Quality of Life and Financial Burden in Duchenne Muscular Dystrophy in Greece: Insights into Health System Performance in the Post-Pandemic Context
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
- (a)
- Parental questionnaire: included demographic, clinical, socioeconomic, and caregiver-related variables, along with the validated PedsQL™ 4.0 Generic Core Scales and PedsQL™ 3.0 DMD Module (proxy version).
- (b)
- Child questionnaire: included the PedsQL™ self-report scales, completed by children aged 8–18 years where feasible. Children aged 5–7 years did not complete self-reports.
2.2. Measures
2.3. Statistical Analysis
3. Results
3.1. Socio-Demographic and Clinical Characteristics
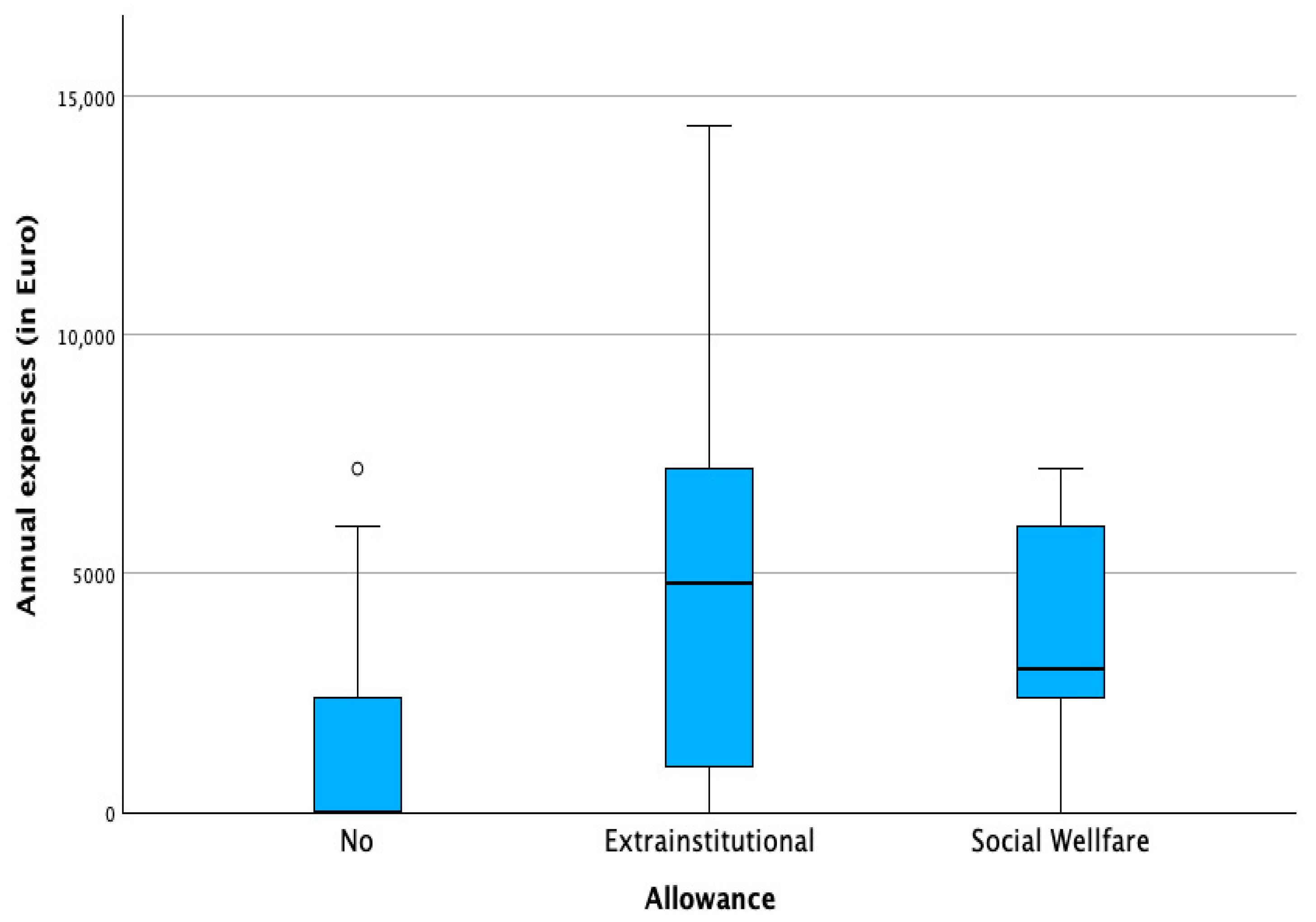
3.2. Financial Burden and Socioeconomic Profile
3.3. Health-Related Quality of Life Outcomes
3.4. Associations Between Socioeconomic and Clinical Variables and HRQoL Domains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryder, S.; Leadley, R.M.; Armstrong, N.; Westwood, M.; De Kock, S.; Butt, T.; Jai, M.; Kleijnen, J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: An evidence review. Orphanet J. Rare Dis. 2017, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; Vita, G.L. Clinical management of Duchenne muscular dystrophy: The state of the art. Neurol. Sci. 2018, 39, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Messina, S.; Trifiro, G. Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 1–20. [Google Scholar] [CrossRef]
- Thongsing, A.; Likasitwattanakul, S.; Sanmaneechai, O. Reliability and validity of the Thai version of the Pediatric Quality of Life inventoryTM 3.0 Duchenne Muscular Dystrophy module in Thai children with Duchenne Muscular Dystrophy. Health Qual. Life Outcomes 2019, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.; Clemens, P.; Hadjigiannakis, S.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.; Cripe, L.; Hadjigiannakis, S.; Olson, A.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Colvin, M.; Cripe, L.; Herron, A.; Kennedy, A.; Kinnet, K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: Primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018, 17, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Clemens, P.R.; Hoffman, E.P. Exon-Skipping in Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8, 343–358. [Google Scholar] [CrossRef]
