Semaglutide in Diabetic Kidney Disease: Integrating Clinical Evidence with Mechanistic Insights
Abstract
1. Introduction
2. Semaglutide
3. Diabetic Kidney Disease
4. Semaglutide’s Effect on Diabetic Kidney Disease
4.1. Mechanistic Basis for Renoprotection
4.2. Clinical Trial Evidence
| Trial | Study Design | Population | Intervention | Duration | Renal Baseline Characteristics | Primary Renal Outcome | Key Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| SUSTAIN-6 | Randomized, double-blind, placebo-controlled | T2DM with high CV risk | Semaglutide 0.5–1.0 mg weekly vs. placebo | Median 2.1 years | Mean eGFR: 75.6 mL/min/1.73 m2; UACR 34 mg/g | Combination of new/worsening nephropathy (≥30% eGFR decline, ESKD, or death from renal causes) | 36% reduction in nephropathy events (HR 0.64, 95% CI 0.46–0.88); 46% lower risk of macroalbuminuria (HR 0.54, 95% CI 0.37–0.77); slower eGFR decline vs. placebo | [9] |
| SUSTAIN 1–7 pooled analysis | A pooled post hoc analysis of the SUSTAIN 1–7 randomized controlled trials | T2DM patients | Semaglutide vs. placebo or active comparators, depending on the specific SUSTAIN trial | Varies by trial | Varies by trial | Changes in eGFR, reductions in urinary albumin-to-creatinine ratio (UACR), and the occurrence of kidney-related adverse events | Initial eGFR dip followed by stabilization, resulting in a net annual slope improvement of ≈0.59 mL/min/1.73 m2 versus comparators; significant reductions in UACR; no new renal safety signals observed | [47] |
| PIONEER-5 | Randomized, double-blind, placebo-controlled phase 3a | T2DM with moderate renal impairment (eGFR 30–59 mL/min/1.73 m2) | Oral semaglutide (14 mg daily) vs. placebo | A 26-week randomized treatment period and a follow-up period of 5 weeks | eGFR 30–59 mL/min/1.73 m2 (moderate CKD) | Exploratory renal safety and albuminuria change (not dedicated renal composite) | eGFR remained generally stable in both groups (no slope provided); UACR decreased numerically in the semaglutide group (magnitude not reported, no formal statistical test); no new renal safety issues were identified | [48] |
| PIONEER-6 | Randomized, double-blind, placebo-controlled CVOT | T2DM with high CV risk (eGFR ≥ 30 mL/min/1.73 m2) | Oral semaglutide (14 mg daily) vs. placebo | Median follow-up = 1.3 years | Mean baseline eGFR = 74 mL/min/1.73 m2 (estimated from pooled data) | Exploratory renal safety outcomes were assessed, but there was no prespecified primary renal endpoint | Exploratory renal outcomes: HRs for persistent eGFR decline <1.0 overall (NS); in patients with baseline eGFR 30–<60, semaglutide significantly reduced the risk of 30% eGFR decline (p = 0.03); no major renal safety concerns; no statistically different interactions between treatment and CKD subgroup were observed | [15] |
