Abstract
Background: Chemotherapy-induced peripheral neuropathy (CIPN) is a common and disabling adverse effect of cancer treatment, affecting up to 65% of patients. It reduces quality of life, increases fall risk, and often leads to chemotherapy dose reduction or discontinuation. Because pharmacological management provides limited relief, non-pharmacological strategies such as exercise and physiotherapy have become increasingly relevant. Methods: A systematic review following the PRISMA 2020 guidelines was conducted to identify randomised controlled trials (RCTs) evaluating exercise and physiotherapy for the prevention or treatment of CIPN. PubMed, Scopus, Web of Science, and Cochrane Library were searched up to May 2025. Methodological quality was assessed with the PEDro scale, and due to heterogeneity, a narrative synthesis was performed. Outcomes included neuropathic symptoms, pain, motor and sensory function, balance, muscle strength, and quality of life. Results: Twenty-six RCTs published between 2017 and 2025 were included. Nineteen assessed exercise-based interventions (aerobic, resistance, sensorimotor, balance, yoga, or multimodal), and seven examined physiotherapy modalities (manual therapy, photobiomodulation, Scrambler therapy, ultrasound, or electrical stimulation). Both approaches improved sensory and motor symptoms, balance, muscle strength, and quality of life. Adherence ranged from 70% to 95%, and no serious adverse events were reported. However, variability in intervention design and outcome measures precluded meta-analysis. Conclusions: Exercise and physiotherapy are safe, feasible, and effective non-pharmacological strategies for managing CIPN. However, heterogeneity in intervention design highlights the need for high-quality RCTs to establish optimal protocols and standardised clinical guidelines.
1. Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the most prevalent and disabling adverse effects of systemic anticancer therapy, with reported incidence rates ranging from 30% to 65%, depending on the neurotoxic agent and cumulative dose [1,2]. CIPN frequently leads to chemotherapy dose modification or discontinuation, compromising treatment efficacy and overall survival [3,4]. Common symptoms include paraesthesia, numbness, neuropathic pain, balance impairment, and reduced muscle strength, which collectively limit patients’ functional independence and quality of life [5,6].
The pathophysiology of CIPN involves axonal degeneration, mitochondrial dysfunction, oxidative stress, altered ion channel activity, and neuroinflammation [7,8]. Pharmacological options such as duloxetine or gabapentinoids provide only partial symptom relief and are often limited by adverse effects [9]. Therefore, non-pharmacological strategies—particularly exercise and physiotherapy—have gained growing attention as complementary approaches.
Exercise exerts neuroprotective, anti-inflammatory, and antioxidant effects, promoting mitochondrial biogenesis, axonal regeneration, and neuromuscular function [10,11]. Similarly, physiotherapy modalities including manual therapy, neuromotor stimulation, and photobiomodulation can enhance proprioception, reduce pain, and improve functional performance [12,13,14].
Despite emerging evidence, methodological heterogeneity and variability in intervention design persist, making it difficult to establish standardised protocols [15]. Such inconsistencies highlight the need for a comprehensive synthesis of randomised controlled trials to clarify the effectiveness of exercise and physiotherapy in chemotherapy-induced peripheral neuropathy (CIPN). This systematic review therefore aims to synthesise current evidence to guide clinical decision-making and the development of standardised exercise and physiotherapy protocols for patients undergoing neurotoxic chemotherapy.
2. Materials and Methods
A systematic review was conducted to identify randomised controlled trials (RCTs) examining the effects of exercise and physiotherapy on chemotherapy-induced peripheral neuropathy (CIPN). The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines and PRISMA 2020 Checklist to ensure transparency and reproducibility [16]. The protocol for this review was retrospectively registered in the Open Science Framework (OSF; registration DOI: https://doi.org/10.17605/OSF.IO/5YJZW).
2.1. Search Strategy
Electronic searches were performed in PubMed, Scopus, Web of Science, and the Cochrane Library from inception to May 2025, complemented by manual searches of reference lists (Table 1). No additional records were identified through manual reference screening.

Table 1.
Search Strategy.
The search strategy combined MeSH terms and free-text keywords using Boolean operators: (“chemotherapy-induced peripheral neuropathy” OR “CIPN”) AND (“exercise” OR “training” OR “physiotherapy” OR “physical therapy”) AND (“cancer” OR “oncology”).
Filters and limits applied included: Humans, Randomised Controlled Trial, English language, and publication years 2015–2025.
