Electromyography as an Objective Outcome Measure for the Therapeutic Effect of Biofeedback Training to Reduce Post-Paralytic Facial Synkinesis
Abstract
1. Introduction
2. Materials and Methods
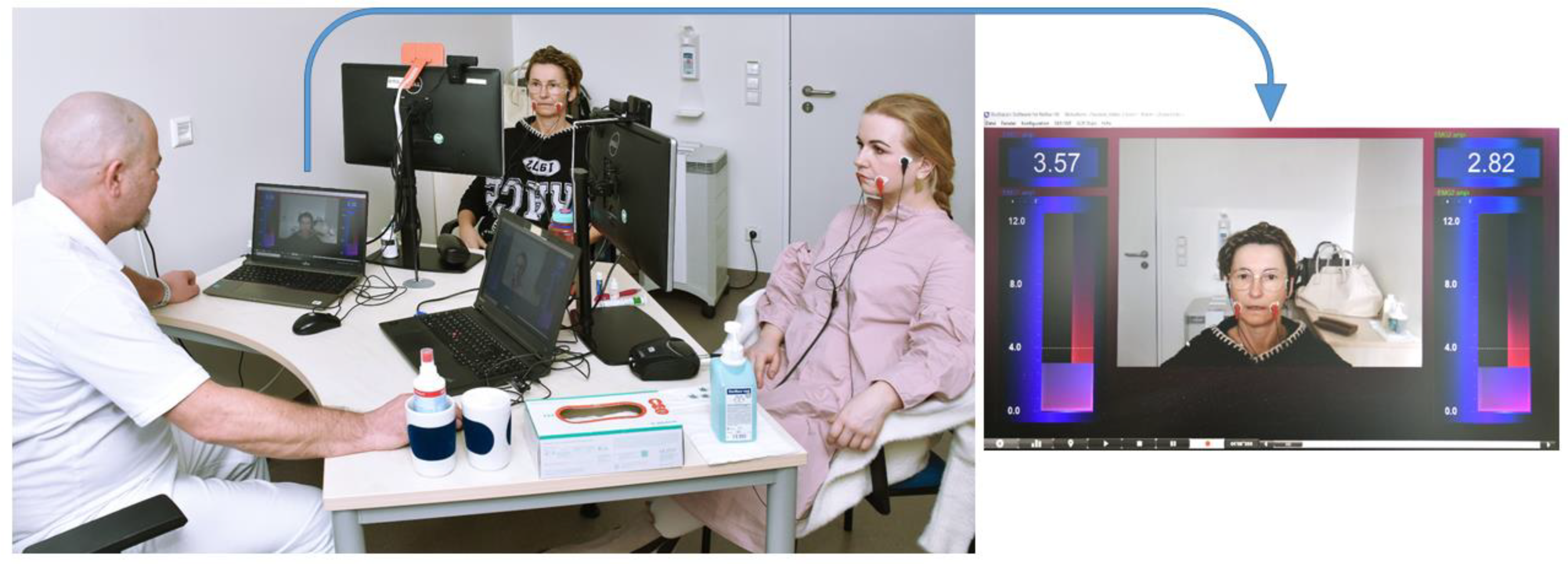
2.1. Study Design, Ethical Approval, Eligibility Criteria and Study Protocol
2.2. Measurement Times and Recorded Parameters
2.3. Facial Clinemetric Evaluation and Facial Disability Index
2.4. Sunnybrook Facial Grading System
2.5. Statistics
3. Results
3.1. Patients’ Characteristics
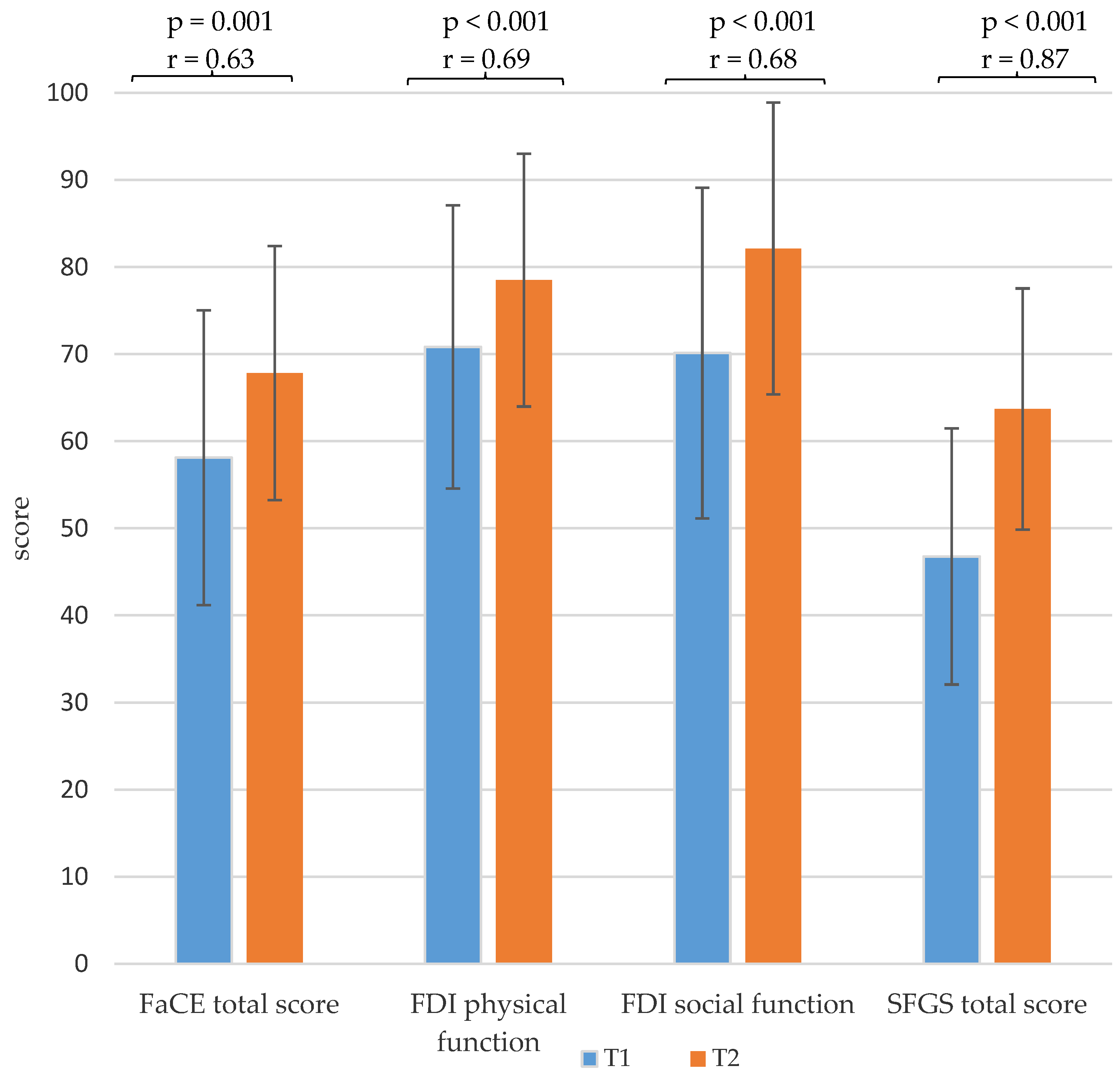
3.2. Facial Grading and Facial Palsy-Related Quality of Life
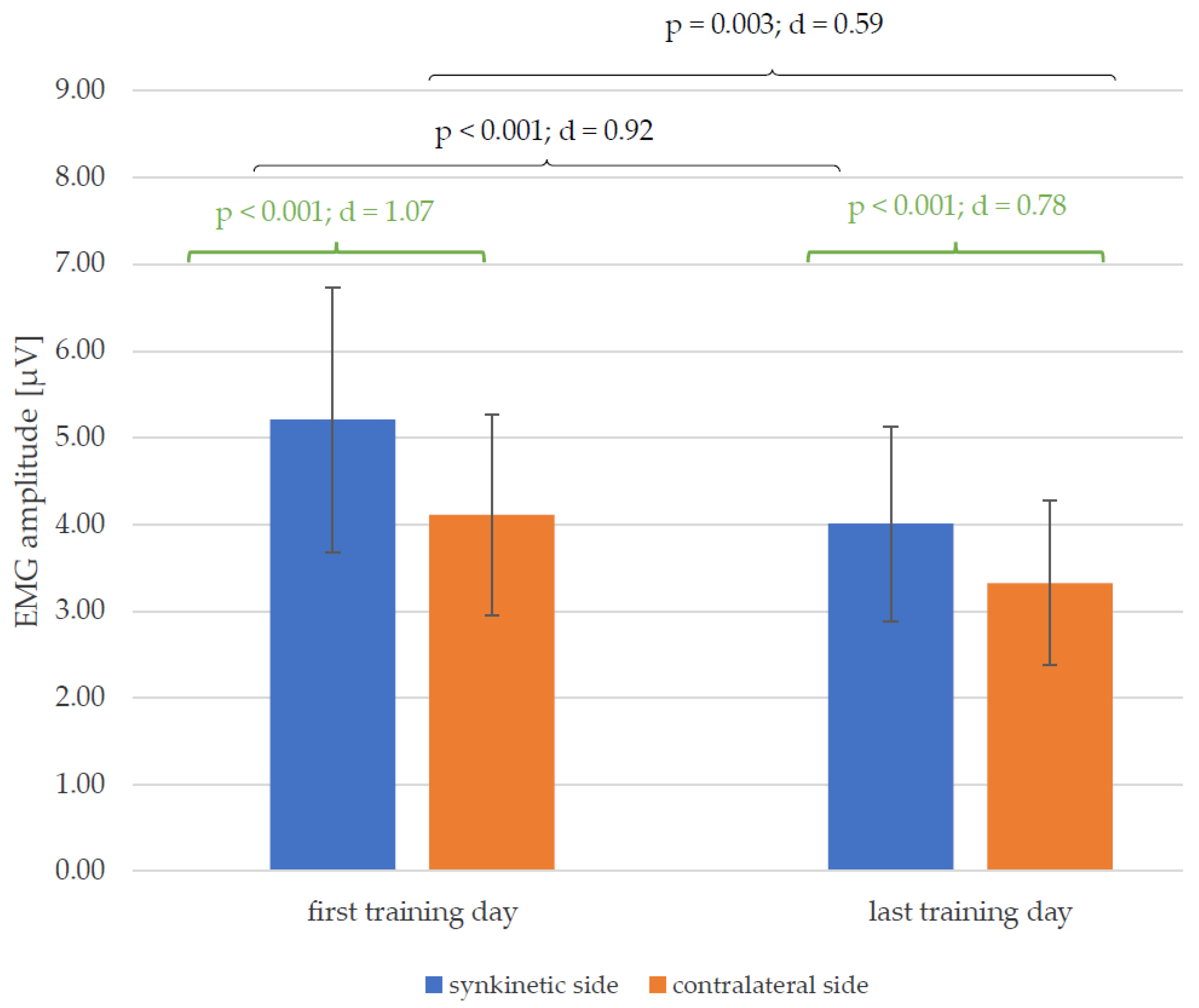
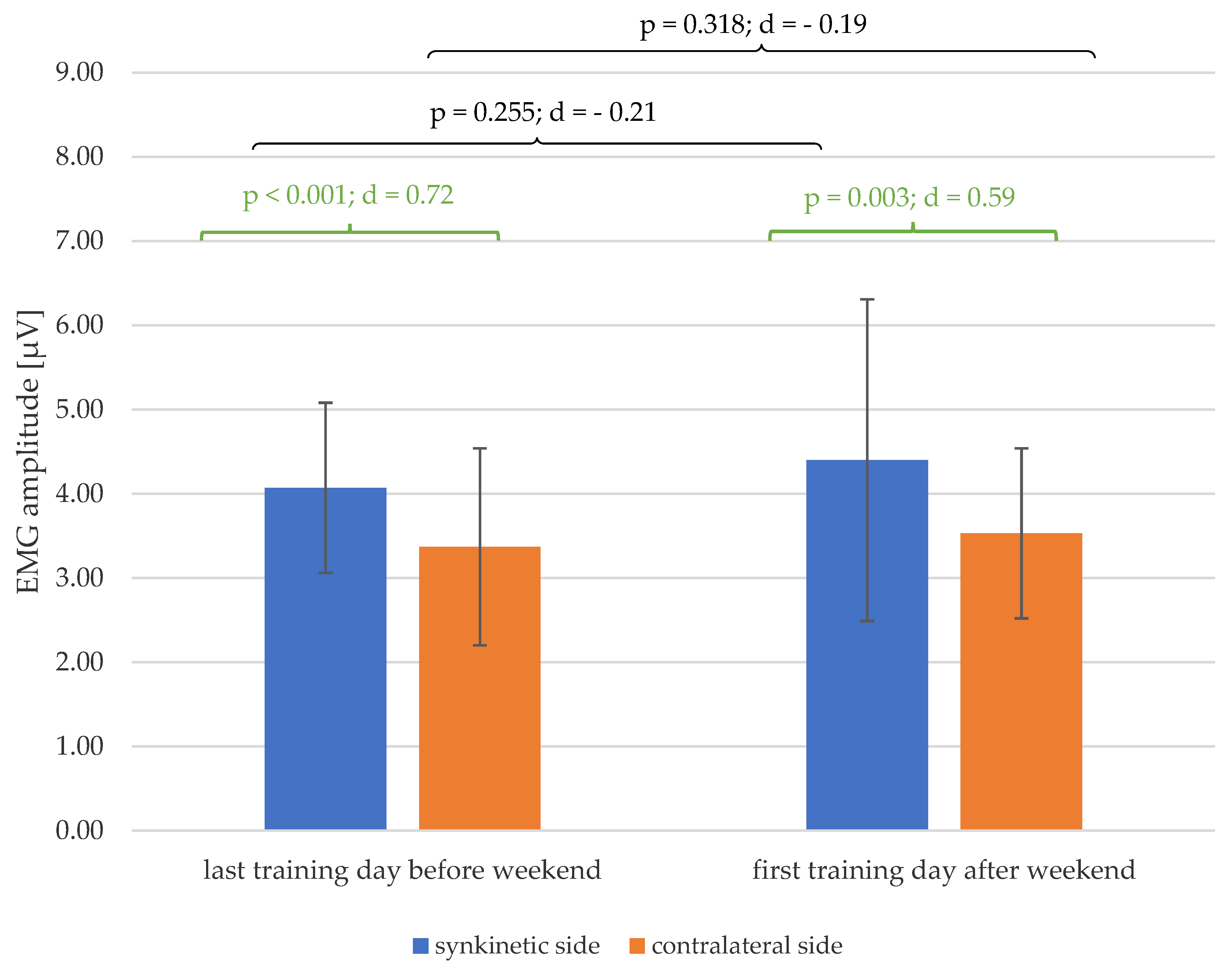
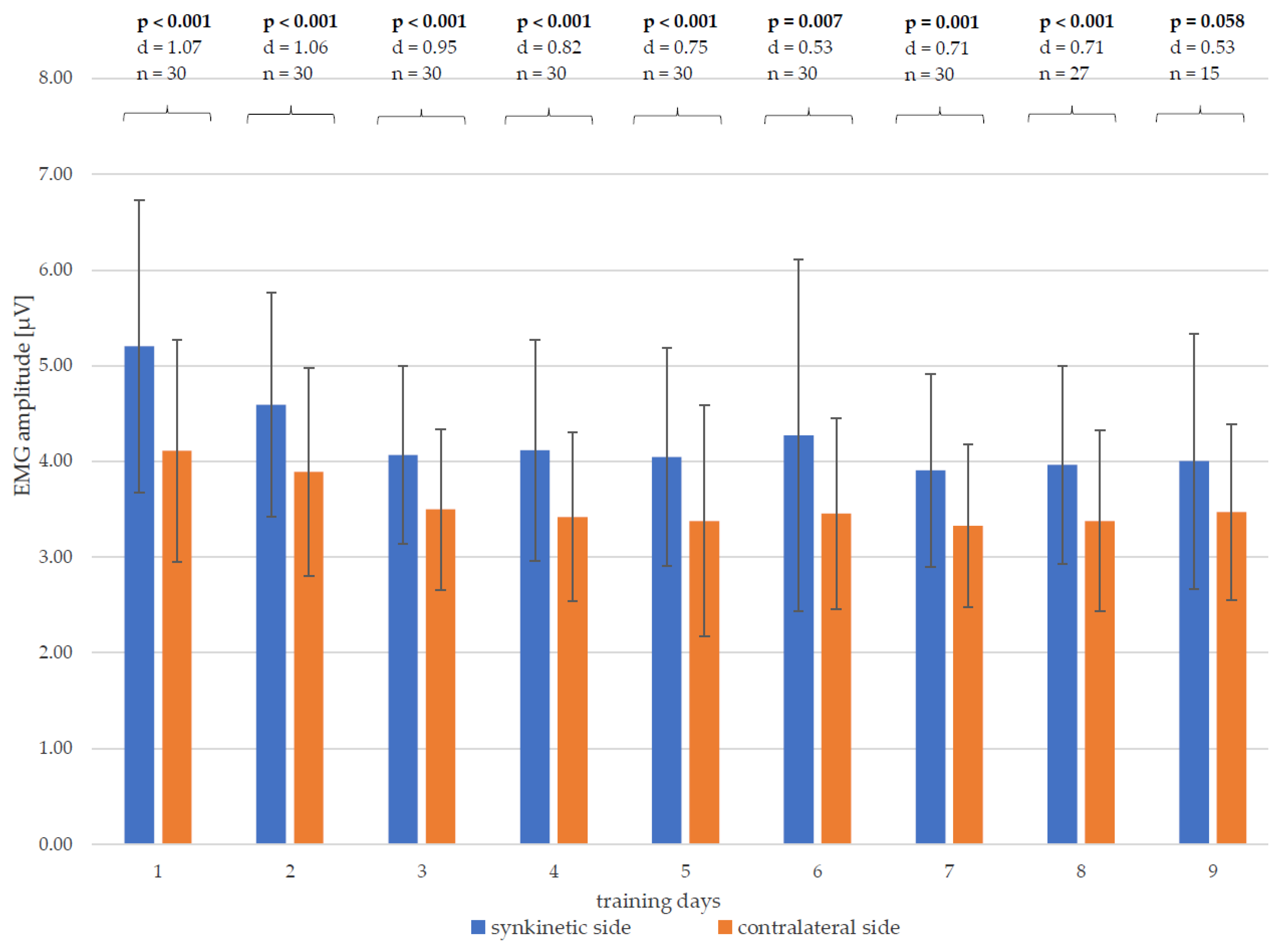
3.3. EMG Amplitudes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Item/Scores | T1 | T2 | T1-T2 | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | p-Value | Effect Size r | |
| FaCE facial movement score | 44.2 ± 19.8 | 51.1 ± 19.0 | 0.015 | 0.445 |
| FaCE facial comfort score | 49.4 ± 25.0 | 62.8 ± 20.3 | 0.005 | 0.512 |
| FaCE oral function score | 74.2 ± 22.5 | 79.58 ± 17.8 | 0.055 | 0.350 |
| FaCE eye comfort score | 52.5 ± 27.9 | 60.4 ± 28.8 | 0.034 | 0.388 |
| FaCE lacrimal control score | 65.8 ± 24.1 | 71.7 ± 28.4 | 0.071 | 0.330 |
| FaCE social function score | 67.9 ± 26.1 | 81.0 ± 19.7 | 0.001 | 0.591 |
| FaCE total score | 58.1 ± 16.9 | 67.8 ± 14.6 | 0.001 | 0.634 |
| FDI physical function score | 70.8 ± 16.2 | 78.5 ± 14.5 | <0.001 | 0.688 |
| FDI social function score | 70.1 ± 19.0 | 82.1 ± 16.8 | <0.001 | 0.682 |
| SFGS resting symmetry score | 10.7 ± 4.3 | 6.3 ± 3.9 | <0.001 | 0.775 |
| SFGS voluntary movement score | 64.8 ± 13.9 | 74.3 ± 11.7 | <0.001 | 0.846 |
| SFGS synkinesis score | 7.4 ± 2.4 | 4.2 ± 1.6 | <0.001 | 0.802 |
| SFGS total score | 46.8 ± 14.7 | 63.7 ± 13.8 | <0.001 | 0.874 |
References
