Medically Tailored Grocery Deliveries to Improve Food Security and Hypertension in Underserved Groups: A Student-Run Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Randomization
2.4. Interventions
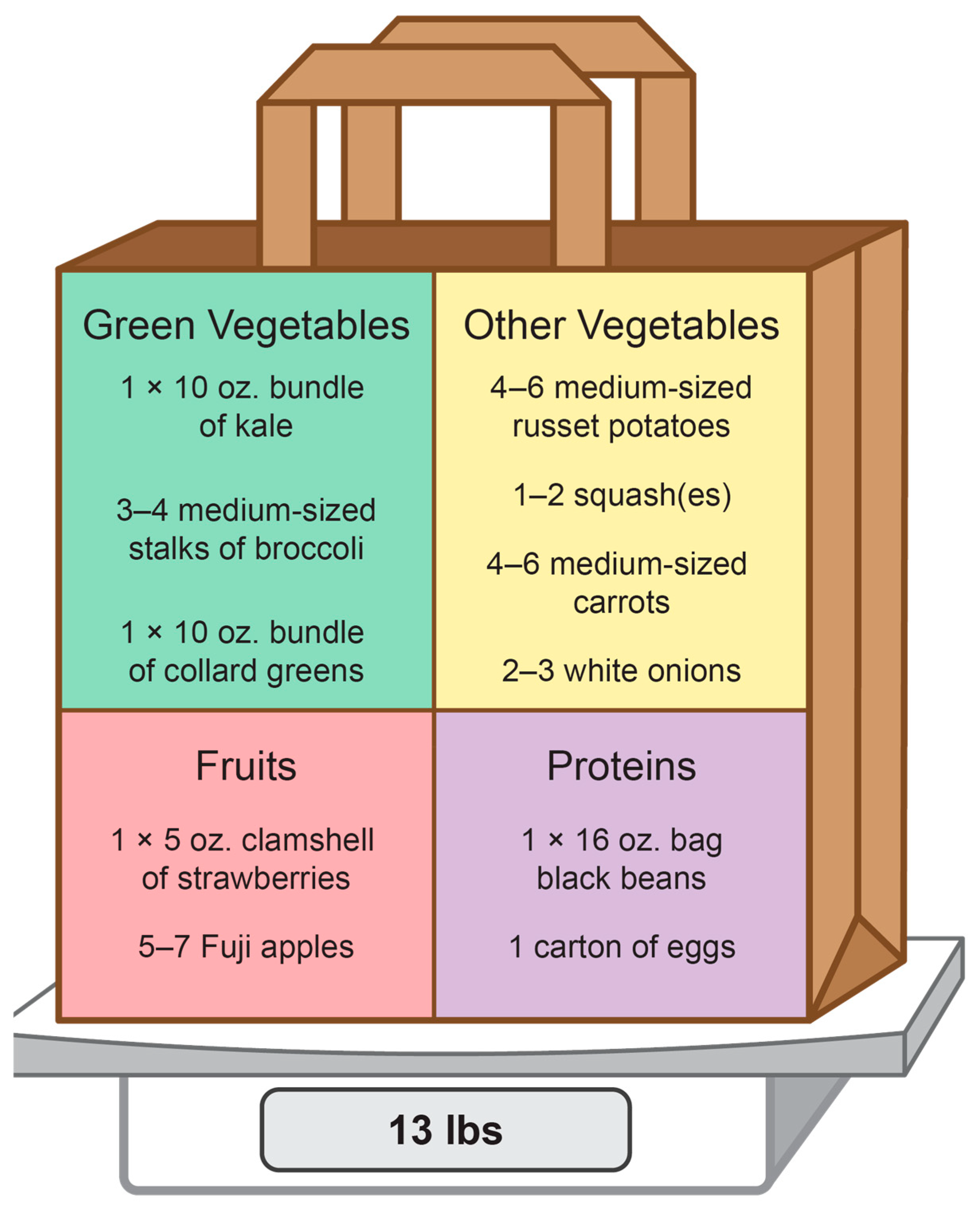
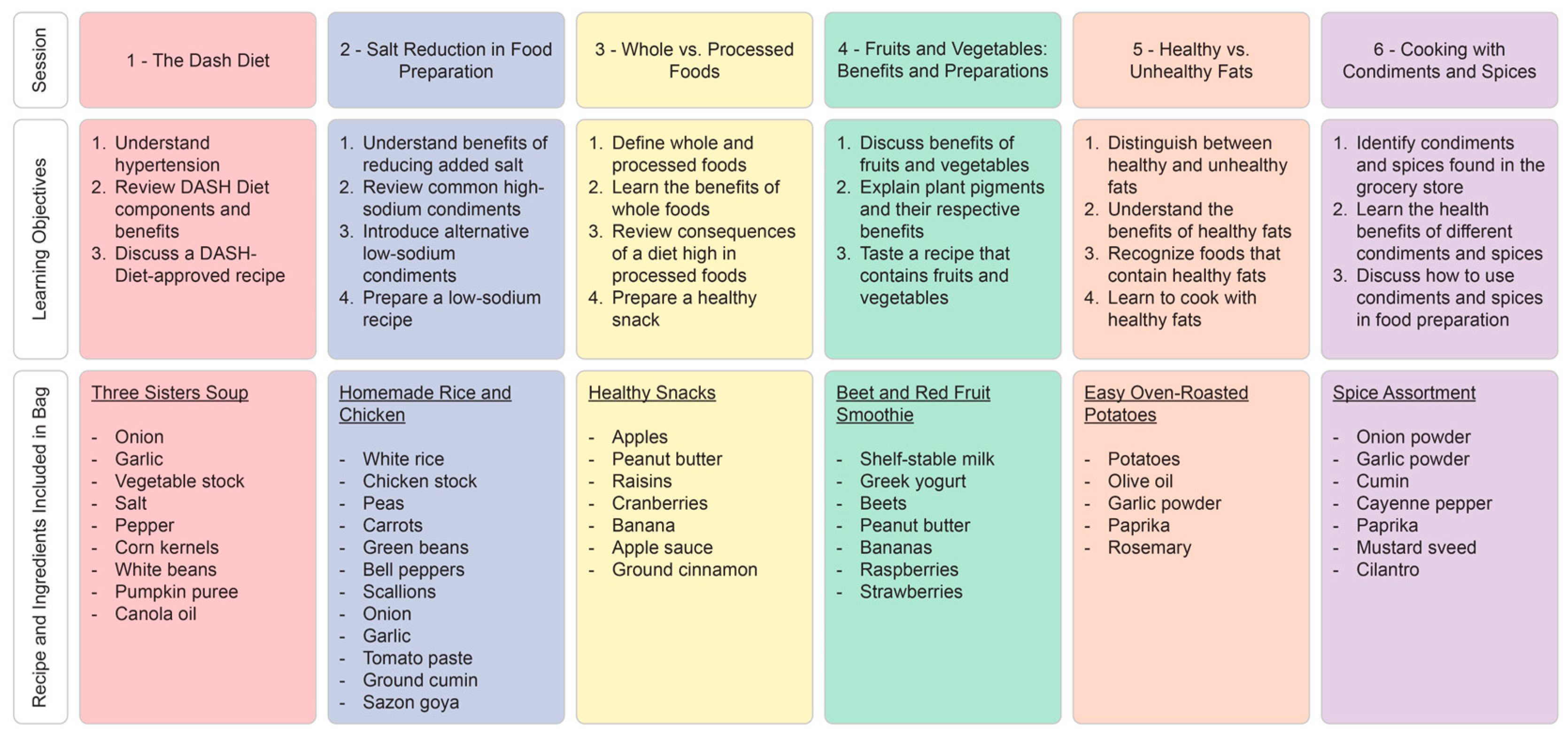
2.4.1. Intervention Group
2.4.2. Control Group
2.4.3. Home Visits
2.4.4. Post-Intervention Period
2.5. Outcomes and Analytical Methods
2.5.1. Statistical Methods
2.5.2. Qualitative Analytical Methods
3. Results
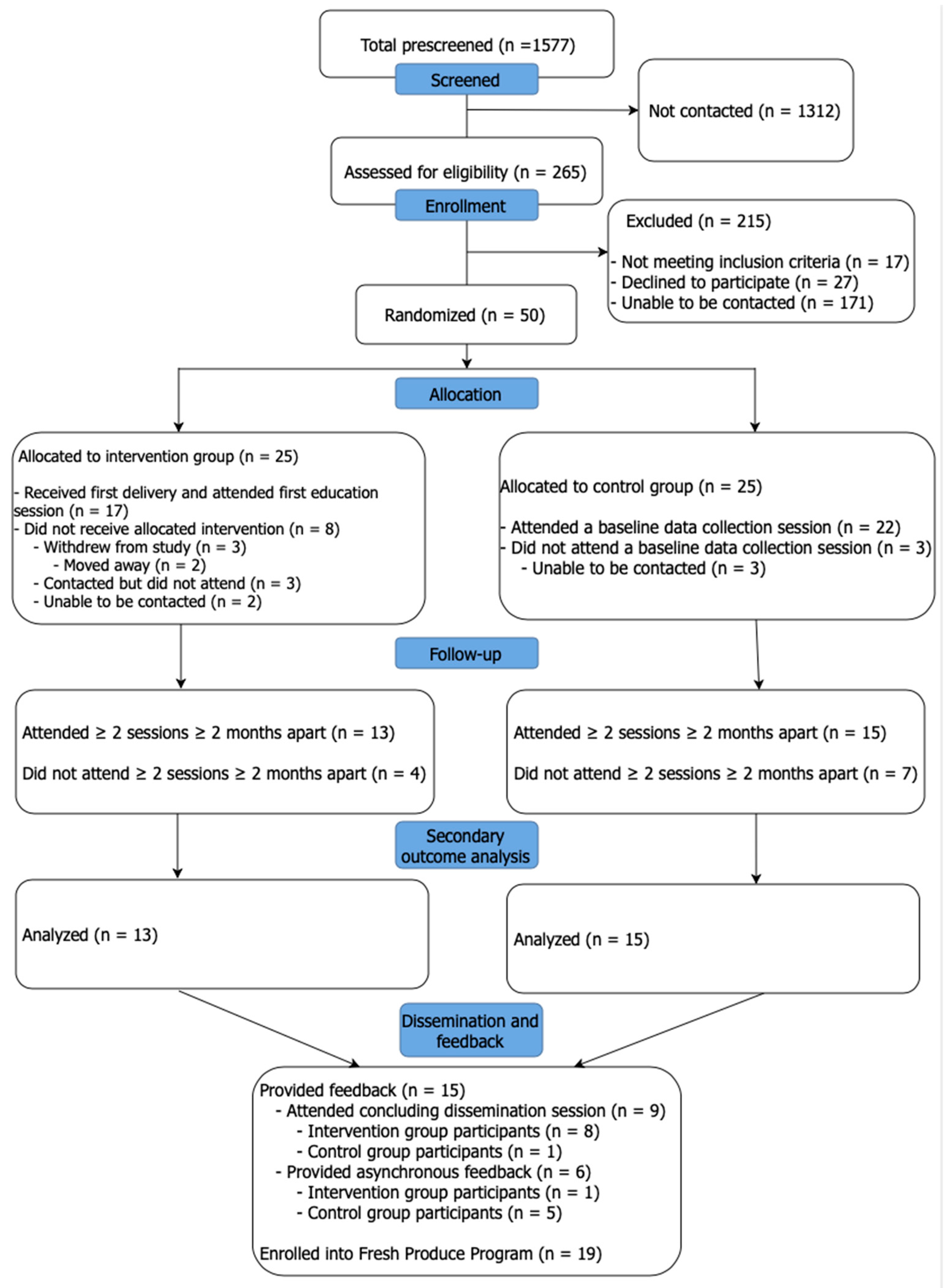
3.1. Participant Recruitment
3.2. Participant Demographics
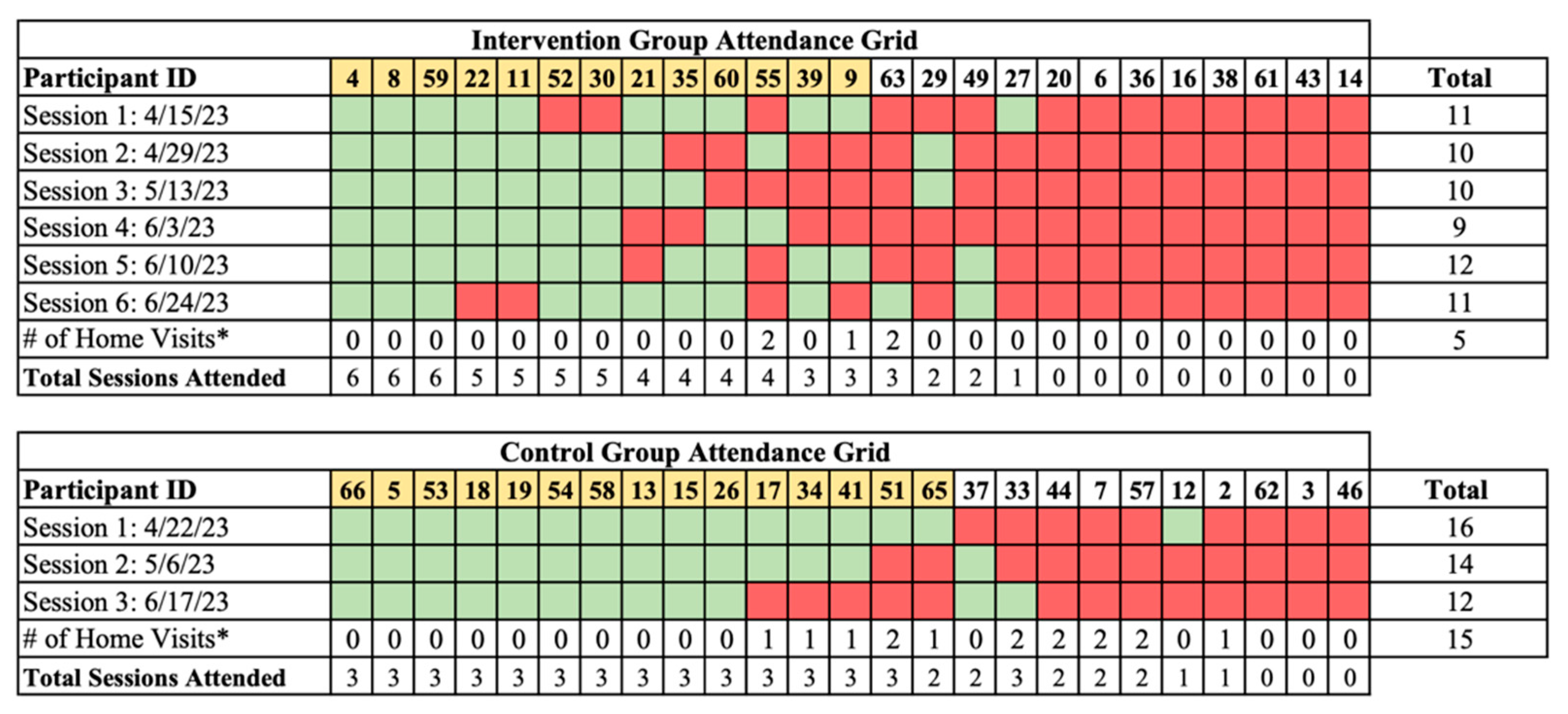
3.3. Participant Retention
3.4. Quantitative and Qualitative Participant Feedback
3.5. Qualitative CBO Feedback
3.6. Analysis of Secondary Outcomes
3.7. Harms
4. Discussion
4.1. Interpretation of Primary Outcomes
4.2. Interpretation of Secondary Outcomes
4.3. Generalizability
4.4. Limitations
4.5. Implications Toward a Definitive Trial
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volpp, K.G.; Berkowitz, S.A.; Sharma, S.V.; Anderson, C.A.M.; Brewer, L.C.; Elkind, M.S.V.; Gardner, C.D.; Gervis, J.E.; Harrington, R.A.; Herrero, M.; et al. Food Is Medicine: A Presidential Advisory from the American Heart Association. Circulation 2023, 148, 1417–1439. [Google Scholar] [CrossRef]
- Hager, K.; Kummer, C.; Lewin-Zwerdling, A.; Li, Z. Food Is Medicine Research Action Plan; Food and Society at Aspen Institute: Aspen, CO, USA, 2024. [Google Scholar]
- Downer, S.; Clippinger, E.; Kummer, C.; Hager, K. Food Is Medicine Research Action Plan; Food and Society at Aspen Institute: Aspen, CO, USA, 2022. [Google Scholar]
- The Joint Commission. New Requirements to Reduce Health Care Disparities. R3 Report: Requirement, Rationale, Reference. Am. J. Prev. Med. 2022, 53, 1–8. [Google Scholar]
