Abstract
Background: Mild cognitive impairment (MCI) represents an early stage of cognitive loss that significantly increases the risk of dementia. The aim of this study was to comprehensively synthesize the current evidence on the effect of combined physical and cognitive therapies in older adults with MCI. Methods: A systematic review with meta-analysis was conducted by searching for specific keywords in the PubMed, Scopus, Cinhal, and Web of Science databases. This meta-analysis included a total of 2256 participants distributed across 21 studies that evaluated the benefits of combining physical exercise with cognitive stimulation. Results: This review revealed that these types of therapies present a significant improvement in memory, attention, and executive functions. Participants showed notable improvements in these cognitive areas, highlighting the synergistic effects of physical exercise and cognitive stimulation, which exceeded the benefits of each therapy separately. These results contribute to the understanding of how these combined therapies can improve cognitive health in this population, offering robust evidence supporting their application in clinical practice. Conclusions: This meta-analysis shows that combined physical exercise and cognitive stimulation interventions may be an effective strategy for improving cognitive health in older adults with MCI. The findings of this study offer a valuable contribution to the field, highlighting the potential of these combined therapies to prevent cognitive decline and improve the quality of life of this population. The results may be of interest to health professionals and guide future research and clinical applications.
1. Introduction
Population aging is a global phenomenon that poses numerous challenges in terms of public health, especially in relation to the increase in cognitive and neurodegenerative disorders in older people [1]. One of these disorders, mild cognitive impairment (MCI), is considered an intermediate phase between normal aging and dementia and is widely recognized as an important risk factor for the development of Alzheimer’s disease and other forms of dementia [2,3]. MCI is characterized by a decline in cognitive abilities, such as memory, attention, and executive functions, without significantly affecting the activities of daily living [4]. The most frequent clinical symptom of MCI is episodic memory impairment, characterized by an accelerated rate of forgetting and difficulties in delayed recall. However, deficits in working memory (WM) and executive functions are also regularly present in individuals with MCI [5]. The first diagnostic criteria for MCI were proposed by Petersen et al. [6], focusing mainly on memory problems. These criteria included memory complaints, the preservation of daily activities, normal general cognitive function, abnormal memory for the patient’s age, and the absence of signs of dementia. However, given the identification of other non-memory problems that may also influence cognitive decline, they revised these criteria by incorporating non-memory aspects [7]. Various tests have been proposed to detect MCI, such as the Mini-Mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA), the Free and Cued Selective Recall Test (FCSRT), the California Verbal Learning Test, and the Boston Naming Test. However, no specific test or cut-off score has been established that is universally accepted for the diagnosis of MCI. The absence of standardized tests and clear cut-off points may affect the accuracy of the diagnosis [8,9].
The prevalence of MCI in people aged 65 years or older ranges between 10% and 20%, increasing with age [10]. In Spain, the prevalence of MCI is 18.5%, with a significantly higher rate in women than in men. Furthermore, this prevalence shows a notable increase with age, reaching 45.3% in individuals over 85 years of age [11]. In light of the growing elderly population and the significant impact of MCI on quality of life, as well as associated healthcare costs, there is an urgent need for interventions that can mitigate or even reverse cognitive decline in this population [12].
Non-pharmacological interventions have garnered interest as effective and safe alternatives for the management of MCI, avoiding the common side effects of pharmacological treatments [13]. Among these, combined physical and cognitive therapies have shown promise. Separately, physical exercise has been shown to effectively prevent and manage MCI, offering benefits such as fewer side effects and greater adherence than pharmacological treatments [14]. However, the literature on cognitive training often presents methodological limitations, such as small sample sizes and uncontrolled designs, which affect the reliability of its conclusions [15]. Although one study shows improvements in specific trained cognitive domains, evidence of these benefits transferring to other functional areas or daily life remains insufficient, which casts doubt on the real effectiveness of the interventions [16]. The combination of cognitive tasks during physical exercise has been shown to enhance these benefits, highlighting the synergy between physical activity and cognitive stimulation [17]. These interventions integrate physical exercise programs (such as walking, stretching, and resistance training) with cognitive stimulation activities (such as memory exercises, attention skill training, and the use of digital technologies) to optimize both physical and cognitive health [18]. It has been postulated that the combination of physical exercise and cognitive stimulation can produce synergistic effects by promoting neuroplasticity and improving cerebral blood flow, two mechanisms that are considered beneficial for maintaining cognitive health, and these results have been found in healthy older populations [19]. Furthermore, these interventions have shown additional benefits in terms of mobility, balance, and strength, key factors in reducing the risk of falls and improving functionality in daily life [20].
In this context, access to integrated interventions faces challenges related to accessibility and personalization [21]. Adapting these programs to meet individual needs, considering factors such as health status, level of cognitive impairment, and personal preferences, could maximize their effectiveness [22]. Likewise, incorporating simple and accessible technological tools, such as mobile applications or online platforms, could facilitate their implementation, especially in areas with limited resources [23].
At a conceptual level, physical exercise improves cardiovascular health and cerebral blood flow, both related to improvements in cognitive status, while cognitive stimulation strengthens neural networks and keeps critical mental functions active [24,25]. The interaction between both components could enhance the individual effects of each one, generating a synergy that favors the recovery or maintenance of cognitive functions in people with MCI [26,27]. This synergy hypothesis is supported by the literature, which suggests that combined interventions can overcome the benefits of single-component interventions. However, research on these combined therapies shows inconsistent results, which may be due to factors such as the duration of the intervention, the frequency of sessions, the specific type of activities used, and the assessment methodologies [28,29]. In addition, individual differences, such as health status, level of cognitive impairment, and lifestyle, can significantly influence the effectiveness of these therapies [30].
In this context, the present systematic review and meta-analysis aims to comprehensively synthesize the current evidence on the effect of combined physical and cognitive therapies on older people with MCI. Through a detailed analysis of recent studies, this review seeks to clarify the benefits of these interventions and determine the key components influencing their effectiveness.
2. Materials and Methods
This review was conducted following the guidelines of the 2020 PRISMA statement [31] and the pre-established protocol registered in PROSPERO (CRD42024620751). Furthermore, the methodological approach adhered to the recommendations outlined in the “Cochrane Manual for the Elaboration of Systematic Reviews of Interventions” [32].
2.1. Sources of Information
A bibliographic search was performed between October and November 2024 using the PubMed, Scopus, CINAHL, and Web of Science (WOS) databases.
2.2. Search Strategy
Different keywords were used in the following search string: (“combined physical” and “cognitive training” OR “cognitive training” OR “cognitive intervention” OR “cognitive therapy”) AND (“physical exercise” OR “physical activity” OR “physical training”) AND (“mild cognitive impairment” OR “mci”) AND (“older adults” OR “elderly” OR “aged”).
2.3. Inclusion Criteria
The selected articles had to meet the following criteria: (i) the studies must be randomized clinical trials (RCTs); (ii) the intervention studied must combine physical exercise and cognitive training; (iii) participants must belong to the older population (aged > 60 years); and (iv) participants must have a diagnosis of mild cognitive impairment (MCI) established using validated diagnostic tools, such as the Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog), among others.
2.4. Exclusion Criteria
Articles were excluded if they met any of the following criteria: (i) studies lacking a non-intervention reference group; (ii) studies not measuring the relevant study variables; (iii) the presence of other conditions, such as cancer, stroke, cardiovascular disease (CVD), lung disease, and/or kidney disease; and (iv) participants not reaching the minimum required attendance percentage at intervention program sessions.
2.5. Study Selection Process
The study selection process began with the elimination of duplicate entries and articles without available abstracts. Titles and abstracts were then thoroughly reviewed to eliminate those that did not meet the established eligibility criteria. Articles that passed this first phase were assessed in full text to determine their suitability for inclusion in the meta-analysis. To ensure objectivity and reduce potential bias, two authors (J.M.M.-P. and A.A.-A.) independently performed the selection. Cases where disagreements arose regarding the eligibility of a study were resolved by consultation with a third author (F.H.-C.), who offered his judgment to reach a consensus. This rigorous procedure ensured that all included studies were relevant and met the predefined criteria.
2.6. Data Extraction
The main variable in this study was cognitive status in older patients with mild cognitive impairment. Data extraction included information on authorship, publication year, study location, population details (sample size, age, and group allocation), study design, outcomes, measurement tools, intervention descriptions, measurement timelines, attrition rates, adverse effects, and key findings.
2.7. Assessment of Methodological Quality
Methodological quality was evaluated using the PEDro scale [33], which consists of an 11-item checklist. The highest possible score is 10 points, as the first item (“eligibility criteria”) is not included in the final scoring. Each item is rated as either “Yes” (1 point) or “No” (0 points). Quality levels are categorized as follows: scores between 0 and 3 indicate “Poor” quality, 4 and 5 represent “Fair” quality, 6 and 8 indicate “Good” quality, and scores above 9 are considered “Excellent” [34].
