Cost-Effectiveness in Critical Care: A Systematic Review of Empirical Evaluations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Registration
2.2. Framework for Review Question Formulation
2.3. Eligibility Criteria
2.4. Search Strategy
2.5. Data Extraction and Processing
2.6. Assessment for Reporting Quality
2.7. Currency Conversion and Cost Standardization
2.8. Data Synthesis and Meta-Analysis
3. Results
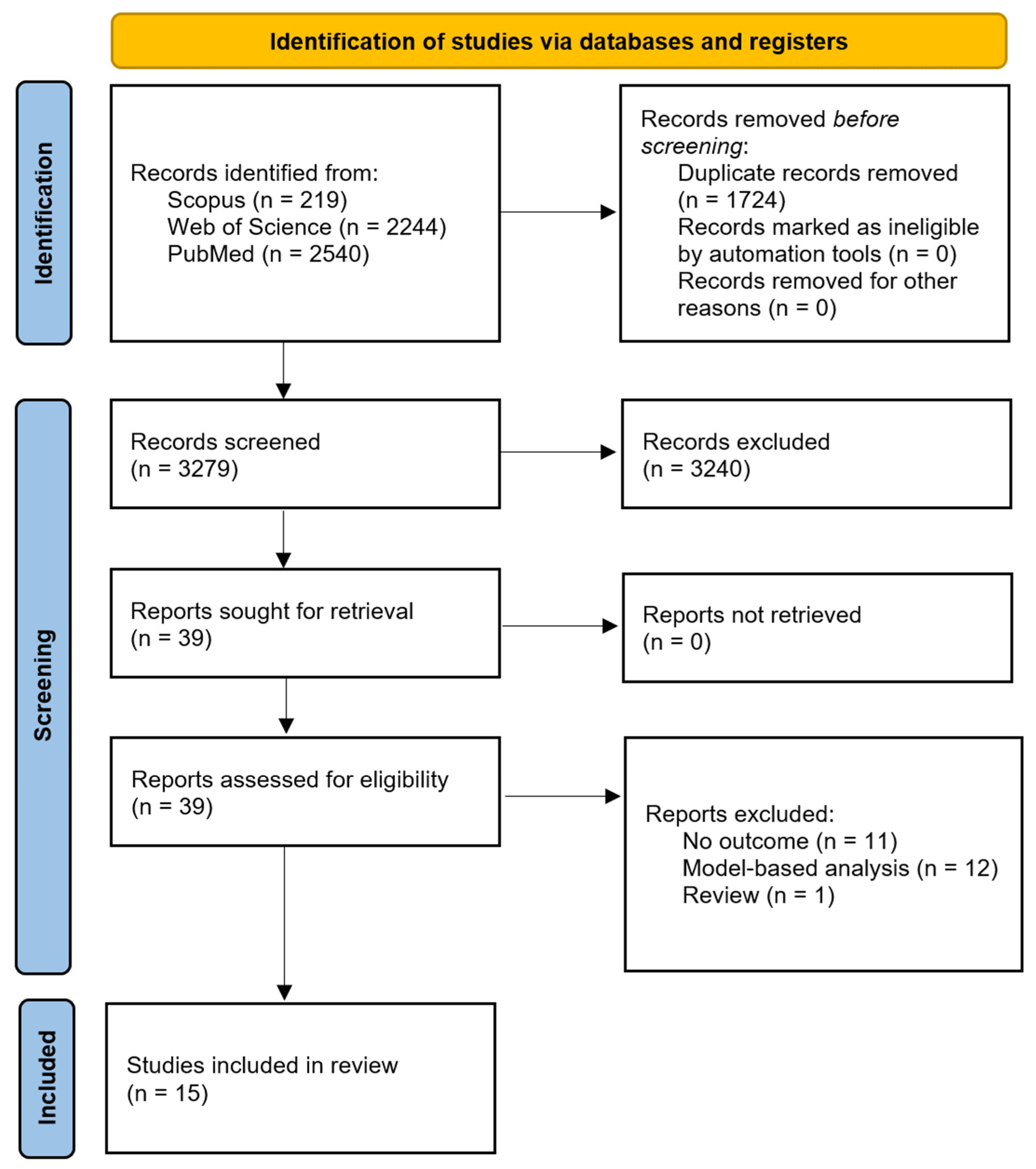
3.1. PRISMA Flow and Study Selection Results
3.2. Characteristics of Included Studies
| Study | Country | Population | Setting | N | Intervention | Comparator | Perspective | Time Horizon |
|---|---|---|---|---|---|---|---|---|
| Berto, 2011 [22] | Italy | Severe sepsis/septic shock patients who had emergency surgery for intra-abdominal infection. | 10 tertiary care ICUs | 64 | Polymyxin B hemoperfusion | Conventional therapy | Hospital | Lifetime |
| Tarricone, 2010 [33] | Italy | ICU patients with central lines | 4 ICUs in an Italian teaching hospital | 1446 | Closed, fully collapsible plastic IV containers | Open glass infusion containers | Hospital | Hospital discharge |
| Heyland, 1998 [30] | Canada | General | 12-bed, adult, medical–surgical ICU | 690 | Continue ICU care >14 days | Withdrawal of support after 14 days | Hospital | 12 months |
| Manns, 2002 [24] | Canada | Severe sepsis | 3 tertiary care hospitals with medical–surgical ICUs. | 787 | Drotrecogin alfa (activated) | Standard care | Healthcare | Lifetime |
| Busse, 2020 [36] | 9 countries (North America, Australasia, and Europe) | Severe distributive shock | 75 centers across 9 countries | 321 | Angiotensin II | Placebo | Healthcare | Lifetime |
| Ersson, 2018 [31] | Sweden | General ICU patients | 12-bed mixed ICU in a 600-bed tertiary teaching hospital | 5950 | Quality improvement (QI) process | Historical cohort | Healthcare | 7 years |
| Lau, 2022 [29] | 3 countries (Canada/USA/Saudi Arabia) | Mechanically ventilated patients | 44 ICUs | 2650 | Probiotics (Lactobacillus rhamnosus GG) | Placebo | Healthcare | Hospital discharge |
| Mayer, 2000 [28] | USA | Patients who are mechanically ventilated | N/A | 510 | Mechanical ventilation | Death | Healthcare | 6 years |
| Dhainaut, 2007 [25] | France | Severe sepsis with multiple organ failure | 85 ICUs | 1096 | Recombinant human activated protein C | Standard care | Hospital | NR |
| El Genedy, 2020 [34] | Germany | Patients at high risk for pressure ulcers | Seven ICUs | 422 | Multi-layered silicone foam dressing | Standard care | Hospital | 12 days |
| Thu, 2015 [35] | Vietnam | ICU patients | 2 main ICUs and 15 CCUs | 984 | Hand hygiene program | Before hand hygiene program | Hospital | NR |