- Trochez, R.J.; Sharma, S.; Stolldorf, D.P.; Mixon, A.S.; Novak, L.L.; Rajmane, A.; Dankwa-Mullan, I.; Kripalani, S. Screening Health-Related Social Needs in Hospitals: A Systematic Review of Health Care Professional and Patient Perspectives. Popul. Health Manag. 2023, 26, 157–167. [Google Scholar] [CrossRef]
- Downer, S.; Berkowitz, S.A.; Berkowitz, S.A.; Harlan, T.S.; Olstad, D.L.; Mozaffarian, D. Food Is Medicine: Actions to Integrate Food and Nutrition into Healthcare. BMJ 2020, 369, m2482. [Google Scholar] [CrossRef]
- Weber, P.; Birkholz, L.; Kohler, S.; Helsper, N.; Dippon, L.; Ruetten, A.; Pfeifer, K.; Semrau, J. Development of a Framework for Scaling Up Community-Based Health Promotion: A Best Fit Framework Synthesis. Int. J. Environ. Res. Public Health 2022, 19, 4773. [Google Scholar] [CrossRef]
- de Leeuw, E. Intersectorality and Health: A Glossary. J. Epidemiol. Community Health 2022, 76, 206–208. [Google Scholar] [CrossRef]
- Lumpkin, J.R.; Taylor, L.H.; Hattori, A.; Jedele, J.M. Impact of Food Delivery and Health Coaching on Outcomes and Costs of Care: A Payer’s Perspective. NEJM Catal. 2023, 4, CAT-22. [Google Scholar] [CrossRef]
- Crusan, A.; Roozen, K.; Godoy-Henderson, C.; Zamarripa, K.; Remache, A. Using Community-Based Participatory Research Methods to Inform the Development of Medically Tailored Food Kits for Hispanic/Latine Adults with Hypertension: A Qualitative Study. Nutrients 2023, 15, 3600. [Google Scholar] [CrossRef]
- Seligman, H.K.; Smith, M.; Rosenmoss, S.; Marshall, M.B.; Waxman, E. Comprehensive Diabetes Self-Management Support from Food Banks: A Randomized Controlled Trial. Am. J. Public Health 2018, 108, 1227–1234. [Google Scholar] [CrossRef]