| Post hoc analysis of SUSTAIN-6 and PIONEER-6 | Pooled post hoc analysis of two cardiovascular outcome trials (SUSTAIN-6 and PIONEER-6) | Patients with T2DM at high cardiovascular risk (n = 6480; semaglutide = 3239; placebo = 3241) | Semaglutide (s.c. and oral) vs. placebo | Median follow-up = 2.1 years (SUSTAIN-6) + 1.3 years (PIONEER-6) | eGFR ≥ 30 mL/min/1.73 m2; subgroup with eGFR 30–<60 mL/min/1.73 m2 analyzed separately; baseline UACR available for SUSTAIN-6 only | Annual eGFR slope; time to persistent eGFR decline (≥30%, ≥40%, ≥50%, ≥57%) | Annual eGFR decline was slower by 0.59 mL/min/1.73 m2/year (95% CI 0.29–0.89) with semaglutide vs. placebo. In patients with baseline eGFR 30–<60, the difference was 1.06 mL/min/1.73 m2/year (95% CI 0.45–1.67). HRs for time to persistent eGFR decline were consistently <1.0, favoring semaglutide, though not all reached statistical significance | [52] |
| Meta-analysis of GLP-1 receptor agonists | Systematic review and meta-analysis of 8 major cardiovascular outcome trials (n = 60,080) | Patients with T2DM; broad cardiovascular risk spectrum | GLP-1 RAs (including liraglutide, semaglutide, dulaglutide, albiglutide, exenatide, lixisenatide) vs. placebo | Median trial duration 1.3–5.4 years | Baseline mean eGFR ~78 mL/min/1.73 m2; ~23% with eGFR < 60 mL/min/1.73 m2 | Combined kidney outcomes (new-onset macroalbuminuria, sustained ≥30–40% decline in eGFR, kidney replacement therapy, or renal death) | GLP-1 receptor agonists reduced the combined kidney outcome by 21% (HR 0.79; 95% CI 0.73–0.87; p < 0.0001), driven largely by a 26% lower risk of new-onset macroalbuminuria (HR 0.74; 95% CI 0.68–0.81). Risk reduction for hard renal endpoints (≥40% eGFR decline, doubling of serum creatinine, end-stage kidney disease, or renal death) was nominal (HR 0.86; 95% CI 0.72–1.02). Effects were consistent across baseline eGFR subgroups | [49] |
| FLOW | Randomized, double-blind, placebo-controlled, event-driven kidney-outcomes trial | 3533 adults with T2DM and CKD (eGFR ~47 mL/min/1.73 m2; median UACR ~568 mg/g) | Semaglutide 1.0 mg weekly vs. placebo (plus standard of care) | Median ~3.4 years | Mean baseline eGFR = 47 mL/min/1.73 m2; median UACR = 568 mg/g | Composite of kidney failure, sustained ≥50% eGFR decline, kidney/cardiovascular death | 24% reduction in primary composite kidney outcome (HR 0.76; 95% CI 0.66–0.88). Annual eGFR decline slowed by ~1.16 mL/min/1.73 m2/yr in the semaglutide group | [50,51] |
4.3. Translational Studies and Real-World Evidence
4.4. Comparative Effectiveness with Other Drug Classes
4.5. Future Directions
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; De Boer, I.H. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic kidney disease: Challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Hoogeveen, E.K. The Epidemiology of Diabetic Kidney Disease. Kidney Dial. 2022, 2, 433–442. [Google Scholar] [CrossRef]
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Ghanim, H.; Arora, P. Improving the residual risk of renal and cardiovascular outcomes in diabetic kidney disease: A review of pathophysiology, mechanisms, and evidence from recent trials. Diabetes Obes. Metab. 2022, 24, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Maiorino, M.I.; Bellastella, G.; Esposito, K. The residual cardiorenal risk in type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 36. