Small Intestine Tumors: Diagnostic Role of Multiparametric Ultrasound
Abstract
1. Introduction
2. Search Strategy
3. Symptoms—When Should Small Intestine Tumors Be Considered?
4. Imaging the Small Intestines
4.1. Device–Assisted Enteroscopy Techniques (DAET)
4.2. Video Capsule Endoscopy (VCE)
4.3. Endoscopic Ultrasound (EUS)
4.4. Abdominal CT, CT-/MR Enterography
4.5. Transabdominal Ultrasound (US)
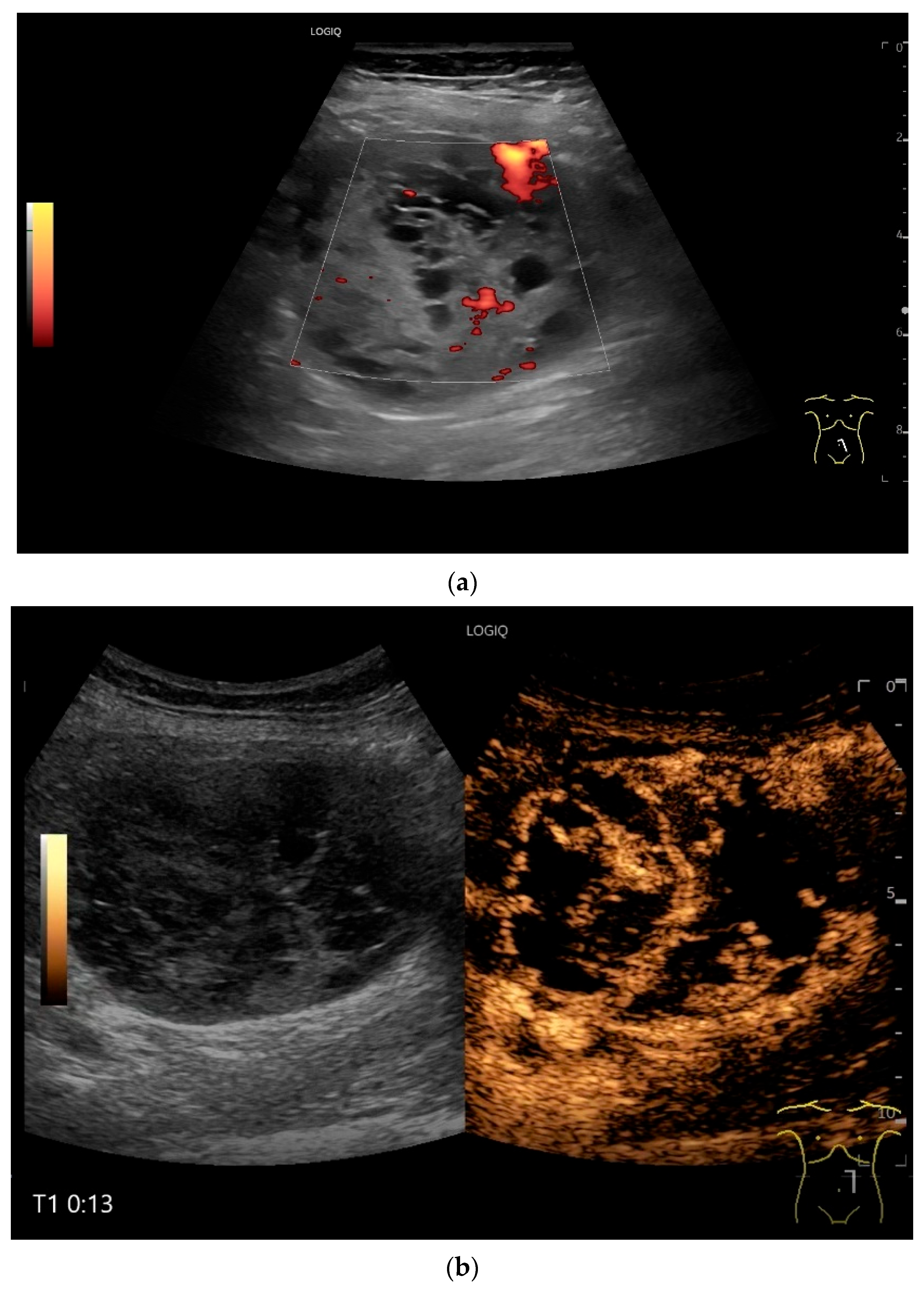
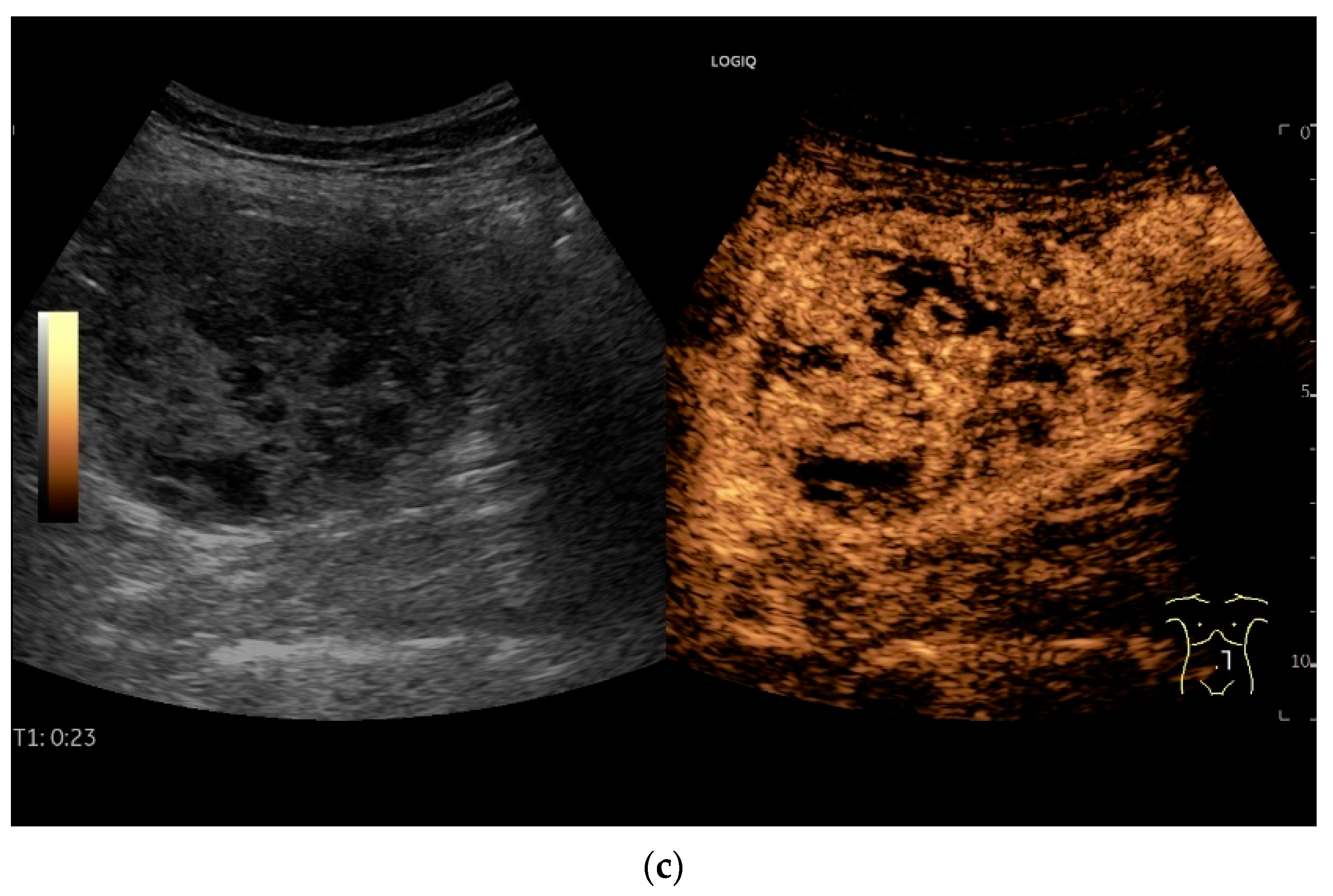
4.6. CEUS
4.7. US-Guided Biopsy
5. Small Intestine Adenomas
6. Intestinal Carcinoma
7. Neuroendocrine Neoplasms (NEN)
Ultrasound Imaging of Small Intestine NEN
8. Lymphoma, Hematolymphoid Tumors
8.1. Diffuse Large B-Cell Lymphoma (DLBCL)
8.2. Extranodal Marginal Zone Lymphoma of Mucosa-Associated Lymphoid Tissue (MALT Lymphoma)
8.3. Follicular Lymphoma
8.4. Duodenal-Type Follicular Lymphoma
8.5. Mantle Cell Lymphoma
8.6. Burkitt Lymphoma
8.7. T-Cell Lymphoma
8.7.1. Enteropathy-Associated T-Cell Lymphoma
8.7.2. Intestinal T-Cell Lymphoma NOS
8.8. Imaging of Lymphomas
9. Mesenchymal Tumors
9.1. Gastrointestinal Stromal Tumor
9.2. Inflammatory Myofibroblastic Tumor (IMFT)
9.3. Inflammatory Fibroid Tumor (Vanek’s Tumor)
9.4. Desmoid Tumors
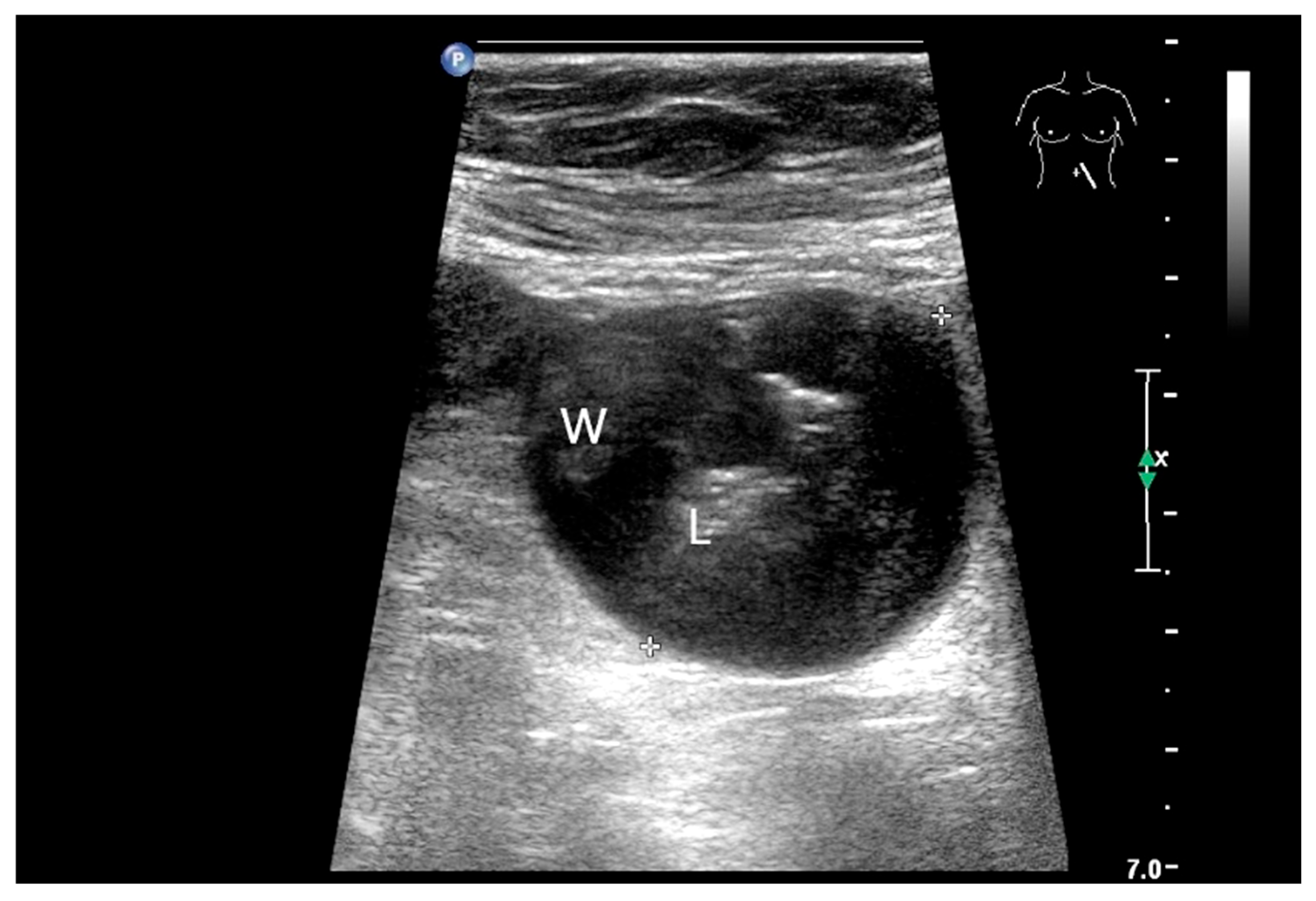
9.5. Lymphangioma
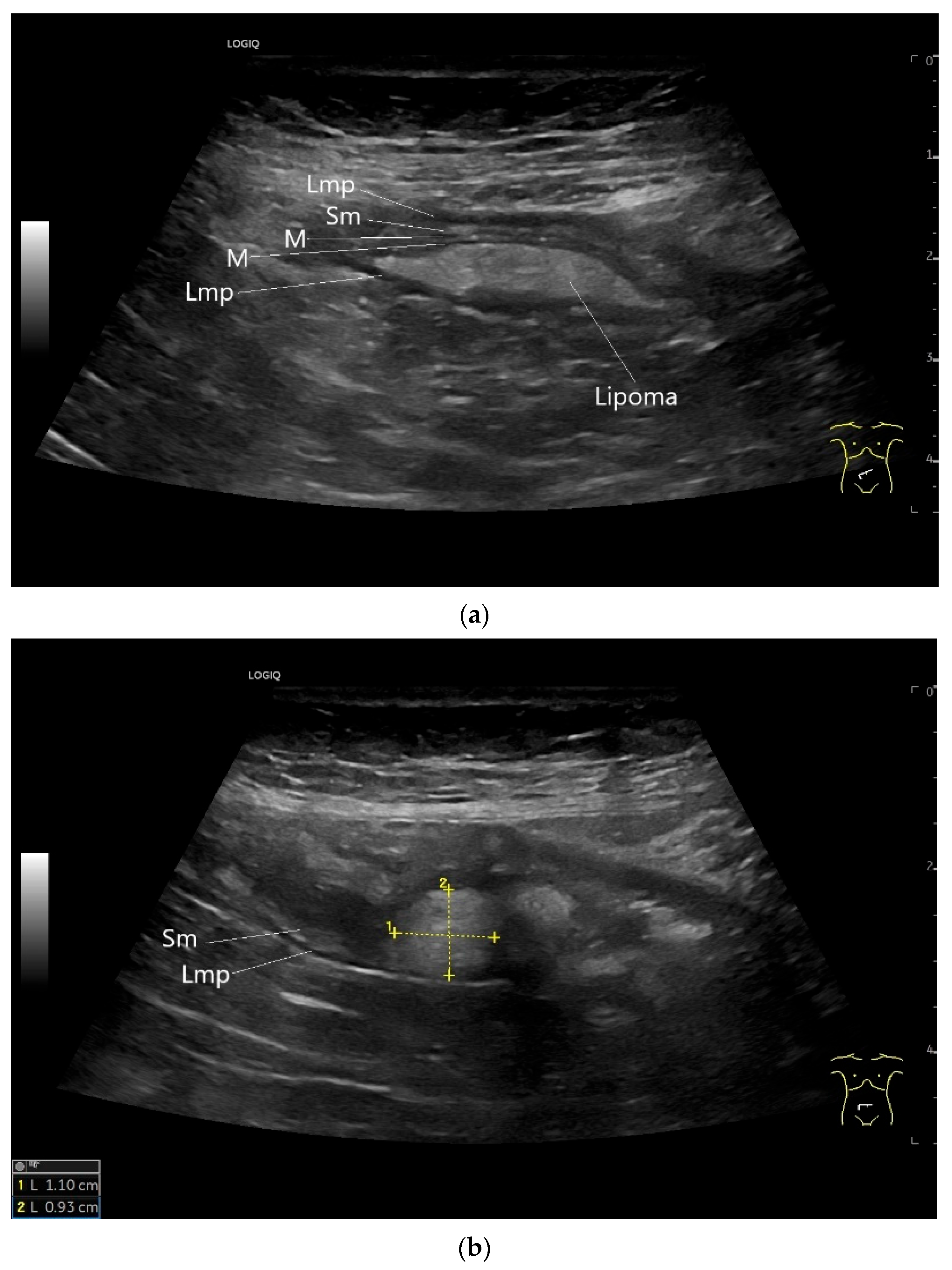
9.6. Lipoma
9.7. Schwannoma
10. Metastases in the Small Intestines
11. Future Directions and Artificial Intelligence (AI)
12. Conclusions
13. Take Home Messages
- Small intestine tumors are rare but clinically significant, especially in patients with unexplained anemia, GI bleeding, or abdominal pain not clarified by endoscopy.
- Transabdominal US is an underutilized but valuable tool for the detection of small bowel tumors, particularly those ≥20 mm in size.
- High-resolution transducers and systematic scanning techniques are essential to visualize bowel wall stratification, segmental thickening, and indirect signs like proximal dilation or intussusception.
- Multiparametric US, including CDI and CEUS, enhances lesion characterization by assessing vascularity and necrosis, especially in GISTs and neuroendocrine tumors.
- Ultrasound-guided biopsy is a reliable diagnostic option for accessible tumors not amenable to endoscopic sampling, particularly in lymphomas and large stromal tumors.
- US can guide further diagnostic decisions, reducing unnecessary CT, MRI, or capsule endoscopy in selected clinical scenarios—provided sufficient time, expertise, and high-quality equipment are available.