| Riou Franca, 2006 [23] | France | Severe sepsis with multiple organ failure | ICU | 9848 | Drotrecogin alfa (activated) | Placebo | Healthcare | Lifetime |
| Thompson, 2022 [27] | New Zealand | Patients with septic shock | 8 medical-surgical ICUs | 419 | Hydrocortisone infusion | Placebo | Healthcare | 2 years |
| Stevens, 2005 [32] | Great Britain | Patients in the ICU | 65 ICU | 1014 | Treatment without using a PAC | Routine PAC use | Healthcare | Lifetime |
| Assuncao, 2014 [26] | Brazil | Severe sepsis and septic shock. | 1 ICU | 414 | Managed protocol | Usual care | Hospital | NR |
3.3. Primary Outcomes
3.4. Secondary Outcome
3.5. Results of Syntheses
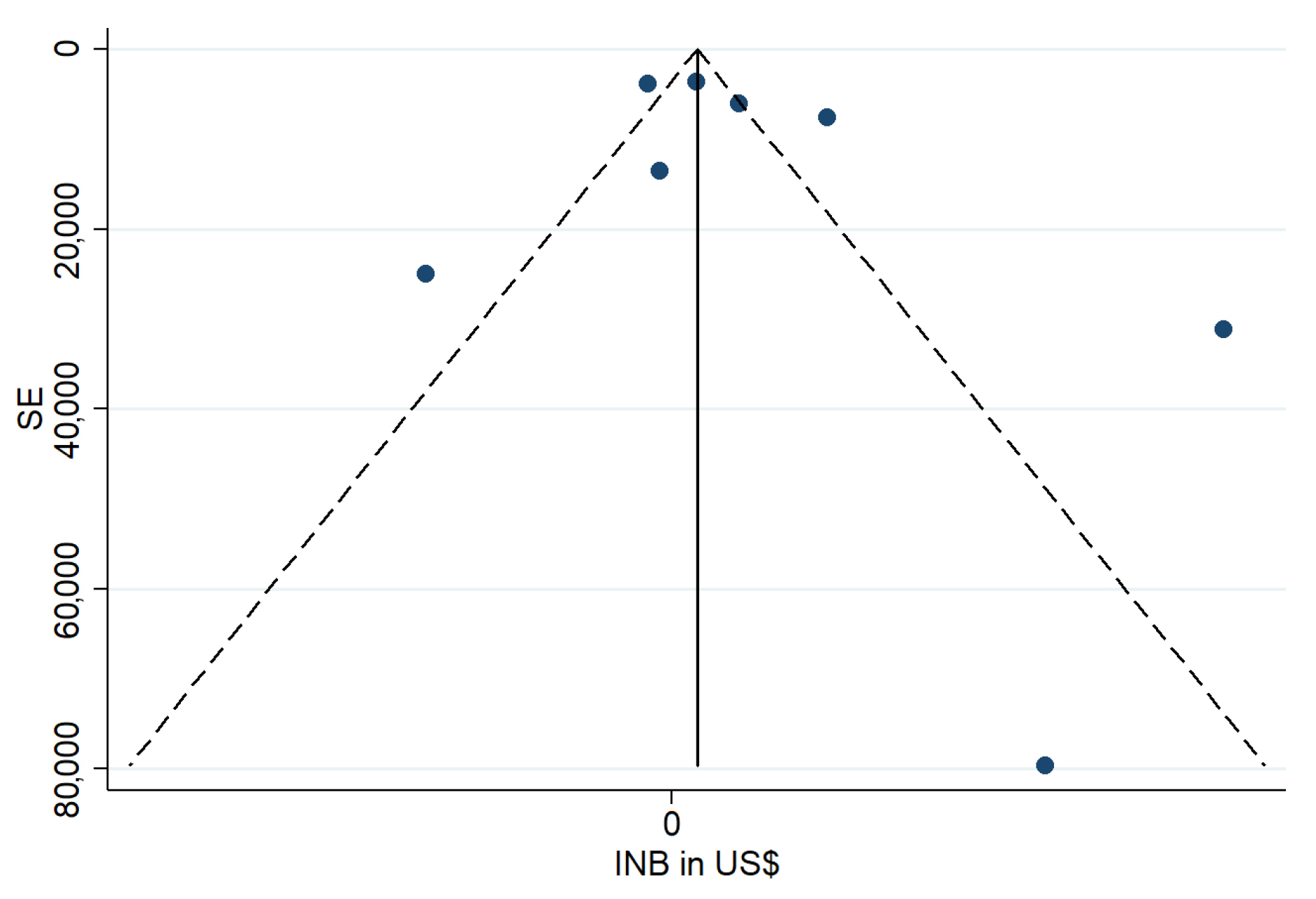
3.6. Study Quality Assessment
4. Discussion
4.1. Comparison with Existing Literature
4.2. Preventive and Quality-Improvement Interventions: High Value at Low Cost
4.3. Interpretation of Cost-Effectiveness Trends
4.4. Pharmacologic and Life-Support Interventions: Substantial Variation in Economic Value
4.5. Meta-Analytic Evidence
4.6. Methodological Quality and Reporting Gaps
4.7. Implications for Practice and Policy
4.8. Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ICU | Intensive Care Unit |
| CEA | Cost-Effectiveness Analysis |
| ICER | Incremental Cost-Effectiveness Ratio |
| LYG | Life-Years Gained |
| QALY | Quality-Adjusted Life Year |
| INB | Incremental Net Benefit |
| CHEERS | Consolidated Health Economic Evaluation Reporting Standards |
| CI | Confidence Interval |
| CEAC | Cost-Effectiveness Acceptability Curve |
| ISPOR | International Society for Pharmacoeconomics and Outcomes Research |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| CRD | Centre for Reviews and Dissemination |
| USD | United States Dollar |
| SD | Standard Deviation |
| SE | Standard Error |
| WTP | Willingness to Pay |
Appendix A
| Dhainaut, 2007 [25] | Berto, 2011 [22] | Busse, 2020 [36] | Ersson, 2018 [31] | Riou Franca, 2006 [23] | El Genedy, 2020 [34] | Heyland, 1998 [30] | Manns, 2002 [24] | Stevens, 2005 [32] | Tarricone, 2010 [33] | Thu, 2015 [35] | Lau, 2022 [29] | Mayer, 2000 [28] | Thompson, 2022 [27] | Assuncao, 2014 [26] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 |
| Abstract | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 0.5 | 1 | 0.5 |
| Background and objectives | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Health economic analysis plan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Study population | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 |
| Setting and Location | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 |
| Comparators | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Perspective | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| Time Horizon | 0.5 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 0.5 | 1 | 0 | 1 | 0.5 | 1 | 0 |