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Drucker, D.J.; Habener, J.F.; Holst, J.J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Investig. 2017, 127, 4217–4227. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Ahmann, A.; Capehorn, M.; Charpentier, G.; Dotta, F.; Henkel, E.; Lingvay, I.; Holst, A.G.; Annett, M.; Aroda, V. Efficacy and safety of once-weekly semaglutide vs exenatide ER after 56 Weeks in subjects with type 2 diabetes (SUSTAIN 3). Diabetes Res. Clin. Pract. 2016, 120, S51. [Google Scholar] [CrossRef]
- Shaman, A.M.; Bain, S.C.; Bakris, G.L.; Buse, J.B.; Idorn, T.; Mahaffey, K.W.; Mann, J.F.; Nauck, M.A.; Rasmussen, S.; Rossing, P.; et al. Effect of the Glucagon-Like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients With Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation 2022, 145, 575–585. [Google Scholar] [CrossRef]
- Lau, J.; Bloch, P.; Schäffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; et al. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Lau, J. The discovery and development of liraglutide and semaglutide. Front. Endocrinol. 2019, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Lüdemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): A randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B.; Buse, J.B. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Jensen, L.; Helleberg, H.; Roffel, A.; van Lier, J.J.; Bjørnsdottir, I.; Pedersen, P.J.; Rowe, E.; Karsbøl, J.D.; Pedersen, M.L. Absorption, metabolism and excretion of the GLP-1 analogue semaglutide in humans and nonclinical species. Eur. J. Pharm. Sci. 2017, 104, 31–41. [Google Scholar] [CrossRef]
- Deacon, C.F.; Knudsen, L.B.; Madsen, K.; Wiberg, F.C.; Jacobsen, O.; Holst, J.J. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologia 1998, 41, 271–278. [Google Scholar] [CrossRef]
- Polonsky, W.H.; Arora, R.; Faurby, M.; Fernandes, J.; Liebl, A. Higher Rates of Persistence and Adherence in Patients with Type 2 Diabetes Initiating Once-Weekly vs Daily Injectable Glucagon-Like Peptide-1 Receptor Agonists in US Clinical Practice (STAY Study). Diabetes Ther. 2022, 13, 175–187. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. 2021, 12, 645563. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, H.W.; Lingvay, I.; Reed, J.; de la Rosa, R.; Rose, L.; Sugimoto, D.; Araki, E.; Chu, P.-L.; Wijayasinghe, N.; Norwood, P. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2291–2301. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; He, X.; Wu, P.; Liu, Y.; Ding, Y.; Zhang, Q. Gastrointestinal adverse events associated with semaglutide: A pharmacovigilance study based on FDA adverse event reporting system. Front. Public Health 2022, 10, 996179. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- FDA. OZEMPIC (Semaglutide) Injection, for Subcutaneous Use Initial U.S. Approval: 2017 [Internet]. FDA-Approved Drugs—Drugs@FDA. 2017; p. 44. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf (accessed on 1 January 2020).
- Ayoub, M.; Chela, H.; Amin, N.; Hunter, R.; Anwar, J.; Tahan, V.; Daglilar, E. Pancreatitis Risk Associated with GLP-1 Receptor Agonists, Considered as a Single Class, in a Comorbidity-Free Subgroup of Type 2 Diabetes Patients in the United States: A Propensity Score-Matched Analysis. J. Clin. Med. 2025, 14, 944. [Google Scholar] [CrossRef]