- We advocate for a broader use of multiparametric ultrasound in the diagnostic workup of patients with suspected small intestinal tumors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klimstra, D.S.N.I.; Rugge, M.; Salto-Tellez, M. Tumours of the small intestine and ampulla; Chapter 4. In WHO Classification of Tumours, 5th ed.; Digestive System Tumors; WHO Classification of Tumours Editorial Board, Ed.; WHO: Geneva, Switzerland, 2019; pp. 111–134. [Google Scholar]
- Chan, J.; Fukayama, M. Haematolymphoid tumours of the digestive system; chapter 11. In WHO Classification of Tumours, 5th ed.; Digestive System Tumours; WHO Classification of Tumours Editorial Board, Ed.; WHO: Geneva, Switzerland, 2019; pp. 372–431. [Google Scholar]
- Fukayama, M.G.J.; Miettinen, M.; Lazar, A.J. Mesenchymal tumours of the digestive system; Chapter 12. In WHO Classification of Tumours, 5th ed.; Digestive Tumours; WHO Classification of Tumours Editorial Board, Ed.; WHO: Geneva, Switzerland, 2019; pp. 432–498. [Google Scholar]
- Paradis, V.S.P.; Singh, R. Other tumours of the digestive system. Chapter 13. In WHO Classification of Tumours, 5th ed.; Digestive System Tumors; WHO Classification of Tumours Editorial Board, Ed.; WHO: Geneva, Switzerland, 2019; pp. 499–510. [Google Scholar]
- Schottenfeld, D.; Beebe-Dimmer, J.L.; Vigneau, F.D. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann. Epidemiol. 2009, 19, 58–69. [Google Scholar] [CrossRef]
- Hatzaras, I.; Palesty, J.A.; Abir, F.; Sullivan, P.; Kozol, R.A.; Dudrick, S.J.; Longo, W.E. Small-bowel tumors: Epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch. Surg. 2007, 142, 229–235. [Google Scholar] [CrossRef]
- Pan, S.Y.; Morrison, H. Epidemiology of cancer of the small intestine. World J. Gastrointest. Oncol. 2011, 3, 33–42. [Google Scholar] [CrossRef]
- Barsouk, A.; Rawla, P.; Barsouk, A.; Thandra, K.C. Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention. Med. Sci. 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.E.; Ilaghi, M.; Mahdavizadeh, V.; Ebrahimi, R.; Aslani, A.; Yekta, Z.; Nejadghaderi, S.A. A population-based study on incidence trends of small intestine cancer in the United States from 2000 to 2020. PLoS ONE 2024, 19, e0307019. [Google Scholar] [CrossRef] [PubMed]
- Nylund, K.; Maconi, G.; Hollerweger, A.; Ripolles, T.; Pallotta, N.; Higginson, A.; Serra, C.; Dietrich, C.F.; Sporea, I.; Saftoiu, A.; et al. EFSUMB Recommendations and Guidelines for Gastrointestinal Ultrasound. Ultraschall Med. 2017, 38, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Nylund, K.; Odegaard, S.; Hausken, T.; Folvik, G.; Lied, G.A.; Viola, I.; Hauser, H.; Gilja, O.H. Sonography of the small intestine. World J. Gastroenterol. 2009, 15, 1319–1330. [Google Scholar] [CrossRef]
- Nylund, K.; Hausken, T.; Odegaard, S.; Eide, G.E.; Gilja, O.H. Gastrointestinal wall thickness measured with transabdominal ultrasonography and its relationship to demographic factors in healthy subjects. Ultraschall Med. 2012, 33, E225–E232. [Google Scholar] [CrossRef]
- Möller, K.; Fischer, P.; Gilja, O.H.; Gottschall, H.; Jenssen, C.; Hollerweger, A.; Lucius, C.; Meier, J.; Rogler, G.; Misselwitz, B.; et al. Gastrointestinal Ultrasound: Measurements and Normal Findings—What Do You Need to Know? Dig. Dis. 2025, 43, 300–335. [Google Scholar] [CrossRef]
- Maconi, G.; Nylund, K.; Ripolles, T.; Calabrese, E.; Dirks, K.; Dietrich, C.F.; Hollerweger, A.; Sporea, I.; Saftoiu, A.; Maaser, C.; et al. EFSUMB Recommendations and Clinical Guidelines for Intestinal Ultrasound (GIUS) in Inflammatory Bowel Diseases. Ultraschall Med. 2018, 39, 304–317. [Google Scholar] [CrossRef]
- Dirks, K.; Calabrese, E.; Dietrich, C.F.; Gilja, O.H.; Hausken, T.; Higginson, A.; Hollerweger, A.; Maconi, G.; Maaser, C.; Nuernberg, D.; et al. EFSUMB Position Paper: Recommendations for Gastrointestinal Ultrasound (GIUS) in Acute Appendicitis and Diverticulitis. Ultraschall Med. 2019, 40, 163–175. [Google Scholar] [CrossRef]
- Hollerweger, A.; Maconi, G.; Ripolles, T.; Nylund, K.; Higginson, A.; Serra, C.; Dietrich, C.F.; Dirks, K.; Gilja, O.H. Gastrointestinal Ultrasound (GIUS) in Intestinal Emergencies—An EFSUMB Position Paper. Ultraschall Med. 2020, 41, 646–657. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Hollerweger, A.; Dirks, K.; Higginson, A.; Serra, C.; Calabrese, E.; Dong, Y.; Hausken, T.; Maconi, G.; Mihmanli, I.; et al. EFSUMB Gastrointestinal Ultrasound (GIUS) Task Force Group: Celiac sprue and other rare gastrointestinal diseases ultrasound features. Med. Ultrason. 2019, 21, 299–315. [Google Scholar] [CrossRef]
- Maconi, G.; Hausken, T.; Dietrich, C.F.; Pallotta, N.; Sporea, I.; Nurnberg, D.; Dirks, K.; Romanini, L.; Serra, C.; Braden, B.; et al. Gastrointestinal Ultrasound in Functional Disorders of the Gastrointestinal Tract—EFSUMB Consensus Statement. Ultrasound Int. Open 2021, 7, E14–E24. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Lembcke, B.; Jenssen, C.; Hocke, M.; Ignee, A.; Hollerweger, A. Intestinal Ultrasound in Rare Gastrointestinal Diseases, Update, Part 2. Ultraschall Med. 2015, 36, 428–456. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, K.; Takedatsu, H.; Yoshioka, S.; Yoshikai, M.; Tomiyasu, K.; Morita, M.; Kuwaki, K.; Mitsuyama, K.; Kawaguchi, T. Symptoms Contributing to the Diagnosis of Small Bowel Tumors. Digestion 2023, 104, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Nishie, H.; Suzuki, T.; Ichikawa, H.; Kataoka, H. Intestinal obstruction caused by small bowel adenocarcinoma misdiagnosed as psychogenic disorder. BMJ Case Rep. 2019, 12, bcr-2018-227326. [Google Scholar] [CrossRef]
- Xin, L.; Liao, Z.; Jiang, Y.P.; Li, Z.S. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: A systematic review of data over the first decade of use. Gastrointest. Endosc. 2011, 74, 563–570. [Google Scholar] [CrossRef]
- Messer, I.; May, A.; Manner, H.; Ell, C. Prospective, randomized, single-center trial comparing double-balloon enteroscopy and spiral enteroscopy in patients with suspected small-bowel disorders. Gastrointest. Endosc. 2013, 77, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yano, T.; Ohmiya, N.; Tanaka, S.; Tanaka, S.; Endo, Y.; Matsuda, T.; Matsui, T.; Iida, M.; Sugano, K. Double-balloon endoscopy is safe and effective for the diagnosis and treatment of small-bowel disorders: Prospective multicenter study carried out by expert and non-expert endoscopists in Japan. Dig. Endosc. 2015, 27, 331–337. [Google Scholar] [CrossRef]
- Beyna, T.; Arvanitakis, M.; Schneider, M.; Gerges, C.; Böing, D.; Devière, J.; Neuhaus, H. Motorised spiral enteroscopy: First prospective clinical feasibility study. Gut 2021, 70, 261–267. [Google Scholar] [CrossRef]
- Buchholz, H.; Mende, M.; Hornoff, S.; Faiss, S. Results of motorized spiral enteroscopy in 83 consecutive patients. Z. Gastroenterol. 2022, 60, 1635–1643. [Google Scholar] [CrossRef]
- Pal, P.; Ramchandani, M.; Banerjee, R.; Viswakarma, P.; Singh, A.P.; Reddy, M.; Rughwani, H.; Patel, R.; Sekaran, A.; Kanaganti, S.; et al. Technical performance and diagnostic yield of motorised spiral enteroscopy compared with single-balloon enteroscopy in suspected Crohn’s disease: A randomised controlled, open-label study (the MOTOR-CD trial). Gut 2023, 72, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.G.; Shan, G.D.; Zhang, H.; Li, L.; Yue, M.; Xiang, Z.; Cheng, Y.; Wu, C.J.; Fang, Y.; Chen, L.H. Double-balloon enteroscopy in small bowel tumors: A Chinese single-center study. World J. Gastroenterol. 2013, 19, 3665–3671. [Google Scholar] [CrossRef]
