Beyond Pain Relief: A Cross-Sectional Study on NSAID Prescribing, Polypharmacy, and Drug Interaction Risks in Community Pharmacies
Highlights
- Celecoxib and ketoprofen were the most commonly prescribed NSAIDs in community pharmacies, while polypharmacy and potential drug–drug interactions (pDDIs) were highly prevalent.
- A significant association (p < 0.05) was found between ibuprofen, diclofenac, aspirin, meloxicam use, and the lack of gastroprotective co-prescription, indicating a need for improved adherence to safety guidelines.
- Reinforcing regulatory frameworks and fortifying medication-use policies, combined with pharmacist-led medication review and continuous monitoring, can mitigate NSAID-related risks.
- The findings provide evidence to inform rational prescribing strategies, strengthen community pharmacy practice, and enhance patient medication safety in the Ras Al Khaimah, one of the Northern Emirates of the United Arab Emirates and similar healthcare settings.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Sample Size
2.3. Sampling Technique
2.4. Criteria for Inclusion and Exclusion
2.5. Study Procedure
2.5.1. Assessment of Prescribing Pattern
2.5.2. Assessment of Polypharmacy
2.5.3. Assessment of pDDIs
2.5.4. Data Analysis
3. Results
- Attributes of the study participants related to demographics and social factors
- Clinical status of the patients under study
- Usage patterns of NSAIDs medications
- Prescribing frequencies of NSAIDs based on the age group and gender
- Co-prescribing Gastroprotective Agents with NSAIDs
- Factors Associated with Gastroprotective (PPI) Co-Prescribing among NSAID Users
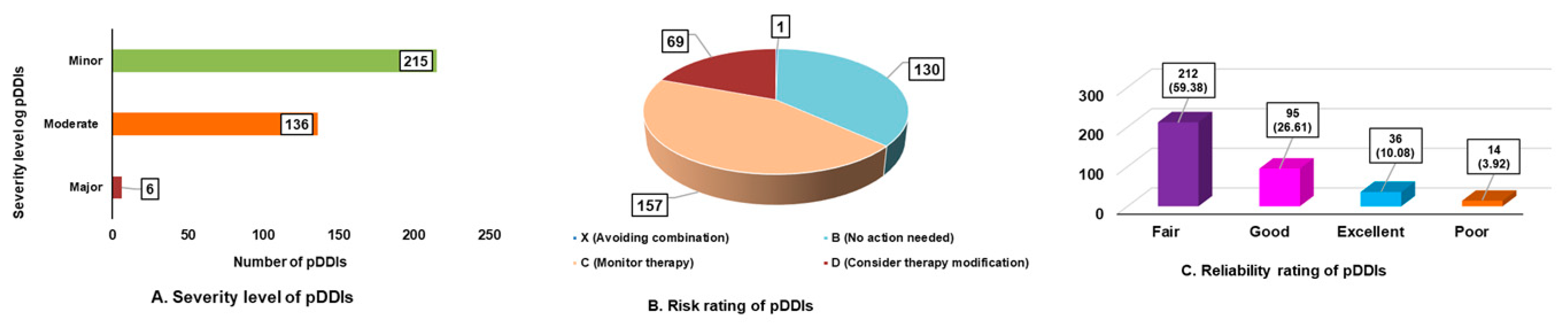
- Prevalence of pDDIs
- Factors Associated with Gastroprotective (PPI) Co-Prescribing
- Nature of pDDIs in study subjects.
- Influence of demographic and clinical characteristics on the likelihood of pDDIs
- Relationship between the number of pDDIs and therapeutic variables
- Binary Logistic Regression Analysis for pDDIs
- Link between demographic, clinical, and treatment-related factors and polypharmacy presence
- Relationship Between Polypharmacy and pDDIs by Severity
4. Discussion
- Prescribing Patterns of NSAIDs
- Drug–Drug Interactions
- Polypharmacy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| pDDIs | Potential drug–drug interactions |
| COX-2 | Cyclooxygenase-2 |
| PPIs | Proton Pump Inhibitors |
| UAE | United Arab Emirates |
| CV | Cardiovascular |
| GI | Gastrointestinal |
References
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Tai, F.W.D.; McAlindon, M.E. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin. Med. 2021, 21, 131–134. [Google Scholar] [CrossRef]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) [Updated 1 May 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547742/ (accessed on 5 May 2025).
