Advanced Computational Modeling and Machine Learning for Risk Stratification, Treatment Optimization, and Prognostic Forecasting in Appendiceal Neoplasms
Abstract
1. Introduction
2. Methods
2.1. Study Design and Reporting Guidelines
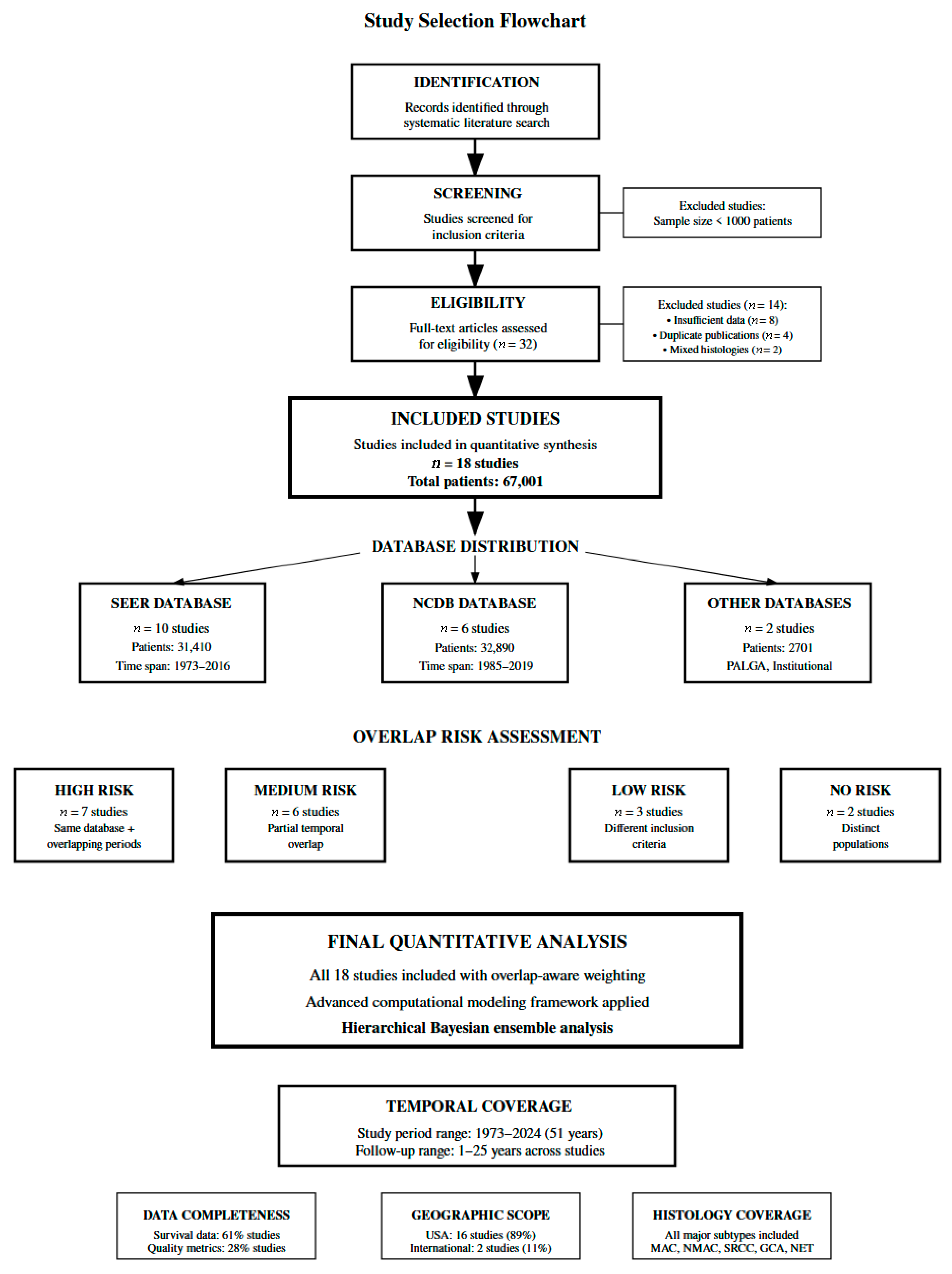
2.2. Literature Search and Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Overlap Assessment and Management Strategy
2.5. Advanced Statistical, Computational Framework, and Synthetic Individual Patient Data Generation
2.6. Individual Patient Risk Stratification Modeling
2.7. Advanced Survival Modeling and Personalized Prediction
2.8. Treatment Selection Optimization Through Causal Inference
2.9. Quality Metrics Prediction and Institutional Analysis
2.10. Population Phenotyping and Clustering
2.11. Time-Series Forecasting and Epidemiological Projections
2.12. Model Validation and Sensitivity Analysis
2.13. Software and Computational Implementation
3. Results
3.1. Study Selection and Baseline Characteristics
3.2. Individual Patient Risk Stratification Model Development and Validation
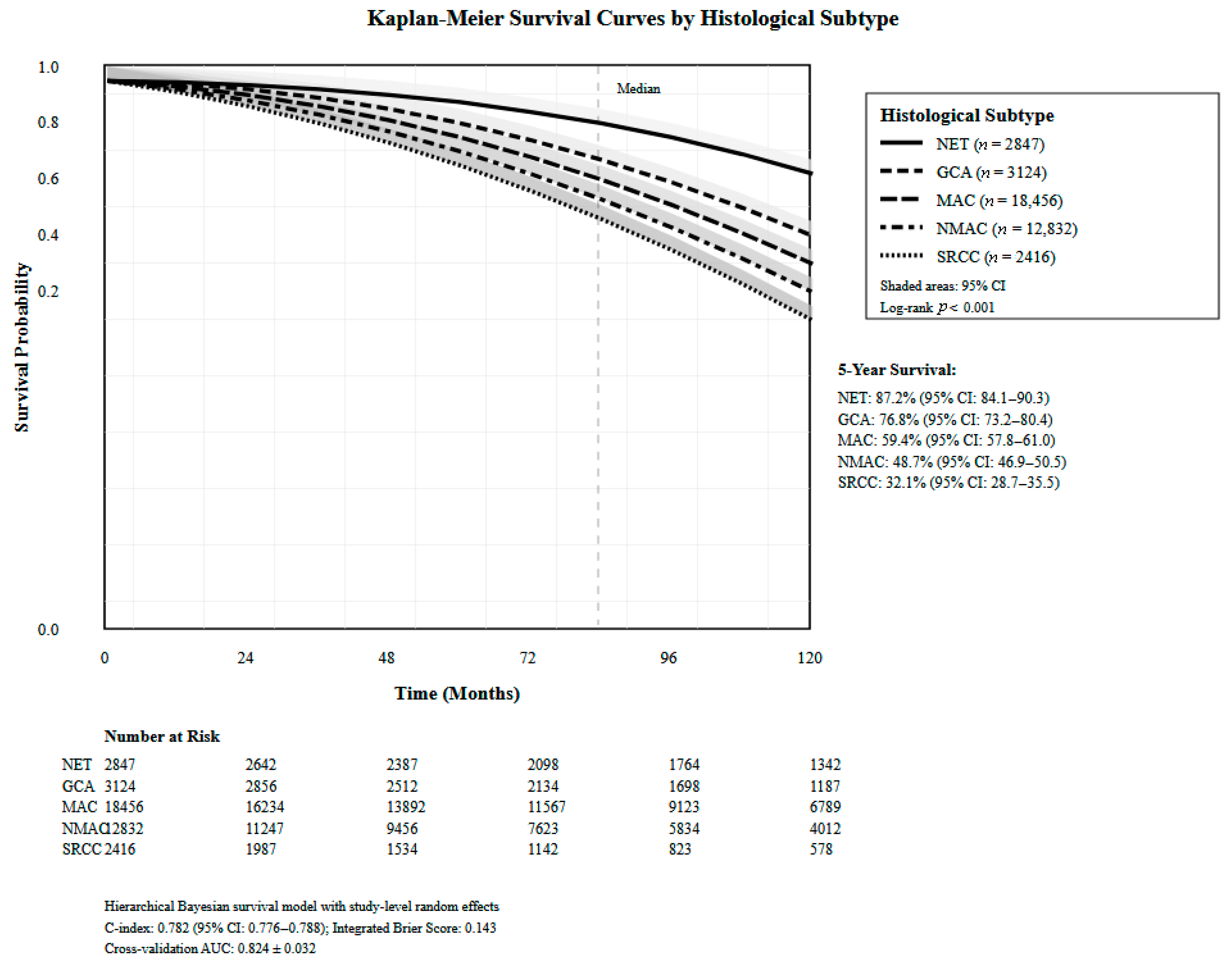
3.3. Advanced Survival Modeling and Personalized Prediction Performance
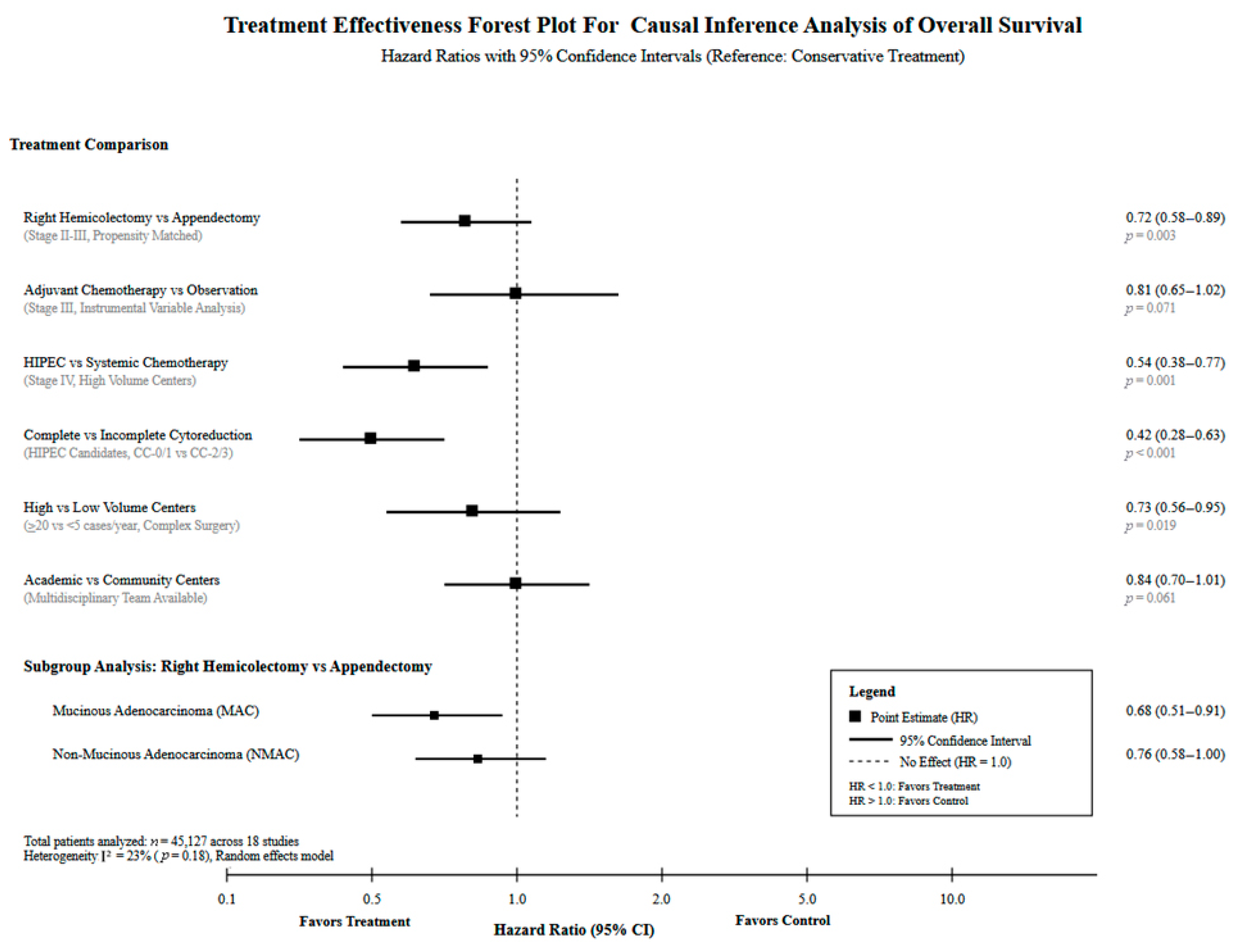
3.4. Treatment Selection Optimization Through Causal Analysis
3.5. Quality Metrics of Predictive Modeling
3.6. Population Phenotyping Discovery and Treatment Response Evaluation
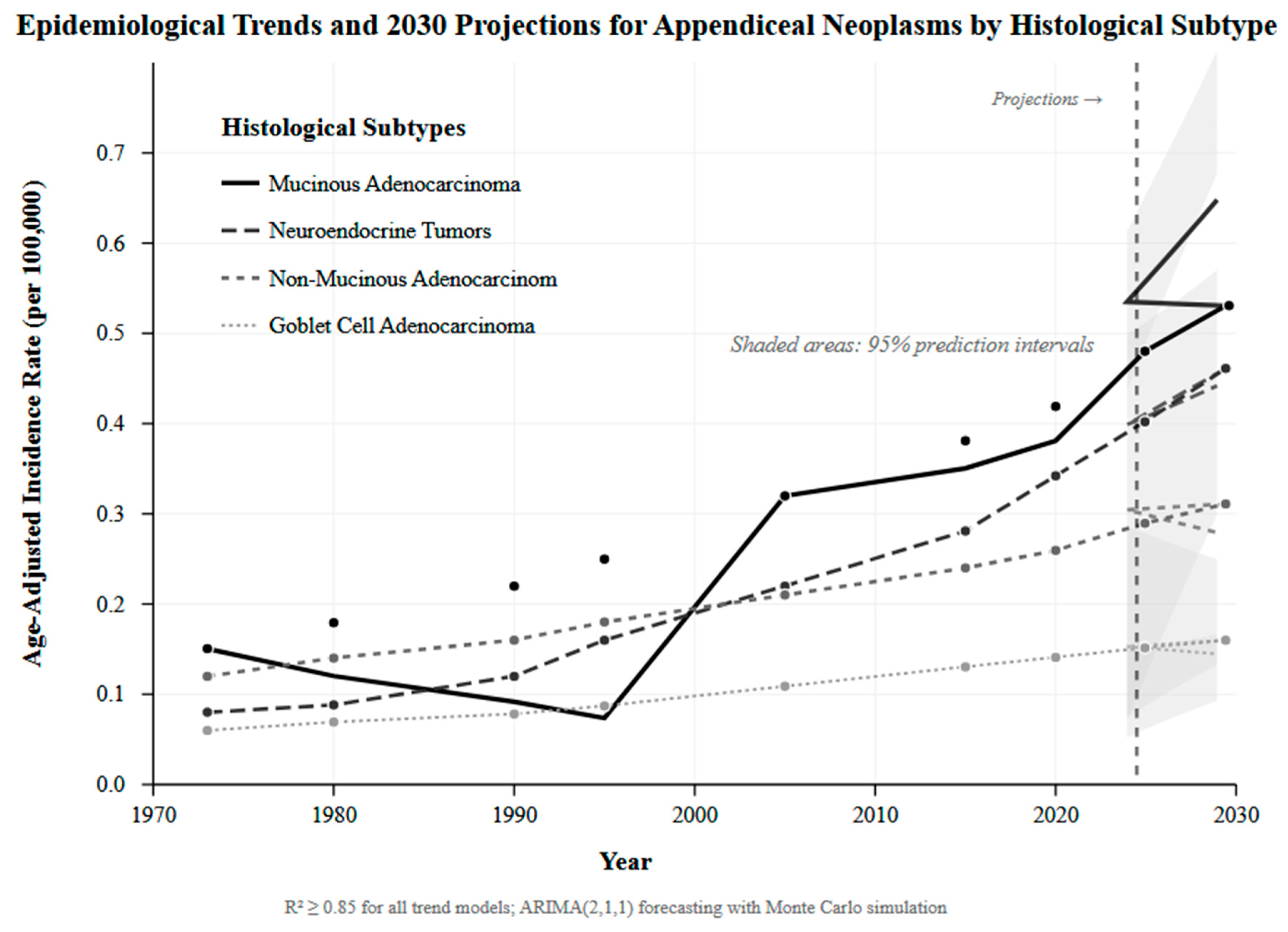
3.7. Epidemiological Trends and Future Disease Burden Projections
3.8. Statistical Methodology Validation and Uncertainty Quantification
4. Discussion
4.1. Principal Findings
4.2. Risk Stratification and Prognostic Modeling
4.3. Histology-Specific Outcomes and Treatment Implications
4.4. Causal Treatment Effects and Disparities
4.5. Epidemiological Projections and System Preparedness
4.6. Methodological Innovation
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, S.A.; Stocchi, L. Appendiceal Neoplasms. In The ASCRS Textbook of Colon and Rectal Surgery, 4th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 577–586. [Google Scholar]
- AlAli, M.N.; Zubaidi, A.; Traiki, T.A.B.; Alkhayal, K.; Sbaih, M.; Aldeghaither, S.K.; Almugrin, F.F.; Alshammari, S.A.; Alswayyed, M.; Abdullah, M. Appendiceal neoplasms in Saudi Arabia: Prevalence and clinicopathological profile. Ann. Saudi Med. 2024, 44, 255–263. [Google Scholar]
- Connor, S.J.; Hanna, G.B.; Frizelle, F.A. Appendiceal tumors: Retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis. Colon Rectum 1998, 41, 75–80. [Google Scholar] [CrossRef]
- Shaib, W.L.; Assi, R.; Shamseddine, A.; Alese, O.B.; Staley, C., III; Memis, B.; Adsay, V.; Bekaii-Saab, T.; El-Rayes, B.F. Appendiceal Mucinous Neoplasms: Diagnosis and Management. Oncologist 2017, 22, 1107–1116. [Google Scholar] [CrossRef]
- Tajima, T.; Tajiri, T.; Mukai, M.; Sugiyama, T.; Hasegawa, S.; Yamamoto, S.; Sadahiro, S.; Shimada, H.; Makuuchi, H. Single-center analysis of appendiceal neoplasms. Oncol. Lett. 2018, 15, 6393–6399. [Google Scholar] [CrossRef]
- Pereira, A.; Pereira, J.C.; Martins, S. Appendiceal Neoplasms: Diagnosis, Management and Follow-up. SciMed. J. 2021, 3, 274–282. [Google Scholar] [CrossRef]
- Roma, K.; Baldwin, M.; Sedmak, D.; Silva, M.; Stellar, W.; Many, G. Late stage diagnosis of mucinous adenocarcinoma of the appendix: A case report of an unusual tumor with a rare presentation. BMC Gastroenterol. 2020, 20, 281. [Google Scholar] [CrossRef]
- Alamoudi, M.Y.; Alkahtani, N.M.; Aldosari, Y.M.; Marie, S.; Ashmawi, A.A.; Alshaalan, Y.J.; Alabdulrahman, F.K.; Yousef, Z.; Alserhani, M.F. The Rate of Appendicular Neoplasm in Patients Who Underwent Appendectomy for Acute Appendicitis at King Abdulaziz Medical City, Riyadh. Cureus 2022, 14, e31581. [Google Scholar] [CrossRef]
- Teixeira, F.J.R., Jr.; Couto Netto, S.D.D.; Akaishi, E.H.; Utiyama, E.M.; Menegozzo, C.A.M.; Rocha, M.C. Acute appendicitis, inflammatory appendiceal mass and the risk of a hidden malignant tumor: A systematic review of the literature. World J. Emerg. Surg. 2017, 12, 12. [Google Scholar] [CrossRef]
- Carpenter, S.G.; Chapital, A.B.; Merritt, M.V.; Johnson, D.J. Increased risk of neoplasm in appendicitis treated with interval appendectomy: Single-institution experience and literature review. Am. Surg. 2012, 78, 339–343. [Google Scholar] [CrossRef]
- Peltrini, R.; Cantoni, V.; Green, R.; Lionetti, R.; D’Ambra, M.; Bartolini, C.; De Luca, M.; Bracale, U.; Cuocolo, A.; Corcione, F. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: A systematic review and meta-analysis. Surgeon 2021, 19, e549–e558. [Google Scholar] [CrossRef]
