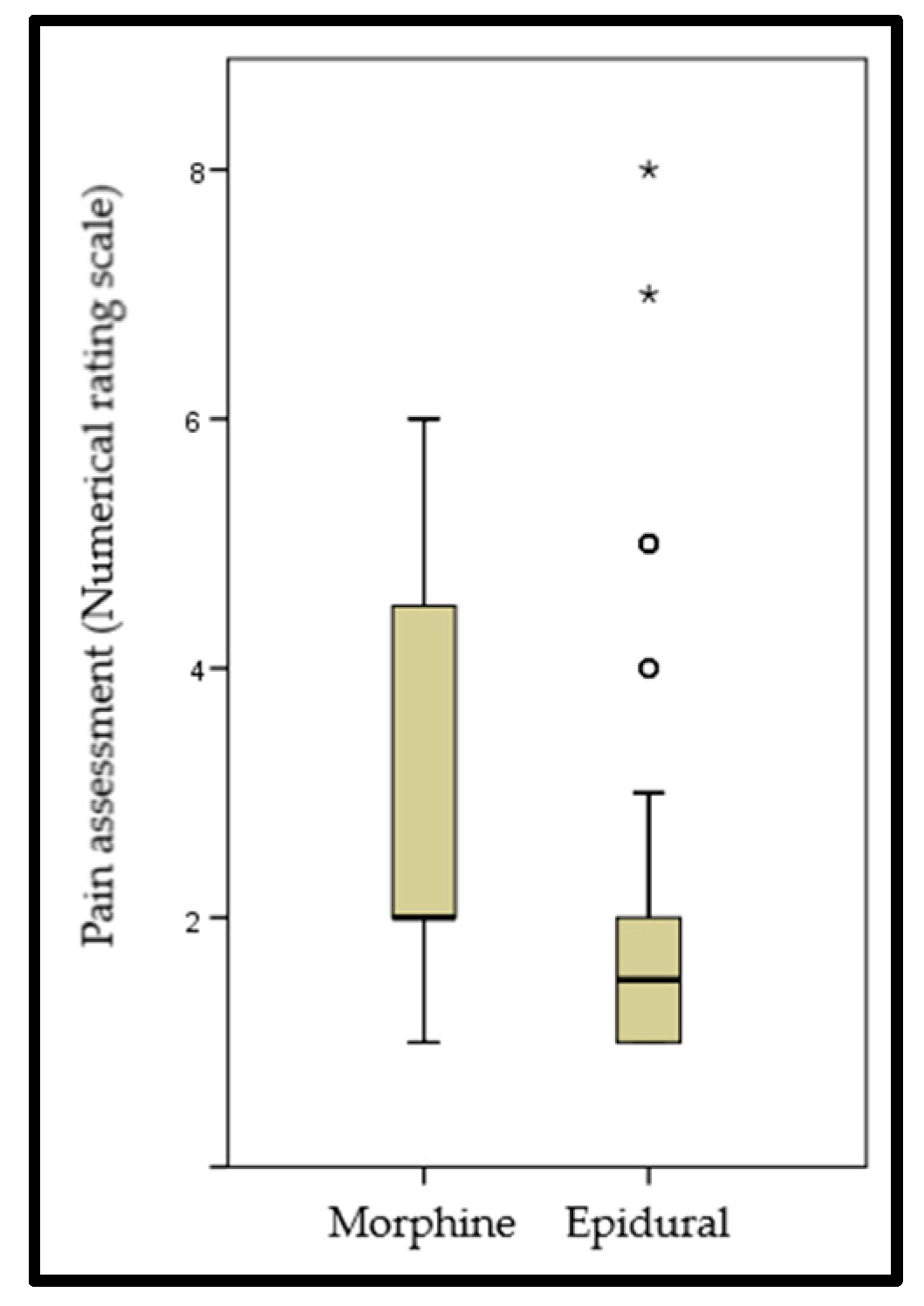
| Pain management | Epidural analgesia, n (%) | 62 (80.5) |
| Morphine, n (%) | 15 (19.5) | |
| Pain subjective assessment | Excellent, n (%) | 59 (76.6) |
| Fair, n (%) | 16 (20.8) | |
| Bad, n (%) | 2 (2.6) | |
| Numerical rating scale, score | 2.32 ± 1.68 | |
| Complications related to pain management | Poor pain control, n (%) | 7 (9.1) |
| Paresthesia, n (%) | 5 (6.5) | |
| Arterial hypotension, n (%) | 5 (6.5) | |
| Pruritus, n (%) | 1 (1.3) | |
| Renal Failure | Respiratory Failure | Adynamic Ileus | Infectious Complication | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No (n = 61) | Yes (n = 16) | p | No (n = 61) | Yes (n = 16) | p | No (n = 61) | Yes (n = 16) | p | No (n = 59) | Yes (n = 18) | p | |
| Female gender, n (%) | 34 (55.7) | 12 (75) | 0.162 | 39 (63.9) | 7 (43.7) | 0.143 | 34 (55.7) | 12 (75) | 0.162 | 37 (62.7) | 9 (50) | 0.336 |
| Age, years | 61.3 ± 8.0 | 62.8 ± 11.3 | 0.549 | 62.3 ± 7.6 | 59.1 ± 12.2 | 0.198 | 61.2 ± 8.7 | 63.4 ± 8.8 | 0.357 | 61.3 ± 9.0 | 62.8 ± 7.8 | 0.510 |
| Body mass index, kg·m−2 | 26.29 ± 4.19 | 26.68 ± 4.57 | 0.743 | 26.09 ± 4.12 | 27.45 ± 4.67 | 0.256 | 26.56 ± 4.16 | 25.67 ± 4.61 | 0.462 | 26.37 ± 4.25 | 26.37 ± 4.35 | 0.997 |
| ASA = 4, n (%) | 23 (37.7) | 5 (31.2) | 0.633 | 23 (37.7) | 5 (31.2) | 0.633 | 23 (37.7) | 5 (31.2) | 0.633 | 22 (37.3) | 6 (33.3) | 0.760 |
| Arterial hypertension, n (%) | 27 (44.3) | 4 (25) | 0.162 | 24 (39.3) | 7 (43.7) | 0.749 | 27 (44.3) | 4 (25) | 0.162 | 24 (40.7) | 7 (38.9) | 0.892 |
| Diabetes mellitus, n (%) | 13 (21.3) | 1 (6.2) | 0.164 | 10 (16.4) | 4 (25.0) | 0.427 | 12 (19.7) | 2 (12.5) | 0.508 | 12 (20.3) | 2 (11.1) | 0.374 |
| Lung disease, n (%) | 4 (6.6) | 3 (18.7) | 0.131 | 5 | 2 | 0.594 | 3 (4.9) | 4 (25) | 0.013 | 6 (10.2) | 1 (5.5) | 0.551 |
| Heart diseases, n (%) | 5 (8.2) | 1 (6.2) | 0.796 | 3 (4.9) | 3 (18.7) | 0.066 | 5 (8.2) | 1 (6.2) | 0.796 | 4 (6.8) | 2 (11.1) | 0.548 |
| Chronic kidney disease, n (%) | 2 (3.3) | 0 (0) | 0.463 | 2 (3.3) | 0 (0) | 0.463 | 2 (3.3) | 0 (0) | 0.463 | 2 (3.4) | 0 (0) | 0.429 |
