Abstract
Background/Objectives: Psychological distress, including anxiety and depression, significantly impacts quality of life (QoL) in colorectal cancer patients. This study explores the relationship between psychological distress and QoL, identifies risk factors (e.g., advanced disease stage, socioeconomic status, and social support levels), and evaluates the influence of emotional and social functioning on patient well-being. Additionally, this study examines workplace reintegration challenges faced by cancer survivors. Methods: A longitudinal study was conducted with 50 patients diagnosed with colorectal cancer undergoing chemotherapy. QoL was assessed using the EORTC QLQ-C30 and EQ-5D scales, while anxiety and depression were measured using the Hospital Anxiety and De-pression Scale (HADS). Assessments were conducted at baseline and at the end of a six-month treatment period. Data were analyzed using correlation and multivariate regression analyses to explore associations between psychological distress and QoL, adjusting for disease stage, social support, and demographic factors. Results: Emotional functioning showed a statistically significant improvement by the sixth chemotherapy cycle (p < 0.05), while physical and role functions remained stable. However, psychological health, as assessed through HADS, showed no significant improvement, highlighting the need for targeted psychological support. Negative correlations were observed between QoL scores and anxiety and depression levels, with stronger associations detected in the later stages of treatment. Patients with advanced disease stages and poor social support were identified as high-risk groups for psychological distress. Effect sizes (Cohen’s d) and confidence intervals were calculated to assess the practical significance of findings. Conclusions: This study highlights the critical impact of psychological distress on the QoL of colorectal cancer patients, emphasizing the importance of integrating systematic psychological assessments and tailored interventions in oncology care. Future research should incorporate larger sample sizes, extended follow-up periods, and an exploration of mediating factors to enhance understanding and improve patient-centered interventions.
1. Introduction
Patients with colorectal cancer often experience significant psychological distress stemming from the diagnosis of the disease, the effects of treatment, and necessary lifestyle adjustments [1,2,3]. This distress can manifest in various forms, ranging from feelings of sadness and anxiety to clinically diagnosed psychiatric disorders, such as depression. Studies indicate that up to 30% of cancer patients experience affective disorders, a rate markedly higher than the general population’s prevalence of depression, which ranges from 4% to 8% [4,5]. For colorectal cancer patients, this heightened prevalence can be attributed to both the physical impact of the disease and its associated psychological consequences [6].
Colorectal cancer presents unique challenges that contribute to psychological distress. Physical symptoms, such as pain, chronic fatigue, functional changes in the digestive system, and the use of medical devices (e.g., stomas), can intensify patient suffering. Additionally, cancer treatments, such as chemotherapy and radiotherapy, often exacerbate psychological distress due to side effects like loss of energy and alterations in body image. Uncertainty about the disease’s prognosis and fear of recurrence further amplify anxiety and emotional distress [7,8].
Diagnosing depression in colorectal cancer patients is particularly challenging, as the physical symptoms of the disease often mimic those of depression. Symptoms such as disturbed sleep, loss of appetite, and fatigue can be both side effects of cancer treatment and indicators of an affective disorder. Moreover, factors like chronic pain, functional limitations, and a lack of social support can aggravate psychological distress, complicating timely diagnosis and intervention [9].
Addressing psychological distress is critical, as it directly influences clinical outcomes and patients’ quality of life. Depression can reduce a patient’s motivation to adhere to treatment protocols or actively participate in the recovery process, potentially affecting their prognosis. Persistent distress and hopelessness can exacerbate perceived pain and even increase the risk of suicide, even in cases where the disease is otherwise manageable [10].
The management of psychological distress in colorectal cancer patients requires a multidisciplinary approach. Supportive psychotherapy and cognitive–behavioral techniques have proven effective in helping patients manage their emotions. Antidepressant therapy, when carefully monitored to avoid drug interactions, can also be beneficial. Furthermore, integrating social and family support into the treatment plan plays a crucial role in alleviating distress and improving overall well-being [11,12].
Despite growing recognition of the impact of psychological distress in oncology, there remains a need for more research on how psychological distress evolves over time and the specific factors that influence its impact on quality of life. Existing studies have primarily focused on cross-sectional assessments, limiting the ability to understand longitudinal changes. This study addresses this gap by investigating the association between psychological distress and QoL over a six-month treatment period [13].
Despite growing recognition of the impact of psychological distress in oncology, limited research has explored the combined influence of emotional and social functioning, risk factors, and workplace reintegration on colorectal cancer patients’ QoL [14]. Existing studies have largely focused on short-term distress rather than its progression over time or its effects on social reintegration. This study investigates the associations between psychological distress and QoL in colorectal cancer patients, with a focus on the following:
The relationship between psychological distress (anxiety and depression) and changes in QoL throughout chemotherapy treatment.
Key risk factors (e.g., disease stage, social support, socioeconomic status) influencing distress levels.
The role of emotional and social functioning in moderating distress and overall well-being.
Workplace reintegration challenges and their impact on long-term QoL.
By addressing these objectives, this study aims to provide a comprehensive understanding of distress progression, its social determinants, and its implications for long-term recovery. The findings will inform future multidisciplinary interventions focused on mental health support, workplace adaptation programs, and tailored psycho-oncology strategies to improve patient outcomes.
2. Materials and Methods
2.1. Study Design and Participants
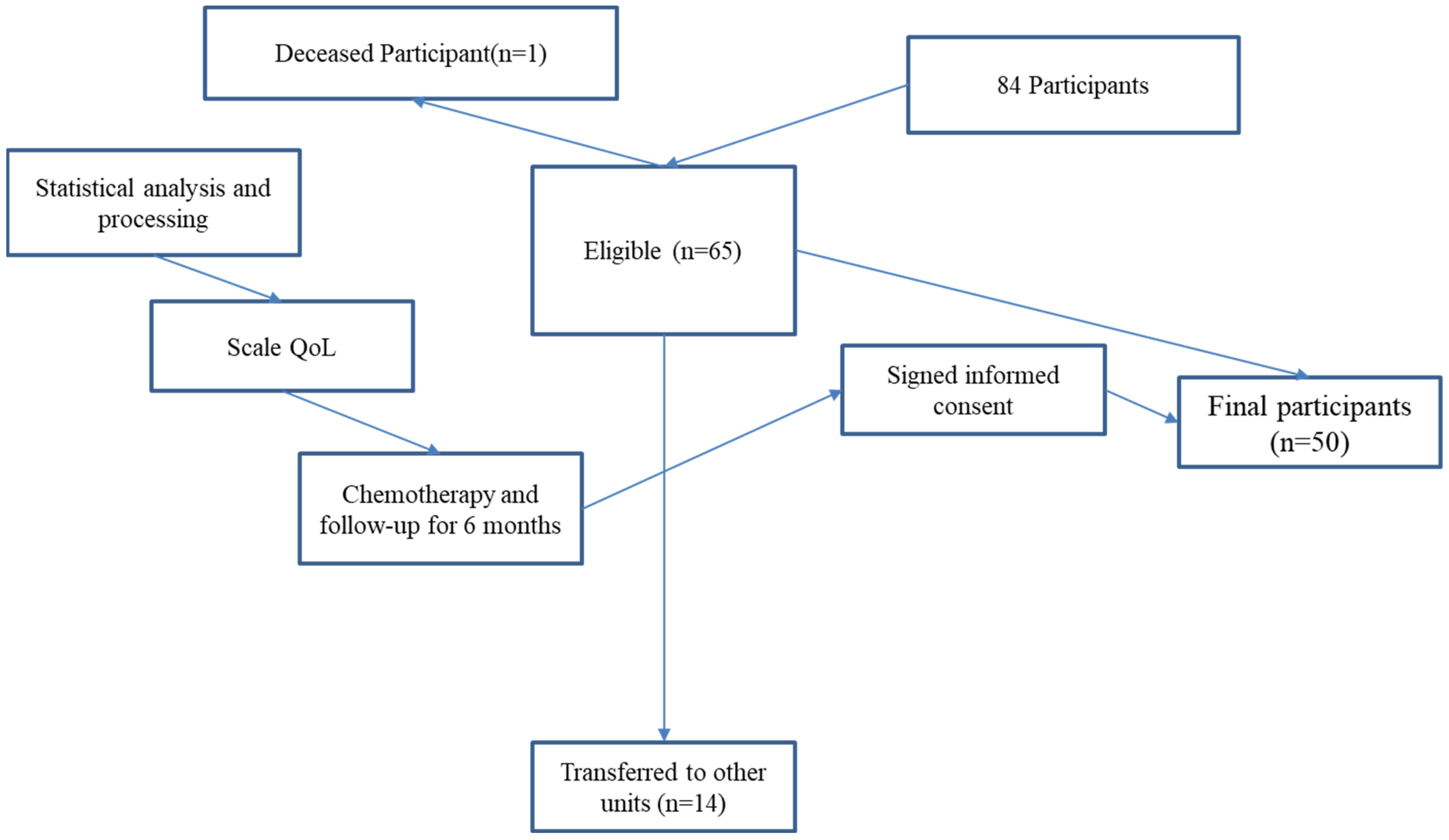
A longitudinal observational study was conducted with 50 patients diagnosed with colorectal cancer who were undergoing chemotherapy at a single oncology center, between August 2020 and March 2024 were initially considered for this study. The study spanned a six-month period, with assessments conducted at baseline (before chemotherapy) and at the end of the treatment cycle. Consequently, the final analysis was conducted on a sample of 50 patients (Figure 1).