- Ferrer, R.L.; Neira, L.M.; De Leon Garcia, G.L.; Cuellar, K.; Rodriguez, J. Primary Care and Food Bank Collaboration to Address Food Insecurity: A Pilot Randomized Trial. Nutr. Metab. Insights 2019, 12, 1178638819866434. [Google Scholar] [CrossRef]
- Ptomey, L.T.; Washburn, R.A.; Goetz, J.R.; Sullivan, D.K.; Gibson, C.A.; Mayo, M.S.; Krebill, R.; Gorczyca, A.M.; Honas, J.J.; Rice, A.M.; et al. A Randomized Trial Comparing Diet and Delivery Strategies for Weight Management in Adolescents with Intellectual Disabilities. Pediatr. Obes. 2023, 18, e12972. [Google Scholar] [CrossRef]
- Rivera, R.L.; Adams, M.; Dawkins, E.; Carter, A.; Zhang, X.; Tu, W.; Peña, A.; Holden, R.J.; Clark, D.O. Delivering Food Resources and Kitchen Skills (FoRKS) to Adults with Food Insecurity and Hypertension: A Pilot Study. Nutrients 2023, 15, 1452. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, M.S.; Chancellor McIntosh, H.; Beachy, C.; Shadid, O. Design and Implementation of a Clinic-Based Food Pharmacy for Food Insecure, Uninsured Patients to Support Chronic Disease Self-Management. J. Nutr. Educ. Behav. 2018, 50, 947–949. [Google Scholar] [CrossRef]
- Hager, K.; Du, M.; Li, Z.; Mozaffarian, D.; Chui, K.; Shi, P.; Ling, B.; Cash, S.B.; Folta, S.C.; Zhang, F.F. Impact of Produce Prescriptions on Diet, Food Security, and Cardiometabolic Health Outcomes: A Multisite Evaluation of 9 Produce Prescription Programs in the United States. Circ. Cardiovasc. Qual. Outcomes 2023, 16, E009520. [Google Scholar] [CrossRef]
- Rocha-Goldberg, M.D.P.; Corsino, L.; Batch, B.; Voils, C.I.; Thorpe, C.T.; Bosworth, H.B.; Svetkey, L.P. Hypertension Improvement Project (HIP) Latino: Results of a Pilot Study of Lifestyle Intervention for Lowering Blood Pressure in Latino Adults. Ethn. Health 2010, 15, 269–282. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Smith, S.A. Community-Based Participatory Research to Promote Healthy Diet and Nutrition and Prevent and Control Obesity Among African-Americans: A Literature Review. J. Racial Ethn. Health Disparities 2017, 4, 259–268. [Google Scholar] [CrossRef]