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Schmidt, M.; Sørensen, H.T.; Pedersen, L. Diclofenac use and cardiovascular risks: Series of nationwide cohort studies. BMJ 2018, 362, k3426. [Google Scholar] [CrossRef]
- Calvo Barbado, D.M.; Saiz Fernández, L.C.; Leache Alegría, L.; Celaya Lecea, M.C.; Gutiérrez-Valencia, M. Acute Kidney Injury associated with “Triple whammy” combination: A protocol for a systematic review. F1000Res 2022, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; Tabeshpour, J.; Maziar, S.V.; Taherzadeh, Z.; Zirak, M.R.; Sent, D.; Azarkhiavi, K.R.; Eslami, S. Prescription Pattern Analysis of Nonsteroidal Anti-inflammatory Drugs in the Northeastern Iranian Population. J. Res. Pharm. Pract. 2017, 6, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Delara, M.; Murray, L.; Jafari, B.; Bahji, A.; Goodarzi, Z.; Kirkham, J.; Chowdhury, M.; Seitz, D.P. Prevalence and factors associated with polypharmacy: A systematic review and Meta-analysis. BMC Geriatr. 2022, 22, 601. [Google Scholar] [CrossRef] [PubMed]
- Pazan, F.; Wehling, M. Polypharmacy in older adults: A narrative review of definitions, epidemiology and consequences. Eur. Geriatr. Med. 2021, 12, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.; Wazaify, M.; Shawabkeh, H.; Boardley, I.; McVeigh, J.; Van Hout, M.C. A Scoping Review of Non-Medical and Extra-Medical Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Drug Saf. 2021, 44, 917–928. [Google Scholar] [CrossRef]
- Tassew, S.G.; Abraha, H.N.; Gidey, K.; Gebre, A.K. Assessment of drug use pattern using WHO core drug use indicators in selected general hospitals: A cross-sectional study in Tigray region, Ethiopia. BMJ Open 2021, 11, e045805. [Google Scholar] [CrossRef]
- Siele, S.M.; Abdu, N.; Ghebrehiwet, M.; Hamed, M.R.; Tesfamariam, E.H. Drug prescribing and dispensing practices in regional and national referral hospitals of Eritrea: Evaluation with WHO/INRUD core drug use indicators. PLoS ONE 2022, 17, e0272936. [Google Scholar] [CrossRef]
- Ye, L.; Yang-Huang, J.; Franse, C.B.; Rukavina, T.; Vasiljev, V.; Mattace-Raso, F.; Verma, A.; Borrás, T.A.; Rentoumis, T.; Raat, H. Factors associated with polypharmacy and the high risk of medication-related problems among older community-dwelling adults in European countries: A longitudinal study. BMC Geriatr. 2022, 22, 841. [Google Scholar] [CrossRef]
- Dervic, E.; Deischinger, C.; Haug, N.; Leutner, M.; Kautzky-Willer, A.; Klimek, P. The Effect of Cardiovascular Comorbidities on Women Compared to Men: Longitudinal Retrospective Analysis. JMIR Cardio 2021, 5, e28015. [Google Scholar] [CrossRef]
- Shuvo, S.D.; Hossen, M.T.; Riazuddin, M.; Hossain, M.S.; Mazumdar, S.; Parvin, R.; Elahi, T. Prevalence of comorbidities and its associated factors among type-2 diabetes patients: A hospital-based study in Jashore District, Bangladesh. BMJ Open 2023, 13, e076261. [Google Scholar] [CrossRef]
- Nasution, E.S.; Mukhtar, R. Pattern of Prescribing NSAIDs Utilisation at Outpatient Pediatric Poly at Universitas Sumatera Utara Hospital. Open Access Maced. J. Med. Sci. 2019, 7, 1631–1634. [Google Scholar] [CrossRef]
- Al-Taie, A.; Hussein, A.N.; Albasry, Z. Prescription pattern of non-steroidal anti-inflammatory drugs (NSAIDs) among community patients with musculoskeletal and co-morbid conditions: A cross sectional study from an Iraqi province. Trop. J. Pharm. Res. 2021, 20, 203–210. [Google Scholar] [CrossRef]
- Ahmed, M.N.J. The Frequency of Prescribing Nonsteroidal Anti-inflammatory Drugs in a Public Hospital. Asian J. Pharm. 2021, 15, 73–76. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, Z.; Li, F.; Li, J.; Wang, N.; Guo, Z.; Wang, J.; Ye, X.; Li, Y. The prescription patterns and safety profiles of oral non-steroidal anti-inflammatory drugs in China: An 8-year real-life analysis. Ann. Palliat. Med. 2021, 10, 2224–2237. [Google Scholar] [CrossRef]
- Qoul, K.; Thuheerat, I.; Abu Ashor, N.; Hakuz, N. Prescribing Patterns Of Non-Steroidal Anti-Inflammatory Drugs In Outpatient Clinics At Royal Rehabilitation Center In King Hussein Medical Center. Zagazig Univ. Med. J. 2014, 20, 4430. [Google Scholar] [CrossRef]
- Medications-Non-Steroidal Anti-Inflammatory Drugs. Available online: https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/medications-non-steroidal-anti-inflammatory-drugs (accessed on 6 January 2025).