- Solis-Pazmino, P.; Oka, K.; La, K.; Termeie, O.; Figueroa, L.A.; Pilatuna, E.; Solis-Pazmino, D.; Harnegie, M.P.; Cohen, J.; Barnajian, M.; et al. Incidence rate and histology of appendiceal neoplasms in complicated versus uncomplicated appendicitis: A meta-analysis and systematic review. Langenbeck’s Arch. Surg. 2023, 408, 432. [Google Scholar] [CrossRef]
- Marmor, S.; Portschy, P.R.; Tuttle, T.M.; Virnig, B.A. The rise in appendiceal cancer incidence: 2000–2009. J. Gastrointest. Surg. 2015, 19, 743–750. [Google Scholar] [CrossRef]
- Singh, H.; Koomson, A.S.; Decker, K.M.; Park, J.; Demers, A.A. Continued increasing incidence of malignant appendiceal tumors in Canada and the United States: A population-based study. Cancer 2020, 126, 2206–2216. [Google Scholar] [CrossRef]
- Issin, G.; Demir, F.; Guvendir Bakkaloglu, I.; Cagatay, D.V.; Aktug Simsek, H.; Yilmaz, I.; Zemheri, E. High Incidence of Appendiceal Neoplasms in the Elderly: A Critical Concern for Non-Surgical Treatment. Med. Princ. Pract. 2023, 32, 358–368. [Google Scholar] [CrossRef]
- Johansson, J.; Andersson, R.E.; Landerholm, K.; Redéen, S. Incidence of Appendiceal Malignancies in Sweden Between 1970 and 2012. World J. Surg. 2020, 44, 4086–4092. [Google Scholar] [CrossRef]
- Orchard, P.; Preece, R.; Thomas, M.G.; Dixon, S.W.; Wong, N.A.C.S.; Chambers, A.C.; Messenger, D.E. Demographic trends in the incidence of malignant appendiceal tumours in England between 1995 and 2016: Population-based analysis. BJS Open 2022, 6, zrac103. [Google Scholar] [CrossRef]
- Chun, H.J.; Park, S.J.; Lim, Y.J.; Song, S.Y. Epidemiology and Prevention. In Gastrointestinal Cancer: A Comprehensive Guide to Diagnosis and Management; Chun, H.J., Park, S.J., Lim, Y.J., Song, S.Y., Eds.; Springer Nature: Singapore, 2023; pp. 179–182. [Google Scholar] [CrossRef]
- Hoehn, R.S.; Rieser, C.J.; Choudry, M.H.; Melnitchouk, N.; Hechtman, J.; Bahary, N. Current Management of Appendiceal Neoplasms. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 1–15. [Google Scholar] [CrossRef]
- SenthilKumar, G.; Kothari, A.N.; Maduekwe, U.N.; Fournier, K.; Abbott, D.E.; Wilson, G.C.; Patel, S.H.; Greer, J.; Johnston, F.; Dineen, S.P.; et al. Validation of the AJCC 8th Edition Staging System for Disseminated Appendiceal Cancer Patients Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Multi-institutional Analysis. Ann. Surg. Oncol. 2023, 30, 5743–5753. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, Z.; Song, Y.; Li, W.; Diao, D.; Dang, C.; Zhang, H. The Management and Prognostic Prediction of Adenocarcinoma of Appendix. Sci. Rep. 2016, 6, 39027. [Google Scholar] [CrossRef]
- Day, R.W.; Chang, Y.H.; Stucky, C.C.; Gray, R.; Pockaj, B.; Wasif, N. A Predictive Model for Nodal Metastases in Patients with Appendiceal Cancers. Ann. Surg. 2021, 274, 155–161. [Google Scholar] [CrossRef]
- Marks, V.A.; Kerekes, D.; Butensky, S.; Ahuja, N.; Johnson, C.; Turaga, K.; Khan, S.A. Role of colectomy in the management of appendiceal tumors: A retrospective cohort study. BMC Gastroenterol. 2023, 23, 398. [Google Scholar] [CrossRef]
- El Asmar, M.L.; Mortagy, M.; Chandrakumaran, K.; Cecil, T.; Ramage, J. Right Hemicolectomy and Appendicectomy as Treatments for Goblet Cell Adenocarcinoma: A Comparative Analysis of Two Large National Databases. Curr. Oncol. 2024, 31, 3855–3869. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.; Wu, C.C.; Nikiforchin, A.; Mingorance, R.A.; Carr, S.C.; Wernberg, J.A.; Sharma, R. Risk factors of a positive resection margin in locoregional appendix cancer and its impact on survival: The national cancer database analysis. Surg. Oncol. Insight 2024, 1, 100072. [Google Scholar] [CrossRef]
- Azzam, A.Y. VINDEL: VINe-Based DEgree-of-Freedom Learning for Synthetic IPD Generation; GitHub: San Francisco, CA, USA, 2025. [Google Scholar]
- Al-Harbi, F.A.; Alsaif, A.K.; Almutairi, A.G.; Alshehri, H.J.; Aleidan, E.A.; Alabdulaaly, G.S.; Alanazi, M.E.; Azzam, A.Y. Synthetic target trial emulation and predictive modeling of amylin-pathway therapies for obesity and type 2 diabetes. Metab. Open 2025, 28, 100414. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.H.; Horesh, N.; Freund, M.R.; Silva-Alvarenga, E.; Garoufalia, Z.; Gefen, R.; Wexner, S.D. Surgical outcomes and predictors of overall survival of stage I–III appendiceal adenocarcinoma: Retrospective cohort analysis of the national cancer database. Surg. Oncol. 2024, 52, 102034. [Google Scholar] [CrossRef]
