Impact of Manual Therapy on Plantar Pressures in Patients with Fibromyalgia: A Single-Arm, Non-Randomized Pilot Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
2.3.1. Manual Therapy Protocol
2.3.2. Treatment Sequence
2.3.3. Technique Applied
2.4. Measurements
2.4.1. Baropodometric Analysis
2.4.2. Variables
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
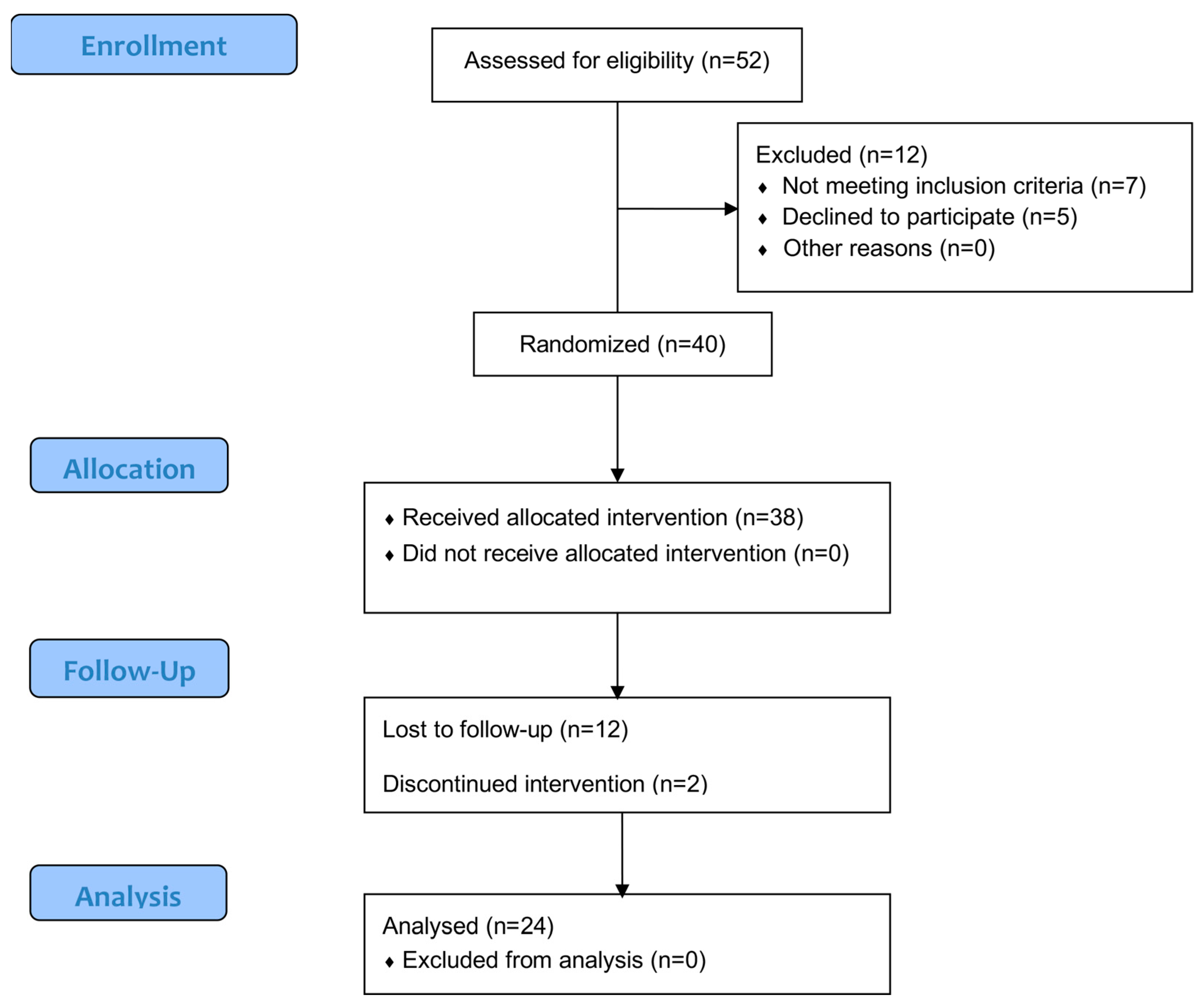
3.1. Participation Flow and Sample Characteristics
3.2. Results of Baropodometric Analysis of the Total Sample Pre-Post
3.3. Results of Baropodometric Analysis Divided into BMI Groups
4. Discussion
Limitations and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| FM | Fibromyalgia |
| MT | Manual Therapy |
| BMI | Body Mass Index |
| PCS | Physical Component Summary |
| MCS | Mental Component Summary |
| RFS | Right Foot Surface |
| LFS | Left Foot Surface |
| P.Max | Max Pressure |
| P.Med | Media Pressure |
| PD | Right Foot |
| PI | Left Foot |
| NCT | National Clinical Trial |
| SPSS | Statistical Package for the Social Sciences |
| JASP | Jeffreys’s Amazing Statistics Program |
| ET | Effect Size |
| ACR | American College of Rheumatology |
| CGCOP | General Council of Official Colleges of Podiatry |
References
- Clauw, D.J.; D’Arcy, Y.; Gebke, K.; Semel, D.; Pauer, L.; Jones, K.D. Normalizing Fibromyalgia as a Chronic Illness. Postgrad. Med. 2018, 130, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H. Controversies and Challenges in Fibromyalgia: A Review and a Proposal. Ther. Adv. Musculoskelet. Dis. 2017, 9, 115–127. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC5394529/ (accessed on 28 February 2025).
