Efficacy of Gut Microbiome-Targeted Interventions on Mental Health Symptoms in Women Across Key Hormonal Life Stages: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy
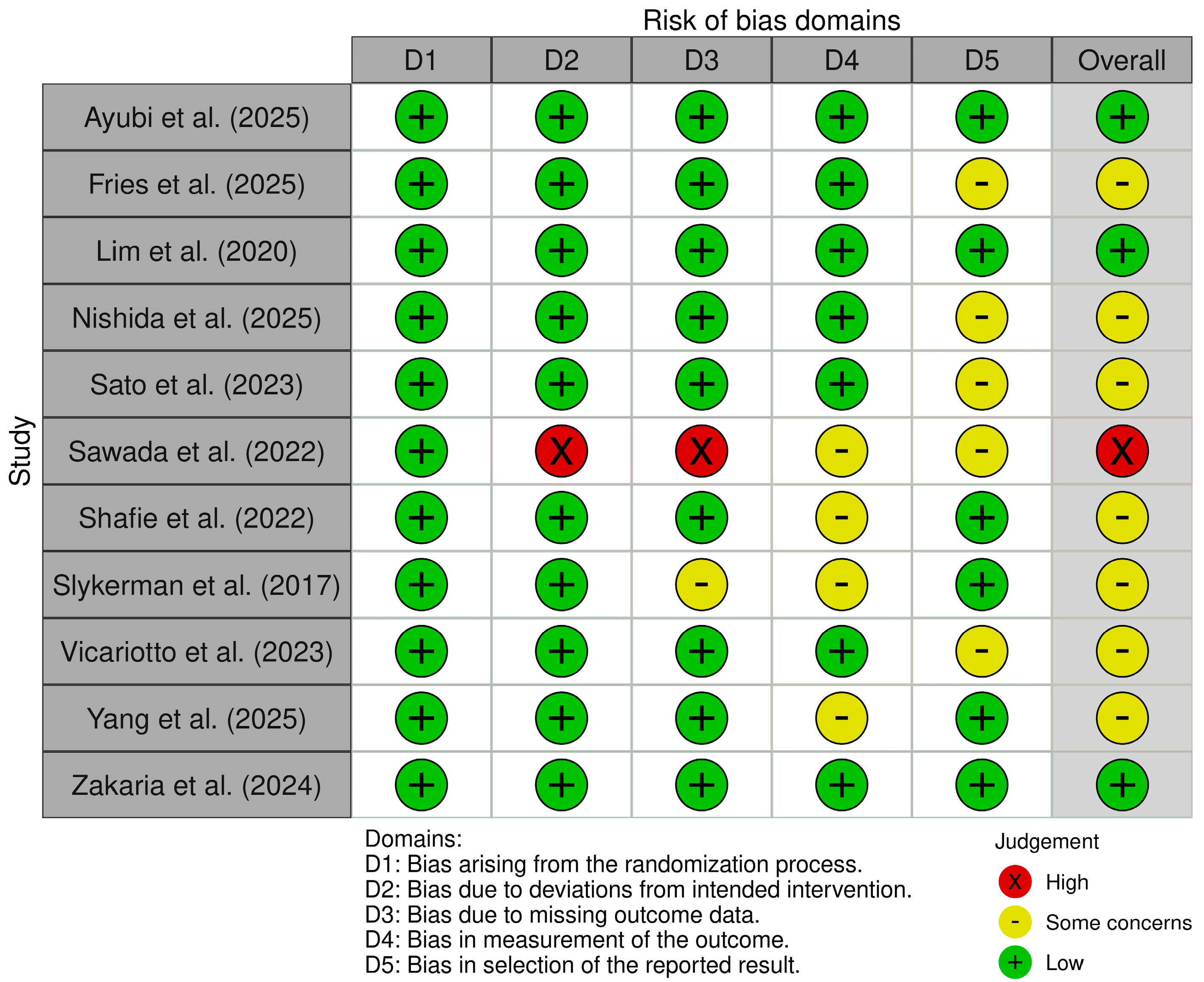
2.3. Methodological Quality Appraisal
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
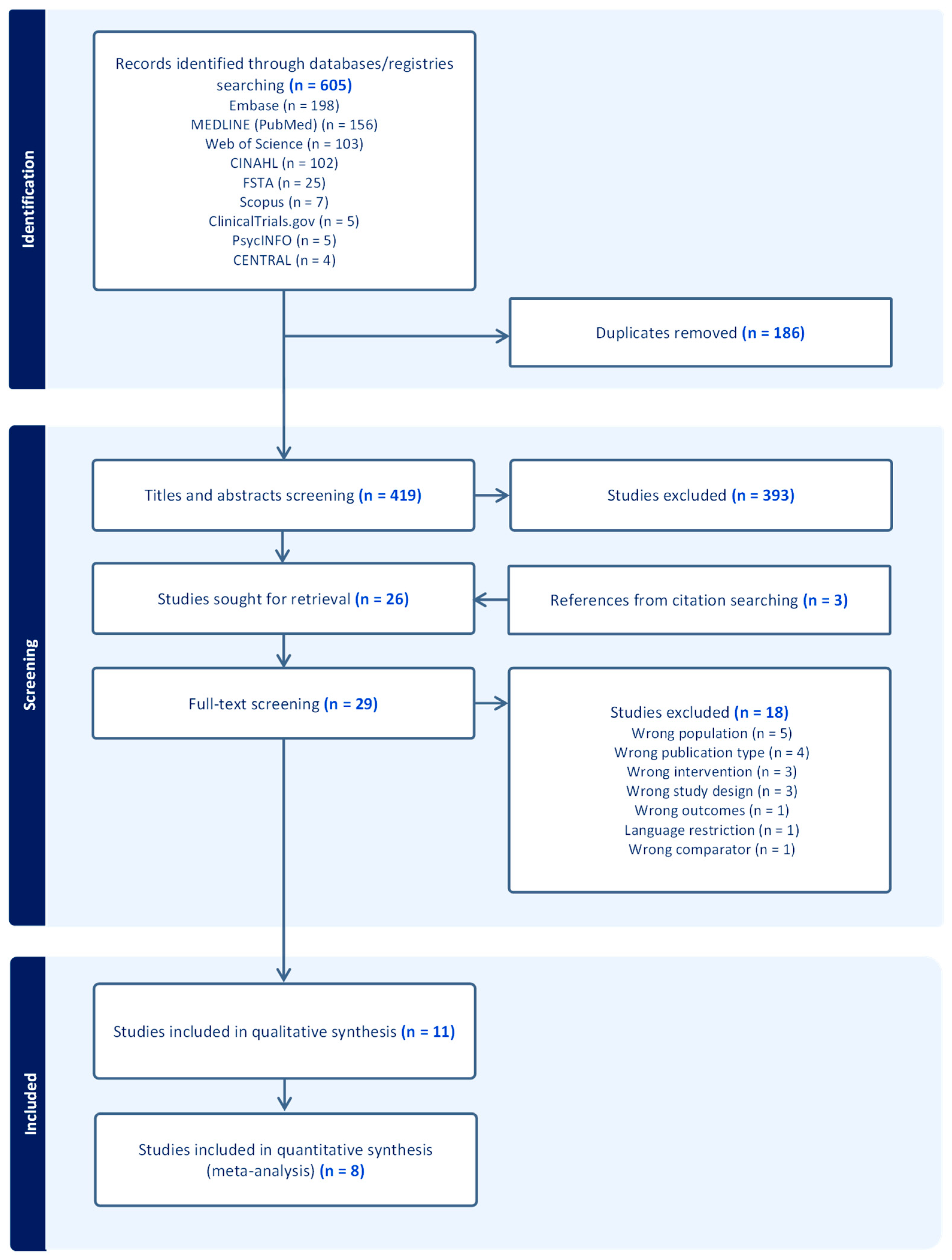
3.1. Study Selection
3.2. Study Characteristics
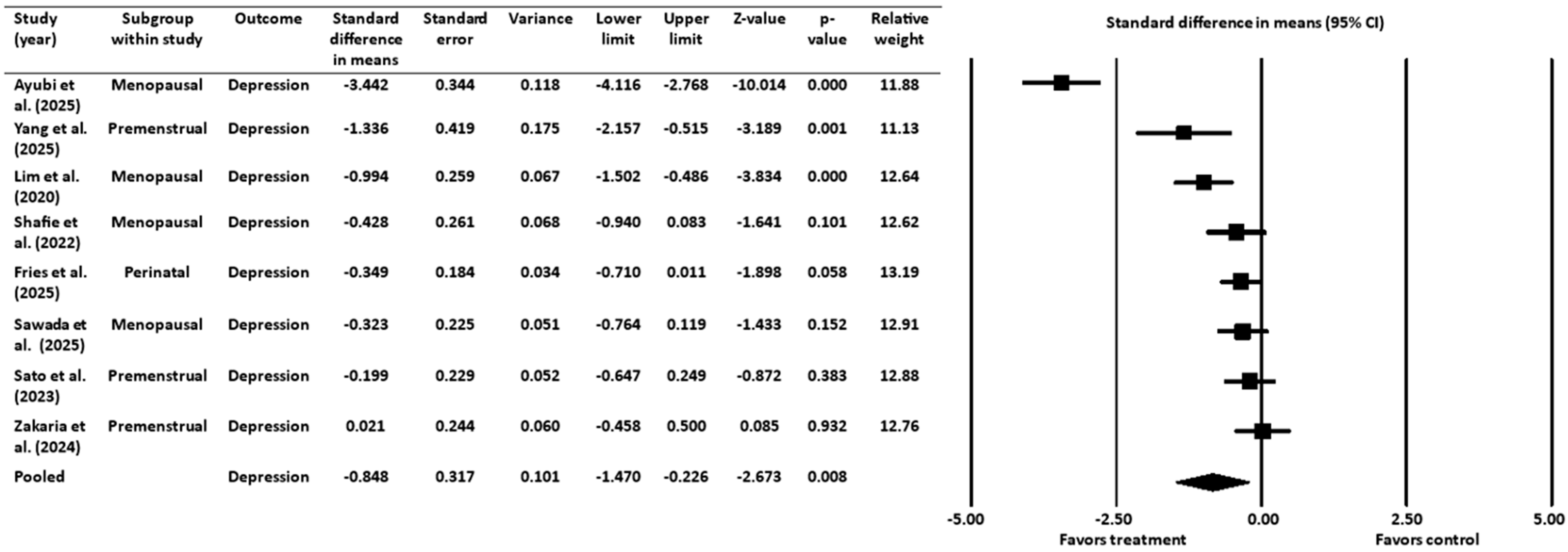
3.3. Effect of Gut-Targeted Interventions on Depression
3.3.1. Heterogeneity and Variation for Depression Outcome
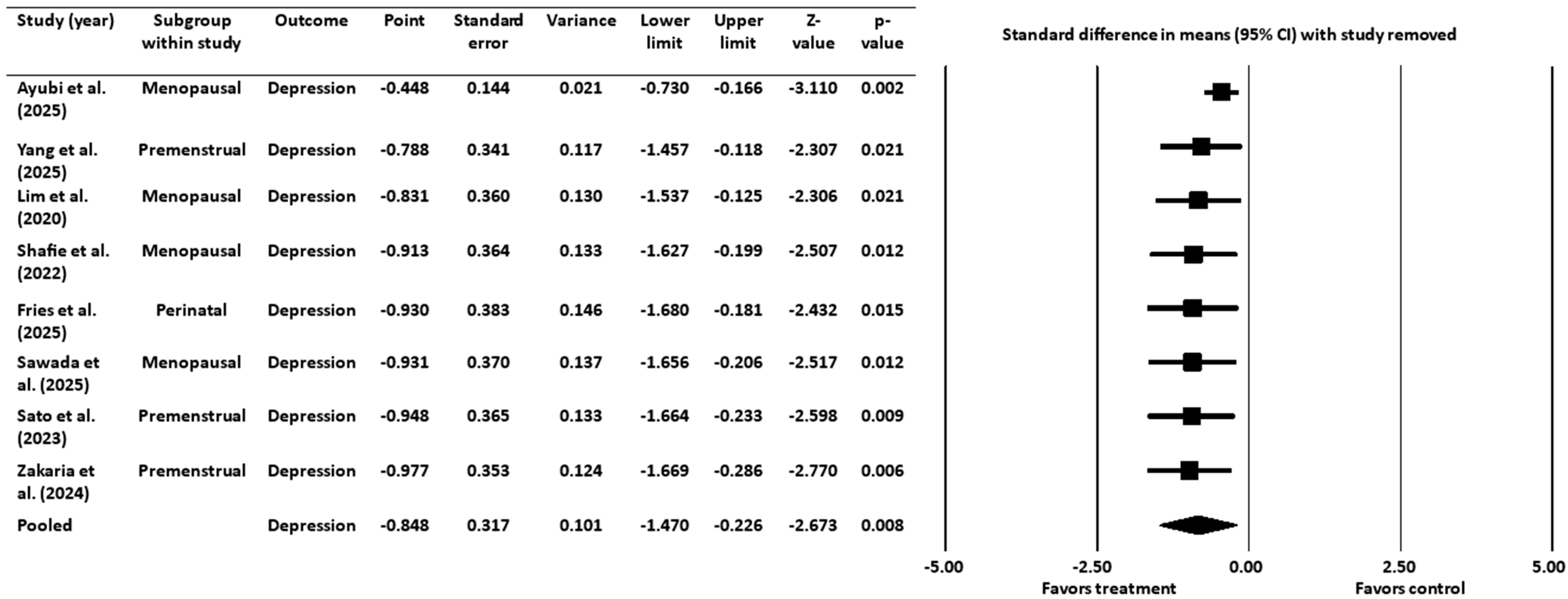
3.3.2. Sensitivity Analysis for Depression Outcome
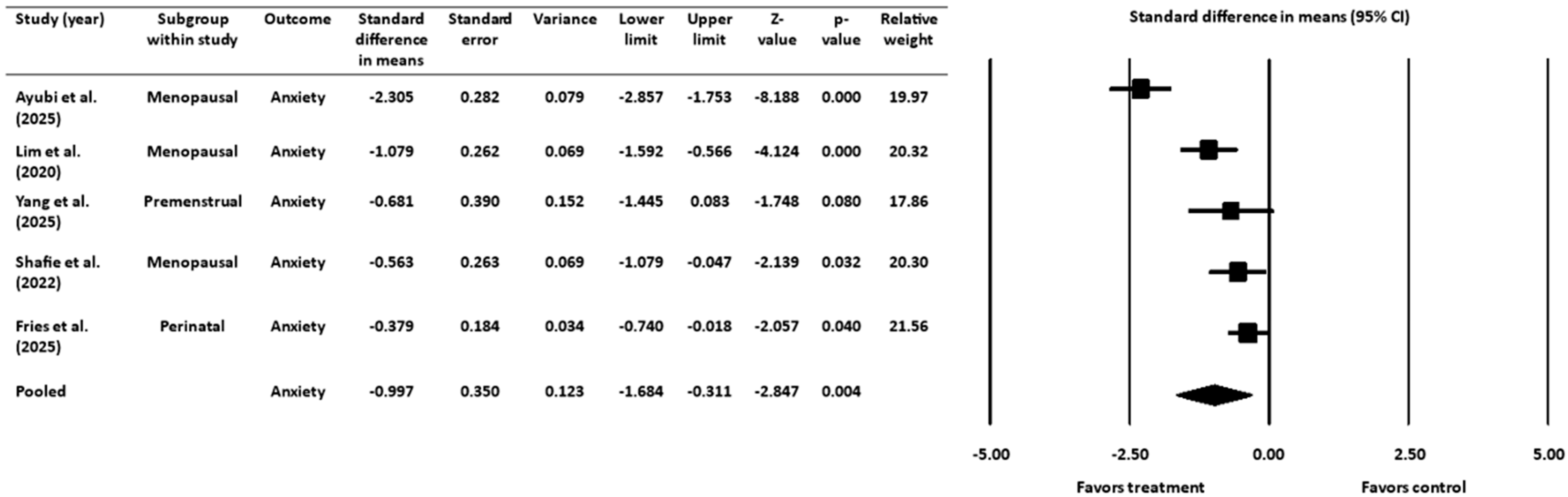
3.4. Effect of Gut-Targeted Interventions on Anxiety
3.4.1. Heterogeneity and Variation for Anxiety Outcome
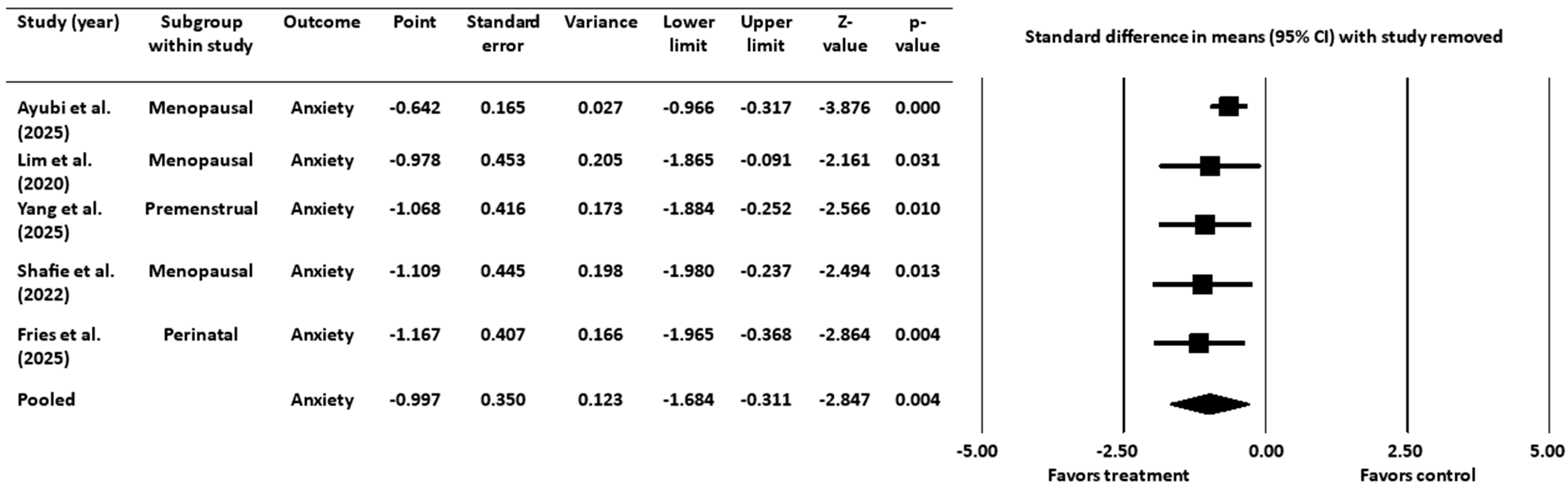
3.4.2. Sensitivity Analysis for Anxiety Outcome
3.5. Meta-Regression Analyses of Gut-Targeted Interventions on Duration of Treatment
3.6. Additional Studies Excluded from the Quantitative Analyses
3.7. Additional Mental Health Outcomes
4. Discussion
4.1. Discussion of Main Findings
4.2. Comparison with Existing Literature
4.3. Potential Mechanisms
4.4. Limitations
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| CMA | Comprehensive Meta-Analysis |
| CNS | Central nervous system |
| ENS | Enteric nervous system |
| FSTA | Food Science and Technology Abstract |
| GABA | Gamma-aminobutyric acid |
| HPA | Hypothalamic–pituitary–adrenal |
| IFN-γ | Interferon-gamma |
| IL-6 | Interleukin-6 |
| MeSH | Medical subject headings |
| PMS | premenstrual syndrome |
| PND | Perinatal depression |
| PPD | Postpartum depression |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| RCT | Randomized controlled trial |
| SMD | Standardized mean difference |
| TNF-α | Tumor necrosis factor-alpha |
| WHO | World Health Organization |
References
- Rong, J.; Wang, X.; Cheng, P.; Li, D.; Zhao, D. Global, Regional and National Burden of Depressive Disorders and Attributable Risk Factors, from 1990 to 2021: Results from the 2021 Global Burden of Disease Study. Br. J. Psychiatry 2025, 227, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, Y.; Zheng, L.; Pang, H.; Zhang, Q.; Lou, L.; Huang, X. Sex Difference in Global Burden of Major Depressive Disorder: Findings from the Global Burden of Disease Study 2019. Front. Psychiatry 2022, 13, 789305. [Google Scholar] [CrossRef]
- Kessler, R.C. Epidemiology of Women and Depression. J. Affect. Disord. 2003, 74, 5–13. [Google Scholar] [CrossRef]
