Association of Systemic Inflammation with Nocturnal Sleeping Time Among Terminally Ill Patients with Cancer: Preliminary Findings
Abstract
1. Introduction
2. Participants and Methods
2.1. Sites and Participants
2.2. Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Lippitz, B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013, 14, 218–228. [Google Scholar] [CrossRef]
- Candido, J.; Hagemann, T. Cancer-related inflammation. J. Clin. Immunol. 2013, 33 (Suppl. S1), 79–84. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.; Diba, P.; Korzun, T.; Marks, D.L. Neural Mechanisms of Cancer Cachexia. Cancers 2021, 13, 3990. [Google Scholar] [CrossRef]
- Stagikas, D.; Simos, Y.V.; Lakkas, L.; Filis, P.; Peschos, D.; Tsamis, K.I. The role of the hypothalamus in the development of cancer cachexia. Physiol. Behav. 2025, 295, 114909. [Google Scholar] [CrossRef]
- Simoes, E.; Uchida, R.; Nucci, M.P.; Duran, F.L.S.; Lima, J.D.C.C.; Gama, L.R.; Costa, N.A.; Otaduy, M.C.G.; Bin, F.C.; Otoch, J.P.; et al. Cachexia Alters Central Nervous System Morphology and Functionality in Cancer Patients. J. Cachexia Sarcopenia Muscle 2025, 16, e13742. [Google Scholar] [CrossRef]
- Zhu, X.A.; Starosta, S.; Ferrer, M.; Hou, J.; Chevy, Q.; Lucantonio, F.; Muñoz-Castañeda, R.; Zhang, F.; Zang, K.; Zhao, X.; et al. A neuroimmune circuit mediates cancer cachexia-associated apathy. Science 2025, 388, eadm8857. [Google Scholar] [CrossRef]
- Greenlund, I.M.; Carter, J.R. Sympathetic neural responses to sleep disorders and insufficiencies. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H337–H349. [Google Scholar] [CrossRef] [PubMed]
- Amano, K.; Hopkinson, J.; Baracos, V. Psychological symptoms of illness and emotional distress in advanced cancer cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Laird, B.J.A.; Scott, A.C.; Colvin, L.A.; McKeon, A.L.; Murray, G.D.; Fearon, K.C.H.; Fallon, M.T. Cancer pain and its relationship to systemic inflammation: An exploratory study. Pain 2011, 152, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Laird, B.J.; McMillan, D.C.; Fayers, P.; Fearon, K.; Kaasa, S.; Fallon, M.T.; Klepstad, P. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist 2013, 18, 1050–1055. [Google Scholar] [CrossRef]
