Abstract
Small intestine tumors are rare. The four main groups include adenocarcinomas, neuroendocrine neoplasms (NEN), lymphomas, and mesenchymal tumors. The jejunum and ileum can only be examined endoscopically with device-assisted enteroscopy techniques (DAET), which are indicated only when specific clinical or imaging findings are present. The initial diagnosis of tumors of the small intestine is mostly made using computed tomography (CT). Video capsule endoscopy (VCE), computed tomography (CT) enterography, and magnetic resonance (MR) enterography are also time-consuming and costly modalities. Modern transabdominal gastrointestinal ultrasound (US) with high-resolution transducers is a dynamic examination method that is underrepresented in the diagnosis of small intestine tumors. US can visualize wall thickening, loss of wall stratification, luminal stenosis, and dilatation of proximal small-intestinal segments, as well as associated lymphadenopathy. This review aims to highlight the role and imaging features of ultrasound in the diagnosis of small-intestinal tumors.
1. Introduction
Small intestine tumors are rare worldwide. There are four main groups: adenocarcinomas, neuroendocrine neoplasms, lymphomas and mesenchymal tumors. Tumors in the small intestine are classified according to the WHO 2019 classification [1,2,3,4].
Although the small intestine has a length of 5–6 m, the global incidence of small intestine tumors is generally less than 1.0 per 100,000 and ranges from 0.3 to 2.0 per 100,000 inhabitants [5]. The most common types were neuroendocrine neoplasms (NEN) (33.2%), followed by adenocarcinomas (30.1%), lymphomas (16.2%), and GIST (7.1%). The remaining 13.5% were a collection of various other entities. The most common tumor in the duodenum was adenocarcinoma (52.8%). Adenocarcinoma was also the most common tumor in the jejunum (38.9%), followed by lymphomas (21.8%), NEN (12.4%), and GIST (9.3%). All other tumor entities accounted for 17.6%. NEN were most common in the ileum (62.8%), followed by lymphomas (15.9%), adenocarcinomas (14.7%), and GISTs (9.0%). The other various entities accounted for 9.6% [6].
The incidence of small intestine tumors and in particular of NEN has increased in recent decades [7]. In the United States, the incidence has more than doubled between 1975 (1.1/100,000) and 2018 (2.4/100,000) with a change in the leading entity from adenocarcinoma (1985: 42%; 2005: 33%) to NEN (1985: 28%; 2005: 44%). The proportion of mesenchymal tumors (in particular GIST; approximately 17%) and lymphomas (approximately 7%) remained stable [8]. More recent data show a continuation of these trends [9]. An increased risk of small intestine neoplasms has been observed in patients with Crohn’s disease, celiac disease, familial adenomatous polyposis and Peutz-Jeghers syndrome [1].
The standardized US-examination of the gastrointestinal tract (GIUS) is described in the EFSUMB guidelines and other publications [10,11,12,13]. EFSUMB GIUS guidelines describe the role of GIUS for chronic inflammatory bowel diseases [14], acute gastrointestinal diseases and emergencies [15,16], rare gastrointestinal diseases [17], and functional gastrointestinal disorders [18]. The US diagnosis of small intestinal tumors is described in the EFSUMB guideline on celiac disease and rare diseases [17,19]. Although US is a dynamic basic examination method with multiparametric procedures, the method is underrepresented in the diagnosis and characterization of small intestine tumors.
The following overview focuses on small intestine tumors, the possibilities for diagnosing them using US, and their specific appearance in US.
2. Search Strategy
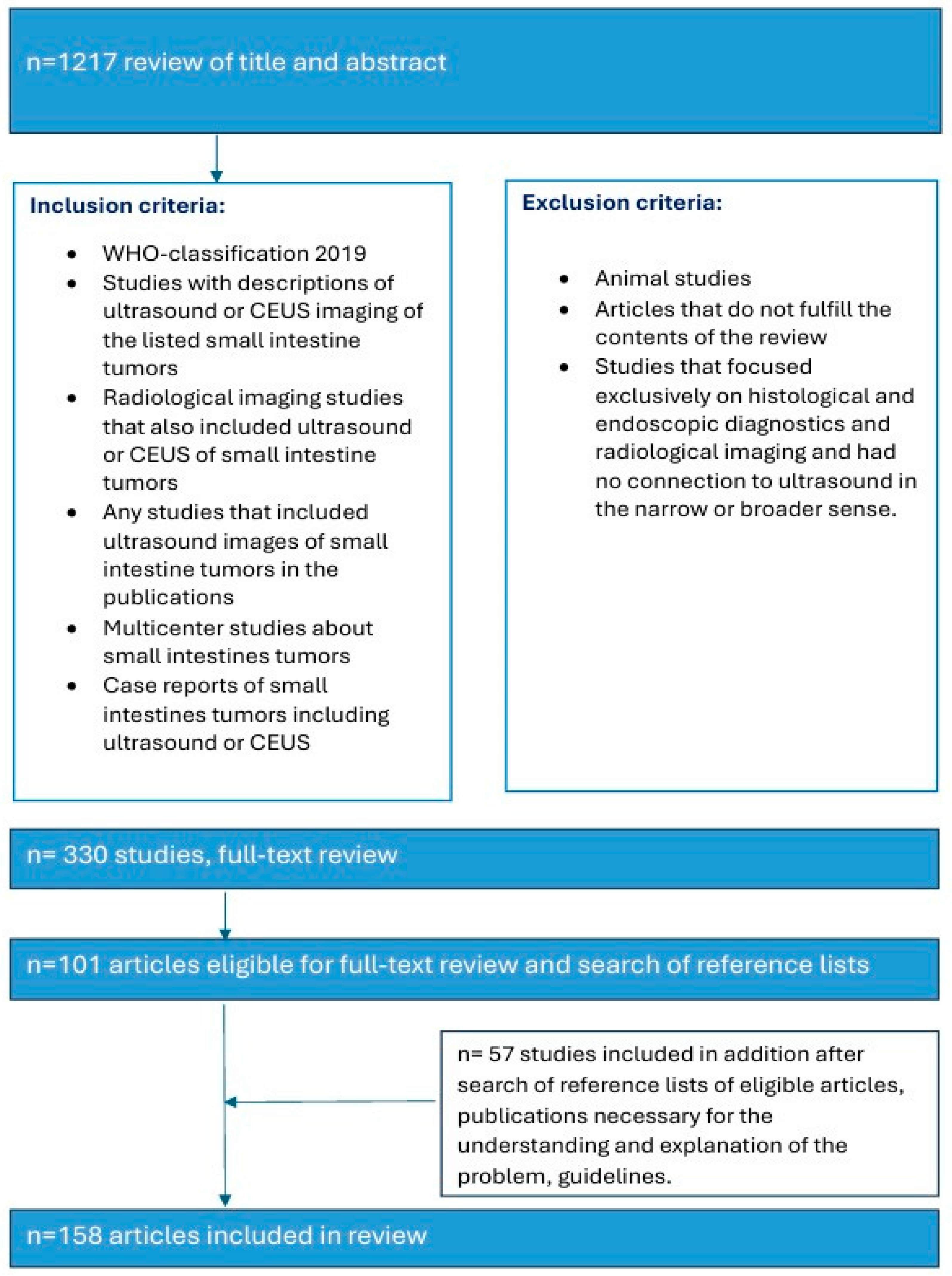
We have based our classification of the various tumors in the small intestine on the 2019 WHO classification. The literature search and analysis were performed specifically for US characteristics of small intestine tumors. PubMed was the database in which entries were searched until 7 June 2025. The keywords and binary operators used are listed in brackets. A total of 3156 titles were reviewed. The abstracts were reviewed, and suitable articles were selected for further analysis. Similar articles on PubMed database were also checked for relevant abstracts: (small intestine adenocarcinoma OR small bowel tumor OR small intestine neuroendocrine tumor OR small intestine lymphoma OR small intestine mesenchymal tumor OR small intestine GIST OR Jejunum GIST OR small intestine leiomyoma OR small intestine Vanek’s tumor OR small intestine inflammatory myofibroblastic tumor OR small intestine lipoma OR secondary small intestine tumors OR small intestine melanoma) AND (Ultrasound OR Ultrasonography OR CEUS) (Figure 1). Further additions were made during the peer review process.
Figure 1.
Search strategy.