- Hyde, J.S.; Mezulis, A.H.; Abramson, L.Y. The ABCs of Depression: Integrating Affective, Biological, and Cognitive Models to Explain the Emergence of the Gender Difference in Depression. Psychol. Rev. 2008, 115, 291–313. [Google Scholar] [CrossRef]
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y. Gender Differences in Depression in Representative National Samples: Meta-Analyses of Diagnoses and Symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef]
- Vesga-López, O.; Schneier, F.R.; Wang, S.; Heimberg, R.G.; Liu, S.M.; Hasin, D.S.; Blanco, C. Gender Differences in Generalized Anxiety Disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J. Clin. Psychiatry 2008, 69, 1606. [Google Scholar] [CrossRef]
- Bebbington, P.; Dunn, G.; Jenkins, R.; Lewis, G.; Brugha, T.; Farrell, M.; Meltzer, H. The Influence of Age and Sex on the Prevalence of Depressive Conditions: Report from the National Survey of Psychiatric Morbidity. Int. Rev. Psychiatry 2003, 15, 74. [Google Scholar] [CrossRef]
- Miller, L.J.; Girgis, C.; Gupta, R. Depression and Related Disorders during the Female Reproductive Cycle. Women’s Health 2009, 5, 577–587. [Google Scholar] [CrossRef]
- Antonelli, A.; Giannini, A.; Chedraui, P.; Monteleone, P.; Caretto, M.; Genazzani, A.D.; Mannella, P.; Simoncini, T.; Genazzani, A.R. Mood Disorders and Hormonal Status Across Women’s Life: A Narrative Review. Gynecol. Endocrinol. 2022, 38, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Wesselhoeft, R.; Pedersen, C.B.; Mortensen, P.B.; Mors, O.; Bilenberg, N. Gender–Age Interaction in Incidence Rates of Childhood Emotional Disorders. Psychol. Med. 2015, 45, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.N.; Zitek, B. Reproductive Hormone Sensitivity and Risk for Depression across the Female Life Cycle: A Continuum of Vulnerability? J. Psychiatry Neurosci. 2008, 33, 331–343. [Google Scholar] [CrossRef]
- Cox, E.; Barker, L.C.; Vigod, S.N.; Meltzer-Brody, S. Premenstrual Dysphoric Disorder, Peripartum (Perinatal) Depression, and Perimenopausal Depression. In Tasman’s Psychiatry; Springer: Cham, Switzerland, 2024; pp. 1881–1916. ISBN 978-3-030-51366-5. [Google Scholar]
- Feld, J.; Halbreich, U.; Karkun, S. The Association of Perimenopausal Mood Disorders with Other Reproductive-Related Disorders. CNS Spectr. 2005, 10, 461–470. [Google Scholar] [CrossRef]