- Amano, K.; Maeda, I.; Morita, T.; Miura, T.; Inoue, S.; Ikenaga, M.; Matsumoto, Y.; Baba, M.; Sekine, R.; Yamaguchi, T.; et al. Clinical implications of C-reactive protein as a prognostic marker in advanced cancer patients in palliative care settings. J. Pain. Symptom Manag. 2016, 51, 860–867. [Google Scholar] [CrossRef]
- Paulsen, Ø.; Laird, B.; Aass, N.; Lea, T.; Fayers, P.; Kaasa, S.; Klepstad, P. The relationship between proinflammatory cytokines and pain, appetite and fatigue in patients with advanced cancer. PLoS ONE 2017, 12, e0177620. [Google Scholar] [CrossRef]
- Kwekkeboom, K.L.; Tostrud, L.; Costanzo, E.; Coe, C.L.; Serlin, R.C.; Ward, S.E.; Zhang, Y. The role of inflammation in the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. J. Pain. Symptom Manag. 2018, 55, 1286–1295. [Google Scholar] [CrossRef]
- Miranda, D.O.; Anatriello, E.; Azevedo, L.R.; Cordeiro, J.F.C.; Peria, F.M.; Flória-Santos, M.; Pereira-da-Silva, G. Elevated serum levels of proinflammatory cytokines potentially correlate with depression and anxiety in colorectal cancer patients in different stages of the antitumor therapy. Cytokine 2018, 104, 72–77. [Google Scholar] [CrossRef]
- Amano, K.; Hatano, Y.; Matsuda, Y.; Maeda, I.; Ishiki, H.; Miura, T.; Imai, K.; Hori, T.; Funaki, H.; Suzuki, K.; et al. C-reactive protein, delirium, and other psychological symptoms among patients with advanced cancer. JCSM Clin. Rep. 2020, 5, 42–51. [Google Scholar] [CrossRef]
- Yule, M.S.; Brown, L.R.; Skipworth, R.J.E.; Laird, B.J.A. Central neural mechanisms of cancer cachexia. Curr. Opin. Support. Palliat. Care 2024, 18, 138–144. [Google Scholar] [CrossRef]