- Volk, G.F.; Klingner, C.; Finkensieper, M.; Witte, O.W.; Guntinas-Lichius, O. Prognostication of recovery time after acute peripheral facial palsy: A prospective cohort study. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Guntinas-Lichius, O.; Prengel, J.; Cohen, O.; Mäkitie, A.A.; Vander Poorten, V.; Ronen, O.; Shaha, A.; Ferlito, A. Pathogenesis, diagnosis and therapy of facial synkinesis: A systematic review and clinical practice recommendations by the international head and neck scientific group. Front. Neurol. 2022, 13, 1019554. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Al-Hassani, F.; Kannan, R. Facial nerve disorder: A review of the literature. IJS Oncol. 2018, 3, e65. [Google Scholar] [CrossRef]
- Moran, C.J.; Neely, J.G. Patterns of facial nerve synkinesis. Laryngoscope 1996, 106, 1491–1496. [Google Scholar] [CrossRef]
- Azizzadeh, B.; Nduka, C. Management of Post-Facial Paralysis Synkinesis; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Mark, V.W.; Taub, E. Constraint-induced movement therapy for chronic stroke hemiparesis and other disabilities. Restor. Neurol. Neurosci. 2004, 22, 317–336. [Google Scholar] [CrossRef]
- Hamlet, C.; Williamson, H.; Hotton, M.; Rumsey, N. ‘Your face freezes and so does your life’: A qualitative exploration of adults’ psychosocial experiences of living with acquired facial palsy. Br. J. Health Psychol. 2021, 26, 977–994. [Google Scholar] [CrossRef]
- Husseman, J.; Mehta, R.P. Management of synkinesis. Facial Plast. Surg. 2008, 24, 242–249. [Google Scholar] [CrossRef]
- Volk, G.F.; Roediger, B.; Geißler, K.; Kuttenreich, A.M.; Klingner, C.M.; Dobel, C.; Guntinas-Lichius, O. Effect of an Intensified Combined Electromyography and Visual Feedback Training on Facial Grading in Patients With Post-paralytic Facial Synkinesis. Front. Rehabil. Sci. 2021, 2, 746188. [Google Scholar] [CrossRef]
- Kondo, K.; Noonan, K.M.; Freeman, M.; Ayers, C.; Morasco, B.J.; Kansagara, D. Efficacy of Biofeedback for Medical Conditions: An Evidence Map. J. Gen. Intern. Med. 2019, 34, 2883–2893. [Google Scholar] [CrossRef]