- Rupert, D.D.; Alvarez, G.V.; Burdge, E.J.; Nahvi, R.J.; Schell, S.M.; Faustino, F.L. Student-Run Free Clinics Stand at a Critical Junction Between Undergraduate Medical Education, Clinical Care, and Advocacy. Acad. Med. 2022, 97, 824–831. [Google Scholar] [CrossRef]
- Krishnan, S.; Sytsma, T.; Wischmeyer, P.E. Addressing the Urgent Need for Clinical Nutrition Education in PostGraduate Medical Training: New Programs and Credentialing. Adv. Nutr. 2024, 15, 100321. [Google Scholar] [CrossRef]
- Castro, L.F.; Adu, Y.; Castro, M.; Palacios, C.; Sheikh, M.; Barrios, Y.; Bennett, K.; Prabhu, F. Investigating Level of Food Security among Patients with Hypertension and Diabetes at a Student-Run Free Clinic. Bayl. Univ. Med. Cent. Proc. 2024, 37, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Dembar, A.; Mell, A.J.; Hsieh, V.; Chandrasekan, S.; Rifkin, R.; Thomas, D.C.; Meah, Y.S. Reducing Food Insecurity Through Personalized Interventions at the East Harlem Health Outreach Partnership. J. Stud.-Run. Clin. 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Fleischman, A.; Vanden Heuvel, C.; Tesfamichael, D.; McGinn Valley, T.; Jones, K. Addressing Social Determinants of Health in a Free Clinic Setting: A Student-Run Free Clinic and Community Resource Navigator Program. J. Stud.-Run. Clin. 2023, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Mckeon, M.; Ufuah, S.; Lloyd, C.; Weaver, E.; Fowler, M.; Miller, R. Descriptive Report Experiences with a Student-Run, In-Clinic Food Donation Program for Uninsured Patients in Nashville. J. Stud.-Run. Clin. 2022, 8, 1–6. [Google Scholar] [CrossRef]