- Sharma, A.; Parachuri, N.; Kumar, N.; Saboo, B.; Tripathi, H.N.; Kuppermann, B.D.; Bandello, F. Semaglutide and the risk of diabetic retinopathy—Current perspective. Eye 2022, 36, 10–11. [Google Scholar] [CrossRef]
- Wang, F.; Mao, Y.; Wang, H.; Liu, Y.; Huang, P. Semaglutide and Diabetic Retinopathy Risk in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Clin. Drug Investig. 2022, 42, 17–28. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.-H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef]
- Afkarian, M.; Zelnick, L.R.; Hall, Y.N.; Heagerty, P.J.; Tuttle, K.; Weiss, N.S.; De Boer, I.H. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA—J. Am. Med. Assoc. 2016, 316, 602–610. [Google Scholar] [CrossRef]
- Fioretto, P.; Mauer, M. Histopathology of Diabetic Nephropathy. Semin. Nephrol. 2007, 27, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Steffes, M.W.; Mauer, M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes 1994, 43, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, Y.S.; Sun, L.; Xie, P.; Liu, F.Y.; Chen, S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 395–423. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Qin, J.; Bo, W.; Li, H.; Jiang, L.; Li, X.; Jiang, J. Prognostic Value of Serum Interleukin-6, NF- κ B plus MCP-1 Assay in Patients with Diabetic Nephropathy. Dis. Markers 2022, 2022, 4428484. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.M.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar] [CrossRef]
- Penno, G.; Solini, A.; Bonora, E.; Fondelli, C.; Orsi, E.; Zerbini, G.; Trevisan, R.; Vedovato, M.; Gruden, G.; Cavalot, F.; et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J. Hypertens. 2011, 29, 1802–1809. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C. The role of inflammatory cytokines in diabetic nephropathy. J. Am. Soc. Nephrol. 2008, 19, 433–442. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Ren, Q.; Niu, S.; Pan, X.; Yue, L.; Li, Z.; Zhu, R.; Jia, Z.; Chen, X.; et al. Metabolomics Provides Insights into Renoprotective Effects of Semaglutide in Obese Mice. Drug Des. Dev. Ther. 2022, 16, 3893–3913. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, S.; Wu, W.; Lin, Y.; Wang, T.; Sun, H.; A-Ni-Wan, A.; Li, Y.; Wang, C.; Li, X.; et al. GLP-1 Receptor Agonists Alleviate Diabetic Kidney Injury via β-Klotho-Mediated Ferroptosis Inhibition. Adv. Sci. 2025, 12, e2409781. [Google Scholar] [CrossRef]
- Dalbøge, L.S.; Christensen, M.; Madsen, M.R.; Secher, T.; Endlich, N.; Drenic’, V.; Manresa-Arraut, A.; Hansen, H.H.; Rune, I.; Fink, L.N.; et al. Nephroprotective Effects of Semaglutide as Mono- and Combination Treatment with Lisinopril in a Mouse Model of Hypertension-Accelerated Diabetic Kidney Disease. Biomedicines 2022, 10, 1661. [Google Scholar] [CrossRef]
- Shan, S.; Alanazi, A.H.; Han, Y.; Zhang, D.; Liu, Y.; Narayanan, S.P.; Somanath, P.R. Pro-Inflammatory Characteristics of Extracellular Vesicles in the Vitreous of Type 2 Diabetic Patients. Biomedicines 2024, 12, 2053. [Google Scholar] [CrossRef]