| Discount Rate | 0.5 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | NA | NA | 1 | 1 | 0 | 0 |
| Selection of Outcomes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Measurement of Outcomes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Valuation of outcomes | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0.5 |
| Measurement and valuation of resources and costs | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 |
| Currency, price date, and conversion | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 |
| Rationale and description of the model | NA | 1 | 0.5 | N | 0 | 1 | 1 | 0.5 | N | 1 | N | N | 0.5 | NA | NA |
| Analytics and assumptions | 1 | 1 | 1 | N | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Characterizing Heterogeneity | 1 | N | 0 | N | 1 | 0.5 | 0 | 1 | 0 | 0 | 0 | 1 | 0.5 | 1 | 0 |
| Characterizing distributional effects | 0 | N | 0 | N | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Characterizing uncertainty | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| Approach to engagement | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Study parameters | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 1 | 0.5 | 0.5 |
| Summary of main results | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 |
| Effect of uncertainty | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 0 | 0.5 | 1 | 0.5 | 1 | 0 |
| Effect of engagement | NA | NA | NA | NA | 0 | NA | NA | NA | NA | 0 | NA | NA | NA | NA | NA |
| Study findings | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Source of funding | 1 | 1 | 1 | 1 | 0.5 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Conflicts of interest | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| % Score | 85% | 92% | 83% | 85% | 73% | 79% | 71% | 81% | 81% | 69% | 70% | 94% | 72% | 83% | 54% |
References
- Halpern, N.A.; Pastores, S.M. Critical Care Medicine in the United States 2000-2005: An Analysis of Bed Numbers, Occupancy Rates, Payer Mix, and Costs. Crit. Care Med. 2010, 38, 65–71. [Google Scholar] [CrossRef]
- Wunsch, H.; Angus, D.C.; Harrison, D.A.; Collange, O.; Fowler, R.; Hoste, E.A.J.; de Keizer, N.F.; Kersten, A.; Linde-Zwirble, W.T.; Sandiumenge, A.; et al. Variation in Critical Care Services across North America and Western Europe. Crit. Care Med. 2008, 36, 2787-e8. [Google Scholar] [CrossRef]
- Kahn, J.M.; Rubenfeld, G.D.; Rohrbach, J.; Fuchs, B.D. Cost Savings Attributable to Reductions in Intensive Care Unit Length of Stay for Mechanically Ventilated Patients. Med. Care 2008, 46, 1226–1233. [Google Scholar] [CrossRef]