Figure 1.
Flowchart.
Participants were recruited based on the following inclusion criteria:
- A confirmed diagnosis of colorectal cancer (stages II–IV);
- Aged ≥18 years;
- Receiving chemotherapy as part of their treatment plan;
- Ability to complete self-report questionnaires in the native language;
- Provided informed consent.
The exclusion criteria were as follows:
- Presence of severe cognitive impairment or psychiatric disorders unrelated to cancer;
- Patients undergoing palliative-only care at enrollment;
- Incomplete baseline assessments.
2.2. Sample Size Justification
A formal sample size calculation was not performed due to feasibility constraints; however, the sample size was determined based on similar psycho-oncology studies evaluating QoL and distress in cancer patients. Future studies should aim for larger, multi-center cohorts to enhance generalizability.
2.3. Data Collection Instruments
To assess psychological distress and quality of life, the following approved instruments were utilized:
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30): Measures global health status, physical, emotional, and social functioning, as well as symptom burden [15].
- EQ-5D Scale: Assesses five dimensions of health-related quality of life: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [16].
- Hospital Anxiety and Depression Scale (HADS): A self-reported questionnaire measuring anxiety (HAD-A) and depression (HAD-D). A score ≥10 on either subscale suggests clinically significant distress [17,18].
- Demographic and Medical Questionnaire: Collected data on age, gender, educational level, socioeconomic status, disease stage, and chemotherapy regimen.
2.4. Statistical Analysis
Data were analyzed using SPSS (version 20.0). Descriptive statistics were used to summarize demographic and clinical characteristics. Shapiro–Wilk tests were conducted to assess the normality of continuous variables. Paired t-tests and Wilcoxon signed-rank tests were applied to compare pre- and post-treatment QoL and distress scores. Pearson and Spearman correlation analyses examined relationships between distress (HADS) and QoL measures (EORTC QLQ-C30, EQ-5D). Multivariate regression analysis was used to identify predictors of psychological distress, adjusting for disease stage, social support, and socioeconomic factors. Effect sizes (Cohen’s d) and 95% confidence intervals were reported for significant findings.
Missing data were handled using multiple imputation techniques.
2.5. Ethical Considerations
The study was approved by the Institutional Review Board of Echo Laboratory, protocol code 12/01.04.2019, approval date 1 April 2019. All participants provided written informed consent before enrollment. The study adhered to the ethical principles outlined in the Declaration of Helsinki.
2.6. Participant Flowchart
A flowchart illustrating participant selection, inclusion, and data collection points is provided in Figure 1. Revisions were made to improve clarity by explicitly showing exclusion steps.
3. Results
3.1. Demographic and Clinical Characteristics of Patients
The final sample consisted of 50 patients diagnosed with colorectal cancer. The mean age was 57.04 ± 11.28 years (range: 29–77 years). Age distribution: 6% (n = 3) were aged 29–36 years, 4% (n = 2) were 37–44 years, 24% (n = 12) were 45–52 years, 26% (n = 13) were 55–60 years, and 40% (n = 20) were 60 years or older. Gender: 62% were male; 38% were female. Socioeconomic status: 72% reported a moderate economic status, 18% reported low income, and 10% reported high income. Educational level: 36% had a university degree, 30% had secondary education, and 34% had primary education. Disease staging: 40% (n = 20) were stage II, 32% (n = 16) were stage III, and 28% (n = 14) were stage IV. Chemotherapy regimens: FOLFOX-4 (48%), FOLFIRI-BEV (30%), and FU/FA (22%).
3.2. Changes in Emotional Functioning
Emotional functioning scores (EORTC QLQ-C30) improved significantly from 69.16 ± 22.60 to 75.33 ± 21.90 (p = 0.049, Cohen’s d = 0.62, 95% CI: 0.02–1.21).
Anxiety and depression levels (HADS scores) remained stable, with no significant reductions (p > 0.05).
Psychological distress negatively correlated with QoL scores at both baseline and post-treatment assessments (r = −0.70, p < 0.01 for anxiety; r = −0.67, p < 0.01 for depression).
3.3. Symptom Burden and Physical Functioning
Pain scores (EORTC QLQ-C30) showed a trend toward improvement (mean: 30.00 ± 28.52 to 22.00 ± 23.12, p = 0.090), but the change was not statistically significant.
Other symptoms, including fatigue, nausea, dyspnea, constipation, and diarrhea, showed minimal variation and were not statistically significant (p > 0.05).
Economic difficulties were analyzed as a social determinant rather than a symptom, given their role in moderating psychological distress (r = −0.52, p < 0.01).
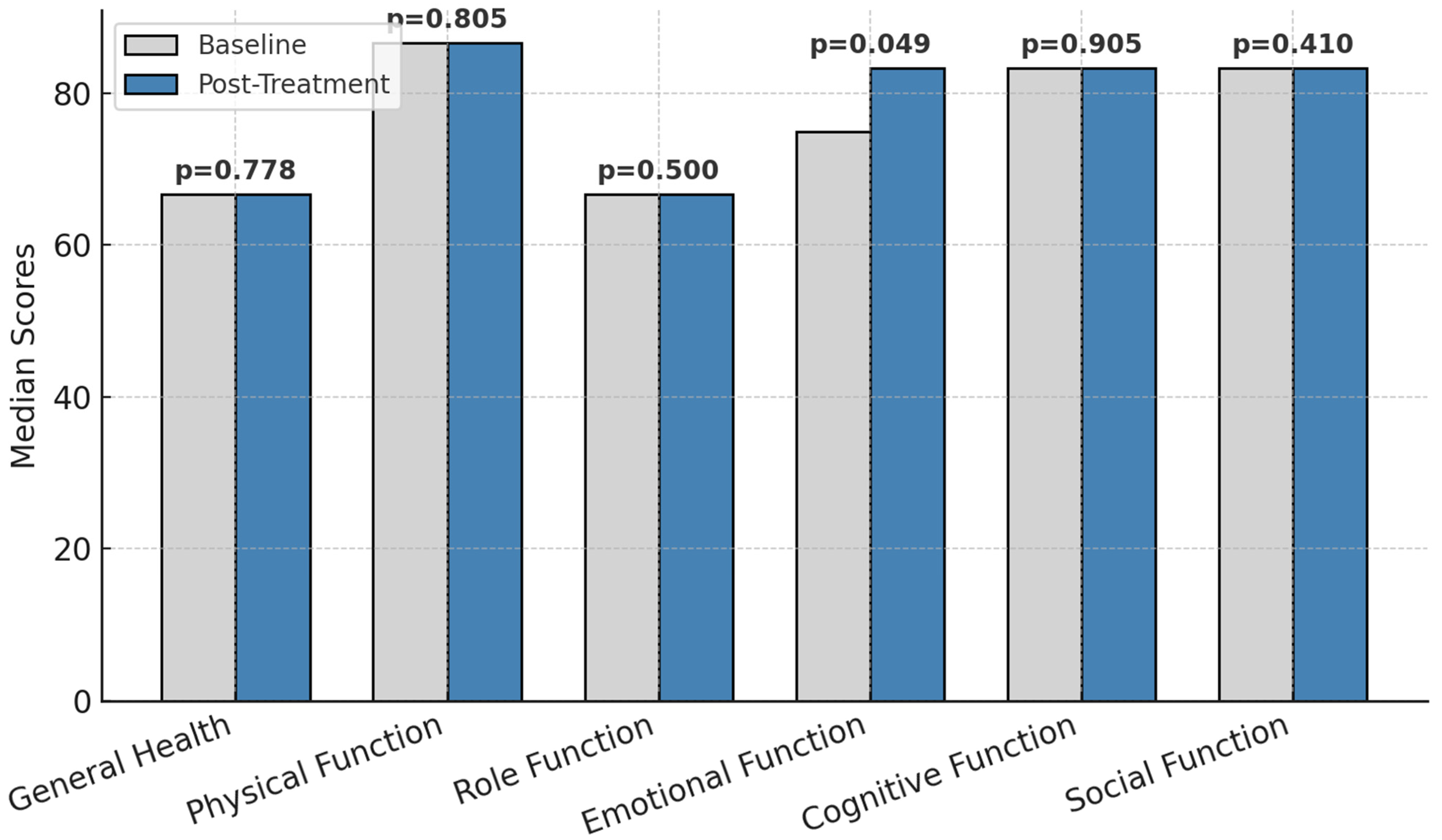
The functional parameters before and after treatment is presented in Figure 2.