- Markati, T.; Oskoui, M.; Farrar, M.A.; Duong, T.; Goemans, N.; Servais, L. Emerging therapies for Duchenne muscular dystrophy. Lancet Neurol. 2022, 21, 814–829. [Google Scholar] [CrossRef]
- Iftikhar, M.; Frey, J.; Shohan, M.J.; Malek, S.; Mousa, S.A. Current and emerging therapies for Duchenne muscular dystrophy and spinal muscular atrophy. Pharmacol. Ther. 2021, 220, 107719. [Google Scholar] [CrossRef]
- Sun, C.; Shen, L.; Zhang, Z.; Xie, X. Therapeutic strategies for duchenne muscular dystrophy: An update. Genes 2020, 11, 837. [Google Scholar] [CrossRef]
- Butterfield, R.J.; Kirkov, S.; Conway, K.M.; Johnson, N.; Matthews, D.; Phan, H.; Cai, B.; Paramsothy, P.; Thomas, S.; Feldkamp, M. Evaluation of effects of continued corticosteroid treatment on cardiac and pulmonary function in non-ambulatory males with Duchenne muscular dystrophy from MD STARnet. Muscle Nerve 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Shieh, P.B.; McIntosh, J.; Jin, F.; Souza, M.; Elfring, G.; Narayanan, S.; Thrifillis, P.; Peltz, S.; McDonald, C.; Darras, B.; et al. Deflazacort versus prednisone/prednisolone for maintaining motor function and delaying loss of ambulation: A Post Hoc analysis from the ACT DMD trial. Muscle Nerve 2018, 58, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Landfeldt, E.; Edström, J.; Buccella, F.; Kirschner, J.; Lochmüller, H. Duchenne muscular dystrophy and caregiver burden: A systematic review. Dev. Med. Child Neurol. 2018, 60, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, L.; Kroksmark, A.K.; Tulinius, M.; Sofou, K. One in five patients with Duchenne muscular dystrophy dies from other causes than cardiac or respiratory failure. Eur. J. Epidemiol. 2022, 37, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Broomfield, J.; Hill, M.; Guglieri, M.; Crowther, M.; Abrams, K. Life expectancy in Duchenne muscular dystrophy. Neurology 2021, 97, 304–314. [Google Scholar] [CrossRef]
- Eu, W.H.; Busse, R.; Klazinga, N.; Panteli, D.; Quentin, W. The editors Improving healthcare quality in Europe Characteristics, effectiveness and implementation of different strategies. Health Policy Ser. 2019, 53, 4–30. [Google Scholar]
- European Commission. The European Health Union: Acting Together for People’s Health COM (2024) 206 Final. Available online: https://health.ec.europa.eu/publications/communication-european-health-union_en#details (accessed on 28 June 2025).