2.8. Analytic Decisions for Meta-Analysis
The results of the meta-analysis are summarized using a forest plot, which shows key details such as the lead author, year of publication, sample size, and individual effect sizes calculated with the Hedge index (g). This index is presented with its 95% confidence interval and corresponding p-value. To ensure the robustness of the results, a sensitivity analysis was performed. This analysis consisted of excluding studies that presented duplicate data, outliers, or single cases that could have affected the overall interpretation. The results of this sensitivity analysis were compared with those obtained in the full meta-analysis, allowing us to verify the stability of the findings and to assess whether the results were sensitive to the inclusion of certain studies. In the subgroup analysis, the studies were classified according to the tools used to assess global cognition (such as MoCA, MMSE, and ADAS-Cog). Separate meta-analyses were performed for each of these subgroups, allowing us to examine the variability of effects within each category and gain a more detailed understanding of how different assessment tools might influence the results. To address heterogeneity between studies, a random-effects model was used, allowing for differences between studies to be considered and for a more generalizable estimate of the effects. This was complemented by assessing heterogeneity through the Q test and I2, which provide information on the variability of effects across included studies. A high I2 value indicates greater heterogeneity, suggesting that studies are not homogeneous and may be influenced by different factors. In addition, publication bias was assessed using a funnel plot. This plot helps to detect potential biases related to studies reporting only positive or significant results, which could affect the validity of the meta-analysis. A visual assessment of the funnel plot and complementary statistical tests (such as Egger’s test) provided information on possible bias in the reviewed literature.
3. Results
3.1. Study Selection Process
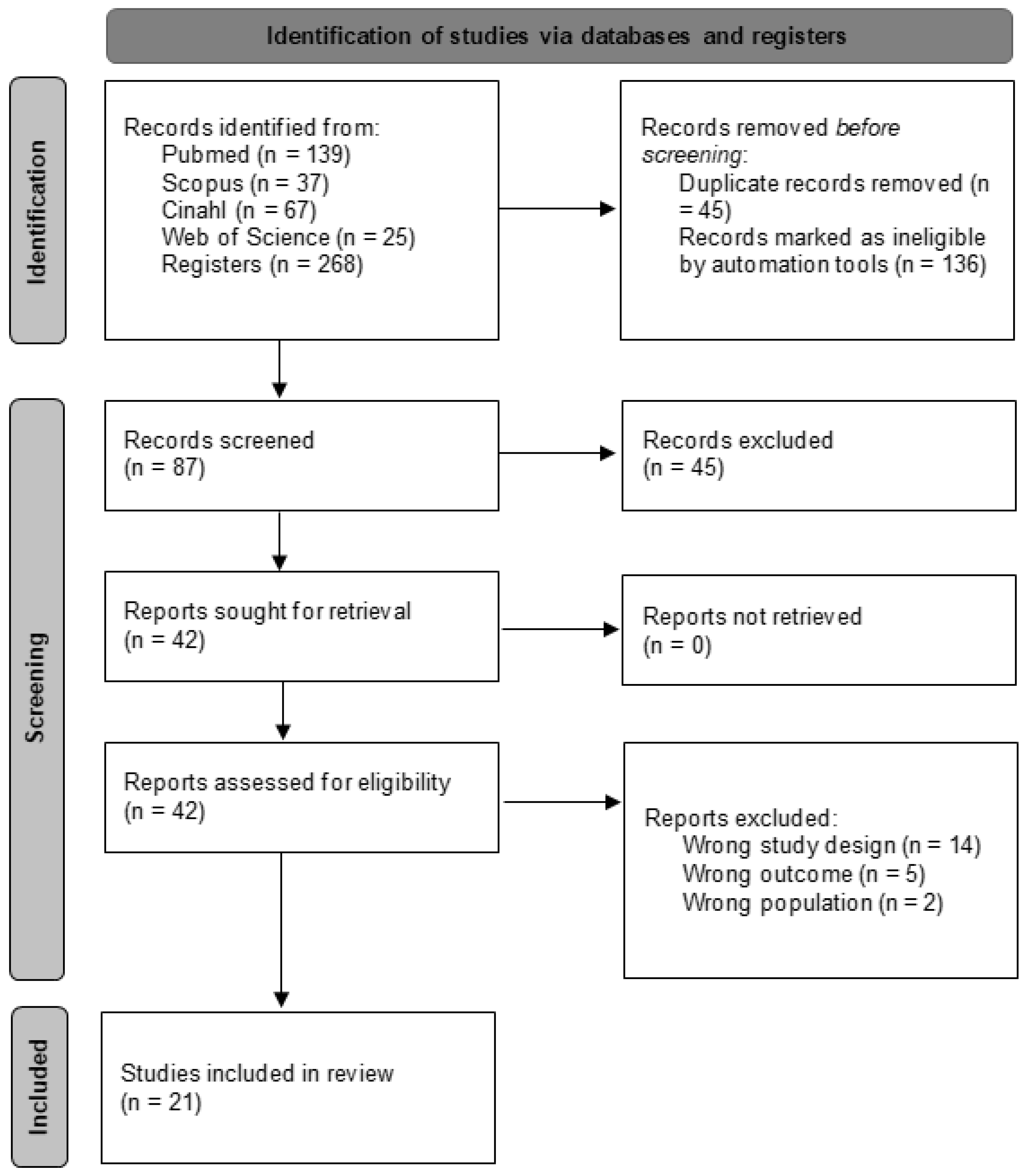
An initial search across various databases identified 268 articles. The search was then refined within the same databases by focusing on specific document types (articles and randomized clinical trials) and filtering for keywords in titles and abstracts, while also eliminating duplicates. This refinement yielded 87 unique articles. These articles were subsequently screened based on their titles and abstracts, narrowing the selection to 42 potential candidates for qualitative evaluation. Ultimately, 21 articles [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] met the inclusion criteria and were included in the meta-analysis, while the remaining 21 were excluded. The selection process is outlined in greater detail in Figure 1.
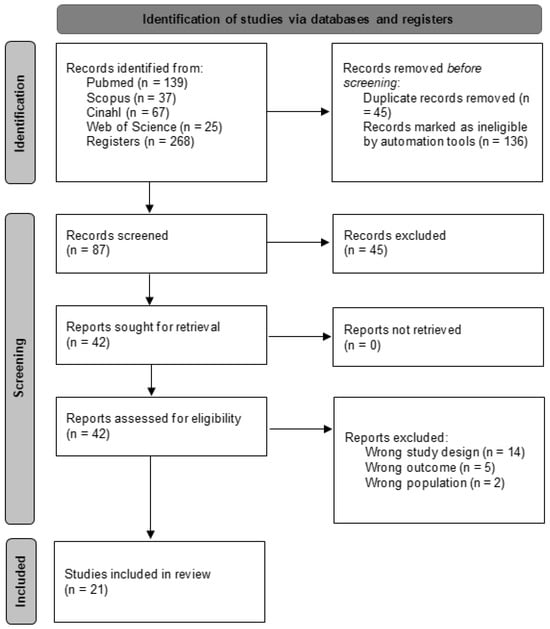
Figure 1.
Study selection process flow chart.
3.2. Methodological Quality
The methodological quality of the included studies was evaluated using the PEDro scale, with the scores sourced from the PEDro web portal. Of the included studies, eighteen were classified as “Good” [35,36,37,38,40,41,42,43,44,46,47,49,50,51,52,53,54,55], while three were rated as “Fair” [39,45,48]. To ensure objectivity and consistency in the assessment, two independent reviewers scored the studies according to the PEDro scale. In cases of discrepancies between the reviewers, a conflict resolution procedure was followed. The reviewers discussed the disagreements and, if a consensus was not reached, the intervention of a third reviewer was requested. This process made it possible to ensure that the grading of the studies was as accurate and reliable as possible. A detailed assessment of the methodological quality is provided in Table 1.

Table 1.
Methodological quality of the included articles.
3.3. Characteristics of the Studies
All studies included in this systematic review and meta-analysis were randomized controlled trials conducted in New Zealand [35], Italy [36], Canada [37,52], Spain [38,44], Iran [39], Taiwan [40,55], Japan [41], China [42,43,46,49,50], Greece [45], Slovakia [47], Australia [48], Turkey [51], the United States [53], and the Philippines [54]. Across these studies, 2256 participants were involved, with 967 in the control group and 1289 in the intervention group, which focused on mind–body training. Women constituted the majority in most of the included studies, with Damirchi et al. [39] reporting an entirely female sample. The overall mean age of participants was 72.4 (Table 2).

Table 2.
Characteristics of the included studies.
Considering these interventions, there was heterogeneity in terms of the training pace and dose employed. Regarding frequency, only one study [41] conducted therapy with one session per week. Six studies [35,38,43,46,47] carried out interventions with two weekly sessions, with the particular case of the study by Mavros et al. [48], where the frequency decreased from three sessions per week to two. Eleven trials [36,37,39,40,44,49,51,52,53,54,55] implemented three weekly sessions. Finally, in three articles [42,45,50], the intervention was applied five days a week.
On the other hand, in other studies [37,45,52], the sample was randomized into five groups. In four other studies [39,48,51,54], four treatment groups were used, while Xu et al. [49] employed three groups.
3.4. Study Results
Of the 21 articles included in this systematic review, five were excluded from the meta-analysis. The article by Bray et al. [47] was excluded because it did not evaluate global cognition but rather assessed changes in functional brain connectivity. A similar situation occurs with [39,40,53], which assessed specific components of executive function, such as attention, decision-making capacity, processing speed, or memory. A separate case is the article by Lipardo et al. [54], which was excluded for not examining cognition.
The main objective of this systematic review and meta-analysis was to evaluate global cognition. Regarding this evaluation, seven articles used the Montreal Cognitive Assessment (MoCA), eight studies employed the Mini-Mental State Examination (MMSE), and five trials used the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog). Among the studies assessing global cognition, 10 reported significant improvements supporting combined therapies [38,42,43,44,45,46,47,50,51,52], achieving a statistical value of p < 0.05. Significant results in cognition were also observed in the studies excluded from the meta-analysis [37,39,40,53], based on their respective scales measuring specific cognitive aspects, favoring combined training. The remaining articles [35,41,48,49,55] did not reach statistical significance (p > 0.05) when measuring changes in global cognition after this type of training.