- Heidari, F.; Afshari, M.; Moosazadeh, M. Prevalence of Fibromyalgia in General Population and Patients, a Systematic Review and Meta-Analysis. Rheumatol. Int. 2017, 37, 1527–1539. Available online: https://link.springer.com/article/10.1007/s00296-017-3725-2 (accessed on 28 February 2025). [PubMed]
- Cheng, C.W.; Wong, C.S.; Hui, G.K.; Chung, E.K.; Wong, S.H. Fibromyalgia: Is It a Neuropathic Pain? Pain Manag. 2018, 8, 377–388. [Google Scholar] [CrossRef]
- Wolfe, F.; Butler, S.H.; Fitzcharles, M.; Häuser, W.; Katz, R.L.; Mease, P.J.; Rasker, J.J.; Russell, A.S.; Russell, I.J.; Walitt, B. Revised Chronic Widespread Pain Criteria: Development from and Integration with Fibromyalgia Criteria. Scand. J. Pain 2020, 20, 77–86. Available online: https://pubmed.ncbi.nlm.nih.gov/31596726/ (accessed on 28 February 2025).
- Bennett, R.M.; Jones, J.; Turk, D.C.; Russell, I.J.; Matallana, L. An Internet Survey of 2,596 People with Fibromyalgia. BMC Musculoskelet. Disord. 2007, 8, 27. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic Encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Sommer, C.; Häuser, W.; Gerhold, K.; Joraschky, P.; Petzke, F.; Tölle, T.; Üçeyler, N.; Winkelmann, A.; Thieme, K. Ätiopathogenese Und Pathophysiologie Des Fibromyalgiesyndroms Und Chronischer Schmerzen in Mehreren Körperregionen. Schmerz 2008, 22, 267–282. Available online: https://link.springer.com/article/10.1007/s00482-008-0672-6 (accessed on 28 February 2025).
- Clauw, D.J. Fibromyalgia and Related Conditions. Mayo Clin. Proc. 2015, 90, 680–692. [Google Scholar] [CrossRef]
- Rehm, S.; Sachau, J.; Hellriegel, J.; Forstenpointner, J.; Børsting Jacobsen, H.; Harten, P.; Gierthmühlen, J.; Baron, R. Pain Matters for Central Sensitization: Sensory and Psychological Parameters in Patients with Fibromyalgia Syndrome. Pain Rep. 2021, 6, e901. [Google Scholar] [CrossRef]
- Mezhov, V.; Guymer, E.; Littlejohn, G. Central Sensitivity and Fibromyalgia. Intern. Med. J. 2021, 51, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Harrison, J.E.; Weber, S.; Jakob, R.; Chute, C.G. ICD-11: An International Classification of Diseases for the Twenty-First Century. BMC Med. Inform. Decis. Mak. 2021, 21, 206. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, F.Y.; Feng, C.Q.; Yang, X.F.; Sun, Y.H. Massage Therapy for Fibromyalgia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2014, 9, e89304. [Google Scholar] [CrossRef]
- Falaguera-Vera, F.J.; Garcia-Escudero, M.; Bonastre-Férez, J.; Zacarés, M.; Oltra, E. Pressure Point Thresholds and ME/CFS Comorbidity as Indicators of Patient’s Response to Manual Physiotherapy in Fibromyalgia. Int. J. Environ. Res. Public Health 2020, 17, 8044. [Google Scholar] [CrossRef]