- Noujaim, M.G.; Dorsey, C.; Parish, A.; Raines, D.; Boudreaux, L.; Hanscom, M.; Cave, D.; Niedzwiecki, D.; Wild, D. Clinical Features and Management of Small Bowel Masses Detected During Device-Assisted Enteroscopy: A Multi-Center Experience. Gastroenterol. Res. 2022, 15, 353–363. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, L.; Zhang, T.; Sun, P.; Sun, J.; Zhou, J.; Wang, L.; Fan, R.; Wang, Z.; Cheng, S.; et al. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: Experience from a Chinese tertiary hospital. Turk. J. Gastroenterol. 2020, 31, 30–35. [Google Scholar] [CrossRef]
- Mitsui, K.; Tanaka, S.; Yamamoto, H.; Kobayashi, T.; Ehara, A.; Yano, T.; Goto, H.; Nakase, H.; Tanaka, S.; Matsui, T.; et al. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: The first Japanese multicenter study. Gastrointest. Endosc. 2009, 70, 498–504. [Google Scholar] [CrossRef]
- Rondonotti, E.; Pennazio, M.; Toth, E.; Menchen, P.; Riccioni, M.E.; De Palma, G.D.; Scotto, F.; De Looze, D.; Pachofsky, T.; Tacheci, I.; et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: A multicenter European study. Endoscopy 2008, 40, 488–495. [Google Scholar] [CrossRef]
- Lewis, B.S.; Eisen, G.M.; Friedman, S. A Pooled Analysis to Evaluate Results of Capsule Endoscopy Trials. Endoscopy 2005, 37, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Murino, A.; Nakamura, M.; Watanabe, O.; Yamamura, T.; Nagura, A.; Yoshimura, T.; Nakano, A.; Goto, H.; Hirooka, Y. Effectiveness of Endoscopic Ultrasonography during Double Balloon Enteroscopy for characterization and management of small bowel submucosal tumours. Dig. Liver Dis. 2016, 48, 1187–1193. [Google Scholar] [CrossRef]
- Zhongcheng, L.; Dongting, C.; Biyao, W.; Xinyu, L.; Qin, G. Application value of small intestinal endoscopic ultrasonography for protruding lesions of the small intestine. Surg. Endosc. 2025, 39, 2902–2910. [Google Scholar] [CrossRef]
- Rangiah, D.S.; Cox, M.; Richardson, M.; Tompsett, E.; Crawford, M. Small bowel tumours: A 10 year experience in four Sydney teaching hospitals. ANZ J. Surg. 2004, 74, 788–792. [Google Scholar] [CrossRef]
- Corvino, A.; Setola, S.V.; Sandomenico, F.; Corvino, F.; Catalano, O. Synchronous tumours detected during cancer patient staging: Prevalence and patterns of occurrence in multidetector computed tomography. Pol. J. Radiol. 2020, 85, e261–e270. [Google Scholar] [CrossRef]
- Masselli, G.; Di Tola, M.; Casciani, E.; Polettini, E.; Laghi, F.; Monti, R.; Bernieri, M.G.; Gualdi, G. Diagnosis of Small-Bowel Diseases: Prospective Comparison of Multi-Detector Row CT Enterography with MR Enterography. Radiology 2016, 279, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Del Gaizo, A.J.; Fletcher, J.G.; Yu, L.; Paden, R.G.; Spencer, G.C.; Leng, S.; Silva, A.M.; Fidler, J.L.; Silva, A.C.; Hara, A.K. Reducing radiation dose in CT enterography. Radiographics 2013, 33, 1109–1124. [Google Scholar] [CrossRef]
- Wang, N.; Cui, X.Y.; Liu, Y.; Long, J.; Xu, Y.H.; Guo, R.X.; Guo, K.J. Adult intussusception: A retrospective review of 41 cases. World J. Gastroenterol. 2009, 15, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, A.; Tanaka, S.; Imagawa, H.; Shishido, T.; Oka, S.; Yoshida, S.; Yamada, H.; Chayama, K. Usefulness and limitations of transabdominal ultrasonography for detecting small-bowel tumors. Scand. J. Gastroenterol. 2009, 44, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Manabe, N.; Honda, K.; Murao, T.; Osawa, M.; Kawai, R.; Akiyama, T.; Shiotani, A.; Haruma, K.; Hata, J. Usefulness of Ultrasonography for Diagnosis of Small Bowel Tumors: A Comparison Between Ultrasonography and Endoscopic Modalities. Medicine 2015, 94, e1464. [Google Scholar] [CrossRef]
- Masselli, G.; Polettini, E.; Casciani, E.; Bertini, L.; Vecchioli, A.; Gualdi, G. Small-bowel neoplasms: Prospective evaluation of MR enteroclysis. Radiology 2009, 251, 743–750. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version). Ultraschall Med. 2018, 39, 154–180. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.A.; et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2018, 4, E2–E15. [Google Scholar] [CrossRef]
- Petersen, F.; Hopfner, M.H.; Jenssen, C.; Nurnberg, D.; Strobel, D.; Vogelpohl, J.; Dietrich, C.F. Why is ultrasound needed in inflammatory bowel disease? Med. Ultrason. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Jenssen, C.; Petersen, F.; Hopfner, M.; Nurnberg, D.; Strobel, D.; Vogelpohl, J.; Dietrich, C.F. Why and when are activity scores and advanced ultrasound techniques needed in inflammatory bowel disease (IBD)? Med. Ultrason. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Petersen, F.; Hopfner, M.; Nurnberg, D.; Strobel, D.; Vogelpohl, J.; Jenssen, C. Ultrasound for detection of complications and evaluation of treatment response in inflammatory bowel disease (IBD)? Med. Ultrason. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Matsui, S.; Kamata, K.; Komaki, T.; Imai, H.; Dote, K.; Kudo, M. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest. Endosc. 2011, 73, 227–237. [Google Scholar] [CrossRef]
- Kannengiesser, K.; Mahlke, R.; Petersen, F.; Peters, A.; Ross, M.; Kucharzik, T.; Maaser, C. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand. J. Gastroenterol. 2012, 47, 1515–1520. [Google Scholar] [CrossRef]
- Kamata, K.; Takenaka, M.; Kitano, M.; Omoto, S.; Miyata, T.; Minaga, K.; Yamao, K.; Imai, H.; Sakurai, T.; Watanabe, T.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of submucosal tumors of the upper gastrointestinal tract. J. Gastroenterol. Hepatol. 2017, 32, 1686–1692. [Google Scholar] [CrossRef]
- Tamura, T.; Kitano, M. Contrast Enhanced EUS Imaging for Gastrointestinal Subepithelial Tumors. Clin. Endosc. 2019, 52, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Saftoiu, A.; Napoleon, B.; Arcidiacono, P.G.; Braden, B.; Burmeister, S.; Carrara, S.; Cui, X.W.; Fusaroli, P.; Gottschalk, U.; Hocke, M.; et al. Do we need contrast agents for EUS? Endosc. Ultrasound 2020, 9, 361–368. [Google Scholar] [CrossRef]
- Yang, Y.T.; Shen, N.; Ao, F.; Chen, W.Q. Diagnostic value of contrast-enhanced harmonic endoscopic ultrasonography in predicting the malignancy potential of submucosal tumors: A systematic review and meta-analysis. Surg. Endosc. 2020, 34, 3754–3765. [Google Scholar] [CrossRef] [PubMed]
- Ignee, A.; Jenssen, C.; Hocke, M.; Dong, Y.; Wang, W.P.; Cui, X.W.; Woenckhaus, M.; Iordache, S.; Saftoiu, A.; Schuessler, G.; et al. Contrast-enhanced (endoscopic) ultrasound and endoscopic ultrasound elastography in gastrointestinal stromal tumors. Endosc. Ultrasound 2017, 6, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Sparchez, Z.; Radu, P.; Zaharia, T.; Kacso, G.; Grigorescu, I.; Badea, R. Contrast enhanced ultrasound guidance: A new tool to improve accuracy in percutaneous biopsies. Med. Ultrason. 2010, 12, 133–138. [Google Scholar]
- Sparchez, Z.; Mocan, T.; Craciun, R.; Sparchez, M.; Nolsoe, C. Contrast enhancement for ultrasound-guided interventions: When to use it and what to expect? Ultrasonography 2022, 41, 263–278. [Google Scholar] [CrossRef]
- Moller, K.; Dietrich, C.F.; Faiss, S.; Mutze, S.; Goelz, L. Alternatives of histological material collection—When and how is histological confirmation by ultrasound (US), computer tomography (CT) or endosonography (EUS) useful? Z. Gastroenterol. 2022, 60, 937–958. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Brabrand, K.; Cantisani, V.; Correas, J.M.; Cui, X.W.; D’Onofrio, M.; Essig, M.; Freeman, S.; Gilja, O.H.; Gritzmann, N. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part II. Diagnostic Ultrasound-Guided Interventional Procedures (Long Version). Ultraschall Med. 2015, 36, E15–E35. [Google Scholar]