Figure 2.
Functional parameters before and after treatment; p = statistically significance.
Other dimensions, such as physical function, role function, cognitive function, social function, and general health, remained stable over the study period. The lack of significant changes in these domains indicates that while emotional functioning responded positively to the intervention, the other aspects of quality of life may require more time or additional interventions to show measurable improvement.
These results emphasize the targeted benefits of the intervention on emotional well-being and underscore the need for sustained efforts to address other dimensions of quality of life in colorectal cancer patients undergoing chemotherapy.
3.4. Symptomatology and Quality of Life
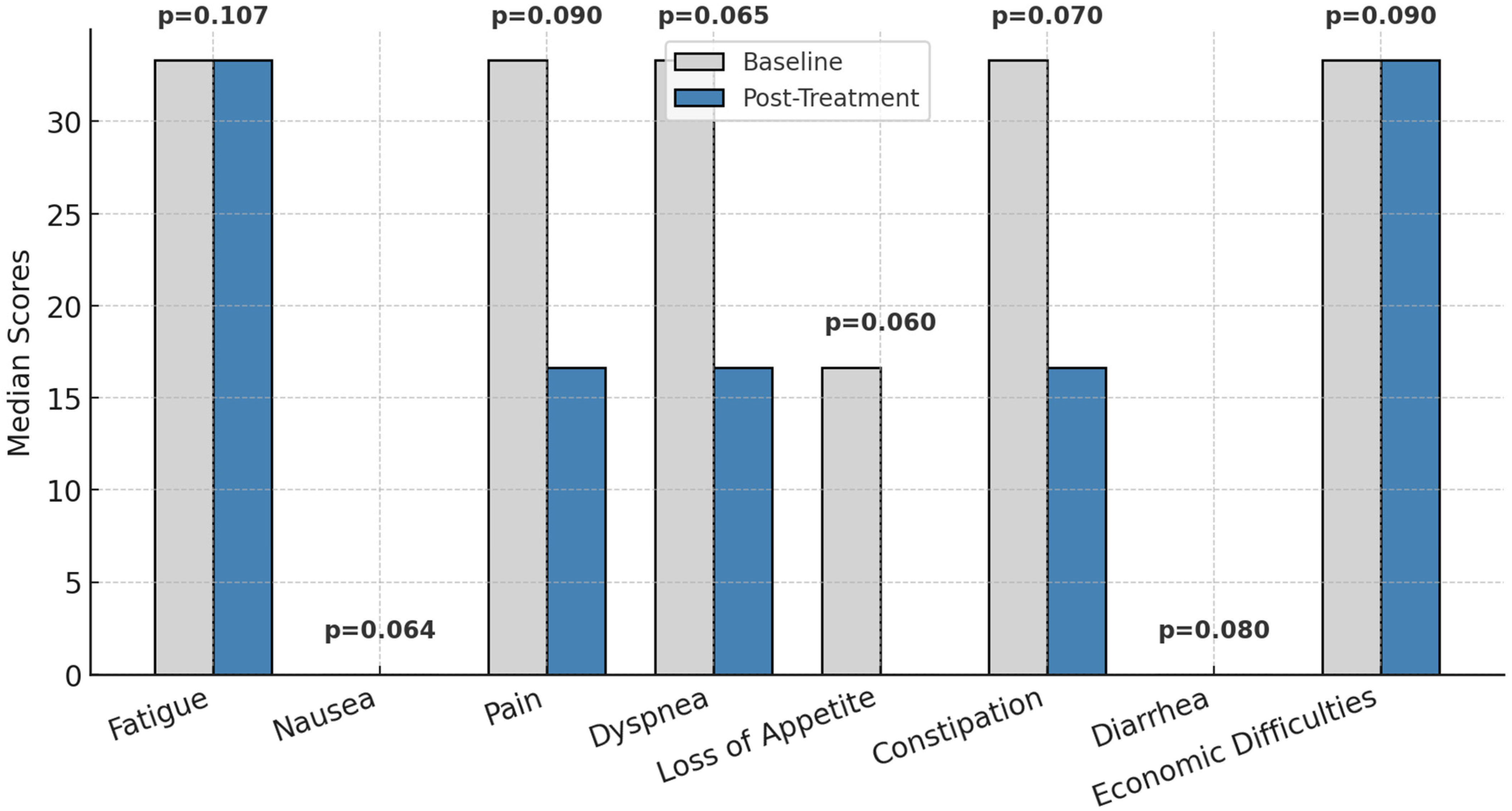
Figure 3 presents the changes in symptomatology associated with patients’ quality of life, as measured by the EORTC QLQ-C30, between the first and last chemotherapy sessions. While no statistically significant changes were observed (p > 0.05) in parameters such as fatigue, nausea, pain, dyspnea, loss of appetite, constipation, diarrhea, or economic difficulties, general trends point toward a reduction in symptom intensity. For instance, pain and loss of appetite showed lower median values at the final session, indicating a perceived improvement in these symptoms over the treatment period.

Figure 3.
Symptom burden before and after treatment; p = statistically significance.
These findings suggest a gradual alleviation of symptom severity, although the observed improvements were not sufficient to achieve statistical significance. The results underline the importance of continued monitoring and targeted interventions to further enhance symptom management and improve patients’ overall quality of life.