- Cavazza, M.; Kodra, Y.; Armeni, P.; De Santis, M.; López-Bastida, J.; Linertová, R.; Oliva-Moreno, J.; Serano-Aguilar, P.; Posata-de la Paz, M.; Taruscio, D.; et al. Social/economic costs and health-related quality of life in patients with Duchenne muscular dystrophy in Europe. Eur. J. Health Econ. 2016, 17, 19–29. [Google Scholar] [CrossRef]
- Teoh, L.J.; Geelhoed, E.A.; Bayley, K.; Leonard, H.; Laing, N.G. Health care utilization and costs for children and adults with duchenne muscular dystrophy. Muscle Nerve 2016, 53, 877–884. [Google Scholar] [CrossRef]
- Thayer, S.; Bell, C.; McDonald, C. The Direct Cost of Managing a Rare Disease: Assessing Medical and Pharmacy Costs Associated with Duchenne Muscular Dystrophy in the United States. J. Manag. Care Spec. Pharm. 2017, 23, 633–641. [Google Scholar] [CrossRef]
- Schreiber-Katz, O.; Klug, C.; Thiele, S.; Schorling, E.; Zowe, J.; Reilich, P.; Nageis, K.; Walter, M. Comparative cost of illness analysis and assessment of health care burden of Duchenne and Becker muscular dystrophies in Germany. Orphanet J. Rare Dis. 2014, 9, 210. [Google Scholar] [CrossRef]
- Larkindale, J.; Yang, W.; Hogan, P.F.; Simon, C.J.; Zhang, Y.; Jain, A.; Habeeb-Louks, E.; Kennedy, A.; Cwik, V. Cost of illness for neuromuscular diseases in the United States. Muscle Nerve 2014, 49, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Shehata, Z.; Rabea, H.; El Sherif, R.; Abdelrahim, M.; Dawoud, D. Estimating Societal Cost of Illness and Patients’ Quality of Life of Duchenne Muscular Dystrophy in Egypt. Value Health Reg. Issues 2023, 33, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Flores, D.; Ribate, M.; Montolio, M.; Ramos, F.; Gómez, M.; García, C. Quantifying the economic impact of caregiving for Duchenne muscular dystrophy (DMD) in Spain. Eur. J. Health Econ. 2020, 7, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Labisa, P.; Andreozzi, V.; Mota, M.; Monteiro, S.; Alves, R.; Almeida, J.; Vandewalle, B.; Felix, J.; Buesch, K.; Canhão, H.; et al. Cost of Illness in Patients with Duchenne Muscular Dystrophy in Portugal: The COIDUCH Study. PharmacoEconomics 2022, 6, 211–218. [Google Scholar] [CrossRef]
- Orso, M.; Migliore, A.; Polistena, B.; Russo, E.; Gatto, F.; Monterubbianesi, M.; d’Angela, D.; Spandonaro, F.; Pane, M. Duchenne muscular dystrophy in Italy: A systematic review of epidemiology, quality of life, treatment adherence, and economic impact. PLoS ONE 2023, 18, e0287774. [Google Scholar] [CrossRef]
- Rudolfsen, J.; Vissingb, J.; Werlauffc, U.; Olesend, C.; Illume, N.; Olsena, J.; Bo Poulsenf, P.; Strandg, M.; Bornh, A. Burden of Disease of Duchenne Muscular Dystrophy in Denmark—A National Register-Based Study of Individuals with Duchenne Muscular Dystrophy and their Closest Relatives. J. Neuromusc Dis. 2024, 11, 443–457. [Google Scholar] [CrossRef]
- Mori-Yoshimura, M.; Ishigaki, K.; Shimizu-Motohashi, Y.; Ishihara, N.; Unuma, A.; Yoshida, S.; Nakamura, H. Social difficulties and care burden of adult Duchenne muscular dystrophy in Japan: A questionnaire survey based on the Japanese Registry of Muscular Dystrophy (Remudy). Orphanet J. Rare Dis. 2024, 19, 182–192. [Google Scholar] [CrossRef]
- Schwartz, C.; Stark, R.; Audhya, I.; Gooch, K. Characterizing the quality-of-life impact of Duchenne muscular dystrophy on caregivers: A case-control investigation. J. Patient Rep. Outcomes 2021, 5, 124–140. [Google Scholar] [CrossRef]