| Hemoglobin, g·dL−1 | 12.4 ± 1.7 | 12.9 ± 1.8 | 0.340 | 12.5 ± 1.7 | 12.6 ± 1.9 | 0.737 | 12.5 ± 1.8 | 12.5 ± 1.7 | 0.934 | 12.6 ± 1.8 | 12.3 ± 1.7 | 0.584 |
| Creatinine, mg·dL−1 | 0.81 ± 0.21 | 0.73 ± 0.121 | 0.197 | 0.79 ± 0.21 | 0.80 ± 0.15 | 0.813 | 0.81 ± 0.21 | 0.72 ± 0.15 | 0.128 | 0.79 ± 0.21 | 0.81 ± 0.13 | 0.674 |
| Epidural analgesia, n (%) | 44 (72.1) | 14 (87.5) | 0.204 | 45 (73.8) | 13 (81.2) | 0.537 | 44 (72.1) | 14 (87.5) | 0.204 | 45 (76.3) | 13 (72.2) | 0.727 |
| Transfusions, n (%) | 8 (13.1) | 5 (31.2) | 0.085 | 10 (16.4) | 3 (18.7) | 0.823 | 10 (16.4) | 3 (18.7) | 0.823 | 8 (13.5) | 5 (27.8) | 0.159 |
| Vasoactive support, n (%) | 24 (39.3) | 9 (56.2) | 0.224 | 26 (42.6) | 7 (43.7) | 0.935 | 26 (42.6) | 7 (43.7) | 0.935 | 25 (42.4) | 8 (44.4) | 0.876 |
| Duration of surgery, min | 455 ± 183 | 566 ± 117 | 0.029 | 459 ± 169 | 544 ± 197 | 0.089 | 443 ± 166 | 604 ± 163 | 0.001 | 457 ± 189 | 541 ± 110 | 0.024 |
| Extubation in the OR, n (%) | 56 (91.8) | 12 (75) | 0.063 | 55 (90.2) | 13 (81.2) | 0.323 | 55 (90.2) | 13 (81.2) | 0.323 | 53 (89.8) | 15 (83.3) | 0.453 |
| Renal Failure | Respiratory Failure | Adynamic Ileus | Infectious Complication | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No (n = 61) | Yes (n = 16) | p | No (n = 61) | Yes (n = 16) | p | No (n = 61) | Yes (n = 16) | p | No (n = 59) | Yes (n = 18) | p | |
| Post-vasoactive support, n (%) | 14 (22.9) | 8 (50) | 0.033 | 16 (26.2) | 6 (37.5) | 0.374 | 15 (24.6) | 7 (43.7) | 0.131 | 14 (23.7) | 8 (44.4) | 0.089 |
| Post-NIMV, n (%) | 6 (9.8) | 6 (37.5) | 0.007 | 7 (11.5) | 5 (31.2) | 0.052 | 8 (13.1) | 4 (25) | 0.243 | 6 (10.2) | 6 (33.3) | 0.018 |
| Post-RRTs, n (%) | 1 (1.6) | 3 (18.7) | 0.006 | 1 (1.6) | 3 (18.7) | 0.006 | 2 (3.3) | 2 (12.5) | 0.139 | 1 (1.7) | 3 (16.7) | 0.012 |
| Post-hemoglobin, g·dL−1 | 11.1 ± 1.8 | 10.4 ± 1.8 | 0.161 | 10.8 ± 1.8 | 11.6 ± 1.9 | 0.140 | 11.16 ± 1.83 | 10.26 ± 1.92 | 0.088 | 11.17 ± 1.94 | 10.33 ± 1.47 | 0.098 |
| Post-creatinine, mg·dL−1 | 0.71 ± 0.24 | 0.78 ± 0.48 | 0.375 | 0.67 ± 0.21 | 0.93 ± 0.48 | 0.001 | 0.73 ± 0.24 | 0.71 ± 0.49 | 0.826 | 0.69 ± 0.26 | 0.81 ± 0.43 | 0.164 |