- Smith, S.; Malinak, D.; Chang, J.; Perez, M.; Perez, S.; Settlecowski, E.; Rodriggs, T.; Hsu, M.; Abrew, A.; Aedo, S. Implementation of a Food Insecurity Screening and Referral Program in Student-Run Free Clinics in San Diego, California. Prev. Med. Rep. 2017, 5, 134–139. [Google Scholar] [CrossRef] [PubMed]
- The Fresh Produce Program Team. 2023 Annual Report Fresh Produce Program; The Fresh Produce Program Team: Durham, NC, USA, 2024. [Google Scholar]
- Durham County Department of Public Health; Partnership for a Healthy Durham; Duke Health Durham County. 2023 Community Health Assessment; Durham County Department of Public Health: Durham, NC, USA, 2024. [Google Scholar]
- Eslava-Schmalbach, J.; Garzón-Orjuela, N.; Elias, V.; Reveiz, L.; Tran, N.; Langlois, E.V. Conceptual Framework of Equity-Focused Implementation Research for Health Programs (EquIR). Int. J. Equity Health 2019, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A.; Altman, D.; Bretz, F.; Campbell, M.; et al. CONSORT 2010 Statement: Extension to Randomised Pilot and Feasibility Trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- Saini, V.; Jaber, T.; Como, J.D.; Lejeune, K.; Bhanot, N. Exploring “Slicer Dicer”, an Extraction Tool in EPIC, for Clinical and Epidemiological Analysis. In Open Forum Infectious Diseases; Oxford University Press: Cary, NC, USA, 2021. [Google Scholar]
- Horvath, M.M.; Rusincovitch, S.A.; Brinson, S.; Shang, H.C.; Evans, S.; Ferranti, J.M. Modular Design, Application Architecture, and Usage of a Self-Service Model for Enterprise Data Delivery: The Duke Enterprise Data Unified Content Explorer (DEDUCE). J. Biomed. Inform. 2014, 52, 231–242. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Moore, T.J.; Appel, L.J.; Obarzanek, E.; Cutler, J.A.; Vollmer, W.M.; Vogt, T.M.; Karanja, N.; Svetkey, L.P.; Lin, P.