3. Symptoms—When Should Small Intestine Tumors Be Considered?
Tumors of the small intestine can be asymptomatic and an incidental finding. When they increase in size and obstruct the bowel lumen, symptoms occur ranging from nonspecific abdominal discomfort to cramp-like pain due to lumen stenosis and even ileus. Just like other gastrointestinal tract tumors, small intestine tumors can manifest with anemia, occult or overt gastrointestinal bleeding. There are few data available on the symptoms leading to diagnosis. In a retrospective analysis of 66 small intestine tumors, 28.8% of cases were discovered by chance. The most common symptom was abdominal pain (44.5%), while 12.1% of cases were presented with hematochezia and 10.6% with anemia [20]. Small bowel intussusception in adults should always be a reason to look for a small intestine tumor distal to the localization of intussusception. It is not uncommon that small intestine tumors cause nonspecific symptoms persisting for several months without diagnostic tests yielding any conclusive findings [21].
4. Imaging the Small Intestines
The role of standard endoscopic examinations is limited: The duodenum until the second portion is examined during every esophago-gastro-duodenoscopy, the terminal ileum during ileocolonoscopy, and the remnant duodenum and the upper jejunum can be viewed during push enteroscopy.
4.1. Device–Assisted Enteroscopy Techniques (DAET)
Device-assisted enteroscopy techniques (DAET), including double-balloon enteroscopy (DBE), single-balloon enteroscopy (SBE), and spiral overtube systems, are well-established methods for evaluating the small intestine [22,23,24]. These are the only endoscopic modalities which allow to examine the lumen and mucosa of the small intestine and to take forceps biopsies for histological evaluation of tumors. Depending on the suspected localization, DAET can be performed perorally or peranally.
In 2017, motorized spiral enteroscopy (MSE) was introduced, showing promise with shorter procedure times, deeper insertion, and higher complete enteroscopy rates than previous methods [25,26,27]. However, in July 2023, the device was withdrawn from the market due to safety concerns after a fatal case of esophageal perforation.
DAET requires laxative measures to prepare a clean intestinal lumen. Single-center studies and a few multicenter studies describe a high diagnostic yield of DAET for the detection and characterization of small intestine tumors [28,29,30,31]. However, the method is time-consuming, and inspection of the entire small intestine is not always possible. Because of its relative invasiveness, less invasive procedures are typically used as the first-line examination of the small intestine. DAET is indicated when findings in the small intestine have been diagnosed in other imaging or VCE examinations and it is advisable to perform a histological examination that is otherwise either not possible or for which primary surgery is not indicated, or for the endoscopic treatment of findings in the small intestine. For example, the treatment of lesions with gastrointestinal bleeding.
4.2. Video Capsule Endoscopy (VCE)
The search for an unclear source of bleeding is a main indication for video capsule endoscopy (VCE). The detection rate for small intestine tumors in VCE was 2.3% in a large study with 5129 patients [32]. In a meta-analysis with 106 small intestine tumors in 691 patients, 18.9% of tumors were missed in VCE [33]. It can be difficult to distinguish subepithelial tumors from extrinsic impressions. Residual staining and intense peristalsis with rapid passage in sections can also be limiting. If occult gastrointestinal bleeding cannot be adequately clarified by gastroscopy, push enteroscopy, ileocolonoscopy, or ultrasound, VCE is indicated. It is important that obstructions are ruled out beforehand, as otherwise capsule retention may occur, requiring surgical removal.
4.3. Endoscopic Ultrasound (EUS)
Mucosal and subepithelial intramural processes in the duodenum can be assessed using EUS. The jejunum and ileum cannot be reached using conventional EUS probes. It is possible to examine protruding impressions in the small intestine lumen using mini probes via DAET [34,35].
4.4. Abdominal CT, CT-/MR Enterography