- Chacko, B.; Ramakrishnan, N.; Peter, J.V. Approach to Intensive Care Costing and Provision of Cost-Effective Care. Indian. J. Crit. Care Med. 2023, 27, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Gooch, R.A.; Kahn, J.M. ICU Bed Supply, Utilization, and Health Care Spending: An Example of Demand Elasticity. JAMA 2014, 311, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Leligdowicz, A.; Adhikari, N.K.J. Intensive Care Unit Capacity in Low-Income Countries: A Systematic Review. PLoS ONE 2015, 10, e0116949. [Google Scholar] [CrossRef] [PubMed]
- Phua, J.; Kulkarni, A.P.; Mizota, T.; Hashemian, S.M.R.; Lee, W.-Y.; Permpikul, C.; Chittawatanarat, K.; Nitikaroon, P.; Arabi, Y.M.; Fang, W.-F.; et al. Critical Care Bed Capacity in Asian Countries and Regions before and during the COVID-19 Pandemic: An Observational Study. Lancet Reg. Health—West. Pac. 2024, 44, 100982. [Google Scholar] [CrossRef]
- Crawford, A.M.; Shiferaw, A.A.; Ntambwe, P.; Milan, A.O.; Khalid, K.; Rubio, R.; Nizeyimana, F.; Ariza, F.; Mohammed, A.D.; Baker, T.; et al. Global Critical Care: A Call to Action. Crit Care 2023, 27, 28. [Google Scholar] [CrossRef]
- Gandjour, A. How Many Intensive Care Beds Are Justifiable for Hospital Pandemic Preparedness? A Cost-Effectiveness Analysis for COVID-19 in Germany. Appl. Health Econ. Health Policy 2021, 19, 181–190. [Google Scholar] [CrossRef]
- Wilcox, M.E.; Vaughan, K.; Chong, C.A.K.Y.; Neumann, P.J.; Bell, C.M. Cost-Effectiveness Studies in the ICU: A Systematic Review. Crit. Care Med. 2019, 47, 1011. [Google Scholar] [CrossRef]
- Dasta, J.F.; McLaughlin, T.P.; Mody, S.H.; Piech, C.T. Daily Cost of an Intensive Care Unit Day: The Contribution of Mechanical Ventilation. Crit. Care Med. 2005, 33, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.-L.; Tse, W.C.; Carney, T.M.; Carrandi, A.; Fagery, M.; Higgins, A.M. Health Economic Evaluations in Intensive Care: An Updated Systematic Review. Crit. Care Explor. 2025, 7, e1288. [Google Scholar] [CrossRef]
- Cuthbertson, B.H.; Roughton, S.; Jenkinson, D.; MacLennan, G.; Vale, L. Quality of Life in the Five Years after Intensive Care: A Cohort Study. Crit. Care 2010, 14, R6. [Google Scholar] [CrossRef]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-966588-4. [Google Scholar]
- Neumann, P.J.; Ganiats, T.G.; Russell, L.B.; Sanders, G.D.; Siegel, J.E. (Eds.) Cost-Effectiveness in Health and Medicine, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2016; ISBN 978-0-19-049293-9. [Google Scholar]
- Briggs, A.; Claxton, K.; Sculpher, M. Decision Modelling for Health Economic Evaluation; Oxford University Press: Oxford, UK, 2006; ISBN 978-0-19-159297-3. [Google Scholar]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement. Value Health 2013, 16, e1–e5. [Google Scholar] [CrossRef]