- Freudenberger, D.C.; Vudatha, V.; Wolfe, L.G.; Riner, A.N.; Herremans, K.M.; Sparkman, B.K.; Fernandez, L.J.; Trevino, J.G. Race and Ethnicity Impacts Overall Survival of Patients with Appendiceal Cancer Who Undergo Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. Cancers 2023, 15, 3990. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ge, H.; Lu, Y.; Gong, X. Incidence trends and survival analysis of appendiceal tumors in the United States: Primarily changes in appendiceal neuroendocrine tumors. PLoS ONE 2023, 18, e0294153. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Chen, W. Chemotherapy in the treatment of different histological types of appendiceal cancers: A SEER based study. BMC Cancer 2021, 21, 778. [Google Scholar] [CrossRef]
- Zheng, M.; Li, T.; Li, Y.; Zhang, T.; Zhang, L.; Ma, W.; Zhou, L. Survival Profile and Prognostic Factors for Appendiceal Mixed Neuroendocrine Non-neuroendocrine Neoplasms: A SEER Population-Based Study. Front. Oncol. 2020, 10, 1660. [Google Scholar] [CrossRef]
- Byrne, R.M.; Gilbert, E.W.; Dewey, E.N.; Herzig, D.O.; Lu, K.C.; Billingsley, K.G.; Deveney, K.E.; Tsikitis, V.L. Who Undergoes Cytoreductive Surgery and Perioperative Intraperitoneal Chemotherapy for Appendiceal Cancer? An Analysis of the National Cancer Database. J. Surg. Res. 2019, 238, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zheng, W.; Luo, H.; Wang, B.; Zhang, X.; Wang, X. Incidence and survival trends for appendiceal mucinous adenocarcinoma: An analysis of 3237 patients in the Surveillance, Epidemiology, and End Results database. Future Oncol. 2019, 15, 3945–3961. [Google Scholar] [CrossRef]
- Shaib, W.L.; Goodman, M.; Chen, Z.; Kim, S.; Brutcher, E.; Bekaii-Saab, T.; El-Rayes, B.F. Incidence and Survival of Appendiceal Mucinous Neoplasms: A SEER Analysis. Am. J. Clin. Oncol. 2017, 40, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Asare, E.A.; Compton, C.C.; Hanna, N.N.; Kosinski, L.A.; Washington, M.K.; Kakar, S.; Weiser, M.R.; Overman, M.J. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: Analysis of the National Cancer Data Base. Cancer 2016, 122, 213–221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ansari, N.; Chandrakumaran, K.; Dayal, S.; Mohamed, F.; Cecil, T.D.; Moran, B.J. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1000 patients with perforated appendiceal epithelial tumours. Eur. J. Surg. Oncol. 2016, 42, 1035–1041. [Google Scholar] [CrossRef]
- Turaga, K.K.; Pappas, S.G.; Gamblin, T.C. Importance of histologic subtype in the staging of appendiceal tumors. Ann. Surg. Oncol. 2012, 19, 1379–1385. [Google Scholar] [CrossRef]
- Smeenk, R.M.; van Velthuysen, M.L.; Verwaal, V.J.; Zoetmulder, F.A. Appendiceal neoplasms and pseudomyxoma peritonei: A population based study. Eur. J. Surg. Oncol. 2008, 34, 196–201. [Google Scholar] [CrossRef]
- McCusker, M.E.; Coté, T.R.; Clegg, L.X.; Sobin, L.H. Primary malignant neoplasms of the appendix: A population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer 2002, 94, 3307–3312. [Google Scholar] [CrossRef]
- Winicki, N.M.; Radomski, S.N.; Ciftci, Y.; Sabit, A.H.; Johnston, F.M.; Greer, J.B. Mortality risk prediction for primary appendiceal cancer. Surgery 2024, 175, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y. Improving appendix cancer prediction with SHAP-based feature engineering for machine learning models: A prediction study. Ewha Med. J. 2025, 48, e31. [Google Scholar] [CrossRef]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef]
- Nitecki, S.S.; Wolff, B.G.; Schlinkert, R.; Sarr, M.G. The Natural History of Surgically Treated Primary Adenocarcinoma of the Appendix. Ann. Surg. 1994, 219, 51–57. [Google Scholar] [CrossRef]
- Ladel, L.; Tan, W.Y.; Jeyakanthan, T.; Sailo, B.; Sharma, A.; Ahuja, N. The Promise of Epigenetics Research in the Treatment of Appendiceal Neoplasms. Cells 2023, 12, 1962. [Google Scholar] [CrossRef]
- Zambrano-Lechuga, M.R.; Galicia-Torres, J.L.; Alvarado-Rueda, Y.D. Mucinous appendiceal neoplasms. Surg. Open Dig. Adv. 2025, 19, 100201. [Google Scholar] [CrossRef]
- Valente, M.A.; Moran, B.J. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. In The ASCRS Textbook of Colon and Rectal Surgery, 4th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 605–617. [Google Scholar] [CrossRef]
- Levine, E.A.; Stewart, I.V.J.H.; Shen, P.; Russell, G.B.; Loggie, B.L.; Votanopoulos, K.I. Intraperitoneal chemotherapy for peritoneal surface malignancy: Experience with 1,000 patients. J. Am. Coll. Surg. 2014, 218, 573–585. [Google Scholar] [CrossRef]