- Baricich, A.; Cabrio, C.; Paggio, R.; Cisari, C.; Aluffi, P. Peripheral Facial Nerve Palsy: How Effective Is. Rehabilitation? Otol. Neurotol. 2012, 33, 1118–1126. [Google Scholar] [CrossRef]
- Cardoso, J.R.; Teixeira, E.C.; Moreira, M.D.; Fávero, F.M.; Fontes, S.V.; Bulle de Oliveira, A.S. Effects of exercises on Bell’s palsy: Systematic review of randomized controlled trials. Otol. Neurotol. 2008, 29, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Neville, C.; Beurskens, C.; Diels, J.; MacDowell, S.; Rankin, S. Consensus Among International Facial Therapy Experts for the Management of Adults with Unilateral Facial Palsy: A Two-Stage Nominal Group and Delphi Study. Facial. Plast. Surg. Aesthet. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S. Veränderungen der Allgemeinen und Krankheitsspezifischen Lebensqualität nach Fazialis-Parese-Training mit EMG-Biofeedback am Universitätsklinikum Jena bei Patienten mit Defektheilung nach Fazialisparese. Ph.D. Dissertation, Friedrich-Schiller-Universität Jena, Jena, Germany, 2023. [Google Scholar]
- Hoche, E. Evaluation Eines Biofeedback-Trainings für Patienten mit Postparalytischen Synkinesien nach Peripherer Fazialisparese Mittels Multikanal-Oberflächen-EMG der Intrinsischen und Extrinsischen Aurikulären Muskulatur. Ph.D. Dissertation, Friedrich-Schiller-Universität Jena, Jena, Germany, 2023. [Google Scholar]
- Volk, G.F.; Steigerwald, F.; Vitek, P.; Finkensieper, M.; Kreysa, H.; Guntinas-Lichius, O. Facial Disability Index and Facial Clinimetric Evaluation Scale: Validation of the German versions. Laryngorhinootologie 2015, 94, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.B.; Gliklich, R.E.; Boyev, K.P.; Stewart, M.G.; Metson, R.B.; McKenna, M.J. Validation of a patient-graded instrument for facial nerve paralysis: The FaCE scale. Laryngoscope 2001, 111, 387–398. [Google Scholar] [CrossRef]
- VanSwearingen, J.M.; Brach, J.S. The Facial Disability Index: Reliability and validity of a disability assessment instrument for disorders of the facial neuromuscular system. Phys. Ther. 1996, 76, 1288–1298; discussion in 1298–1300. [Google Scholar] [CrossRef]