- Turek, J.; Gąsior, Ł. Estrogen Fluctuations During the Menopausal Transition Are a Risk Factor for Depressive Disorders. Pharmacol. Rep. 2023, 75, 32–43. [Google Scholar] [CrossRef]
- Kim, I.B.; Park, S.-C.; Kim, Y.-K. Microbiota-Gut-Brain Axis in Major Depression: A New Therapeutic Approach. In Neuroinflammation, Gut-Brain Axis and Immunity in Neuropsychiatric Disorders; Kim, Y.-K., Ed.; Springer Nature: Singapore, 2023; pp. 209–224. ISBN 978-981-19-7376-5. [Google Scholar]
- Shafie, M.; Homayouni Rad, A.; Mirghafourvand, M. Effects of Prebiotic-Rich Yogurt on Menopausal Symptoms and Metabolic Indices in Menopausal Women: A Triple-Blind Randomised Controlled Trial. Int. J. Food Sci. Nutr. 2022, 73, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Effect of Lactobacillus Rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-Blind Placebo-Controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef]
- Fries, L.R.; Boehme, M.; Lavalle, L.; Sakwinska, O.; Chughlay, F.; Keddani, S.; Porta, N.; Vicario, M.; Bergonzelli, G.; Silva Zolezzi, I.; et al. The Impact of Ingestion of Bifidobacterium Longum NCC3001 on Perinatal Anxiety and Depressive Symptoms: A Randomized Controlled Trial. Sci. Rep. 2025, 15, 11250. [Google Scholar] [CrossRef]
- Ayubi, E.; Abdoli, S.; Mehrpooya, M.; Karami, Z.; Jenabi, E.; Ghaleiha, A.; Soltani, F.; Salehi, A.M. The Effect of Probiotic Administration on the Severity of Menopausal Symptoms and Mental Health of Postmenopausal Women: A Triple-Blind Randomized Controlled Trial in the West of Iran. Menopause 2025, 32, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Fukawa-Nagira, A.; Sashihara, T. Lactobacillus paragasseri OLL2809 Improves Premenstrual Psychological Symptoms in Healthy Women: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 4985. [Google Scholar] [CrossRef] [PubMed]
- Chi, R.; Li, M.; Zhang, M.; Zhang, N.; Zhang, G.; Cui, L.; Ma, G. Exploring the Association Between Anxiety, Depression, and Gut Microbiota During Pregnancy: Findings from a Pregnancy Cohort Study in Shijiazhuang, Hebei Province, China. Nutrients 2024, 16, 1460. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef]
- Eltokhi, A.; Sommer, I.E. A Reciprocal Link Between Gut Microbiota, Inflammation and Depression: A Place for Probiotics? Front. Neurosci. 2022, 16, 852506. [Google Scholar] [CrossRef]
- Mehta, I.; Juneja, K.; Nimmakayala, T.; Bansal, L.; Pulekar, S.; Duggineni, D.; Ghori, H.K.; Modi, N.; Younas, S. Gut Microbiota and Mental Health: A Comprehensive Review of Gut-Brain Interactions in Mood Disorders. Cureus 2025, 17, e81447. [Google Scholar] [CrossRef]