- Kieler, M.; Kössler, P.; Milovic, M.; Meyer, E.; Križanová, K.; Kum, L.; Friedrich, A.; Masel, E.; Bauer, R.; Unseld, M. C-reactive protein and white blood cell count are adverse prognostic markers for patients with advanced cancer on parenteral nutrition in a palliative care unit setting: A retrospective cohort study. Palliat. Med. 2022, 36, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Westgaard, A.; Pirnat, A.; Hjermstad, M.J.; Aass, N.; Kaasa, S.; Dajani, O.F. Prognostic Value of Performance Status, Albumin, and CRP in Last-Line Chemotherapy for Pancreatic vs. Other Gastrointestinal Cancers-Simple Tools Matter. Curr. Oncol. 2024, 31, 5462–5471. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; O’ROurke, F.; Will, S.; Nguyen, H.T.N.; Cranfield, E.; Maseland, C.; MacLeod, N.; Maclay, J.D.; Laird, B.J.; Dolan, R.D.; et al. The prevalence and prognostic value of systemic inflammation in good performance status patients with advanced, inoperable non-small cell lung cancer receiving palliative radiotherapy: Comparison of composite ratios and cumulative scores. Cancer Med. 2024, 13, e70139. [Google Scholar] [CrossRef] [PubMed]
- Yule, M.S.; Machado, A.M.; Brown, L.R.; Rocha, B.M.; Patton, R.; Sayers, J.; Munro, I.; Baxter, J.; McLuskie, A.; Lajolo, P.P.; et al. Dissecting the global leadership initiative on malnutrition criteria in advanced cancer: Reduced intake vs. inflammation. Clin. Nutr. ESPEN 2025, 67, 114–121. [Google Scholar] [CrossRef]
- Pumtako, C.; Dolan, R.D.; Fallon, M.; Sullivan, E.S.; Simmons, C.P.; Ryan, A.M.; McGovern, J.; Power, D.G.; Laird, B.J.; McMillan, D.C. The Global Leadership Initiative on Malnutrition (GLIM) inflammation criteria to predict survival in patients with advanced cancer: A prospective cohort study. Clin. Nutr. ESPEN 2025, 67, 344–352. [Google Scholar] [CrossRef]
- Vagnildhaug, O.M.; Habberstad, R.H.; Salvesen, Ø.; Balstad, T.R.; Bye, A.; Dajani, O.; Kaasa, S.; Klepstad, P.; Solheim, T.S. A comparison of inflammatory markers’ potential to predict weight loss in advanced cancer: A prospective observational study. J. Circ. Biomark. 2025, 14, 12–20. [Google Scholar] [CrossRef]
- Otani, H.; Yokomichi, N.; Imai, K.; Toyota, S.; Yamauchi, T.; Miwa, S.; Yuasa, M.; Okamoto, S.; Kogure, T.; Inoue, S.; et al. A Novel Objective Measure for Terminal Delirium: Activity Scores Measured by a Sheet-Type Sensor. J. Pain. Symptom Manag. 2024, 68, 246–254. [Google Scholar] [CrossRef]