- Tudor, G.R.; Rodgers, P.M.; West, K.P. Bowel lesions: Percutaneous US-guided 18-gauge needle biopsy--preliminary experience. Radiology 1999, 212, 594–597. [Google Scholar] [CrossRef]
- Marco-Domenech, S.F.; Gil-Sanchez, S.; Fernandez-Garcia, P.; De La Iglesia-Carrena, P.; Gonzalez-Anon, M.; Arenas-Jimenez, J.J.; Alonso-Charterina, S.; Piqueras-Olmeda, R.M. Sonographically guided percutaneous biopsy of gastrointestinal tract lesions. Am. J. Roentgenol. 2001, 176, 147–151. [Google Scholar] [CrossRef]
- Minhas, K.; Roebuck, D.J.; Barnacle, A.; De Coppi, P.; Sebire, N.; Patel, P.A. Diagnostic yield and safety of ultrasound-guided bowel mass biopsies in children. Pediatr. Radiol. 2019, 49, 1809–1815. [Google Scholar] [CrossRef]
- Tombesi, P.; Postorivo, S.; Catellani, M.; Tassinari, D.; Abbasciano, V.; Sartori, S. Percutaneous ultrasonography-guided core needle biopsy of gastrointestinal lesions: What’s its actual role in clinical practice? A retrospective study for safety and effectiveness. Ultraschall Med. 2011, 32 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef]
- de Sio, I.; Funaro, A.; Vitale, L.M.; Niosi, M.; Francica, G.; Federico, A.; Sgambato, D.; Loguercio, C.; Romano, M. Ultrasound-guided percutaneous biopsy for diagnosis of gastrointestinal lesions. Dig. Liver Dis. 2013, 45, 816–819. [Google Scholar] [CrossRef]
- Chiorean, L.; Cantisani, V.; Jenssen, C.; Sidhu, P.S.; Baum, U.; Dietrich, C.F. Focal masses in a non-cirrhotic liver: The additional benefit of CEUS over baseline imaging. Eur. J. Radiol. 2015, 84, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Mackey, R.; Brown, N.; Church, J.; Burke, C.; Walsh, R.M. Outcome based on management for duodenal adenomas: Sporadic versus familial disease. J. Gastrointest. Surg. 2010, 14, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Culver, E.L.; McIntyre, A.S. Sporadic duodenal polyps: Classification, investigation, and management. Endoscopy 2011, 43, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Chung, W.C.; Kim, E.J.; Kim, S.H.; Paik, C.N.; Lee, B.I.; Cho, Y.S.; Lee, K.M. Evaluation of non-ampullary duodenal polyps: Comparison of non-neoplastic and neoplastic lesions. World J. Gastroenterol. 2010, 16, 5474–5480. [Google Scholar] [CrossRef]
- Corvino, A.; Corvino, F.; Radice, L.; Catalano, O. Synchronous mucinous colonic adenocarcinoma and multiple small intestinal adenocarcinomas: Report of a case and review of literature. Clin. Imaging 2015, 39, 538–542. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, H.C.; Oh, J.; Won, K.Y.; Park, S.J.; Yang, D.M. Tumors of the jejunum and ileum: A pattern-based imaging approach on CT. Abdom. Radiol. 2019, 44, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Capela, T.; Sousa, P.; Caldeira, A.; Pereira, E. Intestinal Obstruction of Uncommon Cause and Point-of-Care Ultrasonography—Where Do We Stand? GE Port. J. Gastroenterol. 2018, 25, 38–41. [Google Scholar] [CrossRef]
- Wu, S.; Yu, H.; Liu, Y.; Zhou, H.; Zhou, Y. Small bowel adenocarcinoma of the jejunum detected by double contrast enhanced ultrasound: A case report of a novel ultrasound modality. Front. Oncol. 2024, 14, 1288041. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Snorradottir, S.; Asgeirsdottir, A.; Rögnvaldsson, S.; Jonasson, J.G.; Björnsson, E.S. Incidence and prognosis of patients with small intestinal neuroendocrine tumors in a population based nationwide study. Cancer Epidemiol. 2022, 79, 102197. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Sandvik, O.M.; Søreide, K.; Gudlaugsson, E.; Kvaløy, J.T.; Søreide, J.A. Epidemiology and classification of gastroenteropancreatic neuroendocrine neoplasms using current coding criteria. Br. J. Surg. 2016, 103, 226–232. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C.H.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef]
- Palepu, J.; Shrikhande, S.V.; Bhaduri, D.; Shah, R.C.; Sirohi, B.; Chhabra, V.; Dhar, P.; Sastry, R.; Sikora, S. Trends in diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in India: A report of multicenter data from a web-based registry. Indian. J. Gastroenterol. 2017, 36, 445–451. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, L.; Bao, H.; Zhang, J.; Wang, Z.; Gong, P. Clinical, pathological and prognostic characteristics of gastroenteropancreatic neuroendocrine neoplasms in China: A retrospective study. BMC Endocr. Disord. 2014, 14, 54. [Google Scholar] [CrossRef]
- Fan, J.H.; Zhang, Y.Q.; Shi, S.S.; Chen, Y.J.; Yuan, X.H.; Jiang, L.M.; Wang, S.M.; Ma, L.; He, Y.T.; Feng, C.Y.; et al. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in china. Oncotarget 2017, 8, 71699–71708. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.Y.; Kim, J.M.; Sohn, J.H.; Kim, M.J.; Kim, K.M.; Kim, W.H.; Kim, H.; Kook, M.C.; Park, D.Y.; Lee, J.H.; et al. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res. Treat. 2012, 44, 157–165. [Google Scholar] [CrossRef]
- Tsai, H.J.; Wu, C.C.; Tsai, C.R.; Lin, S.F.; Chen, L.T.; Chang, J.S. The epidemiology of neuroendocrine tumors in Taiwan: A nation-wide cancer registry-based study. PLoS ONE 2013, 8, e62487. [Google Scholar] [CrossRef]
- Ellis, L.; Shale, M.J.; Coleman, M.P. Carcinoid tumors of the gastrointestinal tract: Trends in incidence in England since 1971. Am. J. Gastroenterol. 2010, 105, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
- Genus, T.S.E.; Bouvier, C.; Wong, K.F.; Srirajaskanthan, R.; Rous, B.A.; Talbot, D.C.; Valle, J.W.; Khan, M.; Pearce, N.; Elshafie, M.; et al. Impact of neuroendocrine morphology on cancer outcomes and stage at diagnosis: A UK nationwide cohort study 2013–2015. Br. J. Cancer 2019, 121, 966–972. [Google Scholar] [CrossRef]
- Milione, M.; Parente, P.; Grillo, F.; Zamboni, G.; Mastracci, L.; Capella, C.; Fassan, M.; Vanoli, A. Neuroendocrine neoplasms of the duodenum, ampullary region, jejunum and ileum. Pathologica 2021, 113, 12–18. [Google Scholar] [CrossRef]
- Klöppel, G.; Anlauf, M. Gastrinoma--morphological aspects. Wien. Klin. Wochenschr. 2007, 119, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.A.; Chou, Y.H.; Huang, S.H.; Chen, T.J. Primary jejunal gastrinoma: A case report and review of the literature. World J. Surg. Oncol. 2015, 13, 313. [Google Scholar] [CrossRef]
- Sandru, F.; Carsote, M.; Valea, A.; Albu, S.E.; Petca, R.C.; Dumitrascu, M.C. Somatostatinoma: Beyond neurofibromatosis type 1 (Review). Exp. Ther. Med. 2020, 20, 3383–3388. [Google Scholar] [CrossRef]
- Constantinoiu, S.; Constantin, A.; Predescu, D.; Iosif, C.; Hoara, P.; Achim, F.; Surugiu, P.; Bacanu, F.; Cociu, L. Somatostatinoma of the first jejunal loop in a patient with neurofibromatosis von Recklinghausen and bilateral pheochromocytoma. Hepatogastroenterology 2012, 59, 1874–1878. [Google Scholar]
- Maccioni, F.; Rossi, P.; Gourtsoyiannis, N.; Bezzi, M.; Di Nardo, L.; Broglia, L. US and CT findings of small bowel neoplasms. Eur. Radiol. 1997, 7, 1398–1409. [Google Scholar] [CrossRef]
- Giannetti, A.; Randisi, P.; Stumpo, M.; Coratti, F. Diagnosis of one small bowel tumor: The role of conventional ultrasound and elastography. J. Ultrasound 2016, 19, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Grad, S.; Dumitraşcu, D.; Badea, R.; Iobagiu, S.; Pascu, O. Ileal neuroendocrine tumor—Ultrasonographic and capsule endoscopy appearance: A case report. Med. Ultrason. 2010, 12, 245–248. [Google Scholar]
- Tsujimura, K.; Takushi, Y.; Teruya, T.; Iha, K.; Ota, M.; Nakachi, A.; Gakiya, A. Neuroendocrine tumor of the small intestine diagnosed with trans-abdominal ultrasonography: A case report. Int. J. Surg. Case Rep. 2017, 31, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Rioux, M.; Langis, P.; Naud, F. Sonographic appearance of primary small bowel carcinoid tumor. Abdom. Imaging 1995, 20, 37–43. [Google Scholar] [CrossRef]
