Effectiveness of Exercise and Physiotherapy in Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- Randomised controlled trials involving adult patients receiving neurotoxic chemotherapy (taxanes, platinum agents, vinca alkaloids, or proteasome inhibitors).
- Interventions consisting of exercise or physiotherapy, compared with usual care or control.
- Outcomes including CIPN incidence or severity, neuropathic pain, motor and sensory function, balance, muscle strength, or quality of life.
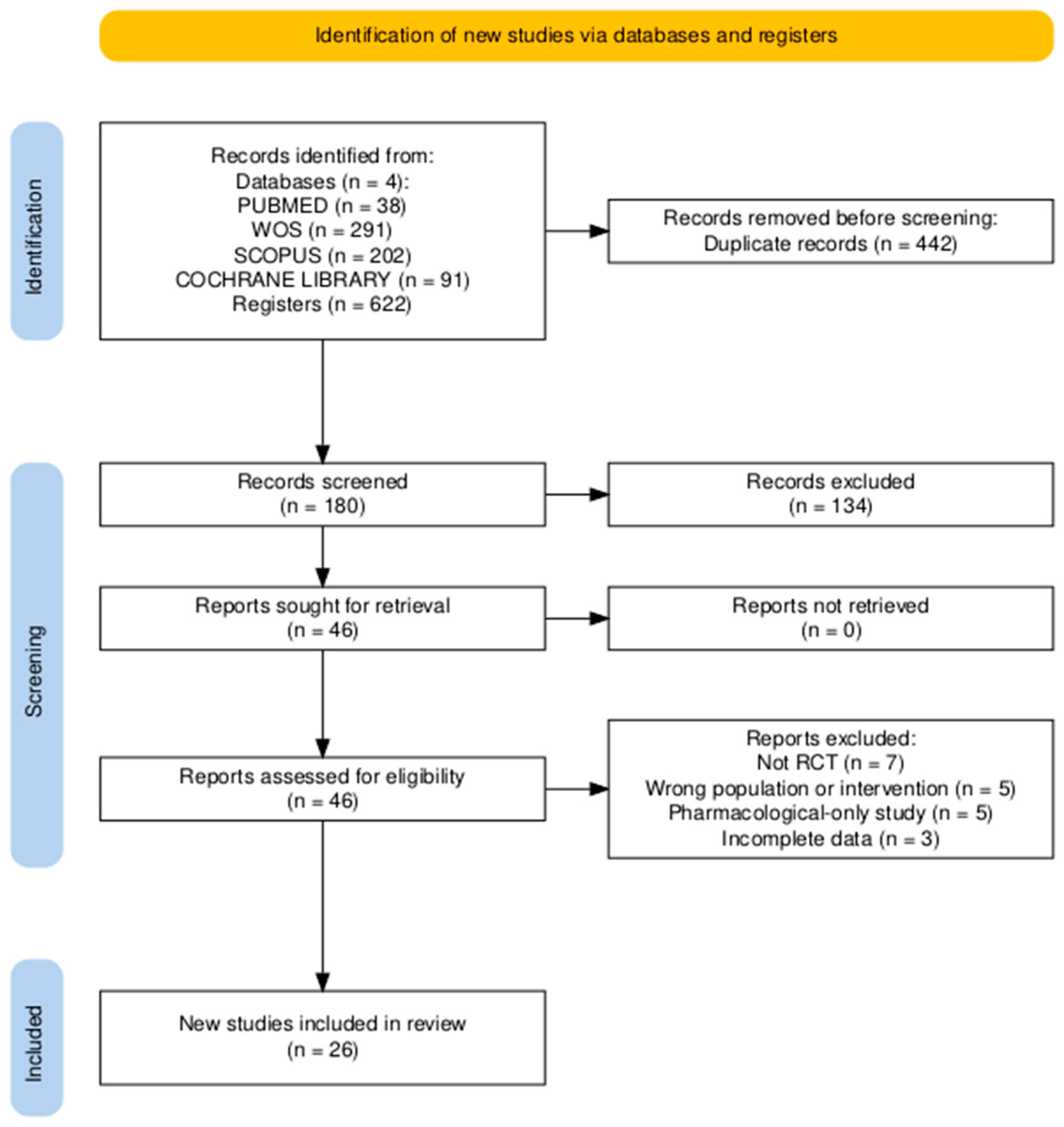
2.3. Selection Process and Data Collection
2.4. Methodological Quality
3. Results
3.1. General Characteristics of the Included Studies
3.2. Exercise-Based Interventions
3.3. Physiotherapy-Based Interventions
3.4. Risk of Bias Assessment
3.5. Synthesis of Results
3.6. Reporting Biases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6MWT | Six-Minute Walk Test |
| ADL-MHQ | Activities of Daily Living—Michigan Hand Outcomes Questionnaire |
| ASCO | American Society of Clinical Oncology |
| BDNF | Brain-Derived Neurotrophic Factor |
| BFI | Brief Fatigue Inventory |
| BOD POD | Air displacement plethysmography system for body composition analysis |
| BR23 | EORTC Quality of Life Questionnaire–Breast Cancer Module |
| CIPN | Chemotherapy-Induced Peripheral Neuropathy |
| CIPNAT | Chemotherapy-Induced Peripheral Neuropathy Assessment Tool |
| CMJ | Countermovement Jump |
| COP | Centre of Pressure |
| CPET | Cardiopulmonary Exercise Test |
| CTCAE/NCI-CTCAE | Common Terminology Criteria for Adverse Events (National Cancer Institute) |
| DASH | Disabilities of the Arm, Shoulder and Hand questionnaire |
| EORTC QLQ-C30 | European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 |
| EORTC QLQ-CIPN20/CIPN15/Taxane/CR29 | EORTC Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy Module (20-, 15-item, Taxane-specific or Colorectal Cancer versions) |
| FAB | Fullerton Advanced Balance scale |
| FACT/GOG-Ntx/FACT/GOG-Taxane | Functional Assessment of Cancer Therapy/Gynaecologic Oncology Group–Neurotoxicity (or Taxane) subscale |
| FES-I | Falls Efficacy Scale–International |
| GGT-Reha | German Geriatric Rehabilitation Test battery |
| h1RM | Half of the one-repetition maximum strength test (50% 1RM) |
| HADS | Hospital Anxiety and Depression Scale |
| HIIT | High-Intensity Interval Training |
| HTEMS | High-Tone External Muscle Stimulation |
| ISI | Insomnia Severity Index |
| LANSS/S-LANSS | Leeds Assessment of Neuropathic Symptoms and Signs (Self-Reported version) |
| MFI-20 | Multidimensional Fatigue Inventory (20-item) |
| MHQ | Michigan Hand Outcomes Questionnaire |
| MICE | Moderate-Intensity Continuous Exercise |
| MDNS | Modified Diabetic Neuropathy Score |
| MLS | Multiwave Locked System (Class IV therapeutic laser) |
| mTNS/TNS/TNSr | (Modified/Revised) Total Neuropathy Score |
| NAS | Numeric Analogue Scale (pain, tingling, numbness) |
| NCV/NCS | Nerve Conduction Velocity/Nerve Conduction Studies |
| NCI-CTC 4.0/5.0 | National Cancer Institute Common Toxicity Criteria (version 4.0/5.0) |
| NPRS/NRS | Numeric Pain Rating Scale/Numeric Rating Scale |
| NTSS-6 | Neuropathy Total Symptom Score–6 |
| PCS | Pain Catastrophising Scale |
| PBM | Photobiomodulation |