- Neumann, T.; Lorenz, A.; Volk, G.F.; Hamzei, F.; Schulz, S.; Guntinas-Lichius, O. Validation of the German Version of the Sunnybrook Facial Grading System. Laryngorhinootologie 2017, 96, 168–174. [Google Scholar] [CrossRef]
- Ross, B.G.; Fradet, G.; Nedzelski, J.M. Development of a sensitive clinical facial grading system. Otolaryngol. Head. Neck Surg. 1996, 114, 380–386. [Google Scholar] [CrossRef]
- Fattah, A.Y.; Gurusinghe, A.D.R.; Gavilan, J.; Hadlock, T.A.; Marcus, J.R.; Marres, H.; Nduka, C.C.; Slattery, W.H.; Snyder-Warwick, A.K. Facial nerve grading instruments: Systematic review of the literature and suggestion for uniformity. Plast. Reconstr. Surg. 2015, 135, 569–579. [Google Scholar] [CrossRef]
- Bortz, J.; Schuster, C. Statistik für Human-und Sozialwissenschaftler: Limitierte Sonderausgabe; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Weiß, C. Basiswissen Medizinische Statistik; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Fischer, T. Oberflächen-EMG-Untersuchungen zum Kontraktionsverhalten der Skelettmuskulatur unter lokaler Wärmeanwendung. Ph.D. Dissertation, Faculty of Medicine, LMU München, München, Germany, 2002. [Google Scholar]
- Nazarpour, K.; Al-Timemy, A.H.; Bugmann, G.; Jackson, A. A note on the probability distribution function of the surface electromyogram signal. Brain Res. Bull. 2013, 90, 88–91. [Google Scholar] [CrossRef]
- Sahib, M. Die Interindividuelle Variabilität der Oberflächen-Elektromyographischen Aktivitätsverteilung von M. Masseter und M. Temporalis Während des Kauvorgangs. Ph.D. Dissertation, Friedrich-Schiller-Universität Jena, Jena, Germany, 2018. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Fahrmeir, L.; Heumann, C.; Künstler, R.; Pigeot, I.; Tutz, G. Statistik: Der weg zur Datenanalyse; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Baba, S.; Kondo, K.; Yoshitomi, A.; Kanemaru, A.; Nakaya, M.; Yamasoba, T. Efficacy of Mirror Biofeedback Rehabilitation on Synkinesis in Acute Stage Facial Palsy in Children. Otol. Neurotol. 