Comparative Efficacy of Pharmacological Interventions for Chronic Prostatitis/Chronic Pelvic Pain Syndrome: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Databases and Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Quality of Studies
2.5. Certainty of Evidence (GRADE Assessment)
2.6. Statistical Analysis
3. Results
3.1. Screening and Flow of Studies
Characteristics of Eligible Studies
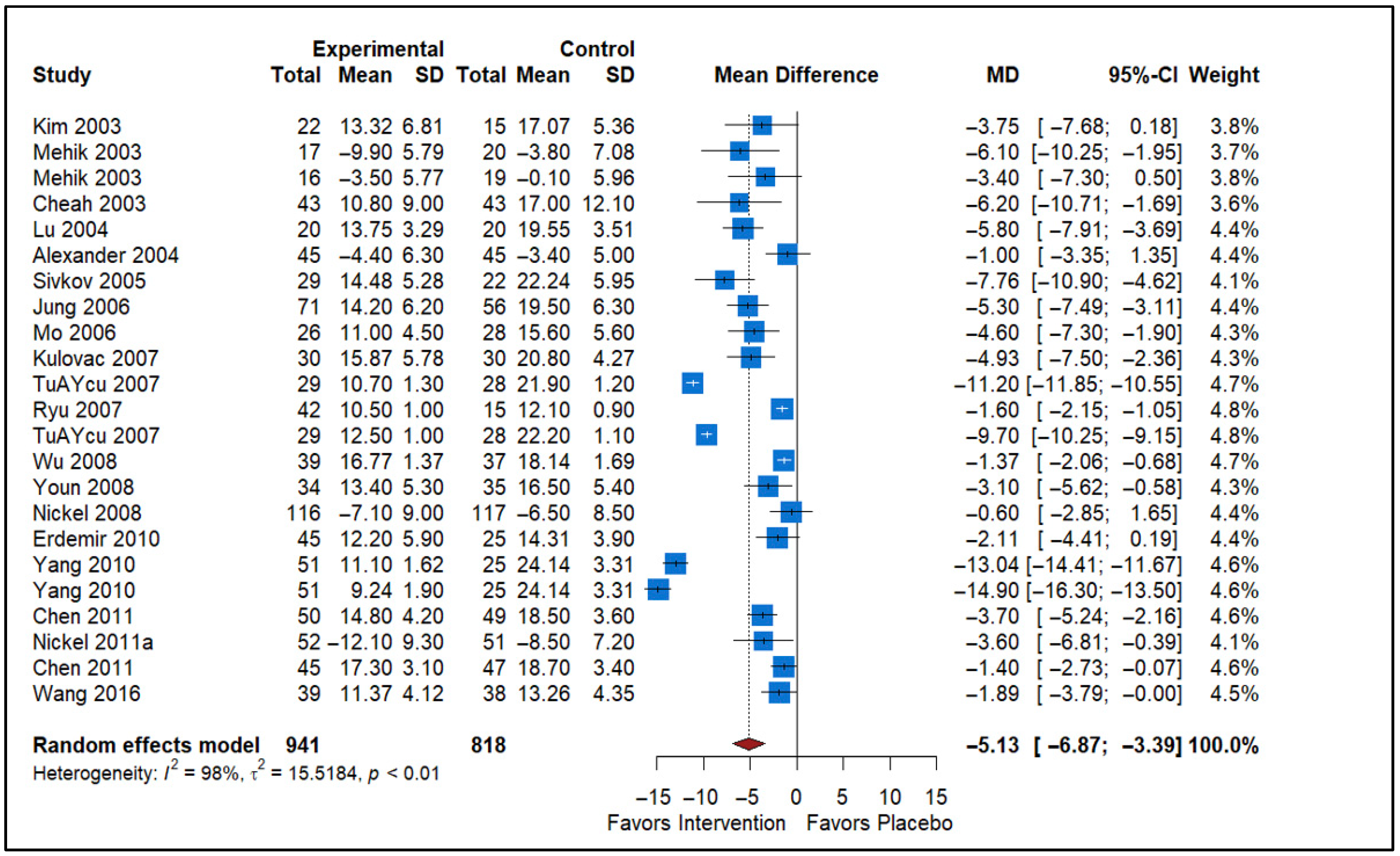
3.2. Effect of Alpha Blockers vs. Placebo
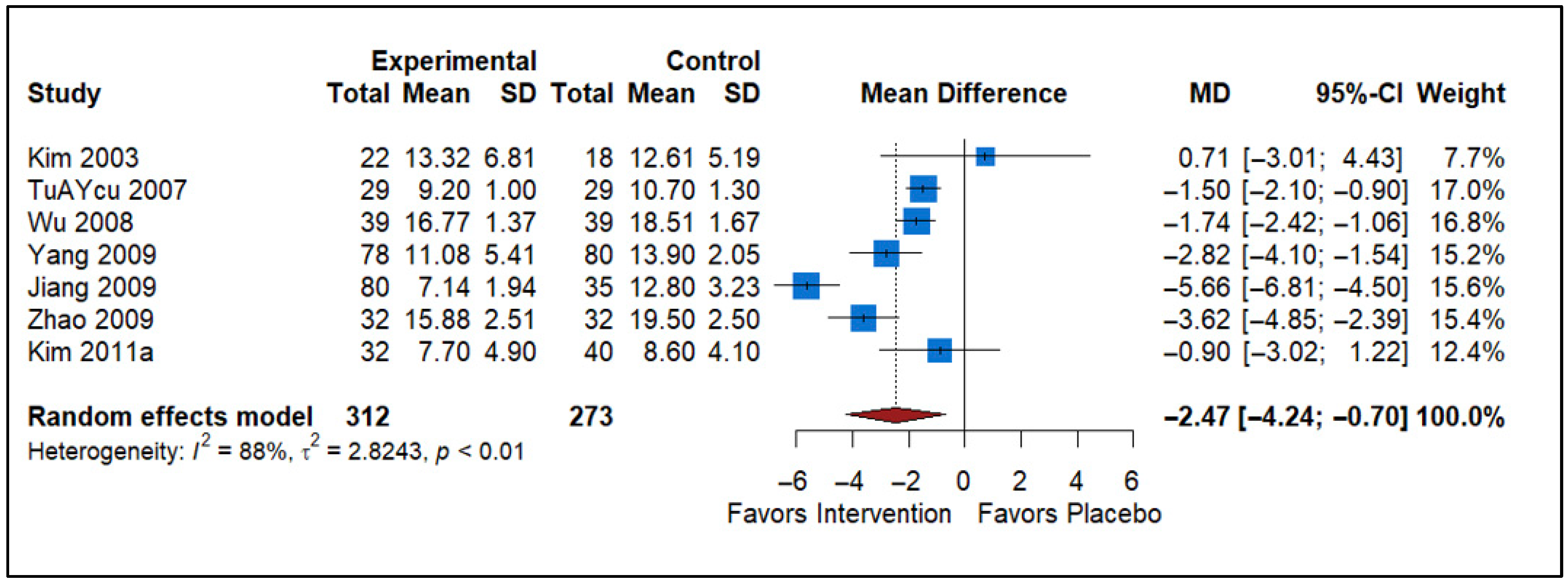
3.3. Effect of Analgesics vs. Placebo
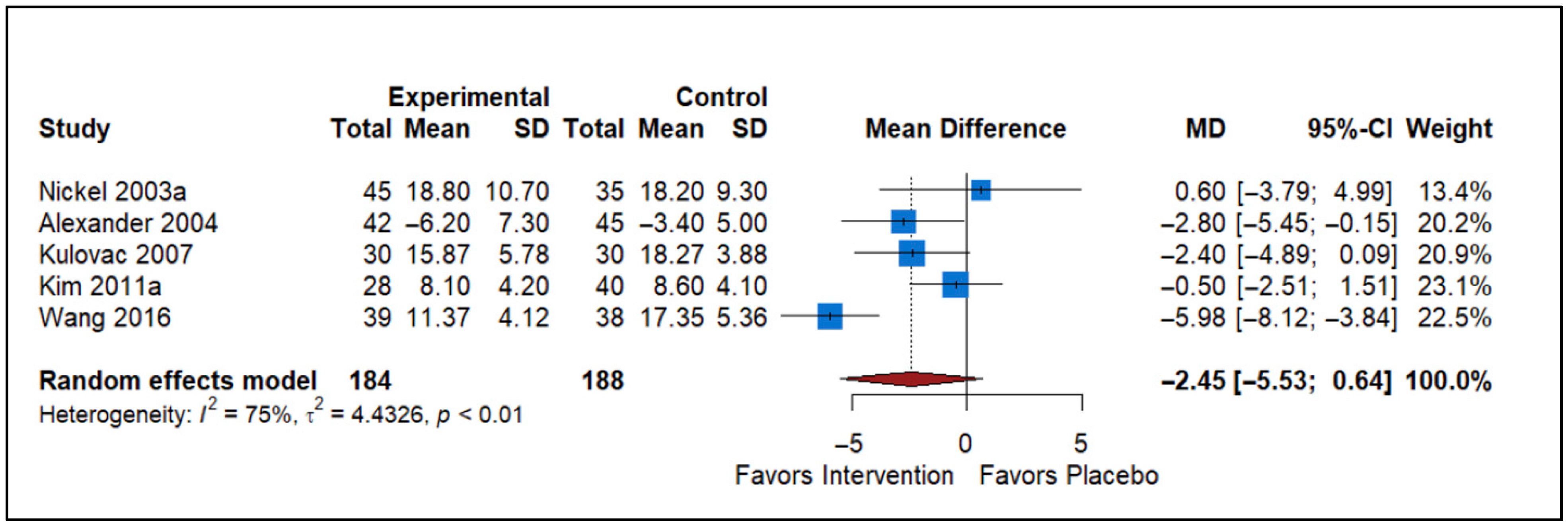
3.4. Effect of Antibiotics vs. Placebo
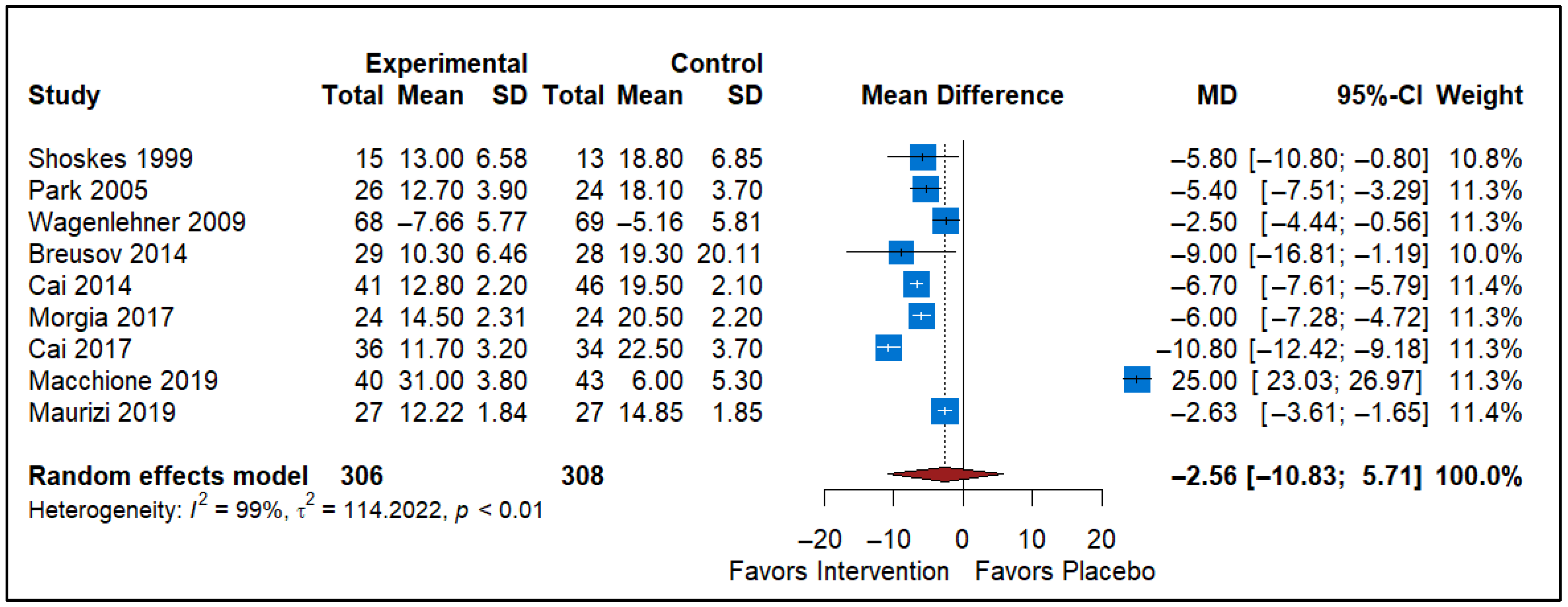
3.5. Effect of Pollen Extract vs. Placebo
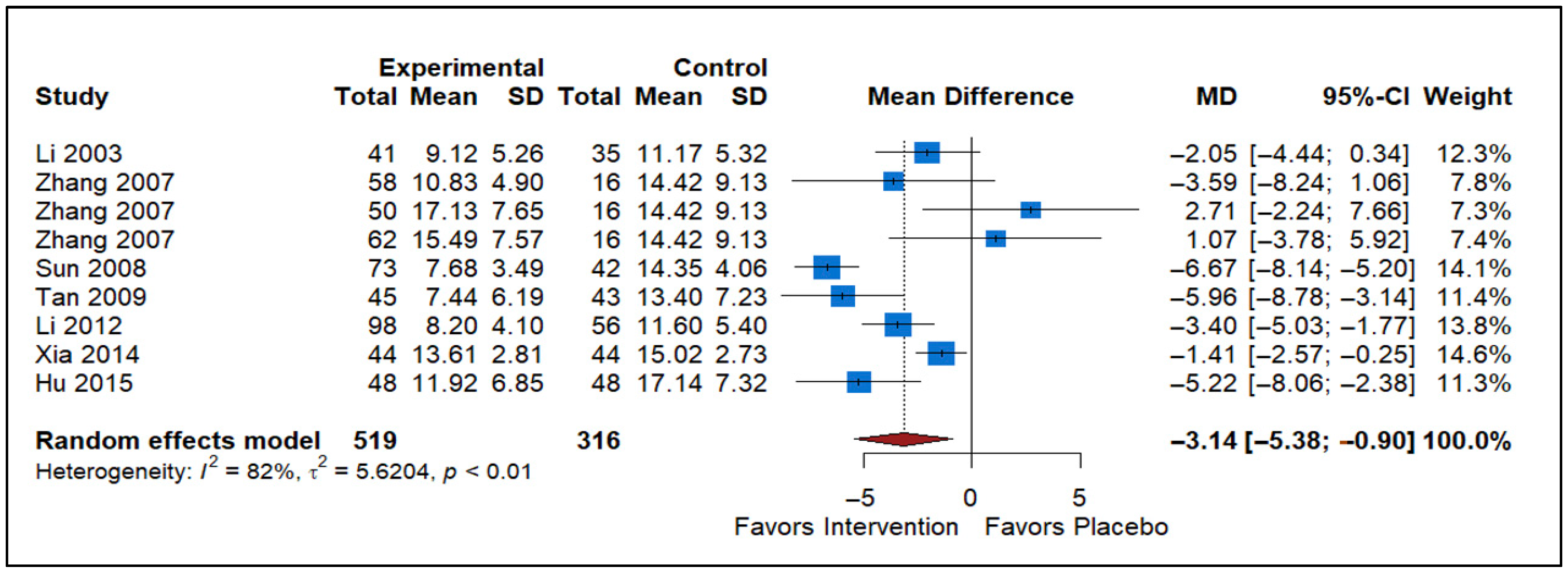
3.6. Effect of Traditional Chinese Medicine vs. Placebo
3.7. Effect of Other Medicines vs. Placebo
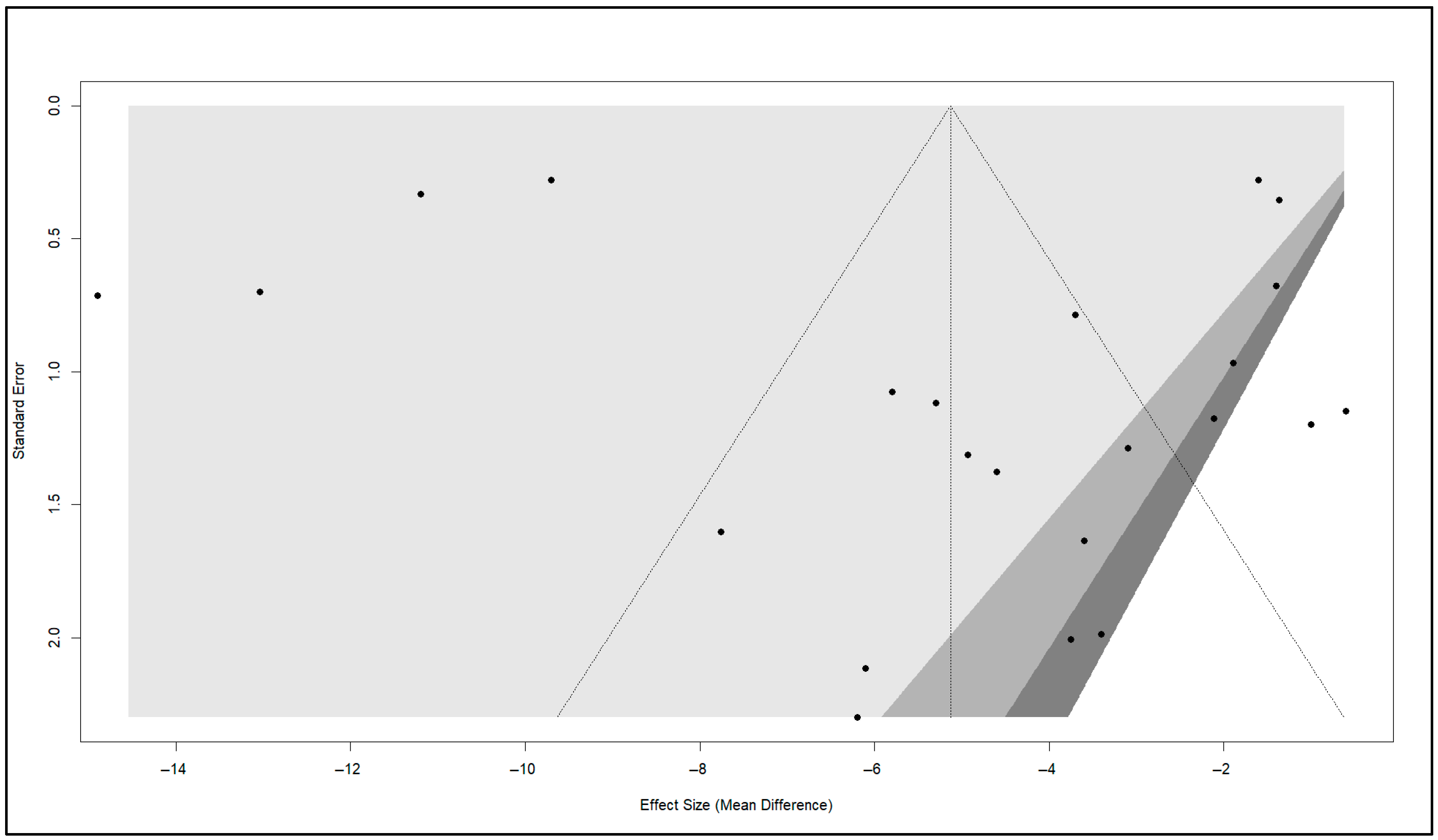
3.8. Publication Bias
3.9. Risk of Bias: Quality of Studies
3.10. Certainty of Evidence (GRADE Assessment)
3.11. Subgroup Analysis
4. Discussion
Strengths and Limitations
5. Conclusions and Clinical Relevance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IQR | Interquartile range |
| g | grams |
| mg | Milligram |
| ml | Milliliter |
| IU | International units |
| BID | bis in die (twice a day) |
| qd | quaque die (every day) |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| NR | Not reported |
| TCM | Traditional Chinese Medicine |
| SD | Standard Deviation |
References
- Zhang, J.; Liang, C.; Shang, X.; Li, H. Chronic prostatitis/chronic pelvic pain syndrome: A disease or symptom? Current perspectives on diagnosis, treatment, and prognosis. Am. J. Men’s Health 2020, 14, 1557988320903200. [Google Scholar] [CrossRef]
- Hedelin, H.; Jonsson, K. Chronic prostatitis/chronic pelvic pain syndrome: Symptoms are aggravated by cold and become less distressing with age and time. Scand. J. Urol. Nephrol. 2007, 41, 516–520. [Google Scholar] [CrossRef]
- Yuen, K.H.; Krieger, J.N.; Riley, D.E.; Cheah, P.Y.; Liong, M.L. Epidemiology of prostatitis: New evidence for a world-wide problem. World J. Urol. 2003, 21, 70–74. [Google Scholar] [CrossRef]
- Alshahrani, S.; Fathi, B.A.; Abouelgreed, T.A.; El-Metwally, A. Prevalence of Sexual Dysfunction with Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS): An Updated Systematic Review and Meta-Analysis. Medicina 2025, 61, 1110. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.; van Till, J.O.; Magri, V.; Perletti, G.; Houbiers, J.G.; Weidner, W.; Nickel, J.C. National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) symptom evaluation in multinational cohorts of patients with chronic prostatitis/chronic pelvic pain syndrome. Eur. Urol. 2013, 63, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Polackwich, A.S.; Shoskes, D.A. Chronic prostatitis/chronic pelvic pain syndrome: A review of evaluation and therapy. Prostate Cancer Prostatic Dis. 2016, 19, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C. Treatment of chronic prostatitis/chronic pelvic pain syndrome. Int. J. Antimicrob. Agents 2008, 31, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Magistro, G.; Wagenlehner, F.M.; Grabe, M.; Weidner, W.; Stief, C.G.; Nickel, J.C. Contemporary management of chronic prostatitis/chronic pelvic pain syndrome. Eur. Urol. 2016, 69, 286–297. [Google Scholar] [CrossRef]