In this systematic review, secondary variables such as physical function, depression, and quality of life are also considered. Physical function was evaluated in thirteen trials [35,37,38,40,43,46,47,48,49,50,51,53,54] using scales such as the 6-Minute Walk Test (6MWT), the Timed Up and Go (TUG) Test, the Sit to Stand Test (STS), the 30-Second Chair Stand Test, and the Short Physical Performance Battery (SPPB), which were among the most frequently used. Statistically significant differences favoring combined training were found in most articles [35,38,40,46,47,48,50,51,53,54], while the remaining studies [37,43,49] did not conclude significant changes.
Additionally, depression was studied in four trials [35,46,49,51] using the Hospital Anxiety and Depression Scale (HADS), the Geriatric Depression Scale (GDS), and the Hamilton Depression Rating Scale (HDRS), with significant improvements observed in almost all included studies [35,46,51]. However, this was not the case for Xu et al. [49], where the GDS results did not show significant differences between groups.
Finally, quality of life was analyzed in five of the included studies [35,46,47,49,51], and like for the previous variable, all studies except Xu et al. [49] achieved significant improvements in quality of life. This was evaluated using scales such as the Quality of Life in Alzheimer’s Disease Scale, the Quality of Life Test, the EuroQoL 5-D Questionnaire (EQ-5D), and the World Health Organization Quality of Life Old Module.
3.5. Meta-Analysis
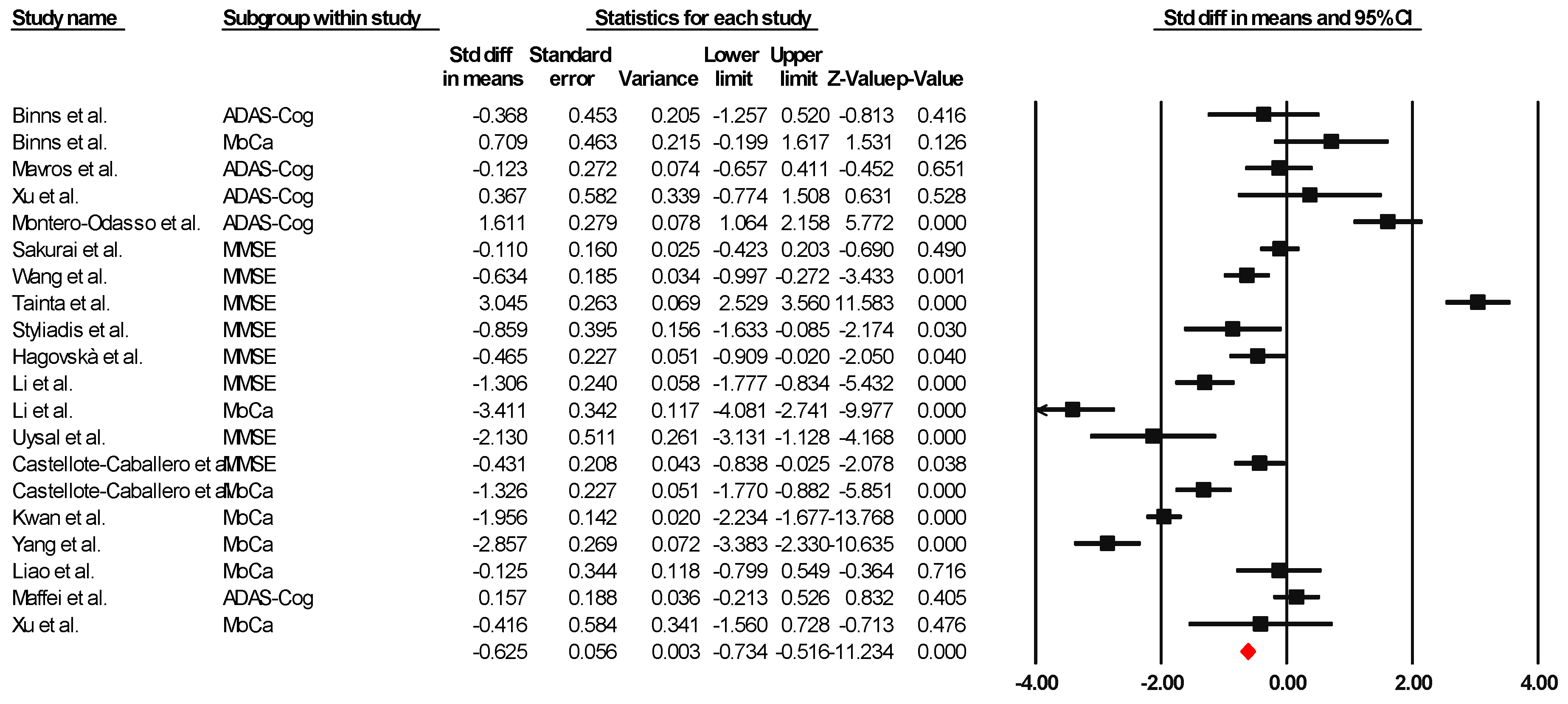
Sixteen of the articles were integrated into the meta-analysis to synthesize the findings on global cognition. The heterogeneity analysis showed that the Q-value was 8.418 with fourteen degrees of freedom. The I-squared statistic, which quantifies the percentage of variability in the observed effects attributed to real effects rather than sampling error, was set to 0%. In addition, we calculated Tau-squared and Tau, which provided information on the variance and standard deviation of the real effect sizes in d units, respectively. Both Tau-squared and Tau were calculated as 0.000, suggesting that all studies shared a common effect size without any dispersion of real effects. Lastly, the prediction interval was not reported because our analysis estimated Tau-squared as zero, reinforcing the notion that all studies exhibited consistent effect sizes without any variability in real effects. This comprehensive analysis incorporating these statistical measures provides valuable information about the results and the observed homogeneity in the effects across the selected studies. Since the I-square, Tau-square, and Tau indices of heterogeneity are minimal, a fixed-effect model was used for the analysis. The effect size index used was the standardized difference between means (g), −0.625, with a 95% confidence interval of −0.734 to −0.516. Importantly, negative effect size values represent an improvement in global cognition attributable to the combined interventions, indicating their success. Figure 2 presents this result through a graph illustrating the consistency of the effects observed across the included studies.
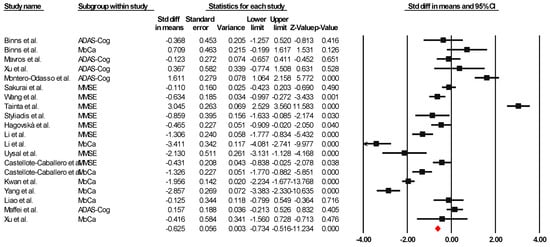
Figure 2.
Forest plot of the overall effect of combined physical and cognitive therapies for the health of older adults with mild cognitive impairment. The black box represents the point estimate for each study, while the box size represents the population size, and the horizontal line is the 95% CI. The diamond-shaped figure represents the estimated point of the mean difference [35,36,38,40,41,42,43,44,45,46,47,48,49,50,51,52].
3.5.1. Subgroup Analysis
A subgroup analysis was performed using the three global cognition measurement tools. The results revealed notable statistical significance, supported by moderate and inversely negative Hedge’s g effect sizes. Subgroup analyses based on this assessment tool demonstrated consistent effect sizes across all cases. This consistency in our results suggests that the choice of assessment tool had a minimal impact on the observed treatment effects.