2021, 42, e936–e941. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Toda, N.; Sakamaki, K.; Kashima, K.; Takeda, N. Biofeedback rehabilitation for prevention of synkinesis after facial palsy. Otolaryngol. Head. Neck Surg. 2003, 128, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Dalla Toffola, E.; Tinelli, C.; Lozza, A.; Bejor, M.; Pavese, C.; Degli Agosti, I.; Petrucci, L. Choosing the best rehabilitation treatment for Bell’s palsy. Eur. J. Phys. Rehabil. Med. 2012, 48, 635–642. [Google Scholar]
- Ross, B.; Nedzelski, J.M.; McLean, J.A. Efficacy of feedback training in long-standing facial nerve paresis. Laryngoscope 1991, 101, 744–750. [Google Scholar] [CrossRef]
- Beurskens, C.H.G.; Heymans, P.G. Positive Effects of Mime Therapy on Sequelae of Facial Paralysis: Stiffness, Lip Mobility, and Social and Physical Aspects of Facial Disability. Otol. Neurotol. 2003, 24, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Franz, L.; de Filippis, C.; Daloiso, A.; Biancoli, E.; Iannacone, F.P.; Cazzador, D.; Tealdo, G.; Marioni, G.; Nicolai, P.; Zanoletti, E. Facial surface electromyography: A systematic review on the state of the art and current perspectives. Am. J. Otolaryngol. 2024, 45, 104041. [Google Scholar] [CrossRef]

| List of Exercises |
|---|
| Long eyelid closure |
| Closed smile |
| Open smile |
| Pursed lips/kissing mouth |
| Closed smile with transition to kissing mouth |
| Pursed lips with transition to showing teeth |
| Open smile with transition to “O”/”U” |
| Letter exercise O/U |
| Letter exercise E/I |
| Letter exercise A |
| Alternating one-sided smile, then both sides |
| Show teeth |
| Wrinkle nose |
| Raise/lower eyebrows |
| Puff out cheeks |
| Parameter | Absolute (N) | Relative (%) |
|---|---|---|
| All | 30 | 100.0 |
| Gender | ||
| Male | 7 | 23.3 |
| Female | 23 | 76.7 |
| Handedness | ||