- Sirari, T.; Renu Suthar, R.; Kansra, P.; Singh, A.; Prinja, S.; Malviya, M.; Chauhan, A.; Viswanathan, V.; Gupta, V.; Sankhayan, N. Socioeconomic determinants of the quality of life in boys suffering from Duchenne muscular dystrophy & their caregivers. Indian J. Med. Res. 2025, 161, 215–225. [Google Scholar] [CrossRef]
- Uttley, L.; Carlton, J.; Buckley Woods, H.; Brazier, J. A review of quality of life themes in Duchenne muscular dystrophy for patients and carers. Health Qual. Life Outcomes 2018, 16, 237–252. [Google Scholar] [CrossRef]
- Gkoltsiou, K.; Dimitrakaki, C.; Tzavara, C.; Papaevangelou, V.; Varni, J.W.; Tountas, Y. Measuring health-related quality of life in Greek children: Psychometric properties of the Greek version of the Pediatric Quality of Life Inventory TM 4.0 Generic Core Scales. Qual. Life Res. 2008, 17, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Katsomiti, E.; Kastanioti, C.; Chroni, E.; Mavridoglou, G. The reliability and validity of the Greek version of the Pediatric Quality of Life InventoryTM 3.0 Duchenne Muscular Dystrophy module in children with Duchenne muscular dystrophy. Paliat. Med. Pract. 2025, 19, 170–181. [Google Scholar] [CrossRef]
- Scaling and Scoring for the Acute and Standard Versions of the Pediatric Quality of Life Inventory™ PedsQL™. Available online: https://www.pedsql.org/PedsQL-Scoring.pdf (accessed on 30 March 2020).
- Angelis, A.; Tordrup, D.; Kanavos, P. Socio-economic burden of rare diseases: A systematic reviewof cost of illness evidence. Health Policy 2015, 119, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Valentin Brodszky, V.; Beretzky, Z.; Baji, P.; Rencz, F.; Péntek, M.; Rotar, A.; Tachkov, K.; Mayer, S.; Judit Simon, J.; Niewada, M.; et al. Cost-of-illness studies in nine Central and Eastern European countries. Eur. J. Health Econ. 2019, 20, 155–172. [Google Scholar] [CrossRef]
- García-Pérez, L.; Linertová, R.; Valcárcel-Nazco, C.; Posada, M.; Gorostiza, I.; Serrano-Aguilar, P. Cost-of-illness studies in rare diseases: A scoping review. Orphanet J. Rare Dis. 2021, 16, 178–189. [Google Scholar] [CrossRef]
- Jo, C. Cost-of-illness studies: Concepts, scopes, and methods. Clin. Mol. Hepatol. 2014, 20, 327–337. [Google Scholar] [CrossRef]
- Conway, K.; Grosse, S.; Ouyang, L.; Street, N.; Romitti, P. Direct costs of adhering to selected Duchenne muscular dystrophy Care Considerations: Estimates from a midwestern state. Muscle Nerve 2022, 65, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Varni, J.W.; Seid, M.; Burwinkle, T.; Skarr, D. The PedsQLTM 4.0 as a pediatric population health measure: Feasibility, Reliability and Validity. Ambul. Pediatr. 2003, 3, 329–341. [Google Scholar] [CrossRef]
- Gocheva, V.; Schmidt, S.; Orsini, A.L.; Hafner, P.; Schaedelin, S.; Rueedi, N.; Weber, P.; Fischer, D. Association Between Health-Related Quality of Life and Motor Function in Ambulant and Nonambulant Duchenne Muscular Dystrophy Patients. J. Child. Neurol. 2019, 34, 873–885. [Google Scholar] [CrossRef]
- Bray, P.; Bundy, A.C.; Ryan, M.M.; North, K.N.; Everett, A. Health-related quality of life in boys with duchenne muscular dystrophy: Agreement between parents and their sons. J. Child. Neurol. 2010, 25, 1188–1194. [Google Scholar] [CrossRef]