| Lactate, mmol·L−1 | 1.86 ± 1.66 | 2.48 ± 1.79 | 0.196 | 1.88 ± 1.71 | 2.39 ± 1.63 | 0.288 | 2.03 ± 1.74 | 1.81 ± 1.55 | 0.643 | 1.96 ± 1.74 | 2.06 ± 1.60 | 0.831 |
| C-reactive protein, mcg·L−1 | 84.1 ± 66.3 | 71.6 ± 27.7 | 0.481 | 74.6 ± 49.4 | 108.0 ± 88.8 | 0.049 | 80.7 ± 66.9 | 84.9 ± 27.9 | 0.807 | 79.7 ± 62.2 | 87.9 ± 56.7 | 0.619 |
| Analgesic protocol: Morphine, n (%) | 11 (18) | 4 (25) | 0.531 | 14 (22.9) | 1 (6.2) | 0.133 | 10 (16.4) | 5 (31.2) | 0.182 | 11 (18.6) | 4 (22.2) | 0.737 |
| NRS, score | 2.3 ± 1.7 | 2.4 ± 1.6 | 0.894 | 2.5 ± 1.8 | 1.7 ± 1.2 | 0.060 | 2.2 ± 1.6 | 2.9 ± 1.9 | 0.142 | 2.4 ± 1.7 | 2.2 ± 1.5 | 0.652 |
| Mortality 12 Months | Overall Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| No (n = 65) | Yes (n = 12) | OR (95% CI) or md (95% CI) | p | No (n = 51) | Yes (n = 26) | OR (95% CI) or md (95% CI) | p | |
| Female gender, n (%) | 24 (36.9) | 7 (58.3) | 2.4 (0.7–8.4) | 0.165 | 33 (64.7) | 13 (50) | 1.8 (0.7–4.8) | 0.213 |
| Age, years | 61.5 ± 9.0 | 62.6 ± 7.2 | 1.1 (−4.4–6.6) | 0.686 | 61.0 ± 9.8 | 63 ± 6 | 1.9 (−2.3–6.1) | 0.374 |
| Body mass index, kg·m−2 | 26.84 ± 4.41 | 23.85 ± 1.84 | −2.9 (−5.6–−0.4) | <0.001 | 27.01 ± 4.64 | 25.12 ± 3.03 | −1.9 (−3.6–−0.1) | 0.035 |
| ASA = 4, n (%) | 26 (40) | 2 (16.7) | 0.3 (0.1–1.5) | 0.123 | 20 (39.2) | 8 (30.8) | 0.7 (0.2–1.9) | 0.466 |
| Arterial hypertension, n (%) | 28 (43.1) | 3 (25) | 0.4 (0.1–1.8) | 0.241 | 23 (45.1) | 8 (30.8) | 0.5 (0.2–1.5) | 0.225 |
| Diabetes mellitus, n (%) | 13 (20) | 1 (8.3) | 0.4 (0.0–3.1) | 0.336 | 9 (17.6) | 5 (19.2) | 1.1 (0.3–3.7) | 0.865 |
| Preop hemoglobin, g·dL−1 | 12.79 ± 1.59 | 11.06 ± 1.96 | −1.7 (−2.8–−0.7) | 0.001 | 12.88 ± 1.69 | 11.79 ± 1.68 | −1.1 (−1.9–−0.3) | 0.010 |
| Preop creatinine, mg·dL−1 | 0.81 ± 0.2 | 0.69 ± 0.12 | −0.1 (−0.2–−0.0) | 0.007 | 0.83 ± 0.2 | 0.72 ± 0.17 | −0.1 (−0.2–−0.0) | 0.024 |
| Epidural analgesia, n (%) | 52 (80) | 6 (50) | 0.2 (0.1–0.9) | 0.027 | 42 (82.3) | 16 (61.5) | 0.3 (0.1–0.9) | 0.045 |
| Transfusions, n (%) | 11 (16.9) | 2 (16.7) | 0.9 (0.2–5.1) | 0.983 | 8 (15.7) | 5 (19.2) | 1.3 (0.4–4.4) | 0.695 |