The number of records retrieved from each database was as follows: PubMed (n = 38), Scopus (n = 202), Web of Science (n = 291), and Cochrane Library (n = 91), for a total of 622 records before removing duplicates.
2.2. Eligibility Criteria
Studies were included if they met the following criteria:
- Randomised controlled trials involving adult patients receiving neurotoxic chemotherapy (taxanes, platinum agents, vinca alkaloids, or proteasome inhibitors).
- Interventions consisting of exercise or physiotherapy, compared with usual care or control.
- Outcomes including CIPN incidence or severity, neuropathic pain, motor and sensory function, balance, muscle strength, or quality of life.
Studies were excluded if they were non-randomised, pharmacological-only trials, preclinical investigations, or paediatric populations.
For synthesis purposes, studies were grouped according to the primary type of intervention: exercise-based programmes and physiotherapy-based modalities. Physiotherapy-based modalities included interventions such as photobiomodulation, manual therapy, Scrambler therapy, transcutaneous electrical nerve stimulation (TENS), and therapeutic ultrasound.
2.3. Selection Process and Data Collection
Titles and abstracts were independently screened by two reviewers (J.A.T.F. and F.L.P.), and potentially eligible full texts were assessed according to the predefined inclusion and exclusion criteria. Data extraction from the included studies was also performed independently by the same reviewers using a predefined data extraction form in Microsoft Excel. Extracted information included authorship, publication year, study design, sample characteristics, intervention type, duration, frequency, outcome measures, main findings, adherence, adverse events, and PEDro score. When specific data were missing or unclear in the original reports, they were recorded as “not reported” (NR) and not imputed. Any discrepancies during study selection or data extraction were resolved through discussion and consensus. No automation tools or machine learning algorithms were used at any stage of the process. The study selection procedure is illustrated in the PRISMA 2020 flow diagram (Figure 1).
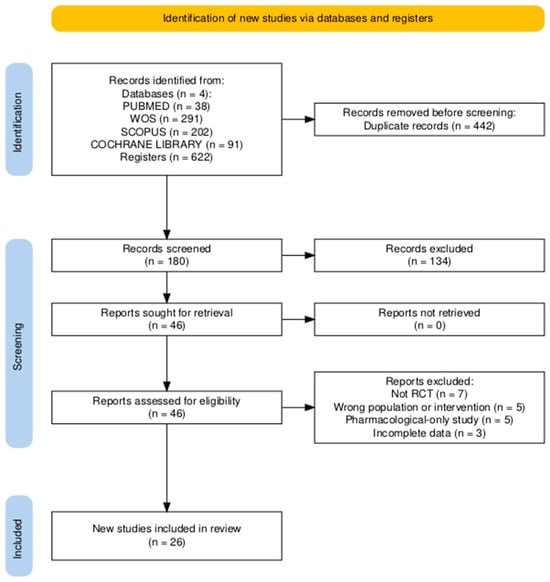
Figure 1.
PRISMA 2020 flow diagram showing the study selection process.
2.4. Methodological Quality
The methodological quality of the included RCTs was assessed using the Physiotherapy Evidence Database (PEDro) scale [17], which rates studies based on 11 items related to internal validity and interpretability. Scores range from 0 to 10, with higher scores indicating better quality. A score ≥6 was considered high quality. Two reviewers (J.A.T.F. and F.L.P.) independently assessed each study, and disagreements were resolved by consensus.
For each outcome, effect measures were extracted as reported in the original studies, typically including mean values, standard deviations, mean differences, percentage changes, and p-values for between-group comparisons. Although many included studies were of moderate-to-high methodological quality, a quantitative meta-analysis was not considered appropriate due to substantial heterogeneity across trials. This heterogeneity involved differences in intervention characteristics (e.g., exercise type, physiotherapy modality, duration, and supervision), outcome measures (e.g., EORTC QLQ-CIPN20, FACT/GOG-NTX, Total Neuropathy Score, or CIPNAT), timing of assessments (during vs. after chemotherapy), and incomplete reporting of variance data in several studies. Therefore, a structured narrative synthesis was performed following the PRISMA 2020 guidance for heterogeneous interventions.
Risk of publication bias was not formally assessed, as no meta-analysis was conducted. However, all available randomised controlled trials meeting the inclusion criteria were included to minimise selection and reporting bias. The overall certainty of evidence was assessed qualitatively, considering study quality, consistency of results, and sample sizes.
3. Results
The selection and inclusion process is summarised in the PRISMA 2020 flow diagram (Figure 1), and the characteristics of the included studies are presented in Table 2.

Table 2.
Included studies and main characteristics.