| PEDro | Physiotherapy Evidence Database |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROMIS Physical Function | Patient-Reported Outcomes Measurement Information System–Physical Function scale |
| QoL | Quality of Life |
| QST | Quantitative Sensory Testing |
| RCT | Randomised Controlled Trial |
| RDI | Rate of Decline Index |
| RT | Resistance Training |
| Rydel-Seiffer tuning fork | Graduated tuning fork used for vibration sensitivity testing |
| SPPB | Short Physical Performance Battery |
| SMT | Sensorimotor Training |
| SWMT | Semmes–Weinstein Monofilament Test |
| TENS | Transcutaneous Electrical Nerve Stimulation |
| TES | Treatment Expectancy Scale |
| UENS | Utah Early Neuropathy Scale |
| VAS | Visual Analogue Scale |
| WBV | Whole-Body Vibration |
References
- Windebank, A.J.; Grisold, W. Chemotherapy-induced neuropathy. J. Peripher. Nerv. Syst. 2008, 13, 27–46. [Google Scholar] [CrossRef]
- Shah, A.; Hoffman, E.M.; Mauermann, M.L.; Loprinzi, C.L.; Windebank, A.J.; Klein, C.J.; Staff, N.P. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J. Neurol. Neurosurg. Psychiatry 2018, 89, 636–641. [Google Scholar] [CrossRef]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2016, 32, 1941–1967. [Google Scholar] [CrossRef]
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Curr. Opin. Neurol. 2015, 28, 500–507. [Google Scholar] [CrossRef]
- Mols, F.; Beijers, T.; Vreugdenhil, G.; van de Poll-Franse, L.V. Chemotherapy-induced peripheral neuropathy and its association with quality of life: A systematic review. Support. Care Cancer 2014, 22, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: A comprehensive literature review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef]
- Lemanne, D.; Cassileth, B.; Gubili, J. The role of physical activity in cancer prevention, treatment, recovery, and survivorship. Oncology 2013, 27, 580–585. [Google Scholar]
- Andersen Hammond, E.; Pitz, M.; Steinfeld, K.; Lambert, P.; Shay, B. An exploratory randomised trial of physical therapy for the treatment of chemotherapy-induced peripheral neuropathy. Neurorehabil. Neural Repair. 2020, 34, 235–246. [Google Scholar] [CrossRef]
- Joy, L.; Jolien, R.; Marithé, C.; Stijn, E.; Laura, S.; Hilde, L.; Sandra, B.; Wendy, N.; Ruth, H.; Liesbeth, R.; et al. The use of photobiomodulation therapy for the prevention of chemotherapy-induced peripheral neuropathy: A randomized, placebo-controlled pilot trial (NEUROLASER trial). Support. Care Cancer 2022, 30, 5509–5517. [Google Scholar] [CrossRef]
- Sassmann, R.; Gampenrieder, S.P.; Rieder, F.; Johansson, T.; Rinnerthaler, G.; Castagnaviz, V.; Lampl, K.; Herfert, J.; Kienberger, Y.T.; Flamm, M.; et al. Electrotherapy as treatment for chemotherapy-induced peripheral neuropathy: A randomized controlled trial. Front. Neurol. 2024, 15, 1451456. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, I.R.; Kamen, C.; Gewandter, J.S.; Mohile, N.A.; Heckler, C.E.; Culakova, E.; Fung, C.; Janelsins, M.C.; Asare, M.; Lin, P.-J.; et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: A multicentre, randomized controlled trial. Support. Care Cancer 2018, 26, 1019–1028. [Google Scholar] [CrossRef]