-H.; et al. A Dietary Approach to Prevent Hypertension: A Review of the Dietary Approaches to Stop Hypertension (DASH) Study. Clin. Cardiol. 1999, 22, 6–10. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory from the American Heart Association. Circulation 2022, 146, E18–E43. [Google Scholar] [CrossRef]
- Whelton, P.; Levine, G.N.; Al-Khatib, S.M.; Beckman, J.A.; Birtcher, K.K.; Bozkurt, B.; Brindis, R.G.; Cigarroa, J.E.; Curtis, L.H.; Deswal, A.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 13–115. [Google Scholar] [CrossRef]
- Economic Research Service. U.S. Household Food Security Survey Module: Six-Item Short Form; Economic Research Service, USDA: Washington, DC, USA, 2012. [Google Scholar]
- North Carolina Department of Health and Human Services. Using Standardized Social Determinants of Health Screening Questions to Identify and Assist Patients with Unmet Health-Related Resource Needs in North Carolina; North Carolina Department of Health and Human Services: Raleigh, NC, USA, 2018. [Google Scholar]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report from the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef]
- Hsieh, H.F.; Shannon, S.E. Three Approaches to Qualitative Content Analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Morse, E.V.; Simon, P.M.; Besch, C.L.; Walker, J. Issues of Recruitment, Retention, and Compliance in Community-Based Clinical Trials with Traditionally Underserved Populations. Appl. Nurs. Res. 1995, 8, 8–14. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Love, P.; Mathers, J.C.; Kirkpatrick, S.I.; Olstad, D.L. Cultural Adaptations and Tailoring of Public Health Nutrition Interventions in Indigenous Peoples and Ethnic Minority Groups: Opportunities for Personalised and