- Kogure, T.; Shirakawa, S.; Shimokawa, M.; Masato, S.; Hosokawa, Y. Automatic sleep/wake scoring from body motion in bed: Validation of a newly developed sensor placed under a mattress. J. Physiol. Anthropol. 2011, 30, 103–109. [Google Scholar] [CrossRef]
- Collins, E.S.; Witt, J.; Bausewein, C.; Daveson, B.A.; Higginson, I.J.; Murtagh, F.E. A Systematic Review of the Use of the Palliative Care Outcome Scale and the Support Team Assessment Schedule in Palliative Care. J. Pain. Symptom Manag. 2015, 50, 842–853. [Google Scholar] [CrossRef]
- Schildmann, E.K.; Groeneveld, E.I.; Denzel, J.; Brown, A.; Bernhardt, F.; Bailey, K.; Guo, P.; Ramsenthaler, C.; Lovell, N.; Higginson, I.J.; et al. Discovering the hidden benefits of cognitive interviewing in two languages: The first phase of a validation study of the Integrated Palliative care Outcome Scale. Palliat. Med. 2016, 30, 599–610. [Google Scholar] [CrossRef]
- Mawatari, H.; Shinjo, T.; Morita, T.; Kohara, H.; Yomiya, K. Revision of Pharmacological Treatment Recommendations for Cancer Pain: Clinical Guidelines from the Japanese Society of Palliative Medicine. J. Palliat. Med. 2022, 25, 1095–1114. [Google Scholar] [CrossRef]
- Ely, E.W.; Truman, B.; Shintani, A.; Thomason, J.W.; Wheeler, A.P.; Gordon, S.; Francis, J.; Speroff, T.; Gautam, S.; Margolin, R.; et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003, 289, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Rosario, M.A.; Castillo-Padrós, M.; Garrido-Bernet, B.; González-Guillermo, T.; Martínez-Castillo, L.P.; González, A. Appropriateness and reliability testing of the modified Richmond Agitation-Sedation Scale in Spanish patients with advanced cancer. J. Pain. Symptom Manag. 2013, 45, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Miyashita, M.; Imai, K.; Miyamoto, S.; Otani, H.; Oishi, A.; Kizawa, Y.; Matsushima, E. Validation of the Integrated Palliative care Outcome Scale (IPOS)—Japanese Version. Jpn. J. Clin. Oncol. 2019, 49, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Morita, T.; Mori, M.; Yokomichi, N.; Fukuta, K. Development and linguistic validation of the Japanese version of the modified Richmond Agitation-Sedation Scale. Palliat. Care Res. 2016, 11, 331–336. (In Japanese) [Google Scholar] [CrossRef]