A total of 622 records were identified through database searches (PubMed, Scopus, Web of Science, and Cochrane Library). After removing 442 duplicates, 180 unique records remained for title and abstract screening. Of these, 134 records were excluded at the title and abstract screening stage, did not meet the inclusion criteria in terms of population, intervention, or study design, and 46 full-text articles were assessed for eligibility. Finally, 26 randomised controlled trials (RCTs) met the inclusion criteria and were included in the qualitative synthesis.
3.1. General Characteristics of the Included Studies
The 26 studies, published between 2017 and 2025, involved approximately 1650 participants receiving neurotoxic chemotherapy. Most trials were conducted in Europe, Asia, and North America using parallel-group RCT designs. Intervention duration ranged from 6 to 24 weeks, with 2–5 sessions per week. Average adherence ranged from 70% to 95%, and no severe adverse events were reported.
The main characteristics and findings of the included studies are summarised in Table 2. Most trials compared an exercise-based or physiotherapy-based intervention with usual care or non-exercise control conditions. The majority reported significant improvements in sensory and motor symptoms, balance, muscle strength, and overall quality of life in participants undergoing neurotoxic chemotherapy. Aerobic, resistance, and sensorimotor exercise programmes were particularly effective in reducing neuropathic symptoms and improving functional performance, while physiotherapy-based modalities such as photobiomodulation, transcutaneous electrical nerve stimulation (TENS), Scrambler therapy, manual therapy, and therapeutic ultrasound also showed positive effects on pain reduction and sensory recovery. No serious adverse events were reported in any study.
3.2. Exercise-Based Interventions
Nineteen studies implemented structured exercise programmes during or after chemotherapy. Common modalities included combined aerobic and resistance training [19,21,38], sensorimotor and balance exercises [32,39], yoga and low-intensity routines [23,35], and multimodal or home-based interventions [22,30].
Assessment tools included EORTC QLQ-CIPN20, FACT/GOG-NTX, Total Neuropathy Score (TNS), SPPB, Six-Minute Walk Test (6MWT), and handgrip dynamometry.
Overall, exercise interventions significantly reduced the sensory and motor symptoms, improved balance, muscle strength, and functional performance, and enhanced quality of life. Preventive effects were reported when exercise was initiated early during chemotherapy [27,31].
3.3. Physiotherapy-Based Interventions
Seven RCTs investigated physiotherapy-based modalities, including photobiomodulation, manual therapy, Scrambler therapy, transcutaneous electrical nerve stimulation (TENS), and therapeutic ultrasound. Studies by Joy et al. [12] and Sassmann et al. [13] demonstrated significant improvements in sensory symptoms, neuropathic pain, and functional capacity, with enhanced EORTC QLQ-CIPN20 scores. Photobiomodulation effectively prevented the progression of taxane-induced neuropathy, while Scrambler therapy and TENS produced benefits in chronic CIPN. No major adverse effects were reported, confirming the safety and tolerability of physiotherapy-based interventions.
3.4. Risk of Bias Assessment
PEDro scale scores ranged from 6 to 10 out of 10, with a mean score of 8.2, indicating overall high methodological quality among the included RCTs (see Table 2 for individual scores). Randomisation and baseline comparability were generally adequate, and most studies clearly reported between-group comparisons and measures of variability. However, allocation concealment and blinding of participants and therapists remained limited due to the nature of physical and exercise-based interventions.
3.5. Synthesis of Results
Both exercise and physiotherapy interventions were effective, safe, and feasible for the management of chemotherapy-induced peripheral neuropathy (CIPN). The most consistent outcomes across trials were reductions in neuropathic symptoms and improvements in balance, muscle strength, and quality of life. The overall findings were coherent among studies of high methodological quality according to the PEDro scale. Due to the heterogeneity of interventions, assessment tools, and follow-up durations, a narrative synthesis was performed rather than a quantitative meta-analysis.
3.6. Reporting Biases
Publication bias was not formally assessed, as no quantitative meta-analysis was performed. Nevertheless, all eligible randomised controlled trials that met the inclusion criteria were incorporated into the synthesis to minimise the risk of selective reporting bias. No evidence of outcome reporting discrepancies was detected among the included studies.
4. Discussion
This systematic review synthesised evidence from 26 randomised controlled trials evaluating the effects of exercise and physiotherapy on chemotherapy-induced peripheral neuropathy (CIPN). The findings consistently demonstrated that both approaches are safe, feasible, and beneficial in alleviating neuropathic symptoms, improving balance and muscle strength, and enhancing the overall quality of life in patients receiving neurotoxic chemotherapy.