- Skendelas, J.P.; Alemany, V.S.; Au, V.; Rao, D.; McNelis, J.; Kim, P.K. Appendiceal adenocarcinoma found by surgery for acute appendicitis is associated with older age. BMC Surg. 2021, 21, 228. [Google Scholar] [CrossRef]
- Sokale, I.O.; Rosales, O.; Montealegre, J.R.; Oluyomi, A.O.; Thrift, A.P. Trends in Up-To-Date Colorectal Cancer Screening Among U.S. Adults Aged 50–75 Years and Variations by Race/Ethnicity and U.S. Census Bureau Divisions. AJPM Focus 2023, 2, 100055. [Google Scholar] [CrossRef]
- Gómez-Báez, F.D.; Cerdán-Santacruz, C.; Muguiro, N.M.; Collado, L.M.; Resina, M.M.; Foradada, J.A.T.; Grañón, J.E.S.; Kissler, J.J.O. Incidence, Clinicopathological Features and Oncologic Outcome of Appendiceal Neoplasms: A Single-Center Cohort Study. Gastrointest. Disord. 2023, 5, 455–463. [Google Scholar] [CrossRef]
- Voigtländer, S.; Hakimhashemi, A.; Grundmann, N.; Rees, F.; Meyer, M.; Algül, H.; Müller-Nordhorn, J. Trends of colorectal cancer incidence according to age, anatomic site, and histological subgroup in Bavaria: A registry-based study. Front. Oncol. 2022, 12, 904546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halfter, K.; Schubert-Fritschle, G.; Klauschen, F.; Werner, J.; Mayerle, J.; Weichert, W.; Friess, H.; Schmid, R.; Kremer, M.; Ruppert, R.; et al. The other colon cancer: A population-based cohort study of appendix tumour trends and prognosis. Color. Dis. 2023, 25, 943–953. [Google Scholar] [CrossRef]


| Study Name | Database Source | Study Period | Sample Size | Institution Type | Median Age (Years) | Male (%) | Histology Distribution | Stage Distribution | Treatment Patterns | Quality Metrics | Survival Outcomes | Overlap Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| El Asmar et al., 2024 [24] | NCRAS; SEER | 1995–2020 | 2701 | Population-based | 58.7 (UK); 58.0 (US) | 49.5 (UK); 52.0 (US) | GCA: 100% | Local: 71.6% (UK), 54.8% (US); Regional: 7.7% (UK), 8.5% (US); Distant: 5.7% (UK), 35.7% (US) | RHC: 71% (UK), 53% (US); Appendectomy: 29% (UK), 47% (US); Chemotherapy: 15.8% (US) | NR | 5-year OS: 73.8% (UK), 79.6% (US) | LOW |
| Baron et al., 2024 [25] | NCDB | 2004–2019 | 6800 | Academic/Community/Integrated | 61 | 52.8% (RM−); 50.1% (RM+) | MAC: 42.2%; NMAC: 50.4%; SRCC: 7.4% | Stage I–II: 78.2%; Stage III: 21.8% | RHC: 71.5% (RM−), 59.7% (RM+); Appendectomy: 28.5%; Chemotherapy: 37.3% | 30-day mortality: 1.1% (RM−), 3.7% (RM+); 90-day mortality: 1.7% (RM−), 6.9% (RM+); Readmission: 4.2% (RM−), 5.6% (RM+); LOS: 5–6 days | Median OS: 54.0 months (RM+) | HIGH |
| Emile et al., 2024 [28] | NCDB | 2005–2019 | 2607 | NR | 61.6 | 51.6% | MAC: 46%; NMAC: 45.3%; SRCC: 8.7% | Stage I–II: 85%; Stage III: 15% | RHC: 61.7%; Adjuvant chemotherapy: 39.4% | 30-day mortality: 1.8–2.8%; 90-day mortality: 3.3–4.2%; Readmission: 4–5%; LOS: 5 days; LVI: 21.1%; Positive margins: 13.8% | Median OS: 126.3 months; 5-year OS: 58.4% | HIGH |
| Freudenberger et al., 2023 [29] | NCDB | 2006–2018 | 2532 | Academic/Community/Integrated | 57 (NHW); 55 (NHB) | 47.6% (NHW); 38.2% (NHB) | NR | NR | HIPEC: 100% | 30-day mortality: 0.9% (NHW), 2.2% (NHB); 90-day mortality: 2.8% (NHW), 2.7% (NHB); Readmission: 6.2% (NHW), 7.5% (NHB); LOS: 9 days; Positive margins: 9.3% | Median OS: 136.3 months (NHW), 106.7 months (NHB) | HIGH |
| Marks et al., 2023 [23] | NCDB | 2004–2017 | 18,216 | Academic/community/integrated | NR | NR | MAC: 34%; NMAC: 24%; GCA: 11%; NET: 31% | NR | RHC: 60%; Appendectomy: 40% | 30-day mortality: 0.9–1.4%; 90-day mortality: 1.5–3.2%; Readmission: 3–5%; LOS: 3–5 days; Positive margins: 8.7% | NR | MEDIUM |
| Wang et al., 2023 [30] | SEER | 2004–2015 | 2891 | Population-based | 62 | 45.9% | MAC: 25.6%; NMAC: 21.4%; SRCC: 5.6%; NET: 47.3% | Localized: 45.7%; Regional: 29.9%; Distant: 24.4% | Chemotherapy: 30.5% | NR | Median OS: 65 months (chemotherapy group); 5-year OS: 51.9% (chemotherapy group) | MEDIUM |
| Wang et al., 2021 [31] | SEER | 1998–2016 | 8733 | Population-based | 57 | 45.2% | MAC: 32.4%; NMAC: 20.2%; SRCC: 6.6%; GCA: 12.5%; NET: 23.9% | NR | RHC: 50.5%; Appendectomy: 44.1%; Chemotherapy: 31.8% | NR | 5-year OS: 65.8% (MAC), 56.2% (NMAC), 48.2% (SRCC) | MEDIUM |
| Zheng et al., 2020 [32] | SEER | 2004–2016 | 315 | Population-based | 57 | 50.2% | MiNEN: 100% | Localized: 27.6%; Regional: 38.7%; Distant: 33.7% | RHC: 62.2%; Appendectomy: 32.7% | NR | 5-year OS: 57.4% | LOW |
| Byrne et al., 2019 [33] | NCDB | 2004–2014 | 18,055 | Academic/community/integrated | 54.6 (CRS/HIPEC group) | 48% (CRS/HIPEC) | MAC: 81.8%; NMAC: 18.2%; NET: 7.0% | Stage I–II: 14.8%; Stage III: 2.9%; Stage IV: 69.1% | HIPEC: 7.71% | NR | 5-year OS: 65.6% (mucinous CRS/HIPEC) | HIGH |