- Chaudhari, D.; Chaudhary, H. Usage of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) And Their Interaction With Other Concurrently Used Drugs in Elderly Patients. J. Surv. Fish. Sci. 2023, 10, 946–953. [Google Scholar] [CrossRef]
- Schjerning Olsen, A.; Gislason, G.H.; McGettigan, P.; Fosbøl, E.; Sørensen, R.; Hansen, M.L.; Køber, L.; Torp-Pedersen, C.; Lamberts, M. Association of NSAID Use with Risk of Bleeding and Cardiovascular Events in Patients Receiving Antithrombotic Therapy After Myocardial Infarction. JAMA 2015, 313, 805–814. [Google Scholar] [CrossRef]
- Sawalha, A.F.; Sweileh, W.M.; Zyoud, S.H.; Al Jabi, S.W.; Bni Shamseh, F.F.; Odah, A. Analysis of prescriptions dispensed at community pharmacies in Nablus, Palestine. EMHJ-East. Mediterr. Health J. 2010, 16, 788–792. Available online: https://apps.who.int/iris/handle/10665/117975 (accessed on 27 October 2025). [CrossRef]
- Gyawali1, M.; Karki, R.; Bhusal, N.; Subedi, S.; Dangi, N.B. Prescribing Practice of NSAIDs in an Orthopedic Department of two Hospitals of Kathmandu Valley, Nepal: A Comparative Study. EASJ Pharm. Pharmacol. 2019, 1, 149–152. [Google Scholar] [CrossRef]
- Neupane, G.P.; Rai, M.; Rokaya, P.K. Patterns of prescription and adverse drug reaction profile of Non- Steroidal Anti-Inflammatory Drugs at orthopedic outpatient’s department. Nepal Med. Coll. J. 2022, 24, 170–175. [Google Scholar] [CrossRef]
- Ahmadi, N.; Jafari, A.; Ghasemnejad-Berenji, M.; Sadeghpour, S. Evaluation of the prescription pattern and drug interactions of non-streoidal anti-inflammatory drugs in patients referred to taleghani and imam khomeini educational pharmacies in urmia in the first 6 months of 2019. Stud. Med. Sci. 2021, 32, 707–714. [Google Scholar] [CrossRef]
- Awodele, O.; Fadipe, A.O.; Adekoya, M.; Adeyemi, O.O. Prescribing Pattern of Non-Steroidal Anti-Inflammatory Drugs at the Outpatient Pharmacy Department of a University Teaching Hospital in Nigeria. Ghana Med. J. 2015, 49, 25–29. [Google Scholar] [CrossRef]
- Bahreini, A.; Koneri, R. Prescription Pattern Analysis of Nonsteroidal Anti-Inflammatory Drugs in the Southeastern Karnataka Population, India. Arch. Pharma. Pract. 2020, 11, 116–119. [Google Scholar]
- Motgahre, V.; Bajait, C.; Turankar, A.; Pimpalkhute, S.; Dholpure, M. Prescription pattern and adverse drug reaction profile of drugs prescribed with focus on NSAIDs for orthopedic indications at a tertiary care hospital. Indian J. Pharm. Pharmacol. 2016, 3, 178–181. [Google Scholar]
- Mehuys, E.; De Backer, T.; De Keyser, F.; Christiaens, T.; Van Hees, T.; Demarche, S.; Van Tongelen, I.; Boussery, K. Prevalence and management of drug interactions between nonsteroidal anti-inflammatory drugs and antithrombotics in ambulatory care. Br. J. Clin. Pharmacol. 2022, 88, 3896–3902. [Google Scholar] [CrossRef]
- Alshakka, M.; Badulla, W.; Alolayan, S.; Mahmoud, M. Prescribing patterns of non-steroidal anti-inflammatory drugs (NSAIDs) at outpatient departments of four hospitals. Biomed. Res. 2018, 29, 3643–3647. [Google Scholar] [CrossRef]
- Amatya, E.; Fois, R.; Williams, K.A.; Pont, L.G. Potential for Detection of Safety Signals for Over-the-Counter Medicines Using National ADR Spontaneous Reporting Data: The Example of OTC NSAID-Associated Gastrointestinal Bleeding. Pharmacy 2020, 8, 174. [Google Scholar] [CrossRef]
- Khalil, V.; Wang, W.; Charlson, L.; Blackley, S. Evaluation of prescribing patterns of nonsteroidal anti-inflammatory agents in a tertiary setting. Int. J. Evid. Based Healthc. 2019, 17, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Almaghaslah, D.; Almanasef, M.; Vasudevan, R.; Alqahtani, A.; Chinnadhurai, M.; Joy, N. A Study of Prescribing Patterns for Non-Steroidal Anti-Inflammatory Drugs in a Tertiary Care Teaching Hospital. Indian J. Pharm. Sci. 2021, 83, 278–283. [Google Scholar] [CrossRef]