- Amarasena, S.; Mayengbam, S. Manipulation of Gut Microbiome to Improve Mental Health. In Anxiety, Gut Microbiome, and Nutraceuticals; CRC Press: Boca Raton, FA, USA, 2023; ISBN 978-1-003-33382-1. [Google Scholar]
- Oake, A.; Nesto, N.; Pathak, Y.V. Gut Microbiota and Mental Health: The Gut–Brain Axis. In Anxiety, Gut Microbiome, and Nutraceuticals; CRC Press: Boca Raton, FA, USA, 2023; ISBN 978-1-003-33382-1. [Google Scholar]
- Ahmed, G.K.; Ramadan, H.K.-A.; Elbeh, K.; Haridy, N.A. Bridging the Gap: Associations between Gut Microbiota and Psychiatric Disorders. Middle East Curr. Psychiatry 2024, 31, 2. [Google Scholar] [CrossRef]
- Leao, L.; Miri, S.; Hammami, R. Gut Feeling: Exploring the Intertwined Trilateral Nexus of Gut Microbiota, Sex Hormones, and Mental Health. Front. Neuroendocrinol. 2025, 76, 101173. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Inslicht, S.S.; Bhargava, A. Gut-Brain Axis: Role of Microbiome, Metabolomics, Hormones, and Stress in Mental Health Disorders. Cells 2024, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef] [PubMed]
- Abdelkawi, A.; Martinez, J.-P.P.; Pathak, S.; Pathak, Y. Synbiotics: Traditional Approach, Present Status, and Future Outlook. In Anxiety, Gut Microbiome, and Nutraceuticals; CRC Press: Boca Raton, FA, USA, 2023; ISBN 978-1-003-33382-1. [Google Scholar]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain–Gut–Microbiome Axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and Paraprobiotics: From Concepts to Applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef]
- Ķimse, L.; Reinis, A.; Miķelsone-Jansone, L.; Gintere, S.; Krūmiņa, A. A Narrative Review of Psychobiotics: Probiotics That Influence the Gut–Brain Axis. Medicina 2024, 60, 601. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Sawada, D.; Yasui, T.; Kuwano, Y.; Rokutan, K. Daily Intake of Lactobacillus Gasseri CP2305 Ameliorates Psychological Premenstrual Symptoms in Young Women: A Randomized, Double-Blinded, Placebo-Controlled Study. J. Funct. Foods 2021, 80, 104426. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–Gut Microbiome Axis: Physiological and Clinical Implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Nabeh, O.A. New Insights on the Impact of Gut Microbiota on Premenstrual Disorders. Will Probiotics Solve This Mystery? Life Sci. 2023, 321, 121606. [Google Scholar] [CrossRef]