Abdominal CT is often the first procedure or follows US in the evaluation of abdominal complaints. In a study by Rangiah et al. [36], CT showed a sensitivity of 87.7% for detecting small intestine tumors (specificity, PPV, and NPV) were not specified. In general, CT is a staging examination used to classify tumors before determining appropriate therapy. Furthermore, the possibility of a secondary tumor should always be considered, especially in older patients [37].
For CT or MR enterography, the lumen of the small intestine must be filled with a neutral contrast medium either by a duodenal catheter or by oral contrast uptake of approximately 1–2 L in 1.5–2 h. In addition to intraluminal contrast agents, intravenous iodinated contrast agents are also administered during CT. Gadolinium-based contrast agents are generally used in MRI for the assessment of inflammatory or potentially malignant small bowel processes. Study data seem to indicate that MRI has a higher diagnostic performance than CT in the specific subgroups of malignant small intestine tumors [38]. The overall sensitivity, specificity and accuracy in the diagnosis of small intestine lesions were 75.9%, 94.8% and 88.0% for CT enterography and 92.6%, 99.0% and 96.7% for MR enterography [38]. CT enterography is associated with radiation exposure. A standard CT enterography protocol (without dose reduction procedure) generally leads to an estimated effective radiation dose of 12 to 20 mSv. The use of low-dose techniques leads to an average dose reduction of 53 to 69% (from 12–20 to 5–7 mSv) for single-phase CT enterography examinations [39]. If unclear obstruction symptoms or occult gastrointestinal bleeding cannot be sufficiently clarified by gastroscopy, push enteroscopy, ileocolonoscopy, and US, MR or CT enterography is the next diagnostic step. MRI is preferable because there is no radiation exposure.
4.5. Transabdominal Ultrasound (US)
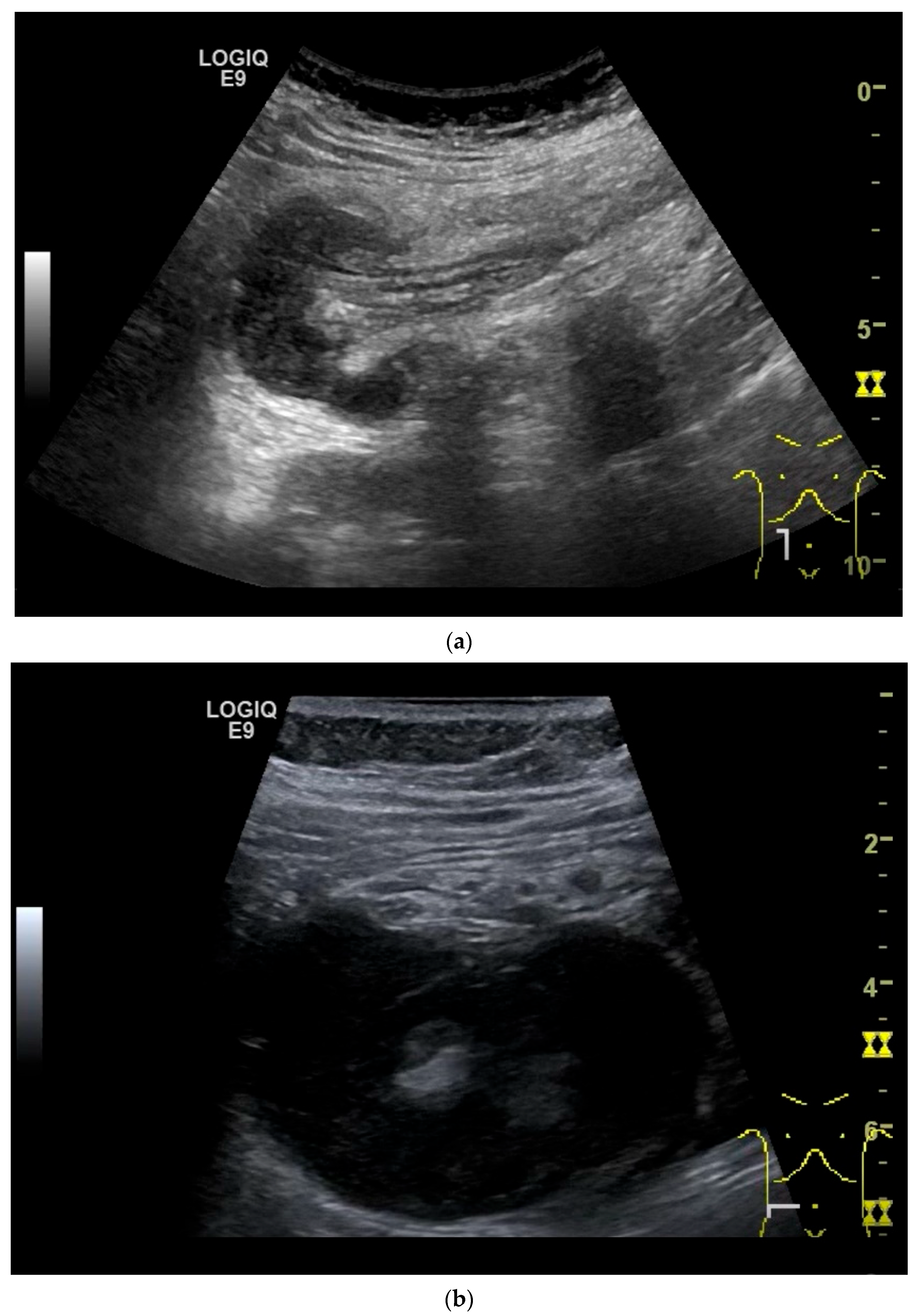
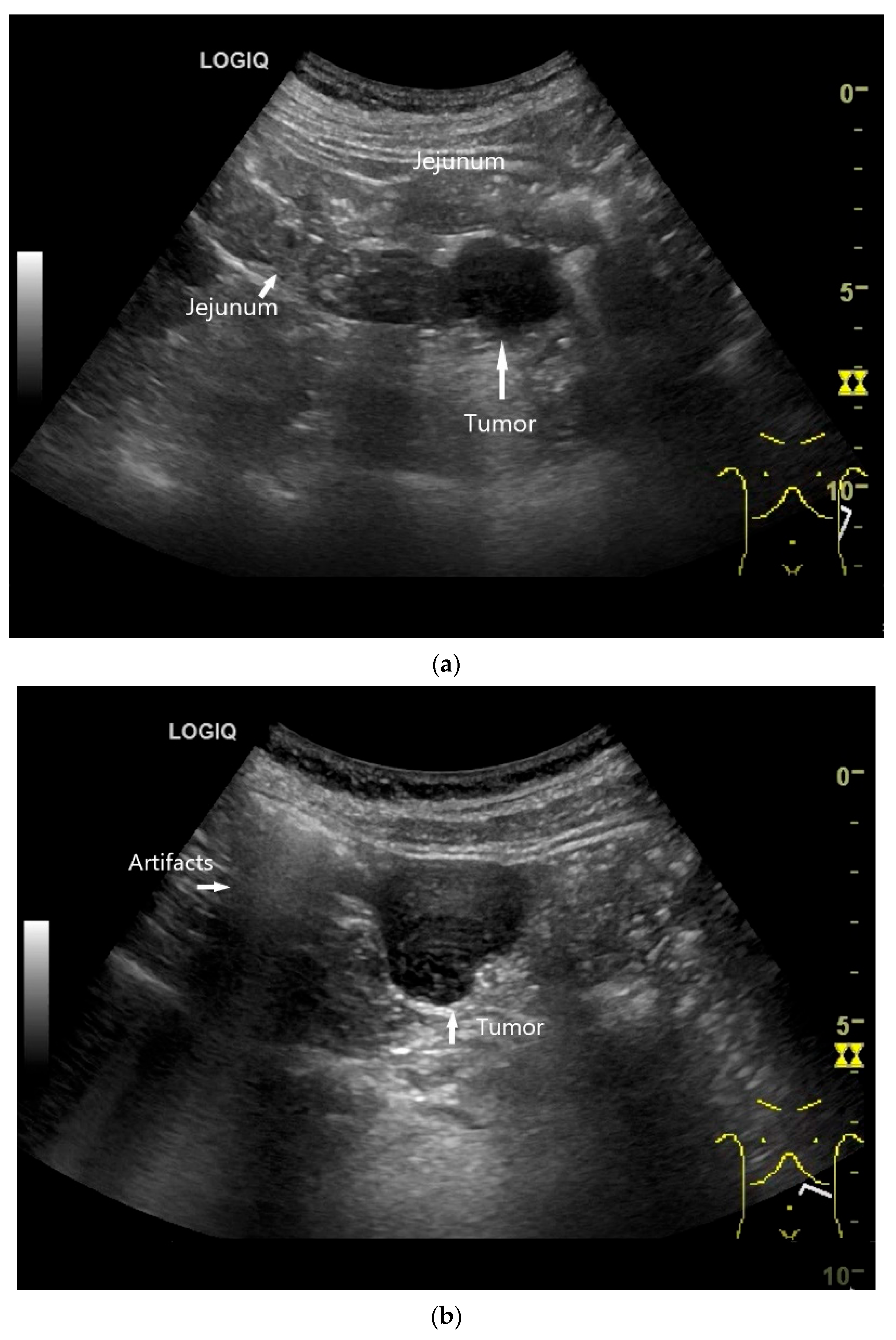
Few publications describe the role of US for the detection and characterization of small intestine tumors. Most cohort studies and registries rely on CT, MRI, or endoscopic findings for initial diagnosis and staging. Case series on small intestine tumors often do not include US, or only a minority of patients undergo US as part of the diagnostic workup. One main reason for underutilization of US is the imaging challenge arising from the fact that the small intestine has a length of 5–6 m and bowel loops lying folded on top of each other. Moreover, the small intestine is mobile, and the position of local findings can vary with intestinal peristalsis. Tumor detection may be limited by small size, deep location, overlying gas, and patient body habits. However, a standardized, systematic approach and the combined use of curvilinear abdominal sector transducers (2–6 MHz) and high-resolution linear transducers (9–12 MHz) for gastrointestinal US may overcome some of these limitations [13]. High-resolution transducers allow visualization of the intestinal wall layers, adjacent mesentery, vessels, and mesenteric lymph nodes. The wall thickness, filling status and peristalsis are assessed with US. The thickness of the wall of small intestine is up to 2 mm. Tumors typically present as segmental wall thickening of the intestinal wall, intraluminal polypoid or nodular masses, or extraluminal masses. Depending on the stage of the tumor, the stratification is usually lost, or individual layers are thickened. The transition to the normal intestinal wall with five layers can be seen at the edge of the tumor. In the area of wall thickening, the lumen reflex is usually narrowed. Luminal obstruction results in dilatation and sometimes hypertrophy of the muscle layer in the proximal intestine. If the small intestine is increasingly filled and shows hyperperistalsis, a pathological intestinal wall finding should be looked for further distally (Figure 2). The localization and ultrasound morphology of the intestinal wall thickening depends on the underlying neoplasia. In adults, intussusceptions can be a concomitant phenomenon of small intestine tumors [40] (Figure 3). Intraluminal gas and intestinal contents can lead to artifacts in US (Figure 4).