- Liang, R.; Chan, S.H.S.; Ho, F.K.W.; Tang, O.C.; Cherk, S.W.W.; Ip, P.; Ying Lau, E.Y. Health-related quality of life in Chinese boys with Duchenne muscular dystrophy and their families. J. Child. Health Care 2019, 23, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Speechley, K.N.; Zou, G.; Campbell, C. Factors Associated with Health-Related Quality of Life in Children with Duchenne Muscular Dystrophy. J. Child. Neurol. 2016, 31, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Uzark, K.; King, E.; Cripe, L.; Spicer, R.; Sage, J.; Kinnett, K.; Wong, B.; Pratt, J.; Varni, J. Health-related quality of life in children and adolescents with Duchenne muscular dystrophy. Pediatrics 2012, 130, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.E.; Hynan, L.S.; Limbers, C.A.; Andersen, C.M.; Greene, M.C.; Varni, J.W.; Iannaccone, S. The PedsQLTM in Pediatric Patients with Duchenne Muscular Dystrophy: Feasibility, Reliability, and Validity of the Pediatric Quality of Life Inventory Neuromuscular Module and Generic Core Scales. J. Clin. Neuromuscul. Dis. 2010, 11, 97–109. [Google Scholar] [CrossRef]

| Variable | Mean | SD | Range |
|---|---|---|---|
| Age at onset (years) | 3.58 | 2.83 | 0–10 |
| Age at time of the evaluation (years) | 12.52 | 2.97 | 8–19 |
| Variable | N | % | |
| Age distribution(years) | |||
| 5–7 | 9 | 18.0% | |
| 8–12 | 24 | 48.0% | |
| 13–18 | 17 | 34.0% | |
| Non-ambulatory patients | 15 | 30.0% | |
| Current steroid use | 23 | 46.0% | |
| Physiotherapy | 27 | 54.0% | |
| Family history | 34 | 68.0% | |
| Place of residence | |||
| Attica | 11 | 22% | |
| Thessaloniki | 16 | 32% | |
| Other | 23 | 46% |
| Extra Institutional Allowance | Social Wellfare Allowance | No Allowance | ||
|---|---|---|---|---|
| 22 | 9 | 12 | ||
| Monthly out-of-pocket expenditures | yes | 17 | 9 | 5 |
| no | 5 | 0 | 7 | |
| Monthly out-of-pocket-money not covered by amount of the allowance | 3 | 4 | 5 | |
| Use of wheelchair | no | 6 | 8 | 12 |
| partly | 4 | 0 | 0 | |
| always | 12 | 1 | 0 | |
| Physiotherapy covered by public or private insurance | yes | 15 | 5 | 6 |
| no | 7 | 4 | 6 | |
| Annual income | Up to €15.000 | 7 | 3 | 3 |
| €15.001–€25.000 | 9 | 4 | 6 | |
| More than €25.001 | 6 | 2 | 3 | |
| Place of residense | Attica | 8 | 0 | 0 |
| Thessaloniki | 6 | 3 | 6 | |
| Other | 8 | 6 | 6 | |
| Mother’s labour | full-time | 10 | 5 | 6 |
| part-time | 2 | 2 | 1 | |
| no | 8 | 2 | 5 | |
| pension | 2 | 0 | 0 |
| Annual Unexpected Costs | Annual Unexpected Costs as Percentage of Annual Income | Annual Regular Expenses (Monthly out-of-Pocket Costs × 12) |
|---|---|---|
| Age | Age | |
| Wheelchair | Wheelchair | |
| Allowance | Allowance | Allowance |
| Region | Region |
| 5–7 (Years) | 8–12 (Years) | 13–18 (Years) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Child self–report | (Ν = 14) | (Ν = 11) | ||||
| Total Score GC | 59.86 | 15.10 | 61.65 | 18.47 | ||
| Physical Health Summary Score | 46.21 | 25.04 | 39.77 | 29.42 | ||
| Emotional Score | 67.14 | 15.65 | 72.39 | 17.68 | ||
| Social Score | 66.79 | 14.22 | 71.82 | 17.65 | ||
| School Score | 67.50 | 19.19 | 76.70 | 17.59 | ||
| Psychosocial Score | 67.14 | 12.06 | 73.64 | 14.66 | ||
| Parent proxy report | (Ν = 7) | (Ν = 20) | (Ν = 15) | |||