- Crawford, C.; Boyd, C.; Paat, C.F.; Price, A.; Xenakis, L.; Yang, E.M.; Zhang, W.; Buckenmaier, C.; Buckenmaier, P.; Cambron, J.; et al. The Impact of Massage Therapy on Function in Pain Populations—A Systematic Review and Meta-Analysis of Randomized Controlled Trials: Part I, Patients Experiencing Pain in the General Population. Pain. Med. 2016, 17, 1353–1375. [Google Scholar] [CrossRef]
- Metyas, C.; Aung, T.T.; Cheung, J.; Joseph, M.; Ballester, A.M.; Metyas, S. Diet and Lifestyle Modifications for Fibromyalgia. Curr. Rheumatol. Rev. 2024, 20, 405–413. [Google Scholar] [CrossRef]
- Collado, A.; Gomez, E.; Coscolla, R.; Sunyol, R.; Solé, E.; Rivera, J.; Altarriba, E.; Carbonell, J.; Castells, X. Work, Family and Social Environment in Patients with Fibromyalgia in Spain: An Epidemiological Study: EPIFFAC Study. BMC Health Serv. Res. 2014, 14, 513. [Google Scholar] [CrossRef]
- Buskila, D.; Neumann, L.; Odes, L.R.; Schleifer, E.; Depsames, R.; Abu-Shakra, M. The Prevalence of Musculoskeletal Pain and Fibromyalgia in Patients Hospitalized on Internal Medicine Wards. Semin. Arthritis Rheum. 2001, 30, 411–417. [Google Scholar] [CrossRef]
- Almenar-Pérez, E.; Sánchez-Fito, T.; Ovejero, T.; Nathanson, L.; Oltra, E. Impact of Polypharmacy on Candidate Biomarker MiRNomes for the Diagnosis of Fibromyalgia and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Striking Back on Treatments. Pharmaceutics 2019, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Flub, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR Revised Recommendations for the Management of Fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.A.; Ste-Marie, P.A.; Goldenberg, D.L.; Pereira, J.X.; Abbey, S.; Choinière, M.; Ko, G.; Moulin, D.E.; Panopalis, P.; Proulx, J.; et al. 2012 Canadian Guidelines for the Diagnosis and Management of Fibromyalgia Syndrome: Executive Summary. Pain Res. Manag. 2013, 18, 119–126. [Google Scholar] [CrossRef]
- Bonastre-Férez, J.; Giménez-Orenga, K.; Falaguera-Vera, F.J.; Garcia-Escudero, M.; Oltra, E. Manual Therapy Improves Fibromyalgia Symptoms by Downregulating SIK1. Int. J. Mol. Sci. 2024, 25, 9523. [Google Scholar] [CrossRef]
- Carrasco-Vega, E.; Guiducci, S.; Nacci, F.; Randone, S.B.; Bevilacqua, C.; Gonzalez-Sanchez, P.M.; Barni, L. Efficacy of Physiotherapy Treatment in Medium and Long Term in Adults with Fibromyalgia: An Umbrella of Systematic Reviews. Clin. Exp. Rheumatol. 2024, 42, 1248–1261. [Google Scholar] [CrossRef]
- Qureshi, A.G.; Jha, S.K.; Iskander, J.; Avanthika, C.; Jhaveri, S.; Patel, V.H.; Rasagna Potini, B.; Talha Azam, A. Diagnostic Challenges and Management of Fibromyalgia. Cureus 2021, 13, 10. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Pellegrino, G.; Giorgi, V.; Bongiovanni, S.F.; Varrassi, G.; Di Lascio, S.; Fornasari, D.; Sirotti, S.; Di Carlo, M.; Salaffi, F. Inflammatory or Non-Inflammatory Pain in Inflammatory Arthritis–How to Differentiate It? Best. Pract. Res. Clin. Rheumatol. 2024, 38, 101970. [Google Scholar] [CrossRef]