- Streckmann, F.; Lehmann, H.C.; Balke, M.; Schenk, A.; Oberste, M.; Heller, A.; Schürhörster, A.; Elter, T.; Bloch, W.; Baumann, F.T. Sensorimotor training and whole-body vibration training have the potential to reduce motor and sensory symptoms of chemotherapy-induced peripheral neuropathy—A randomized controlled pilot trial. Support. Care Cancer 2019, 27, 2471–2478. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomised controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Al Onazi, M.M.; Yurick, J.L.; Harris, C.; Nishimura, K.; Suderman, K.; Pituskin, E.; Chua, N.; McNeely, M.L. Therapeutic ultrasound for chemotherapy-related pain and sensory disturbance in the hands and feet in patients with colorectal cancer: A pilot randomised controlled trial. J. Pain Symptom Manag. 2021, 61, 1127–1138. [Google Scholar] [CrossRef]
- Argenta, P.A.; Ballman, K.V.; Geller, M.A.; Carson, L.F.; Ghebre, R.; Mullany, S.A.; Teoh, D.G.; Winterhoff, B.J.; Rivard, C.L.; Erickson, B.K. The effect of photobiomodulation on chemotherapy-induced peripheral neuropathy: A randomised, sham-controlled clinical trial. Gynecol. Oncol. 2017, 144, 159–166. [Google Scholar] [CrossRef]
- Bao, T.; Zhi, I.; Baser, R.; Hooper, M.; Chen, C.; Piulson, L.; Li, Q.S.; Galantino, M.L.; Blinder, V.; Robson, M.; et al. Yoga for chemotherapy-induced peripheral neuropathy and fall risk: A randomized controlled trial. JNCI Cancer Spectr. 2020, 4, pkaa048. [Google Scholar] [CrossRef] [PubMed]
- Bland, K.A.; Kirkham, A.A.; Bovard, J.; Shenkier, T.; Zucker, D.; McKenzie, D.C.; Davis, M.K.; Gelmon, K.A.; Campbell, K.L. Effect of exercise on taxane chemotherapy-induced peripheral neuropathy in women with breast cancer: A randomised controlled trial. Clin. Breast Cancer 2019, 19, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Cartmel, B.; Li, F.Y.; Gottlieb, L.T.; Harrigan, M.; Ligibel, J.A.; Gogoi, R.; Schwartz, P.E.; Esserman, D.A.; Irwin, M.L.; et al. Effect of exercise on chemotherapy-induced peripheral neuropathy among patients treated for ovarian cancer: A secondary analysis of a randomised clinical trial. JAMA Netw. Open 2023, 6, e2326463. [Google Scholar] [CrossRef]
- Dhawan, S.; Andrews, R.; Kumar, L.; Wadhwa, S.; Shukla, G. A randomized controlled trial to assess the effectiveness of muscle strengthening and balancing exercises on chemotherapy-induced peripheral neuropathic pain and quality of life among cancer patients. Cancer Nurs. 2020, 43, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, İ.; Kutlutürkan, S. The effect of hand-foot exercises on chemotherapy-induced peripheral neuropathy-related pain, falls, and quality of life in colorectal cancer: A randomised controlled trial. Eur. J. Oncol. Nurs. 2024, 71, 102641. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Kim, I.Y.; Hur, H.K.; Lee, J.Y.; Park, S.M. The Effects of an app-based physical activity programme on colorectal cancer patients undergoing chemotherapy: A randomised controlled trial. Cancer Nurs. 2025, 48, E90–E97. [Google Scholar] [CrossRef]
- Ikio, Y.; Sagari, A.; Nakashima, A.; Matsuda, D.; Sawai, T.; Higashi, T. Efficacy of combined hand exercise intervention in patients with chemotherapy-induced peripheral neuropathy: A pilot randomised controlled trial. Support. Care Cancer 2022, 30, 4981–4992. [Google Scholar] [CrossRef] [PubMed]
- Izgu, N.; Metin, Z.G.; Karadas, C.; Ozdemir, L.; Çetin, N.; Demirci, U. Prevention of chemotherapy-induced peripheral neuropathy with classical massage in breast cancer patients receiving paclitaxel: An assessor-blinded randomised controlled trial. Eur. J. Oncol. Nurs. 2019, 40, 36–43. [Google Scholar] [CrossRef]