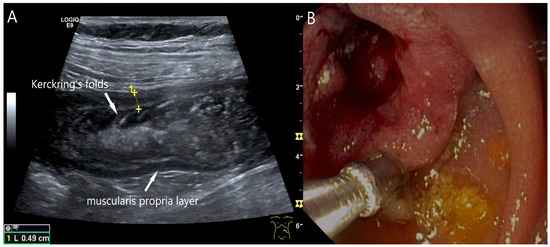
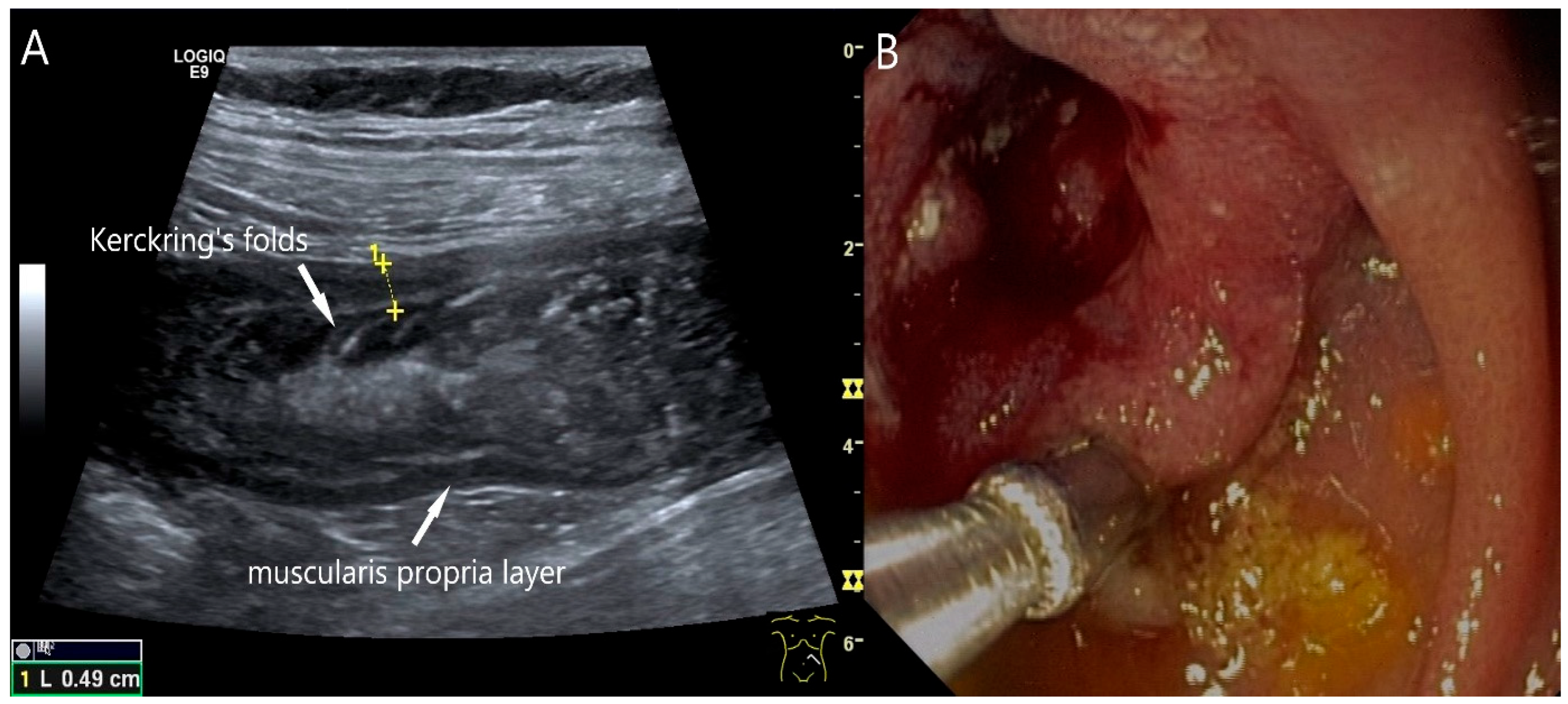
Figure 2.
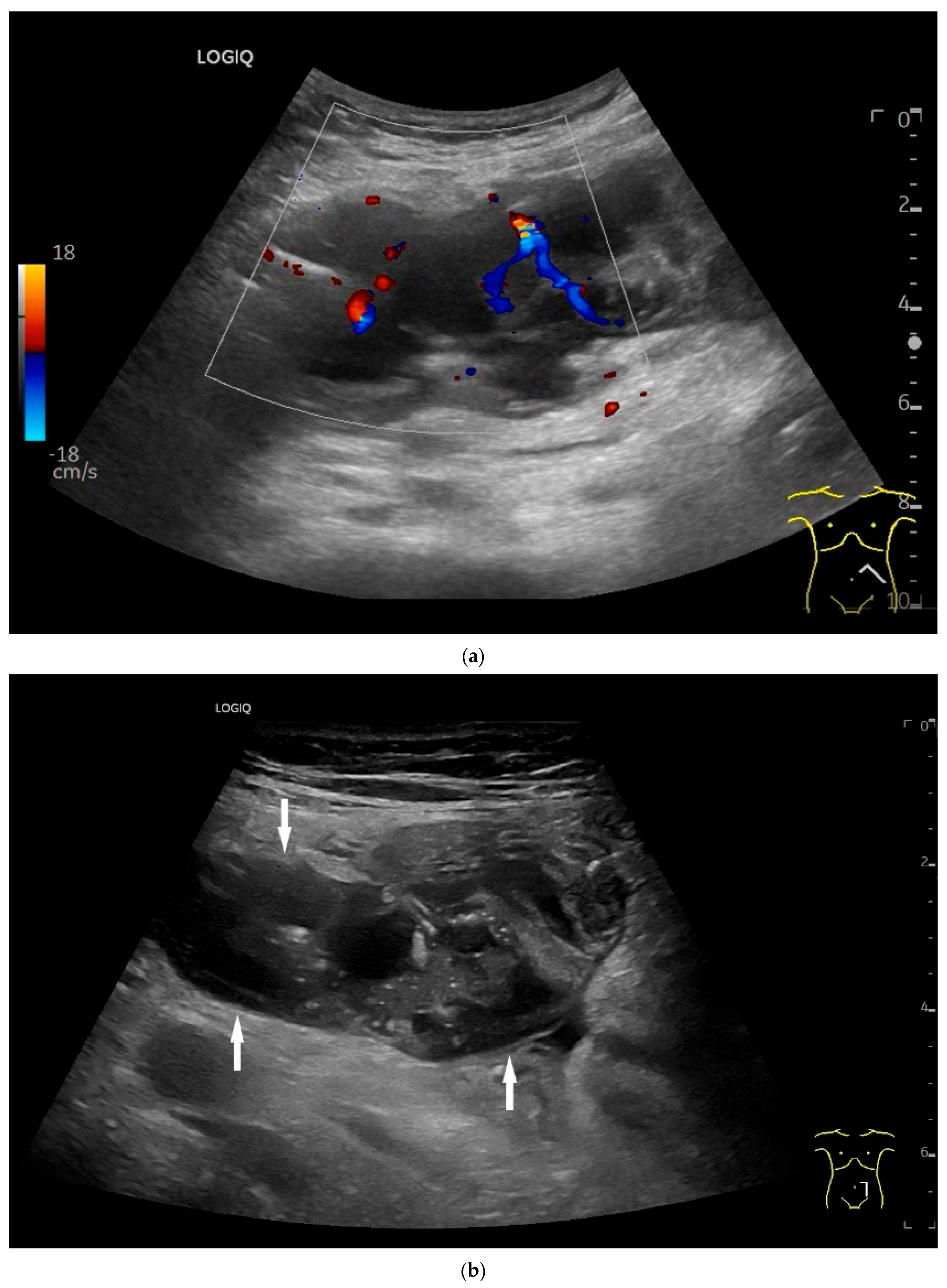
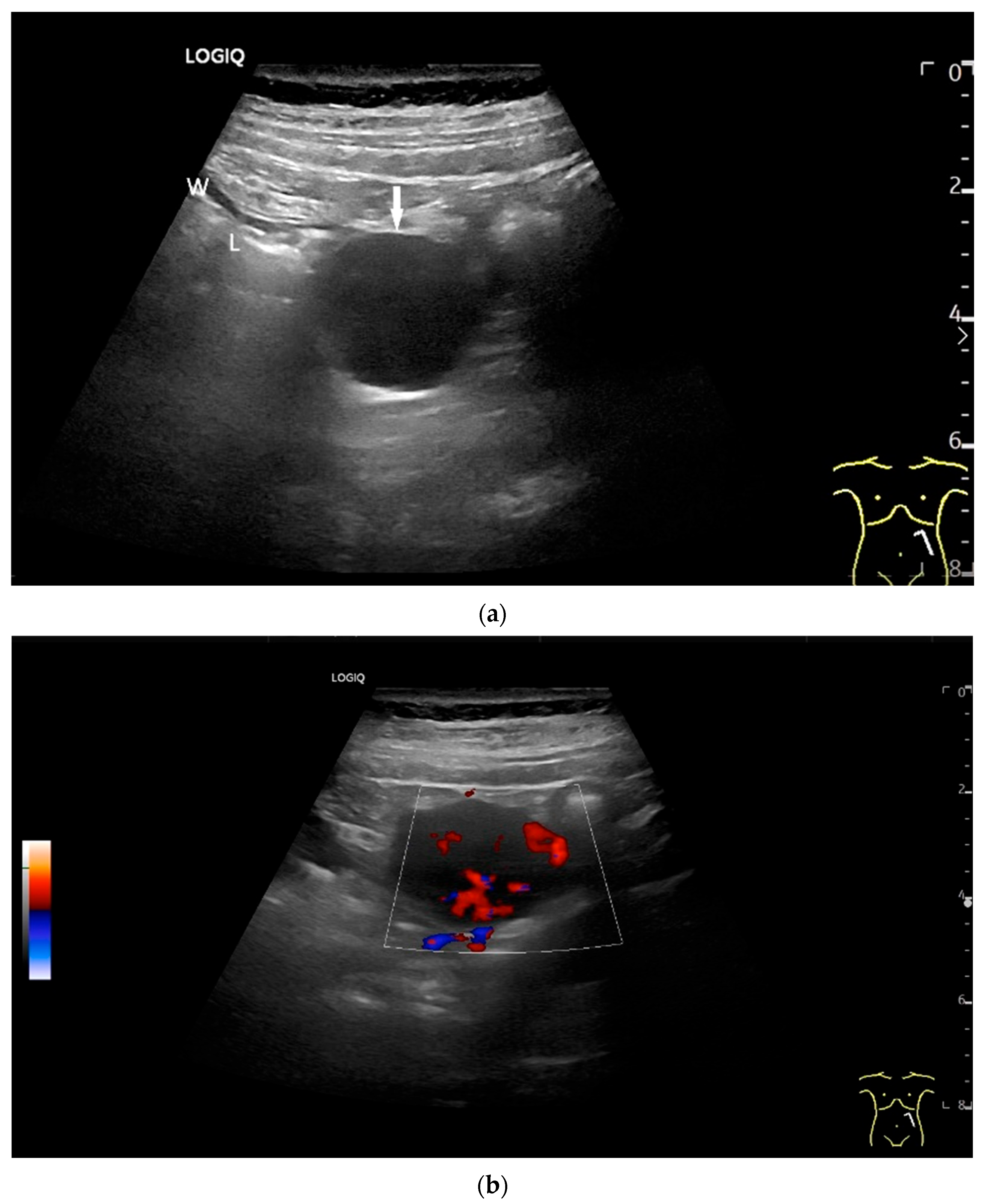
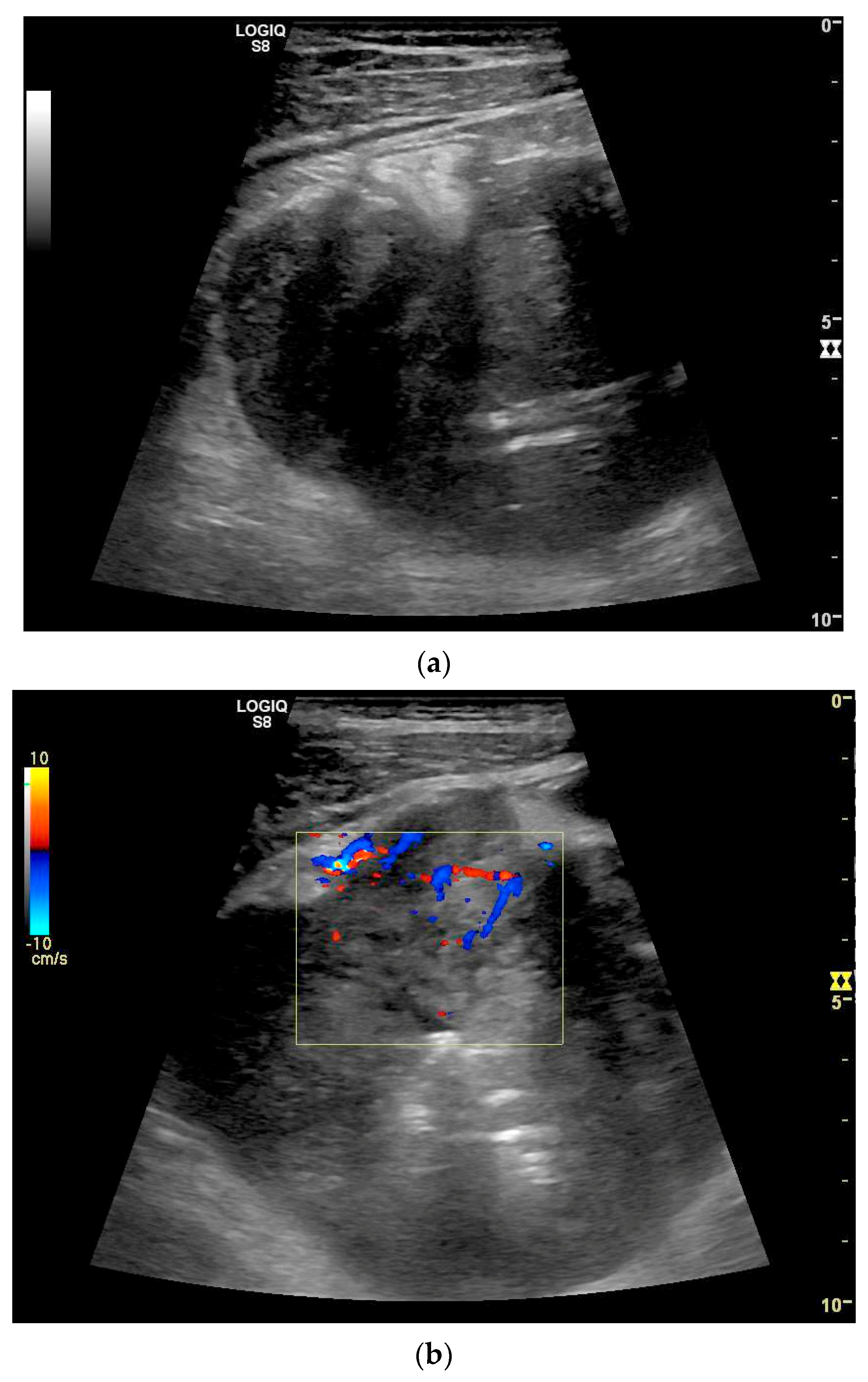
Adenocarcinoma of the Jejunum. Unclear cramp-like abdominal pain, inconclusive gastroscopy and ileocolonoscopy. US shows dilation of the jejunum with active peristalsis. The wall is thickened to 4.9 mm (between the markers). The lamina muscularis propria (white arrow) is prominent, hypertrophic with visibility of the radial and longitudinal muscle layer with echogenic collagen fibers in between (A). Although the US findings did not reveal the cause, they prompted further diagnostics. Push enteroscopy detected a circumferential growing and stenosing polypoid tumor (B).