These results are consistent with previous systematic reviews and international clinical practice guidelines recommending exercise and rehabilitation as supportive interventions in oncology care. In particular, the ASCO Clinical Practice Guideline and the ESMO–EONS–EANO guideline on therapy-induced neurotoxicity emphasise the role of structured exercise and physical rehabilitation in the management of chemotherapy-induced peripheral neuropathy [3,6]. Likewise, the findings of this review are consistent with previous systematic reviews that also reported improvements in neuropathic symptoms and physical function following structured exercise interventions in patients receiving neurotoxic chemotherapy [3,40]. More recently, Amarelo et al. [41] conducted a meta-analysis confirming that exercise significantly alleviates chemotherapy-induced peripheral neuropathy and enhances quality of life. Together, these findings reinforce the growing evidence supporting exercise and physiotherapy as effective strategies for the prevention and management of CIPN. Multimodal exercise programmes that combine aerobic, resistance, and balance training appear particularly effective, supporting the neuroprotective, anti-inflammatory, and antioxidant effects of regular exercise [26,29]. Early initiation of exercise during chemotherapy, as reported by Kleckner et al. [14] and Streckmann et al. [15], may mitigate nerve toxicity and prevent functional decline.
Physiotherapy-based modalities such as photobiomodulation and transcutaneous electrical stimulation have also shown promising outcomes. Studies by Joy et al. [12] and Sassmann et al. [13] demonstrated significant reductions in neuropathic pain and improvements in sensory function without adverse effects. The potential mechanisms involve enhanced microcirculation, reduced inflammation, and modulation of nociceptive signalling pathways [25,37].
High adherence rates (70–95%) across studies confirm the feasibility of implementing these interventions within oncology settings [25]. Adherence was likely facilitated by professional supervision, individualised programming, and the perceived benefit of symptom relief. Importantly, no severe adverse events were reported, reinforcing the safety profile of both exercise and physiotherapy approaches.
Compared with earlier literature, recent trials demonstrate greater methodological rigour and more diverse intervention designs, evolving from traditional aerobic exercise towards functional, resistance, and sensorimotor training [20]. Similarly, physiotherapy interventions have advanced towards active, integrative modalities that combine manual therapy and neuromuscular re-education, providing broader rehabilitative benefits.
The physiological mechanisms underlying these effects may include improved peripheral nerve perfusion, modulation of oxidative stress and inflammatory mediators, and upregulation of neurotrophic factors such as brain-derived neurotrophic factor (BDNF), which supports axonal regeneration and neural plasticity [28]. Collectively, these findings highlight the potential of physical rehabilitation not only as symptom management but also as a neuroprotective therapeutic strategy.
Despite the encouraging evidence, several limitations should be acknowledged. Considerable heterogeneity exists in intervention type, duration, and intensity, which limits comparability and standardisation. Small sample sizes in some trials may have reduced statistical power and inflated treatment effects. Furthermore, blinding of participants and therapists remains challenging in non-pharmacological interventions. Variability in assessment tools—such as EORTC QLQ-CIPN20, FACT/GOG-NTX, and TNS—also hinders quantitative synthesis.
Another important limitation is the lack of long-term follow-up in most trials, making it difficult to determine the durability of treatment effects. Future research should therefore prioritise multicentre RCTs with longer follow-up periods and standardised intervention protocols. The inclusion of objective biomarkers, such as neurophysiological or inflammatory markers, could further elucidate the mechanisms of neuroprotection and functional recovery. Additionally, this review was limited to studies published in English and may therefore be subject to language or publication bias despite comprehensive database searches.
Furthermore, certain methodological constraints of this review should be acknowledged. The protocol was retrospectively registered in the Open Science Framework (OSF; registration DOI: https://doi.org/10.17605/OSF.IO/5YJZW) to ensure methodological transparency. Grey literature was not systematically searched, which may have led to the omission of unpublished or non-indexed studies. However, multiple databases were comprehensively searched, and a rigorous two-reviewer selection process was employed to minimise bias.
Notwithstanding these limitations, this review possesses notable strengths, including a comprehensive multi-database search strategy, exclusive inclusion of RCTs, and methodological appraisal using the PEDro scale. The incorporation of recent studies up to 2025 enhances the relevance of the findings for current clinical practice.
From a clinical perspective, the evidence supports integrating structured exercise and physiotherapy into standard oncology care. Exercise programmes should be initiated early, adapted to individual tolerance and functional capacity, and supervised by trained professionals. Physiotherapy modalities provide additional benefits by targeting sensory symptoms and pain relief, contributing to greater functional independence, psychological well-being, and quality of life.