- Schwarze, V.; Marschner, C.; Grosu, S.; Rübenthaler, J.; Knösel, T.; Clevert, D.A. Modern sonographic imaging of abdominal neuroendocrine tumors. Radiologe 2019, 59, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Smereczyński, A.; Starzyńska, T.; Kołaczyk, K. Mesenteric changes in an ultrasound examination can facilitate the diagnosis of neuroendocrine tumors of the small intestine. J. Ultrason. 2015, 15, 274–282. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Zhuang, H.; Luo, Y.; Su, M.G.; Xiong, M.L.; Wu, Y.T. Double contrast-enhanced ultrasonography of a small intestinal neuroendocrine tumor: A case report of a recommendable imaging modality. Precis. Clin. Med. 2020, 3, 147–152. [Google Scholar] [CrossRef]
- Yin, L.; Chen, C.Q.; Peng, C.H.; Chen, G.M.; Zhou, H.J.; Han, B.S.; Li, H.W. Primary small-bowel non-Hodgkin’s lymphoma: A study of clinical features, pathology, management and prognosis. J. Int. Med. Res. 2007, 35, 406–415. [Google Scholar] [CrossRef]
- Iwamuro, M.; Tanaka, T.; Okada, H. Review of lymphoma in the duodenum: An update of diagnosis and management. World J. Gastroenterol. 2023, 29, 1852–1862. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Olszewska-Szopa, M.; Wróbel, T. Gastrointestinal non-Hodgkin lymphomas. Adv. Clin. Exp. Med. 2019, 28, 1119–1124. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.; Deshmukh, A.; Chandrashekhara, S.H. Imaging of Bowel Lymphoma: A Pictorial Review. Dig. Dis. Sci. 2022, 67, 1187–1199. [Google Scholar] [CrossRef]
- Chowdhury, M.; Endo, M.; Chiba, T.; Kudara, N.; Oana, S.; Sato, K.; Akasaka, R.; Tomita, K.; Fujiwara, S.; Mizutani, T.; et al. Characterization of follicular lymphoma in the small intestine using double-balloon endoscopy. Gastroenterol. Res. Pract. 2009, 2009, 835258. [Google Scholar] [CrossRef]
- Tormane, M.A.; Laamiri, G.; Beji, H.; Gazzeh, H.; Bouassida, M.; Touinsi, H. Primary mantle-cell lymphoma of small intestine presenting with intussusceptions: A case report and review of the literature. Int. J. Surg. Case Rep. 2024, 121, 109963. [Google Scholar] [CrossRef]
- Kella, V.K.; Constantine, R.; Parikh, N.S.; Reed, M.; Cosgrove, J.M.; Abo, S.M.; King, S. Mantle cell lymphoma of the gastrointestinal tract presenting with multiple intussusceptions--case report and review of literature. World J. Surg. Oncol. 2009, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.G.; Xu, X.M.; Wei, S.M. Multiple Lymphomatous Polyposis of the Intestine with Ileocecal Intussusception Due to Mantle Cell Lymphoma: A Case Report of a 34-Year-Old Man. Am. J. Case Rep. 2018, 19, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Aukhojee, V.; Gilong, C.M.; Seewoogoolam, G.; Strauss, P.N. Rare presentation of ileocolic intussusception secondary to mantle cell lymphoma. BMJ Case Rep. 2019, 12, e229425. [Google Scholar] [CrossRef] [PubMed]
- Kasparian, S.; Burns, E.; Shehabeldin, A.; Awar, M.; Pingali, S.R. Recurrent small bowel obstruction caused by Burkitt lymphoma in an elderly man: A case report and review of the literature. J. Med. Case Rep. 2020, 14, 127. [Google Scholar] [CrossRef]
- Takayama, Y.; Saito, M.; Ichida, K.; Muto, Y.; Tanaka, A.; Rikiyama, T. Intestinal perforation secondary to intestinal Burkitt lymphoma. Int. J. Surg. Case Rep. 2021, 79, 417–420. [Google Scholar] [CrossRef]
- Grajo, J.R.; Kayton, M.L.; Steffensen, T.S.; Dragicevic, N.; Guidi, C.B. Presentation of ileal Burkitt lymphoma in children. J. Radiol. Case Rep. 2012, 6, 27–38. [Google Scholar] [CrossRef]
- Mago, S.; Mavilia, M.; Forouhar, F.; Vaziri, H. Small bowel T-cell lymphoma: A MEITL-ing diagnosis. Clin. J. Gastroenterol. 2021, 14, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Sumimura, J.; Mizutani, S.; Tazuke, Y.; Okuda, S.; Dezawa, T. The doughnut sign: An ultrasound finding in pediatric intestinal Burkitt’s lymphoma. Pediatr. Surg. Int. 1998, 13, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Hasaballah, M.; Abdel-Malek, R.; Zakaria, Z.; Marie, M.S.; Naguib, M.S. Transabdominal ultrasonographic features in the diagnosis of gastrointestinal lymphoma. J. Gastrointest. Oncol. 2018, 9, 1190–1197. [Google Scholar] [CrossRef]
- Goerg, C.; Schwerk, W.B.; Goerg, K. Gastrointestinal lymphoma: Sonographic findings in 54 patients. Am. J. Roentgenol. 1990, 155, 795–798. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, B.; Cai, S.; Jiang, Y.X.; Li, W.B.; Yang, X.; Zhao, R.N. Ultrasonographic and general pathologic features assessment of small intestinal lymphoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2013, 35, 318–321. [Google Scholar] [CrossRef]
- Cui, N.Y.; Gong, X.T.; Tian, Y.T.; Wang, Y.; Zhang, R.; Liu, M.J.; Han, J.; Wang, B.; Yang, D. Contrast-enhanced ultrasound imaging for intestinal lymphoma. World J. Gastroenterol. 2021, 27, 5438–5447. [Google Scholar] [CrossRef]
- Starczewska Amelio, J.M.; Cid Ruzafa, J.; Desai, K.; Tzivelekis, S.; Muston, D.; Khalid, J.M.; Ashman, P.; Maguire, A. Prevalence of gastrointestinal stromal tumour (GIST) in the United Kingdom at different therapeutic lines: An epidemiologic model. BMC Cancer 2014, 14, 364. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Ota, S.; Yamasaki, M.; Batsaikhan, B.; Furukawa, A.; Watanabe, Y. Gastrointestinal stromal tumors: A comprehensive radiological review. Jpn. J. Radiol. 2022, 40, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Tryggvason, G.; Gíslason, H.G.; Magnússon, M.K.; Jónasson, J.G. Gastrointestinal stromal tumors in Iceland, 1990–2003: The icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int. J. Cancer 2005, 117, 289–293. [Google Scholar] [CrossRef]
- Nilsson, B.; Bümming, P.; Meis-Kindblom, J.M.; Odén, A.; Dortok, A.; Gustavsson, B.; Sablinska, K.; Kindblom, L.G. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005, 103, 821–829. [Google Scholar] [CrossRef]
- Miettinen, M.; Makhlouf, H.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors of the jejunum and ileum: A clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am. J. Surg. Pathol. 2006, 30, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Z.; Wang, W.; Zhao, W.; Tuo, S.; Shi, Y.; Zhang, W.; Chen, L.; Hong, L.; Yang, J.; et al. Current characteristics on small intestinal stromal tumor-a case control study. Ann. Palliat. Med. 2020, 9, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liao, Y.; Wu, J.; Yang, J.; Zhang, H.; Wang, X.; Sun, S. Small bowel gastrointestinal stromal tumor: A retrospective study of 32 cases at a single center and review of the literature. Ther. Clin. Risk Manag. 2018, 14, 1467–1481. [Google Scholar] [CrossRef]
- Basukala, S.; Karki, S.; Maharjan, S.; Banmala, S.; Shrestha, M.; Jayswal, M.; Shrestha, K. Small bowel intussusception in an adult secondary to gastrointestinal stromal tumor: A rare case report. Ann. Med. Surg. 2023, 85, 1952–1955. [Google Scholar] [CrossRef]
- Mishra, A.; Gyawali, S.; Kharel, S.; Mishra, A.; Pathak, N.; Subedi, N.; Gaire, P. Multiple jejunal gastrointestinal stromal tumors and Neurofibromatosis type 1: A rare association. Int. J. Surg. Case Rep. 2021, 85, 106178. [Google Scholar] [CrossRef]
- Bozkurt, T.; Butsch, B.; Schmiegelow, P.; Lux, G. Sonographische Darstellung mesenchymaler Dünndarmtumoren in der Diagnostik von unklaren, gastrointestinalen Blutungen. Ultraschall Med. 1993, 14, 264–268. [Google Scholar] [CrossRef]