- McLean, B.A.; Wong, C.K.; Kaur, K.D.; Seeley, R.J.; Drucker, D.J. Differential importance of endothelial and hematopoietic cell GLP-1Rs for cardiometabolic versus hepatic actions of semaglutide. JCI Insight 2021, 6, e153732. [Google Scholar] [CrossRef]
- Farah, L.X.S.; Valentini, V.; Pessoa, T.D.; Malnic, G.; McDonough, A.A.; Girardi, A.C.C. The physiological role of glucagon-like peptide-1 in the regulation of renal function. Am. J. Physiol. Physiol. 2015, 310, F123–F127. [Google Scholar] [CrossRef]
- Mann, J.F.E.; Hansen, T.; Idorn, T.; A Leiter, L.; Marso, S.P.; Rossing, P.; Seufert, J.; Tadayon, S.; Vilsbøll, T. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: A post-hoc analysis of the SUSTAIN 1–7 randomised controlled trials. Lancet Diabetes Endocrinol. 2020, 8, 880–893. [Google Scholar] [CrossRef]
- Mosenzon, O.; Blicher, T.M.; Rosenlund, S.; Eriksson, J.W.; Heller, S.; Hels, O.H.; Pratley, R.; Sathyapalan, T.; Desouza, C.; Abramof, R.; et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): A placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019, 7, 515–527. [Google Scholar] [CrossRef]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Rossing, P.; Baeres, F.M.M.; Bakris, G.; Bosch-Traberg, H.; Gislum, M.; Gough, S.C.L.; Idorn, T.; Lawson, J.; Mahaffey, K.W.; E Mann, J.F.; et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol. Dial. Transplant. 2023, 38, 2041–2051. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.; Bakris, G.; Baeres, F.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Bosch-Traberg, H.; Cherney, D.Z.; Hadjadj, S.; Lawson, J.; Mosenzon, O.; Rasmussen, S.; Bain, S.C. Post hoc analysis of SUSTAIN 6 and PIONEER 6 trials suggests that people with type 2 diabetes at high cardiovascular risk treated with semaglutide experience more stable kidney function compared with placebo. Kidney Int. 2023, 103, 772–781. [Google Scholar] [CrossRef]
- Luna, E.; Álvarez, Á.; Rodriguez-Sabiñón, J.; Villa, J.; Giraldo, T.; Martín, M.V.; Vázquez, E.; Fernández, N.; Ruiz, B.; Garcia-Pino, G.; et al. Long-Term Effect of Semaglutide on the Glomerular Filtration Rate Slope in High-Risk Patients with Diabetic Nephropathy: Analysis in Real-World Clinical Practice. Pharmaceutics 2025, 17, 943. [Google Scholar] [CrossRef]
- Bueno, B.A.; Soler, M.J.; Perez-Belmonte, L.; Millan, A.J.; Ruiz, F.R.; de Lucas, M.D.G. Semaglutide in type 2 diabetes with chronic kidney disease at high risk progression-real-world clinical practice. Clin. Kidney J. 2022, 15, 1593–1600. [Google Scholar] [CrossRef]
- ADVANCE Collaborative Group. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- ACCORD Study Group. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N. Engl. J. Med. 2008, 8, 2545–2559. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Muskiet, M.H.A.; Tonneijck, L.; Smits, M.M.; Van Baar, M.J.B.; Kramer, M.H.H.; Hoorn, E.J.; Joles, J.A.; Van Raalte, D.H. GLP-1 and the kidney: From physiology to pharmacology and outcomes in diabetes. Nat. Rev. Nephrol. 2017, 13, 605–628. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef]