Overall, this synthesis underscores the growing evidence that structured exercise and physiotherapy represent safe, feasible, and effective components of supportive cancer rehabilitation. Their integration into routine oncology care may help reduce the burden of chemotherapy-induced neuropathy and improve patient outcomes.
Taken together, the consistency of results, high methodological quality of the included RCTs, and absence of major adverse events support a moderate-to-high level of certainty in the evidence that exercise and physiotherapy are effective and safe approaches for managing CIPN.
Future studies should also evaluate cost-effectiveness and implementation strategies to facilitate the integration of these interventions into routine oncology care.
5. Conclusions
This systematic review demonstrates that both exercise and physiotherapy are safe, feasible, and effective interventions for the prevention and management of chemotherapy-induced peripheral neuropathy (CIPN). Programmes combining aerobic, resistance, and sensorimotor training consistently improved sensory and motor symptoms, balance, muscle strength, and overall quality of life in patients undergoing neurotoxic chemotherapy.
Physiotherapy modalities such as photobiomodulation and transcutaneous electrical stimulation also produced clinically meaningful benefits without adverse effects, confirming their feasibility as complementary strategies within comprehensive oncology rehabilitation programmes.
Although variability in intervention design and outcome measures limits direct comparison, the overall evidence supports the systematic incorporation of structured exercise and physiotherapy into cancer care. Future research should aim to establish standardised and reproducible protocols, determine optimal therapeutic exercise doses, incorporate objective neurophysiological and biochemical markers, and extend follow-up periods to confirm the durability of observed effects.
In conclusion, exercise and physiotherapy represent key non-pharmacological approaches for improving function, reducing neuropathic symptoms, and enhancing quality of life in individuals affected by chemotherapy-induced peripheral neuropathy. These findings provide a moderate-to-high level of confidence supporting their integration into routine oncology rehabilitation practice.
Author Contributions
Conceptualisation, J.A.T.F. and F.L.P.; Methodology, J.A.T.F. and F.L.P.; Formal Analysis, J.A.T.F. and F.L.P.; Writing—original draft preparation, J.A.T.F. and F.L.P.; Writing—review and editing, J.A.T.F. and F.L.P.; Supervision, J.A.T.F. and F.L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 6MWT | Six-Minute Walk Test |
| ADL-MHQ | Activities of Daily Living—Michigan Hand Outcomes Questionnaire |
| ASCO | American Society of Clinical Oncology |
| BDNF | Brain-Derived Neurotrophic Factor |
| BFI | Brief Fatigue Inventory |
| BOD POD | Air displacement plethysmography system for body composition analysis |
| BR23 | EORTC Quality of Life Questionnaire–Breast Cancer Module |
| CIPN | Chemotherapy-Induced Peripheral Neuropathy |
| CIPNAT | Chemotherapy-Induced Peripheral Neuropathy Assessment Tool |
| CMJ | Countermovement Jump |
| COP | Centre of Pressure |
| CPET | Cardiopulmonary Exercise Test |
| CTCAE/NCI-CTCAE | Common Terminology Criteria for Adverse Events (National Cancer Institute) |
| DASH | Disabilities of the Arm, Shoulder and Hand questionnaire |
| EORTC QLQ-C30 | European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 |
| EORTC QLQ-CIPN20/CIPN15/Taxane/CR29 | EORTC Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy Module (20-, 15-item, Taxane-specific or Colorectal Cancer versions) |
| FAB | Fullerton Advanced Balance scale |
| FACT/GOG-Ntx/FACT/GOG-Taxane | Functional Assessment of Cancer Therapy/Gynaecologic Oncology Group–Neurotoxicity (or Taxane) subscale |
| FES-I | Falls Efficacy Scale–International |
| GGT-Reha | German Geriatric Rehabilitation Test battery |