- Morley, J.J.; Kushner, I. Serum C-reactive protein levels in disease. Ann. N. Y. Acad. Sci. 1982, 389, 406–418. [Google Scholar] [CrossRef]
- Merker, M.; Felder, M.; Gueissaz, L.; Bolliger, R.; Tribolet, P.; Kägi-Braun, N.; Gomes, F.; Hoess, C.; Pavlicek, V.; Bilz, S.; et al. Association of Baseline Inflammation With Effectiveness of Nutritional Support Among Patients With Disease-Related Malnutrition: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e200663. [Google Scholar] [CrossRef]
- Admane, S.; Pasyar, S.; Bassett RJr Bruera, E.; Hui, D. Circadian rhythm in end-of-life delirium: A secondary analysis of two randomized controlled trials. J. Pain. Symptom Manag. 2025, 70, 131–139.e2. [Google Scholar] [CrossRef]
- Shakhar, K.; Shakhar, G. Why Do We Feel Sick When Infected—Can Altruism Play a Role? PLoS Biol. 2015, 13, e1002276. [Google Scholar] [CrossRef]
- Kelley, K.W.; Kent, S. The Legacy of Sickness Behaviors. Front. Psychiatry 2020, 11, 607269. [Google Scholar] [CrossRef]
- Eba, J.; Nakamura, K. Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn. J. Clin. Oncol. 2022, 52, 539–544. [Google Scholar] [CrossRef]
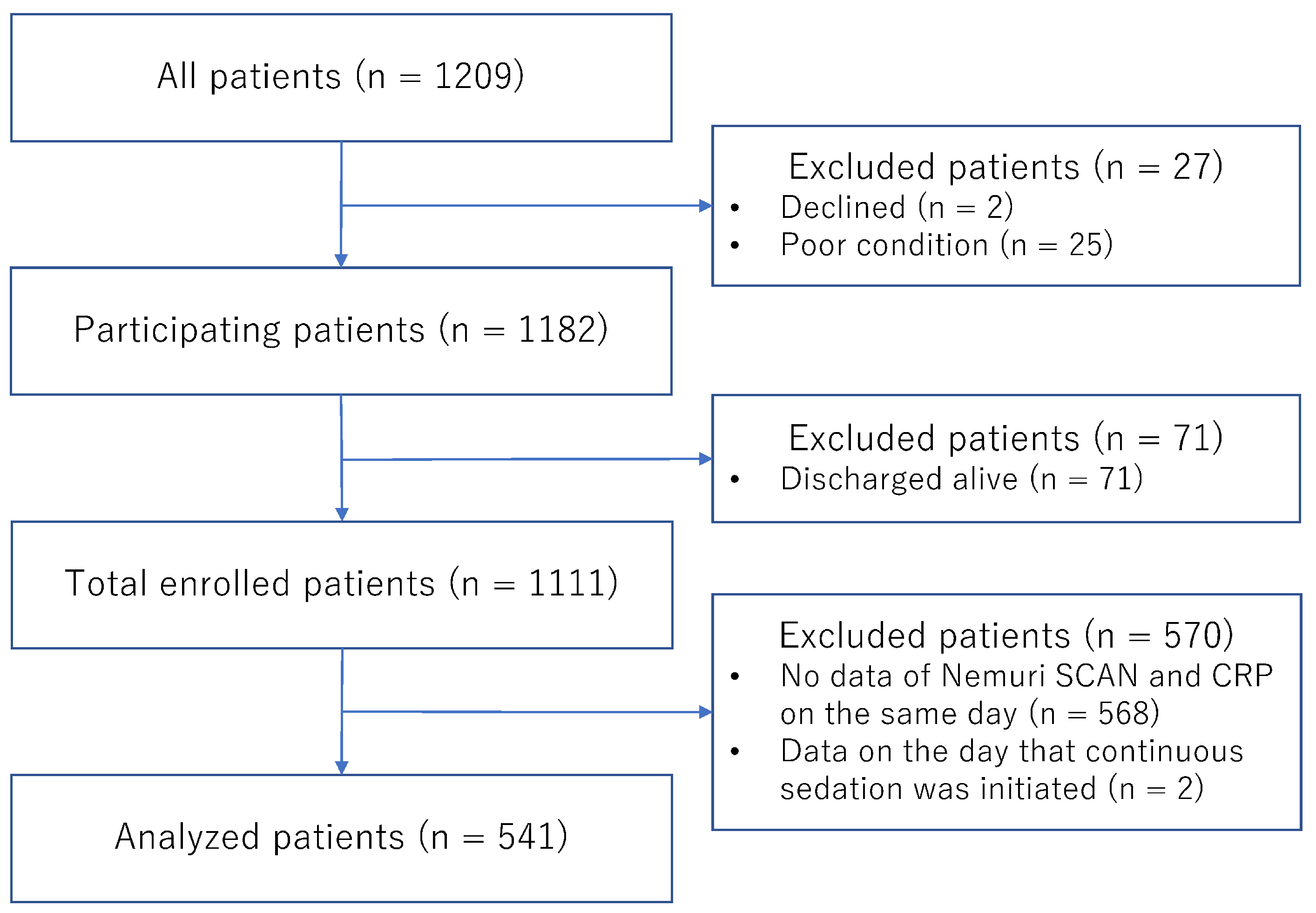
| Age in years | 75 (69, 82) |
| Sex | |
| Male | 302 (55.8) |
| Female | 239 (44.2) |
| Primary cancer site | |
| Upper and lower gastrointestinal tracts | 134 (24.8) |
| Liver, biliary system, and pancreas | 118 (21.8) |
| Lung | 114 (21.1) |
| Urological | 42 (7.8) |
| Gynecologic | 26 (4.8) |
| Breast | 25 (4.6) |
| Hematological | 16 (3.0) |
| Others | 66 (12.2) |
| Metastasis | |
| Lung | 227 (42.0) |
| Liver | 201 (37.2) |
| Bone | 117 (21.6) |
| Central nervous system | 67 (12.4) |
| Total (n = 541) | CRP < 1 (n = 66) | 1 ≤ CRP < 10 (n = 290) | 10 ≤ CRP (n = 185) | p-Value | |
|---|---|---|---|---|---|