- Franco, J.V.A.; Turk, T.; Jung, J.H.; Xiao, Y.T.; Iakhno, S.; Tirapegui, F.I.; Garrote, V.; Vietto, V. Pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database Syst. Rev. 2019, 10, CD012552. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Yang, M.; Chen, Y.; Liu, Z. Factors related to acupuncture response in patients with chronic prostatitis/chronic pelvic pain syndrome: Secondary analysis of a randomized controlled trial. World J. Urol. 2024, 42, 112. [Google Scholar] [CrossRef]
- Qin, P.; Cao, X.; Ni, H.; Yang, L.; Tong, Y.; Dang, M.; Xu, J. Efficacy of different acupuncture therapies for chronic prostatitis/chronic pelvic pain syndrome: A network meta-analysis. J. Pain Res. 2025, 18, 3653–3673. [Google Scholar] [CrossRef]
- Chi, C.-C.; Shao, S.-C.; Kuo, L.-T.; Huang, Y.-T.; Lai, P.-C. Using Grading of Recommendations Assessment, Development, and Evaluation (GRADE) to rate the certainty of evidence of study outcomes from systematic reviews: A quick tutorial. Dermatol. Sin. 2023, 41, 3–7. [Google Scholar] [CrossRef]
- Cheah, P.Y.; Liong, M.L.; Yuen, K.H.; Teh, C.L.; Khor, T.; Yang, J.R.; Yap, H.W.; Krieger, J.N. Initial, long-term, and durable responses to terazosin, placebo, or other therapies for chronic prostatitis/chronic pelvic pain syndrome. Urology 2004, 64, 881–886. [Google Scholar] [CrossRef]
- Erdemir, F.; Firat, F.; Atilgan, D.; Uluocak, N.; Parlaktas, B.S.; Yasar, A. The comparison of the efficacy of three different treatment protocols on the type 3 chronic prostatitis (chronic pelvic pain syndrome). J. Clin. Anal. Med. 2010, 1, 26–30. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, J.G.; Cho, I.R. The efficacy of terazosin in the management of chronic pelvic pain syndrome (CPPS): Comparison between category IIIa and IIIb. Korean J. Urol. 2006, 47, 1191–1196. [Google Scholar] [CrossRef]
- Wang, J.; Yan, D.; Liang, K.; Xu, Z. A randomized controlled trial of levofloxacin, terazosin, and combination therapy in patients with category III chronic prostatitis/chronic pelvic pain syndrome. Int. Urol. Nephrol. 2016, 48, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zheng, Z.; Zhuang, Z.; Lin, H.; Xu, Z.; Zhang, C.; Zhao, X. Tamsulosin therapy for chronic nonbacterial prostatitis: A randomized double-blind placebo controlled clinical trial. Chin. J. Androl. 2010, 24, 32–35. [Google Scholar]
- Kulovac, B.; Aganović, D.; Prcić, A.; Hadžiosmanović, O. Management of chronic nonbacterial prostatitis/chronic pelvic pain syndrome. Bosn. J. Basic Med. Sci. 2007, 7, 245–249. [Google Scholar] [CrossRef]
- Tuğcu, V.; Taşçı, A.İ.; Fazlıoğlu, A.; Gürbüz, G.; Özbek, E.; Şahin, S.; Kurtuluş, F.; Çek, M. A placebo-controlled comparison of the efficiency of triple-and monotherapy in category III B chronic pelvic pain syndrome (CPPS). Eur. Urol. 2007, 51, 1113–1118. [Google Scholar] [CrossRef]