Precision Nutrition. Proc. Nutr. Soc. 2023, 82, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Sholle, E.T.; Pinheiro, L.C.; Adekkanattu, P.; Davila, M.A.; Johnson, S.B.; Pathak, J.; Sinha, S.; Li, C.; Lubansky, S.A.; Safford, M.M.; et al. Underserved Populations with Missing Race Ethnicity Data Differ Significantly from Those with Structured Race/Ethnicity Documentation. J. Am. Med. Inform. Assoc. 2019, 26, 722–729. [Google Scholar] [CrossRef]
- Gordon, A.M.; Mendes, W.B. A Large-Scale Study of Stress, Emotions, and Blood Pressure in Daily Life Using a Digital Platform. Proc. Natl. Acad. Sci. USA 2021, 118, e2105573118. [Google Scholar] [CrossRef] [PubMed]
- Niiranen, T.J.; Hänninen, M.R.; Johansson, J.; Reunanen, A.; Jula, A.M. Home-Measured Blood Pressure Is a Stronger Predictor of Cardiovascular Risk than Office Blood Pressure: The Finn-Home Study. Hypertension 2010, 55, 1346–1351. [Google Scholar] [CrossRef]
- Goodwin, M.A.; Stange, K.C.; Zyzanski, S.J.; Crabtree, B.F.; Borawski, E.A.; Flocke, S.A. The Hawthorne Effect in Direct Observation Research with Physicians and Patients. J. Eval. Clin. Pract. 2017, 23, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Shavers, V.L.; Lynch, C.F.; Burmeister, L.F. Racial Differences in Factors That Influence the Willingness to Participate in Medical Research Studies. Ann. Epidemiol. 2002, 12, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Howe, C.J.; Robinson, W.R. Survival-Related Selection Bias in Studies of Racial Health Disparities. Epidemiology 2018, 29, 521–524. [Google Scholar] [CrossRef]
| Variable | Intervention (n = 25) | Control (n = 25) | p-Value |
|---|---|---|---|
| Age (years) | |||
| Median (Q1, Q3) | 55 (48, 62) | 57 (49, 63) | 0.75 |
| Range | 40–73 | 38–73 | |
| Gender (%) | 0.56 | ||
| Female | 14 (56) | 17 (68) | |