MoCa
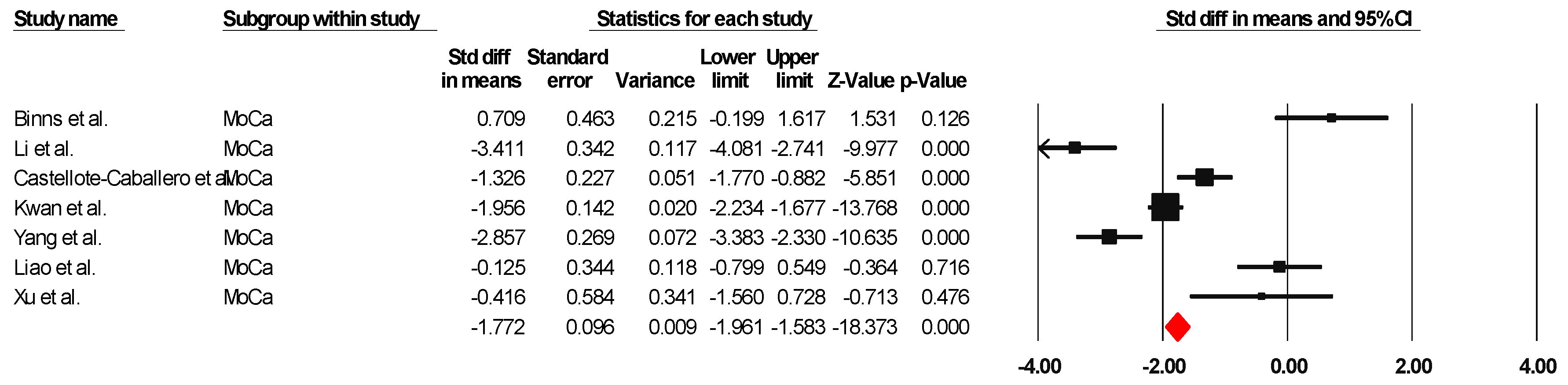
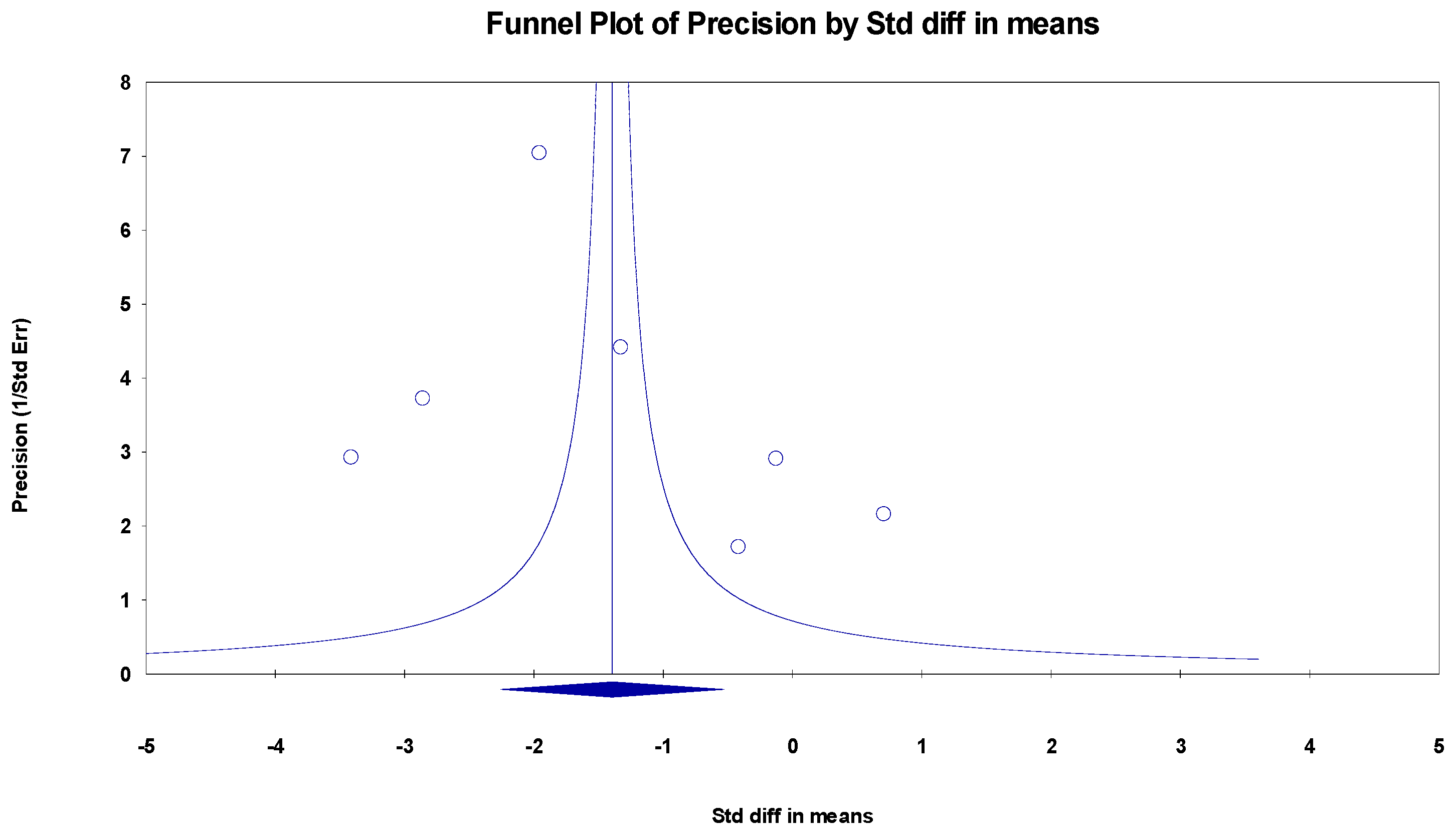
The results indicated an effect size of −1.774 for the MoCa, showing the substantial impact of this measurement tool on the observed outcomes. Independent Q tests were performed, showing evidence of significant heterogeneity, as the Q-value was 5.802 with three degrees of freedom (df) and a p-value of 0.000 (Figure 3), without risk of publication bias (Egger p = 0.20) (Figure 4).
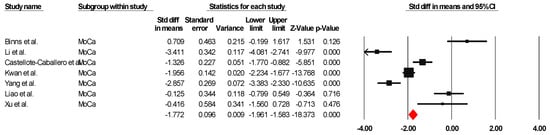
Figure 3.
Forest plot of the effectiveness of combined physical and cognitive therapies for the health of older adults with mild cognitive impairment [35,38,40,43,46,49,50].
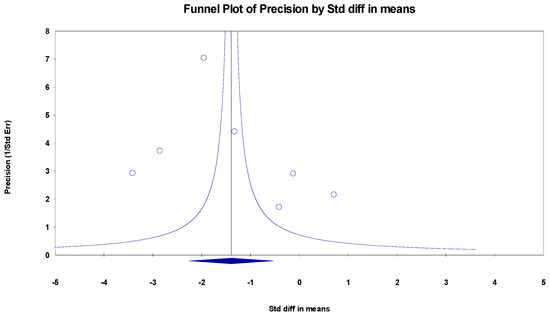
Figure 4.
Funnel plot for MoCa.
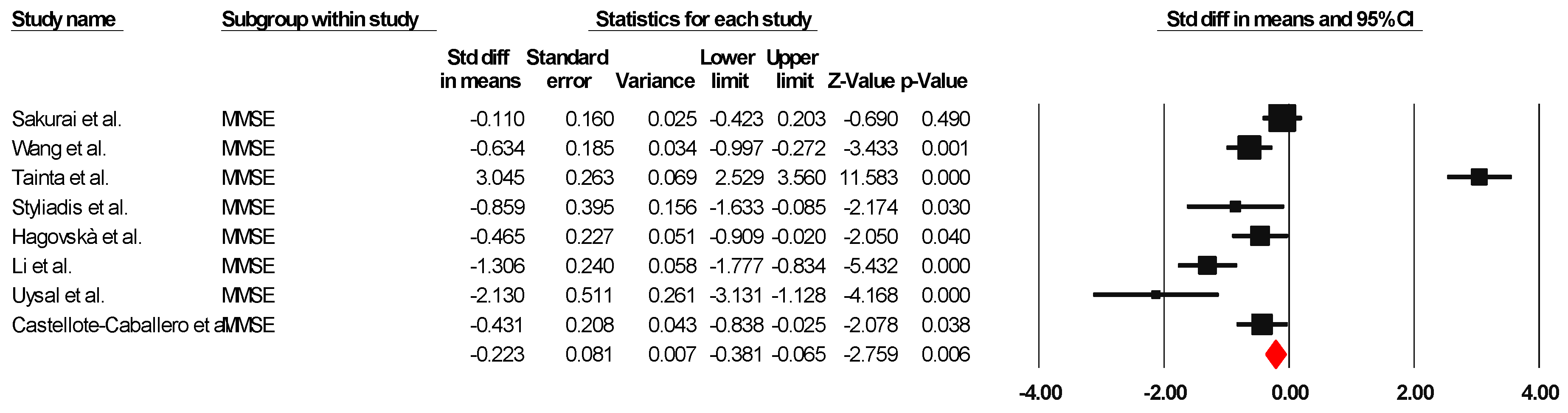
MMSE
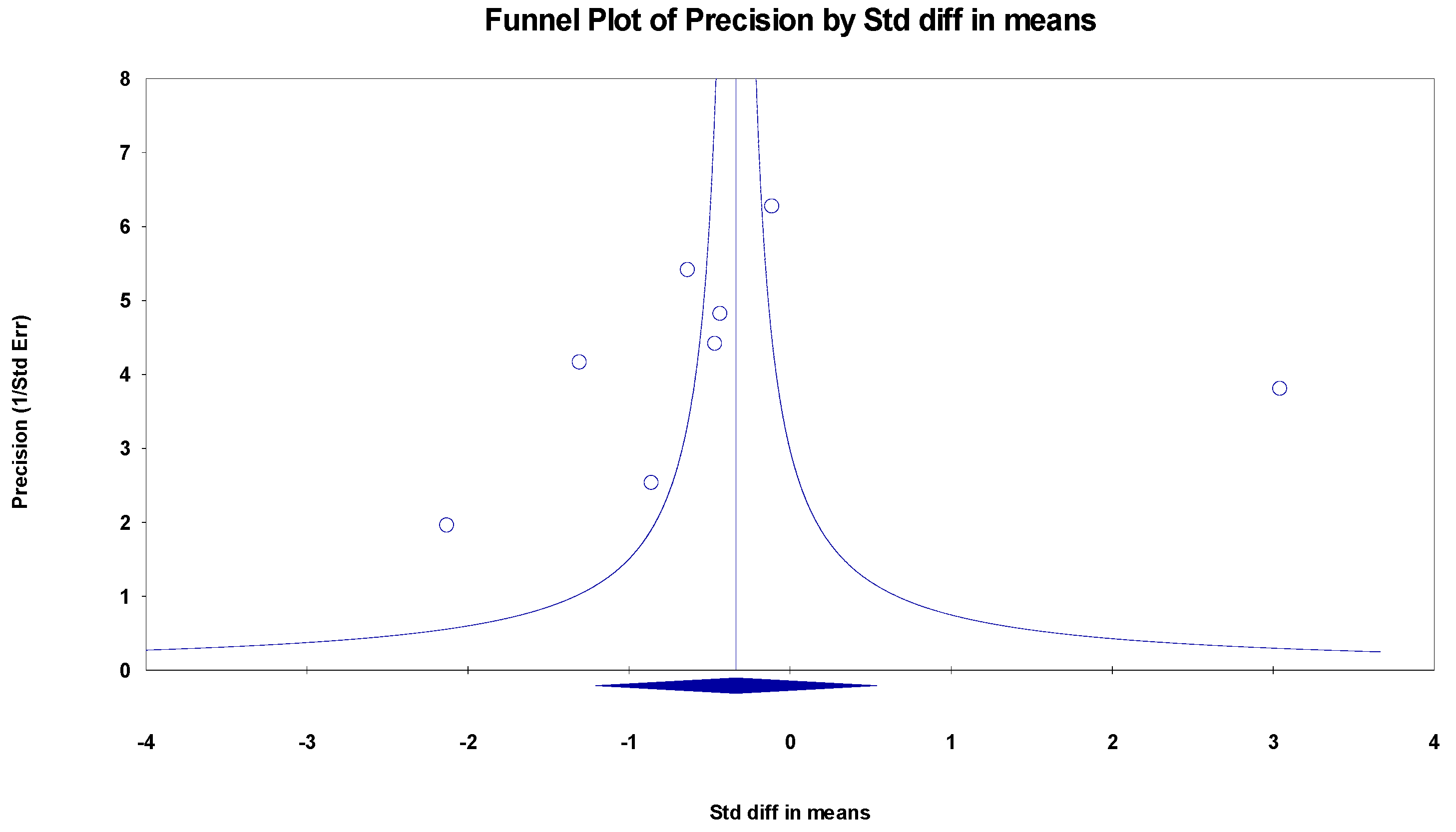
The results indicated an effect size of −0.187 for the MMSE, showing the substantial impact of this measurement tool on the observed outcomes. Independent Q tests were performed, showing evidence of significant heterogeneity, as the Q-value was 7.587 with six degrees of freedom (df) and a p-value of 0.000 (Figure 5), without risk of publication bias (Egger p = 0.42) (Figure 6).