- Manca, A.; Sculpher, M.J.; Goeree, R. The Analysis of Multinational Cost-Effectiveness Data for Reimbursement Decisions. Pharmacoeconomics 2010, 28, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Ades, A.E.; Lu, G.; Claxton, K. Expected Value of Sample Information Calculations in Medical Decision Modeling. Med. Decis. Mak. 2004, 24, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. BMC Med. 2022, 20, 23. [Google Scholar] [CrossRef]
- Shemilt, I.; James, T.; Marcello, M. A Web-Based Tool for Adjusting Costs to a Specific Target Currency and Price Year. Evid. Policy 2010, 6, 51–59. [Google Scholar] [CrossRef]
- Berto, P.; Ronco, C.; Cruz, D.; Melotti, R.M.; Antonelli, M. Cost-Effectiveness Analysis of Polymyxin-B Immobilized Fiber Column and Conventional Medical Therapy in the Management of Abdominal Septic Shock in Italy. Blood Purif. 2011, 32, 331–340. [Google Scholar] [CrossRef]
- Riou França, L.; Launois, R.; Le Lay, K.; Aegerter, P.; Bouhassira, M.; Meshaka, P.; Guidet, B. Cost-Effectiveness of Drotrecogin Alfa (Activated) in the Treatment of Severe Sepsis with Multiple Organ Failure. Int. J. Technol. Assess. Health Care 2006, 22, 101–108. [Google Scholar] [CrossRef][Green Version]
- Manns, B.J.; Lee, H.; Doig, C.J.; Johnson, D.; Donaldson, C. An Economic Evaluation of Activated Protein C Treatment for Severe Sepsis. N. Engl. J. Med. 2002, 347, 993–1000. [Google Scholar] [CrossRef]
- Dhainaut, J.-F.; Payet, S.; Vallet, B.; França, L.R.; Annane, D.; Bollaert, P.-E.; Tulzo, Y.L.; Runge, I.; Malledant, Y.; Guidet, B.; et al. Cost-Effectiveness of Activated Protein C in Real-Life Clinical Practice. Crit. Care 2007, 11, R99. [Google Scholar] [CrossRef] [PubMed]
- Assuncao, M.S.C.; Teich, V.; Shiramizo, S.C.P.L.; Araújo, D.V.; Carrera, R.M.; Serpa Neto, A.; Silva, E. The Cost-Effectiveness Ratio of a Managed Protocol for Severe Sepsis. J. Crit. Care 2014, 29, 692.e1–692.e6. [Google Scholar] [CrossRef]
- Thompson, K.J.; Young, P.J.; Venkatesh, B.; Cohen, J.; Finfer, S.R.; Grattan, S.; Hammond, N.E.; Jan, S.; Li, Q.; Tanna, G.L.D.; et al. Long-Term Costs and Cost-Effectiveness of Adjunctive Corticosteroids for Patients with Septic Shock in New Zealand. Aust. Crit. Care 2022, 35, 241–250. [Google Scholar] [CrossRef]
- Mayer, S.A.; Copeland, D.; Bernardini, G.L.; Boden-Albala, B.; Lennihan, L.; Kossoff, S.; Sacco, R.L. Cost and Outcome of Mechanical Ventilation for Life-Threatening Stroke. Stroke 2000, 31, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Lau, V.I.; Xie, F.; Fowler, R.A.; Rochwerg, B.; Johnstone, J.; Lauzier, F.; Marshall, J.C.; Basmaji, J.; Henderson, W.; Khwaja, K.; et al. Health Economic Evaluation alongside the Probiotics to Prevent Severe Pneumonia and Endotracheal Colonization Trial (E-PROSPECT): A Cost-Effectiveness Analysis. Can. J. Anesth 2022, 69, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Konopad, E.; Noseworthy, T.W.; Johnston, R.; Gafni, A. Is It ‘Worthwhile’ To Continue Treating Patients with a Prolonged Stay (>14 Days) in the ICU?: An Economic Evaluation. CHEST 1998, 114, 192–198. [Google Scholar] [CrossRef][Green Version]