- Dowse, E.; Chan, S.; Ebert, L.; Wynne, O.; Thomas, S.; Jones, D.; Fealy, S.; Evans, T.-J.; Oldmeadow, C. Impact of Perinatal Depression and Anxiety on Birth Outcomes: A Retrospective Data Analysis. Matern. Child Health J. 2020, 24, 718–726. [Google Scholar] [CrossRef]
- Rogers, A.; Obst, S.; Teague, S.J.; Rossen, L.; Spry, E.A.; Macdonald, J.A.; Sunderland, M.; Olsson, C.A.; Youssef, G.; Hutchinson, D. Association Between Maternal Perinatal Depression and Anxiety and Child and Adolescent Development: A Meta-Analysis. JAMA Pediatr. 2020, 174, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Vicariotto, F.; Malfa, P.; Torricelli, M.; Lungaro, L.; Caio, G.; De Leo, V. Beneficial Effects of Limosilactobacillus reuteri Pbs072 and Bifidobacterium breve Bb077 on Mood Imbalance, Self-Confidence, and Breastfeeding in Women during the First Trimester Postpartum. Nutrients 2023, 15, 3513. [Google Scholar] [CrossRef]
- Burger, H.G. Physiology and Endocrinology of the Menopause. Medicine 2006, 34, 27–30. [Google Scholar] [CrossRef]
- Talaulikar, V. Menopause Transition: Physiology and Symptoms. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 81, 3–7. [Google Scholar] [CrossRef]
- Lim, E.Y.; Lee, S.-Y.; Shin, H.S.; Lee, J.; Nam, Y.-D.; Lee, D.O.; Lee, J.Y.; Yeon, S.H.; Son, R.H.; Park, C.L.; et al. The Effect of Lactobacillus Acidophilus YT1 (MENOLACTO) on Improving Menopausal Symptoms: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. J. Clin. Med. 2020, 9, 2173. [Google Scholar] [CrossRef] [PubMed]
- Sawada, D.; Sugawara, T.; Hirota, T.; Nakamura, Y. Effects of Lactobacillus gasseri CP2305 on Mild Menopausal Symptoms in Middle-Aged Women. Nutrients 2022, 14, 1695. [Google Scholar] [CrossRef]
- Shafie, M.; Homayouni Rad, A.; Mohammad-Alizadeh-Charandabi, S.; Mirghafourvand, M. The Effect of Probiotics on Mood and Sleep Quality in Postmenopausal Women: A Triple-Blind Randomized Controlled Trial. Clin. Nutr. ESPEN 2022, 50, 15–23. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis, Version 4; Biostat, Inc.: Englewood, NJ, USA, 2022.
- Borenstein, M. Common Mistakes in Meta-Analysis and How to Avoid Them; Biostat, Inc.: Englewood, NJ, USA, 2019; ISBN 978-1-7334367-0-0. [Google Scholar]
- Page, M.; Higgins, J.; Sterne, J. Chapter 13: Assessing Risk of Bias Due to Missing Evidence in a Meta-Analysis | Cochrane. Available online: https://www.cochrane.org/authors/handbooks-and-manuals/handbook/current/chapter-13 (accessed on 23 September 2025).