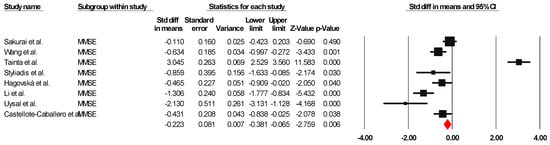
Figure 5.
Forest plot of the effectiveness of combined physical and cognitive therapies for the health of older adults with mild cognitive impairment [38,41,42,44,45,47,50,51].
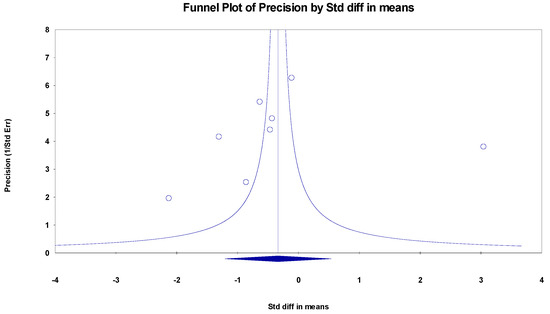
Figure 6.
Funnel plot for MMSE.
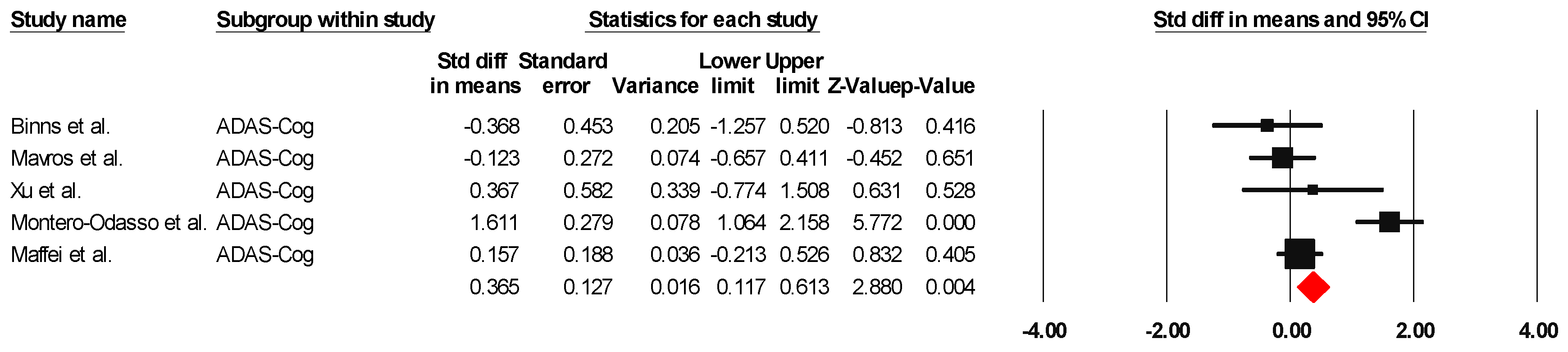
ADAS-Cog
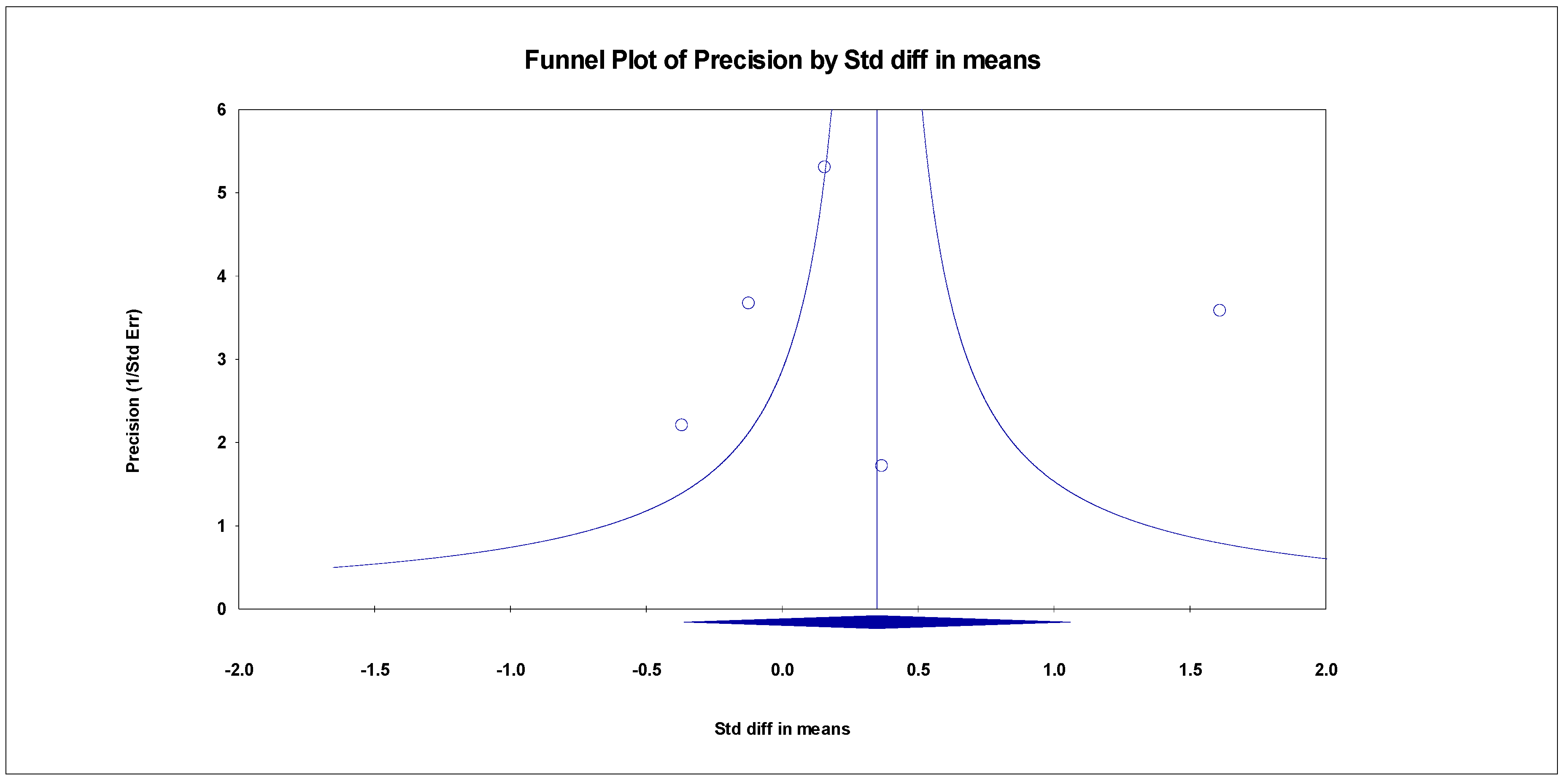
The results indicated an effect size of 0.518 for the ADAS-Cog, showing the substantial impact of this measurement tool on the observed outcomes. Independent Q tests were performed, showing evidence of significant heterogeneity, as the Q value was 4.978 with three degrees of freedom (df) and a p-value of 0.000 (Figure 7), without risk of publication bias (Egger p = 0.49) (Figure 8).