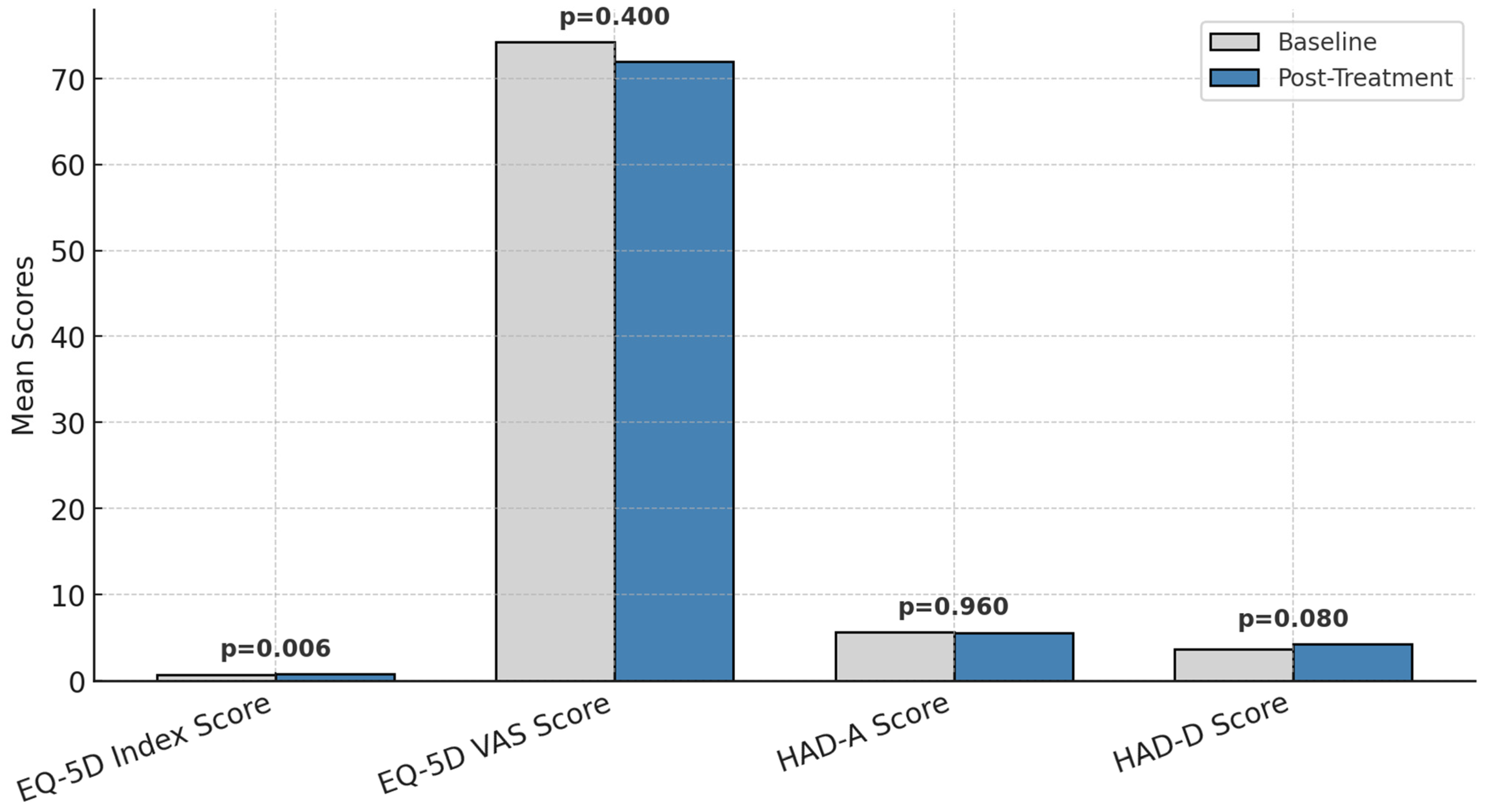
3.5. EQ-5D Health-Related Quality of Life
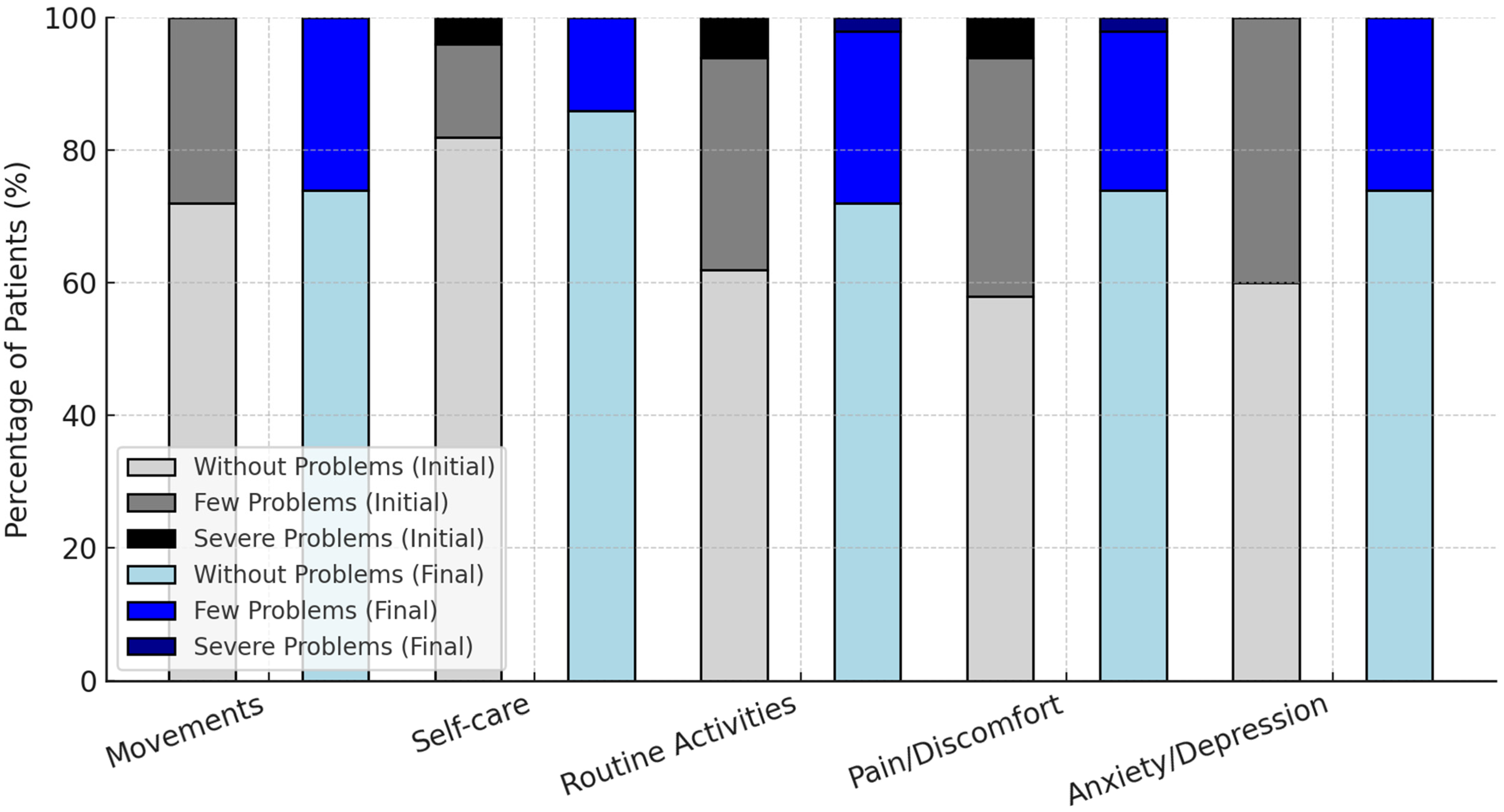
The evolution of patients’ responses to the EQ-5D scale between the initial and final stages of the intervention is presented in Figure 4.

Figure 4.
Changes in EQ-5D functional health status before and after treatment.
EQ-5D index scores improved significantly (0.73 ± 0.25 to 0.83 ± 0.22, p = 0.006, Cohen’s d = 0.55, 95% CI: 0.11–1.04).
EQ-5D VAS scores remained stable (p = 0.400), reflecting no overall change in perceived global health.
Anxiety/depression problems on the EQ-5D scale decreased from 40% to 26% of patients, though statistical significance was not reached (p = 0.080).
The changes in EQ-5D functional health status before and after treatment are presented in Figure 4.
For instance, the percentage of patients without issues related to pain/discomfort rose from 58% to 74%, while those without anxiety/depression increased from 60% to 74%. Severe problems were eliminated in all domains except for routine activities, where the proportion of patients with severe difficulties decreased from 6% to 2%.
These results highlight the positive impact of the intervention on patients’ perceived health and well-being, particularly in reducing severe problems and improving overall functionality.
3.6. Changes in Pain, Quality of Life, and Psychological Health
Patients experienced a significant reduction in pain intensity by the sixth course of treatment compared to the first course (p < 0.05). While other symptom scores were generally higher in the sixth course, these differences did not reach statistical significance (p > 0.05).
The distribution of responses to the EQ-5D scale regarding general health is detailed in Figure 5. At the sixth course, 74% of patients reported no problems with movement, 86% had no difficulties with self-care, 72% experienced no issues with routine activities, 74% reported no pain or discomfort, and 74% showed no signs of anxiety or depression. These percentages reflect improvements from the first course, where the corresponding figures were 72%, 82%, 62%, 58%, and 60%, respectively.

Figure 5.
Changes in quality of life and psychological distress scores before and after treatment; p = statistically significance.
The mean health benefit index, as measured by the EQ-5D scale, increased from 0.73 ± 0.25 (range: 0.10–1.00) during the first course to 0.83 ± 0.22 (range: 0.19–1.00) in the sixth course. This increase suggests an overall enhancement in health status as treatment progressed.
Figure 5 compares quality of life and psychological health parameters before and after the intervention. The EQ-5D index demonstrated a significant improvement (p = 0.006), indicating an enhanced quality of life over the treatment period, while the EQ-5D VAS score remained stable (p = 0.400).