- Öz, N.; Özer, A.; Duruöz, M.T. Central Sensitization and Its Role in Persistent Pain Among Spondyloarthritis Patients on Biological Treatments. Medicina 2025, 61, 319. [Google Scholar] [CrossRef]
- Mesci, E.; Mesci, N.; Karatekin, B.D.; İçağasıoğlu, A. Can Early Fatigue in Leg Muscles After Exercise Cause Postural Instability in Women With Fibromyalgia? J. Musculoskelet. Neuronal Interact. 2023, 23, 338. [Google Scholar]
- Aoyagi, K.; Sharma, N.K. Correlation Between Central Sensitization and Remote Muscle Performance in Individuals With Chronic Low Back Pain. J. Manipulative Physiol. Ther. 2021, 44, 14–24. [Google Scholar] [CrossRef]
- Carrasco-Vega, E.; Ruiz-Muñoz, M.; Cuesta-Vargas, A.; Romero-Galisteo, R.P.; González-Sánchez, M. Individuals with Fibromyalgia Have a Different Gait Pattern and a Reduced Walk Functional Capacity: A Systematic Review with Meta-Analysis. PeerJ 2022, 10, e12908. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Fuentes, D.; Obrero-Gaitán, E.; Zagalaz-Anula, N.; Ibáñez-Vera, A.J.; Achalandabaso-Ochoa, A.; López-Ruiz, M.D.C.; Rodríguez-Almagro, D.; Lomas-Vega, R. Alteration of Postural Balance in Patients with Fibromyalgia Syndrome—A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Tornero-Caballero, M.C.; Salom-Moreno, J.; Cigarán-Méndez, M.; Morales-Cabezas, M.; Madeleine, P.; Fernándezde-Las-Peñas, C. Muscle Trigger Points and Pressure Pain Sensitivity Maps of the Feet in Women with Fibromyalgia Syndrome. Pain Med. 2016, 17, 1923–1932. [Google Scholar] [CrossRef]
- Ciaffi, J.; Brognara, L.; Gangemi, G.; Vanni, E.; Assirelli, E.; Neri, S.; Casadei, G.; Mazzotti, A.; Di Martino, A.; Faldini, C.; et al. Prevalence and Characteristics of Fibromyalgia in Patients with Foot and Ankle Pain: The Experience of an Academic Podiatry Clinic. Medicina 2022, 59, 58. [Google Scholar] [CrossRef]
- Padín Galea, J.M.; Fernández-Aceñero, M.J.; de la Fuente, J.L.M. Characteristics of Patients with Fibromyalgia. Foot 2017, 32, 27–29. [Google Scholar] [CrossRef]
- López-Muñoz, S.; Gracia-Vesga, M.Á.; Gracia-Sánchez, A.; Zúnica-Garcia, S.; Gijón-Nogueron, G.; Chicharro-Luna, E. Impact of Fibromyalgia and Related Factors on Foot Function and Quality of Life: Cross-Sectional Study. Foot Ankle Surg. 2023, 29, 627–632. [Google Scholar] [CrossRef]
- Reddy, R.S.; Alkhamis, B.A.; Kirmani, J.A.; Uddin, S.; Ahamed, W.M.; Ahmad, F.; Ahmad, I.; Raizah, A. Age-Related Decline in Cervical Proprioception and Its Correlation with Functional Mobility and Limits of Stability Assessed Using Computerized Posturography: A Cross-Sectional Study Comparing Older (65+ Years) and Younger Adults. Healthcare 2023, 11, 1924. [Google Scholar] [CrossRef]