- Hocke, M.; Braden, B.; Jenssen, C.; Dietrich, C.F. Present status and perspectives of endosonography 2017 in gastroenterology. Korean J. Intern. Med. 2018, 33, 36–63. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Wu, T.; Qin, L. Case report: Ultrasound and contrast-enhanced ultrasound findings of pediatric small intestinal inflammatory myofibroblastic tumor. Front. Oncol. 2025, 15, 1512402. [Google Scholar] [CrossRef]
- Rispo, A.; De Sire, R.; D’Armiento, M.; De Bonis, L.; Tropeano, F.P.; Ricciolino, S.; Nardone, G.; Luglio, G. Ultrasonographic diagnosis of ileo-ileal intussusception secondary to Vanek’s tumor. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 350–353. [Google Scholar] [CrossRef]
- Shinagare, A.B.; Ramaiya, N.H.; Jagannathan, J.P.; Krajewski, K.M.; Giardino, A.A.; Butrynski, J.E.; Raut, C.P. A to Z of desmoid tumors. Am. J. Roentgenol. 2011, 197, W1008–W1014. [Google Scholar] [CrossRef]
- Eswaravaka, S.K.; Deshpande, S.H.; Chiranjeev, R.; Pandya, J.S. Intra-abdominal small intestinal desmoid tumour mimicking GIST. BMJ Case Rep. 2021, 14, e237032. [Google Scholar] [CrossRef]
- Möller, K.; Holz, T.; Jenssen, C.; Braden, B.; Hocke, M.; On, W.; Everett, S.M.; Dong, Y.; Ge, N.; Sun, S.; et al. Comments and illustrations of the European Federation of Societies for Ultrasound in Medicine contrast-enhanced ultrasonography guidelines: Multiparametric imaging and EUS-guided sampling in rare pancreatic tumors. Mesenchymal pancreatic tumors of intermediate biological behaviour. Endosc. Ultrasound 2024, 13, 145–153. [Google Scholar] [CrossRef]
- Al-Obeed, O.A.; Abdulla, M.H. Lymphangioma of the ileocecal valve clinically masquerading as a submucosal small intestinal GIST: Report of a case and literature review. Saudi J. Gastroenterol. 2014, 20, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.-L.; Lee, T.-Y.; Hung, C.-F.; Ng, K.-K. Ultrasound and CT findings of a cecal lymphangioma presenting with intussusception. Eur. J. Radiol. 1998, 27, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Limaiem, F.; Khalfallah, T.; Marsaoui, L.; Bouraoui, S.; Lahmar, A.; Mzabi, S. Jejunal lymphangioma: An unusual cause of intussusception in an adult patient. Pathologica 2015, 107, 19–21. [Google Scholar]
- Zhao, N.; Fu, Y.; Wang, Z.; An, Q.; Jia, W.-Z. Case report: Submucosal cavernous lymphangioma causing jejuno-jejunal intussusception in an adult. Front. Surg. 2022, 9, 953840. [Google Scholar] [CrossRef]
- Fukushima, N.; Aoki, H.; Fukazawa, N.; Ogawa, M.; Yoshida, K.; Yanaga, K. Schwannoma of the Small Intestine. Case Rep. Gastroenterol. 2019, 13, 294–298. [Google Scholar] [CrossRef]
- Onishi, S.; Ibuka, T.; Uno, Y.; Kojima, K.; Takada, J.; Kubota, M.; Shimizu, M. A rare case of schwannoma of the small intestine discovered during intussusception. JGH Open 2024, 8, e13078. [Google Scholar] [CrossRef] [PubMed]
- Jezovit, M.; Bakirli, H.; Bakirov, I.; Hureibi, K.; Bakirova, G.; Okolicany, R.; Janac, P.; Meciarova, I.; Alhwaymel, N.; Bakirli, I.; et al. Ileal Schwannoma: A Rare Cause of Pelvic Mass. Case Rep. Surg. 2024, 2024, 5572087. [Google Scholar] [CrossRef]
- Wippold, F.J., 2nd; Lubner, M.; Perrin, R.J.; Lammle, M.; Perry, A. Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns. Am. J. Neuroradiol. 2007, 28, 1633–1638. [Google Scholar] [CrossRef]
- Möller, K.; Batali, A.; Jenssen, C.; Braden, B.; Hocke, M.; On, W.; Everett, S.M.; Dong, Y.; Ge, N.; Sun, S.; et al. Comments and illustrations of the European Federation of Societies for Ultrasound in Medicine contrast-enhanced ultrasound guidelines: Multiparametric imaging and EUS-guided sampling in rare pancreatic tumors. Benign mesenchymal pancreatic tumors. Endosc. Ultrasound 2024, 13, 218–231. [Google Scholar] [CrossRef]
- Dwivedi, R.C.; Kazi, R.; Agrawal, N.; Chisholm, E.; St Rose, S.; Elmiyeh, B.; Rennie, C.; Pepper, C.; Clarke, P.M.; Kerawala, C.J.; et al. Comprehensive review of small bowel metastasis from head and neck squamous cell carcinoma. Oral. Oncol. 2010, 46, 330–335. [Google Scholar] [CrossRef]
- Di, J.Z.; Peng, J.Y.; Wang, Z.G. Prevalence, clinicopathological characteristics, treatment, and prognosis of intestinal metastasis of primary lung cancer: A comprehensive review. Surg. Oncol. 2014, 23, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Gakuhara, A.; Kitani, K.; Terashita, D.; Tomihara, H.; Fukuda, S.; Ota, K.; Hashimoto, K.; Ishikawa, H.; Hida, J.; Wakasa, T.; et al. Small Bowel Metastasis of Breast Cancer-A Case Report. Gan To Kagaku Ryoho 2021, 48, 1737–1739. [Google Scholar] [PubMed]
- Zhang, Z.; Liu, J.; Yu, P.F.; Yang, H.R.; Li, J.Y.; Dong, Z.W.; Shi, W.; Gu, G.L. Clinicopathological analysis of small intestinal metastasis from extra-abdominal/extra-pelvic malignancy. World J. Gastrointest. Oncol. 2024, 16, 4138–4145. [Google Scholar] [CrossRef]
- Đokić, M.; Badovinac, D.; Petrič, M.; Trotovšek, B. An unusual presentation of metastatic malignant melanoma causing jejuno-jejunal intussusception: A case report. J. Med. Case Rep. 2018, 12, 337. [Google Scholar] [CrossRef]
- Hsu, Y.F.; Huang, C.Y.; Chen, T.J.; Chou, Y.H. Small bowel intussusception due to metastatic intestinal carcinosarcoma from a pulmonary primary. Int. Med. Case Rep. J. 2011, 4, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.Q.; Ren, J.Y.; Xu, X.L.; Xiong, L.Y.; Peng, Y.X.; Pan, X.F.; Dietrich, C.F.; Cui, X.W. Ultrasound-based artificial intelligence in gastroenterology and hepatology. World J. Gastroenterol. 2022, 28, 5530–5546. [Google Scholar] [CrossRef]
- Graumann, O.; Cui Xin, W.; Goudie, A.; Blaivas, M.; Braden, B.; Campbell Westerway, S.; Chammas, M.C.; Dong, Y.; Gilja, O.H.; Hsieh, P.C.; et al. Artificial Intelligence in Abdominal, Gynecological, Obstetric, Musculoskeletal, Vascular and Interventional Ultrasound. Ultrasound Med. Biol. 2025, 51, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.A.; Kallenbach, M.; Radu, I.P.; Berzigotti, A.; Dietrich, C.F.; Kather, J.N.; Luedde, T.; Seraphin, T.P. Artificial Intelligence for Contrast-Enhanced Ultrasound of the Liver: A Systematic Review. Digestion 2025, 106, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Okagawa, Y.; Abe, S.; Yamada, M.; Oda, I.; Saito, Y. Artificial Intelligence in Endoscopy. Dig. Dis. Sci. 2022, 67, 1553–1572. [Google Scholar] [CrossRef] [PubMed]

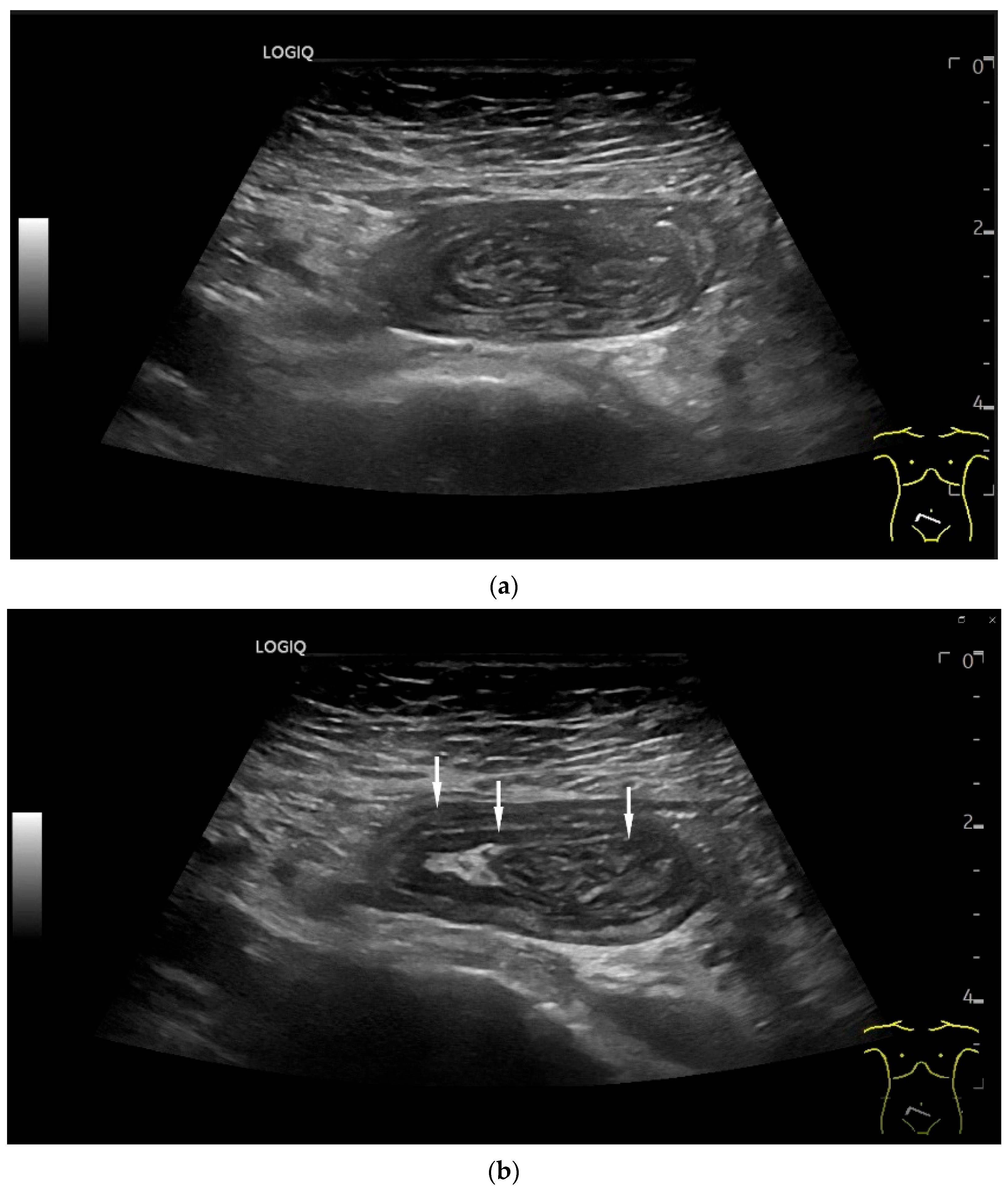
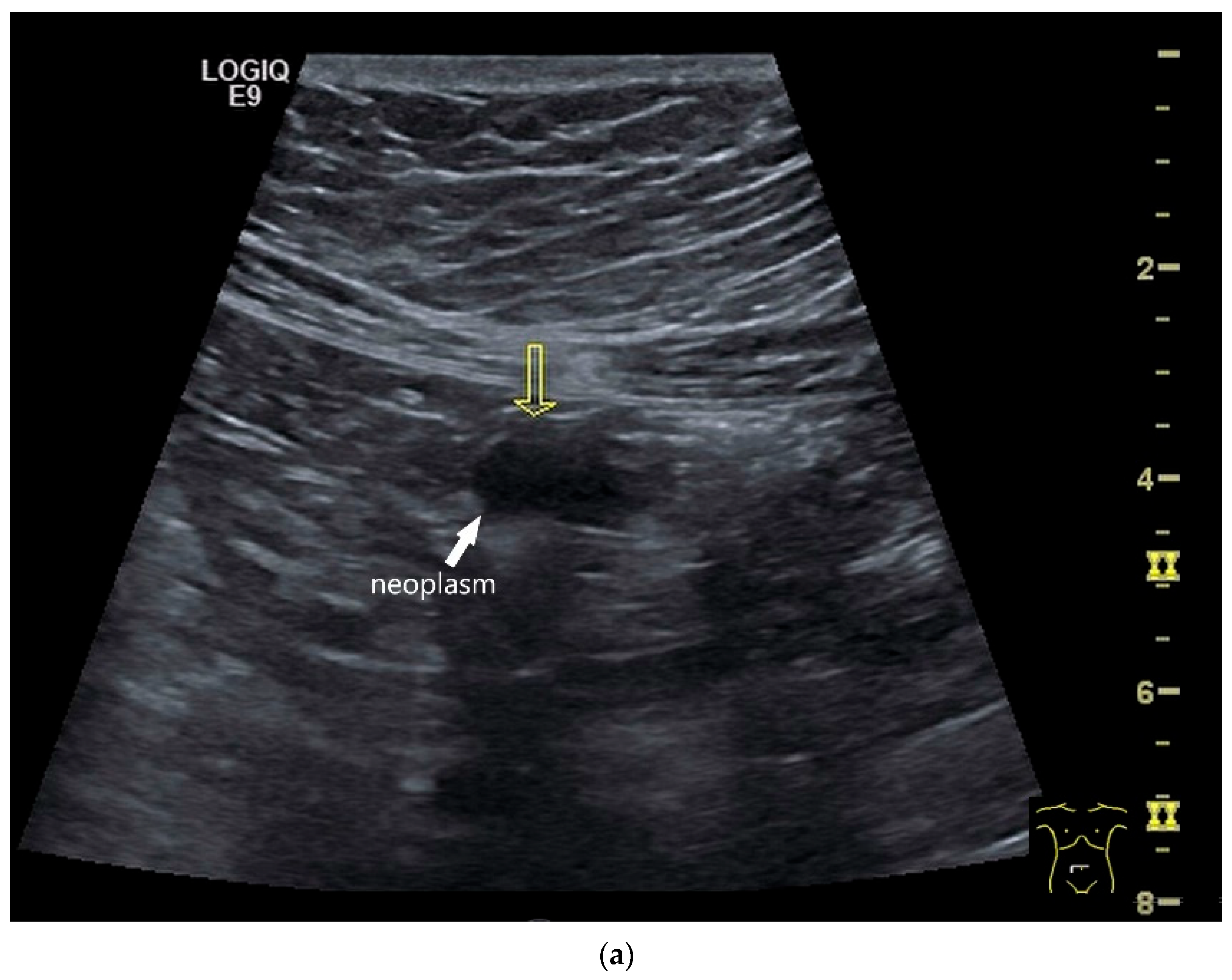
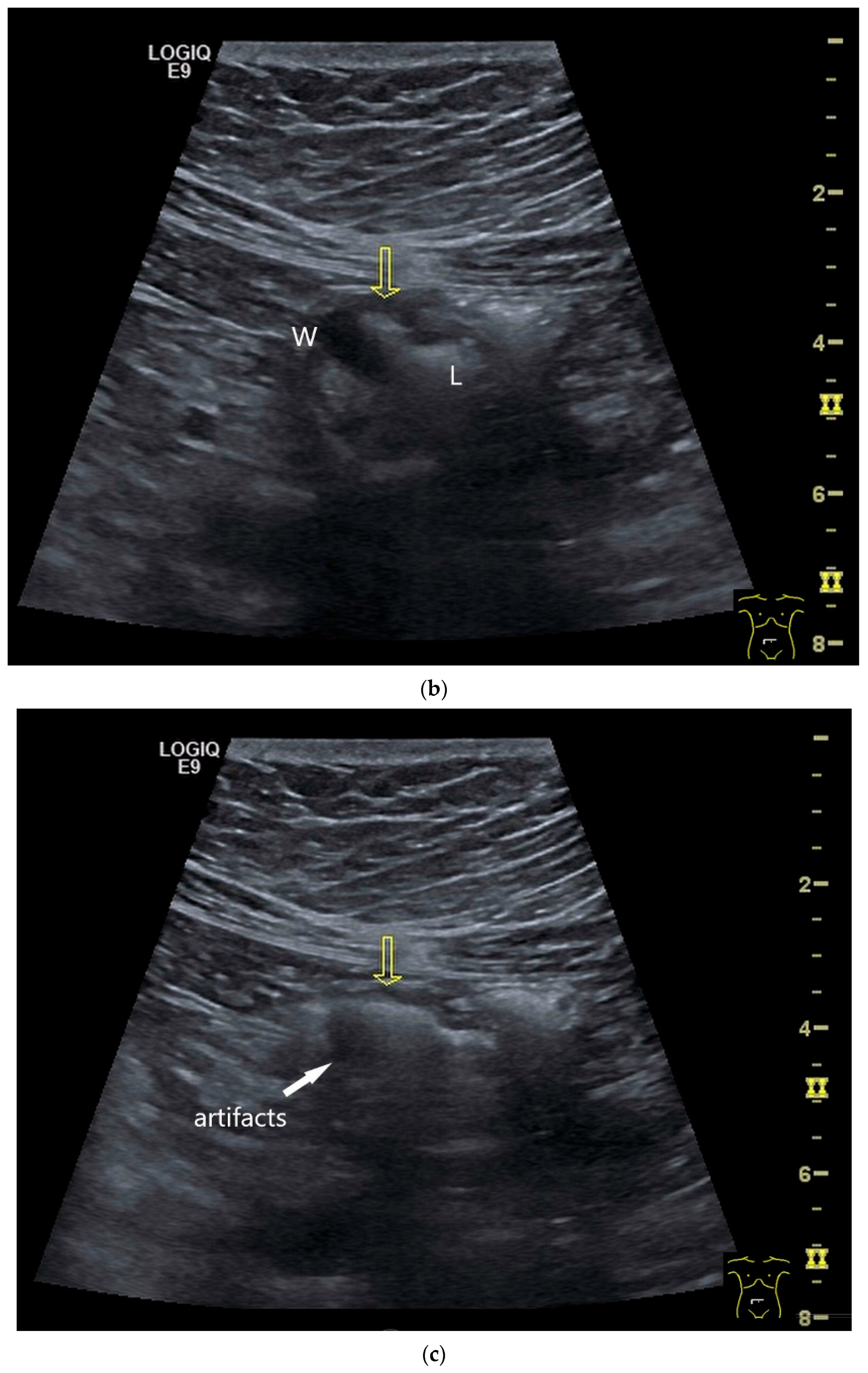

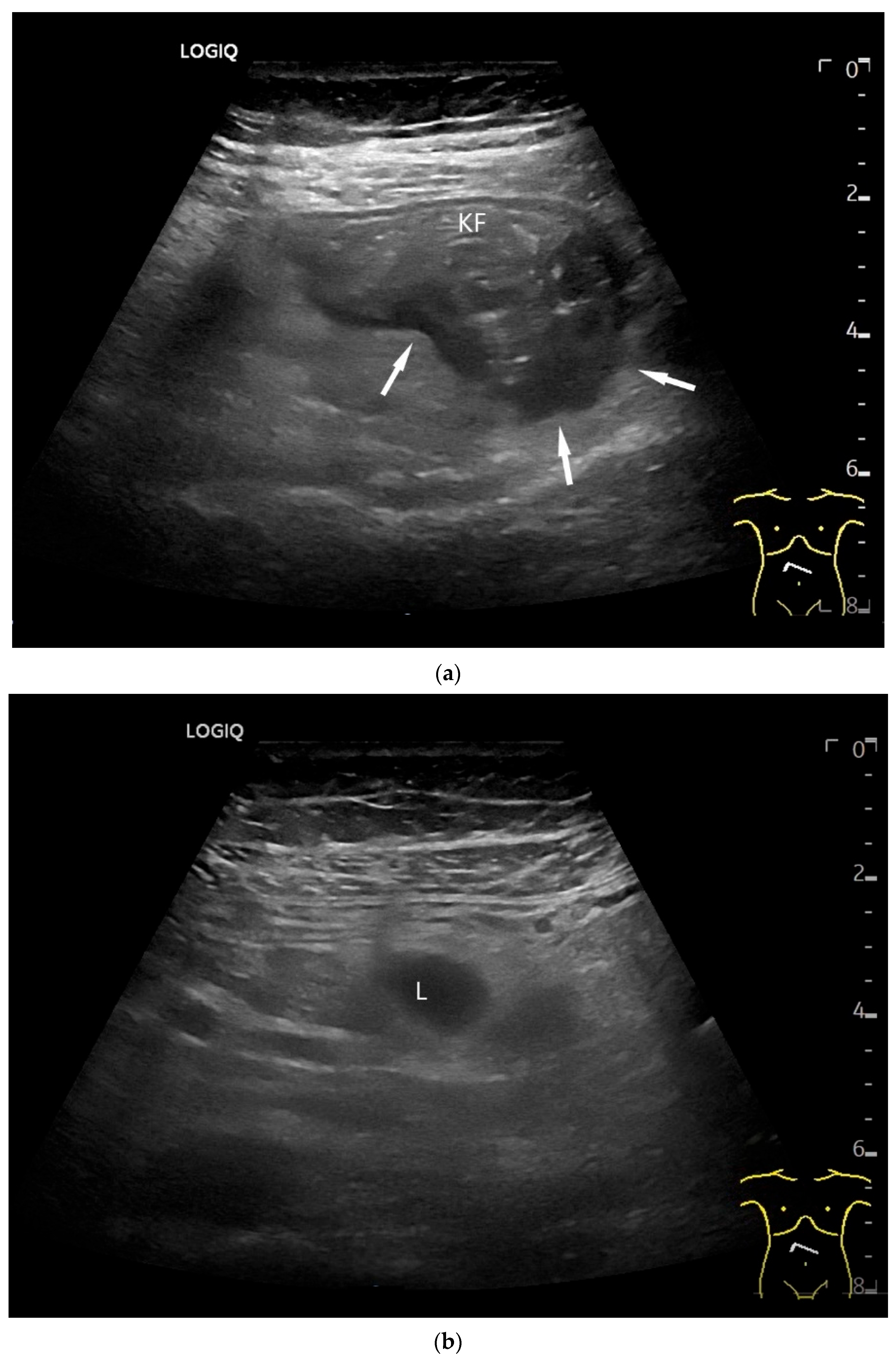
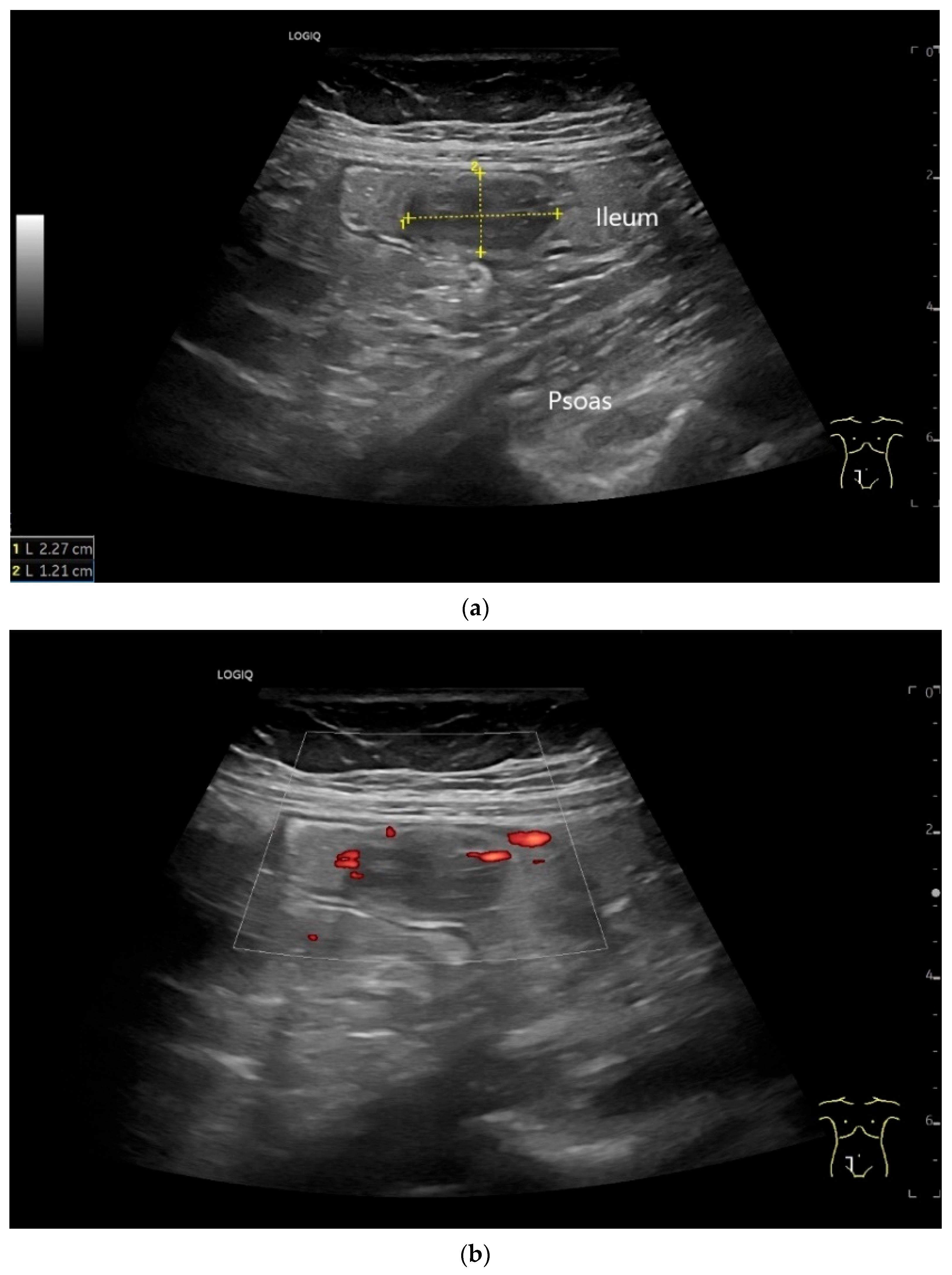
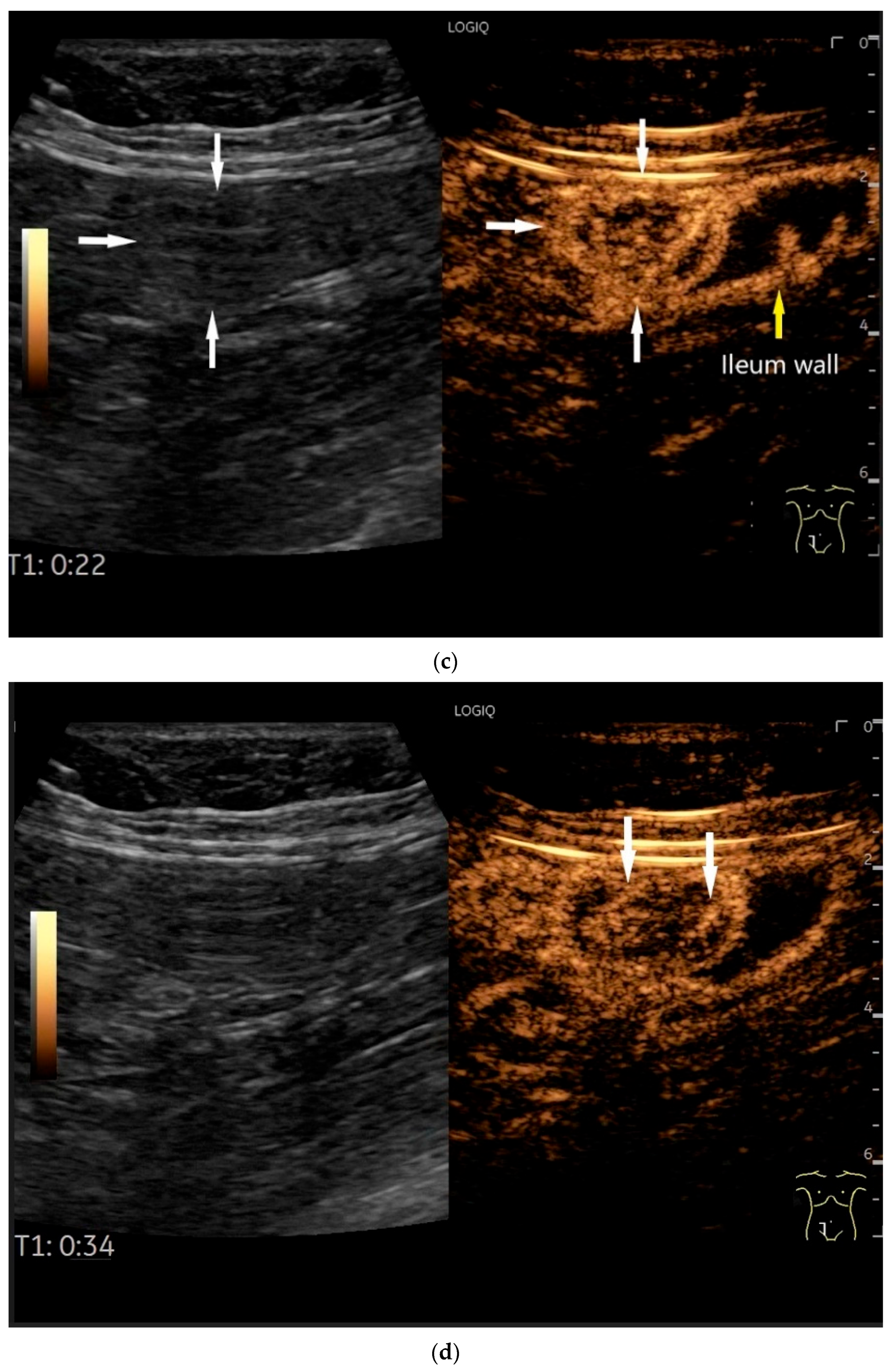
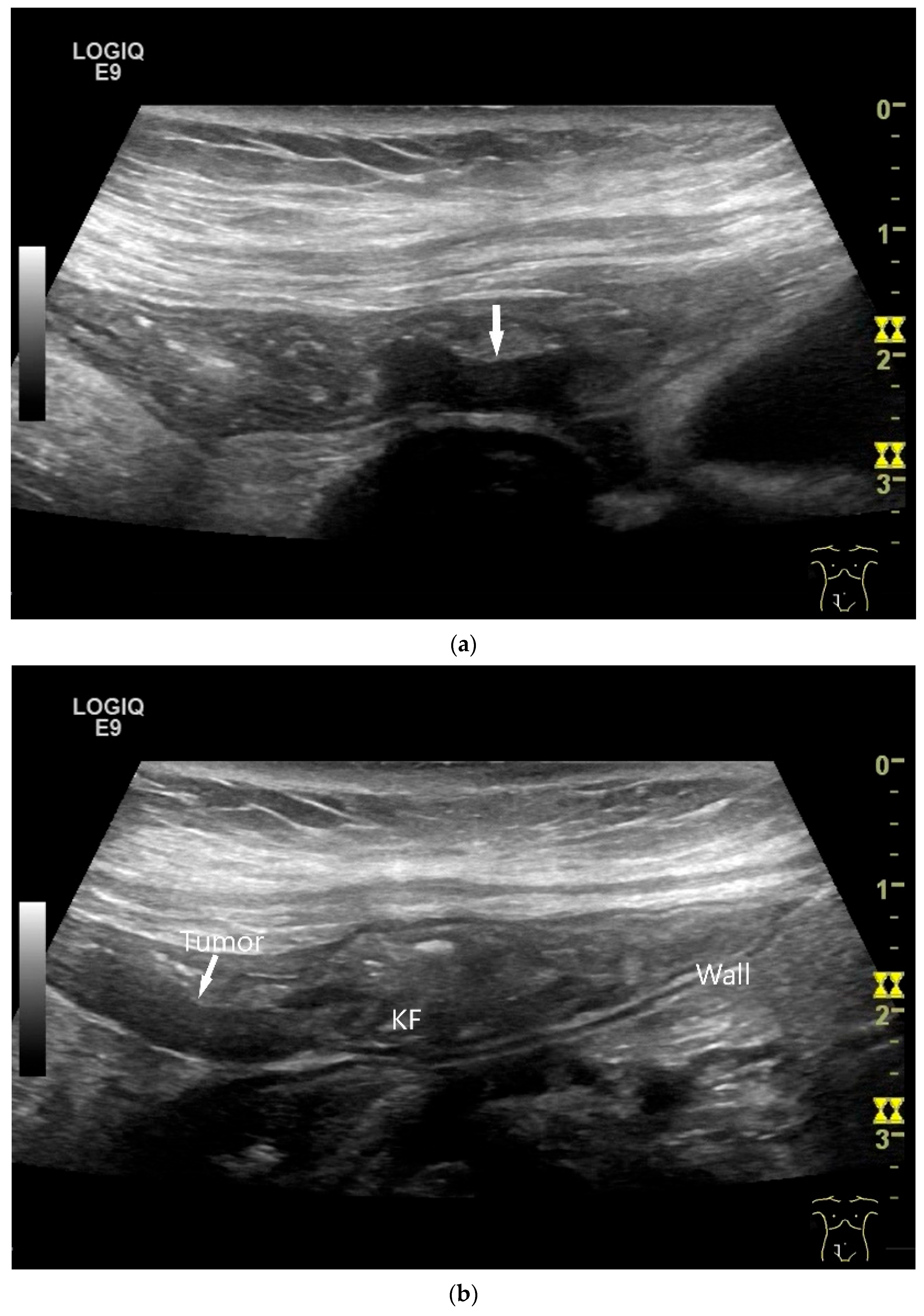
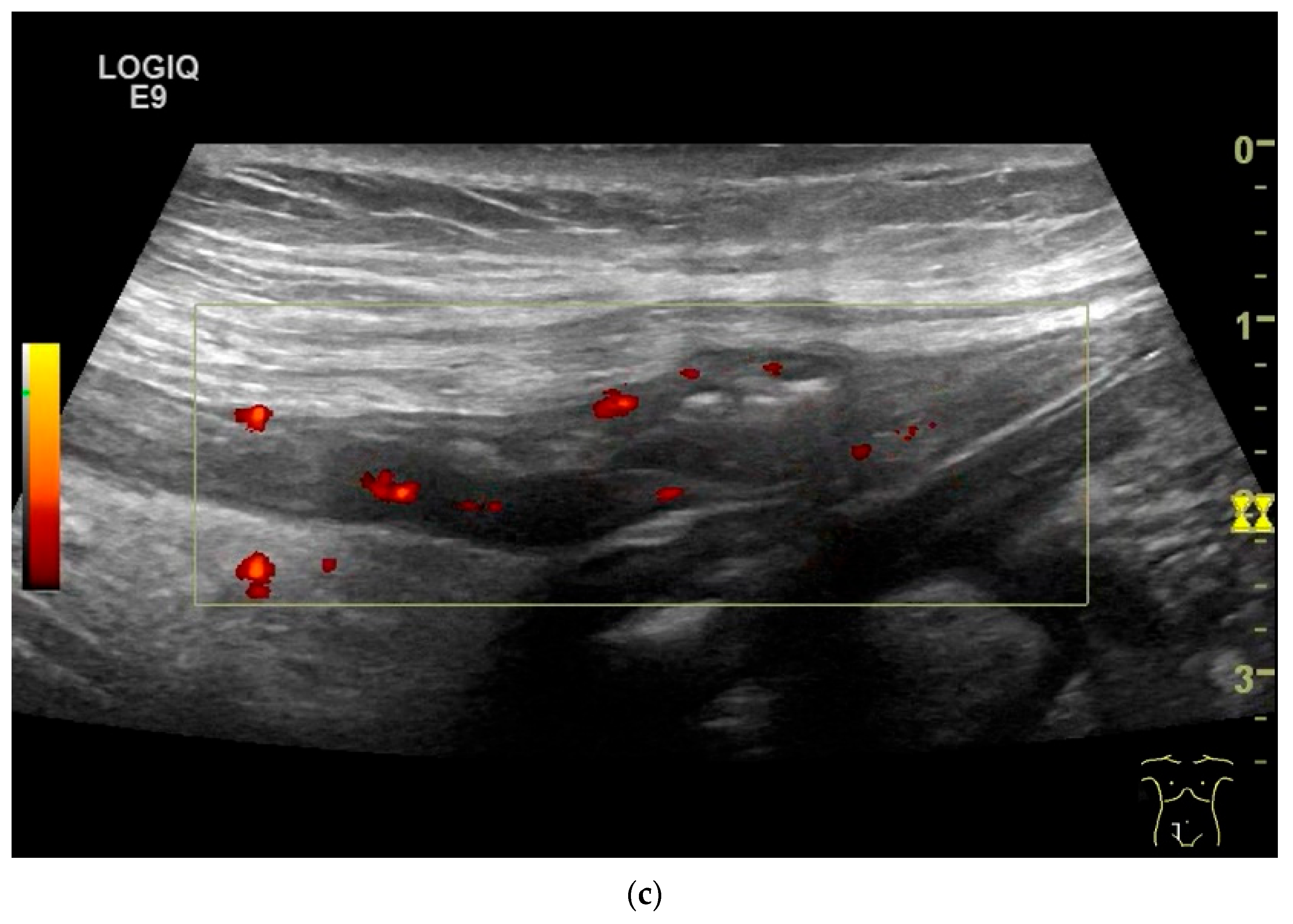
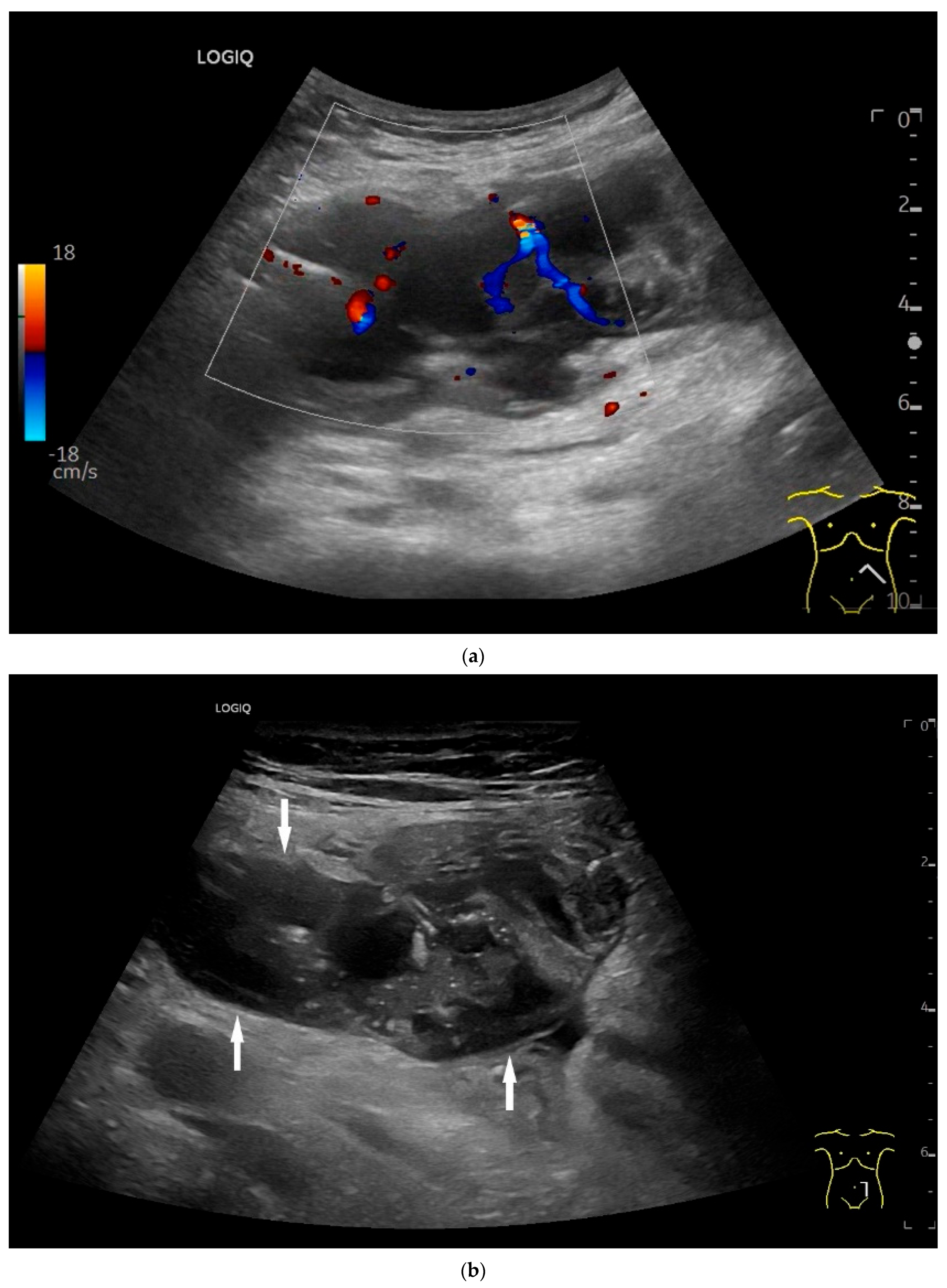
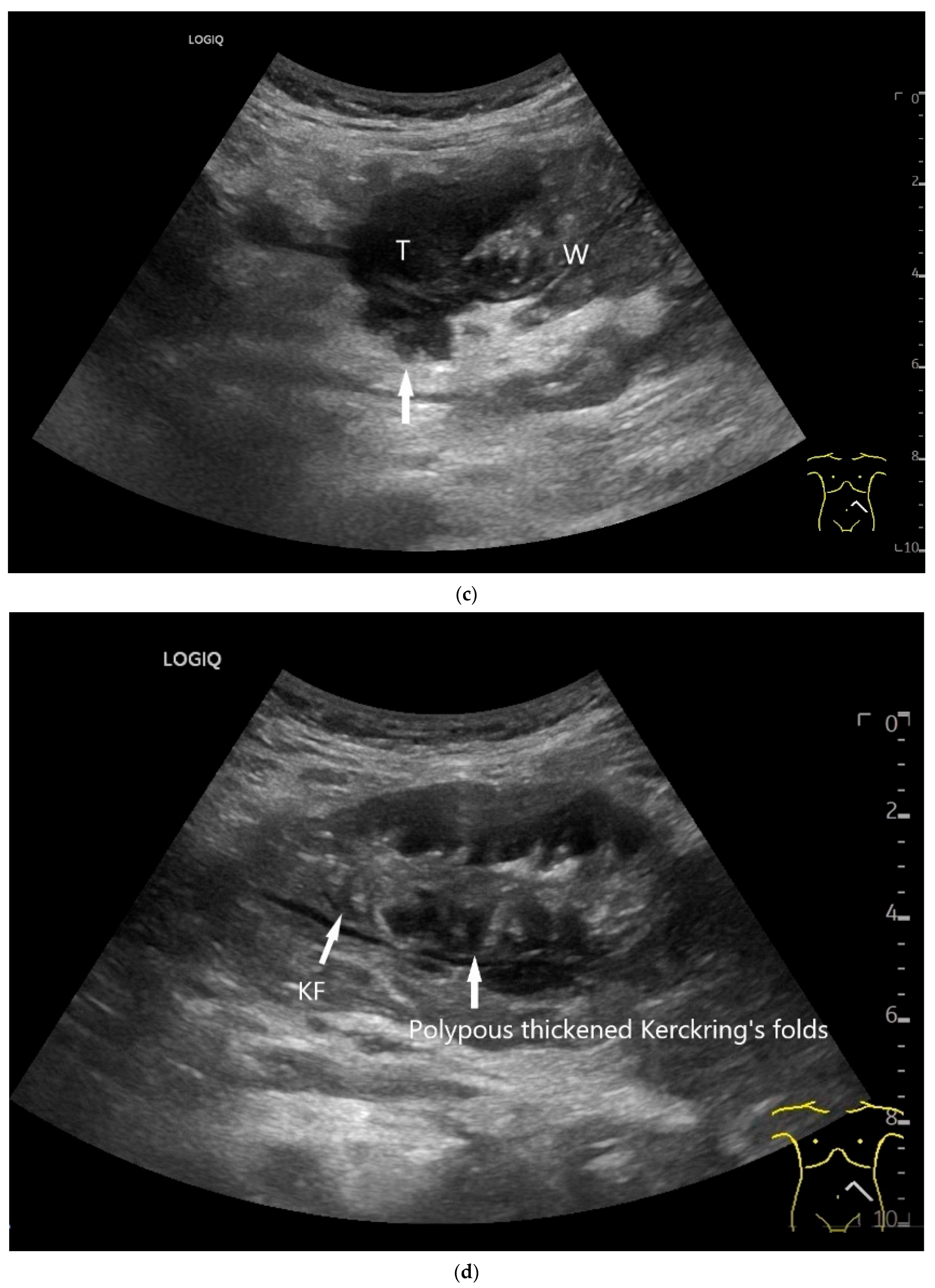
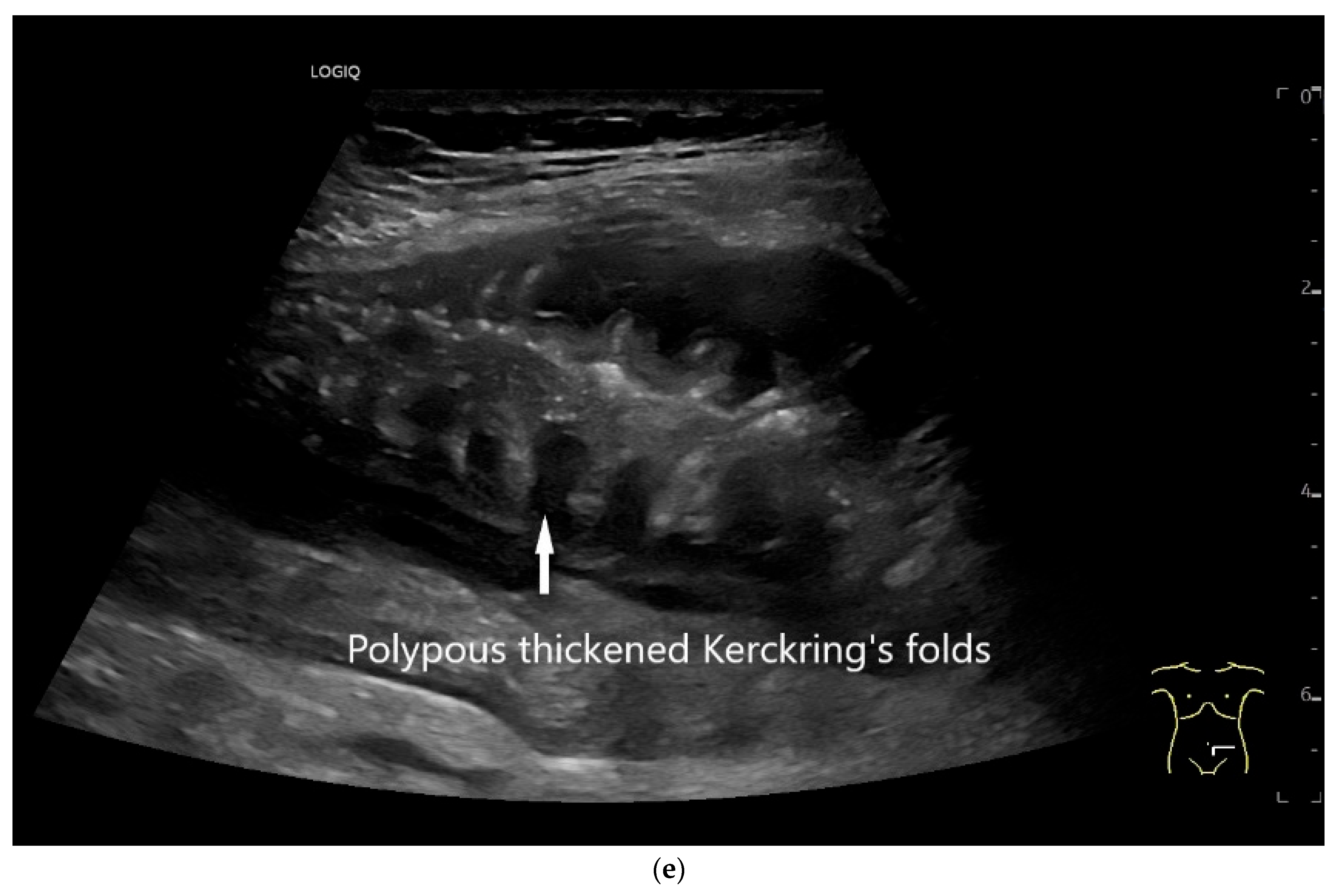


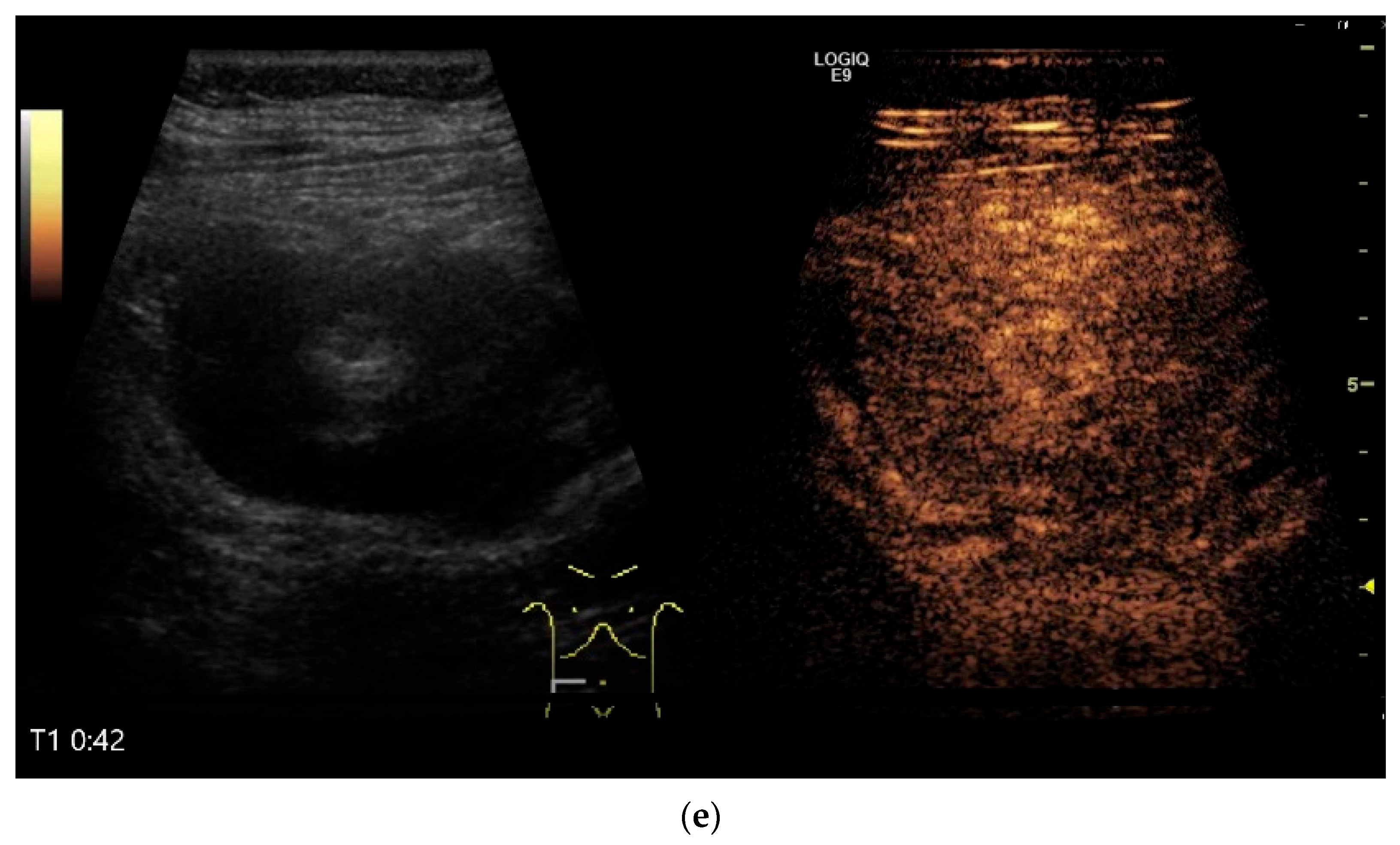


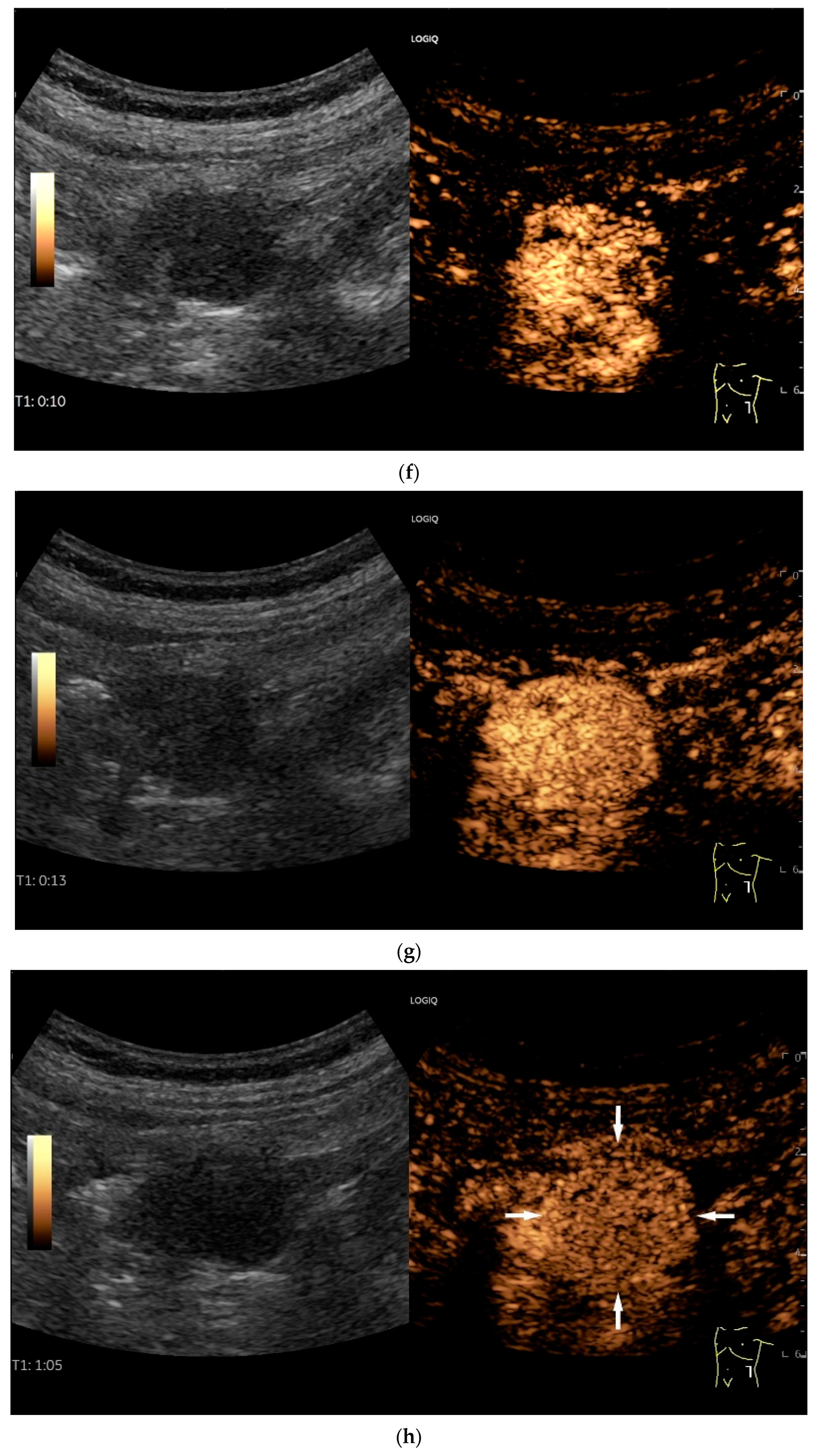
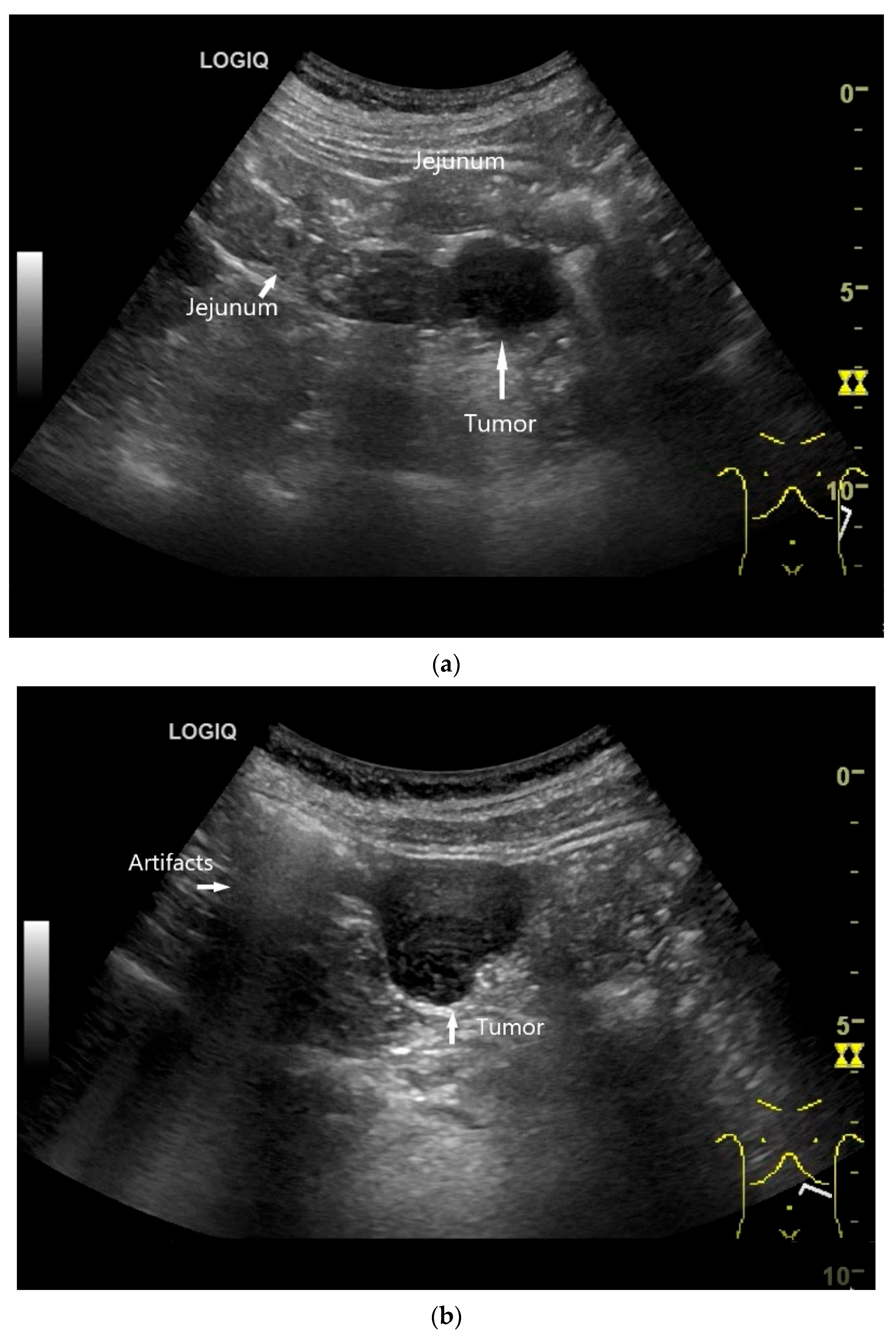


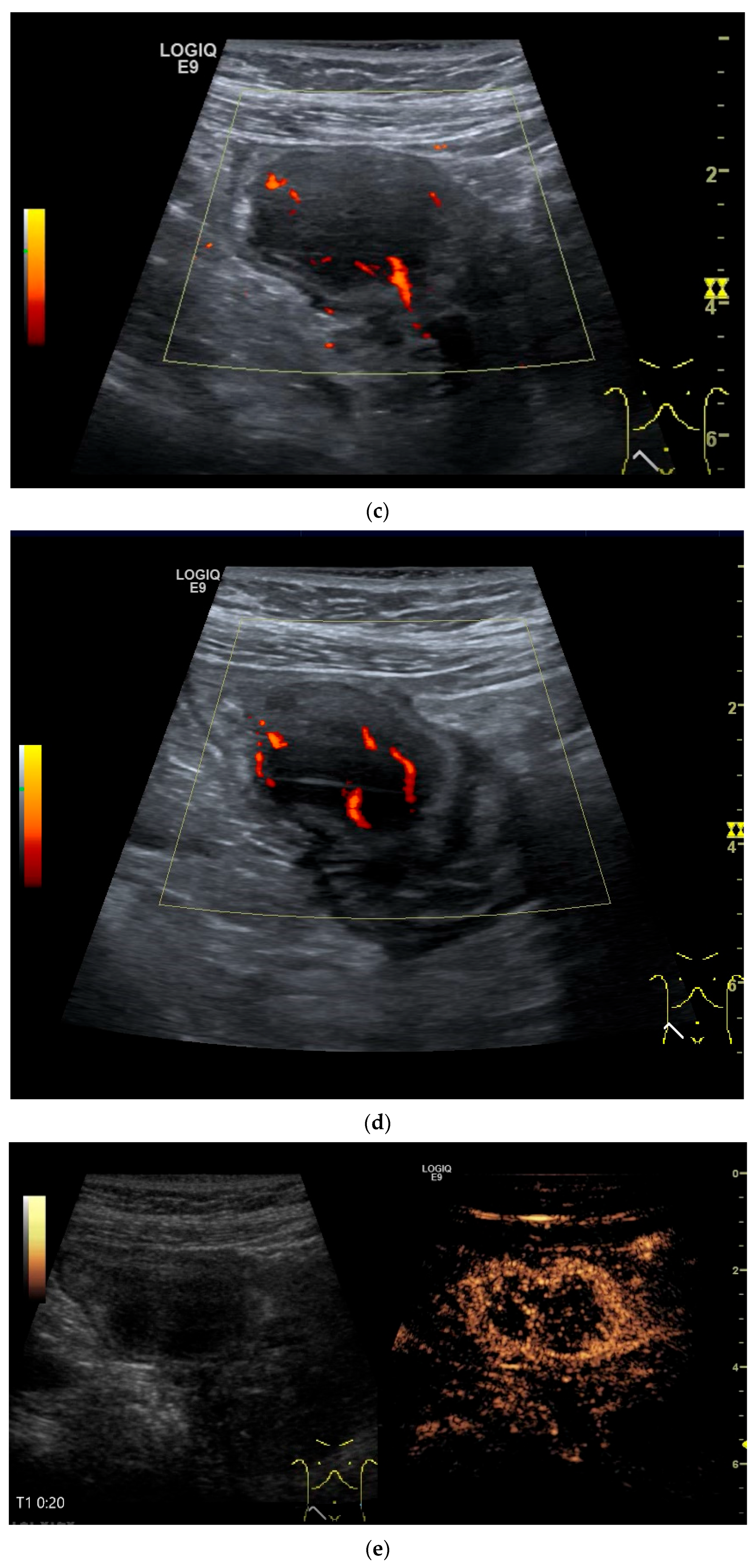
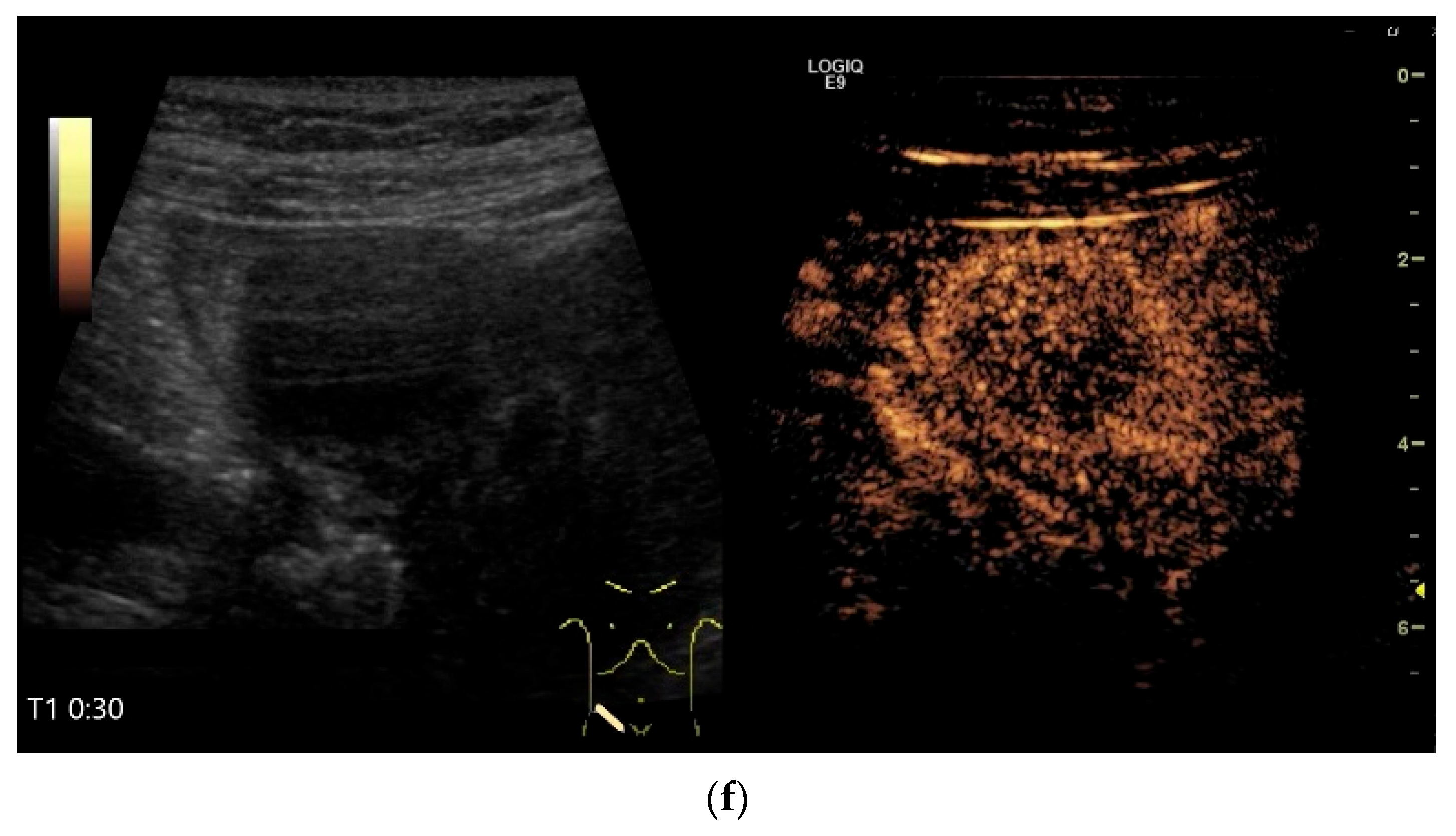
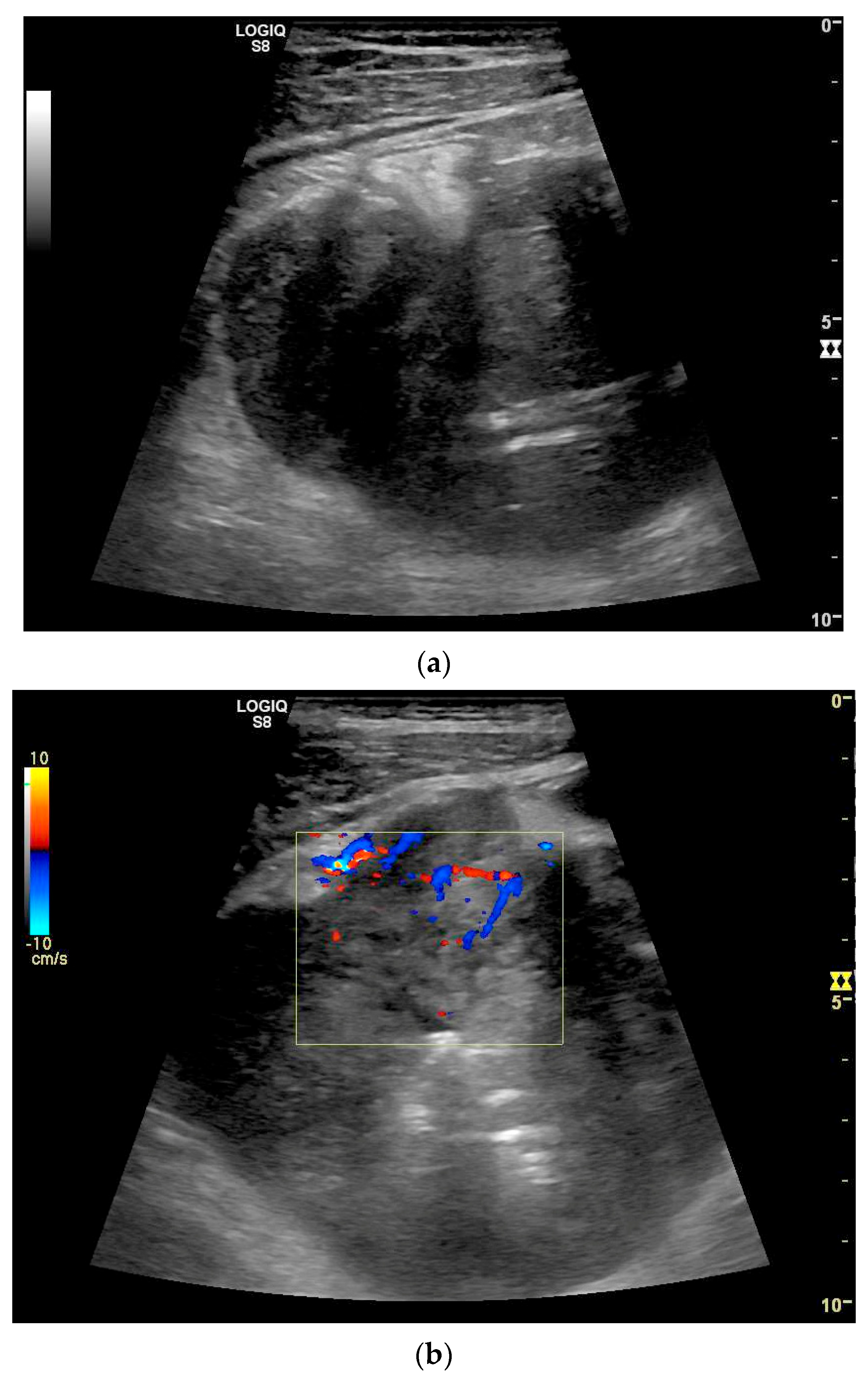
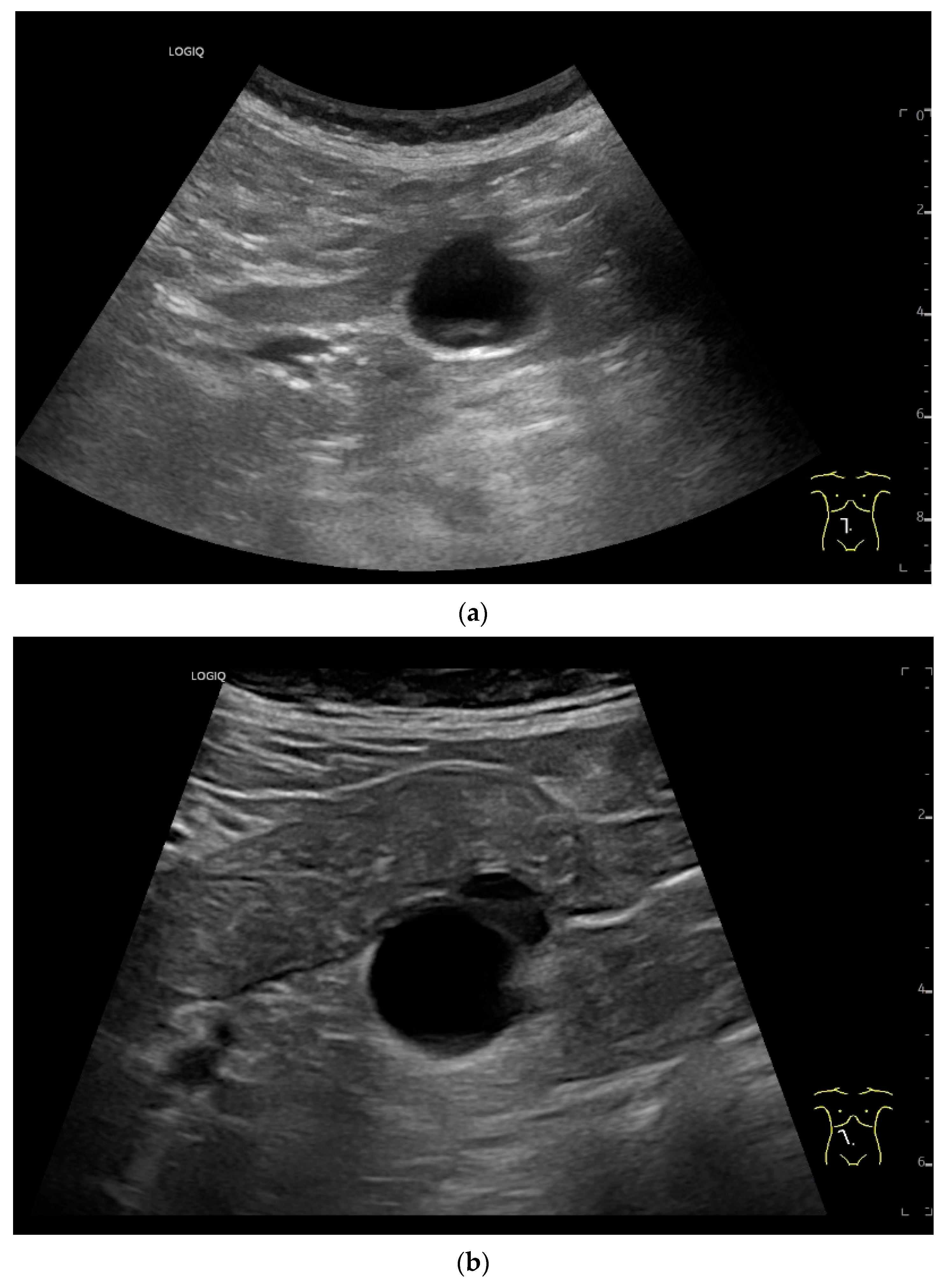
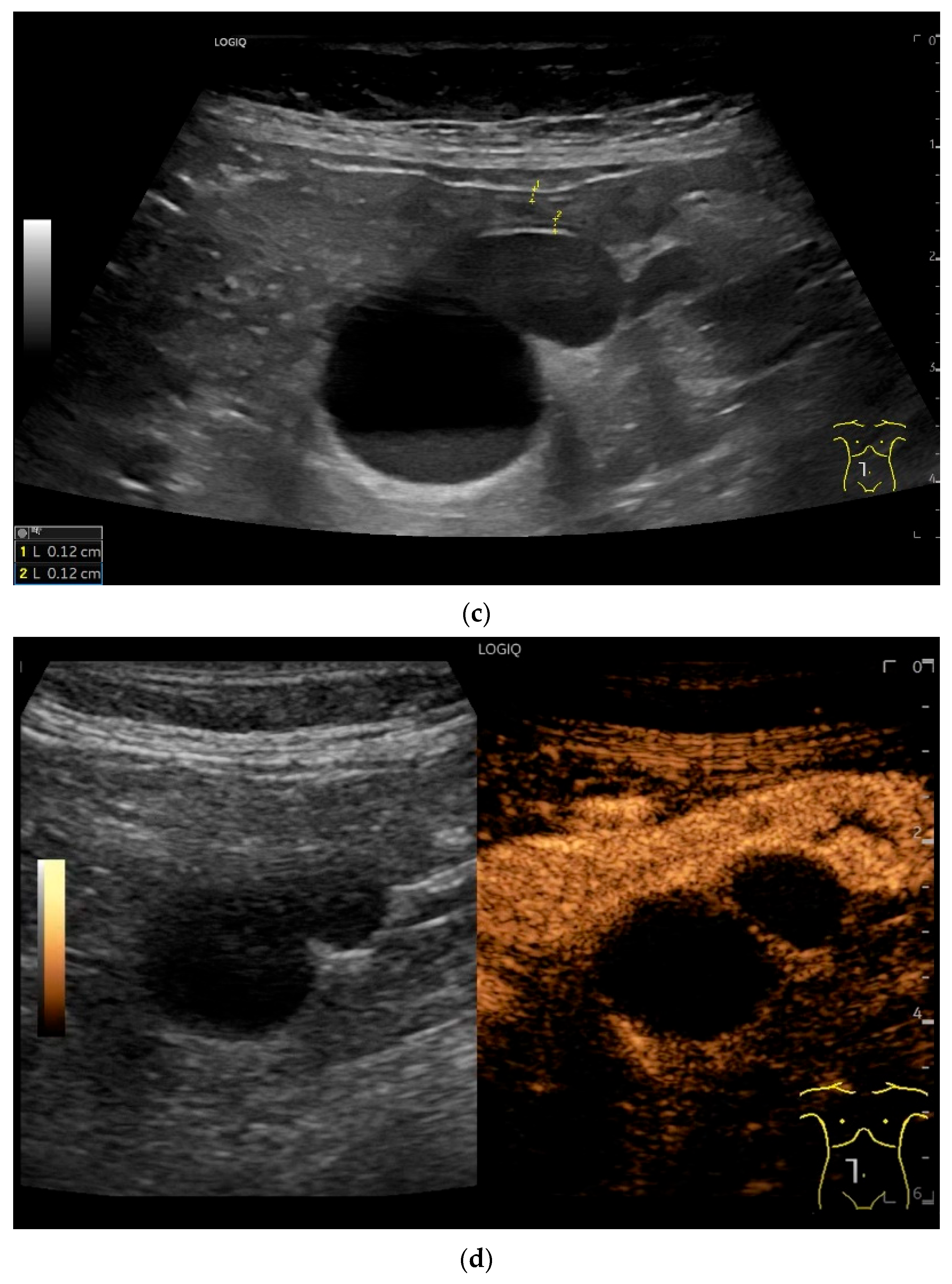
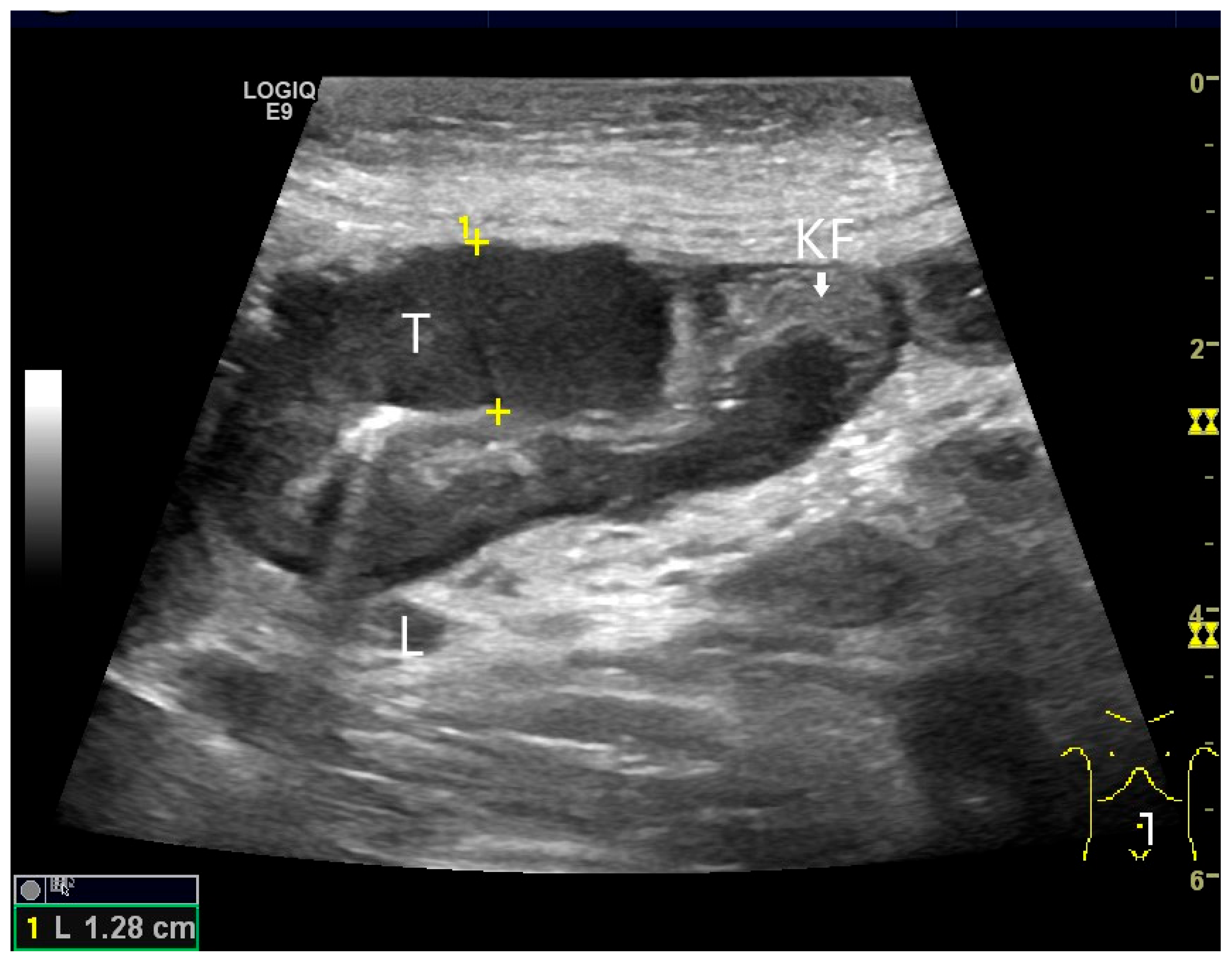
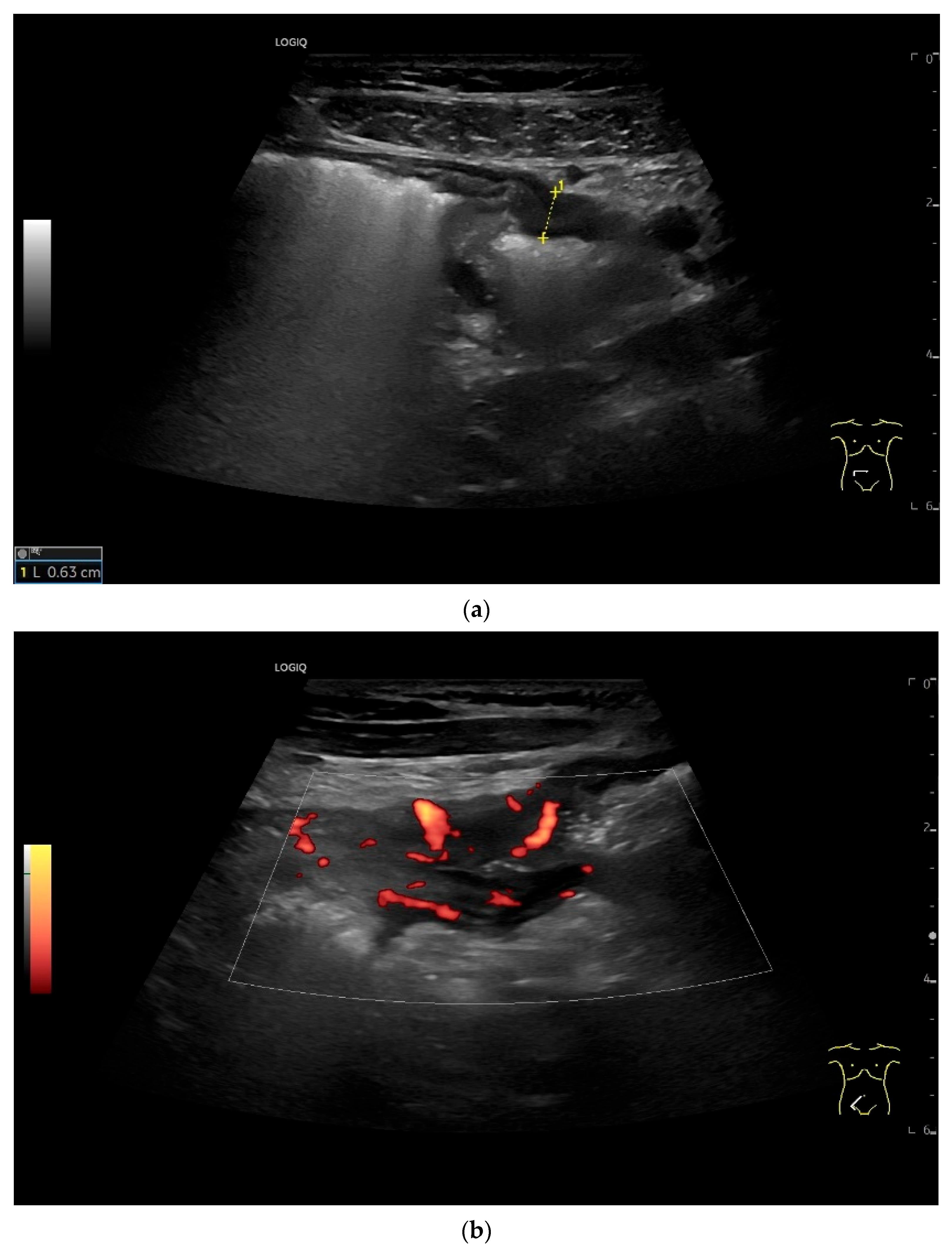
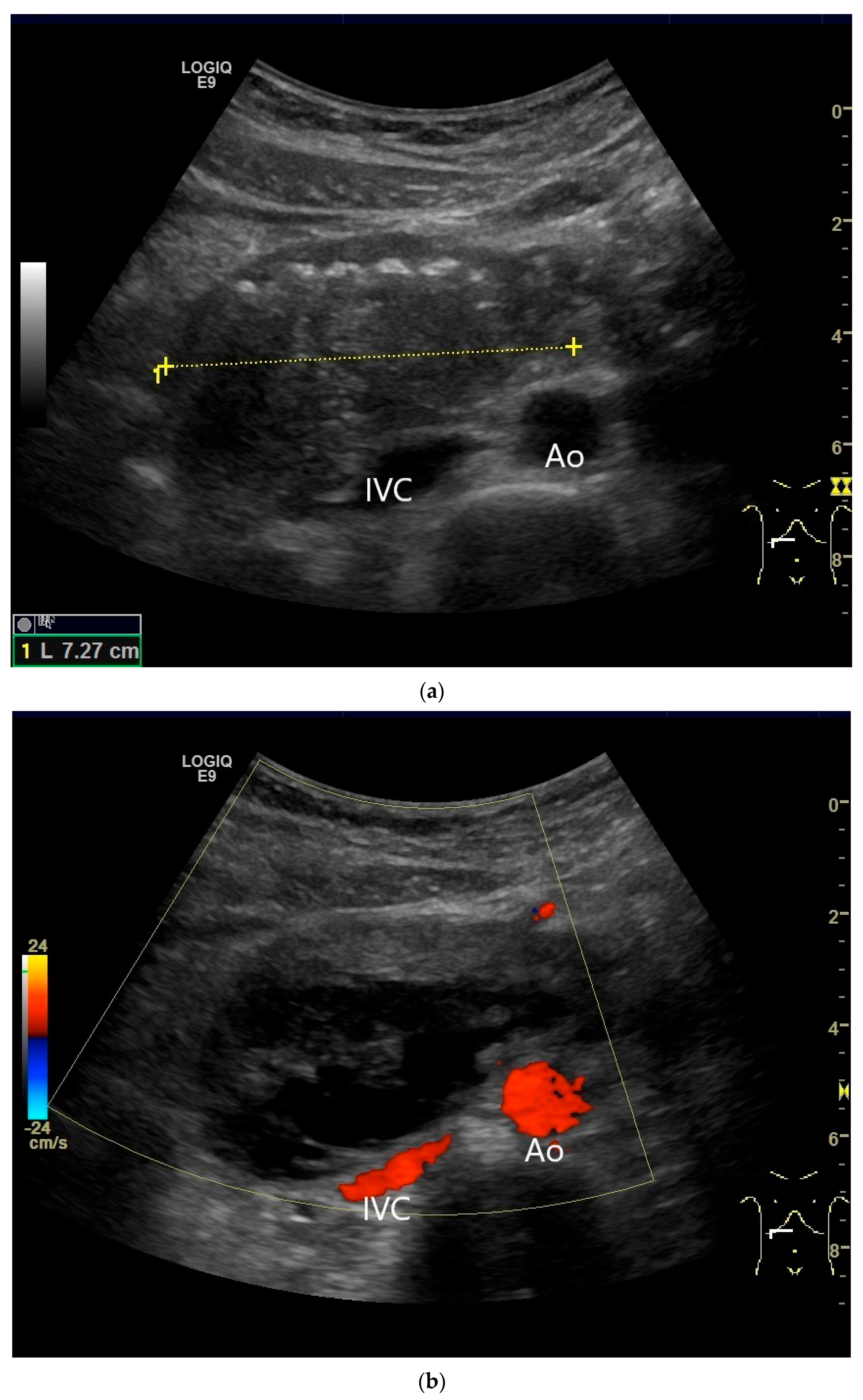



| Fukumoto et al. 2009 [41] | Fujita et al. 2015 [42] | |
|---|---|---|
| Number of small intestine tumors | 87 | 97 |
| Mean tumor size | 20.0 mm (range 1–150 mm) | N/A |
| Transducer | 3.5 MHz and 7.0 MHz | 3.5 MHz curved and 7.5 MHz linear array transducers |
| Sensitivity of US | 26.4% | 50.5% |
| Specificity of US | 98.6% | 100% |
| PPV | N/A | 100% |
| NPV | N/A | 90.9% |
| Detection rate | ||
| All lesions | 25% | 52.6% |
| Lesions < 20 mm | 1.8% | 14.3% |
| Lesions > 20 mm | 59.5% | 91.7% |
| Factors favoring detection |
|
|
| Factors favoring non-detection |
|