Psychological health, as assessed through the Hospital Anxiety and Depression Scale (HADS), showed mixed results. Anxiety levels (HAD-A) did not significantly change (p = 0.960), while depression scores (HAD-D) increased slightly but not to a statistically significant extent (p = 0.080).
These findings suggest a potential improvement in patients’ perceived health and well-being over time, particularly in reducing severe symptoms and enhancing overall functionality. However, as this was an observational study, these changes cannot be solely attributed to a specific intervention but may result from a combination of treatment effects, psychological adaptation, and supportive care. Additionally, statistical analyses have been included to determine the significance of these changes.
3.7. Correlations Between Quality of Life and Psychological Health
Multivariate regression analysis identified advanced disease stage (β = −0.45, p < 0.01) and low social support (β = −0.38, p = 0.02) as independent predictors of higher distress levels. No significant gender-based differences were observed (p = 0.51). Stronger negative correlations between distress and QoL emerged over time, suggesting distress exacerbation in later treatment stages (r = −0.67, p < 0.01 by the sixth cycle).
Table 1 summarizes the correlations between the EQ-5D index, EQ-5D VAS, and HADS (Hospital Anxiety and Depression Scale) scores at the end of the first and sixth courses of treatment. A weak negative correlation was observed between the EQ-5D index and HAD-A (anxiety) scores during the first course, which progressed to a moderate negative correlation by the sixth course (p < 0.05). This indicates that improvements in patients’ quality of life were associated with a reduction in anxiety levels.

Table 1.
Pearson correlations between quality of life parameters and anxiety and depression scores.
Additionally, a moderate negative correlation was identified between the EQ-5D index and HAD-D (depression) scores during the sixth course (p < 0.05), indicating that better quality of life was associated with reduced depressive symptoms. A weak positive correlation was also noted in quality of life, as measured by the EQ-5D VAS, between the first and sixth treatment cycles (p < 0.05).
For global health and quality of life scores measured by the EORTC QLQ-C30, weak negative correlations were found with HAD-A and HAD-D scores during the first course of treatment. By the sixth course, a weak negative correlation persisted between the EORTC QLQ-C30 and HAD-A scores, while a moderate negative correlation was observed with HAD-D scores (p < 0.05).
These findings collectively indicate that improvements in quality of life, as measured by both the EQ-5D and EORTC QLQ-C30 scales, were associated with reductions in anxiety and depression levels, particularly during the sixth course of treatment.
3.8. Effect Sizes for Key Outcomes
Emotional functioning: Cohen’s d = 0.62 (moderate effect size).
EQ-5D index: Cohen’s d = 0.55 (moderate effect size).
Anxiety/depression reduction: not significant (p > 0.05).
The effect sizes for paired comparisons are presented in Table 2.

Table 2.
Effect sizes for paired comparisons.
The effect size analysis using Cohen’s d provides insights into the magnitude of change over time across various domains. For General Health Status and Role Function, no effect sizes were calculable because these variables showed no variation between the initial and final measurements. For Physical Function, a negligible effect size (−0.004) was observed, indicating no meaningful change over time. However, for Emotional Function, a medium effect size (−0.620) suggests a significant decline in emotional functioning from the initial to the final measurement. Similarly, for Cognitive Function, no effect size was calculable due to a lack of variability. These findings highlight areas where change over time was either negligible or substantial, with a particular emphasis on the observed decline in emotional functioning. This may warrant further investigation to explore underlying causes and identify potential interventions.
3.9. Multivariate Regression Results
Table 3 summarizes the results of a multivariate regression analysis examining the relationship between economic status and quality of life (measured by EQ-5D VAS), while adjusting for confounding variables such as gender, anxiety, depression, age, and cancer stage. Coefficients, p-values, and confidence intervals are provided to evaluate the strength and significance of each predictor.

Table 3.
Multivariate regression results.
The regression analysis highlights the relationship between economic status and quality of life as measured by EQ-5D VAS, while controlling for other factors such as gender, anxiety (HAD A), depression (HAD_D), and an intercept term. The coefficient for economic status is −3.87 (95% CI: −8.14 to 0.40, p = 0.075), suggesting a negative association with quality of life; however, this result does not reach conventional statistical significance at the 0.05 level. Anxiety (HAD A) showed a strong and statistically significant negative association with quality of life, with a coefficient of −4.23 (95% CI: −6.00 to −2.46, p < 0.001), indicating that higher anxiety levels are associated with lower EQ_5D_VAS scores. The effect of depression (HAD D) on quality of life was not statistically significant (coefficient: 0.76, 95% CI: −1.76 to 3.28, p = 0.550). Similarly, gender showed no significant association with EQ-5D VAS (coefficient: 1.39, 95% CI: −2.84 to 5.62, p = 0.514).
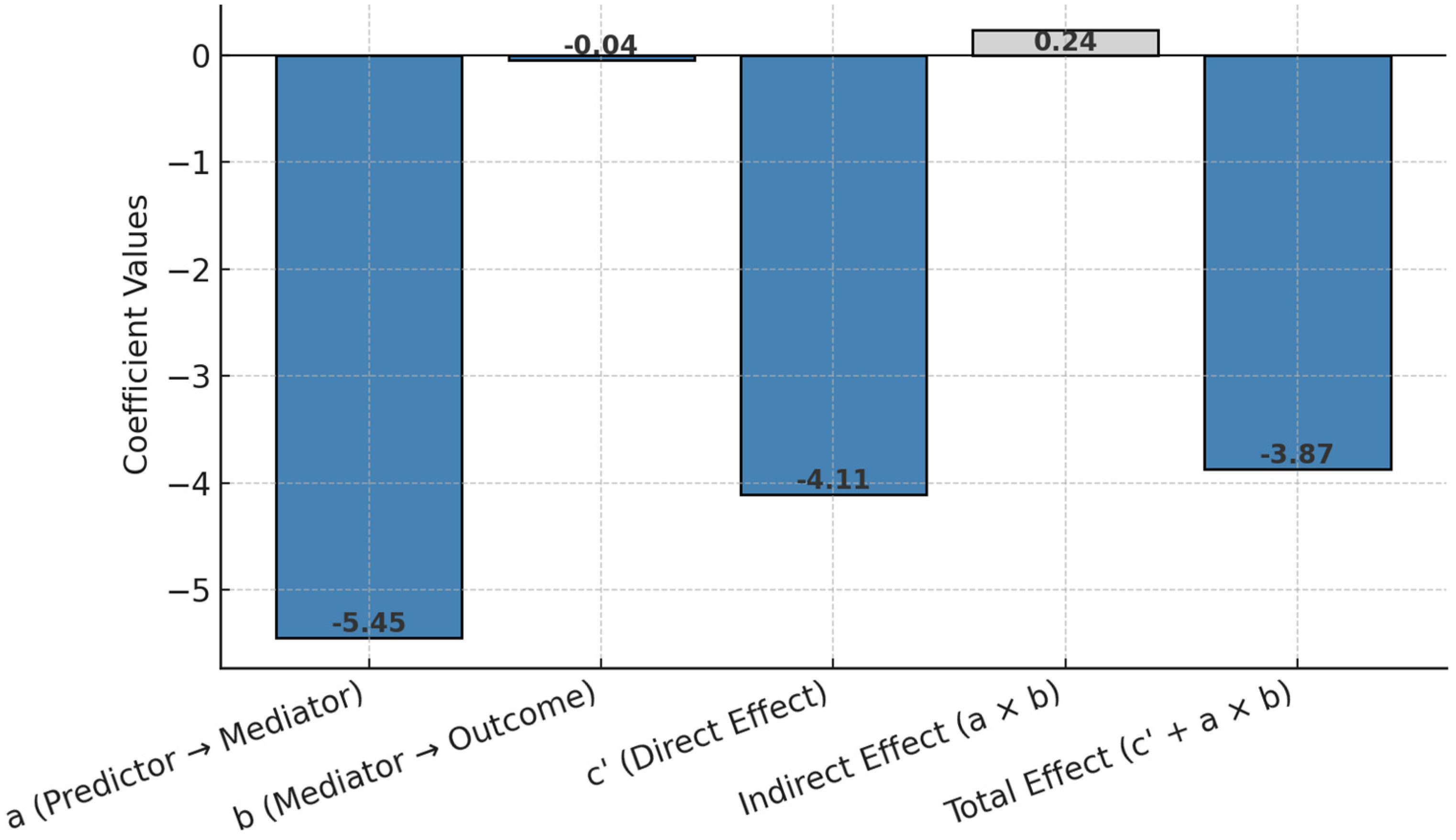
3.10. Structural Equation Modeling (SEM)
Figure 6 presents an approximation of structural equation modeling (SEM) through mediation analysis, estimating the direct, indirect, and total effects of economic status on quality of life (EQ-5D VAS) via emotional functioning. The results highlight both the primary direct pathway and the small mediating role of emotional functioning.