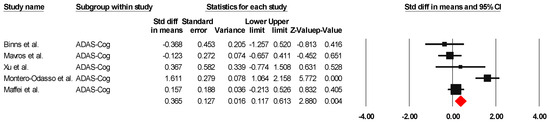
Figure 7.
The forest plot of the effectiveness of combined physical and cognitive therapies for the health of older adults with mild cognitive impairment [35,36,48,49,52].
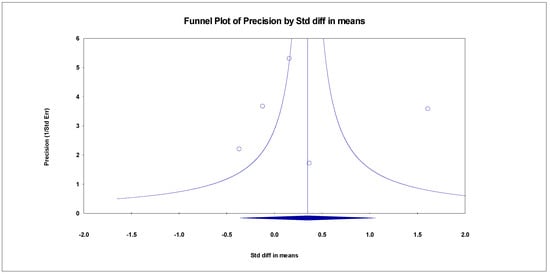
Figure 8.
Funnel plot for ADAS-Cog.
4. Discussion
The main objective of this study was to evaluate the effectiveness of combining physical exercise and cognitive training in improving cognitive performance in older adults with mild cognitive impairment (MCI). Through a meta-analysis of the available randomized clinical trials, we sought to determine whether this combined intervention could generate significant benefits compared with traditional treatments or individual interventions, as well as to evaluate the sustainability of these effects in the long term.
In terms of methodological quality, most of the studies analyzed [35,36,37,38,40,41,42,43,44,46,47,49,50,51,52,53,54,55] showed good quality in design and execution. However, three [39,45,48] presented an average methodological quality. None of the included studies achieved an excellent rating. Notably, the main limitations in these studies were a lack of blinding of participants or therapists and an inadequate assignment of interventions. These methodological deficiencies may have significantly impacted the observed results. Previous research has shown that a lack of blinding and an inadequate assignment of tasks can increase the possibility of results being exaggerated by up to 13% and 7%, respectively [56]. Furthermore, one aspect that should be noted in the reviewed studies is a lack of detailed analysis isolating the specific effects of physical exercise and cognitive training. Although the combination of both interventions is the main hypothesis of this review, some studies may not have separately evaluated the effects of each component due to the inherent complexity of intervention designs. However, this should not be interpreted as a flaw in the design but rather as an inherent characteristic of multifaceted interventions, in which the interaction between multiple components is sought to be exploited to obtain global benefits. The fact that the effects of each intervention cannot be broken down independently does not invalidate the results obtained but rather reflects the integrative nature of combined therapies. Indeed, many clinical interventions specifically seek synergistic effects that cannot be observed by implementing isolated interventions. Furthermore, in the context of MCI, the combined approach may be more representative of real interventions, which typically include both physical exercise and cognitive training together. Despite the absence of a detailed analysis of the separate effects, the relevance of this work lies in its ability to provide empirical evidence on the efficacy of combined therapies in a realistic context. Rather than focusing solely on isolated components, this approach reflects a strategy closer to clinical and rehabilitation practices, where the aim is to improve the overall well-being of patients by combining various therapeutic modalities.
Several recent systematic reviews have evaluated the effects of physical exercise on cognitive function in older adults, both with and without cognitive deficits. In general, these reviews have found that physical exercise can have positive effects on different cognitive domains. However, some studies have focused exclusively on people with MCI. In this regard, more recent research has documented that physical exercise improves global cognitive function in older adults with MCI, specifically in areas such as working memory and executive cognition [57,58,59,60,61]. Although the results have been promising, some studies have included samples that were not diagnosed with MCI, which could influence the generalizability of the observed effects [58,59,60,61,62].
Despite advances, there are still aspects to be evaluated. In particular, several studies have not considered important moderating factors, such as duration, modality, and intensity of physical exercise, limiting our understanding of the specific mechanisms underlying the observed cognitive benefits [57,61]. Indeed, previous meta-analyses have shown that physical exercise has a more robust effect than pharmacological treatments in improving cognition in patients with MCI, with longer interventions (at least six months) showing greater benefits; however, these studies did not analyze key parameters such as intensity and type of exercise in detail, which is crucial to understanding how physical exercise influences cognitive function [61,63]. That said, some reviews have indicated that, although physical exercise improves global cognitive function in older adults with MCI, it does not necessarily significantly affect other cognitive domains [60,64]. Recently, the work of Law et al. [59] demonstrated that the positive effects of physical exercise on cognition could be especially related to working memory, suggesting that this domain is the most sensitive to physical interventions in older people with MCI. Low-impact physical interventions, such as walking or tai chi, have also shown positive effects on the cognition of older adults, especially in terms of reducing the risk of cognitive decline and improving overall quality of life [65,66]. These approaches, although valuable, tend to offer more limited benefits than more intensive or combined exercise and cognitive stimulation programs [67].
However, cognitive training has also shown positive effects on cognitive function in individuals with MCI. Previous reviews such as that of Wei et al. [68] have shown that cognitive training significantly benefits general cognitive function in individuals with cognitive impairment, as well as other cognitive variables, such as attention, orientation, delayed memory, and language skills. Similarly, Lampit et al. [69], in their systematic review, documented that cognitive behavioral therapy has moderate effectiveness in improving cognitive abilities, albeit in healthy older adults.
Furthermore, cognitive training through games, mental tasks, or even reminiscence therapy has been shown to offer improvements in memory, processing speed, and attention span in older adults with mild cognitive impairment [70,71]. These cognitive stimulation programs are often accessible and relatively easy to implement, making them valuable in both clinical and community settings. However, while these cognitive stimulation programs can be effective, the reviewed studies suggest that the combination of physical exercise and cognitive training is even more effective, as it amplifies the benefits of each intervention separately [54,72]. This combination significantly impacts the cognitive areas most affected in older adults with MCI, such as memory, attention, and executive functions. Physical exercise can help maintain neuroplasticity, providing a solid foundation on which cognitive training can work more effectively [73].
Combined interventions integrating moderate physical exercise and cognitive training have emerged as some of the most promising strategies to address MCI. The results of this systematic review and meta-analysis confirm that combined physical exercise and cognitive training interventions offer significant benefits for improving global cognition, supporting previous findings from other systematic reviews such as that by Reiker et al. [19], in which they observed improvements in cognitive functioning after combined cognitive and physical interventions; however, unlike our study, these interventions were carried out in healthy older adults. Our findings also support those of Lauenroth et al. [74], who analyzed studies with samples ranging from healthy older adults to people with Alzheimer’s and concluded that, although it seems that combined training positively influences cognition, due to the heterogeneity of studies with respect to a series of vital methodological parameters, such results should be interpreted with caution.
In our analysis, 10 of the 21 articles that assessed global cognition using scales such as MoCA, MMSE, or ADAS-Cog reported significant improvements (p < 0.05), which is consistent with the evidence that these multifaceted strategies improve key cognitive functions such as memory and executive functions. Furthermore, although not included in the meta-analysis because they sampled healthy older adults, studies that assessed specific aspects of cognition also favor combined interventions. This reinforces the findings of Castaño et al. [75], who showed that resistance training combined with cognitive training increases brain-derived neurotrophic factor and improves cognitive function.
Furthermore, although not all studies in this systematic review managed to achieve statistical significance in global cognition, the positive results observed in secondary variables such as physical function, quality of life, and depression add value to the comprehensive approach of these interventions. Most studies on physical function, for example, show significant improvements with scales such as the 6MWT, TUG, and SPPB. This supports the claim of Aminirakan et al. [76], who argued that these interventions not only improve cognitive aspects but also optimize functional capacities, which is essential for the daily life of patients with MCI.
Regarding depression and quality of life, the results are consistent with the review by Karssemeijer et al. [77], which noted that combined programs reduce symptoms of depression and promote improvements in psychological well-being. In the present review, three of the four studies assessing depression found significant differences, and four of five studies on quality of life also demonstrated notable benefits. These improvements could be due, in part, to the positive impact of physical exercise on the hippocampus–amygdala axis and emotional responses, as well as to cognitive stimulation, which promotes greater control and sense of purpose in patients. This is particularly relevant for healthcare professionals working with older adults at risk of dementia, as it suggests that a holistic approach that addresses both the body and mind offers amplified benefits.
Despite the positive results, this meta-analysis has several limitations that need to be considered. First, the heterogeneity of the included studies, both in terms of the types of exercise and cognitive tasks used, makes it difficult to generalize the results. Some studies used brief or low-intensity interventions, which could have limited the magnitude of the observed effects. Furthermore, the methodological quality of the included studies varied considerably, introducing a possible bias in the final results. Future studies should use more homogeneous protocols and better control for confounding variables, such as the initial level of cognition and the presence of comorbidities that could affect the results.
5. Conclusions
This review and meta-analysis provides strong evidence that combined physical and cognitive exercise therapy is an effective strategy for improving cognition in older adults with MCI. However, further research with high-quality studies using standardized protocols is needed to better understand the underlying mechanisms and optimize these interventions. It is also crucial to consider individual variations in participant characteristics, such as prior physical activity levels and comorbidities, to design more personalized interventions. The results of this meta-analysis support the idea that combined interventions have significant therapeutic potential, which can be a useful tool in preventing and managing cognitive decline in the aging population.