| Male | 11 (44) | 8 (32) | |
| Race/ethnicity (%) | 1.00 | ||
| Black | 13 (52) | 12 (48) | |
| Hispanic/Latinx | 12 (48) | 13 (52) | |
| Primary language (%) | 0.39 | ||
| English | 17 (68) | 13 (52) | |
| Spanish | 8 (32) | 12 (48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapay, E.R.; Sytsma, T.M.; Hutchinson, H.M.; Yoon, E.J.; Brummel, S.A.; Tang, L.Y.; Suarez, E.G.; Mitra, K.; Kane, R.M.; Hemming, J.P. Medically Tailored Grocery Deliveries to Improve Food Security and Hypertension in Underserved Groups: A Student-Run Pilot Randomized Controlled Trial. Healthcare 2025, 13, 253. https://doi.org/10.3390/healthcare13030253
Lapay ER, Sytsma TM, Hutchinson HM, Yoon EJ, Brummel SA, Tang LY, Suarez EG, Mitra K, Kane RM, Hemming JP. Medically Tailored Grocery Deliveries to Improve Food Security and Hypertension in Underserved Groups: A Student-Run Pilot Randomized Controlled Trial. Healthcare. 2025; 13(3):253. https://doi.org/10.3390/healthcare13030253
Chicago/Turabian StyleLapay, Elaijah R., Trevor M. Sytsma, Haley M. Hutchinson, Elliot J. Yoon, Scott A. Brummel, Linda Y. Tang, Elena G. Suarez, Kishen Mitra, Ryan M. Kane, and J. Patrick Hemming. 2025. "Medically Tailored Grocery Deliveries to Improve Food Security and Hypertension in Underserved Groups: A Student-Run Pilot Randomized Controlled Trial" Healthcare 13, no. 3: 253. https://doi.org/10.3390/healthcare13030253
APA StyleLapay, E. R., Sytsma, T. M., Hutchinson, H. M., Yoon, E. J., Brummel, S. A., Tang, L. Y., Suarez, E. G., Mitra, K., Kane, R. M., & Hemming, J. P. (2025). Medically Tailored Grocery Deliveries to Improve Food Security and Hypertension in Underserved Groups: A Student-Run Pilot Randomized Controlled Trial. Healthcare, 13(3), 253. https://doi.org/10.3390/healthcare13030253


_Rachiotis.png)