| Symptom, Integrated Palliative Care Outcome Scale (0–4) | |||||
| Pain | 1 (0, 1) | 0 (0, 1) | 0 (0, 1) | 1 (0, 1) | 0.014 |
| Dyspnea | 0 (0, 1) | 0 (0, 0) | 0 (0, 1) | 0 (0, 1) | 0.067 |
| Fatigue | 1 (1, 1) | 1 (0, 1) | 1 (1, 1) | 1 (1, 1) | 0.096 |
| Nausea | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.234 |
| Opioid oral morphine milligram equivalent (mg/day) | 24.0 (0.0, 60.0) | 10.6 (0.0, 30.0) | 24.0 (0.0, 48.0) | 24.0 (10.0, 84.0) | <0.001 |
| Benzodiazepine and/or psychotropic drug | 291 (53.8) | 35 (53.0) | 142 (49.0) | 114 (61.6) | 0.022 |
| Modified Richmond Agitation-Sedation Scale (−5 to +4) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.480 |
| Survival time (days) | 11.0 (6.0, 19.0) | 13.0 (6.8, 21.0) | 11.0 (6.0, 20.0) | 10.0 (5.0, 16.0) | 0.079 |
| Total (n = 541) | CRP < 1 (n = 66) | 1 ≤ CRP < 10 (n = 290) | 10 ≤ CRP (n = 185) | p-Value | |
|---|---|---|---|---|---|
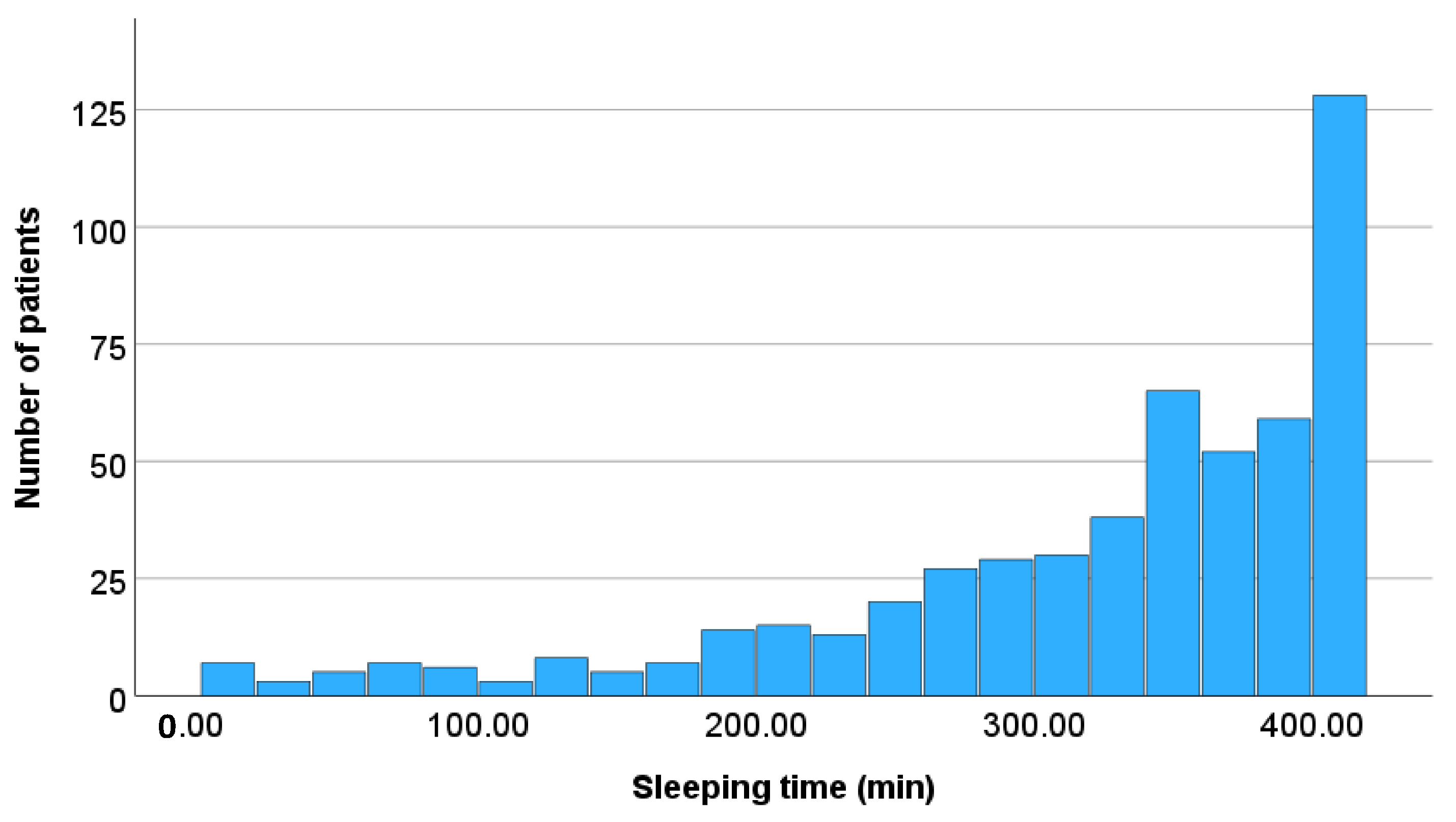
| Total sleeping time (min/day) | 350.0 (275.0, 397.0) 321.5 ± 96.9 | 340.5 (265.3, 384.3) 314.0 ± 95.7 | 347.5 (264.5, 398.3) 319.1 ± 98.0 | 352.0 (301.5, 398.5) 327.8 ± 95.8 | 0.487 |
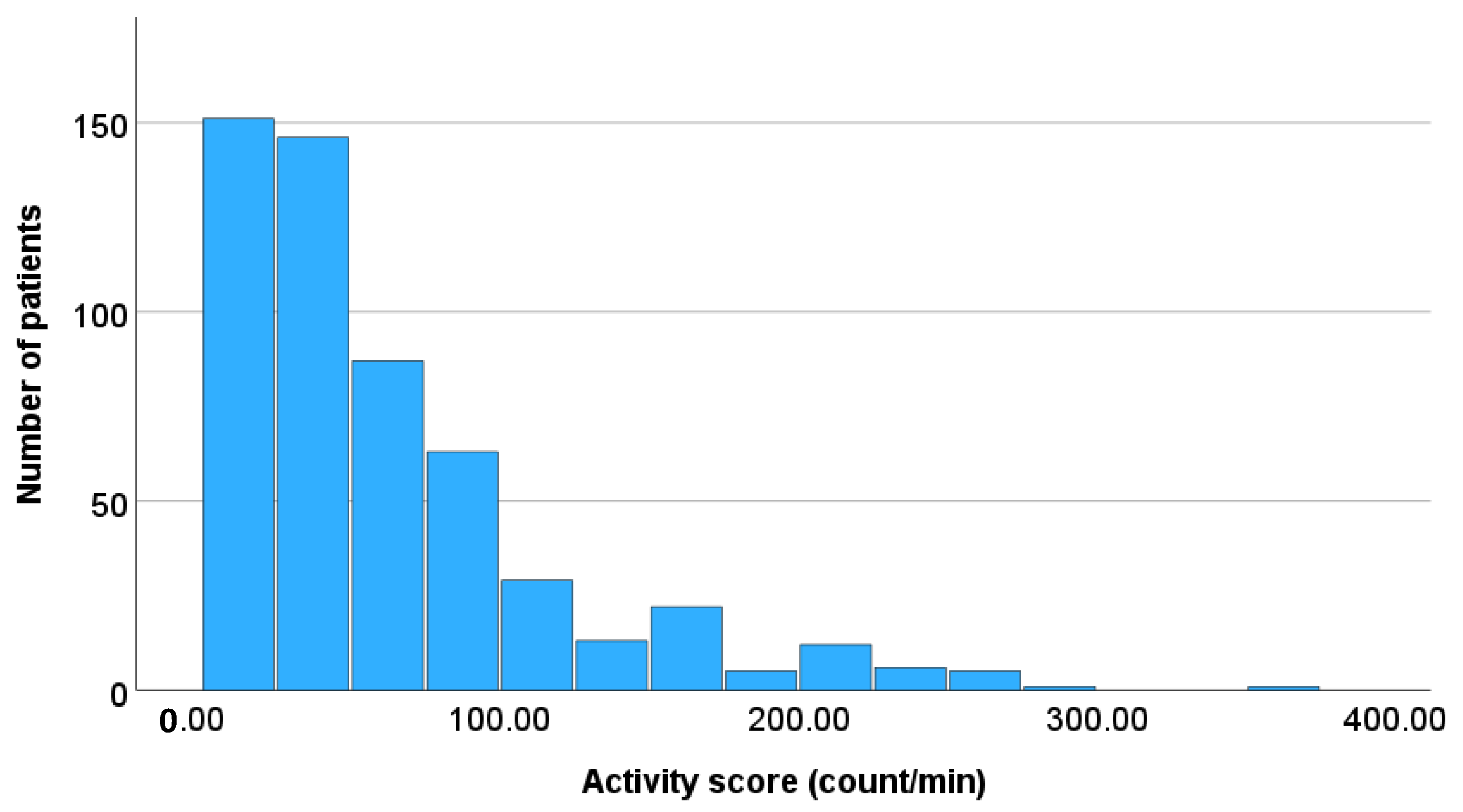
| Activity score (count/min) | 45.1 (22.5, 81.5) 61.6 ± 56.5 | 54.5 (22.5, 88.1) 65.1 ± 53.8 | 45.8 (22.2, 87.8) 63.1 ± 58.7 | 41.2 (22.6, 71.6) 58.1 ± 53.9 | 0.556 |
| Time away from bed (min) | 0.0 (0.0, 11.0) 10.4 ± 23.1 | 0.0 (0.0, 13.5) 9.0 ± 13.8 | 0.0 (0.0, 11.3) 11.2 ± 24.4 | 0.0 (0.0, 9.0) 9.7 ± 23.6 | 0.620 |
| Number of times away from bed (count) | 0.0 (0.0, 2.0) 1.4 ± 2.4 | 0.0 (0.0, 2.0) 1.2 ± 1.5 | 0.0 (0.0, 2.0) 1.4 ± 2.5 | 0.0 (0.0, 2.0) 1.3 ± 2.4 | 0.643 |
| Respiratory rate (count/min) | 13.9 (11.9, 16.4) 14.5 ± 3.3 | 14.0 (11.8, 16.2) 14.3 ± 3.1 | 13.7 (11.7, 16.1) 14.2 ± 3.1 | 14.1 (12.1, 17.0) 14.9 ± 3.7 | 0.182 |
| Heart rate (count/min) | 83.1 (72.8, 94.6) 83.9 ± 15.5 | 75.9 (67.2, 89.4) 77.8 ± 14.5 | 80.4 (71.3, 92.5) 81.4 ± 14.9 | 91.3 (78.8, 98.1) 90.0 ± 14.7 | <0.001 |
| Long-Survival Group (n = 273) | Short-Survival Group (n = 262) | |||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| CRP (mg/dL) | ||||