- Ersson, A.; Beckman, A.; Jarl, J.; Borell, J. Effects of a Multifaceted Intervention QI Program to Improve ICU Performance. BMC Health Serv. Res. 2018, 18, 838. [Google Scholar] [CrossRef]
- Stevens, K.; McCabe, C.; Jones, C.; Ashcroft, J.; Harvey, S.; Rowan, K. The Incremental Cost Effectiveness of Withdrawing Pulmonary Artery Catheters from Routine Use in Critical Care. Appl. Health Econ. Health Policy 2005, 4, 257–264. [Google Scholar] [CrossRef]
- Tarricone, R.; Torbica, A.; Franzetti, F.; Rosenthal, V.D. Hospital Costs of Central Line-Associated Bloodstream Infections and Cost-Effectiveness of Closed vs. Open Infusion Containers. The Case of Intensive Care Units in Italy. Cost Eff. Resour. Alloc. 2010, 8, 8. [Google Scholar] [CrossRef]
- El Genedy, M.; Hahnel, E.; Tomova-Simitchieva, T.; Padula, W.V.; Hauß, A.; Löber, N.; Blume-Peytavi, U.; Kottner, J. Cost-Effectiveness of Multi-Layered Silicone Foam Dressings for Prevention of Sacral and Heel Pressure Ulcers in High-Risk Intensive Care Unit Patients: An Economic Analysis of a Randomised Controlled Trial. Int. Wound J. 2020, 17, 1291–1299. [Google Scholar] [CrossRef]
- Thu, L.T.A.; Thoa, V.T.H.; Trang, D.T.V.; Tien, N.P.; Van, D.T.; Anh, L.T.K.; Wertheim, H.F.L.; Son, N.T. Cost-Effectiveness of a Hand Hygiene Program on Health Care–Associated Infections in Intensive Care Patients at a Tertiary Care Hospital in Vietnam. Am. J. Infect. Control 2015, 43, e93–e99. [Google Scholar] [CrossRef]
- Busse, L.W.; Nicholson, G.; Nordyke, R.J.; Lee, C.-H.; Zeng, F.; Albertson, T.E. Angiotensin II for the Treatment of Distributive Shock in the Intensive Care Unit: A US Cost-Effectiveness Analysis. Int. J. Technol. Assess. Health Care 2020, 36, 145–151. [Google Scholar] [CrossRef]
- Ruiz-Ramos, J.; Frasquet, J.; Romá, E.; Poveda-Andres, J.L.; Salavert-Leti, M.; Castellanos, A.; Ramirez, P. Cost-Effectiveness Analysis of Implementing an Antimicrobial Stewardship Program in Critical Care Units. J. Med. Econ. 2017, 20, 652–659. [Google Scholar] [CrossRef]
- Møller, A.H.; Hansen, L.; Jensen, M.S.; Ehlers, L.H. A Cost-Effectiveness Analysis of Reducing Ventilator-Associated Pneumonia at a Danish ICU with Ventilator Bundle. J. Med. Econ. 2012, 15, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhao, Q.; Guo, S.; Bai, L.; Yang, S.; Zhao, Y.; Xu, Y.; Zhou, X. Efficacy of Preventive Interventions against Ventilator-Associated Pneumonia in Critically Ill Patients: An Umbrella Review of Meta-Analyses. J. Hosp. Infect. 2024, 145, 174–186. [Google Scholar] [CrossRef]