- Soriano-Maldonado, A.; Henriksen, M.; Segura-Jiménez, V.; Aparicio, V.A.; Carbonell-Baeza, A.; Delgado-Fernández, M.; Amris, K.; Ruiz, J.R. Association of Physical Fitness with Fibromyalgia Severity in Women: The al-Ándalus Project. Arch. Phys. Med. Rehabil. 2015, 96, 1599–1605. [Google Scholar] [CrossRef]
- Silva, A.P.; Chagas, D.d.V.; Cavaliere, M.L.; Pinto, S.; de Oliveira Barbosa, J.S.; Batista, L.A. Kinematic Analysis of Subtalar Eversion during Gait in Women with Fibromyalgia. Foot 2016, 28, 42–46. [Google Scholar] [CrossRef]
- Serpas, D.G.; Zettel-Watson, L.; Cherry, B.J. Pain Intensity and Physical Performance among Individuals with Fibromyalgia in Mid-to-Late Life: The Influence of Depressive Symptoms. J. Health Psychol. 2022, 27, 1723–1737. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Begg, C.; Cho, M.; Eastwood, S.; Horton, R.; Moher, D.; Olkin, I.; Pitkin, R.; Rennie, D.; Schulz, K.F.; Simel, D.; et al. Improving the Quality of Reporting of Randomized Controlled Trials. The CONSORT Statement. JAMA 1996, 276, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; Von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lemer, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Field, T.; Diego, M.; Cullen, C.; Hernandez-Reif, M.; Sunshine, W.; Douglas, S. Fibromyalgia Pain and Substance P Decrease and Sleep Improves after Massage Therapy. J. Clin. Rheumatol. 2002, 8, 72–76. [Google Scholar] [CrossRef]
- Yuan, S.L.K.; Matsutani, L.A.; Marques, A.P. Effectiveness of Different Styles of Massage Therapy in Fibromyalgia: A Systematic Review and Meta-Analysis. Man. Ther. 2015, 20, 257–264. [Google Scholar] [CrossRef]
- Espejo, J.A.; García-Escudero, M.; Oltra, E. Unraveling the Molecular Determinants of Manual Therapy: An Approach to Integrative Therapeutics for the Treatment of Fibromyalgia and Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. Int. J. Mol. Sci. 2018, 19, 2673. [Google Scholar] [CrossRef]
- Waters-Banker, C.; Butterfield, T.A.; Dupont-Versteegden, E.E. Immunomodulatory Effects of Massage on Nonperturbed Skeletal Muscle in Rats. J. Appl. Physiol. (1985) 2014, 116, 164–175. [Google Scholar] [CrossRef]
- Gravante, G.; Russo, G.; Pomara, F.; Ridola, C. Comparison of Ground Reaction Forces between Obese and Control Young Adults during Quiet Standing on a Baropodometric Platform. Clin. Biomech. 2003, 18, 780–782. [Google Scholar] [CrossRef]
- Fabris, S.M.; Valezi, A.C.; Fabris De Souza, S.A.; Faintuch, J.; Cecconello, I.; Pedroni, M. Computerized Baropodometry in Obese Patients. Obes. Surg. 2006, 16, 1574–1578. [Google Scholar] [CrossRef]
- de Bengoa Vallejo, R.B.; Iglesias, M.E.L.; Zeni, J.; Thomas, S. Reliability and Repeatability of the Portable EPS-Platform Digital Pressure-Plate System. J. Am. Podiatr. Med. Assoc. 2013, 103, 197–203. [Google Scholar] [CrossRef]