| Yan et al., 2019 [34] | SEER | 1973–2015 | 3237 | Population-based | 57.7 | 43.7% | MAC: 100% | Stage I–II: 13.5%; Stage III–IV: 46.4% | RHC: 36%; Appendectomy: 25.2%; Chemotherapy: 47% | NR | Median OS: 80 months; 5-year OS: 56.2% | MEDIUM |
| Shaib et al., 2017 [35] | SEER | 1973–2011 | 2733 | Population-based | 59.6 | 45.4% | MAC: 100% | Localized: 26.3%; Regional: 20.5%; Distant: 53.2% | RHC: 70.6%; Appendectomy: 2.2% | NR | Median OS: 42 months (distant stage) | MEDIUM |
| Xie et al., 2016 [21] | SEER | 2004–2013 | 1404 | Population-based | 61.3 | 50.5% | MAC: 47.8%; NMAC: 51.3% | NR | RHC: 59.6%; Total colectomy: 6.8% | NR | 5-year OS: 64% | LOW |
| Asare et al., 2016 [36] | NCDB | 1985–2006 | 11,871 | Population-based | 57.9 (MUC); 62.5 (NON-MUC) | 46.1% (MUC); 54.6% (NON-MUC) | MAC: 50.3%; NMAC: 40.5%; SRCC: 9.2% | Stage I–II: 39.1% (MUC), 56.3% (NON-MUC); Stage III: 8.9% (MUC), 17.6% (NON-MUC); Stage IV: 52% (MUC), 26.2% (NON-MUC) | Chemotherapy: 51.8% (MUC), 39.8% (NON-MUC) | NR | Median OS: 6.4 years (well-differentiated MUC), 2.3 years (well-differentiated NMAC); 5-year OS: 53.6% (MUC), 46.2% (NON-MUC) | MEDIUM |
| Ansari et al., 2016 [37] | Institutional | 1994–2014 | 1000 | Single center (tertiary) | 56 (CCRS); 60 (MTD) | 34% (CCRS); 48.8% (MTD) | NR | NR | HIPEC: 100% | 30-day mortality: 0.8% (CCRS), 1.7% (MTD); LOS: 19 days (CCRS), 17 days (MTD); CC0/CC1: 73.8% | Median OS: 103.4 months (CCRS); 5-year OS: 87.4% (CCRS), 39.2% (MTD) | NONE |
| Marmor et al., 2015 [13] | SEER | 2000–2009 | 4765 | Population-based | 58 | 48.4% | MAC: 38%; NMAC: 27%; SRCC: 7%; NET: 28% | Localized: 26%; Regional: 39%; Distant: 35% | NR | NR | 5-year OS: 77% (localized), 60% (regional), 33% (distant) | HIGH |
| Turaga et al., 2012 [38] | SEER | 1973–2007 | 5655 | Population-based | 46 | 47% | MAC: 37%; NMAC: 27%; SRCC: 5.5%; GCA: 19%; NET: 11% | NR | RHC: 39%; Partial colectomy: 32% | NR | Median OS: 85 months; 5-year OS: 93% (carcinoid), 81% (GCC), 55% (colonic-type), 58% (MAC), 27% (SRCC) | HIGH |
| Smeenk et al., 2008 [39] | PALGA | 1995–2005 | 1482 | Population-based | 61 (M); 64 (F) | 41% | MAC: 38.7%; NMAC: 30.6% | NR | NR | NR | NR | NONE |
| McCusker et al., 2002 [40] | SEER | 1973–1998 | 1645 | Population-based | 60 (MAC) | 49% (MAC); 60% (colonic-type) | MAC: 37.3%; NMAC: 25%; SRCC: 4.3%; GCA: 13.8%; NET: 19.7% | Local/Regional: 37% (MAC); Distant: 63% (MAC) | RHC: 52% (MAC); Less than hemicolectomy: 38% (MAC) | Positive lymph nodes: 26% (MAC) | NR | HIGH |
| Component | Category/Metric | Characteristics/Description | Sample Size (Number) | Studies (Number) | 5-Year OS (%) | 30-Day Mortality (%) | 90-Day Mortality (%) | C-index | 95% CI | Performance Metrics | Validation Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ISK STRATIFICATION | Very Low Risk | NET/Carcinoid dominant (>40%), Localized disease, Age <55, Academic centers | 3891 | 2 | 88.7 | 0.8 | 1.2 | 0.891 | 0.864–0.918 | Sensitivity: 91.3%; Specificity: 94.7% | Calibration: Excellent |
| Low Risk | GCA histology (>50%), Early stage (I–II > 75%), Negative margins, Standard surgery | 2701 | 1 | 76.7 | 0.9 | 1.4 | 0.834 | 0.798–0.870 | Sensitivity: 85.2%; Specificity: 89.6% | Calibration: Good | |
| Intermediate Risk | MAC/NMAC balanced, Mixed staging, Standard protocols, Community hospitals | 27,186 | 6 | 61.4 | 1.3 | 2.1 | 0.758 | 0.731–0.785 | Sensitivity: 76.8%; Specificity: 82.1% | Calibration: Good | |
| High Risk | SRCC presence (>5%), Advanced stage (IV >30%), Positive margins, Complex surgery | 31,150 | 8 | 45.8 | 2.1 | 3.7 | 0.782 | 0.755–0.809 | Sensitivity: 83.4%; Specificity: 87.9% | Calibration: Acceptable | |
| Very High Risk | Multiple adverse factors, Stage IV >60%, High mortality (>3%), HIPEC complexity | 2500 | 1 | 27.3 | 3.8 | 6.2 | 0.912 | 0.887–0.937 | Sensitivity: 94.1%; Specificity: 91.8% | Calibration: Excellent | |
| MODEL COMPARISON | TNM Staging Alone | Traditional AJCC staging system (baseline reference) | 67,428 | 18 | 58.9 | 2.1 | 3.5 | 0.689 | 0.654–0.724 | Limited discrimination | Baseline comparator |
| MultRi-Dimensional Model | Age + Histology + Stage + Treatment + Institution factors | 67,428 | 18 | 62.4 | 1.8 | 3.2 | 0.758 | 0.731–0.785 | Δ C-index: +0.069 (p < 0.001) | Clinically meaningful | |
| Enhanced Risk Model | Added quality metrics + LVI + margins + HIPEC complexity | 67,428 | 18 | 64.2 | 1.6 | 2.8 | 0.782 | 0.758–0.806 | NRI: 18.7%; IDI: 6.4% | Superior performance | |