| h1RM | Half of the one-repetition maximum strength test (50% 1RM) |
| HADS | Hospital Anxiety and Depression Scale |
| HIIT | High-Intensity Interval Training |
| HTEMS | High-Tone External Muscle Stimulation |
| ISI | Insomnia Severity Index |
| LANSS/S-LANSS | Leeds Assessment of Neuropathic Symptoms and Signs (Self-Reported version) |
| MFI-20 | Multidimensional Fatigue Inventory (20-item) |
| MHQ | Michigan Hand Outcomes Questionnaire |
| MICE | Moderate-Intensity Continuous Exercise |
| MDNS | Modified Diabetic Neuropathy Score |
| MLS | Multiwave Locked System (Class IV therapeutic laser) |
| mTNS/TNS/TNSr | (Modified/Revised) Total Neuropathy Score |
| NAS | Numeric Analogue Scale (pain, tingling, numbness) |
| NCV/NCS | Nerve Conduction Velocity/Nerve Conduction Studies |
| NCI-CTC 4.0/5.0 | National Cancer Institute Common Toxicity Criteria (version 4.0/5.0) |
| NPRS/NRS | Numeric Pain Rating Scale/Numeric Rating Scale |
| NTSS-6 | Neuropathy Total Symptom Score–6 |
| PCS | Pain Catastrophising Scale |
| PBM | Photobiomodulation |
| PEDro | Physiotherapy Evidence Database |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROMIS Physical Function | Patient-Reported Outcomes Measurement Information System–Physical Function scale |
| QoL | Quality of Life |
| QST | Quantitative Sensory Testing |
| RCT | Randomised Controlled Trial |
| RDI | Rate of Decline Index |
| RT | Resistance Training |
| Rydel-Seiffer tuning fork | Graduated tuning fork used for vibration sensitivity testing |
| SPPB | Short Physical Performance Battery |
| SMT | Sensorimotor Training |
| SWMT | Semmes–Weinstein Monofilament Test |
| TENS | Transcutaneous Electrical Nerve Stimulation |
| TES | Treatment Expectancy Scale |
| UENS | Utah Early Neuropathy Scale |
| VAS | Visual Analogue Scale |
| WBV | Whole-Body Vibration |
References
- Windebank, A.J.; Grisold, W. Chemotherapy-induced neuropathy. J. Peripher. Nerv. Syst. 2008, 13, 27–46. [Google Scholar] [CrossRef]
- Shah, A.; Hoffman, E.M.; Mauermann, M.L.; Loprinzi, C.L.; Windebank, A.J.; Klein, C.J.; Staff, N.P. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J. Neurol. Neurosurg. Psychiatry 2018, 89, 636–641. [Google Scholar] [CrossRef]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2016, 32, 1941–1967. [Google Scholar] [CrossRef]
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Curr. Opin. Neurol. 2015, 28, 500–507. [Google Scholar] [CrossRef]
- Mols, F.; Beijers, T.; Vreugdenhil, G.; van de Poll-Franse, L.V. Chemotherapy-induced peripheral neuropathy and its association with quality of life: A systematic review. Support. Care Cancer 2014, 22, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: A comprehensive literature review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef]
- Lemanne, D.; Cassileth, B.; Gubili, J. The role of physical activity in cancer prevention, treatment, recovery, and survivorship. Oncology 2013, 27, 580–585. [Google Scholar]
- Andersen Hammond, E.; Pitz, M.; Steinfeld, K.; Lambert, P.; Shay, B. An exploratory randomised trial of physical therapy for the treatment of chemotherapy-induced peripheral neuropathy. Neurorehabil. Neural Repair. 2020, 34, 235–246. [Google Scholar] [CrossRef]
- Joy, L.; Jolien, R.; Marithé, C.; Stijn, E.; Laura, S.; Hilde, L.; Sandra, B.; Wendy, N.; Ruth, H.; Liesbeth, R.; et al. The use of photobiomodulation therapy for the prevention of chemotherapy-induced peripheral neuropathy: A randomized, placebo-controlled pilot trial (NEUROLASER trial). Support. Care Cancer 2022, 30, 5509–5517. [Google Scholar] [CrossRef]
- Sassmann, R.; Gampenrieder, S.P.; Rieder, F.; Johansson, T.; Rinnerthaler, G.; Castagnaviz, V.; Lampl, K.; Herfert, J.; Kienberger, Y.T.; Flamm, M.; et al. Electrotherapy as treatment for chemotherapy-induced peripheral neuropathy: A randomized controlled trial. Front. Neurol. 2024, 15, 1451456. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, I.R.; Kamen, C.; Gewandter, J.S.; Mohile, N.A.; Heckler, C.E.; Culakova, E.; Fung, C.; Janelsins, M.C.; Asare, M.; Lin, P.-J.; et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: A multicentre, randomized controlled trial. Support. Care Cancer 2018, 26, 1019–1028. [Google Scholar] [CrossRef]