| CRP < 1 | 1.00 (reference) | 1.00 (reference) | ||
| 1 ≤ CRP < 10 | 1.93 (0.87, 4.26) | 0.104 | 0.79 (0.33, 1.89) | 0.596 |
| 10 ≤ CRP | 2.81 (1.19, 6.65) | 0.019 | 0.94 (0.38, 2.32) | 0.886 |
| Age, years | 1.02 (0.99, 1.04) | 0.159 | 1.01 (0.98, 1.03) | 0.709 |
| Sex | ||||
| Male | 1.00 (reference) | 1.00 (reference) | ||
| Female | 1.29 (0.75, 2.22) | 0.364 | 1.73 (0.98, 3.05) | 0.060 |
| Primary cancer site | ||||
| Lung | 1.00 (reference) | 1.00 (reference) | ||
| Upper and lower gastrointestinal tracts | 0.62 (0.28, 1.35) | 0.226 | 0.86 (0.41, 1.79) | 0.683 |
| Liver, biliary system, and pancreas | 1.12 (0.51, 2.47) | 0.781 | 0.56 (0.26, 1.21) | 0.143 |
| Breast | 2.30 (0.59, 8.95) | 0.230 | 0.43 (0.11, 1.65) | 0.216 |
| Gynecologic | 1.26 (0.33, 4.78) | 0.731 | 0.97 (0.25, 3.76) | 0.965 |
| Urological | 1.23 (0.40, 3.75) | 0.716 | 1.74 (0.62, 4.88) | 0.293 |
| Hematological | 1.52 (0.30, 7.61) | 0.610 | 1.80 (0.31, 10.34) | 0.512 |
| Others | 0.47 (0.17, 1.30) | 0.145 | 0.79 (0.35, 1.80) | 0.571 |
| Opioid oral morphine milligram equivalent (per 10 mg) | 0.96 (0.92, 0.99) | 0.024 | 0.98 (0.94, 1.01) | 0.145 |
| Benzodiazepine and/or psychotropic drug | ||||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 0.84 (0.50, 1.43) | 0.520 | 1.04 (0.61, 1.76) | 0.896 |
| Long-Survival Group (n = 273) | Short-Survival Group (n = 262) | |||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| CRP (mg/dl) | ||||
| CRP < 1 | 1.00 (reference) | 1.00 (reference) | ||
| 1 ≤ CRP < 10 | 0.66 (0.30, 1.45) | 0.300 | 0.74 (0.31, 1.78) | 0.507 |
| 10 ≤ CRP | 0.46 (0.20, 1.08) | 0.076 | 0.65 (0.26, 1.61) | 0.351 |
| Age, years | 0.99 (0.97, 1.02) | 0.510 | 1.00 (0.97, 1.02) | 0.746 |
| Sex | ||||
| Male | 1.00 (reference) | 1.00 (reference) | ||
| Female | 0.77 (0.45, 1.35) | 0.364 | 0.80 (0.45, 1.43) | 0.450 |
| Primary cancer site | ||||
| Lung | 1.00 (reference) | 1.00 (reference) | ||
| Upper and lower gastrointestinal tracts | 1.68 (0.77, 3.67) | 0.195 | 1.57 (0.74, 3.34) | 0.240 |