- Yang, M.-Y.; Chen, H.-Y.; Ho, C.-H.; Huang, W.-C. Impact of Probiotic Supplementation and High-Intensity Interval Training on Primary Dysmenorrhea: A Double-Blind, Randomized Controlled Trial Investigating Inflammation and Hormonal Modulation. Nutrients 2025, 17, 622. [Google Scholar] [CrossRef]
- Zakaria, I.A.; Mohammed Zain, N.A.; Teik, C.K.; Abu, M.A.; Zainuddin, A.A.; Abdul Aziz, N.H.; Safian, N.; Mohd Mokhtar, N.; Raja Ali, R.A.; Beng Kwang, N.; et al. The Role of Probiotics in Improving Menstrual Health in Women with Primary Dysmenorrhoea: A Randomized, Double-Blind, Placebo-Controlled Trial (the PERIOD Study). Women’s Health 2024, 20, 17455057241234524. [Google Scholar] [CrossRef]
- Simon, G.E.; Moise, N.; Mohr, D.C. Management of Depression in Adults: A Review. JAMA 2024, 332, 141–152. [Google Scholar] [CrossRef]
- Jespersen, C.; Lauritsen, M.P.; Frokjaer, V.G.; Schroll, J.B. Selective Serotonin Reuptake Inhibitors for Premenstrual Syndrome and Premenstrual Dysphoric Disorder. Cochrane Database Syst. Rev. 2024. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, J.; Song, X.; Lai, S.; Zhong, S.; Jia, Y. The Effect of Exogenous Estrogen on Depressive Mood in Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Psychiatr. Res. 2023, 162, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.V.E.; Wilson, C.A.; Ayre, K.; Robertson, L.; South, E.; Molyneaux, E.; Trevillion, K.; Howard, L.M.; Khalifeh, H. Antidepressant Treatment for Postnatal Depression. Cochrane Database Syst. Rev. 2021. [Google Scholar] [CrossRef]
- Arroll, B.; Elley, C.R.; Fishman, T.; Goodyear-Smith, F.A.; Kenealy, T.; Blashki, G.; Kerse, N.; MacGillivray, S. Antidepressants versus Placebo for Depression in Primary Care. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef] [PubMed]
- Halemani, K.; Shetty, A.P.; Thimmappa, L.; Issac, A.; Dhiraaj, S.; Radha, K.; Mishra, P.; Mathias, E.G. Impact of Probiotic on Anxiety and Depression Symptoms in Pregnant and Lactating Women and Microbiota of Infants: A Systematic Review and Meta-Analysis. J. Glob. Health 2023, 13, 04038. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.; Kozyrskyj, A.L.; Lau, S.; Sanni, O.; Dennett, L.; Walter, J.; Ospina, M.B. Effectiveness of Probiotic, Prebiotic, and Synbiotic Supplementation to Improve Perinatal Mental Health in Mothers: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 622181. [Google Scholar] [CrossRef]
- Alemu, B.K.; Wu, L.; Azeze, G.G.; Lau, S.L.; Wang, Y.; Wang, C.C. Microbiota-Targeted Interventions and Clinical Implications for Maternal-Offspring Health: An Umbrella Review of Systematic Reviews and Meta-Analyses of Randomised Controlled Trials. J. Glob. Health 2024, 14, 04177. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.A.F.; Lacey, A.; Roach, H.; Tomlinson, R.; Kidd, E.J.; Bache, K. Investigating the Effects of Probiotics During the Menopause Transition: A Systematic Review & Meta-Analysis. Clin. Nutr. ESPEN 2025, 69, 241–256. [Google Scholar] [CrossRef]
- Takeda, T.; Yoshimi, K.; Kai, S.; Ozawa, G.; Yamada, K.; Hiramatsu, K. Characteristics of the Gut Microbiota in Women with Premenstrual Symptoms: A Cross-Sectional Study. PLoS ONE 2022, 17, e0268466. [Google Scholar] [CrossRef]
- Okuma, K.; Kono, K.; Otaka, M.; Ebara, A.; Odachi, A.; Tokuno, H.; Masuyama, H. Characteristics of the Gut Microbiota in Japanese Patients with Premenstrual Syndrome. Int. J. Women’s Health 2022, 14, 1435–1445. [Google Scholar] [CrossRef]
- Yao, Y.; Hu, H.; Chen, L.; Zheng, H. Association Between Gut Microbiota and Menstrual Disorders: A Two-Sample Mendelian Randomization Study. Front. Microbiol. 2024, 15, 1321268. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, X.; Yu, Z.; Zhang, Z.; Deng, M.; Zhao, J.; Ruan, B. Altered Gut Microbiota Profile in Patients with Generalized Anxiety Disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, J.; Wu, D.; Yu, S.; Qiang, X.; Bai, H.; Wang, H.; Peng, Z. Association Between Fecal Microbiota and Generalized Anxiety Disorder: Severity and Early Treatment Response. J. Affect. Disord. 2019, 259, 56–66. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; He, Y. The Variation Characteristics of Fecal Microbiota in Remission UC Patients with Anxiety and Depression. Front. Microbiol. 2023, 14, 1237256. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, B.; Zhang, J.; Dong, J.; Ma, J.; Zhang, Y.; Jin, K.; Lu, J. Effect of Prebiotics, Probiotics, Synbiotics on Depression: Results from a Meta-Analysis. BMC Psychiatry 2023, 23, 477. [Google Scholar] [CrossRef]
- Asad, A.; Kirk, M.; Zhu, S.; Dong, X.; Gao, M. Effects of Prebiotics and Probiotics on Symptoms of Depression and Anxiety in Clinically Diagnosed Samples: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2025, 83, e1504–e1520. [Google Scholar] [CrossRef]
- Moshfeghinia, R.; Nemati, H.; Ebrahimi, A.; Shekouh, D.; Karami, S.; Eraghi, M.M.; Mohagheghzadeh, H.; Hunter, J.; Pasalar, M. The Impact of Probiotics, Prebiotics, and Synbiotics on Depression and Anxiety Symptoms of Patients with Depression: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2025, 188, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Z.; Gu, L.; Zhang, K. Fermented Dairy Foods Consumption and Depressive Symptoms: A Meta-Analysis of Cohort Studies. PLoS ONE 2023, 18, e0281346. [Google Scholar] [CrossRef] [PubMed]
- Anguiano Morán, A.C.; de León Castañeda, C.D.; Rodríguez Orozco, A.R.; Valtierra Oba, E.R.; Lemus Loeza, B.M.; Galván Villalobos, G. Efficacy of Probiotics, Prebiotics, and Symbiotics for the Treatment of Depression: A Meta-Review. Salud Ment. 2025, 48, 31–46. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.-J. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: New York, NY, USA, 2014; pp. 39–71. ISBN 978-1-4939-0897-4. [Google Scholar]
- Holzer, P.; Farzi, A. Neuropeptides and the Microbiota-Gut-Brain Axis. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: New York, NY, USA, 2014; pp. 195–219. ISBN 978-1-4939-0897-4. [Google Scholar]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/Brain Axis and the Microbiota. J. Clin. Invest. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Toader, C.; Dobrin, N.; Costea, D.; Glavan, L.-A.; Covache-Busuioc, R.-A.; Dumitrascu, D.-I.; Bratu, B.-G.; Costin, H.-P.; Ciurea, A.V. Mind, Mood and Microbiota—Gut–Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2024, 25, 3340. [Google Scholar] [CrossRef]