- Wu, Z.; Xia, S.; Geng, H.; Zhu, H.; Tang, J.; Zhang, T.; Feng, C. Combined therapy for chronic prostatitis/chronic pelvic pain syndrome with doxazosin and diclofenac. Chin. J. Androl. 2008, 22, 20–22. [Google Scholar]
- Youn, C.W.; Son, K.-C.; Choi, H.-S.; Kwon, D.D.; Park, K.; Ryu, S.B. Comparison of the efficacy of antibiotic monotherapy and antibiotic plus alpha-blocker combination therapy for patients with inflammatory chronic prostatitis/chronic pelvic pain syndrome. Korean J. Urol. 2008, 49, 72–76. [Google Scholar] [CrossRef]
- Lü, M.; Zhao, S.-T.; Wang, S.-M.; Shi, B.-K.; Fan, Y.-D.; Wang, J.-Z. Alpha-blockers and bioflavonoids in men with chronic nonbacterial prostatitis (NIH-IIIa): A prospective, placebo-controlled trial. Zhonghua Liu Xing Bing Xue Za Zhi 2004, 25, 169–172. [Google Scholar] [PubMed]
- Alexander, R.B.; Propert, K.J.; Schaeffer, A.J.; Landis, J.R.; Nickel, J.C.; O’LEary, M.P.; Pontari, M.A.; McNaughton-Collins, M.; Shoskes, D.A.; Comiter, C.V.; et al. Ciprofloxacin or tamsulosin in men with chronic prostatitis/chronic pelvic pain syndrome: A randomized, double-blind trial. Ann. Intern. Med. 2004, 141, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, X.; Liu, J.; Tang, W.; Zhao, T.; Zhang, J. Effects of a 6-month course of tamsulosin for chronic prostatitis/chronic pelvic pain syndrome: A multicenter, randomized trial. World J. Urol. 2011, 29, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Ha, J.S.; Lee, S.J.; Cho, Y.H.; Yoon, M.S. Clinical effect of tamsulosin in noninflammatory chronic pelvic pain syndrome. Korean J. Urol. 2003, 44, 120–123. [Google Scholar]
- Mehik, A.; Alas, P.; Nickel, J.; Sarpola, A.; Helström, P.J. Alfuzosin treatment for chronic prostatitis/chronic pelvic pain syndrome: A prospective, randomized, double-blind, placebo-controlled, pilot study. Urology 2003, 62, 425–429. [Google Scholar] [CrossRef]
- Mo, K.I.; Lee, K.S.; Kim, D.G. Efficacy of combination therapy for patients with chronic prostatitis/chronic pelvic pain syndrome: A prospective study. Korean J. Urol. 2006, 47, 536–540. [Google Scholar] [CrossRef]
- Ryu, Y.-G.; Kim, H.-J.; Park, H.-J. The efficacy of alfuzosin for chronic prostatitis/chronic pelvic pain syndrome in young and middle aged patients. Korean J. Urol. 2007, 48, 858–862. [Google Scholar] [CrossRef]
- Nickel, J.C.; O’Leary, M.; Lepor, H.; Caramelli, K.; Thomas, H.; Hill, L.A.; Hoel, G. 1433 Effects of Silodosin in Men with Moderate or Severe Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A Double-Blind, Placebo-Controlled Phase 2 Study. J. Urol. 2011, 185, e574. [Google Scholar] [CrossRef]
- Sivkov, A.; Oschepkov, V.; Egorov, A. Double-blind placebo-controlled study of terazosin in patients with chronic abacterial prostatitis. Urologiia 2005, 47–53. [Google Scholar]
- Kim, T.H.; Lee, K.S.; Kim, J.H.; Jee, J.Y.; Seo, Y.E.; Choi, D.W.; Sung, Y.G.; Kong, G.S.; Kim, D.W.; Cho, W.Y. Tamsulosin monotherapy versus combination therapy with antibiotics or anti-inflammatory agents in the treatment of chronic pelvic pain syndrome. Int. Neurourol. J. 2011, 15, 92–96. [Google Scholar] [CrossRef]