| Liver, biliary system, and pancreas | 0.97 (0.44, 2.14) | 0.932 | 3.17 (1.44, 6.97) | 0.004 |
| Breast | 0.95 (0.25, 3.58) | 0.938 | 6.70 (1.50, 29.98) | 0.013 |
| Gynecologic | 0.80 (0.21, 3.09) | 0.749 | 2.43 (0.62, 9.49) | 0.200 |
| Urological | 1.03 (0.34, 3.13) | 0.960 | 0.86 (0.30, 2.50) | 0.786 |
| Hematological | 0.66 (0.13, 3.39) | 0.623 | 0.77 (0.13, 4.48) | 0.773 |
| Others | 3.44 (1.17, 10.14) | 0.025 | 1.66 (0.72, 3.83) | 0.238 |
| Opioid oral morphine milligram equivalent (per 10 mg) | 1.05 (1.01, 1.10) | 0.016 | 1.05 (1.01, 1.09) | 0.018 |
| Benzodiazepine and/or psychotropic drug | ||||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 1.73 (1.02, 2.94) | 0.044 | 0.87 (0.50, 1.49) | 0.608 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amano, K.; Imai, K.; Toyota, S.; Yamauchi, T.; Miwa, S.; Yuasa, M.; Okamoto, S.; Inoue, S.; Kogure, T.; Morita, T. Association of Systemic Inflammation with Nocturnal Sleeping Time Among Terminally Ill Patients with Cancer: Preliminary Findings. Healthcare 2025, 13, 2959. https://doi.org/10.3390/healthcare13222959
Amano K, Imai K, Toyota S, Yamauchi T, Miwa S, Yuasa M, Okamoto S, Inoue S, Kogure T, Morita T. Association of Systemic Inflammation with Nocturnal Sleeping Time Among Terminally Ill Patients with Cancer: Preliminary Findings. Healthcare. 2025; 13(22):2959. https://doi.org/10.3390/healthcare13222959
Chicago/Turabian StyleAmano, Koji, Kengo Imai, Saori Toyota, Toshihiro Yamauchi, Satoru Miwa, Misuzu Yuasa, Soichiro Okamoto, Satoshi Inoue, Takamasa Kogure, and Tatsuya Morita. 2025. "Association of Systemic Inflammation with Nocturnal Sleeping Time Among Terminally Ill Patients with Cancer: Preliminary Findings" Healthcare 13, no. 22: 2959. https://doi.org/10.3390/healthcare13222959
APA StyleAmano, K., Imai, K., Toyota, S., Yamauchi, T., Miwa, S., Yuasa, M., Okamoto, S., Inoue, S., Kogure, T., & Morita, T. (2025). Association of Systemic Inflammation with Nocturnal Sleeping Time Among Terminally Ill Patients with Cancer: Preliminary Findings. Healthcare, 13(22), 2959. https://doi.org/10.3390/healthcare13222959