- Hassan, N.A.G.M.; Gacem, S.A.; Al-Qaysi, A.A.H.; Alaani, M.J. Prevalence of pain and self-medication pattern of paracetamol and nsaids among university students in United Arab Emirates. Res. J. Pharm. Technol. 2021, 14, 3393–3398. [Google Scholar] [CrossRef]
- Ayenew, W.; Asmamaw, G.; Issa, A. Prevalence of potential drug-drug interactions and associated factors among outpatients and inpatients in Ethiopian hospitals: A systematic review and meta-analysis of observational studies. BMC Pharmacol. Toxicol. 2020, 21, 63. [Google Scholar] [CrossRef]
- Dagnew, E.M.; Ergena, A.E.; Wondm, S.A.; Sendekie, A.K. Potential drug-drug interactions and associated factors among admitted patients with psychiatric disorders at selected hospitals in Northwest Ethiopia. BMC Pharmacol. Toxicol. 2022, 23, 88. [Google Scholar] [CrossRef]
- Kabakama, C.C.; Pydimarri, R.; Ponnusankar, S. A prospective study on assessment of clinically potential drug-drug interactions in hospital and community pharmacy prescriptions. Afr. J. Pharm. Pharmacol. 2021, 15, 118–125. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Shaibi, S.; Sankaralingam, S.; Awaisu, A.; Kattezhathu, V.S.; Wongwiwatthananukit, S.; Owusu, Y.B. Determination of potential drug–drug interactions in prescription orders dispensed in a community pharmacy setting using Micromedex® and Lexicomp®: A retrospective observational study. Int. J. Clin. Pharm. 2022, 44, 348–356. [Google Scholar] [CrossRef]
- Pappala, R.T.; Sandiri, B.; Peddi, L.P.; Pappala, R.K.; Pravallika, M.R.P.L.; Prashanthi, G. A Clinical Study on Prescribing Patterns of NSAIDs and Assessment of Drug Interactions. Acta Sci. Pharm. Sci. 2020, 4, 83–87. [Google Scholar] [CrossRef]
- Alhumaidi, R.M.; Bamagous, G.A.; Alsanosi, S.M.; Alqashqari, H.S.; Qadhi, R.S.; Alhindi, Y.Z.; Ayoub, N.; Falemban, A.H. Risk of Polypharmacy and Its Outcome in Terms of Drug Interaction in an Elderly Population: A Retrospective Cross-Sectional Study. J. Clin. Med. 2023, 12, 3960. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Gong, H.; Li, C.; Wu, J.; Xia, T.; Li, C.; Li, S.; Chen, M. Prevalence and associated factors of drug-drug interactions in elderly outpatients in a tertiary care hospital: A cross-sectional study based on three databases. Ann. Transl. Med. 2023, 11, 17. [Google Scholar] [CrossRef]
- Sheikh-Taha, M.; Asmar, M. Polypharmacy and severe potential drug-drug interactions among older adults with cardiovascular disease in the United States. BMC Geriatr. 2021, 21, 233. [Google Scholar] [CrossRef]
- Shetty, V.; Chowta, M.N.; Chowta, K.N.; Shenoy, A.; Kamath, A.; Kamath, P. Evaluation of Potential Drug-Drug Interactions with Medications Prescribed to Geriatric Patients in a Tertiary Care Hospital. J. Aging Res. 2018, 2018, 5728957. [Google Scholar] [CrossRef] [PubMed]
- Samardžić, I.; Marinović, I.; Kuča, N.; Vrca, V.B. Potential clinically significant drug-drug interactions in prescribed pharmacotherapy in an outpatient setting. Pharmazie 2021, 76, 390–395. [Google Scholar] [CrossRef]
- Abdu, N.; Mosazghi, A.; Teweldemedhin, S.; Asfaha, L.; Teshale, M.; Kibreab, M.; Anand, I.S.; Tesfamariam, E.H.; Russom, M. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs): Usage and co-prescription with other potentially interacting drugs in elderly: A cross-sectional study. PLoS ONE 2020, 15, e0238868. [Google Scholar] [CrossRef]