- Nickel, J.; Downey, J.; Clark, J.; Casey, R.W.; Pommerville, P.J.; Barkin, J.; Steinhoff, G.; Brock, G.; Patrick, A.B.; Flax, S.; et al. Levofloxacin for chronic prostatitis/chronic pelvic pain syndrome in men: A randomized placebo-controlled multicenter trial. Urology 2003, 62, 614–617. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, X.; Wu, Z.; Xiao, N.; Lü, C.; Hou, Y. Corticoid combined with an antibiotic for chronic nonbacterial prostatitis. Zhonghua Nan Ke Xue = Natl. J. Androl. 2009, 15, 237–240. [Google Scholar]
- Jiang, M.-H.; Wu, G.-C.; Liu, H.-L. Dexketoprofen trometamol in the treatment of chronic prostatitis/chronic pelvic pain syndrome. Zhonghua Nan Ke Xue = Natl. J. Androl. 2009, 15, 825–828. [Google Scholar]
- Zhao, W.; Zhang, Z.; Li, X.; Yu, D.; Rui, X.; Li, G.; Ding, G. Celecoxib reduces symptoms in men with difficult chronic pelvic pain syndrome (Category IIIA). Braz. J. Med. Biol. Res. 2009, 42, 963–967. [Google Scholar] [CrossRef]
- Falahatkar, S.; Shahab, E.; Moghaddam, K.G.; Kazemnezhad, E. Transurethral intraprostatic injection of botulinum neurotoxin type A for the treatment of chronic prostatitis/chronic pelvic pain syndrome: Results of a prospective pilot double-blind and randomized placebo-controlled study. BJU Int. 2015, 116, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, H.; Berger, R.; Miller, J.; Yang, C. Pelvic Floor Injection of Botulinum Toxin A for Pelvic Pain: A Randomized, Controlled Pilot Study: 1041. J. Urol. 2010, 183, e405. [Google Scholar] [CrossRef]
- Li, N.; Zhang, K.; Xiao, H.; Liu, C.-Z.; Liu, Z.-G.; Wang, Y.-H.; Wang, J.-H.; Liu, Z.-J.; Liu, F. The efficacy and safety of Prostant in the treatment of chronic prostatitis: A multi center, randomized, double-blind, placebo-controlled clinical trial. Chin. J. Urol. 2003, 24, 780–782. [Google Scholar]
- Tan, Y.; Zhu, X.; Liu, Y. Clinical efficiency of Tamsulosin combining with Prostant in the treatment of patients with chronic abacterial prostatitis. Chin. J. Androl. 2009, 23, 44–47. [Google Scholar]
- Li, J.-P.; Chong, T.; Chen, H.-W.; Li, H.-C.; Cao, J.; Zhang, P.; Li, H.-L. Qianlieping capsule plus alpha-blocker for chronic non-bacterial prostatitis: Analysis of 220 cases. Zhonghua Nan Ke Xue = Natl. J. Androl. 2012, 18, 856–858. [Google Scholar]
- Sun, Y.-Y.; Chen, Z.-G. Clinical observation of Qianlieantong tablet combined with terazosin hydrochlorid in treating chronic nonbacterial prostatitis. Chin. J. Inf. Tradit. Chin. Med. 2008, 15, 65–66. [Google Scholar]
- Xia, Y.-G.; Zeng, W.-T.; Mei, X.-F.; Liu, X.; Li, G.-S.; Cai, J.; Wu, B.; Gao, P. Yuleshu oral mixture combined with conventional therapy for chronic prostatitis. Zhonghua Nan Ke Xue = Natl. J. Androl. 2014, 20, 177–180. [Google Scholar]
- Zhang, M.-J.; Chu, K.-D.; Shi, Y.-L. Clinical study on treatment of chronic prostatitis/chronic pelvic pain syndrome by three different TCM principles. Zhongguo Zhong Xi Yi Jie He Za Zhi = Chin. J. Integr. Tradit. West. Med. 2007, 27, 989–992. [Google Scholar]