|
| Detection according to shape | ||
| Granular lateral spreading | 0% (0/24) | 42.8% (3/7) |
| Flat elevated | 33.3% (1/3) | N/A |
| Polypoid | 50.0% (4/8) | 22.0% (4/41) |
| Submucosal | 16.6% (4/42) | 47.1% (8/17)) |
| Partial ulcerative | 33.3% (2/6) | 92.3% (12/13) |
| Circumferential ulcerative | 100% (9/9) | 100% (19/19) |
| Method | Strengths | Weaknesses/Limitations | Sensitivity/ Specificity |
|---|---|---|---|
| US | Performable at any time, widely available, can be performed bedside. Dynamic and real-time examination allowing for detection of pathological peristalsis phenomena. Highest spatial and temporal resolution of all cross-sectional imaging modalities using high-frequency transducers, allowing delineation of the bowel wall layering and vascularization as well as assessment of the surrounding structures, Combination with CEUS to differentiate between nonvascularized and vascularized lesions and to delineate necrosis. Percutaneous US-guided sampling is possible if a histological diagnosis is required. | Detection depends on the size and shape of the small intestine tumor. Considerable examiner experience is required. Patient limitations include obesity, air overlap, and artifacts-causing from bowel contents. Deep-seated findings may be obscured and less visible. Systematic examination of the small bowel is time-consuming. | 26.4%/98.6% [41] 53.1%/100%/PPV 100%/NPV 90.9% [42] |
| CT | Also a primary examination, readily available, not time-consuming. Allows excellent overview and multiplanar reconstruction. Can be used as an acute examination. Contrast enhancement allows assessment of vascularity | Restrictions due to elevated renal retention parameters. Radiation exposure. Limited spatial resolution. Limited soft-tissue contrast. | Sensitivity 87.7%/N/A [36] |
| CT enterography | High detection rate. Imaging of the intestinal wall and surrounding area. | Filling of the small intestine with contrast medium required. Limitations in elevated renal retention parameters. High radiation exposure. | 75.9%/94.8% Accuracy 88.0% [38] |
| MR enterography | High detection rate. Imaging of the intestinal wall and surrounding area. No radiation exposure. | Filling of the small intestine with contrast medium required. Limited availability, time-consuming and relatively costly. | 92.6%/99.0% Accuracy 96.7% [38] |
| VCE | Indicated in particular for the search for causative intestinal lesions in obscure bleeding | Bowel preparation/laxative measures, exclusion of stenosis by means of US or CT/CT-/MR enterography to reduce the risk of capsule retention. Subepithelial lesions can be overlooked, and external impressions can be misinterpreted. Rapid peristaltic waves can lead to forced capsule passage in the area of lesions. Bowel contents and blood impair the assessment. | Lesions in patients with suspicion on small intestine tumors: Compared to MRI 86%/98%, Accuracy 97% [43] |
| DAET | The only endoscopic modality which allows to examine the lumen and mucosa of the small intestine and to take forceps biopsies for histological evaluation of tumors | Not the entire small intestine is possible to be examined. Peroral (prograde) access or peranal (retrograde) access often have to be combined. Time-consuming, costly, limited availability. Laxative measures for bowel cleansing. High level of examination expertise required. | Based on surgery results, the accuracy rates of prograde and retrograde DAET for locating small intestinal tumors (adenocarcinoma, GIST and lymphoma) were 94.4%, 100% and 100%, respectively [28] |
| Lymphoma | Most Common Localization in the Gastrointestinal Tract | Appearance |
|---|---|---|
| B-Cell-Lymphoma | ||
| Diffuse large B-cell lymphoma | Any part of the digestive tract, most commonly stomach and ileocecal region. | Often solitary, infiltrating tumor mass with ulceration of the mucosa. Mostly transmural infiltration. |
| Extranodal marginal zone lymphoma | At any site of the digestive system, most common stomach and small intestine. | Arising in mucosal or glandular tissues. Thickening of the mucosa. Mass-forming lesions occur. |
| Follicular lymphoma | Most often in the small intestine and colon. | Multiple nodular/polypoid lesions and elevated white patches |
| Duodenal-type follicular lymphoma | Most commonly in the second portion of the duodenum; commonly simultaneous involvement of the jejunum or ileum. | Located in the mucosa/submucosa and often result in a polypoid architecture. Nodular white submucosal deposits or white villus enlargement. Mostly without lymph node involvement. |
| Mantle cell lymphoma | Lymph nodes and any site of gastrointestinal tract. | Subclinical tumor cell infiltration of the gastrointestinal tract is possible, superficial ulcers, large tumor masses and diffuse thickening of the gastrointestinal mucosa. Presentation as multiple intestinal polyps (multiple lymphomatous polyposis). |
| Burkitt lymphoma | In the gastrointestinal tract the ileocecal region, stomach and small intestine are the most common. | Bulky disease and high tumor burden. |
| Plasmablastic lymphoma | Most commonly in the oral cavity of HIV-positive patients. The digestive tract is affected in 20% of cases, including the small intestine. | Thickening of the intestinal wall, multiple tumor nodules in the intestinal wall and mesentery of the small intestine and surrounding organs. |
| Posttransplant lymphoproliferative disorders (PTLD) | A spectrum of abnormal lymphatic hyperplasia to open neoplasia that occurs after organ transplantation. Often associated with Epstein-Barr virus (EBV) infection, but this is not mandatory. Most frequent in the distal jejunum and ileum. | Wall thickening and dilatation, eccentric mass, luminal ulceration, and short-segment intussusceptions |
| T-Cell-Lymphoma | ||
| Enteropathy-associated T-cell lymphoma | Jejunum is most often involved, but other segments of the small intestine, and less commonly the stomach and colon, may be affected. | Often multifocal, with circumferential ulcers, ulcerated nodules, plaques and strictures. The mesentery and mesenteric lymph nodes may be involved. |
| Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) | Mostly ulcerated masses in the small intestine or (more rarely) in the large intestine, duodenum or stomach. A small subset of patients may be present with multifocal gastrointestinal lesions. In addition to the mesenteric lymph nodes, manifestations in the liver, lungs and brain are possible. | Multiple elevated and/or ulcerated lesions. |
| Intestinal T-cell lymphoma NOS (ITCL) | The colon and the small intestine are the organs most frequently affected. Dissemination to regional lymph nodes and extra gastrointestinal sites is not uncommon. | Often an ulcerated, plaque-like appearance; sometimes protruding luminal masses. |
| Indolent T-cell lymphoproliferative disorder of the gastrointestinal tract | Mostly small intestine or colon. However, all sites of GI may be involved. | The affected mucosa is thickened, with conspicuous folds or nodules. In some tumors, the infiltrate gives rise to intestinal polyps that resemble lymphatic polyposis. |
| Extranodal NK/T-cell lymphoma, nasal-type | The gastrointestinal tract is the third most common site after the nasal cavity and the skin. Predominantly in the small and large intestine. | Gastrointestinal tract manifestations with tumor infiltration, mass formation, often accompanied by necrosis, ulceration, and/or perforation. |
| Mesenchymal Tumor | Most Common Localization in the GIT | Appearance |
|---|---|---|
| Benign | ||