- Adem, L.; Tegegne, G.T. Medication Appropriateness, Polypharmacy, and Drug-Drug Interactions in Ambulatory Elderly Patients with Cardiovascular Diseases at Tikur Anbessa Specialized Hospital, Ethiopia. Clin. Interv. Aging 2022, 17, 509–517. [Google Scholar] [CrossRef]
- Barakat, H.E.; Aziz, C.N.; Abougalambou, S.S.I. Evaluation of knowledge, practices, and attitudes of community pharmacists toward adverse effects of NSAIDs: A cross-sectional study in Iraq. J. Pharm. Policy Pract. 2023, 16, 132. [Google Scholar] [CrossRef]
- Jaber, D.; Al Shihab, A.; Tamimi, L.N. Efficacy and safety of pharmacist managed NSAIDs deprescribing: A Jordanian outpatient study. J. Clin. Pharm. Ther. 2024, 49, e5874686. [Google Scholar] [CrossRef]
- Husain, W.A.; Moosa, R.M.; Awadh, A.M.; Aladraj, F.M.; Mahdi, B.; Shehata, M.H. The prevalence and contributing factors of NSAIDs prescribing among chronic kidney disease patients in primary care: A mixed methods study from Kingdom of Bahrain. BMC Prim. Care 2025, 26, 116. [Google Scholar] [CrossRef] [PubMed]
- Al-Shargi, O.Y. Prescribing Pattern of Non-steroidal Anti-inflammatory Drugs (NSAIDs) in the Middle East Region: A Critical Review. Curr. Drug Saf. 2024, 19, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Al-Azayzih, A.; Al-Azzam, S.I.; Alzoubi, K.H.; Jarab, A.S.; Kharaba, Z.; Al-Rifai, R.H.; Alnajjar, M.S. Nonsteroidal anti-inflammatory drugs utilization patterns and risk of adverse events due to drug drug interactions among elderly patients: A study from Jordan. Saudi Pharm. J. 2020, 28, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Malebari, A.M.; Khayyat, A.N.; Mahdali, R.A.; Alamoudi, J.S.; Alsayed, B.Y.; Alrasheed, S.A. Evaluation of the community pharmacists’ performance in screening NSAID risks in Saudi Arabia. Saudi Med. J. 2020, 41, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Farah, R.I.; Khatib, A.E.; Abu Ziyad, H.J.; Jiad, D.K.; Al Qusous, L.R.; Ababneh, A.J.; Ajarmeh, S. Pattern of use and awareness of side-effects of non-steroidal anti-inflammatory drugs in the Jordanian population. Ann. Med. 2023, 55, 2242248. [Google Scholar] [CrossRef] [PubMed]
| Variable | n = 600 (%) | 95%Confidence Interval |
|---|---|---|
| Gender | ||
| Female | 352 (58.7) | 54.7–62.6 |
| Male | 248 (41.3) | 37.4–45.3 |
| Age (In Years) | ||
| ≤25 | 84 (14.0) | 11.0–16.8 |
| 26–50 | 409 (68.20) | 64.3–72.0 |
| 51–75 | 99 (16.50) | 13.7–19.5 |
| >75 | 08 (1.30) | 0.5–2.3 |
| Nationality | ||
| Asians | 355 (59.2) | 55.3–63.0 |
| Arab Countries other than UAE | 197 (32.8) | 29.0–36.7 |
| UAE Nationals | 42 (7.0) | 5.0–9.0 |
| Others | 06 (1.0) | 0.2–1.8 |
| Number of comorbidities | ||
| 0 | 26 (4.3) | 2.8–6.6 |
| 1–2 | 481 (80.2) | 76.8–83.2 |
| 3–4 | 80 (13.3) | 10.8–15.8 |
| ≥5 | 13 (2.2) | 1.2–3.5 |
| Comorbidities | ||
| Diabetes mellitus | ||
| Yes | 24 (4.0) | 2.5–5.7 |
| No | 576 (96.0) | 94.3–97.5 |
| Hypertension | ||
| Yes | 102 (17.0) | 13.8–20.0 |
| No | 498 (83.0) | 80.0–86.2 |
| Dyslipidemia | ||
| Yes | 176 (29.3) | 25.8–33.0 |
| No | 424 (70.7) | 67.0–74.2 |
| Thyroid disorders | ||
| Yes | 48 (8.0) | 5.8–10.2 |
| No | 552 (92.0) | 89.8–94.2 |
| Gastrointestinal diseases | ||
| Yes | 81(13.5) | 10.8–16.5 |
| No | 519 (86.5) | 83.5–89.2 |
| Kidney diseases | ||
| Yes | 26 (4.3) | 2.8–6.2 |
| No | 574 (95.7) | 93.8–97.2 |
| Anemia | ||
| Yes | 13 (2.2) | 1.0–3.3 |
| No | 587 (97.8) | 96.7–99 |
| Asthma | ||
| Yes | 53 (8.8) | 6.5–11.2 |
| No | 547 (91.2) | 88.8–93.5 |
| Chronic Obstructive pulmonary Disease | ||
| Yes | 5 (0.8) | 0.2–1.5 |
| No | 595 (99.2) | 98.5–99.8 |
| Orthopedic | ||