- Kneis, S.; Wehrle, A.; Müller, J.; Maurer, C.; Ihorst, G.; Gollhofer, A.; Bertz, H. It’s never too late-balance and endurance training improves functional performance, quality of life, and alleviates neuropathic symptoms in cancer survivors suffering from chemotherapy-induced peripheral neuropathy: Results of a randomized controlled trial. BMC Cancer 2019, 19, 414. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Le-Rademacher, J.G.; Majithia, N.; McMurray, R.P.; O’Neill, C.R.; Bendel, M.A.; Beutler, A.; Lachance, D.H.; Cheville, A.; Strick, D.M.; et al. Scrambler therapy for chemotherapy neuropathy: A randomised phase II pilot trial. Support. Care Cancer 2020, 28, 1183–1197. [Google Scholar] [CrossRef]
- Moraitis, A.M.; Rose, N.B.; Johnson, A.F.; Dunston, E.R.; Garrido-Laguna, I.; Hobson, P.; Barber, K.; Basen-Engquist, K.; Coletta, A.M. Feasibility and acceptability of an mHealth, home-based exercise intervention in colorectal cancer survivors: A pilot randomised controlled trial. PLoS ONE 2023, 18, e0287152. [Google Scholar] [CrossRef]
- Müller, J.; Weiler, M.; Schneeweiss, A.; Haag, G.M.; Steindorf, K.; Wick, W.; Wiskemann, J. Preventive effect of sensorimotor exercise and resistance training on chemotherapy-induced peripheral neuropathy: A randomised controlled trial. Br. J. Cancer 2021, 125, 955–965. [Google Scholar] [CrossRef]
- Saraboon, C.; Siriphorn, A. Effects of foam pad balance exercises on cancer patients undergoing chemotherapy: A randomized controll trial. J. Bodyw. Mov. Ther. 2021, 28, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Uysal, N.; Ünal Toprak, F. The effect of hand and foot exercises on peripheral neuropathy and quality of life in women with breast cancer: A randomised controlled trial. Support. Care Cancer 2025, 33, 83. [Google Scholar] [CrossRef]
- Vollmers, P.L.; Mundhenke, C.; Maass, N.; Bauerschlag, D.; Kratzenstein, S.; Röcken, C.; Schmidt, T. Evaluation of the effects of sensorimotor exercise on physical and psychological parameters in breast cancer patients undergoing neurotoxic chemotherapy. J. Cancer Res. Clin. Oncol. 2018, 144, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Waibel, S.; Wehrle, A.; Müller, J.; Bertz, H.; Maurer, C. Type of exercise may influence postural adaptations in chemotherapy-induced peripheral neuropathy. Ann. Clin. Transl. Neurol. 2021, 8, 1680–1694. [Google Scholar] [CrossRef]
- Xiaoqian, Y.; Jiwei, H.; Lizhi, Z.; Baojia, G.; Luyan, G.; Huiqian, X.; Hong, L.; Yijing, F. A randomized controlled trial: Effects of compression therapy combined with exercise on chemotherapy-induced peripheral neuropathy in patients with breast cancer. Cancer Treat. Res. Commun. 2025, 42, 100871. [Google Scholar] [CrossRef]
- Zhi, W.I.; Baser, R.E.; Zhi, L.M.; Talukder, D.; Li, Q.S.; Paul, T.; Patterson, C.; Piulson, L.; Seluzicki, C.; Galantino, M.L.; et al. Yoga for cancer survivors with chemotherapy-induced peripheral neuropathy: Health-related quality of life outcomes. Cancer Med. 2021, 10, 5456–5465. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Trebing, S.; Timmers-Trebing, U.; Schenk, A.; Paust, R.; Bloch, W.; Rudolph, R.; Streckmann, F.; Baumann, F.T. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: A randomized controlled trial. Support. Care Cancer 2018, 26, 615–624. [Google Scholar] [CrossRef]