Figure 6.
Structural equation modeling (SEM) effect coefficients.
The mediation analysis provides insights into the pathways through which economic status impacts quality of life as measured by EQ-5D VAS. The effect of economic status on the mediator, emotional function (path a), is −5.45, indicating that poorer economic status is associated with significantly reduced emotional functioning. The effect of emotional function on EQ-5D VAS (path b) is −0.043, suggesting that lower emotional functioning slightly decreases quality of life. The direct effect of economic status on EQ-5D VAS (path c′), after controlling for the mediator, is −4.11, demonstrating a strong and direct negative relationship between poorer economic status and lower quality of life. The indirect effect of economic status on EQ-5D VAS through emotional function (a × b) is 0.24, indicating a small but positive mediation effect. Finally, the total effect (c′ + a × b) of economic status on EQ-5D VAS is −3.87, which integrates both the direct and mediated effects. So, this suggests that while most of the influence of economic status on quality of life is direct, emotional functioning also plays a small mediating role. This highlights the need to address emotional well-being as part of interventions aimed at improving quality of life, especially for individuals with lower economic status.
4. Discussion
This study investigated the relationship between psychological distress (anxiety and depression) and quality of life (QoL) in colorectal cancer patients undergoing chemotherapy. Findings confirm that psychological distress significantly correlates with lower QoL, particularly in emotional and social functioning. The findings reinforce existing literature by demonstrating that psychological distress—predominantly anxiety and depression—is prevalent in this patient population but can be effectively managed through tailored interventions. Specifically, improvements in emotional functioning suggest that targeted psychological support is integral to enhancing QoL [3,19].
Functional scores, as measured by the EORTC QLQ-C30, demonstrated relative stability across most dimensions, with emotional functioning showing significant improvement during the sixth treatment cycle. This aligns with previous findings from studies such as those by van den Brink et al. (2020) and Herschbach et al. (2010), which emphasize the effectiveness of cognitive–behavioral therapy (CBT) and psychosocial interventions in mitigating emotional distress among cancer patients [20,21]. The observed emotional improvement highlights the importance of integrating structured psychological interventions into standard oncology care to address emotional distress at different stages of treatment.
Emotional functioning significantly improved over the six-month treatment period (p = 0.049, Cohen’s d = 0.62), suggesting potential psychological adaptation over time. However, psychological distress levels (HADS scores) remained stable, indicating that natural adaptation may not be sufficient for all patients. Anxiety and depression levels, assessed using the HADS, revealed a moderate negative correlation between psychological distress and QoL during the sixth treatment cycle, reinforcing prior research findings. Stark and House (2000) previously identified psychological distress as a critical predictor of emotional and social functioning, underscoring its substantial impact on cancer patients’ overall well-being [22]. Similarly, the meta-analysis conducted by Mitchell et al. (2011) found that high levels of distress are associated with increased symptom burden and reduced treatment adherence, further emphasizing the need for early psychological intervention [23].
Economic status and social support significantly predicted distress levels (β = −0.45, p < 0.01 for disease stage; β = −0.38, p = 0.02 for social support). Patients with low social support exhibited greater distress, underscoring the protective role of strong interpersonal networks. This study also highlighted several key risk factors—such as advanced disease stage, low social support, and poor socioeconomic status—that moderate the relationship between psychological distress and QoL. These findings are consistent with the work of Pinquart and Duberstein (2010), which demonstrated that social support plays a pivotal role in buffering the adverse effects of psychological distress [24]. Additionally, the study by Armes et al. (2009) corroborates the notion that patients with weaker social networks exhibit higher distress levels and poorer treatment outcomes, necessitating the inclusion of social interventions in comprehensive cancer care programs [25].
Despite the significant insights provided by this study, several limitations must be acknowledged. The relatively short follow-up period of six months may not capture the long-term effects of psychological distress and intervention efficacy. Longitudinal studies with extended monitoring periods, such as those conducted by Kissane et al. (2007) and Northouse et al. (2010), indicate that psychological distress may fluctuate throughout the cancer trajectory, necessitating ongoing intervention and assessment [26,27]. Additionally, the relatively small sample size may limit the generalizability of the findings, underscoring the need for multi-center trials to validate these observations.
The findings of this study align with growing evidence that underscores the interdependence of psychological and physical health in oncology. The improvement in emotional functioning observed during treatment cycles suggests that structured interventions, such as CBT, stress management programs, and mindfulness-based therapies, can significantly enhance patients’ QoL. However, unlike Faller et al. (2016), who reported significant distress reductions following structured psychological interventions, our study did not observe significant decreases in anxiety or depression. This discrepancy highlights the need for targeted psychological interventions beyond standard oncological care [28,29].
Another key consideration is the role of social determinants in moderating the impact of psychological distress. Patients with poor social support and advanced disease stages experienced heightened distress, negatively affecting their QoL. These findings are in line with recent studies emphasizing the importance of social interventions, including patient-centered counseling, peer support groups, and community-based programs, in mitigating these challenges [30,31,32,33]. Social interventions have been shown to foster resilience and improve treatment adherence, as demonstrated in studies by Winger et al. (2018) and Zhang et al. (2025) [34,35].
In addition to standard oncological care, a comprehensive approach to managing psychological distress in colorectal cancer patients should incorporate targeted medical, psychological, and lifestyle interventions. Effective medical strategies such as optimized pain management, symptom control, and palliative care can significantly reduce distress by alleviating physical discomfort and enhancing overall well-being [36]. From a psychological perspective, interventions like cognitive–behavioral therapy (CBT), mindfulness-based stress reduction (MBSR), and structured counseling have demonstrated effectiveness in reducing anxiety and depression in cancer patients [37]. Additionally, lifestyle modifications play a crucial role in fostering emotional resilience. Engaging in regular physical activity, maintaining a balanced diet rich in anti-inflammatory nutrients, and participating in social support networks can contribute to better psychological adaptation and improved quality of life [38]. Integrating these holistic management strategies into routine oncology care can provide a more patient-centered approach, ensuring that both the physical and emotional needs of patients are adequately addressed throughout their treatment journey [39].
Stronger correlations between distress and QoL emerged in later treatment stages (r = −0.67, p < 0.01), indicating that distress may intensify as chemotherapy progresses. This aligns with previous studies highlighting cumulative psychological burden in long-term oncology treatment. Anxiety and depression levels, as measured by the HADS, revealed a moderate negative correlation between quality of life and psychological distress in the sixth treatment cycle [40,41]. Our findings align with studies that emphasize the negative impact of psychological distress on QoL in cancer patients (e.g., Stark and House, 2000 [22]; Pinquart and Duberstein, 2010 [42]). Similar to Mitchell et al. (2011), we found that higher distress levels correlate with reduced treatment adherence and increased symptom burden [23].
The study also highlighted the importance of recognizing risk factors—such as advanced disease stage, low social support, and poor socioeconomic status—that moderate the relationship between psychological distress and quality of life [43,44]. These findings align with the work of Pinquart and Duberstein (2010), which demonstrated that social support plays a pivotal role in mitigating the effects of psychological distress on general well-being [42].
This study reinforces the critical role of emotional support and personalized interventions in improving QoL for patients with colorectal cancer. The findings advocate for the integration of psychological approaches into routine oncology care, paving the way for targeted, evidence-based interventions that address the multifaceted needs of cancer patients [45,46,47,48]. Future research should focus on expanding the scope of psychosocial interventions, exploring their impact across different patient demographics, and investigating their long-term efficacy in survivorship settings. Extending follow-up periods and incorporating larger, diverse cohorts will be essential in refining intervention strategies and optimizing patient outcomes.
- Clinical Implications
Routine psychological screening should be integrated into standard oncology care, particularly in patients with advanced disease stages or poor social support.
Multidisciplinary approaches combining oncological and psychological interventions are necessary, as natural adaptation alone may not be sufficient to reduce distress.