- Hu, A.; Hu, M.; Wang, B.; Wang, Y. Chinese and Western medicine in treatment of chronic nonbacterial prostatitis. Liaoning J. Tradit. Chin. Med. 2015, 42, 353–354. [Google Scholar]
- Wagenlehner, F.; Schneider, H.; Ludwig, M.; Horstmann, A.; Schnitker, J.; Weidner, W. 559 Long Term Efficacy of Cernilton in Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome Type Nih Iiia. Eur. Urol. Suppl. 2009, 8, 260. [Google Scholar] [CrossRef]
- Breusov, A.; Kulchavenya, E. Influence of combined phytotherapy on sexual function in patients with chronic abacterial prostatitis. Urologiia 2014, 24–26. [Google Scholar]
- Morgia, G.; Russo, G.I.; Urzì, D.; Privitera, S.; Castelli, T.; Favilla, V.; Cimino, S. A phase II, randomized, single-blinded, placebo-controlled clinical trial on the efficacy of Curcumina and Calendula suppositories for the treatment of patients with chronic prostatitis/chronic pelvic pain syndrome type III. Arch. Ital. Urol. Androl. 2017, 89, 110–113. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Zeitlin, S.I.; Shahed, A.; Rajfer, J. Quercetin in men with category III chronic prostatitis: A preliminary prospective, double-blind, placebo-controlled trial. Urology 1999, 54, 960–963. [Google Scholar] [CrossRef]
- Park, S.-J.; Yoon, H.-N.; Shim, B.-S. Prevention of relapse with the cranberry juice in chronic pelvic pain syndrome. Korean J. Urol. 2005, 46, 63–67. [Google Scholar]
- Cai, T.; Verze, P.; La Rocca, R.; Palmieri, A.; Tiscione, D.; Luciani, L.G.; Mazzoli, S.; Mirone, V.; Malossini, G. The clinical efficacy of pollen extract and vitamins on chronic prostatitis/chronic pelvic pain syndrome is linked to a decrease in the pro-inflammatory cytokine interleukin-8. World J. Men’s Health 2017, 35, 120–128. [Google Scholar] [CrossRef]
- Macchione, N.; Bernardini, P.; Piacentini, I.; Mangiarotti, B.; Del Nero, A. Flower pollen extract in association with vitamins (Deprox 500®) versus Serenoa repens in chronic prostatitis/chronic pelvic pain syndrome: A comparative analysis of two different treatments. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2019, 18, 151–161. [Google Scholar] [CrossRef]
- Maurizi, A.; De Luca, F.; Zanghi, A.; Manzi, E.; Leonardo, C.; Guidotti, M.; Antonaccio, F.; Olivieri, V.; De Dominicis, C. The role of nutraceutical medications in men with non bacterial chronic prostatitis and chronic pelvic pain syndrome: A prospective non blinded study utilizing flower pollen extracts versus bioflavonoids. Arch. Ital. Urol. Androl. 2018, 90, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Wagenlehner, F.M.; Luciani, L.G.; Tiscione, D.; Malossini, G.; Verze, P.; Mirone, V.; Bartoletti, R. Pollen extract in association with vitamins provides early pain relief in patients affected by chronic prostatitis/chronic pelvic pain syndrome. Exp. Ther. Med. 2014, 8, 1032–1038. [Google Scholar] [CrossRef]
- Nickel, J.C.; O’Leary, M.P.; Lepor, H.; Caramelli, K.E.; Thomas, H.; Hill, L.A.; Hoel, G.E. Silodosin for men with chronic prostatitis/chronic pelvic pain syndrome: Results of a phase II multicenter, double-blind, placebo controlled study. J. Urol. 2011, 186, 125–131. [Google Scholar] [CrossRef]