| Right | 30 | 100.0 |
| Left | 0 | 0 |
| Etiology | ||
| idiopathic | 12 | 40.0 |
| postoperative with benign tumor | 9 | 30.0 |
| Ramsay Hunt syndrome | 6 | 20.0 |
| traumatic | 3 | 10.0 |
| congenital | 0 | 0 |
| postoperative with malignant tumor | 0 | 0 |
| cerebral insult | 0 | 0 |
| Localization of the paresis | ||
| right | 15 | 50.0 |
| left | 15 | 50.0 |
| Presence of oro-ocular synkinesis | ||
| yes | 30 | 100.0 |
| no | 0 | 0 |
| Therapist week 1 | ||
| Therapist A | 10 | 33.3 |
| Therapist B | 20 | 66.7 |
| Therapist week 2 | ||
| Therapist A | 19 | 63.3 |
| Therapist B | 11 | 36.7 |
| Number of training days completed between the first (T1) and second (T2) measurement point | ||
| 9 training days | 15 | 50.0 |
| 8 training days | 12 | 40.0 |
| 7 training days | 3 | 10.0 |
| Mean ± SD | Median, range | |
| Age at beginning of facial palsy in years | 45.88 ± 13.71 | 47.33; 18.45–70.31 |
| Age at start of training in years | 48.62 ± 12.41 | 48.84; 24.05–71.43 |
| Height in m | 1.71 ± 0.09 | 1.70; 1.56–1.89 |
| Weight in kg | 78.90 ± 17.84 | 72.5; 48.0–150.0 |
| BMI in kg/m2 | 26.08 ± 6.43 | 24.95; 16.61–43.36 |
| Period between beginning of facial palsy and reinnervation in years (n = 28 *) | 0.33 ± 0.25 | 0.27; 0.04–1.03 |
| Time between beginning of facial palsy and first contact with Facial-Nerve-Center in years | 2.11 ± 2.30 | 1.21; 0.44–9.79 |
| Time between beginning of facial palsy and first measurement (T1) in years | 2.80 ± 2.41 | 1.84; 1.12–10.99 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.017 (0.2–1.7) | 0.001 (0.6–1.9) | 0.018 (0.2–2.1) | 0.002 (0.6–2.2) | 0.011 (0.3–1.9) | 0.003 (0.6–2.4) | 0.001 (0.7–2.2) | 0.001 (0.7–2.1) | |
| 2 | 0.017 (0.2–1.7) | 0.202 (−0.8–0.2) | 0.549 (−1.0–0.6) | 0.118 (−1.0–0.1) | 0.570 (−0.8–0.5) | 0.121 (−1.3–0.2) | 0.066 (−1.1–0.0) | 0.097 (−1.0–0.1) | |
| 3 | 0.001 (0.6–1.9) | 0.202 (−0.2–0.8) | 0.764 (−0.5–0.7) | 0.483 (−0.5–0.2) | 0.536 (−0.3–0.6) | 0.382 (−0.8–0.3) | 0.172 (−0.5–0.1) | 0.564 (−0.7–0.4) | |