- EMPA-Kidney Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes. Metab. 2017, 19, 1353–1362. [Google Scholar] [CrossRef]
- Gourdy, P.; Darmon, P.; Dievart, F.; Halimi, J.M.; Guerci, B. Combining glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM). Cardiovasc. Diabetol. 2023, 22, 79. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S144–S174. [Google Scholar] [CrossRef]
- Rossing, P. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. Suppl. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Greene, T.; Tighiouart, H.; Gansevoort, R.T.; Coresh, J.; Simon, A.L.; Chan, T.M.; Hou, F.F.; Locatelli, F.; Praga, M.; et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: A meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019, 7, 128–139. [Google Scholar] [CrossRef] [PubMed]
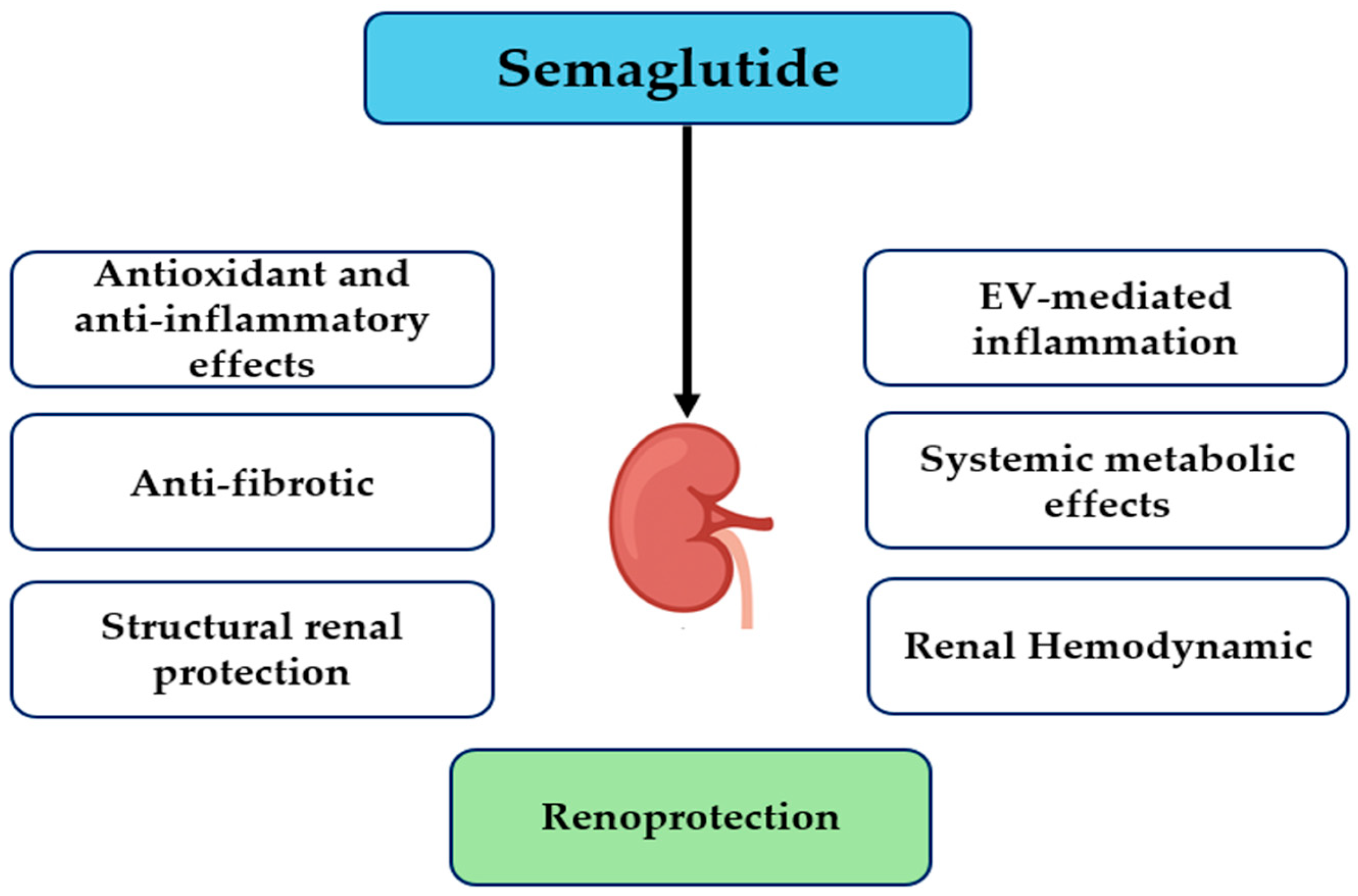
| Mechanism | Key Effects | Experimental Model/Evidence | Reference |
|---|---|---|---|
| Oxidative stress and inflammation | ↓ ROS, ↑ SOD, ↓ TNF-α, IL-1β, IL-6 | Obese mice with renal injury | [42] |
| Ferroptosis modulation | ↑ β-Klotho, ↑ AMPK, modulates iron metabolism, fatty acid synthesis, lipid peroxidation; ↓ ferroptosis | DKD mouse model | [43] |
| Structural renal protection | ↑ podocyte slit density, ↓ glomerulosclerosis, ↓ KIM-1 | Mouse model of hypertension-accelerated DKD | [44] |
| EV-mediated inflammation | ↓ macrophage activation, ↓ pro-inflammatory cytokines | Human vitreous samples from type 2 diabetic and non-diabetic donors | [45] |
| Systemic metabolic effects | ↓ glucose, ↓ lipid toxicity, ↓ inflammation | Transgenic mouse models with endothelial and hematopoietic cell-specific GLP-1 receptor deletion | [46] |
| Renal hemodynamics | ↑ natriuresis, ↑ diuresis via NHE3 inhibition | Male Wistar rats | [47] |
| Feature | Randomized Controlled Trials (RCTs) | Real-World Observational Studies (RWE) |
|---|---|---|
| Study Design | Prospective, randomized, controlled (e.g., SUSTAIN-6, PIONEER-5, PIONEER-6) | Observational, non-randomized, pragmatic (e.g., Spanish multicenter cohort, CKD real-life study) |
| Population | Narrow inclusion criteria; selected T2DM patients with CVD or CKD | Broad, heterogeneous T2DM and CKD populations, including multiple comorbidities |
| Sample Size | Typically large for efficacy endpoints (e.g., >3000 participants in SUSTAIN-6) | Often smaller cohorts (e.g., 122–156 patients in Luna et al. [53]), but can vary widely |
| Follow-up | Usually limited to 1–2 years | Can be extended depending on clinical practice (up to 24 months in real-world studies) |
| Endpoints | Hard clinical endpoints: ESKD, eGFR decline, macroalbuminuria | Less standardized endpoints |
| Limitations | May not reflect the general population; strict inclusion criteria | Confounding factors, lack of randomization, and smaller sample sizes |
| Strengths | High internal validity; robust causal inference | Reflects clinical practice, real-world effectiveness, and external validity |
| Drug Class/Agent | Mechanisms of Action | Renal Outcomes | Safety Concerns |
|---|---|---|---|