- Rode, J.; Edebol Carlman, H.M.T.; König, J.; Hutchinson, A.N.; Thunberg, P.; Persson, J.; Brummer, R.J. Multi-Strain Probiotic Mixture Affects Brain Morphology and Resting State Brain Function in Healthy Subjects: An RCT. Cells 2022, 11, 2922. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Wall, R.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.F.; Dinan, T.G.; Stanton, C. Bacterial Neuroactive Compounds Produced by Psychobiotics. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: New York, NY, USA, 2014; pp. 221–239. ISBN 978-1-4939-0897-4. [Google Scholar]
- Reigstad, C.S.; Salmonson, C.E.; Iii, J.F.R.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut Microbes Promote Colonic Serotonin Production through an Effect of Short-Chain Fatty Acids on Enterochromaffin Cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Willner, P.; Scheel-Krüger, J.; Belzung, C. The Neurobiology of Depression and Antidepressant Action. Neurosci. Biobehav. Rev. 2013, 37, 2331–2371. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.; Farzi, A.; Zhu, W. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-Associated Depression: From Serotonin to Kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Ying, S. Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites 2023, 13, 1166. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Krog, M.C.; Hugerth, L.W.; Bashir, Z.; Fransson, E.; Boulund, F.; Belibasakis, G.N.; Wannerberger, K.; Engstrand, L.; Nielsen, H.S.; et al. Dysbiosis of the Human Oral Microbiome During the Menstrual Cycle and Vulnerability to the External Exposures of Smoking and Dietary Sugar. Front. Cell Infect. Microbiol. 2021, 11, 625229. [Google Scholar] [CrossRef]
- Pugh, J.N.; Lydon, K.M.; O’Donovan, C.M.; O’Sullivan, O.; Madigan, S.M. More than a Gut Feeling: What Is the Role of the Gastrointestinal Tract in Female Athlete Health? Eur. J. Sport Sci. 2022, 22, 755–764. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Li, X.; Sun, Q.; Qin, P.; Wang, Q. Compositional and Functional Features of the Female Premenopausal and Postmenopausal Gut Microbiota. FEBS Lett. 2019, 593, 2655–2664. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Carvalho, A.F.; Geffard, M.; Maes, M. The Menstrual Cycle May Not Be Limited to the Endometrium but Also May Impact Gut Permeability. Acta Neuropsychiatr. 2019, 31, 294–304. [Google Scholar] [CrossRef]
- Fasano, A.; Chassaing, B.; Haller, D.; Flores Ventura, E.; Carmen-Collado, M.; Pastor, N.; Koren, O.; Berni Canani, R. Microbiota During Pregnancy and Early Life: Role in Maternal−Neonatal Outcomes Based on Human Evidence. Gut Microbes 2024, 16, 2392009. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Kling Bäckhed, H.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes During Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shaw, G.; et al. Temporal and Spatial Variation of the Human Microbiota During Pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, B.; Wang, G. The Role of Gut Microbiota in the Pathogenesis and Treatment of Postpartum Depression. Ann. Gen. Psychiatry 2023, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.T.; Castelo, P.M.; Ribeiro, D.A.; Ferreira, C.M. Influence of Oral and Gut Microbiota in the Health of Menopausal Women. Front. Microbiol. 2017, 8, 1884. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Ma, M.; Zhang, W.; Bi, Y.; Cheng, P.; Yu, X.; Fu, Y.; Chao, Y.; Ji, T.; Li, J.; et al. The Gut Microbiota During the Progression of Atherosclerosis in the Perimenopausal Period Shows Specific Compositional Changes and Significant Correlations with Circulating Lipid Metabolites. Gut Microbes 2021, 13, 1880220. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Mao, T.; Huang, Y.; Liang, J.; Zhu, M.; Yao, P.; Zong, Y.; Lang, J.; Zhang, Y. The Relationship Between Menopausal Syndrome and Gut Microbes. BMC Women’s Health 2022, 22, 437. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Arnoriaga-Rodríguez, M.; Luque-Córdoba, D.; Priego-Capote, F.; Pérez-Brocal, V.; Moya, A.; Burokas, A.; Maldonado, R.; Fernández-Real, J.-M. Gut Microbiota Steroid Sexual Dimorphism and Its Impact on Gonadal Steroids: Influences of Obesity and Menopausal Status. Microbiome 2020, 8, 136. [Google Scholar] [CrossRef]
- Gadek-Michalska, A.; Tadeusz, J.; Rachwalska, P.; Bugajski, J. Cytokines, Prostaglandins and Nitric Oxide in the Regulation of Stress-Response Systems. Pharmacol. Rep. 2013, 65, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Zhang, M.; Tu, Q.; Li, J.; Wang, Y.; Wan, S.; Li, D.; Qian, Q.; Xia, L. From Gut Inflammation to Psychiatric Comorbidity: Mechanisms and Therapies for Anxiety and Depression in Inflammatory Bowel Disease. J. Neuroinflamm. 2025, 22, 149. [Google Scholar] [CrossRef]
- Ge, L.; Liu, S.; Li, S.; Yang, J.; Hu, G.; Xu, C.; Song, W. Psychological Stress in Inflammatory Bowel Disease: Psychoneuroimmunological Insights into Bidirectional Gut–Brain Communications. Front. Immunol. 2022, 13, 1016578. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So Depression Is an Inflammatory Disease, but Where Does the Inflammation Come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Elgellaie, A.; Thomas, S.J.; Kaelle, J.; Bartschi, J.; Larkin, T. Pro-Inflammatory Cytokines IL-1α, IL-6 and TNF-α in Major Depressive Disorder: Sex-Specific Associations with Psychological Symptoms. Eur. J. Neurosci. 2023, 57, 1913–1928. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, J.K.; Kim, J.-K.; Kim, D.-H.; Jang, S.W.; Han, S.-W.; Yoon, I.-Y. Effects of Probiotic Nvp-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2660. [Google Scholar] [CrossRef]
- Lew, L.-C.; Hor, Y.-Y.; Yusoff, N.A.A.; Choi, S.-B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Probiotic Lactobacillus Plantarum P8 Alleviated Stress and Anxiety While Enhancing Memory and Cognition in Stressed Adults: A Randomised, Double-Blind, Placebo-Controlled Study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Description |
|---|---|
| Population (P) | Inclusion criteria: Healthy women aged 18–65 years. Participants in one of three hormonal life stages: 1. Premenstrual group: women of reproductive age experiencing premenstrual syndrome or menstrual-related symptoms. 2. Perinatal group: pregnant women or women up to 12 months postpartum. 3. Menopausal group: women experiencing menopausal symptoms during perimenopause or postmenopause. Exclusion criteria: Men or mixed-gender studies without sex-specific data. Women with major psychiatric disorders requiring medication. Women with comorbid conditions affecting gut microbiome (e.g., inflammatory bowel disease, diabetes, obesity). |