Author Contributions
Conceptualization, J.M.M.-P. and M.d.C.C.-F.; methodology, J.C.-S. and Y.C.-C.; writing—original draft preparation, A.A.-A. and A.G.-G.; writing—review and editing, F.H.-C. and M.A.M.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cahill, S. WHO’s global action plan on the public health response to dementia: Some challenges and opportunities. Aging Ment. Health 2020, 2, 197–199. [Google Scholar] [CrossRef]
- Roberts, R.; Knopman, D.S. Classification and epidemiology of MCI. Clin. Geriatr. Med. 2013, 29, 753–772. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment--beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef]
- Aurtenetxe, S.; García-Pacios, J.; Del Río, D.; López, M.E.; Pineda-Pardo, J.A.; Marcos, A.; Delgado Losada, M.L.; López-Frutos, J.M.; Maestú, F. Interference Impacts Working Memory in Mild Cognitive Impairment. Front. Neurosci. 2016, 10, 443. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Writing Group of Chinese Guidelines for Diagnosis and Treatment of Dementia and Cognitive Impairment, Chinese Medical Doctor Association Neurologist Branch Cognitive Disorders Professional, Committee. Chinese guidelines for diagnosis and treatment of dementia and cognitive impairment in 2018 (five): Diagnosis and treatment of mild cognitive impairment. Natl. Med. J. China 2018, 17, 1294–1301. [Google Scholar]
- Ishikawa, K.M.; Davis, J.; Chen, J.J.; Lim, E. The prevalence of mild cognitive impairment by aspects of social isolation. PLoS ONE 2022, 17, e0269795. [Google Scholar] [CrossRef]
- Franco-García, J.M.; Denche-Zamorano, Á.; Carlos-Vivas, J.; Castillo-Paredes, A.; Mendoza-Holgado, C.; Pérez-Gómez, J. Subjective Cognitive Impairment and Physical Activity: Investigating Risk Factors and Correlations among Older Adults in Spain. J. Funct. Morphol. Kinesiol. 2024, 9, 150. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; Izquierdo, M.; Serra-Rexach, J.A.; Santos-Lozano, A.; Lucia, A. Physical Exercise in the Oldest Old. Compr. Physiol. 2019, 9, 1281–1304. [Google Scholar]
- Lautenschlager, N.T.; Cox, K.L.; Flicker, L.; Foster, J.K.; Van Bockxmeer, F.M.; Xiao, J.; Greenop, K.R.; Almeida, O.P. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA 2008, 300, 1027–1037. [Google Scholar] [CrossRef]
- Di Lorito, C.; Bosco, A.; Booth, V.; Goldberg, S.; Harwood, R.H.; Van der Wardt, V. Adherence to exercise interventions in older people with mild cognitive impairment and dementia: A systematic review and meta-analysis. Prev. Med. Rep. 2020, 19, 101139. [Google Scholar] [CrossRef] [PubMed]
- Gates, N.J.; Rutjes, A.W.; Di Nisio, M.; Karim, S.; Chong, L.Y.; March, E.; Martínez, G.; Vernooij, R.W. Computerised cognitive training for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database Syst. Rev. 2019, 3, CD012277. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.; Yoon, S.; Lee, J.; Kurtz, M.M.; Choi, K. A meta-analysis of the effects of social-cognitive training in schizophrenia: The role of treatment characteristics and study quality. Br. J. Clin. Psychol. 2021, 61, 1. [Google Scholar] [CrossRef] [PubMed]
- Bherer, L.; Gagnon, C.; Langeard, A.; Lussier, M.; Desjardins-Crépeau, L.; Berryman, N.; Bosquet, L.; Vu, T.T.M.; Fraser, S.; Li, K.Z.H.; et al. Synergistic Effects of Cognitive Training and Physical Exercise on Dual-Task Performance in Older Adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, 1533–1541. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Rieker, J.A.; Reales, J.M.; Muiños, M.; Ballesteros, S. The Effects of Combined Cognitive-Physical Interventions on Cognitive Functioning in Healthy Older Adults: A Systematic Review and Multilevel Meta-Analysis. Front. Hum. Neurosci. 2022, 16, 838968. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Sanjuán, M.; Navarro, E.; Calero, M.D. Effectiveness of Cognitive Interventions in Older Adults: A Review. Eur. J. Investig. Health Psychol. Educ. 2020, 10, 876–898. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, P.J. Improving health literacy using the power of digital communications to achieve better health outcomes for patients and practitioners. Front. Digit. Health 2023, 5, 1264780. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Gildengers, A.G.; Butters, M.A. Physical activity and brain plasticity in late adulthood. Dialogues Clin. Neurosci. 2013, 15, 99–108. [Google Scholar] [CrossRef]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. The Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- Song, D.; Yu, D.S.F. Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: A randomised controlled trial. Int. J. Nurs. Stud. 2019, 9, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.Y.; Ku, Y.; Zanto, T.P.; Gazzaley, A. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: A systematic review and meta-analysis. Neurobiol. Aging 2015, 36, 2348–2359. [Google Scholar] [CrossRef]
- Jacova, C.; Kertesz, A.; Blair, M.; Fisk, J.D.; Feldman, H.H. Neuropsychological testing and assessment for dementia. Alzheimers Dement. 2007, 3, 299–317. [Google Scholar] [CrossRef]
- Zhu, X.; Yin, S.; Lang, M.; He, R.; Li, J. The more the better? A meta-analysis on effects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Res. Rev. 2016, 31, 67–79. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Dong, C.; Minkov, R.; Bahar-Fuchs, A.; Ellis, K.A.; Lautenschlager, N.T.; Mellow, M.L.; Wade, A.T.; Smith, A.E.; Finke, C.; et al. Combined physical and cognitive training for older adults with and without cognitive impairment: A systematic review and network meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 66, 101232. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023); Cochrane: London, UK, 2023; Available online: www.training.cochrane.org/handbook (accessed on 22 November 2023).
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy evidence database (pedro) scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- de Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Binns, E.; Kerse, N.; Peri, K.; Cheung, G.; Taylor, D. Combining cognitive stimulation therapy and fall prevention exercise (CogEx) in older adults with mild to moderate dementia: A feasibility randomised controlled trial. Pilot Feasibility Stud. 2020, 25, 6. [Google Scholar] [CrossRef]
- Train the Brain Consortium. Randomized trial on the effects of a combined physical/cognitive training in aged MCI subjects: The Train the Brain study. Sci. Rep. 2017, 7, 39471. [Google Scholar]
- Bray, N.W.; Pieruccini-Faria, F.; Witt, S.T.; Bartha, R.; Doherty, T.J.; Nagamatsu, L.S.; Almeida, Q.J.; Liu-Ambrose, T.; Middleton, L.E.; Bherer, L.; et al. Combining exercise with cognitive training and vitamin D3 to improve functional brain connectivity (FBC) in older adults with mild cognitive impairment (MCI). Results from the SYNERGIC trial. Geroscience 2023, 45, 1967–1985. [Google Scholar] [CrossRef]
- Castellote-Caballero, Y.; del Carmen Carcelén Fraile, M.; Aibar-Almazán, A.; Afanador-Restrepo, D.F.; González-Martín, A.M. Effect of combined physical–cognitive training on the functional and cognitive capacity of older people with mild cognitive impairment: A randomized controlled trial. BMC Med. 2024, 22, 281. [Google Scholar] [CrossRef] [PubMed]
- Damirchi, A.; Hosseini, F.; Babaei, P. Mental Training Enhances Cognitive Function and BDNF More Than Either Physical or Combined Training in Elderly Women With MCI: A Small-Scale Study. Am. J. Alzheimer’s Dis. Other Demen. 2018, 33, 20–29. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Hsuan Chen, I.; Lin, Y.J.; Chen, Y.; Hsu, W.C. Effects of virtual reality-based physical and cognitive training on executive function and dual-task gait performance in older adults with mild cognitive impairment: A randomized control trial. Front. Aging Neurosci. 2019, 11, 162. [Google Scholar] [CrossRef]
- Sakurai, T.; Sugimoto, T.; Akatsu, H.; Doi, T.; Fujiwara, Y.; Hirakawa, A.; Kinoshita, F.; Kuzuya, M.; Lee, S.; Matsumoto, N.; et al. Japan-Multimodal Intervention Trial for the Prevention of Dementia: A randomized controlled trial. Alzheimer’s Dement. 2024, 20, 3918–3930. [Google Scholar] [CrossRef]
- Wang, P.; Yang, T.; Peng, W.; Wang, M.; Chen, X.; Yang, Y.; Huang, Y.; Jiang, Y.; Wang, F.; Sun, S.; et al. Effects of a Multicomponent Intervention With Cognitive Training and Lifestyle Guidance for Older Adults at Risk of Dementia: A Randomized Controlled Trial. J. Clin. Psychiatry 2024, 85, 23. [Google Scholar] [CrossRef] [PubMed]
- Kwan, R.Y.C.; Liu, J.; Sin, O.S.K.; Fong, K.N.; Qin, J.; Wong, J.C.Y.; Lai, C. Effects of Virtual Reality Motor-Cognitive Training for Older People with Cognitive Frailty: Multicentered Randomized Controlled Trial. J. Med. Internet Res. 2024, 26, e57809. [Google Scholar] [CrossRef]
- Tainta, M.; Ecay-Torres, M.; de Arriba, M.; Barandiaran, M.; Otaegui-Arrazola, A.; Iriondo, A.; Garcia-Sebastian, M.; Estanga, A.; Saldias, J.; Clerigue, M.; et al. GOIZ ZAINDU study: A FINGER-like multidomain lifestyle intervention feasibility randomized trial to prevent dementia in Southern Europe. Alzheimers Res. Ther. 2024, 16, 44. [Google Scholar]