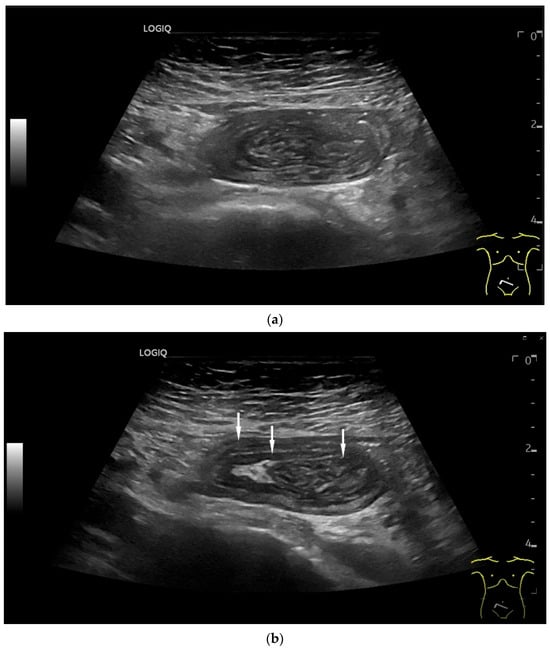
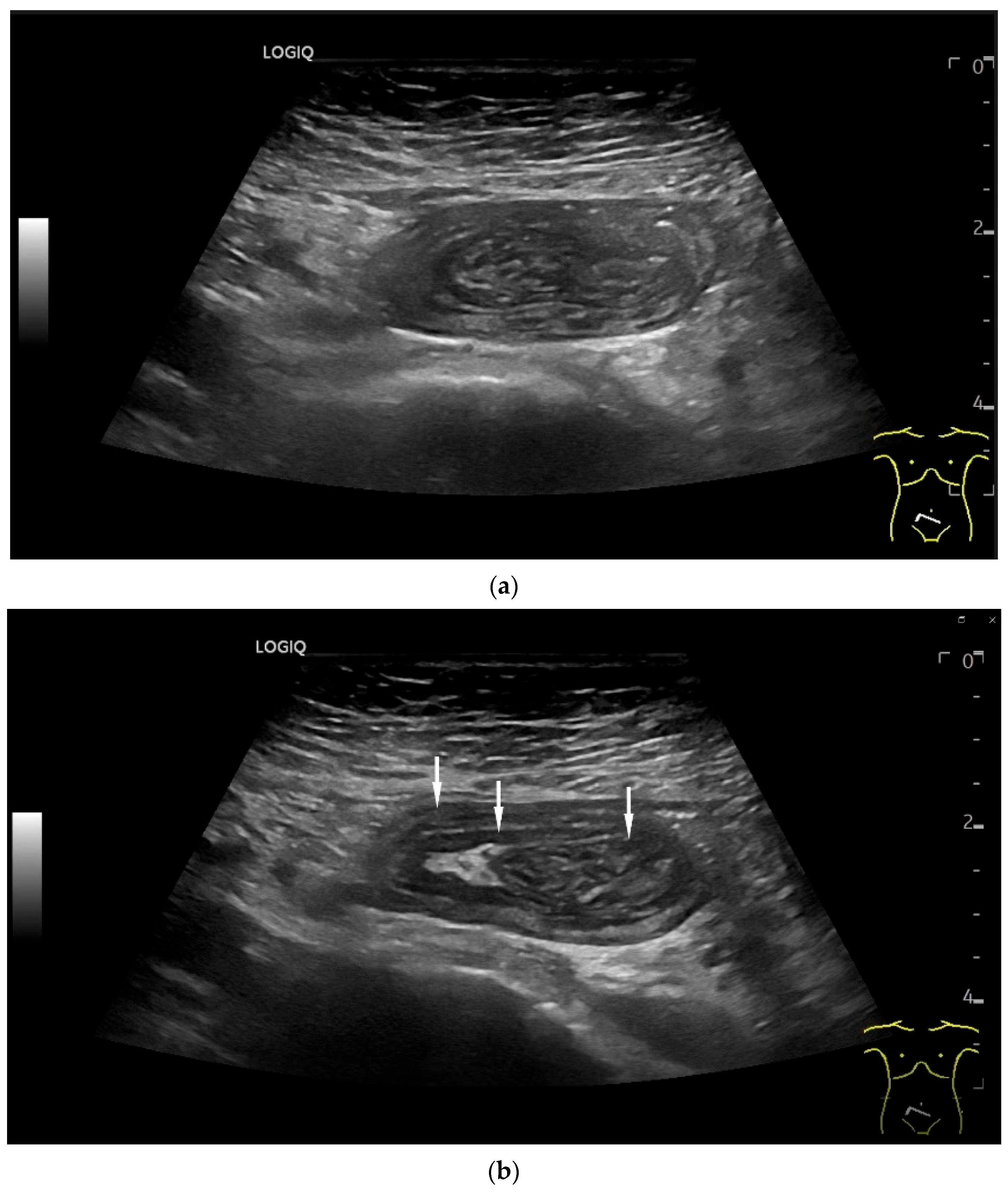
Figure 3.
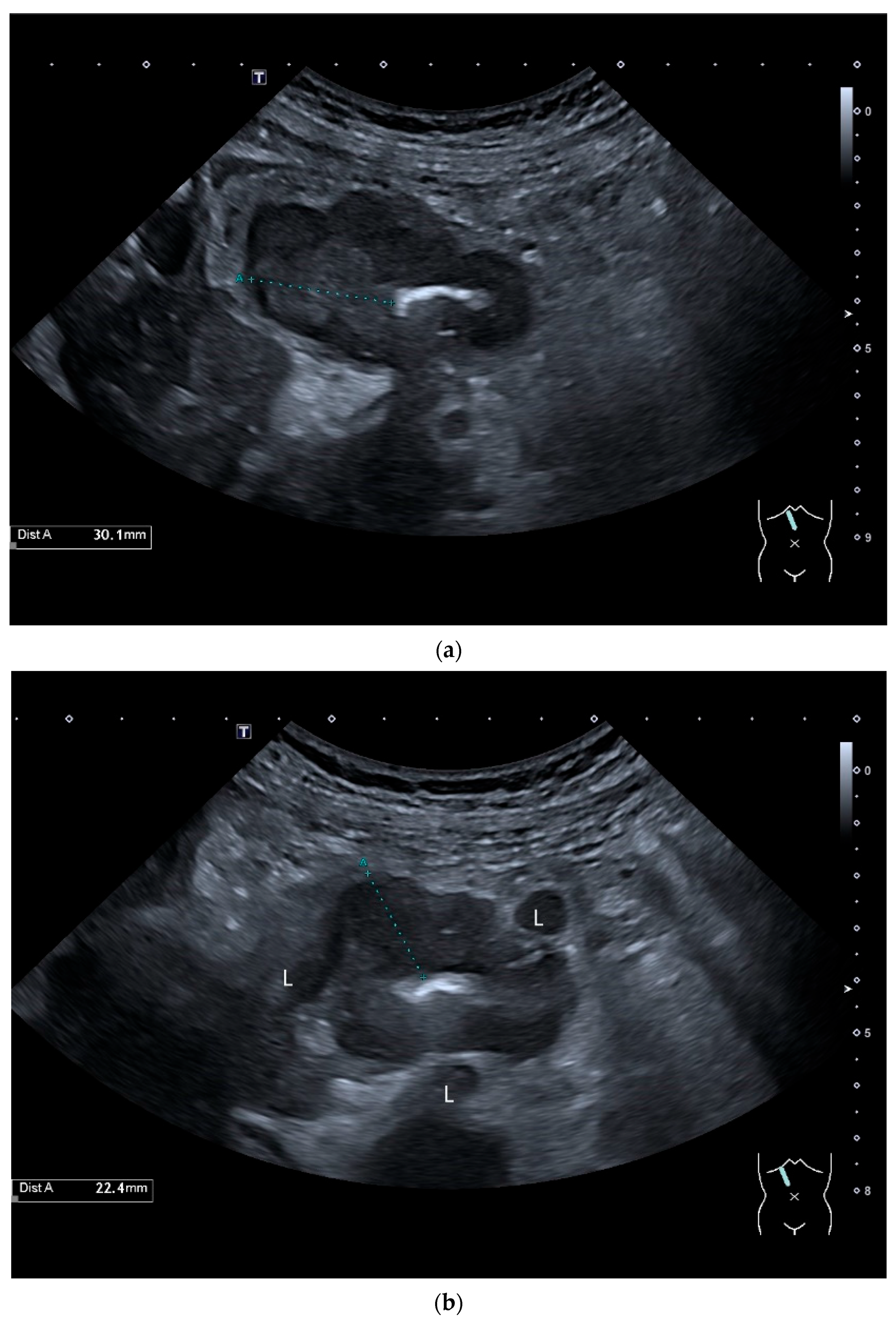
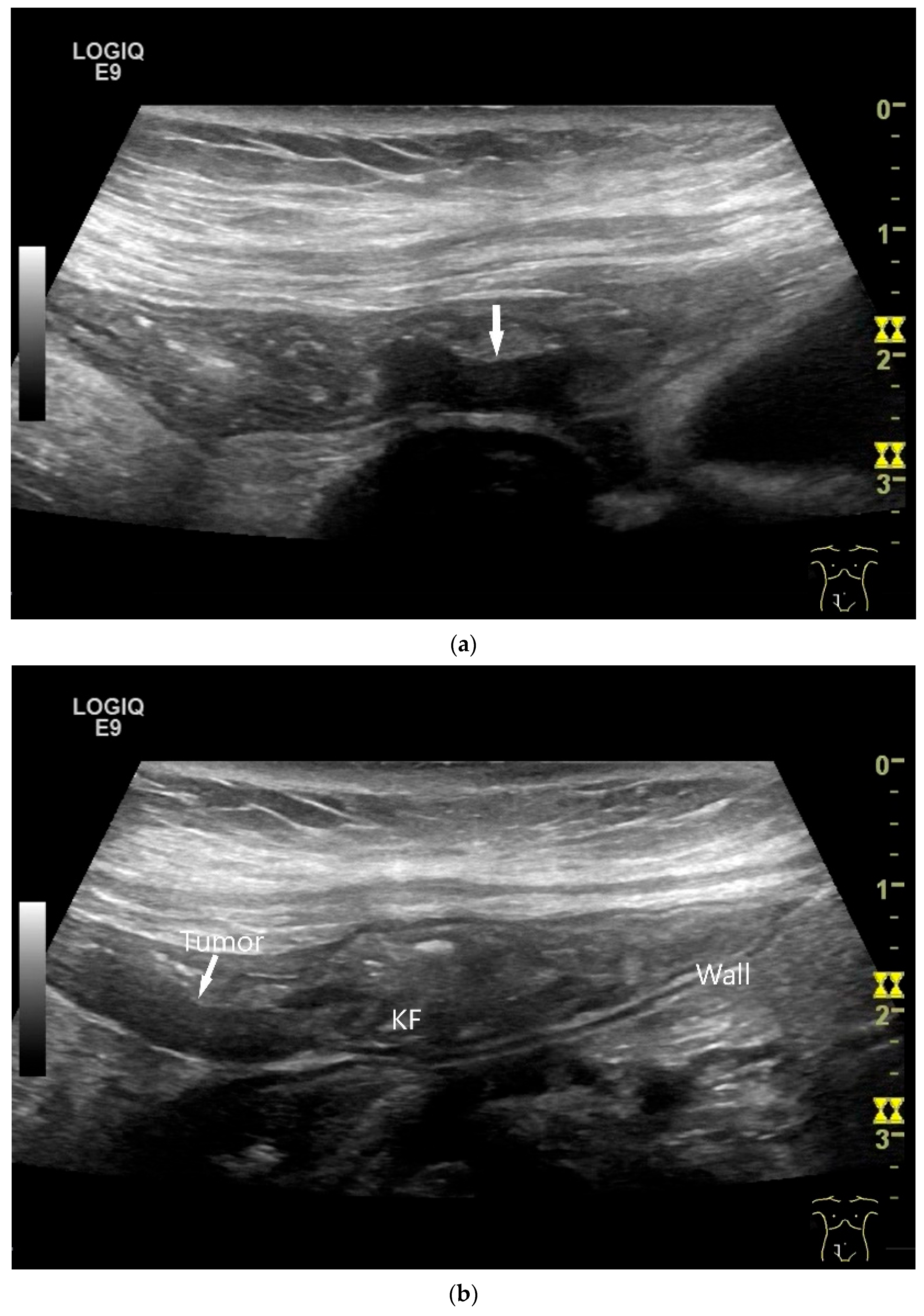
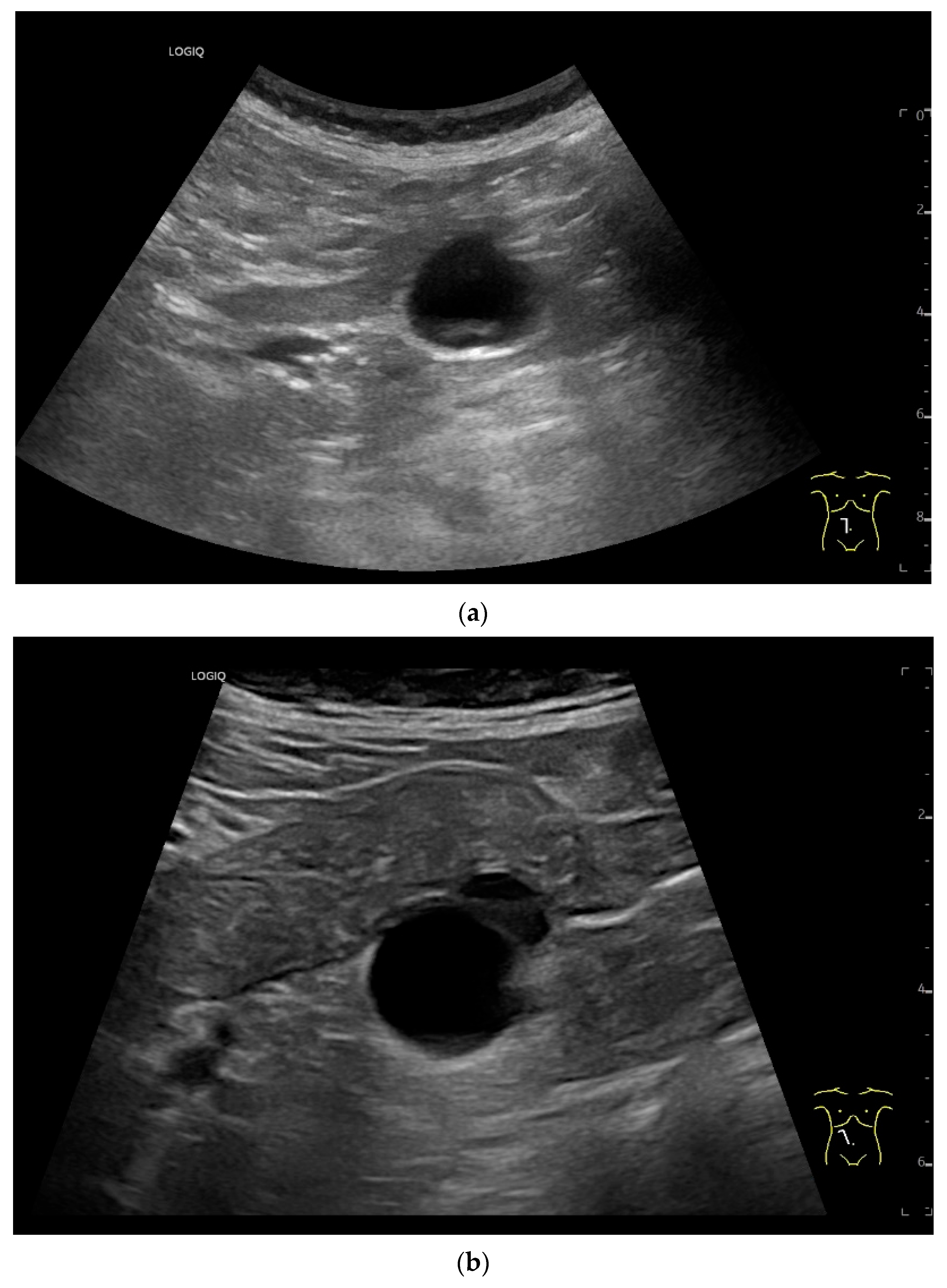
Intussusception. In the ileum, the intestine is inverted. Instead of the usual five layers, there are many onion-skin-like layers (a). The intestine is folded in on itself several times. The wall is marked with arrows (b).