- Cobos-Moreno, P.; Astasio-Picado, Á.; Martínez- Nova, A.; Sánchez- Rodríguez, R.; Escamilla-Martínez, E.; Gómez-Martín, B. The Podoprint® Plantar Pressure Platform: Evaluation of Reliability and Repeatability, and Determination of the Normality Parameters. J. Tissue Viability 2022, 31, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Available online: https://iris.who.int/handle/10665/42330 (accessed on 28 February 2025).
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Weng, C.; Yan, J.; Fang, Y. Epidemiological Characteristics and Factors Influencing Falls among Elderly Adults in Long-Term Care Facilities in Xiamen, China. Medicine 2019, 98, e14375. [Google Scholar] [CrossRef]
- Jurado-Priego, L.N.; Cueto-Ureña, C.; Ramírez-Expósito, M.J.; Martínez-Martos, J.M. Fibromyalgia: A Review of the Pathophysiological Mechanisms and Multidisciplinary Treatment Strategies. Biomedicines 2024, 12, 1543. [Google Scholar] [CrossRef]
- Ahn, J.H.; Seon, C.W.; Kim, B.J.; Park, I.H.; Cha, Y.Y. Efficacy of Manual Therapy for Fibromyalgia Syndrome: A Systematic Review and Meta-Analysis. J. Churna Manual Med. Spine Nerves 2023, 18, 29–42. [Google Scholar] [CrossRef]
- Hernando-Garijo, I.; Jiménez-Del-Barrio, S.; Mingo-Gómez, T.; Medrano-De-La-Fuente, R.; Ceballos-Laita, L. Effectiveness of Non-Pharmacological Conservative Therapies in Adults with Fibromyalgia: A Systematic Review of High-Quality Clinical Trials. J. Back. Musculoskelet. Rehabil. 2022, 35, 3–20. [Google Scholar] [CrossRef]
- Duymaz, T.; Sakınç, G.T. AB0931 Effects of Manual Therapy on Pain, Posture, Flexibility, Quality of Sleep and Depressive Symptoms in Fibromyalgia Syndrome. Ann. Rheum. Dis. 2017, 76, 1381. [Google Scholar] [CrossRef]
- Ornek, C.; Coskun Benlidayi, I.; Sariyildiz, A. Uncovering the Alterations in Extrinsic Foot Muscle Mechanical Properties and Foot Posture in Fibromyalgia: A Case-Control Study. Rheumatol. Int. 2024, 44, 2997–3008. [Google Scholar] [CrossRef]
- Cathcart, E.; McSweeney, T.; Johnston, R.; Young, H.; Edwards, D.J. Immediate Biomechanical, Systemic, and Interoceptive Effects of Myofascial Release on the Thoracic Spine: A Randomised Controlled Trial. J. Bodyw. Mov. Ther. 2019, 23, 74–81. [Google Scholar] [CrossRef]
- Tavares, L.F.; Germano Maciel, D.; Pereira Barros da Silva, T.Y.; Brito Vieira, W.H. de Comparison of Functional and Isokinetic Performance between Healthy Women and Women with Fibromyalgia. J. Bodyw. Mov. Ther. 2020, 24, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Offenbächer, M.; Stucki, G. Physical Therapy in the Treatment of Fibromyalgia. Scand. J. Rheumatol. Suppl. 