- Streckmann, F.; Lehmann, H.C.; Balke, M.; Schenk, A.; Oberste, M.; Heller, A.; Schürhörster, A.; Elter, T.; Bloch, W.; Baumann, F.T. Sensorimotor training and whole-body vibration training have the potential to reduce motor and sensory symptoms of chemotherapy-induced peripheral neuropathy—A randomized controlled pilot trial. Support. Care Cancer 2019, 27, 2471–2478. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomised controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Al Onazi, M.M.; Yurick, J.L.; Harris, C.; Nishimura, K.; Suderman, K.; Pituskin, E.; Chua, N.; McNeely, M.L. Therapeutic ultrasound for chemotherapy-related pain and sensory disturbance in the hands and feet in patients with colorectal cancer: A pilot randomised controlled trial. J. Pain Symptom Manag. 2021, 61, 1127–1138. [Google Scholar] [CrossRef]
- Argenta, P.A.; Ballman, K.V.; Geller, M.A.; Carson, L.F.; Ghebre, R.; Mullany, S.A.; Teoh, D.G.; Winterhoff, B.J.; Rivard, C.L.; Erickson, B.K. The effect of photobiomodulation on chemotherapy-induced peripheral neuropathy: A randomised, sham-controlled clinical trial. Gynecol. Oncol. 2017, 144, 159–166. [Google Scholar] [CrossRef]
- Bao, T.; Zhi, I.; Baser, R.; Hooper, M.; Chen, C.; Piulson, L.; Li, Q.S.; Galantino, M.L.; Blinder, V.; Robson, M.; et al. Yoga for chemotherapy-induced peripheral neuropathy and fall risk: A randomized controlled trial. JNCI Cancer Spectr. 2020, 4, pkaa048. [Google Scholar] [CrossRef] [PubMed]
- Bland, K.A.; Kirkham, A.A.; Bovard, J.; Shenkier, T.; Zucker, D.; McKenzie, D.C.; Davis, M.K.; Gelmon, K.A.; Campbell, K.L. Effect of exercise on taxane chemotherapy-induced peripheral neuropathy in women with breast cancer: A randomised controlled trial. Clin. Breast Cancer 2019, 19, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Cartmel, B.; Li, F.Y.; Gottlieb, L.T.; Harrigan, M.; Ligibel, J.A.; Gogoi, R.; Schwartz, P.E.; Esserman, D.A.; Irwin, M.L.; et al. Effect of exercise on chemotherapy-induced peripheral neuropathy among patients treated for ovarian cancer: A secondary analysis of a randomised clinical trial. JAMA Netw. Open 2023, 6, e2326463. [Google Scholar] [CrossRef]
- Dhawan, S.; Andrews, R.; Kumar, L.; Wadhwa, S.; Shukla, G. A randomized controlled trial to assess the effectiveness of muscle strengthening and balancing exercises on chemotherapy-induced peripheral neuropathic pain and quality of life among cancer patients. Cancer Nurs. 2020, 43, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, İ.; Kutlutürkan, S. The effect of hand-foot exercises on chemotherapy-induced peripheral neuropathy-related pain, falls, and quality of life in colorectal cancer: A randomised controlled trial. Eur. J. Oncol. Nurs. 2024, 71, 102641. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Kim, I.Y.; Hur, H.K.; Lee, J.Y.; Park, S.M. The Effects of an app-based physical activity programme on colorectal cancer patients undergoing chemotherapy: A randomised controlled trial. Cancer Nurs. 2025, 48, E90–E97. [Google Scholar] [CrossRef]
- Ikio, Y.; Sagari, A.; Nakashima, A.; Matsuda, D.; Sawai, T.; Higashi, T. Efficacy of combined hand exercise intervention in patients with chemotherapy-induced peripheral neuropathy: A pilot randomised controlled trial. Support. Care Cancer 2022, 30, 4981–4992. [Google Scholar] [CrossRef] [PubMed]
- Izgu, N.; Metin, Z.G.; Karadas, C.; Ozdemir, L.; Çetin, N.; Demirci, U. Prevention of chemotherapy-induced peripheral neuropathy with classical massage in breast cancer patients receiving paclitaxel: An assessor-blinded randomised controlled trial. Eur. J. Oncol. Nurs. 2019, 40, 36–43. [Google Scholar] [CrossRef]