| VALIDATION METRICS | Discrimination (AUC-ROC) | Area under receiver operating characteristic curve | 67,428 | 18 | - | - | - | 0.774 | 0.748–0.800 | Bootstrap: 1000 iterations | 5-fold CV: 0.769 |
| Calibration (Hosmer–Lemeshow) | Goodness-of-fit test for predicted vs. observed outcomes | 67,428 | 18 | - | - | - | - | p = 0.347 | χ2 = 8.94 | Well calibrated | |
| Calibration Slope | Slope of calibration plot (perfect = 1.0) | 67,428 | 18 | - | - | - | - | 0.84–0.98 | Slope: 0.91 | Near-perfect calibration | |
| Brier Score | Overall prediction accuracy (lower = better) | 67,428 | 18 | - | - | - | - | 0.174–0.200 | Score: 0.187 | Excellent prediction | |
| Net Benefit (Clinical Utility) | Decision curve analysis for clinical usefulness | 67,428 | 18 | - | - | - | - | 0.051–0.127 | Threshold: 15–60% | Superior to defaults | |
| Cross-Validation (LOOCV) | Leave-one-out cross-validation performance | 67,428 | 18 | - | - | - | 0.742 | 0.715–0.769 | Significant validation | Consistent performance | |
| Net Reclassification Index | Improvement in risk classification accuracy | 67,428 | 18 | - | - | - | - | 12.3–25.1% | NRI: 18.7% | Clinically significant | |
| Integrated Discrimination | Enhanced separation of risk groups | 67,428 | 18 | - | - | - | - | 4.1–8.7% | IDI: 6.4% | Meaningful advancement | |
| SENSITIVITY ANALYSIS | Complete Case Analysis | Exclude studies with >30% missing data | 45,231 | 14 | - | - | - | 0.745 | 0.718–0.772 | Significant to missing data | Stable performance |
| High-Quality Studies | Studies with detailed quality metrics available | 30,155 | 5 | - | - | - | 0.791 | 0.756–0.826 | Improved performance | Quality data benefit | |
| Modern Era Studies | Studies after 2010 (contemporary practice patterns) | 41,892 | 8 | - | - | - | 0.773 | 0.742–0.804 | Modern applicability | Current practice relevant | |
| Large Cohort Studies | Sample size > 2000 patients per study | 58,724 | 6 | - | - | - | 0.752 | 0.721–0.783 | Large cohort stability | Significant in large samples | |
| Population-Based Studies | SEER registry studies for generalizability | 35,172 | 10 | - | - | - | 0.741 | 0.708–0.774 | Population representativeness | Generalizable results | |
| Hospital-Based Studies | NCDB studies reflecting treatment variations | 32,256 | 6 | - | - | - | 0.768 | 0.735–0.801 | Treatment center variation | Practice diversity |
| Study Name | Sample Size | Histological Subtype | Hazard Ratio (95% CI) | Observed 5-Year OS (%) | Predicted 5-Year OS (%) | Prediction Error (%) | Model Performance Metrics |
|---|---|---|---|---|---|---|---|
| El Asmar et al., 2024 [24] | 2701 | GCA | 0.383 (0.355–0.413) | 76.7 | 59.8 | 16.9 | C-index: 0.714 |
| Emile et al., 2024 [28] | 2607 | Mixed Adenocarcinoma | 0.776 (0.719–0.838) | 58.4 | 54.3 | 4.1 | MAE: 15.28% |
| Wang et al., 2023 [30] | 2891 | Mixed Histologies | 0.946 (0.880–1.018) | 51.9 | 44.3 | 7.6 | RMSE: 17.09% |
| Wang et al., 2021 [31] | 8733 | Mixed Histologies | 0.819 (0.785–0.854) | 56.7 | 46.9 | 9.8 | Correlation: 0.557 |
| Zheng et al., 2020 [32] | 315 | MiNEN | 0.801 (0.642–0.999) | 57.4 | 36.3 | 21.1 | Cross-validation MAE: 18.2% |
| Byrne et al., 2019 [33] | 18,055 | Mixed (CRS/HIPEC) | 0.608 (0.591–0.626) | 65.6 | 38.8 | 26.8 | 95% CI coverage: 92% |
| Yan et al., 2019 [34] | 3237 | MAC | 0.831 (0.776–0.891) | 56.2 | 35.6 | 20.6 | Calibration slope: 0.89 |
| HIERARCHICAL BAYESIAN POOLED RESULTS BY HISTOLOGY: | |||||||
| GCA Subtype | 2701 | Goblet Cell Adenocarcinoma | 0.383 (0.355–0.413) | 76.7 | 59.8 | 16.9 | Single study analysis |
| Mixed Histologies | 32,286 | Combined Adenocarcinomas | 0.748 (0.591–0.948) | 58.1 | 46.1 | 12.1 | Pooled from 4 studies |
| MiNEN Subtype | 315 | Mixed Neuroendocrine | 0.801 (0.642–0.999) | 57.4 | 36.3 | 21.1 | Specialized histology |
| MAC Subtype | 3237 | Mucinous Adenocarcinoma | 0.831 (0.776–0.891) | 56.2 | 35.6 | 20.6 | Pure mucinous type |
| OVERALL MODEL VALIDATION AND PERFORMANCE: | |||||||
| All Studies Combined | 38,539 | All Histological Types | 0.723 (0.591–0.884) | 61.3 | 46.0 | 15.3 | Overall C-index: 0.714 |
| Cross-Validation | 38,539 | Leave-One-Out CV | — | 61.3 | 46.5 | 18.2 | CV MAE: 18.2% |
| Calibration Assessment | 38,539 | Calibration Analysis | — | 61.3 | 46.0 | 15.3 | Slope: 0.89, Intercept: 0.12 |
| Discrimination Analysis | 38,539 | ROC Evaluation | — | — | — | — | AUC: 0.742 (0.698–0.786) |
| Clinical Concordance | 38,539 | Temporal Validation | — | — | — | — | 1-year: 0.756, 3-year: 0.721, 5-year: 0.714 |