- Nickel, J.; Downey, J.; Pontari, M.; Shoskes, D.; Zeitlin, S. A randomized placebo-controlled multicentre study to evaluate the safety and efficacy of finasteride for male chronic pelvic pain syndrome (category IIIA chronic nonbacterial prostatitis). BJU Int. 2004, 93, 991–995. [Google Scholar] [CrossRef]
- Pontari, M.A.; Krieger, J.N.; Litwin, M.S.; White, P.C.; Anderson, R.U.; McNaughton-Collins, M.; Nickel, J.C.; Shoskes, D.A.; Alexander, R.B.; O’leary, M.; et al. Pregabalin for the treatment of men with chronic prostatitis/chronic pelvic pain syndrome: A randomized controlled trial. Arch. Intern. Med. 2010, 170, 1586–1593. [Google Scholar] [CrossRef]
- Shim, K.H.; Kim, T.W.; Chung, B.H.; Lee, S.W.; Park, J.K.; Park, K.; Cheon, J.; Lee, K.S.; Kim, H.-J.; Seong, D.-H.; et al. Changes in autonomic nervous system activity after treatment with alpha-blocker in men with lower urinary tract symptoms. Investig. Clin. Urol. 2018, 59, 49–54. [Google Scholar] [CrossRef]
- Gowtham, P.; Girigoswami, K.; Thirumalai, A.; Harini, K.; Pallavi, P.; Girigoswami, A. Association of TIMP2 418 G/C and MMP Gene Polymorphism with Risk of Urinary Cancers: Systematic Review and Meta-analysis. Genet. Test. Mol. Biomark. 2024, 28, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhang, C.; Guo, J.; Kwong, J.S.; Li, X.; Pang, R.; Doiron, R.; Nickel, J.; Wu, J. Oral pharmacological treatments for chronic prostatitis/chronic pelvic pain syndrome: A systematic review and network meta-analysis of randomised controlled trials. eClinicalMedicine 2022, 48, 101457. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Fagin, A.P.; Hariton, E.; Niska, J.R.; Pierce, M.W.; Kuriyama, A.; Whelan, J.S.; Jackson, J.L.; Dimitrakoff, J.D. Therapeutic intervention for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): A systematic review and meta-analysis. PLoS ONE 2012, 7, e41941. [Google Scholar] [CrossRef]
- Liu, S.; Kelliher, L. Physiology of pain—A narrative review on the pain pathway and its application in the pain management. Dig. Med. Res. 2022, 5, 56. [Google Scholar] [CrossRef]
- Stein, C.; Clark, J.D.; Oh, U.; Vasko, M.R.; Wilcox, G.L.; Overland, A.C.; Vanderah, T.W.; Spencer, R.H. Peripheral mechanisms of pain and analgesia. Brain Res. Rev. 2009, 60, 90–113. [Google Scholar] [CrossRef]
- Lee, S.W.; Liong, M.L.; Yuen, K.H.; Leong, W.S.; Chee, C.; Cheah, P.Y.; Choong, W.P.; Wu, Y.; Khan, N.; Choong, W.L.; et al. Acupuncture versus sham acupuncture for chronic prostatitis/chronic pelvic pain. Am. J. Med. 2008, 121, 79.e1–79.e7. [Google Scholar] [CrossRef]
- Ohlsen, B.A. Acupuncture and traditional Chinese medicine for the management of a 35-year-old man with chronic prostatitis with chronic pelvic pain syndrome. J. Chiropr. Med. 2013, 12, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Antolak, S.J. Acupuncture ameliorates symptoms in men with chronic prostatitis/chronic pelvic pain syndrome. Urology 2004, 63, 212. [Google Scholar] [CrossRef]