| Yes | 184 (30.7) | 26.8–34.2 |
| No | 416 (69.3) | 65.8–73.2 |
| Neurological | ||
| Yes | 22 (3.7) | 2.3–5.3 |
| No | 578 (96.3) | 94.7–97.7 |
| Psychiatric | ||
| Yes | 25 (4.2) | 2.7–6.0 |
| No | 575 (95.8) | 94.0–97.3 |
| Dermatological | ||
| Yes | 19 (3.2) | 1.8–4.8 |
| No | 581 (96.8) | 95.2–98.2 |
| Muscle spasm | ||
| Yes | 37 (6.2) | 4.2–8.2 |
| No | 563 (93.8) | 91.8–95.8 |
| Respiratory infections | ||
| Yes | 94 (15.7) | 12.8–18.8 |
| No | 506 (84.3) | 81.2–87.2 |
| Glaucoma | ||
| Yes | 34 (5.7) | 3.8–7.7 |
| No | 566 (94.3) | 92.3–96.2 |
| Sl No | Diagnosis | Frequency (n = 600) | % |
|---|---|---|---|
| Pain-related conditions | |||
| 1 | Pain in shoulder/joint/elbow/low back/body ache | 273 | 45.5 |
| 2 | Spondylosis/radiculopathy | 156 | 26.0 |
| 3 | Arthritis related pain | 101 | 16.83 |
| 4 | Dental/tooth/gingivitis | 21 | 3.50 |
| 5 | Pain unspecified disease/disorder | 11 | 1.83 |
| 6 | Chronic diseases related pain (gout, inflammatory bowel disease, diabetes mellitus, iron deficiency anemia) | 14 | 2.33 |
| 7 | Osteoporosis/hemorrhoids | 08 | 1.33 |
| 8 | Neuropathic pain | 07 | 1.16 |
| 9 | Diagnosis unspecified | 09 | 1.50 |
| Common comorbidities | |||
| 1. Cardiovascular diseases | 302 | 50.3 | |
| 2. Musculoskeletal disorders | 201 | 33.5 | |
| 3. Respiratory disorders | 152 | 25.3 | |
| 4. Diabetes mellitus | 24 | 4.0 | |
| 5. Hypertension | 102 | 17.0 | |
| 6. Dyslipidaemia | 176 | 29.3 |
| Drugs Category | ATC Code | n = 908 | % | CI 95% |
|---|---|---|---|---|
| Non-selective COX inhibitors | ||||
| Aspirin | B01AC06 | 75 | 12.5 | 10.0–15.3 |
| Ibuprofen | M01AE01 | 90 | 15.0 | 12.2–18.3 |
| Indomethacin | M02AA23 | 18 | 3.0 | 1.7–4.5 |
| Ketoprofen | M02AA10 | 224 | 37.3 | 33.5–41.3 |
| Piroxicam | M01AC01 | 58 | 9.7 | 7.3–12.2 |
| Preferential COX-2 Inhibitors | ||||
| Diclofenac | M01AB05 | 126 | 21.0 | 17.5–24.3 |
| Meloxicam | M01AC06 | 95 | 15.8 | 13.0–18.7 |
| Selective COX-2 inhibitors | ||||
| Celecoxib | M01AH01 | 220 | 36.7 | 33.2–40.7 |
| NSAIDs | ≤25 Years | 26–50 Years | 51–75 Years | >75 Years | Total (n = 908) | p-Value |
|---|---|---|---|---|---|---|
| Aspirin | 4 (5.33) | 50 (66.66) | 21 (28.0) | 0 (0.0) | 75 | 0.008 |
| Celecoxib | 19 (8.63) | 158 (71.81) | 37 (16.81) | 6 (2.72) | 220 | 0.004 |
| Diclofenac | 19 (15.07) | 88 (69.84) | 17 (13.49) | 02 (1.58) | 126 | 0.769 |
| Ibuprofen | 27 (30.0) | 56 (62.22) | 7 (7.77) | 0 (0.0) | 90 | <0.0001 ** |
| Indomethacin | 3 (16.66) | 12 (66.66) | 3 (16.66) | 0 (0.0) | 18 | 0.947 ** |
| Ketoprofen | 17 (7.58) | 161(71.87) | 42 (18.75) | 4 (1,78) | 224 | 0.004 |
| Meloxicam | 17 (17.89) | 66 (69.47) | 12 (12,63) | 0 (0.0) | 95 | 0.274 |
| Piroxicam | 4 (6.89) | 31 (53.44) | 21 (36.20) | 2 (3.44) | 58 | 0.001 |
| Variables | Co-Prescription with Gastro-Protective Agents | p-Value | ||
|---|---|---|---|---|
| Yes | No | Total (n = 908) | ||
| Age group (years) | ||||
| ≤25 | 12 (14.3) | 72 (85.7) | 84 | 0.021 |
| 26–50 | 82 (20.0) | 327 (80.0) | 409 | |
| 51–75 | 52 (31.3) | 114 (68.7) | 166 | |
| >75 | 8 (40.0) | 12 (60.0) | 20 | |
| Comorbidities | ||||
| Present | 128 (26.6) | 353 (73.4) | 481 | 0.033 |
| Absent | 20 (15.9) | 106 (84.1) | 126 | |
| Type of NSAIDs | ||||
| Ibuprofen | 9 (10.0) | 81 (90.0) | 90 | <0.0001 |
| Celecoxib | 70 (31.81) | 150 (68.18) | 220 | 0.071 |
| Diclofenac | 23 (18.25) | 103 (81.74) | 126 | 0.013 |
| Piroxicam | 20 (34.48) | 38 (65.51) | 58 | 0.216 |
| Ketoprofen | 70 (31.25) | 154 (68.75) | 224 | 0.108 |
| Aspirin | 37 (49.33) | 38 (50.66) | 75 | <0.001 |
| Indomethacin | 02 (11.11) | 16 (88.88) | 18 | 0.177 |