- Stuecher, K.; Bolling, C.; Vogt, L.; Niederer, D.; Schmidt, K.; Dignaß, A.; Banzer, W. Exercise improves functional capacity and lean body mass in patients with gastrointestinal cancer during chemotherapy: A single-blind randomised controlled trial. Support. Care Cancer 2019, 27, 2159–2169. [Google Scholar] [CrossRef]
- Streckmann, F.; Zopf, E.M.; Lehmann, H.C.; May, K.; Rizza, J.; Zimmer, P.; Gollhofer, A.; Bloch, W.; Baumann, F.T. Exercise intervention studies in patients with peripheral neuropathy: A systematic review. Sports Med. 2014, 44, 1289–1304. [Google Scholar] [CrossRef]
- Amarelo, A.; da Mota, M.C.C.; Amarelo, B.L.P.; Ferreira, M.C.; Fernandes, C.S. Effects of physical exercise on chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain Manag. Nurs. 2025, 26, 212–221. [Google Scholar] [CrossRef] [PubMed]
| Database | Search | Filters/Limits | Date of Last Search |
|---|---|---|---|
| PubMed | (“chemotherapy-induced peripheral neuropathy” [MeSH Terms] OR “CIPN” OR “peripheral neuropathy secondary to chemotherapy”) AND (“exercise” OR “training” OR “physiotherapy” OR “physical therapy”) AND (“cancer” OR “oncology” OR “neoplasm”) | Humans, Randomised Controlled Trial, English, Publication years 2015–2025 | May 2025 |
| Scopus | TITLE-ABS-KEY (“chemotherapy-induced peripheral neuropathy” OR “CIPN”) AND TITLE-ABS-KEY (“exercise” OR “physiotherapy” OR “training” OR “physical therapy”) AND TITLE-ABS-KEY (“cancer” OR “oncology”) | Article type: randomised controlled trial; Language: English | May 2025 |
| Web of Science | (“chemotherapy-induced peripheral neuropathy” OR “CIPN”) AND (“exercise” OR “physiotherapy” OR “training” OR “physical therapy”) AND (“cancer” OR “oncology”) | Document type: Article; Language: English | May 2025 |
| Cochrane Library | (“chemotherapy-induced peripheral neuropathy” OR “CIPN”) AND (“exercise” OR “training” OR “physiotherapy” OR “physical therapy”) AND (“cancer” OR “oncology” OR “neoplasm”) | Trials; Humans; English; Publication years 2015–2025 | May 2025 |
| Main Author (Year) | Sample and Groups (Number of Participants) | Programme Duration (Frequency) | Intensity | Measurement Instruments | Main Outcomes | Adherence | Adverse Effects | PEDro Score |
|---|---|---|---|---|---|---|---|---|
| Al Onazi et al. (2021) [18] | Therapeutic ultrasound (n = 16) vs. Control (n = 15) | 2 weeks (10 ultrasound sessions) + 6 weeks of home-based exercise | Continuous ultrasound, 0.7–0.8 W/cm2, 3 MHz | FACT/GOG-NTX, QLQ-CIPN20, Semmes-Weinstein monofilament, 128 Hz vibration, Achilles tendon reflex, balance | Significant improvement in symptoms at 2 weeks (p = 0.003), with no differences at 6 weeks. | 100% | None | 8/10 |
| Andersen-Hammond et al. (2020) [11] | Physiotherapy (n = 22) vs. Control (n = 26) | Four sessions with a physiotherapist + daily home exercises | Moderate intensity | Numeric Pain Rating Scale (NPRS), DASH, S-LANSS, Pressure Pain Threshold Test, Handgrip Dynamometry, Quantitative Sensory Testing (QST) | Reduction in neuropathic pain, improved handgrip strength and pain pressure threshold | Not reported (NR) | None | 10/10 |