| Semaglutide (GLP-1RA) | Anti-inflammatory, anti-fibrotic, and natriuretic effects; weight loss, appetite regulation; improved glycemic control; reduction in oxidative stress | Decreases albuminuria; potential slowing of DKD progression; direct eGFR protection less established | Low risk of electrolyte imbalance; gastrointestinal side effects; rare risk pancreatitis |
| SGLT2 inhibitors (canagliflozin, dapagliflozin, empagliflozin) | Inhibits renal glucose reabsorption leading to glycosuria; reduces glomerular hyperfiltration via afferent arteriolar vasoconstriction; lowers intraglomerular pressure and systemic BP; reduces renal hypoxia | Robust reduction in risk of ≥50% eGFR decline, ESKD, renal or cardiovascular death (e.g., CREDENCE, DAPA-CKD, EMPA-KIDNEY) | Genital infections; risk of volume depletion; rare diabetic ketoacidosis; modest effects on weight and glycemia |
| Non-steroidal MRAs (finerenone) | Mineralocorticoid receptor antagonism → antifibrotic and anti-inflammatory effects; reduces renal and cardiovascular damage | Decreased risk of CKD progression and cardiovascular events; ~18% reduction in ESKD or persistent eGFR decline | Hyperkalemia requires monitoring; less pronounced metabolic effects |
| Gap | Research Priority | Expected Impact |
|---|---|---|
| Long-term renal outcomes | FLOW trial and its published results assessing eGFR slope, ESKD, and renal mortality | Establish definitive renoprotective efficacy |
| Advanced CKD/ESRD | Dose-finding, tolerability, and safety studies in eGFR < 30 mL/min/1.73 m2 and dialysis populations | Guide safe use in high-risk patients |
| Biomarker-guided response | Validation of urinary (KIM-1, NGAL) and plasma (TGF-β) biomarkers; multi-omics and imaging studies | Enable precision medicine and monitor treatment response |
| Mechanistic understanding | Clinical studies on anti-inflammatory, antifibrotic, and hemodynamic effects; renal biopsy and tissue-level imaging | Clarify mechanisms and optimize therapy targeting |
| Combination therapy | RCTs with SGLT2 inhibitors + MRAs and/or GLP-1RA + SGLT2i to assess additive or synergistic benefits | Inform clinical decision-making for dual/triple therapy |
| Real-world evidence | Observational studies in diverse, comorbid populations | Ensure generalizability and safety across populations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Dayel, F.F. Semaglutide in Diabetic Kidney Disease: Integrating Clinical Evidence with Mechanistic Insights. Healthcare 2025, 13, 2922. https://doi.org/10.3390/healthcare13222922
Bin Dayel FF. Semaglutide in Diabetic Kidney Disease: Integrating Clinical Evidence with Mechanistic Insights. Healthcare. 2025; 13(22):2922. https://doi.org/10.3390/healthcare13222922
Chicago/Turabian StyleBin Dayel, Faten F. 2025. "Semaglutide in Diabetic Kidney Disease: Integrating Clinical Evidence with Mechanistic Insights" Healthcare 13, no. 22: 2922. https://doi.org/10.3390/healthcare13222922
APA StyleBin Dayel, F. F. (2025). Semaglutide in Diabetic Kidney Disease: Integrating Clinical Evidence with Mechanistic Insights. Healthcare, 13(22), 2922. https://doi.org/10.3390/healthcare13222922