- Kneis, S.; Wehrle, A.; Müller, J.; Maurer, C.; Ihorst, G.; Gollhofer, A.; Bertz, H. It’s never too late-balance and endurance training improves functional performance, quality of life, and alleviates neuropathic symptoms in cancer survivors suffering from chemotherapy-induced peripheral neuropathy: Results of a randomized controlled trial. BMC Cancer 2019, 19, 414. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Le-Rademacher, J.G.; Majithia, N.; McMurray, R.P.; O’Neill, C.R.; Bendel, M.A.; Beutler, A.; Lachance, D.H.; Cheville, A.; Strick, D.M.; et al. Scrambler therapy for chemotherapy neuropathy: A randomised phase II pilot trial. Support. Care Cancer 2020, 28, 1183–1197. [Google Scholar] [CrossRef]
- Moraitis, A.M.; Rose, N.B.; Johnson, A.F.; Dunston, E.R.; Garrido-Laguna, I.; Hobson, P.; Barber, K.; Basen-Engquist, K.; Coletta, A.M. Feasibility and acceptability of an mHealth, home-based exercise intervention in colorectal cancer survivors: A pilot randomised controlled trial. PLoS ONE 2023, 18, e0287152. [Google Scholar] [CrossRef]
- Müller, J.; Weiler, M.; Schneeweiss, A.; Haag, G.M.; Steindorf, K.; Wick, W.; Wiskemann, J. Preventive effect of sensorimotor exercise and resistance training on chemotherapy-induced peripheral neuropathy: A randomised controlled trial. Br. J. Cancer 2021, 125, 955–965. [Google Scholar] [CrossRef]
- Saraboon, C.; Siriphorn, A. Effects of foam pad balance exercises on cancer patients undergoing chemotherapy: A randomized controll trial. J. Bodyw. Mov. Ther. 2021, 28, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Uysal, N.; Ünal Toprak, F. The effect of hand and foot exercises on peripheral neuropathy and quality of life in women with breast cancer: A randomised controlled trial. Support. Care Cancer 2025, 33, 83. [Google Scholar] [CrossRef]
- Vollmers, P.L.; Mundhenke, C.; Maass, N.; Bauerschlag, D.; Kratzenstein, S.; Röcken, C.; Schmidt, T. Evaluation of the effects of sensorimotor exercise on physical and psychological parameters in breast cancer patients undergoing neurotoxic chemotherapy. J. Cancer Res. Clin. Oncol. 2018, 144, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Waibel, S.; Wehrle, A.; Müller, J.; Bertz, H.; Maurer, C. Type of exercise may influence postural adaptations in chemotherapy-induced peripheral neuropathy. Ann. Clin. Transl. Neurol. 2021, 8, 1680–1694. [Google Scholar] [CrossRef]
- Xiaoqian, Y.; Jiwei, H.; Lizhi, Z.; Baojia, G.; Luyan, G.; Huiqian, X.; Hong, L.; Yijing, F. A randomized controlled trial: Effects of compression therapy combined with exercise on chemotherapy-induced peripheral neuropathy in patients with breast cancer. Cancer Treat. Res. Commun. 2025, 42, 100871. [Google Scholar] [CrossRef]
- Zhi, W.I.; Baser, R.E.; Zhi, L.M.; Talukder, D.; Li, Q.S.; Paul, T.; Patterson, C.; Piulson, L.; Seluzicki, C.; Galantino, M.L.; et al. Yoga for cancer survivors with chemotherapy-induced peripheral neuropathy: Health-related quality of life outcomes. Cancer Med. 2021, 10, 5456–5465. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Trebing, S.; Timmers-Trebing, U.; Schenk, A.; Paust, R.; Bloch, W.; Rudolph, R.; Streckmann, F.; Baumann, F.T. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: A randomized controlled trial. Support. Care Cancer 2018, 26, 615–624. [Google Scholar] [CrossRef]
- Stuecher, K.; Bolling, C.; Vogt, L.; Niederer, D.; Schmidt, K.; Dignaß, A.; Banzer, W. Exercise improves functional capacity and lean body mass in patients with gastrointestinal cancer during chemotherapy: A single-blind randomised controlled trial. Support. Care Cancer 2019, 27, 2159–2169. [Google Scholar] [CrossRef]
- Streckmann, F.; Zopf, E.M.; Lehmann, H.C.; May, K.; Rizza, J.; Zimmer, P.; Gollhofer, A.; Bloch, W.; Baumann, F.T. Exercise intervention studies in patients with peripheral neuropathy: A systematic review. Sports Med. 2014, 44, 1289–1304. [Google Scholar] [CrossRef]
- Amarelo, A.; da Mota, M.C.C.; Amarelo, B.L.P.; Ferreira, M.C.; Fernandes, C.S. Effects of physical exercise on chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain Manag. Nurs. 2025, 26, 212–221. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).