| Argenta et al. (2017) [19] | Photobiomodulation (PBM) (n = 30) vs. Sham (n = 40) + crossover to PBM/PT | 6 weeks (PBM 3 sessions per week) | Not applicable (Class IV laser photobiomodulation) | Modified Total Neuropathy Score (mTNS), adherence log | Significant reduction in neuropathic symptoms (−52.6% in mTNS, p < 0.001) | >95% | None | 10/10 |
| Bao et al. (2020) [20] | Yoga (n = 21) vs. Usual care (n = 20) | 8 weeks (2 supervised sessions per week + 5 home-based practices per week) | Moderate intensity | Numeric Rating Scale (NRS), FACT/GOG-Ntx, Functional Reach Test, Chair Stand Test, 4-Meter Gait Speed Test | Improvement in chemotherapy-induced neuropathic pain and functional performance (p = 0.035) | 87.8% | Four mild adverse events (grade 1) in the yoga group | 8/10 |
| Bland et al. (2019) [21] | Immediate exercise (n = 12) vs. Delayed exercise (n = 15) | 8–12 weeks (3 sessions per week) | Moderate intensity | EORTC QLQ-CIPN20, EORTC QLQ-C30, Vibration Test, Pain Perception Test | Less progression of sensory neuropathy before the final chemotherapy cycle and greater adherence to treatment (p < 0.05) | 78–93% | None | 10/10 |
| Cao et al. (2023) [22] | Aerobic exercise (n = 69) vs. Control (n = 65) | 6 months (home-based exercise with weekly telephone supervision) | Moderate intensity | FACT/GOG-Ntx, Physical Activity Questionnaire | Significant reduction in CIPN (−1.6 points, p = 0.03), with no changes observed in the control group | 83.8% | None | 10/10 |
| Dhawan et al. (2020) [23] | Exercise (n = 22) vs. Control (n = 23) | 10 weeks (daily home-based exercise, 30 min/day) | Moderate intensity | CIPNAT, NCV, LANSS, EORTC QLQ-C30 | Reduction in neuropathic pain (p < 0.0001) and improvement in quality of life (p < 0.004) | 68% | None | 7/10 |
| Eroğlu & Kutlutürkan (2024) [24] | Hand and foot exercises (n = 19) vs. Control (n = 20) | 8 weeks (3 sessions per day, 3 days per week) | Moderate intensity | NRS, CIPNAT, Fall Follow-Up Form, EORTC QLQ-C30, EORTC QLQ-CR29 | Significant reduction in neuropathic pain and improvement in quality of life (p < 0.05), with no differences in fall incidence | Not reported (NR) | None | 7/10 |
| Hwang et al. (2025) [25] | Exercise app (n = 17) vs. Control with educational brochure (n = 17) | 6 weeks (home-based exercise using the app) | Moderate intensity | CIPNAT, Interference with Activities Scale, EORTC QLQ-C30, Exercise Adherence Log | Fewer neuropathic symptoms (p = 0.002), improved quality of life (p = 0.003), and greater exercise adherence (p < 0.001) | Not reported (NR) | None | 8/10 |
| Ikio et al. (2022) [26] | Hand exercise (n = 21) vs. Control (n = 21) | 6–8 weeks (3 or more sessions per week, 30 min per session) | Moderate intensity | ADL-MHQ, SWMT, Purdue Pegboard Test, VAS, PCS, FACT/GOG-Ntx, Hand Dynamometer | Less decline in ADL-MHQ (p = 0.0397), greater pinch strength (p = 0.0007), and reduced pain (p = 0.0083) | 73.3% | None | 8/10 |
| Izgu et al. (2019) [27] | Classical massage (n = 19) vs. Control (n = 21) | 12 weeks (1 session per week, 30 min) | Low to moderate intensity | S-LANSS, EORTC QLQ-CIPN20, Nerve Conduction Studies (NCS) | Reduction in neuropathic pain and improvement in quality of life (p < 0.05), with positive changes in nerve conduction studies | 100% | None | 6/10 |