| Intervention (I) | Inclusion criteria: Probiotics, prebiotics, synbiotics, psychobiotics, paraprobiotics, postbiotics, or fermented foods with documented probiotic content. Exclusion criteria: Interventions combined with other treatments (e.g., pharmacological) where effects cannot be separated. Interventions involving antibiotics or other gut-depleting interventions. |
| Comparison (C) | Placebo, no intervention, standard care. |
| Outcome (O) | Primary outcomes: changes in scores on validated scales measuring symptoms of depression and anxiety. Secondary outcomes: changes in other mental health-related outcomes. |
| Study design (S) | Inclusion criteria: Randomized controlled trials (RCTs) Exclusion criteria: Unpublished trials, pilot studies, non-RCT designs. |
| Study, Country | Hormonal Life Stage Group | Specific Period Covered | Study Design | Participants | Average Age (T, C) | Sample Size (T, C) | Intervention Type | Comparator | Duration of Treatment (Weeks) | Mental Health Outcome(s) Measured | Measurement Tool(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nishida et al. (2021) [37] Japan | Premenstrual | N/A | Double-blind RCT | Female students | T = ~21; C = ~22 | T = 25; C = 31 | Paraprobiotics | Placebo | ~26 | Depression, Anxiety, Irritability, Mood swings | PMTS-VAS |
| Sato et al. (2023) [20] Japan | Premenstrual | N/A | Double-blind RCT | Women with menstrual-related symptoms | T = ~32; C = ~32 | T = 39; C = 38 | Probiotics | Placebo | ~12 | Depression, Irritability, Mood swings | MDQ, VAS |
| Yang et al. (2025) [52] Taiwan | Premenstrual | N/A | Double-blind RCT | Women with primary dysmenorrhea | T = ~22; C = ~25 | T = 15; C = 13 | Probiotics | Placebo | 10 | Depression, Anxiety, Irritability | PSST |
| Zakaria et al. (2024) [53] Malaysia | Premenstrual | N/A | Double-blind RCT | Women with primary dysmenorrhea | T = ~25; C = ~26 | T = 34; C = 33 | Probiotics | Placebo | 12 | Depression | SF12v2 |
| Fries et al. (2025) [18] China | Perinatal | Pregnancy and Postpartum | Double-blind RCT | Pregnant women | T = ~32; C = ~32 | T = 61; C = 59 | Probiotics | Placebo | ~22 | Depression, Anxiety | EPDS, STAI-S |
| Slykerman et al. (2017) [17] New Zealand | Perinatal | Pregnancy and postpartum | Double-blind RCT | Pregnant women | T = ~34; C = ~34 | Depression: T = 194; C = 187 Anxiety: T = 192; C = 187 | Probiotics | Placebo | ~52 | Depression, Anxiety | EPDS, STAI6 |
| Vicariotto et al. (2023) [42] Italy | Perinatal | Postpartum | Double-blind RCT | Women in their first trimester postpartum | T = ~33; C = ~33 | T = 95; C = 95 | Probiotics | Placebo | ~13 | Depression | EPDS |
| Ayubi et al. (2025) [19] Iran | Menopausal | Postmenopause | Triple-blind RCT | Menopausal women | T = ~51; C = ~51 | T = 42; C = 42 | Probiotics | Placebo | 6 | Depression, Anxiety, Stress | DASS21 |
| Lim et al. (2020) [45] Korea | Menopausal | Postmenopause | Double-blind RCT | Menopausal women | T = ~54; C = ~52 | T = 32; C = 35 | Probiotics | Placebo | 12 | Depression, Anxiety | KMI |
| Sawada et al. (2022) [46] Japan | Menopausal | Perimenopause | Double-blind RCT | Perimenopausal women | T = ~46; C = ~45 | T = 40; C = 40 | Paraprobiotics | Placebo | ~24 | Depression | GCS |
| Shafie et al. (2022) [16] Iran | Menopausal | Postmenopause | Triple-blind RCT | Menopausal women | T = ~52; C = ~52 | T = 30; C = 30 | Prebiotics | Placebo | 6 | Depression, Anxiety | GCS |
| Study | Group | Intervention Type | Strain(s)/Product | Daily Dosage | Delivery Form |
|---|---|---|---|---|---|
| Nishida et al. (2021) [37] | Premenstrual | Paraprobiotics | Lactobacillus gasseri CP2305 | 1 × 1010 BC | Tablet |
| Sato et al. (2023) [20] | Premenstrual | Probiotics | Lactobacillus paragasseri OLL2809 | 1 × 1010 BC | Tablet |
| Yang et al. (2025) [52] | Premenstrual | Probiotics | Bifidobacterium longum subsp. longum OLP-01, L. plantarum PL-02, and Lactococcus lactis LY-66 | 1.5 × 1010 CFU | Capsule |
| Zakaria et al. (2024) [53] | Premenstrual | Probiotics | Lactobacillus acidophilus BCMC 12130, Lactobacillus casei subsp BCMC 12313, Lactobacillus lactis BCMC 12451, Bifidobacterium bifidum BCMC 02290, Bifidobacterium longum BCMC 02120, and Bifidobacterium infantis BCMC 02129 | 1 × 1010 CFU | Sachet |
| Fries et al. (2025) [18] | Perinatal | Probiotics | Bifidobacterium longum NCC3001 | 1 × 1010 CFU | Sachet |
| Slykerman et al. (2017) [17] | Perinatal | Probiotics | Lactobacillus rhamnosus HN001 | 6 × 109 CFU | Capsule |
| Vicariotto et al. (2023) [42] | Perinatal | Probiotics | Limosilactobacillus reuteri PBS072 and Bifidobacterium breve BB077 | 4 × 109 CFU | Capsule |
| Ayubi et al. (2025) [19] | Menopausal | Probiotics | Lactobacillus plantarum, Lactobacillus casei, L. acidophilus, L. bulgaricus, Bifidobacterium infantis, Bifidobacterium longum, Bifidobacterium breve, and S. thermophilus | 4.5 × 1011 CFU | Capsule |
| Lim et al. (2020) [45] | Menopausal | Probiotics | Lactobacillus acidophilus YT1 | 1 × 108 CFU | Sachet |
| Sawada et al. (2022) [46] | Menopausal | Paraprobiotics | Lactobacillus gasseri CP2305 | 1 × 1010 BC | Tablet |
| Shafie et al. (2022) [16] | Menopausal | Prebiotics | Long-chain Inulin | 1.5 g | Yogurt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubois, N.; Vincent, C.; Giroux, I. Efficacy of Gut Microbiome-Targeted Interventions on Mental Health Symptoms in Women Across Key Hormonal Life Stages: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare 2025, 13, 2851. https://doi.org/10.3390/healthcare13222851
Dubois N, Vincent C, Giroux I. Efficacy of Gut Microbiome-Targeted Interventions on Mental Health Symptoms in Women Across Key Hormonal Life Stages: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare. 2025; 13(22):2851. https://doi.org/10.3390/healthcare13222851
Chicago/Turabian StyleDubois, Naika, Coralie Vincent, and Isabelle Giroux. 2025. "Efficacy of Gut Microbiome-Targeted Interventions on Mental Health Symptoms in Women Across Key Hormonal Life Stages: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Healthcare 13, no. 22: 2851. https://doi.org/10.3390/healthcare13222851
APA StyleDubois, N., Vincent, C., & Giroux, I. (2025). Efficacy of Gut Microbiome-Targeted Interventions on Mental Health Symptoms in Women Across Key Hormonal Life Stages: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare, 13(22), 2851. https://doi.org/10.3390/healthcare13222851