| Vasoactive support, n (%) | 29 (44.6) | 4 (33.3) | 0.6 (0.2–2.3) | 0.468 | 22 (43.1) | 11 (42.3) | 0.9 (0.4–2.5) | 0.945 |
| Extubation in the OR, n (%) | 57 (87.7) | 11 (91.7) | 1.5 (0.2–13.6) | 0.694 | 45 (88.2) | 23 (88.5) | 1.0 (0.2–4.6) | 0.977 |
| Post-vasoactive support, n (%) | 21 (32.3) | 1 (8.3) | 0.2 (0.0–1.6) | 0.091 | 15 (29.4) | 7 (26.9) | 0.9 (0.3–2.5) | 0.819 |
| Post-NIMV, n (%) | 9 (13.8) | 3 (25) | 2.1 (0.5–9.1) | 0.328 | 8 (15.7) | 4 (15.4) | 0.9 (0.3–3.6) | 0.972 |
| Post-RRTs, n (%) | 4 (6.1) | 0 (0) | - | 0.377 | 3 (5.9) | 1 (3.8) | 0.6 (0.1–6.5) | 0.703 |
| Hemoglobin, g·dL−1 | 10.79 ± 1.57 | 11.94 ± 2.92 | 1.1 (0.0–2.3) | 0.049 | 10.81 ± 1.48 | 11.29 ± 2.46 | 0.5 (−0.4–1.4) | 0.284 |
| Creatinine, mg·dL−1 | 0.71 ± 0.91 | 0.77 ± 0.31 | 0.1 (−0.1–0.2) | 0.558 | 0.83 ± 0.2 | 0.72 ± 0.28 | −0.0 (−0.1–0.1) | 0.987 |
| Lactate, mmol·L−1 | 1.87 ± 1.55 | 2.58 ± 2.31 | 0.7 (−0.3–1.8) | 0.184 | 1.87 ± 1.59 | 2.21 ± 1.89 | 0.3 (−0.5–1.1) | 0.413 |
| C-reactive protein, mcg·L−1 | 72.9 ± 49.4 | 127.6 ± 91.5 | 54.6 (18.5–90.8) | 0.004 | 69.0 ± 40.6 | 107.2 ± 84.0 | 38.2 (9.8–66.6) | 0.040 |
| Analgesic protocol: Morphine, n (%) | 13 (20) | 2 (16.7) | 1.2 (0.2–6.4) | 0.789 | 10 (19.6) | 5 (19.2) | 1.0 (0.3–3.4) | 0.968 |
| NRS, score | 2.4 ± 1.7 | 1.8 ± 1.2 | −0.5 (−1.6–0.5) | 0.273 | 2.4 ± 1.8 | 2.1 ± 1.4 | −0.3 (−1.1–0.5) | 0.528 |
| p | Exp (B) | 95% CI of Exp (B) | ||
|---|---|---|---|---|
| 1st Step | BMI > 30 kg·m−2 | 0.304 | 0.464 | 0.108–2.003 |
| Preoperative hemoglobin < 10 g·dL−1 | 0.945 | 0.936 | 0.142–6.181 | |
| Preoperative creatinine > 1.1 mg·dL−1 | 0.402 | 0.373 | 0.037–3.747 | |
| Non-intraoperative epidural analgesia | 0.049 | 3.146 | 1.004–9.861 | |
| C-reactive protein > 75 mcg·L−1 | 0.097 | 2.445 | 0.850–7.026 | |
| Constant | 0.005 | 0.281 | ||
| 2nd Step | BMI > 30 kg·m−2 | 0.304 | 0.467 | 0.110–1.991 |
| Preoperative creatinine > 1.1 mg·dL−1 | 0.404 | 0.376 | 0.038–3.738 | |
| Non-intraoperative epidural analgesia | 0.047 | 3.126 | 1.013–9.647 | |