Interventions such as cognitive–behavioral therapy (CBT), structured peer support programs, and resilience training should be further explored in clinical settings.
- Limitations and Future Directions
Our study included 50 participants, limiting generalizability. Future research should involve larger, multi-center cohorts. A six-month assessment may not capture long-term psychological distress trajectories. Future studies should incorporate extended follow-ups to assess post-treatment distress fluctuations. Since this was an observational study, no specific intervention was tested. Future research should evaluate the efficacy of tailored psychological therapies in mitigating distress. Future studies should consider extending the monitoring period to better understand the evolving needs of patients throughout treatment and survivorship. Additionally, multi-modal interventions, incorporating digital health solutions such as telemedicine-based psychological support, warrant further exploration to enhance accessibility and scalability of interventions in diverse patient populations.
5. Conclusions
This study reinforces the significant impact of psychological distress on quality of life (QoL) in colorectal cancer patients, particularly in those with advanced disease stages and low social support. While emotional functioning showed improvements over time (p = 0.049, Cohen’s d = 0.62), psychological distress levels (anxiety and depression) remained stable, underscoring the need for additional psychological support.
Findings highlight that patients with lower social support and poorer economic conditions exhibited higher distress levels, emphasizing the role of psychosocial determinants in oncology care. Routine psychological screening should be integrated into oncology protocols to identify at-risk patients early.
Although no formal interventions were tested in this observational study, our results suggest that targeted interventions such as cognitive–behavioral therapy (CBT), resilience training, and structured social support programs should be explored in future trials to mitigate distress.
Final Thoughts
Integrating psychological care into oncology practice is essential for optimizing patient outcomes. By addressing psychological distress proactively, multidisciplinary care teams can significantly enhance the well-being and overall treatment experience of colorectal cancer patients (Figure 7).

Figure 7.
Summary of key study findings.
Author Contributions
Conceptualization, L.A.R. and T.C.G.; methodology, L.A.R. and T.C.G.; software, L.A.R. and T.C.G.; validation, L.A.R. and T.C.G.; formal analysis L.A.R. and T.C.G.; investigation, L.A.R. and T.C.G.; resources, L.A.R. and T.C.G.; data curation, L.A.R. and T.C.G.; writing—original draft preparation, L.A.R. and T.C.G.; writing—review and editing, L.A.R. and T.C.G.; visualization, L.A.R. and T.C.G.; supervision, A.M.M.; project administration, L.A.R. and T.C.G.; funding acquisition, L.A.R. and T.C.G. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by University of Oradea, Oradea, Romania.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Echo Laboratory (protocol code 12/01.04.2019, approval date: 1 April 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank to the University of Oradea for supporting the payment of the invoice through an internal project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lloyd, S.; Baraghoshi, D.; Tao, R.; Garrido-Laguna, I.; Gilcrease, G.W., 3rd; Whisenant, J.; Weis, J.R.; Scaife, C.; Pickron, T.B.; Huang, L.C.; et al. Mental Health Disorders are More Common in Colorectal Cancer Survivors and Associated with Decreased Overall Survival. Am. J. Clin. Oncol. 2019, 42, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, B.A.; Rat, L.A.; Moldovan, A.F.; Mihancea, P.; Mariș, L. An Open-Label Trial Study of Quality-of-Life Assessment in Irritable Bowel Syndrome and Their Treatment. Medicina 2022, 58, 763. [Google Scholar] [CrossRef]
- Rat, L.A.; Moldovan, A.F.; Trifan, D.F.; Matiș, L.; Murvai, G.F.; Maris, L.; Ghitea, T.C.; Maghiar, M.A. Can the Correlation of Periodontopathies with Gastrointestinal Diseases Be Used as Indicators in Severe Colorectal Diseases? Biomedicines 2023, 11, 402. [Google Scholar] [CrossRef]
- Niedzwiedz, C.L.; Knifton, L.; Robb, K.A.; Katikireddi, S.V.; Smith, D.J. Depression and anxiety among people living with and beyond cancer: A growing clinical and research priority. BMC Cancer 2019, 19, 943. [Google Scholar] [CrossRef]
- PeaceHealth, M.; a Bill, P. Depression (PDQ®): Supportive Care-Health Professional Information [NCI]. Available online: https://www.peacehealth.org/medical-topics/id/ncicdr0000062739 (accessed on 16 November 2024).
- Shalata, W.; Gothelf, I.; Bernstine, T.; Michlin, R.; Tourkey, L.; Shalata, S.; Yakobson, A. Mental Health Challenges in Cancer Patients: A Cross-Sectional Analysis of Depression and Anxiety. Cancers 2024, 16, 2827. [Google Scholar] [CrossRef]
- Denlinger, C.S.; Engstrom, P.F. Colorectal cancer survivorship: Movement matters. Cancer Prev. Res. 2011, 4, 502–511. [Google Scholar]
- Han, C.J.; Yang, G.S.; Syrjala, K. Symptom Experiences in Colorectal Cancer Survivors After Cancer Treatments: A Systematic Review and Meta-analysis. Cancer Nurs. 2020, 43, E132–E158. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.R. Depression in cancer patients: Pathogenesis, implications and treatment (Review). Oncol. Lett. 2015, 9, 1509–1514. [Google Scholar] [CrossRef]
- Maj, M.; Stein, D.J.; Parker, G.; Zimmerman, M.; Fava, G.A.; De Hert, M.; Demyttenaere, K.; McIntyre, R.S.; Widiger, T.; Wittchen, H.U. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry 2020, 19, 269–293. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Liu, T.; Du, C.; Luo, Q.; Song, L.; Liu, X.; Zhou, Y. Effect of multidisciplinary collaborative empowerment education on psychological distress and quality of life in patients with colorectal cancer undergoing chemotherapy. Support. Care Cancer 2023, 31, 116. [Google Scholar] [CrossRef]
- Dekker, J.; Graves, K.D.; Badger, T.A.; Diefenbach, M.A. Management of Distress in Patients with Cancer-Are We Doing the Right Thing? Ann. Behav. Med. 2020, 54, 978–984. [Google Scholar] [CrossRef] [PubMed]
- El-Shami, K.; Oeffinger, K.C.; Erb, N.L.; Willis, A.; Bretsch, J.K.; Pratt-Chapman, M.L.; Cannady, R.S.; Wong, S.L.; Rose, J.; Barbour, A.L.; et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J. Clin. 2015, 65, 428–455. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, X.; Yang, X.; Mao, J.; Li, Q. Factors Influencing Social Isolation among Cancer Patients: A Systematic Review. Healthcare 2024, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Groenvold, M.; Klee, M.C.; Sprangers, M.A.; Aaronson, N.K. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J. Clin. Epidemiol. 1997, 50, 441–450. [Google Scholar]
- Parra, R.S.; Chebli, J.M.F.; Amarante, H.; Flores, C.; Parente, J.M.L.; Ramos, O.; Fernandes, M.; Rocha, J.J.R.; Feitosa, M.R.; Feres, O.; et al. Quality of life, work productivity impairment and healthcare resources in inflammatory bowel diseases in Brazil. World J. Gastroenterol. 2019, 25, 5862–5882. [Google Scholar] [CrossRef]
- Williet, N.; Sarter, H.; Gower-Rousseau, C.; Adrianjafy, C.; Olympie, A.; Buisson, A.; Beaugerie, L.; Peyrin-Biroulet, L. Patient-reported Outcomes in a French Nationwide Survey of Inflammatory Bowel Disease Patients. J. Crohns Colitis 2017, 11, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, P.; Perrone, M.; Nisi, E.; Garufi, C.; Giannarelli, D.; Bottomley, A.; Terzoli, E. An integrated psychological strategy for advanced colorectal cancer patients. Health Qual. Life Outcomes 2006, 4, 9. [Google Scholar] [CrossRef]