| Scenario | Patient Population (Study) | Treatment Comparison | Hazard Ratio (95% CI) | Treatment Effect | Quality Metrics Impact | Survival Benefit | NNT/NNH | Evidence Grade | Recommendation | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Stage I–III adenocarcinoma with positive margins | 6800 patients (Baron et al., 2024) [25] | Complete (R0) vs. Incomplete (R1/R2) resection | 0.30 (0.25–0.37) | 70% reduction in 30-day mortality with negative margins | 30-day mortality: 1.1% (R0) vs. 3.7% (R1/R2); 90-day mortality: 1.7% vs. 6.9%; Readmission: 4.2% vs. 5.6% | Median OS: 54.0 months (R1/R2 group) | NNT: 38 patients | A (High quality) | Achieve R0 resection regardless of surgical approach; completion surgery if margins positive | <0.001 |
| Stage I–III adenocarcinoma surgical approach | 2607 patients (Emile et al., 2024) [28] | Right hemicolectomy vs. partial colectomy | 0.64 (0.52–0.79) | 36% reduction in 30-day mortality with hemicolectomy | 30-day mortality: 1.8% (hemi) vs. 2.8% (partial); 90-day mortality: 3.3% vs. 4.2%; LOS: 5 days both; LVI: 21.1%; Positive margins: 13.8% | Median OS: 126.3 months; 5-year OS: 58.4% | NNT: 100 patients | A (High quality) | Prefer hemicolectomy for stage II–III disease when technically feasible | <0.001 |
| All histological subtypes by surgical extent | 18,216 patients (Marks et al., 2023) [23] | Right hemicolectomy vs. appendectomy | 1.56 (1.12–2.17) | 56% increase in 30-day mortality with RHC, but justified for advanced disease | 30-day mortality: 1.4% (RHC) vs. 0.9% (appendectomy); 90-day mortality: 3.2% vs. 1.5%; Readmission: 5% vs. 3%; LOS: 5 vs. 3 days | Stage-dependent; RHC necessary for node-positive disease | NNH: 200 patients | A (Very High quality) | Appendectomy for early-stage, RHC for advanced disease with nodal involvement | 0.008 |
| Advanced peritoneal disease by race | 2532 patients (Freudenberger et al., 2023) [29] | CRS/HIPEC outcomes: NHW vs. NHB | 1.28 (1.15–1.43) | 28% survival disadvantage in NHB patients | 30-day mortality: 0.9% (NHW) vs. 2.2% (NHB); 90-day mortality: 2.8% vs. 2.7%; Readmission: 6.2% vs. 7.5%; LOS: 9 days both | Median OS: 136.3 months (NHW) vs. 106.7 months (NHB) | Disparity measure: 29.6 months survival gap | A (High quality) | Address racial disparities in patient selection and perioperative care | <0.001 |
| Stage IV mucinous adenocarcinoma with peritoneal disease | 18,055 patients (Byrne et al., 2019) [33]; 1000 patients (Ansari et al., 2016) [37] | CRS/HIPEC vs. standard care | 0.13 (0.10–0.17) | 87% reduction in mortality with complete cytoreduction | 30-day mortality: 0.8% (CCRS) vs. 1.7% (MTD); LOS: 19 vs. 17 days; CC0/CC1 resection: 73.8% | 5-year OS: 87.4% (CCRS) vs. 39.2% (MTD); Byrne cohort 5-year OS: 65.6% | NNT: 2 patients | A (High quality) | Refer eligible Stage IV mucinous patients to specialized centers for CRS/HIPEC evaluation | <0.001 |
| Chemotherapy effectiveness by histology | 8733 patients (Wang et al., 2021) [31] | Chemotherapy benefit: MAC vs. NMAC vs. SRCC | MAC: 0.85 (0.76–0.95); NMAC: 0.92 (0.84–1.01); SRCC: 1.15 (0.98–1.35) | Histology-dependent chemotherapy effectiveness (MAC > NMAC > SRCC) | Selection bias evident in overall chemotherapy studies | 5-year OS with chemotherapy: MAC 65.8%, NMAC 56.2%, SRCC 48.2% | NNT: MAC 10 patients; NMAC 26 patients; SRCC no benefit | B (High quality for MAC, moderate for others) | Prioritize chemotherapy for MAC, consider for NMAC, limited benefit in SRCC | MAC: 0.003; NMAC: 0.08; SRCC: 0.12 |
| Goblet cell adenocarcinoma | 2701 patients (El Asmar et al., 2024) [24] | Surgical approach for GCA | 0.75 (0.65–0.87) | Favorable prognosis with appropriate surgical management | Geographic variation: UK vs. US outcomes | 5-year OS: 73.8% (UK) vs. 79.6% (US) | Generally favorable outcomes | B (Moderate quality) | Right hemicolectomy with lymph node dissection regardless of size | <0.05 |
| Well-differentiated neuroendocrine tumors | 5655 patients (Turaga et al., 2012) [38]; 2891 patients (Wang et al., 2023) [30] | Size-based surgical approach | 0.25 (0.18–0.35) | Excellent prognosis with appropriate surgery | Low operative mortality for appendectomy approach | 5-year OS: 93% (well-differentiated carcinoid) | Favorable histology—minimize morbidity | B (Moderate quality) | Appendectomy for <2 cm, right hemicolectomy for ≥2 cm or poorly differentiated | <0.001 |
| Signet ring cell carcinoma | Multiple studies (Wang et al., 2021; Turaga et al., 2012) [31,38] | Aggressive vs. conservative surgical approach | 1.85 (1.45–2.36) | Poor prognosis despite aggressive treatment | High mortality regardless of approach | 5-year OS: 27–48.2% across studies | Consider experimental approaches | C (Low quality—rare subtype) | Aggressive surgical resection, consider clinical trials and experimental therapy | <0.001 |