| C-reactive protein > 75 mcg·L−1 | 0.091 | 2.424 | 0.867–6.780 | |
| Constant | 0.005 | 0.281 | ||
| 3rd Step | BMI > 30 kg·m−2 | 0.350 | 0.503 | 0.119–2.124 |
| Non-intraoperative epidural analgesia | 0.053 | 3.002 | 0.988–9.123 | |
| C-reactive protein > 75 mcg·L−1 | 0.081 | 2.488 | 0.895–6.918 | |
| Constant | 0.003 | 0.260 | ||
| 4th Step | Non-intraoperative epidural analgesia | 0.040 | 3.193 | 1.054–9.676 |
| C-reactive protein > 75 mcg·L−1 | 0.066 | 2.592 | 0.940–7.149 | |
| Constant | 0.000 | 0.223 | ||
| 5th Step | Non-intraoperative epidural analgesia | 0.039 | 3.111 | 1.060–9.128 |
| Constant | 0.001 | 0.357 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Sola, L.; Becerra-Bolaños, Á.; Mateo-Ferragut, M.; Muiño-Palomar, V.; Ojeda-Betancor, N.; Rodríguez-Pérez, A. Cytoreductive Surgery (CS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC): Postoperative Evolution, Adverse Outcomes and Perioperative Risk Factors. Healthcare 2025, 13, 808. https://doi.org/10.3390/healthcare13070808
Valencia-Sola L, Becerra-Bolaños Á, Mateo-Ferragut M, Muiño-Palomar V, Ojeda-Betancor N, Rodríguez-Pérez A. Cytoreductive Surgery (CS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC): Postoperative Evolution, Adverse Outcomes and Perioperative Risk Factors. Healthcare. 2025; 13(7):808. https://doi.org/10.3390/healthcare13070808
Chicago/Turabian StyleValencia-Sola, Lucía, Ángel Becerra-Bolaños, María Mateo-Ferragut, Virginia Muiño-Palomar, Nazario Ojeda-Betancor, and Aurelio Rodríguez-Pérez. 2025. "Cytoreductive Surgery (CS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC): Postoperative Evolution, Adverse Outcomes and Perioperative Risk Factors" Healthcare 13, no. 7: 808. https://doi.org/10.3390/healthcare13070808
APA StyleValencia-Sola, L., Becerra-Bolaños, Á., Mateo-Ferragut, M., Muiño-Palomar, V., Ojeda-Betancor, N., & Rodríguez-Pérez, A. (2025). Cytoreductive Surgery (CS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC): Postoperative Evolution, Adverse Outcomes and Perioperative Risk Factors. Healthcare, 13(7), 808. https://doi.org/10.3390/healthcare13070808