2000, 113, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, V.F.; Sforza, C.; Schmitz, J.H.; Taroni, A. Occlusion and Center of Foot Pressure Variation: Is There a Relationship? J. Prosthet. Dent. 1996, 76, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Carda-Navarro, I.; Lacort-Collado, L.; Fernández-Ehrling, N.; Lanuza-Garcia, A.; Ferrer-Torregrosa, J.; Guinot-Barona, C. Relationship between Body Posture Assessed by Dynamic Baropodometry and Dental Occlusion in Patients with and without Dental Pathology. Sensors 2024, 24, 1921. [Google Scholar] [CrossRef]
- Yunus, M.B.; Arslan, S.; Aldag, J.C. Relationship between Body Mass Index and Fibromyalgia Features. Scand. J. Rheumatol. 2002, 31, 27–31. [Google Scholar] [CrossRef]
- Kurt, G.; Kiloatar, H.; Akdeniz Leblebicier, M.; Saraçoğlu, İ. Effects of Manual Lymphatic Drainage on Pain Intensity, Impact of Disease and Quality of Life in Women with Fibromyalgia Syndrome: A Double-Blind Randomized Sham-Controlled Trial. Disabil. Rehabil. 2025, 1–7. [Google Scholar] [CrossRef]
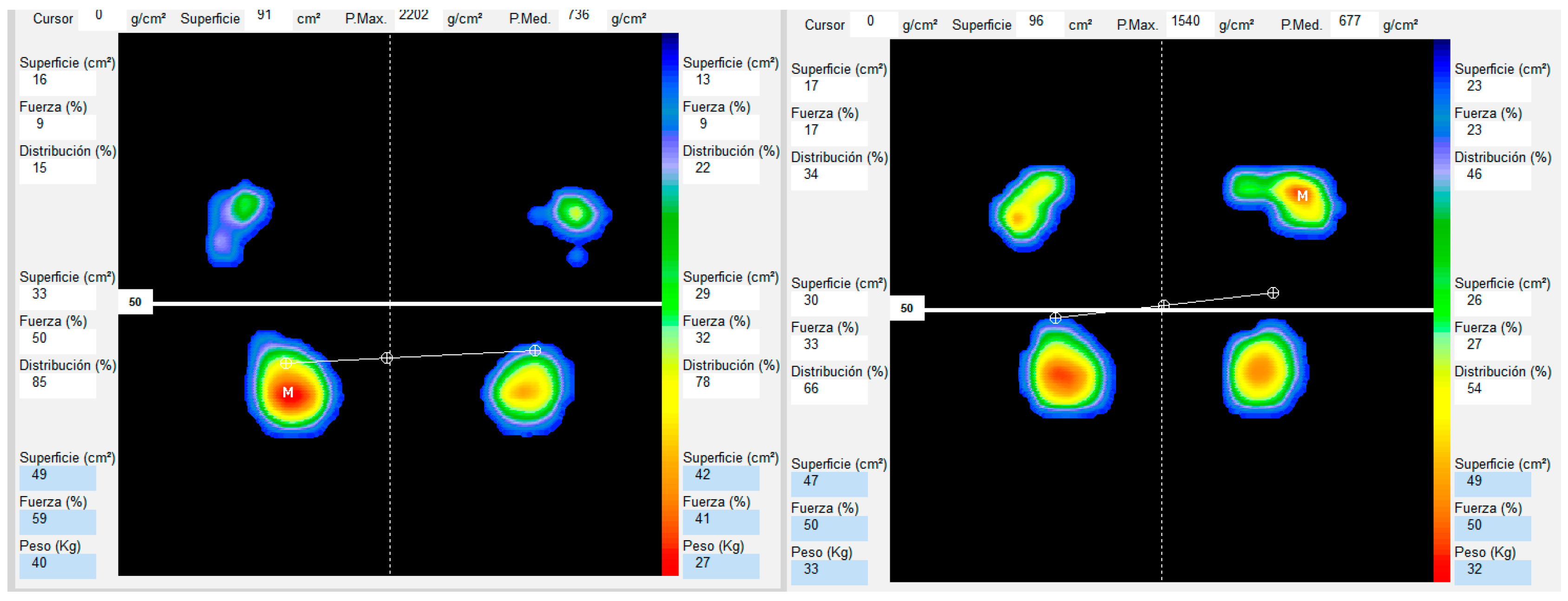
| Outcome | All Participants (n = 24) | Overweight (n = 10) | Normality (n = 14) | p-Value |
|---|---|---|---|---|
| Age (years) | 56.83 ± 6.89 | 58.77 ± 7.27 | 54.30 ± 5.76 | 0.18 |
| Height (cm) | 162.83 ± 4.76 | 162.00 ± 5.11 | 164.20 ± 4.18 | 0.28 |
| Weight (Kg) | 71.08 ± 9.19 | 77.29 ± 5.31 | 62.40 ± 5.62 | 0.001 * |
| Body mass index. (kg/m2) | 26.87 ± 3.79 | 29.54 ± 2.28 | 23.12 ± 1.54 | 0.001 * |
| Shoe size | 38.40 ± 1.05 | 38.21 ± 1.25 | 38.65 ± 0.67 | 0.33 |
| Short Form-36 Health Survey | 38.33 ± 14.87 | 38.00 ± 19.75 | 38.57 ± 10.99 | 0.52 |
| P.Max Pre (gr/cm2) | 1885.00 ± 352.66 | 1957.21 ± 329.36 | 1783.90 ± 376.39 | 0.24 |
| P.Max Post (gr/cm2) | 1811.00 ± 324.29 | 1918.71 ± 353.66 | 1660.20 ± 211.59 | 0.05 * |
| Surface Pre (cm2) | 134.08 ± 32.28 | 142.14 ± 34.74 | 122.80 ± 26.03 | 0.15 |
| Surface Post (cm2) | 144.33 ± 33.24 | 157.93 ± 32.70 | 125.30 ± 24.28 | 0.01 * |
| RF Force Pre | 50.00 ± 6.21 | 51.07 ± 5.51 | 48.50 ± 7.11 | 0.33 |
| RF Force Post | 49.75 ± 6.85 | 49.21 ± 7.26 | 51.07 ± 5.51 | 0.66 |
| LF Force Pre | 50.00 ± 6.21 | 48.93 ± 5.51 | 51.50 ± 7.11 | 0.33 |
| LF Force Post | 50.25 ± 6.85 | 50.79 ± 7.26 | 49.50 ± 6.54 | 0.66 |
| Measure 1 | Measure 2 | t | gl | p | D de Cohen | ET D de Cohen |
|---|---|---|---|---|---|---|
| Surface pre | Surface post | −3.01 | 23 | 0.006 * | −0.61 | 0.11 |
| LF Surface pre | LF Surface post | −2.65 | 23 | 0.01 * | −0.54 | 0.14 |
| LF Force pre | LF Force post | −0.20 | 23 | 0.85 | −0.04 | 0.19 |
| RF Surface pre | RF Surface post | −2.91 | 23 | 0.007 * | −0.59 | 0.10 |
| RF Force pre | RF Force post | 0.20 | 23 | 0.85 | 0.04 | 0.19 |
| P.Max pre | P.Max post | 1.25 | 23 | 0.22 | 0.25 | 0.18 |
| Pre | Post | t | gl | p | D de Cohen | ET D de Cohen | |
|---|---|---|---|---|---|---|---|
| Surface | Surface | Normal | −3.45 | 13 | 0.004 * | −0.92 | 0.16 |