- Liddle, C.E.; Harris, R.E. Cellular reorganization plays a vital role in acupuncture analgesia. Med. Acupunct. 2018, 30, 15–20. [Google Scholar] [CrossRef]
- Xue, Y.; Duan, Y.; Gong, X.; Zheng, W.; Li, Y. Traditional Chinese medicine on treating chronic prostatitis/chronic pelvic pain syndrome: A systematic review and meta-analysis. Medicine 2019, 98, e16136. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Du, X.; Zhou, W.; Mei, X.; Tian, Y.; Chen, L.; Xia, Y.; Zhang, O. Systematic review and meta-analysis: α-adrenergic receptor blockers in chronic prostatitis. Ann. Palliat. Med. 2021, 10, 9870–9878. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.C.; Browne, J.; Emberton, M. Role of α-blockers in type III prostatitis: A systematic review of the literature. J. Urol. 2007, 177, 25–30. [Google Scholar] [CrossRef]


| Study | Year | Country | Risk of Bias |
|---|---|---|---|
| Cheah 2003 [13] | 2004 | Malaysia and United States of America | Unclear risk |
| Erdemir 2010 [14] | 2010 | Turkey | High risk |
| Jung 2006 [15] | 2006 | South Korea | Unclear risk |
| Wang 2016 [16] | 2016 | China | Low risk |
| Yang 2010 [17] | 2010 | China | Unclear risk |
| Kulovac 2007 [18] | 2007 | Bosnia and Herzegovina | High risk |
| TuAYcu 2007 [19] | 2007 | Turkey | Unclear risk |
| Wu 2008 [20] | 2008 | China | Unclear risk |
| Youn 2008 [21] | 2008 | South Korea | Unclear risk |
| Lu 2004 [22] | 2004 | China | High risk |
| Alexander 2004 [23] | 2004 | United States of America and Canada | Low risk |
| Chen 2011 [24] | 2011 | China | Low risk |
| Kim 2003 [25] | 2003 | South Korea | Unclear risk |
| Yang 2010 [17] | 2010 | China | Unclear risk |
| Mehik 2003 [26] | 2003 | Finland | Low risk |
| Mo 2006 [27] | 2006 | South Korea | Unclear risk |
| Nickel 2008 [7] | 2008 | United States of America, Canada and Malaysia | Low risk |
| Ryu 2007 [28] | 2007 | South Korea | Unclear risk |
| Nickel 2011a [29] | 2011 | Canada | Low risk |
| Sivkov 2005 [30] | 2005 | Russia | Unclear risk |
| Chen 2011 [24] | 2011 | China | Low risk |
| Alexander 2004 [23] | 2004 | United States of America and Canada | Low risk |
| Kim 2011a [31] | 2011 | Republic of Korea | High risk |
| Kulovac 2007 [18] | 2007 | Bosnia and Herzegovina | High risk |
| Nickel 2003a [32] | 2003 | Canada | Low risk |
| Wang 2016 [16] | 2016 | China | Low risk |
| Yang 2009 [33] | 2009 | China | Unclear risk |
| Jiang 2009 [34] | 2009 | China | Unclear risk |
| Kim 2003 [25] | 2003 | South Korea | Unclear risk |
| Kim 2011a [31] | 2011 | Republic of Korea | High risk |
| Wu 2008 [20] | 2008 | China | Unclear risk |
| Zhao 2009 [35] | 2009 | China | Unclear risk |
| TuAYcu 2007 [19] | 2007 | Turkey | Unclear risk |
| Falahatkar 2015 [36] | 2015 | Iran | Low risk |
| Gottsch 2011 [37] | 2011 | United States of America | High risk |
| Li 2003 [38] | 2003 | China | Unclear risk |
| Tan 2009 [39] | 2009 | China | High risk |
| Li 2012 [40] | 2012 | China | High risk |
| Sun 2008 [41] | 2008 | China | Unclear risk |
| Xia 2014 [42] | 2014 | China | Unclear risk |
| Zhang 2007 [43] | 2007 | China | High risk |
| Hu 2015 [44] | 2015 | China | Low risk |
| Zhang 2007 [43] | 2007 | China | Low risk |