| Inflammatory fibroid polyp | Most commonly in the stomach, followed by the ileum, but can develop throughout the GI tract. | Usually found in the submucosa but can also be localized in the mucosa. Ileal tumors are often larger. |
| Hemangioma | The small intestine is the most common location for hemangiomas and vascular malformations. Otherwise, they can occur anywhere. | Gastrointestinal tract hemangiomas grow either polypoid and intraluminal or are diffusely infiltrating submucosal lesions. They are purplish red to blue, soft and compressible. |
| Lipoma | Found in any part of the gastrointestinal tract. Rare in the small intestine and there predominantly located in the ileum. | Smoothly bordered oval/round lipomatous lesions, usually originating in the submucosa. Yellowish shimmering impressions on endoscopy, soft on contact. |
| Leiomyoma | Predominantly in the esophagus, colon and rectum and rare in the stomach and small intestine. | Smoothly bordered intramural tumors of different sizes. |
| Schwannoma | Mostly found in the stomach, very rare in other gastrointestinal areas including the small intestine. | Well-defined lesions originating in the submucosa and lamina muscularis propria. Solid and cystic parts. |
| Lymphangioma/lymphangiomatosis | Most commonly in the small intestine, followed by the colon and esophagus. | Either small (<2 cm) polypoid white or yellow mucosal lesions, usually discovered as incidentalomas, or larger masses with transmural spread or origin in the mesenterial adipose tissue. |
| PEComa/Angiomyolipoma | Rare tumors with occurrence in the colon, liver, small intestine, and pancreas. | Circumscribed unencapsulated masses may extend into the adjacent mesenteric tissue. Tumor size ranges from <1 cm to >20 cm. |
| Granular cell tumor | Most common in esophagus, colon and perianal region, very rare in the small intestine. | Small lesions of 0.1–3.0 cm, yellowish shimmering through. |
| Perineurinoma/benign fibroblastic polyp | May occur in the colon, only very rarely in the small intestine or stomach. | Small (0.2–0.6 cm) colonic polyps. More rarely non-polypoid lesions in the submucosa. |
| Intermediate | ||
| Inflammatory myofibroblastic tumor | Most commonly located in the small intestine and colon. | Different sizes. The submucosa, the muscularis propria or the mesentery may be affected. |
| Desmoid fibromatosis | Most commonly in the mesentery of the small bowel, followed by the mesentery of the ileocolic region and the mesocolon. | Tends to be a large mass. |
| Solitary fibrous tumor | At any anatomical location including the small bowel mesentery. | Well circumscribed, unencapsulated, may become very large. |
| Kaposi sarcoma | It can manifest at any location within the gastrointestinal tract, most common in the stomach and duodenum. | Usually affects the mucosa at several localizations, reddish-blue or brown flat lesions, luminal polypoid nodules or ulcerating and hemorrhagic lesions on the mucosa, rarely as a transmural mass. |
| Malignant | ||
| Gastrointestinal stromal tumor | Approximately 30% in the small intestine, 50–70% in the stomach. | Round, hypoechoic lesions with reference to the lamina muscularis propria. |
| Gastrointestinal clear cell sarcoma (CCS)/malignant gastrointestinal neuroectodermal tumor (GNET) | Most common locations are the small intestine, stomach, and colon. | Variable size, polypoid masses, often lobulated, often ulcerations |
| Leiomyosarcoma | Since GISTs have been classified, there have been only a few reports of leiomyosarcomas in the GI tract since 2000. | Polypoid intraluminal tumors or ulcerating, solid or cystic masses |
| Small Intestinal Neoplasm | Appearance on US |
|---|---|
| Local Findings | |
| Adenocarcinoma | Hypoechoic wall thickening with infiltration into the surrounding mesentery depending on the stage. Loss of stratification depending on tumor stage. There is insufficient data to describe vascularization in CDI and CEUS. Focally enlarged (tumor) lymph nodes, distant (tumor) lymph nodes. |
| Neuroendocrine tumor | Small, nodular hypoechoic wall thickenings, mostly in the submucosa with spreading into the other layers. Usually with small vessels on CDI. Regionally enlarged lymph nodes. Multilocular manifestations are possible. |
| Lymphoma | Very pronounced wall thickening with marked hypoechogenicity. Large regional and distant lymph nodes. Look for splenic infiltration. Tumor vessels on CDI and hyperenhancement on CEUS. Heterogeneous hyperechogenicity of the mesentery with walling of the mesenteric vessels. Multiple localizations are possible. |
| GIST | Round hypoechoic masses, homogeneous or heterogeneous depending on size. They usually originate from the muscularis propria, which can be difficult to distinguish in US. Small vessels on CDI, hyperenhancement on CEUS. They move with the small intestine and can change position. |
| Lymphangioma | Anechoic cystic lesions related to the small intestine wall. No vessels on CDI, Nonenhancement on CEUS. They move with the small intestine. |
| Intestinal metastases | Round lesions or wall thickening. Take medical history into account. There is insufficient data to describe vascularization in CDI and CEUS. Assigning them to the small intestinal wall can be difficult. Round intestinal metastases must be differentiated from mesenchymal tumors and enlarged or tumorous lymph nodes. |
| Indirect Signs | |
| Intussusception | The small intestine proximal to the tumor is invaginated. More than five wall layers are seen in an onion-skin shape. |
| Proximal to the tumor | Dilated small bowel lumen, possibly hypertrophy of the muscle layer and hyperperistalsis of this bowel segment |
| Differential Diagnoses | |
| Crohn’s disease | Hypoechoic wall thickening, lumen obstruction, infiltration into surrounding tissue, lymph nodes. Very difficult or impossible to distinguish from inflammatory wall infiltration. Lack of response to anti-inflammatory/immunosuppressive therapy. |
| Tuberculosis | Asymmetric wall thickening with preserved but often blurred stratification. Intramural caseous necrosis/abscesses, inflammation extending beyond the wall. Enlarged hypoechoic mesenteric lymph nodes, ascites with fibrin strands, adhesions, hyperechoic thickening of the omentum and peritoneum; usually terminal ileum, ileocecal valve, and cecum. Patients from endemic areas, with immunosuppression/HIV. |
| Hematoma | Sudden severe pain, following abdominal trauma or anticoagulation therapy. Hypoechoic/nonechoic wall thickening. Lumen obstruction with dilatation of the proximal small intestine. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, K.; Jenssen, C.; Dirks, K.; Hollerweger, A.; Gottschall, H.; Faiss, S.; Dietrich, C.F. Small Intestine Tumors: Diagnostic Role of Multiparametric Ultrasound. Healthcare 2025, 13, 2776. https://doi.org/10.3390/healthcare13212776
Möller K, Jenssen C, Dirks K, Hollerweger A, Gottschall H, Faiss S, Dietrich CF. Small Intestine Tumors: Diagnostic Role of Multiparametric Ultrasound. Healthcare. 2025; 13(21):2776. https://doi.org/10.3390/healthcare13212776
Chicago/Turabian StyleMöller, Kathleen, Christian Jenssen, Klaus Dirks, Alois Hollerweger, Heike Gottschall, Siegbert Faiss, and Christoph F. Dietrich. 2025. "Small Intestine Tumors: Diagnostic Role of Multiparametric Ultrasound" Healthcare 13, no. 21: 2776. https://doi.org/10.3390/healthcare13212776
APA StyleMöller, K., Jenssen, C., Dirks, K., Hollerweger, A., Gottschall, H., Faiss, S., & Dietrich, C. F. (2025). Small Intestine Tumors: Diagnostic Role of Multiparametric Ultrasound. Healthcare, 13(21), 2776. https://doi.org/10.3390/healthcare13212776