| 4 | 0.018 (0.2–2.1) | 0.549 (−0.6–1.0) | 0.764 (−0.7–0.5) | 0.374 (−0.7–0.3) | 0.879 (−0.7–0.8) | 0.327 (−1.0–0.3) | 0.305 (−0.9–0.3) | 0.516 (−1.0–0.5) | |
| 5 | 0.002 (0.6–2.2) | 0.118 (−0.1–1.0) | 0.483 (−0.2–0.5) | 0.374 (−0.3–0.7) | 0.340 (−0.3–0.8) | 0.688 (−0.7–0.5) | 0.601 (−0.4–0.3) | 0.938 (−0.6–0.6) | |
| — | 0.011 (0.3–1.9) | 0.570 (−0.5–0.8) | 0.536 (−0.6–0.3) | 0.879 (−0.8–0.7) | 0.340 (−0.8–0.3) | 0.052 (−0.7–0.0) | 0.117 (−0.8–0.1) | 0.232 (−0.8–0.2) | |
| 7 | 0.003 (0.6–2.4) | 0.121 (−0.2–1.3) | 0.382 (−0.3–0.8) | 0.327 (−0.3–1.0) | 0.688 (−0.5–0.7) | 0.052 (0.0–0.7) | 0.900 (−0.4–0.5) | 0.669 (−0.3–0.5) | |
| 8 | 0.001 (0.7–2.2) | 0.066 (0.0–1.1) | 0.172 (−0.1–0.5) | 0.305 (−0.3–0.9) | 0.601 (−0.3–0.4) | 0.117 (−0.1–0.8) | 0.900 (−0.5–0.4) | 0.795 (−0.4–0.6) | |
| 9 | 0.001 (0.7–2.1) | 0.097 (−0.1–1.0) | 0.564 (−0.4–0.7) | 0.516 (−0.5–1.0) | 0.938 (−0.6–0.6) | 0.232 (−0.2–0.8) | 0.669 (−0.5–0.3) | 0.795 (−0.6–0.4) |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.075 (−0.1–1.0) | 0.005 (0.2–1.0) | 0.050 (0.0–1.2) | 0.006 (0.3–1.6) | 0.128 (−0.2–1.2) | 0.018 (0.2–1.4) | 0.009 (0.2–1.3) | 0.006 (0.3–1.4) | |
| 2 | 0.075 (−0.1–1.0) | 0.608 (−0.6–0.4) | 0.652 (−0.8–0.5) | 0.031 (−0.9–0.0) | 0.916 (−0.6–0.5) | 0.198 (−0.9–0.2) | 0.084 (−0.7–0.0) | 0.059 (−0.8–0.0) | |
| 3 | 0.005 (0.2–1.0) | 0.608 (−0.4–0.6) | 0.935 (−0.4–0.4) | 0.147 (−0.8–0.1) | 0.714 (−0.4–0.6) | 0.409 (−0.7–0.3) | 0.377 (−0.6–0.2) | 0.158 (−0.7–0.1) | |
| 4 | 0.050 (0.0–1.2) | 0.652 (−0.5–0.8) | 0.935 (−0.4–0.4) | 0.076 (−0.7–0.0) | 0.694 (−0.5–0.7) | 0.460 (−0.7–0.3) | 0.507 (−0.7–0.4) | 0.210 (−0.7–0.2) | |
| 5 | 0.006 (0.3–1.6) | 0.031 (0.0–0.9) | 0.147 (−0.1–0.8) | 0.076 (0.0–0.7) | 0.039 (0.0–0.8) | 0.467 (−0.2–0.5) | 0.262 (−0.1–0.5) | 0.584 (−0.2–0.3) | |
| 6 | 0.128 (−0.2–1.2) | 0.916 (−0.5–0.6) | 0.714 (−0.6–0.4) | 0.694 (−0.7–0.5) | 0.039 (−0.8–0.0) | 0.052 (−0.6–0.0) | 0.153 (−0.7–0.1) | 0.033 (−0.7–0.0) | |