| Zhang 2007 [43] | 2007 | China | Low risk |
| Wagenlehner 2009 [45] | 2009 | Germany | High risk |
| Breusov 2014 [46] | 2014 | Russia | Unclear risk |
| Morgia 2017 [47] | 2017 | Italy | Unclear risk |
| Shoskes 1999 [48] | 1999 | United States of America | Unclear risk |
| Park 2005 [49] | 2005 | South Korea | High risk |
| Cai 2017 [50] | 2017 | Europe | Unclear risk |
| Macchione 2019 [51] | 2019 | Europe | Unclear risk |
| Maurizi 2019 [52] | 2019 | Europe | Unclear risk |
| Cai 2014 [53] | 2014 | Europe | Unclear risk |
| Nickel 2011b [54] | 2011 | North America | Low risk |
| Nickel 2004 [55] | 2004 | North America | Unclear risk |
| Pontari 2010 [56] | 2012 | North America | Low risk |
| Comparison | Number of Studies (N = 56 Total) | Pooled Effect (MD, 95% CI) | Effect Magnitude & Significance | Downgrading Factors | Certainty of Evidence |
|---|---|---|---|---|---|
| Alpha-blockers vs. Placebo | 22 | −5.13 (−6.87 to −3.39) | Significant reduction in NIH-CPSI | Risk of Bias, Inconsistency (I2 = 98%) | Low |
| Analgesics vs. Placebo | 7 | −2.47 (−4.24 to −0.70) | Significant reduction in NIH-CPSI | Risk of Bias, Inconsistency (I2 = 88%) | Low |
| Antibiotics vs. Placebo | 5 | −2.45 (−5.53 to 0.64) | Non-significant reduction in NIH-CPSI | Risk of Bias, Inconsistency (I2 = 75%), Imprecision (CI crosses null) | Very Low |
| Pollen Extract vs. Placebo | 9 | −2.56 (−10.83 to 5.71) | Non-significant | Risk of Bias, Inconsistency (I2 = 99%), Imprecision (Wide CI/Null) | Very Low |
| TCM vs. Placebo | 9 | −3.14 (−5.38 to −0.90) | Significant reduction in NIH-CPSI | Risk of Bias, Inconsistency (I2 = 82%) | Low |
| Other Medications vs. Placebo | 5 | −6.94 (−19.79 to 5.91) | Non-significant | Risk of Bias, Inconsistency (I2 = 96%), Imprecision (Wide CI/Null) | Very Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshahrani, S.; Fathi, B.A.; Abouelgreed, T.A.; El-Metwally, A. Comparative Efficacy of Pharmacological Interventions for Chronic Prostatitis/Chronic Pelvic Pain Syndrome: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare 2025, 13, 2956. https://doi.org/10.3390/healthcare13222956
Alshahrani S, Fathi BA, Abouelgreed TA, El-Metwally A. Comparative Efficacy of Pharmacological Interventions for Chronic Prostatitis/Chronic Pelvic Pain Syndrome: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare. 2025; 13(22):2956. https://doi.org/10.3390/healthcare13222956
Chicago/Turabian StyleAlshahrani, Saad, Basem A. Fathi, Tamer A. Abouelgreed, and Ashraf El-Metwally. 2025. "Comparative Efficacy of Pharmacological Interventions for Chronic Prostatitis/Chronic Pelvic Pain Syndrome: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials" Healthcare 13, no. 22: 2956. https://doi.org/10.3390/healthcare13222956
APA StyleAlshahrani, S., Fathi, B. A., Abouelgreed, T. A., & El-Metwally, A. (2025). Comparative Efficacy of Pharmacological Interventions for Chronic Prostatitis/Chronic Pelvic Pain Syndrome: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare, 13(22), 2956. https://doi.org/10.3390/healthcare13222956