- Burchardi, H.; Schneider, H. Economic Aspects of Severe Sepsis. PharmacoEconomics 2004, 22, 793–813. [Google Scholar] [CrossRef] [PubMed]
- Pradelli, L.; Klek, S.; Mayer, K.; Omar Alsaleh, A.J.; Rosenthal, M.D.; Heller, A.R.; Muscaritoli, M. Omega-3 Fatty Acid-Containing Parenteral Nutrition in ICU Patients: Systematic Review with Meta-Analysis and Cost-Effectiveness Analysis. Crit. Care 2020, 24, 634. [Google Scholar] [CrossRef]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health Care–Associated Infections: A Meta-Analysis of Costs and Financial Impact on the US Health Care System. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- Umscheid, C.A.; Mitchell, M.D.; Doshi, J.A.; Agarwal, R.; Williams, K.; Brennan, P.J. Estimating the Proportion of Healthcare-Associated Infections That Are Reasonably Preventable and the Related Mortality and Costs. Infect. Control Hosp. Epidemiol. 2011, 32, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of Endemic Health-Care-Associated Infection in Developing Countries: Systematic Review and Meta-Analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- dos Santos, W.M.; Aromataris, E.; Secoli, S.R.; Matuoka, J.Y. Cost-Effectiveness of Antimicrobial Treatment for Inpatients with Carbapenem-Resistant Klebsiella Pneumoniae Infection: A Systematic Review of Economic Evidence. JBI Evid. Synth. 2019, 17, 2417. [Google Scholar] [CrossRef]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef]
- Bernard, G.R.; Vincent, J.-L.; Laterre, P.-F.; LaRosa, S.P.; Dhainaut, J.-F.; Lopez-Rodriguez, A.; Steingrub, J.S.; Garber, G.E.; Helterbrand, J.D.; Ely, E.W.; et al. Efficacy and Safety of Recombinant Human Activated Protein C for Severe Sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-Effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016, 316, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Garrison, L.P.; Neumann, P.J.; Willke, R.J.; Basu, A.; Danzon, P.M.; Doshi, J.A.; Drummond, M.F.; Lakdawalla, D.N.; Pauly, M.V.; Phelps, C.E.; et al. A Health Economics Approach to US Value Assessment Frameworks—Summary and Recommendations of the ISPOR Special Task Force Report [7]. Value Health 2018, 21, 161–165. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Primary Outcome ICER |
|---|---|---|
| Berto, 2011 [22] | Italy | Cost per LYG: 6904 |
| Tarricone, 2010 [33] | Italy | Cost per CLABSI prevented: Dominant |
| Heyland, 1998 [30] | Canada | Cost per LYG: 7176 Cost per Life Saved: 107,592 |
| Manns, 2002 [24] | Canada | Cost per LYG: 47,231 |
| Busse, 2020 [36] | 9 countries (North America, Australasia, and Europe) | Cost per LYG: 10,756 |
| Ersson, 2018 [31] | Sweden | Cost per LYG: Dominant |