- Styliadis, C.; Kartsidis, P.; Paraskevopoulos, E.; Ioannides, A.A.; Bamidis, P.D. Neuroplastic effects of combined computerized physical and cognitive training in elderly individuals at risk for dementia: An eLORETA controlled study on resting states. Neural Plast. 2015, 2015, 172192. [Google Scholar] [PubMed]
- Yang, Q.H.; Lyu, X.; Lin, Q.R.; Wang, Z.W.; Tang, L.; Zhao, Y.; Lyu, Q.Y. Effects of a multicomponent intervention to slow mild cognitive impairment progression: A randomized controlled trial. Int. J. Nurs. Stud. 2022, 125, 104110. [Google Scholar] [PubMed]
- Hagovská, M.; Olekszyová, Z. Impact of the combination of cognitive and balance training on gait, fear and risk of falling and quality of life in seniors with mild cognitive impairment. Geriatr. Gerontol. Int. 2016, 16, 1043–1050. [Google Scholar] [CrossRef]
- Mavros, Y.; Gates, N.; Wilson, G.C.; Jain, N.; Meiklejohn, J.; Brodaty, H.; Wen, W.; Singh, N.; Baune, B.T.; Suo, C.; et al. Mediation of Cognitive Function Improvements by Strength Gains After Resistance Training in Older Adults with Mild Cognitive Impairment: Outcomes of the Study of Mental and Resistance Training. J. Am. Geriatr. Soc. 2017, 65, 550–559. [Google Scholar]
- Xu, Z.; Zhang, D.; Lee, A.T.; Sit, R.W.; Wong, C.; Lee, E.K.; Yip, B.H.; Tiu, J.Y.; Lam, L.C.; Wong, S.Y.; et al. A pilot feasibility randomized controlled trial on combining mind-body physical exercise, cognitive training, and nurse-led risk factor modification to reduce cognitive decline among older adults with mild cognitive impairment in primary care. PeerJ 2020, 8, e9845. [Google Scholar] [CrossRef]
- Li, L.; Liu, M.; Zeng, H.; Pan, L. Multi-component exercise training improves the physical and cognitive function of the elderly with mild cognitive impairment: A six-month randomized controlled trial. Ann. Palliat. Med. 2021, 10, 8919–8929. [Google Scholar] [CrossRef]
- Uysal, İ.; Başar, S.; Aysel, S.; Kalafat, D.; Büyüksünnetçi, A.Ö. Aerobic exercise and dual-task training combination is the best combination for improving cognitive status, mobility and physical performance in older adults with mild cognitive impairment. Aging Clin. Exp. Res. 2023, 35, 271–281. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Zou, G.; Speechley, M.; Almeida, Q.J.; Liu-Ambrose, T.; Middleton, L.E.; Camicioli, R.; Bray, N.W.; Li, K.Z.; Fraser, S.; et al. Effects of Exercise Alone or Combined with Cognitive Training and Vitamin D Supplementation to Improve Cognition in Adults with Mild Cognitive Impairment: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, E2324465. [Google Scholar] [CrossRef]
- Fairchild, J.K.; Myers, J.; Louras, P.; Jo, B.; McNerney, M.W.; Hallmayer, J.; Yesavage, J. Multimodal Exercise and Cognitive Training Program Improves Cognitive Function in Amnestic Mild Cognitive Impairment. Am. J. Geriatr. Psychiatry 2024, 32, 463–474. [Google Scholar] [CrossRef]
- Lipardo, D.S.; Tsang, W.W.N. Effects of combined physical and cognitive training on fall prevention and risk reduction in older persons with mild cognitive impairment: A randomized controlled study. Clin. Rehabil. 2020, 34, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.Y.; Tseng, H.Y.; Lin, Y.J.; Wang, C.J.; Hsu, W.C. Using virtual reality-based training to improve cognitive function, instrumental activities of daily living and neural efficiency in older adults with mild cognitive impairment. Eur. J. Phys. Rehabil. Med. 2020, 56, 47–57. [Google Scholar] [CrossRef]
- Savović, J.; Jones, H.E.; Altman, D.G.; Harris, R.J.; Jüni, P.; Pildal, J.; Als-Nielsen, B.; Balk, E.M.; Gluud, C.; Gluud, L.L.; et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann. Intern. Med. 2012, 157, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Falck, R.S.; David, J.C.; Best, J.R.; Crockett, R.A.; Liu-Ambrose, T. Impact of exercise training on physical and cognitive function among older adults: A systematic review and meta-analysis. Neurobiol. Aging 2019, 79, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Gates, N.; Singh, M.A.F.; Sachdev, P.S.; Valenzuela, M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2013, 21, 1086–1097. [Google Scholar] [CrossRef]
- Law, C.; Lam, F.M.H.; Chung, R.C.K.; Pang, M.Y.C. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: A systematic review. J. Physiother. 2020, 66, 9–18. [Google Scholar] [CrossRef]
- Sanders, L.M.J.; Hortobágyi, T.; la Bastide-van Gemert, S.; van der Zee, E.A.; van Heuvelen, M.J.G. Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0210036. [Google Scholar] [CrossRef]
- Zheng, G.; Xia, R.; Zhou, W.; Tao, J.; Chen, L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2016, 50, 1443–1450. [Google Scholar] [CrossRef]
- Öhman, H.; Savikko, N.; Strandberg, T.E.; Pitkälä, K.H. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: A systematic review. Dement. Geriatr. Cogn. Disord. 2014, 38, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Ströhle, A.; Schmidt, D.K.; Schultz, F.; Fricke, N.; Staden, T.; Hellweg, R.; Priller, J.; Rapp, M.A.; Rieckmann, N. Drug and exercise treatment of alzheimer disease and mild cognitive impairment: A systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2015, 23, 1234–1249. [Google Scholar] [CrossRef]
- Song, D.; Yu, D.S.F.; Li, P.W.C.; Lei, Y. The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2018, 79, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.B.L.D.; Santos, G.D.; Moreira, A.P.B.; Ishibashi, G.A.; Verga, C.E.R.; Moraes, L.C.D.; Lessa, P.P.; Cardoso, N.P.; Ordonez, T.N.; Brucki, S.M.D. Cognitive interventions in mature and older adults, benefits for psychological well-being and quality of life: A systematic review study. Dement. Neuropsychol. 2021, 15, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.C.; Chau, R.C.; Wong, B.M.; Fung, A.W.; Tam, C.W.; Leung, G.T.; Kwok, T.C.; Leung, T.Y.; Ng, S.P.; Chan, W.M. A 1-year randomized controlled trial comparing mind body exercise (Tai Chi) with stretching and toning exercise on cognitive function in older Chinese adults at risk of cognitive decline. J. Am. Med. Dir. Assoc. 2012, 13, 568.e15–568.e20. [Google Scholar] [CrossRef]
- Sungkarat, S.; Boripuntakul, S.; Chattipakorn, N.; Watcharasaksilp, K.; Lord, S.R. Effects of Tai Chi on Cognition and Fall Risk in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2017, 65, 721–727. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, X.; Liu, Y. A meta-analysis of the consequences of cognitive training on the cognitive function of aged mild cognitive impairment patients. Psychogeriatrics 2024, 24, 1371–1388. [Google Scholar] [CrossRef]
- Lampit, A.; Hallock, H.; Valenzuela, M. Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PLoS Med. 2014, 11, e1001756. [Google Scholar] [CrossRef]
- Chen, X.; Maguire, B.; Brodaty, H.; O’Leary, F. Dietary Patterns and Cognitive Health in Older Adults: A Systematic Review. J. Alzheimer’s Dis. 2019, 67, 583–619. [Google Scholar] [CrossRef]
- Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Casas-Herrero, Á.; Izquierdo, M. Role of physical exercise on cognitive function in healthy older adults: A systematic review of randomized clinical trials. Ageing Res. Rev. 2017, 37, 117–134. [Google Scholar] [CrossRef]
- Guo, W.; Zang, M.; Klich, S.; Kawczyński, A.; Smoter, M.; Wang, B. Effect of Combined Physical and Cognitive Interventions on Executive Functions in OLDER Adults: A Meta-Analysis of Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6166. [Google Scholar] [CrossRef] [PubMed]
- Stillman, C.M.; Cohen, J.; Lehman, M.E.; Erickson, K.I. Mediators of Physical Activity on Neurocognitive Function: A Review at Multiple Levels of Analysis. Front. Hum. Neurosci. 2016, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Lauenroth, A.; Ioannidis, A.E.; Teichmann, B. Influence of combined physical and cognitive training on cognition: A systematic review. BMC Geriatr. 2016, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Castaño, L.A.A.; Castillo de Lima, V.; Barbieri, J.F.; de Lucena, E.G.P.; Gáspari, A.F.; Arai, H.; Teixeira, C.V.L.; Coelho-Júnior, H.J.; Uchida, M.C. Resistance Training Combined With Cognitive Training Increases Brain Derived Neurotrophic Factor and Improves Cognitive Function in Healthy Older Adults. Front. Psychol. 2022, 13, 870561. [Google Scholar] [CrossRef]
- Aminirakan, D.; Losekamm, B.; Wollesen, B. Effects of combined cognitive and resistance training on physical and cognitive performance and psychosocial well-being of older adults ≥65: Study protocol for a randomised controlled trial. BMJ Open 2024, 14, e082192. [Google Scholar] [CrossRef]
- Karssemeijer, E.G.A.; Aaronson, J.A.; Bossers, W.J.; Smits, T.; Olde Rikkert, M.G.M.; Kessels, R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017, 40, 75–83. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).