| Meloxicam | 44 (46.31) | 51(53.68) | 95 | <0.0001 |
| Sl No. | Drug Pairs | n = 357 (%) | Risk Rating | Severity | Reliability Rating | Mechanism of Interaction and Effect | Action |
|---|---|---|---|---|---|---|---|
| 1 | Clopidogrel and Esomeprazole | 01 (0.28) | X | Major | Fair | Esomeprazole may diminish the antiplatelet effect of Clopidogrel. | Avoiding concurrent use due to decreased clopidogrel effectiveness. |
| 2 | Calcium carbonate and Multivitamins | 03 (0.84) | D | Major | Fair | Multivitamins/Fluoride (with ADE) may increase the serum concentration of Calcium Salts. | Avoid consuming dairy products, vitamins, or supplements with calcium salts |
| 3 | Clopidogrel and Pantoprazole | 02 (0.56) | C | Major | Fair | Pantoprazole may decrease the serum concentration of clopidogrel’s active metabolite. | Use pantoprazole with clopidogrel only when GI protection is clearly needed. |
| 4 | Metformin and Perindopril | 14 (3.92) | C | Moderate | Poor | ACEIs may enhance the adverse/toxic effect of Metformin. | Monitor patient response to metformin closely if using these drugs concurrently. |
| 5 | Aspirin and Celecoxib | 27 (7.56) | D | Moderate | Good | Aspirin may enhance the adverse/toxic effect of NSAIDs (COX-2 Selective). | High-dose aspirin is not recommended with COX-2 inhibitors beyond cardioprotective use. |
| 6 | Aspirin (Salicylates) and Metformin | 21 (5.88) | C | Moderate | Fair | Salicylates may enhance the hypoglycaemic effects of blood glucose-lowering agents. | Monitor for excessive pharmacological effects (e.g., hypoglycaemia). |
| 7 | Bisoprolol and metformin | 13 (3.64) | C | Moderate | Fair | Beta-blockers (Beta1 Selective) may enhance the hypoglycaemic effect of Antidiabetic Agents. | Monitor and educate patients treated with antidiabetic agents regarding the risk of masked hypoglycaemia symptoms. |
| 8 | Celecoxib and perindopril | 11 (3.08) | C | Minor | Excellent | Aspirin may enhance the adverse/toxic effect of NSAIDs. | In CHF patients, consider alternative anti-inflammatory therapy to avoid NSAID-related fluid retention and edema. |
| 9 | Diclofenac (topical) and valsartan | 18 (5.04) | C | Minor | Fair | NSAIDs (Topical) may diminish the therapeutic effect of ARBs. | Monitor for reduced ARB efficacy (e.g., BP, edema, renal function) when coadministered with topical NSAIDs. |
| 10 | Ketoprofen and losartan | 14 (3.92) | C | Minor | Good | ARBs may enhance the adverse/toxic effect of NSAIDs. | Monitor blood pressure and renal function when ARBs are used with NSAIDs. |
| 11 | Bisoprolol and celecoxib | 14 (3.92) | C | Minor | Fair | NSAIDs may diminish the antihypertensive effect of Beta-Blockers. | Monitor blood pressure changes when NSAID therapy is started, stopped, or adjusted. |
| 12 | Amlodipine and clopidogrel | 12 (3.36) | C | Minor | Fair | Calcium Channel Blockers may diminish the therapeutic effect of Clopidogrel. | Monitor clopidogrel response when used with calcium channel blockers, as clinical impact and risks vary. |
| 13 | Celecoxib and valsartan | 11 (3.08) | C | Minor | Excellent | ARBs may enhance the adverse/toxic effect of NSAIDs. | Consider alternative anti-inflammatory treatments in CHF patients to prevent NSAID-related fluid retention and edema. |
| 14 | Lisinopril and celecoxib | 12 (3.36) | C | Minor | Excellent | ACEIs may enhance the adverse/toxic effect of NSAIDs. | Consider alternative anti-inflammatory therapy in CHF patients to avoid NSAID-related fluid retention and edema. |
| 15 | Atorvastatin and clopidogrel | 14 (3.92) | B | Minor | Good | Atorvastatin may reduce clopidogrel’s antiplatelet effect. | No action is needed beyond standard clinical care measures. |