| Total Score GC | 65.83 | 20.94 | 60.82 | 16.90 | 49.47 | 20.42 |
| Physical Health Summary Score | 30.77 | 48.44 | 26.41 | 25.22 | 29.15 | |
| Emotional Score | 75.00 | 21.41 | 69.25 | 18.23 | 64.64 | 27.42 |
| Social Score | 64.11 | 20.70 | 62.00 | 23.92 | 56.61 | 17.64 |
| School Score | 71.43 | 19.30 | 71.00 | 17.74 | 75.23 | 19.12 |
| Psychosocial Score | 70.22 | 19.01 | 67.42 | 15.75 | 64.83 | 19.53 |
| 5–7 (Years) | 8–12 (Years) | 13–18 (Years) | All | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Child self–report | (Ν = 18) | (Ν = 13) | (Ν = 31) | |||||
| Total (18) | 70.85 | 11.55 | 71.02 | 22.92 | 70.92 | 16.91 | ||
| Daily Activities (5) | 74.44 | 16.71 | 67.69 | 29.41 | 71.61 | 22.71 | ||
| Treatment Barriers (4) | 75.46 | 21.63 | 71.35 | 22.69 | 73.82 | 21.77 | ||
| Worry (6) | 66.69 | 17.56 | 77.63 | 15.17 | 71.28 | 17.23 | ||
| Communication (3) | 66.67 | 21.77 | 63.46 | 38.12 | 65.32 | 29.19 | ||
| Parent proxy report | (Ν = 8) | (Ν = 24) | (Ν = 16) | (Ν = 48) | ||||
| Total (18) | 76.18 | 20.69 | 66.21 | 18.73 | 56.74 | 25.62 | 64.71 | 22.14 |
| Daily Activities (5) | 56.88 | 33.16 | 60.83 | 25.62 | 50.00 | 38.21 | 56.56 | 31.22 |
| Treatment Barriers (4) | 82.81 | 21.84 | 71.20 | 20.62 | 63.28 | 32.02 | 70.48 | 25.59 |
| Worry (6) | 84.38 | 19.13 | 64.90 | 20.79 | 56.04 | 28.49 | 65.19 | 24.82 |
| Communication (3) | 84.38 | 16.33 | 69.10 | 30.74 | 59.90 | 36.80 | 68.58 | 31.66 |
| x/y | Allowance * | Annual Unexpected Expenses ** | Provision by Insurance ** | Monthly Expenses ** | Use of Wheelchair ** | Age Group * | Corticoids ** |
|---|---|---|---|---|---|---|---|
| Daily activities | Yes | Yes | Yes | ||||
| Treatment | Yes | Yes | |||||
| Worry | Yes | Yes | Yes | Yes | Yes | ||
| Communication | Yes | ||||||
| Total Score DMD | Yes | ||||||
| Physical Health Summary Score | Yes | Yes | Yes | Yes | |||
| Emotional Score | Yes | Yes | Yes | ||||
| Social Score | Yes | ||||||
| School Score | Yes | ||||||
| Psychosocial Score | Yes | Yes | |||||
| Total Score GC | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsomiti, E.; Kastanioti, C.; Chroni, E.; Mavridoglou, G.; Pavlou, E. Quality of Life and Financial Burden in Duchenne Muscular Dystrophy in Greece: Insights into Health System Performance in the Post-Pandemic Context. Healthcare 2025, 13, 2835. https://doi.org/10.3390/healthcare13222835
Katsomiti E, Kastanioti C, Chroni E, Mavridoglou G, Pavlou E. Quality of Life and Financial Burden in Duchenne Muscular Dystrophy in Greece: Insights into Health System Performance in the Post-Pandemic Context. Healthcare. 2025; 13(22):2835. https://doi.org/10.3390/healthcare13222835
Chicago/Turabian StyleKatsomiti, Eleni, Catherine Kastanioti, Elisabeth Chroni, George Mavridoglou, and Evangelos Pavlou. 2025. "Quality of Life and Financial Burden in Duchenne Muscular Dystrophy in Greece: Insights into Health System Performance in the Post-Pandemic Context" Healthcare 13, no. 22: 2835. https://doi.org/10.3390/healthcare13222835
APA StyleKatsomiti, E., Kastanioti, C., Chroni, E., Mavridoglou, G., & Pavlou, E. (2025). Quality of Life and Financial Burden in Duchenne Muscular Dystrophy in Greece: Insights into Health System Performance in the Post-Pandemic Context. Healthcare, 13(22), 2835. https://doi.org/10.3390/healthcare13222835