| Overweight | −0.60 | 9 | 0.57 | −0.19 | 0.17 | ||
| LF Surface | LF Surface | Normal | −3.45 | 13 | 0.004 * | −0.92 | 0.22 |
| Overweight | −0.08 | 9 | 0.94 | −0.02 | 0.16 | ||
| LF Force | LF Force | Normal | −1.42 | 13 | 0.18 | −0.38 | 0.20 |
| Overweight | 0.86 | 9 | 0.41 | 0.27 | 0.35 | ||
| RF Force | RF Force | Normal | −2.98 | 13 | 0.01 * | −0.80 | 0.12 |
| Overweight | −0.98 | 9 | 0.35 | −0.31 | 0.20 | ||
| RF Force | RF Force | Normal | 1.42 | 13 | 0.18 | 0.38 | 0.20 |
| Overweight | −0.86 | 9 | 0.41 | −0.27 | 0.35 | ||
| P.Max | P.Max | Normal | 0.50 | 13 | 0.63 | 0.13 | 0.23 |
| Overweight | 1.30 | 9 | 0.22 | 0.41 | 0.29 |
| Overweight (n = 10) | Normal Range (n = 14) | p-Value | |
|---|---|---|---|
| Delta Surface | 15.79 ± 17.11 | 2.50 ± 13.25 | 0.05 |
| Delta P.Max | −38.50 ± 289.72 | −123.70 ± 300.06 | 0.49 |
| Delta LF Surface | 9.29 ± 10.06 | 0.20 ± 8.04 | 0.003 |
| Delta RF Surface | 6.50 ± 8.17 | 2.30 ± 7.42 | 0.21 |
| Delta RF force | −1.86 ± 4.90 | 2.00 ± 7.35 | 0.14 |
| Delta LF force | 1.86 ± 4.90 | −2.00 ± 7.35 | 0.14 |
| t | gl | p | D de Cohen | ET D de Cohen | |
|---|---|---|---|---|---|
| Delta Surface | −2.05 | 22 | 0.05 * | −0.85 | 0.46 |
| Delta P.Max | −0.70 | 22 | 0.49 | −0.29 | 0.42 |
| Delta P.Med | 1.78 | 22 | 0.09 | 0.74 | 0.45 |
| Delta PI Suface | −2.36 | 22 | 0.03 * | −0.98 | 0.47 |
| Delta PD Surface | −1.29 | 22 | 0.21 | −0.53 | 0.43 |
| Delta PD Force | 1.55 | 22 | 0.14 | 0.64 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falaguera-Vera, F.J.; Torralba-Estellés, J.; Vicente-Mampel, J.; Ferrer-Torregrosa, J.; Oltra, E.; Garcia-Escudero, M. Impact of Manual Therapy on Plantar Pressures in Patients with Fibromyalgia: A Single-Arm, Non-Randomized Pilot Clinical Trial. Healthcare 2025, 13, 764. https://doi.org/10.3390/healthcare13070764
Falaguera-Vera FJ, Torralba-Estellés J, Vicente-Mampel J, Ferrer-Torregrosa J, Oltra E, Garcia-Escudero M. Impact of Manual Therapy on Plantar Pressures in Patients with Fibromyalgia: A Single-Arm, Non-Randomized Pilot Clinical Trial. Healthcare. 2025; 13(7):764. https://doi.org/10.3390/healthcare13070764
Chicago/Turabian StyleFalaguera-Vera, Francisco J., Javier Torralba-Estellés, Juan Vicente-Mampel, Javier Ferrer-Torregrosa, Elisa Oltra, and María Garcia-Escudero. 2025. "Impact of Manual Therapy on Plantar Pressures in Patients with Fibromyalgia: A Single-Arm, Non-Randomized Pilot Clinical Trial" Healthcare 13, no. 7: 764. https://doi.org/10.3390/healthcare13070764
APA StyleFalaguera-Vera, F. J., Torralba-Estellés, J., Vicente-Mampel, J., Ferrer-Torregrosa, J., Oltra, E., & Garcia-Escudero, M. (2025). Impact of Manual Therapy on Plantar Pressures in Patients with Fibromyalgia: A Single-Arm, Non-Randomized Pilot Clinical Trial. Healthcare, 13(7), 764. https://doi.org/10.3390/healthcare13070764