| Lau, 2022 [29] | 3 countries (Canada/USA/Saudi Arabia) | Cost per Life Saved: 476,499 |
| Mayer, 2000 [28] | USA | Cost per LYG: 69,346 |
| Dhainaut, 2007 [25] | France | Cost per LYG: 36,042 |
| El Genedy, 2020 [34] | Germany | Cost per PU avoided: 3091 |
| Thu, 2015 [35] | Vietnam | Cost per HAI prevented: Dominant |
| Riou Franca, 2006 [23] | France | Cost per LYG: 19,664 |
| Thompson, 2022 [27] | New Zealand | Cost per LYG: Dominant |
| Stevens, 2005 [32] | Great Britain | Cost per Life Saved: 51,664 |
| Assuncao, 2014 [26] | Brazil | Cost per LYG: Dominant Cost per Life Saved: Dominant |
| Study | Country | ICER |
|---|---|---|
| Manns, 2002 [24] | Canada | Cost per QALY: 78,719 |
| Busse, 2020 [36] | 9 countries (North America, Australasia, and Europe) | Cost per QALY: 15,700 |
| Ersson, 2018 [31] | Sweden | Cost per QALY: Dominant |
| Mayer, 2000 [28] | USA | Cost per QALY: 321,280 |
| Dhainaut, 2007 [25] | France | Cost per QALY: 60,070 |
| RiouFranca, 2006 [23] | France | Cost per QALY 32,772 |
| Thompson, 2022 [27] | New Zealand | Cost per QALY Dominated |
| Stevens, 2005 [32] | Great Britain | Cost per QALY: 7059 |
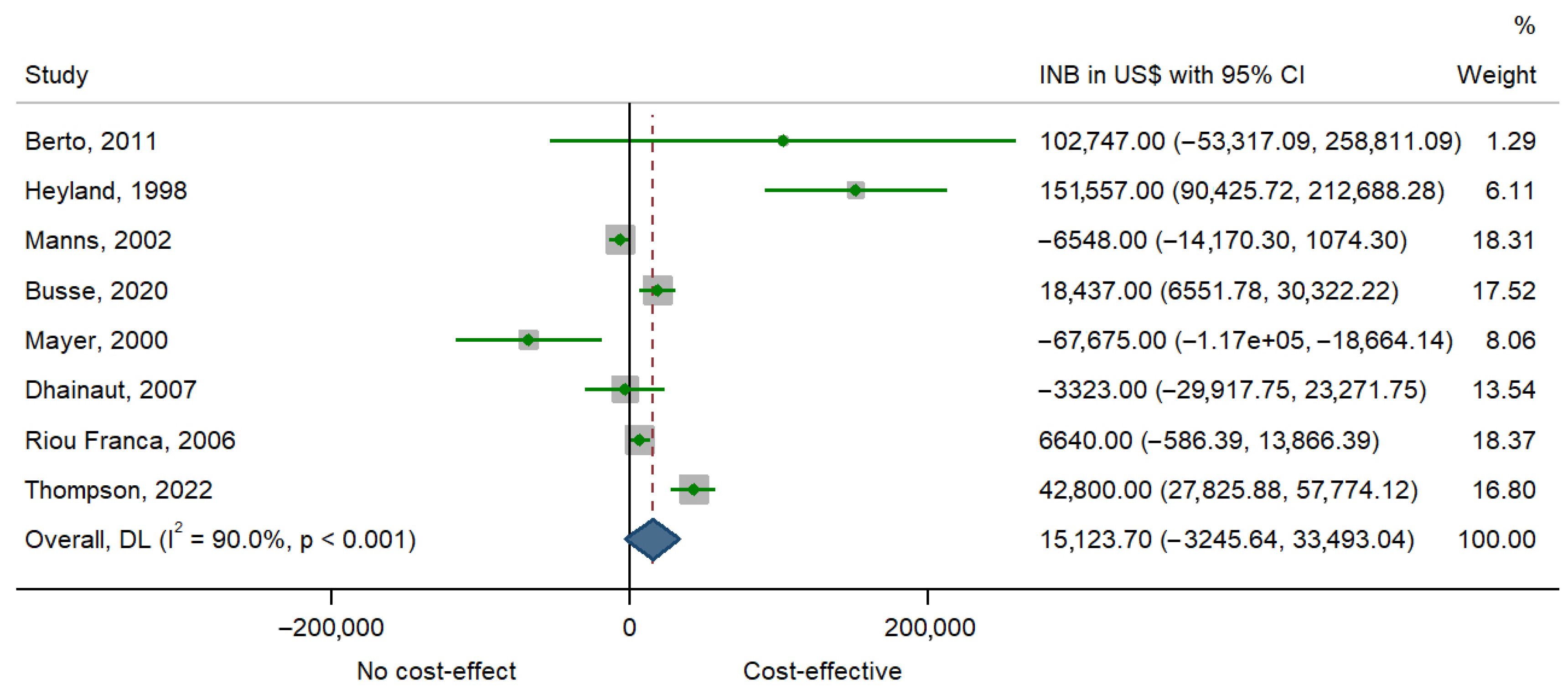
| Study | INB ($) | 95% CI |
|---|---|---|
| Berto, 2011 [22] | 102,747 | −53,320 to 258,814 |
| Heyland, 1998 [30] | 151,557 | 90,422 to 212,691 |
| Manns, 2002 [24] | −6548 | −14,172 to 1076 |
| Busse, 2020 [36] | 18,437 | 6549 to 30,324 |
| Mayer, 2000 [28] | −67,675 | −116,687 to −18,662 |
| Dhainaut, 2007 [25] | −3323 | −29,918 to 23,272 |
| RiouFranca, 2006 [23] | 6640 | −588 to 13,868 |
| Thompson, 2022 [27] | 42,800 | 27,825 to 57,774 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatsis, F.; Gouva, M.; Dragioti, E.; Veroniki, F.; Stamatis, K.; Papathanakos, G.; Koulouras, V. Cost-Effectiveness in Critical Care: A Systematic Review of Empirical Evaluations. Healthcare 2025, 13, 2783. https://doi.org/10.3390/healthcare13212783
Tatsis F, Gouva M, Dragioti E, Veroniki F, Stamatis K, Papathanakos G, Koulouras V. Cost-Effectiveness in Critical Care: A Systematic Review of Empirical Evaluations. Healthcare. 2025; 13(21):2783. https://doi.org/10.3390/healthcare13212783
Chicago/Turabian StyleTatsis, Fotios, Mary Gouva, Elena Dragioti, Foteini Veroniki, Konstantinos Stamatis, Georgios Papathanakos, and Vasilios Koulouras. 2025. "Cost-Effectiveness in Critical Care: A Systematic Review of Empirical Evaluations" Healthcare 13, no. 21: 2783. https://doi.org/10.3390/healthcare13212783
APA StyleTatsis, F., Gouva, M., Dragioti, E., Veroniki, F., Stamatis, K., Papathanakos, G., & Koulouras, V. (2025). Cost-Effectiveness in Critical Care: A Systematic Review of Empirical Evaluations. Healthcare, 13(21), 2783. https://doi.org/10.3390/healthcare13212783