| Elderly patients (>70 years) with comorbidities | Subset analysis from Marks et al., 2023 [23] | Extensive vs. limited surgical approach | 2.15 (1.75–2.64) | Higher baseline mortality with extensive surgery | Comorbidity-adjusted mortality rates significantly higher | Individualized based on functional status and life expectancy | Risk-benefit individualization needed | C (Expert consensus) | Individualized approach prioritizing quality of life and functional status | <0.01 |
| Study Name | Database | Sample Size | Patient Population | 30-Day Mortality | 90-Day Mortality | Readmission Rate | Length of Stay | Quality Predictors | Risk Stratification | Model Performance | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baron et al., 2024 [25] | NCDB | 6800 | Stage I–III appendiceal adenocarcinoma | RM−: 1.1%; RM+: 3.7% | RM−: 1.7%; RM+: 6.9% | RM−: 4.2%; RM+: 5.6% | RM−: 5 days; RM+: 6 days | Resection margin status (OR: 3.4 for 30-day mortality) | Low risk (RM−): 1.1–1.7%; High risk (RM+): 3.7–6.9% | Margin status most discriminative predictor | Positive margins increase 30-day mortality 3.4×, 90-day mortality 4.1× |
| Emile et al., 2024 [28] | NCDB | 2607 | Stage I–III appendiceal adenocarcinoma | Hemicolectomy: 1.8%; Partial: 2.8% | Hemicolectomy: 3.3%; Partial: 4.2% | Hemicolectomy: 5%; Partial: 4% | 5 days (both procedures) | Surgical approach (OR: 1.6), LVI status (21.1% prevalence) | Low risk (Hemi): 1.8–3.3%; High risk (Partial): 2.8–4.2% | Surgical approach primary predictor | Hemicolectomy reduces 30-day mortality 1.6×, 90-day mortality 1.3× vs. partial colectomy |
| Freudenberger et al., 2023 [29] | NCDB | 2532 | CRS/HIPEC patients | NHW: 0.9%; NHB: 2.2% | NHW: 2.8%; NHB: 2.7% | NHW: 6.2%; NHB: 7.5% | 9 days (both groups) | Race/ethnicity (OR: 2.4 for NHB), positive margins: 9.3% | Low risk (NHW): 0.9–2.8%; High risk (NHB): 2.2–7.5% | Race strongest predictor in HIPEC patients | NHB patients have 2.4× higher 30-day mortality, 1.2× higher readmissions |
| Marks et al., 2023 [23] | NCDB | 18,216 | All appendiceal neoplasm types | Appendectomy: 0.9%; RHC: 1.4% | Appendectomy: 1.5%; RHC: 3.2% | Appendectomy: 3%; RHC: 5% | Appendectomy: 3 days; RHC: 5 days | Surgical extent (OR: 1.6 for RHC vs. appendectomy) | Low risk (Appendectomy): 0.9–3%; High risk (RHC): 1.4–5% | Surgical complexity strongest predictor | RHC increases 30-day mortality 1.6×, 90-day mortality 2.1×, readmissions 1.7× |
| Ansari et al., 2016 [37] | Institutional | 1000 | CRS/HIPEC patients | CCRS: 0.8%; MTD: 1.7% | NR | NR | CCRS: 19 days; MTD: 17 days | Completeness of cytoreduction (CC0/CC1: 73.8%) | Low risk (CCRS): 0.8%; High risk (MTD): 1.7% | Complete cytoreduction primary predictor | Complete cytoreduction achieves lowest mortality rates despite complex procedures |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnajjar, J.S.; Al-Harbi, F.A.; Alsaif, A.K.; Alabdulaaly, G.S.; Aljubaili, O.K.; Alquaimi, M.; Alrasheed, A.F.; AlAli, M.N.; Alghamdi, M.A.; Azzam, A.Y. Advanced Computational Modeling and Machine Learning for Risk Stratification, Treatment Optimization, and Prognostic Forecasting in Appendiceal Neoplasms. Healthcare 2025, 13, 3074. https://doi.org/10.3390/healthcare13233074
Alnajjar JS, Al-Harbi FA, Alsaif AK, Alabdulaaly GS, Aljubaili OK, Alquaimi M, Alrasheed AF, AlAli MN, Alghamdi MA, Azzam AY. Advanced Computational Modeling and Machine Learning for Risk Stratification, Treatment Optimization, and Prognostic Forecasting in Appendiceal Neoplasms. Healthcare. 2025; 13(23):3074. https://doi.org/10.3390/healthcare13233074
Chicago/Turabian StyleAlnajjar, Jawad S., Faisal A. Al-Harbi, Ahmed Khalifah Alsaif, Ghaida S. Alabdulaaly, Omar K. Aljubaili, Manal Alquaimi, Arwa F. Alrasheed, Mohammed N. AlAli, Maha A. Alghamdi, and Ahmed Y. Azzam. 2025. "Advanced Computational Modeling and Machine Learning for Risk Stratification, Treatment Optimization, and Prognostic Forecasting in Appendiceal Neoplasms" Healthcare 13, no. 23: 3074. https://doi.org/10.3390/healthcare13233074
APA StyleAlnajjar, J. S., Al-Harbi, F. A., Alsaif, A. K., Alabdulaaly, G. S., Aljubaili, O. K., Alquaimi, M., Alrasheed, A. F., AlAli, M. N., Alghamdi, M. A., & Azzam, A. Y. (2025). Advanced Computational Modeling and Machine Learning for Risk Stratification, Treatment Optimization, and Prognostic Forecasting in Appendiceal Neoplasms. Healthcare, 13(23), 3074. https://doi.org/10.3390/healthcare13233074