| 16 | Amlodipine and atorvastatin | 21 (5.88) | B | Minor | Fair | Amlodipine may increase atorvastatin levels, while atorvastatin may reduce amlodipine levels. | No action is required beyond standard clinical care measures. |
| Variable | Total Number of Patients (n = 600) | Chi-Square Test | ||
|---|---|---|---|---|
| DDI Present (n = 190) | DDI Absent (n = 410) | X2 | p-Value | |
Gender
| 94 (37.9%) 96 (27.3%)) | 154 (62.1%) 256 (72.7%) | 7.598 | 0.007 |
Age Distribution (In years)
| 28 (33.3%) 137 (33.5%) 25 (25.3) 00 (0.0) | 56 (66.7) 272 (66.5) 74 (74.7) 08 (100%) | 6.330 | 0.094 |
Nationality
| 84 (23.7%) 90 (45.7%) 15 (35.7%) 01 (16.7%) | 271 (76.3%) 107 (54.3%) 27 (64.3%) 5 (83.3%) | 29.345 | <0.0001 |
Total Number of Prescribed Drugs
| 108 (27.1%) 82 (40.8%) | 291(72.9%) 119 (59.2%) | 11.642 | 0.001 |
Presence of Comorbidities
| 182 (31.7) 08 (30.8) | 392 (68.3%) 18 (69.2%) | 23.058 | <0.0001 |
| Coefficients | |||||||
|---|---|---|---|---|---|---|---|
| Models | Unstandardized Coefficients | Standardized Coefficients | Sig | OR (Exp(B)) | 95% CI | ||
| B | Std. Error | Beta | Lower | Upper | |||
| Constant | −3.669 | 0.461 | 63.426 | ||||
| Gender | 0.272 | 0.190 | 2.060 | 0.151 | 1.313 | 0.905 | 1.903 |
| Age | 0.478 | 0.262 | 3.334 | 0.068 | 1.613 | 0.966 | 2.693 |
| Nationality | 0.724 | 0.189 | 14.606 | 0.000 | 2.062 | 1.423 | 2.989 |
| No. of Comorbidities | 0.628 | 0.183 | 11.773 | 0.001 | 1.875 | 1.309 | 2.684 |
| Total no. of drugs | 0.448 | 0.179 | 6.286 | 0.012 | 1.565 | 1.103 | 2.221 |
| Variables | Total Number of Patients n = 600 | Chi-Square Test | ||
|---|---|---|---|---|
| Polypharmacy Present (n = 201) | Polypharmacy Absent (n = 399) | X2 | p-Value | |
Gender
| 105 (52.23) 96 (47.76) | 143 (35.83) 256 (64.16) | 14.824 | <0.0001 |
Age Distribution (In years)
| 22 (10.94) 142 (70.64) 34 (16.91) 03 (1.49) | 62 (15.53) 267 (66.91) 65 (16.29) 05 (1.25) | 2.376 | 0.500 |
Nationality
| 99 (49.25) 84 (41.79) 17 (8.45) 01 (0.49) | 256 (64.16) 113 (28.32) 25 (6.26) 05 (1.25) | 14.087 | 0.002 |
Comorbidities
| 190 (94.52) 11 (5.47) | 384 (96.24) 15 (3.75) | 8.704 | 0.032 |
| pDDIs | Polypharmacy | Chi-Square Test | ||
|---|---|---|---|---|
| Present | Absent | X2 | p-Value | |
| Minor | 134 | 66 | 8.855 | 0.107 |
| Moderate | 78 | 122 | 14.311 | 0.024 * |
| Severe | 08 | 192 | 3.206 | 0.244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shareef, J.; Sridhar, S.B.; Al Naqbi, S.H.; Bakshi, A.I. Beyond Pain Relief: A Cross-Sectional Study on NSAID Prescribing, Polypharmacy, and Drug Interaction Risks in Community Pharmacies. Healthcare 2025, 13, 3264. https://doi.org/10.3390/healthcare13243264
Shareef J, Sridhar SB, Al Naqbi SH, Bakshi AI. Beyond Pain Relief: A Cross-Sectional Study on NSAID Prescribing, Polypharmacy, and Drug Interaction Risks in Community Pharmacies. Healthcare. 2025; 13(24):3264. https://doi.org/10.3390/healthcare13243264
Chicago/Turabian StyleShareef, Javedh, Sathvik Belagodu Sridhar, Saeed Humaid Al Naqbi, and Adyan Iftekhar Bakshi. 2025. "Beyond Pain Relief: A Cross-Sectional Study on NSAID Prescribing, Polypharmacy, and Drug Interaction Risks in Community Pharmacies" Healthcare 13, no. 24: 3264. https://doi.org/10.3390/healthcare13243264
APA StyleShareef, J., Sridhar, S. B., Al Naqbi, S. H., & Bakshi, A. I. (2025). Beyond Pain Relief: A Cross-Sectional Study on NSAID Prescribing, Polypharmacy, and Drug Interaction Risks in Community Pharmacies. Healthcare, 13(24), 3264. https://doi.org/10.3390/healthcare13243264