- Mystakidou, K.; Eleni, T.; Efi, P.; Ourania, K.; Vassilios, S.; Lambros, V. The EORTC Core Quality of Life Questionnaire (QLQ-C30, Version 3.0) in terminally ill cancer patients under palliative care: Validity and reliability in a Hellenic sample. Int. J. Cancer 2001, 94, 135–139. [Google Scholar] [CrossRef]
- Brink, V.; van Driel, C.; El Bouhaddani, S.; Wardenaar, K.J.; van Domburgh, L.; Schaefer, B.; van Beilen, M.; Bartels-Velthuis, A.A.; Veling, W. Spontaneous discontinuation of distressing auditory verbal hallucinations in a school-based sample of adolescents: A longitudinal study. Eur. Child Adolesc. Psychiatry 2020, 29, 777–790. [Google Scholar] [CrossRef]
- Herschbach, P.; Book, K.; Dinkel, A.; Berg, P.; Waadt, S.; Duran, G.; Engst-Hastreiter, U.; Henrich, G. Evaluation of two group therapies to reduce fear of progression in cancer patients. Support. Care Cancer 2010, 18, 471–479. [Google Scholar]
- Stark, D.P.H.; House, A. Anxiety in cancer patients. Br. J. Cancer 2000, 83, 1261–1267. [Google Scholar] [PubMed]
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [PubMed]
- Pinquart, M.; Duberstein, P.R. Associations of social networks with cancer mortality: A meta-analysis. Crit. Rev. Oncol./Hematol. 2010, 75, 122–137. [Google Scholar] [PubMed]
- Armes, J.; Crowe, M.; Colbourne, L.; Morgan, H.; Murrells, T.; Oakley, C.; Palmer, N.; Ream, E.; Young, A.; Richardson, A. Patients’ supportive care needs beyond the end of cancer treatment: A prospective, longitudinal survey. J. Clin. Oncol. 2009, 27, 6172–6179. [Google Scholar] [CrossRef]
- Kissane, D.W.; Grabsch, B.; Clarke, D.M.; Smith, G.C.; Love, A.W.; Bloch, S.; Snyder, R.D.; Li, Y. Supportive-expressive group therapy for women with metastatic breast cancer: Survival and psychosocial outcome from a randomized controlled trial. Psycho-Oncol. J. Psychol. Soc. Behav. Dimens. Cancer 2007, 16, 277–286. [Google Scholar]
- Northouse, L.L.; Katapodi, M.C.; Song, L.; Zhang, L.; Mood, D.W. Interventions with family caregivers of cancer patients: Meta-analysis of randomized trials. CA Cancer J. Clin. 2010, 60, 317–339. [Google Scholar] [CrossRef]
- Faller, H.; Weis, J.; Koch, U.; Brähler, E.; Härter, M.; Keller, M.; Schulz, H.; Wegscheider, K.; Boehncke, A.; Hund, B.; et al. Perceived need for psychosocial support depending on emotional distress and mental comorbidity in men and women with cancer. J. Psychosom. Res. 2016, 81, 24–30. [Google Scholar] [CrossRef]
- Hsiao, H.J.; Chen, S.H.; Jaing, T.H.; Yang, C.P.; Chang, T.Y.; Li, M.Y.; Chiu, C.H.; Huang, J.L. Psychosocial interventions for reduction of distress in children with leukemia during bone marrow aspiration and lumbar puncture. Pediatr. Neonatol. 2019, 60, 278–284. [Google Scholar] [CrossRef]
- Schuck, S.; Loussikian, P.; Mebarki, A.; Malaab, J.; Foulquié, P.; Talmatkadi, M.; Kearney, M. Perceived unmet needs and impact on quality of life of patients living with advanced bladder cancer and their caregivers: Results of a social media listening study conducted in five European countries. BMC Cancer 2024, 24, 1444. [Google Scholar] [CrossRef]
- Hosseini, Z.; Homayuni, A.; Etemadifar, M. Barriers to quality of life in patients with multiple sclerosis: A qualitative study. BMC Neurol. 2022, 22, 174. [Google Scholar] [CrossRef]
- Haskamp, A.; Haase, J.; Azzouz, F.; Monahan, P. Social Support and Symptom Distress in Adolescents/Young Adults with Cancer. J. Pediatr. Oncol. Nurs. Off. J. Assoc. Pediatr. Oncol. Nurses 2008, 25, 275–284. [Google Scholar] [CrossRef]
- Zebrack, B.; Corbett, V.; Embry, L.; Aguilar, C.; Meeske, K.; Hayes-Lattin, B.; Block, R.; Zeman, D.; Cole, S. Psychological distress and unsatisfied need for psychosocial support in adolescent and young adult cancer patients during the first year following diagnosis. Psycho-Oncol. 2014, 23, 1267–1275. [Google Scholar] [CrossRef]
- Winger, J.G.; Rand, K.L.; Hanna, N.; Jalal, S.I.; Einhorn, L.H.; Birdas, T.J.; Ceppa, D.P.; Kesler, K.A.; Champion, V.L.; Mosher, C.E. Coping Skills Practice and Symptom Change: A Secondary Analysis of a Pilot Telephone Symptom Management Intervention for Lung Cancer Patients and Their Family Caregivers. J. Pain Symptom Manag. 2018, 55, 1341–1349.e4. [Google Scholar] [CrossRef]
- Zhang, T.; Ren, Z.; Wakefield, C.E.; Hui, B.P.H.; Akechi, T.; Shi, C.; Du, X.; Chen, W.; Lai, L.; Zhao, C.; et al. Are digital psychological interventions for psychological distress and quality of life in cancer patients effective? A systematic review and network meta-analysis. Clin. Psychol. Rev. 2025, 115, 102520. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.M.; Wahba, N.M.I.; Zaghamir, D.E.F.; Mersal, N.A.; Mersal, F.A.; Ali, R.A.E.; Eltaib, F.A.; Mohamed, H.A.H. Impact of a comprehensive rehabilitation palliative care program on the quality of life of patients with terminal cancer and their informal caregivers: A quasi-experimental study. BMC Nurs. 2024, 23, 357. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Gómez, A.F. Mindfulness-Based Interventions for Anxiety and Depression. Psychiatr. Clin. N. Am. 2017, 40, 739–749. [Google Scholar] [CrossRef]
- Duan, A.; Zhao, H.; Zhou, C. The Effects of a Healthy Lifestyle on Depressive Symptoms in Older Chinese Adults: The Mediating Role of Psychological Resilience. Cureus 2024, 16, e57258. [Google Scholar] [CrossRef]
- Cadet, T.; Davis, C.; Elks, J.; Wilson, P. A Holistic Model of Care to Support Those Living with and beyond Cancer. Healthcare 2016, 4, 88. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Govina, O.; Tsatsou, I.; Mantzorou, M.; Mantoudi, A.; Tsiou, C.; Adamakidou, T. Quality of life, distress, anxiety and depression of ambulatory cancer patients receiving chemotherapy. Med. Pharm. Rep. 2022, 95, 418–429. [Google Scholar] [CrossRef]
- Faller, H.; Schuler, M.; Richard, M.; Heckl, U.; Weis, J.; Küffner, R. Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: Systematic review and meta-analysis. J. Clin. Oncol. 2013, 31, 782–793. [Google Scholar] [CrossRef]
- Pinquart, M.; Duberstein, P. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [PubMed]
- Alegría, M.; NeMoyer, A.; Falgàs Bagué, I.; Wang, Y.; Alvarez, K. Social Determinants of Mental Health: Where We Are and Where We Need to Go. Curr. Psychiatry Rep. 2018, 20, 95. [Google Scholar] [CrossRef]
- Kirkbride, J.B.; Anglin, D.M.; Colman, I.; Dykxhoorn, J.; Jones, P.B.; Patalay, P.; Pitman, A.; Soneson, E.; Steare, T.; Wright, T.; et al. The social determinants of mental health and disorder: Evidence, prevention and recommendations. World Psychiatry 2024, 23, 58–90. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Bone, L.; Forte, J.; Kirley, E.; Lynch, T.; Aboumatar, H. The benefits and challenges of established peer support programmes for patients, informal caregivers, and healthcare providers. Fam. Pract. 2022, 39, 903–912. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Hu, X. Peer support interventions on quality of life, depression, anxiety, and self-efficacy among patients with cancer: A systematic review and meta-analysis. Patient Educ. Couns. 2022, 105, 3213–3224. [Google Scholar] [CrossRef] [PubMed]
- Beard, D.; Cottam, C.; Painter, J. Evaluation of the Perceived Benefits of a Peer Support Group for People with Mental Health Problems. Nurs. Rep. 2024, 14, 1661–1675. [Google Scholar] [CrossRef]
- Tripathi, P.; Research, B.; Bijnr; James, A. Peer Support Programs in Mental Health Nursing: Harnessing Lived Experience for Recovery. Int. J. Nurs. Res. 2024, 5, 234. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).