| 7 | 0.018 (0.2–1.4) | 0.198 (−0.2–0.9) | 0.409 (−0.3–0.7) | 0.460 (−0.3–0.7) | 0.467 (−0.5–0.2) | 0.052 (0.0–0.6) | 0.851 (−0.3–0.3) | 0.672 (−0.4–0.3) | |
| 8 | 0.009 (0.2–1.3) | 0.084 (0.0–0.7) | 0.377 (−0.2–0.6) | 0.507 (−0.4–0.7) | 0.262 (−0.5–0.1) | 0.153 (−0.1–0.7) | 0.851 (−0.3–0.3) | 0.441 (−0.4–0.2) | |
| 9 | 0.006 (0.3–1.4) | 0.059 (0.0–0.8) | 0.158 (−0.1–0.7) | 0.210 (−0.2–0.7) | 0.584 (−0.3–0.2) | 0.033 (0.0–0.7) | 0.672 (−0.3–0.4) | 0.441 (−0.2–0.4) |
| p-Value | Partial η² | |
|---|---|---|
| Interaction between the change in EMG amplitude of the synkinetic side and therapist A, therapist B and change of therapists | 0.891 | 0.365 |
| Interaction between the change in EMG amplitude of the contralateral side and therapist A, therapist B and change of therapists | 0.450 | 0.603 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahnemann, I.; Fron, J.; Ballmaier, J.; Guntinas-Lichius, O.; Volk, G.F. Electromyography as an Objective Outcome Measure for the Therapeutic Effect of Biofeedback Training to Reduce Post-Paralytic Facial Synkinesis. Healthcare 2025, 13, 550. https://doi.org/10.3390/healthcare13050550
Hahnemann I, Fron J, Ballmaier J, Guntinas-Lichius O, Volk GF. Electromyography as an Objective Outcome Measure for the Therapeutic Effect of Biofeedback Training to Reduce Post-Paralytic Facial Synkinesis. Healthcare. 2025; 13(5):550. https://doi.org/10.3390/healthcare13050550
Chicago/Turabian StyleHahnemann, Isabell, Julia Fron, Jonas Ballmaier, Orlando Guntinas-Lichius, and Gerd Fabian Volk. 2025. "Electromyography as an Objective Outcome Measure for the Therapeutic Effect of Biofeedback Training to Reduce Post-Paralytic Facial Synkinesis" Healthcare 13, no. 5: 550. https://doi.org/10.3390/healthcare13050550
APA StyleHahnemann, I., Fron, J., Ballmaier, J., Guntinas-Lichius, O., & Volk, G. F. (2025). Electromyography as an Objective Outcome Measure for the Therapeutic Effect of Biofeedback Training to Reduce Post-Paralytic Facial Synkinesis. Healthcare, 13(5), 550. https://doi.org/10.3390/healthcare13050550