| Kneis et al. (2019) [28] | Balance + resistance training (n = 25) vs. Resistance training only (n = 25) | 12 weeks (2 sessions per week) | Moderate intensity | Force Platform, Countermovement Jump (CMJ), EORTC QLQ-CIPN20, Cardiopulmonary Exercise Test (CPET) | Significant reduction in motor and autonomic symptoms, and improvement in postural control | 92–100% | None | 10/10 |
| Kleckner et al. (2018) [14] | Exercise (n = 170) vs. Control (n = 185) | 6 weeks (home-based walking and resistance exercise) | Moderate intensity | Neuropathy Rating Scale (0–10), Walk4Life Pedometer, Activity Log | Less neuropathy (p = 0.045) and improvement in sensory symptoms (p = 0.061) | 77% | None | 10/10 |
| Joy et al. (2022) [12] | Photobiomodulation (PBM) (n = 16) vs. Placebo (n = 16) | 12–18 weeks (2 sessions per week) | Class IV MLS Laser (905/808 nm, 4 J/cm2, 0.168 W/cm2) | mTNS, FACT/GOG-Taxane, 6MWT, NRS | Less symptom progression, better quality of life, reduced pain, and improved functional capacity in the PBM group | Not reported (NR) | None | 9/10 |
| Loprinzi et al. (2020) [29] | Scrambler therapy (n = 25) vs. TENS (n = 25) | 2 weeks of treatment with 8-week follow-up | Not applicable (cutaneous electrical stimulation therapy) | EORTC QLQ-CIPN20, NAS (pain, tingling, numbness), Global Impression of Change | Greater reduction in neuropathic symptoms in the Scrambler group (40% vs. 20% with TENS, p = 0.12) | Not reported (NR) | Mild skin irritation observed in both groups | 7/10 |
| Moraitis et al. (2023) [30] | HIIT (n = 2) vs. MICE (n = 5) | 12 weeks (4–5 sessions per week) | HIIT: High intensity (85–90% HRmax); MICE: Moderate intensity (50–70% HRmax) | Polar A370, Polar H10, SPPB, PROMIS Physical Function, NTSS-6, UENS, BOD POD | High adherence and improvements in quality of life, strength, and physical function, with no significant differences between groups | 88.6% | None | 9/10 |
| Müller et al. (2021) [31] | Sensorimotor training (n = 52) vs. Resistance training (n = 60) vs. Control (n = 58) | 20 weeks (3 sessions per week) | Moderate to high intensity (70–80% 1RM for resistance training) | TNSr, EORTC QLQ-CIPN15, EORTC QLQ-C30, COP, Isokinetic Dynamometer, FES-I, RDI | Less progression of sensory symptoms in the feet (p = 0.039), improved strength (p < 0.001), and enhanced quality of life (p = 0.005) | 55% SMT, 49% RT | None | 10/10 |
| Saraboon & Siriphorn (2021) [32] | Balance exercise (n = 15) vs. Control (n = 15) | 6 weeks (60 min, 2 sessions per week) | Moderate intensity | FAB, SPPB, MDNS, FACT-Taxane | Improved balance (p < 0.01), better physical function (p = 0.03), and higher quality of life (p < 0.01), with no changes in neuropathy | Not reported (NR) | None | 7/10 |
| Sassmann et al. (2024) [13] | HTEMS (n = 25) vs. TENS (n = 25) vs. Control (n = 17) | 8 weeks of home-based electrotherapy treatment | Moderate intensity | EORTC QLQ-CIPN20, EORTC QLQ-C30, CTCAE v4, clinical sensory and motor examinations | Significant improvement in sensory and motor scores (EORTC QLQ-CIPN20: TENS −12.3/−8.2; HTEMS −14.7/−8.2); CIPN grade improved in both intervention groups, while physical function improved only in HTEMS (+7.9 points) | Not reported (NR) | None | 6/10 |
| Streckmann et al. (2024) [15] | SMT (n = 55) vs. WBV (n = 53) vs. Control (n = 50) | During chemotherapy (2 sessions per week, 15–30 min each) | Moderate intensity | Force platform, Rydel-Seiffer tuning fork, FACT/GOG-Ntx, EORTC QLQ-C30, Pain-DETECT | Lower incidence of CIPN (50–70%), improved balance and neuropathic pain, and reduced need for chemotherapy dose reductions | 72.8% | None | 10/10 |
| Uysal & Toprak (2025) [33] | Massage ball (n = 26) vs. Stress ball (n = 26) vs. Control (n = 27) | 8 weeks (daily home-based exercise) | Low to moderate intensity | NCI-CTCAE v5.0, EORTC QLQ-C30, EORTC QLQ-CIPN20 | Significant reduction in CIPN (p < 0.001), with improvements in quality of life and reductions in pain and fatigue | >90% | None | 8/10 |
| Vollmers et al. (2018) [34] | Sensorimotor exercise (n = 17) vs. Control (n = 19) | During chemotherapy plus 6 weeks post-treatment (2 sessions per week) | Moderate intensity | Force platform, FAB, Hand Dynamometry, Chair Rising Test, EORTC QLQ-C30, BR23, CIPN20, MFI-20 | Improved postural stability and prevention of strength loss (p < 0.001), with no significant changes in quality of life | High (not quantified) | None | 6/10 |
| Waibel et al. (2021) [35] | Balance + resistance training (n = 16) vs. Resistance training only (n = 15) | 12 weeks (2 sessions per week) | Moderate intensity | Force platform, Motion Capture System, EORTC QLQ-CIPN20 | Reduced postural sway, improved stability, and decreased CIPN symptoms | ≥70% | None | 7/10 |
| Xiaoqian et al. (2025) [36] | Compression + exercise (n = 36) vs. Compression only (n = 36) vs. Control (n = 36) | 5 chemotherapy cycles (daily exercise) | Low to moderate intensity | NCI-CTC v4.0, CIPNAT, ADL | Significant reduction in CIPN and improvement in quality of life (p < 0.001) | High (no dropouts) | None | 7/10 |
| Zhi et al. (2021) [37] | Yoga (n = 21) vs. Waitlist control (n = 20) | 8 weeks (60 min/day, 2 in-person sessions per week) | Low to moderate intensity | HADS, BFI, ISI, TES | Reduced anxiety at 12 weeks (p = 0.017), with no significant improvements in fatigue, insomnia, or depression | >85% | None | 6/10 |
| Zimmer et al. (2018) [38] | Multimodal exercise (n = 17) vs. Control (n = 13) | 8 weeks (2 sessions per week, 60 min each) | Moderate intensity | FACT/GOG-NTX, GGT-Reha, h1RM, 6MWT | Prevention of CIPN deterioration (p = 0.028), improvements in balance and strength (p < 0.05), with no changes in aerobic capacity | 88.3% | None | 10/10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamayo Fajardo, J.A.; León Parejo, F. Effectiveness of Exercise and Physiotherapy in Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review. Healthcare 2025, 13, 2973. https://doi.org/10.3390/healthcare13222973
Tamayo Fajardo JA, León Parejo F. Effectiveness of Exercise and Physiotherapy in Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review. Healthcare. 2025; 13(22):2973. https://doi.org/10.3390/healthcare13222973
Chicago/Turabian StyleTamayo Fajardo, Javier Antonio, and Francisco León Parejo. 2025. "Effectiveness of Exercise and Physiotherapy in Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review" Healthcare 13, no. 22: 2973. https://doi.org/10.3390/healthcare13222973
APA StyleTamayo Fajardo, J. A., & León Parejo, F. (2025). Effectiveness of Exercise and Physiotherapy in Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review. Healthcare, 13(22), 2973. https://doi.org/10.3390/healthcare13222973