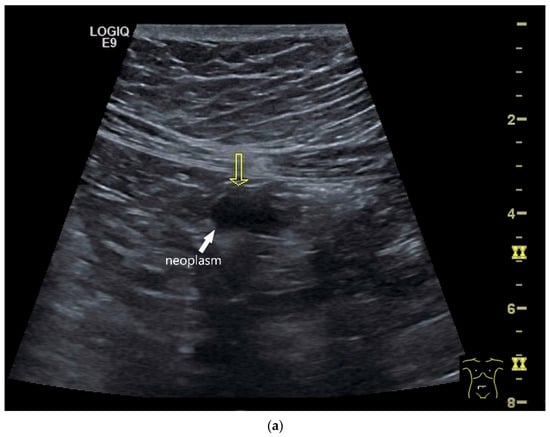
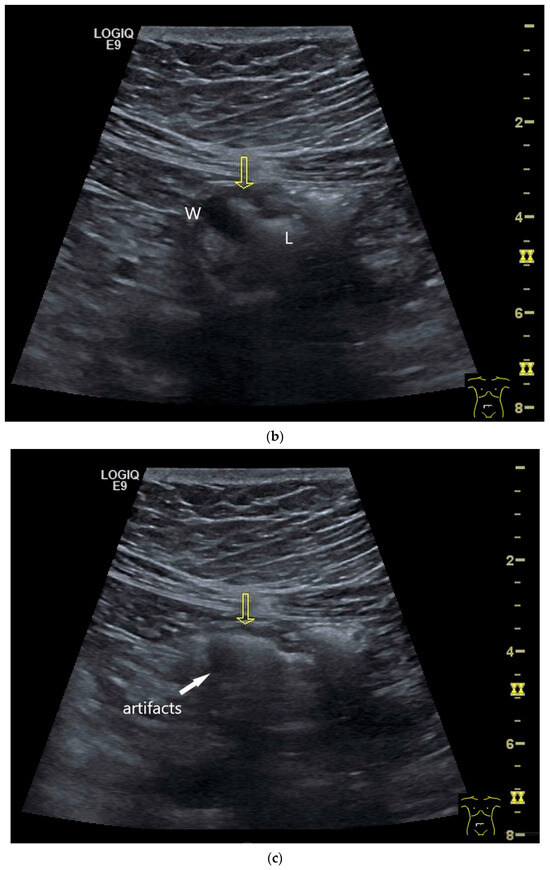
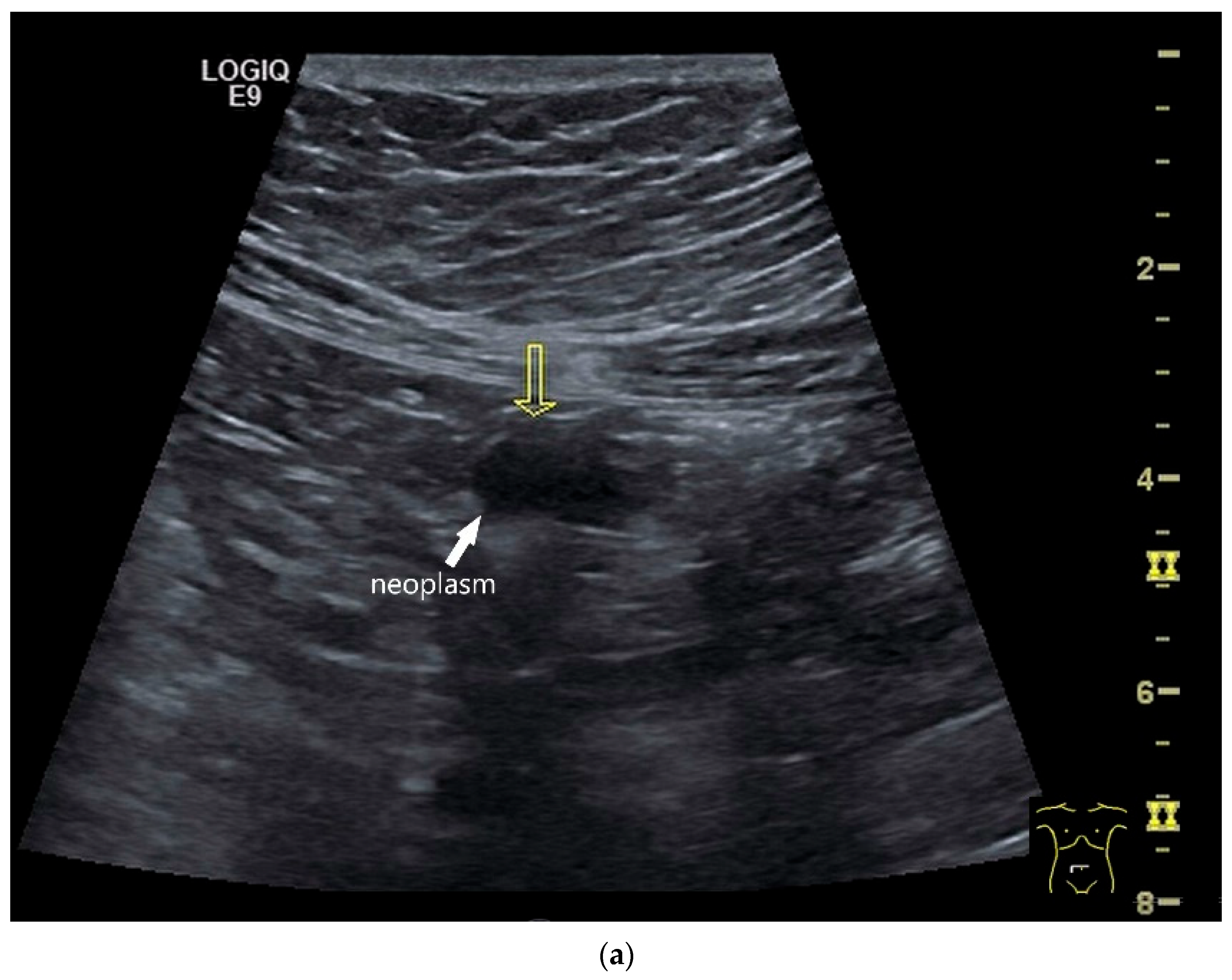
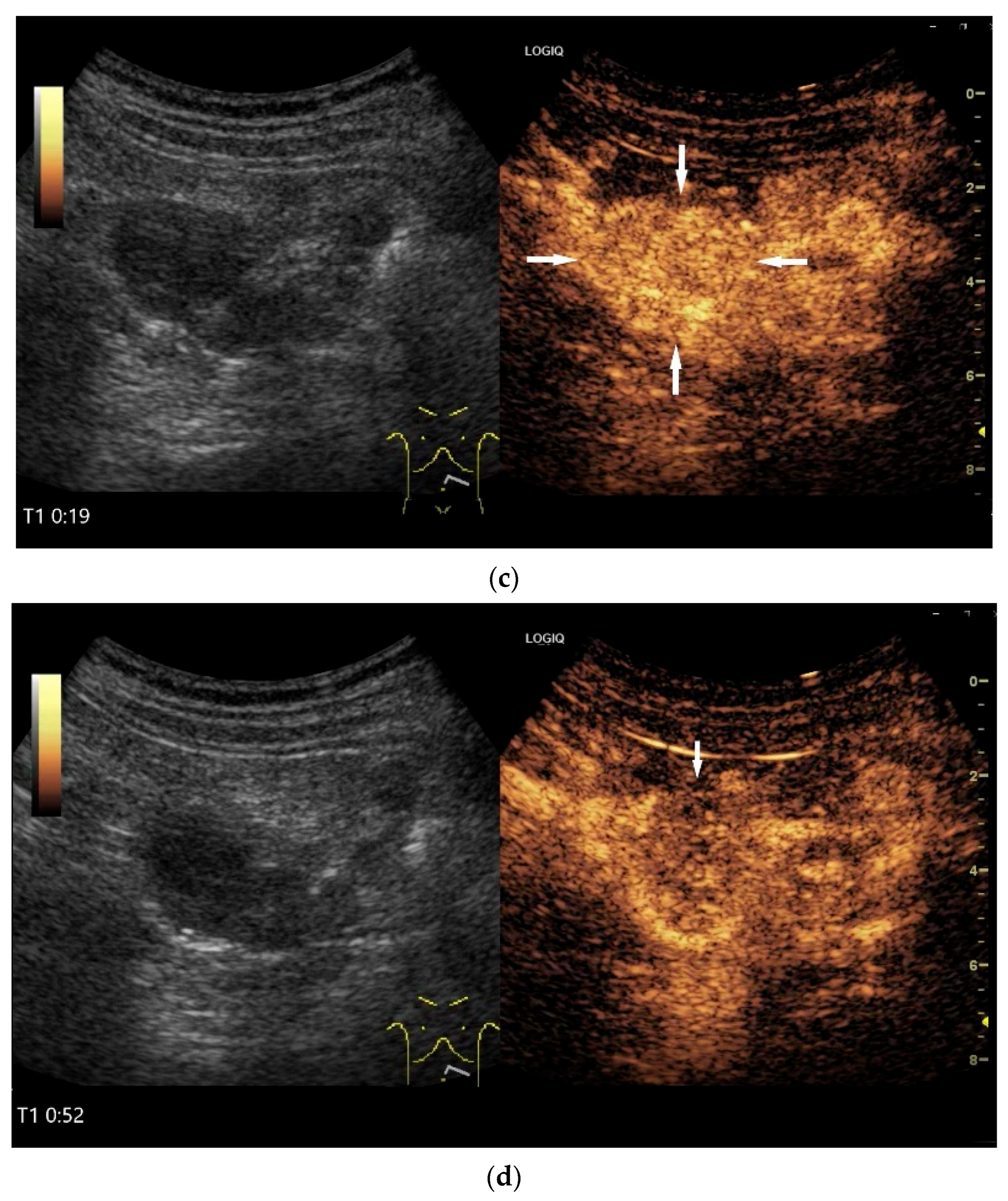
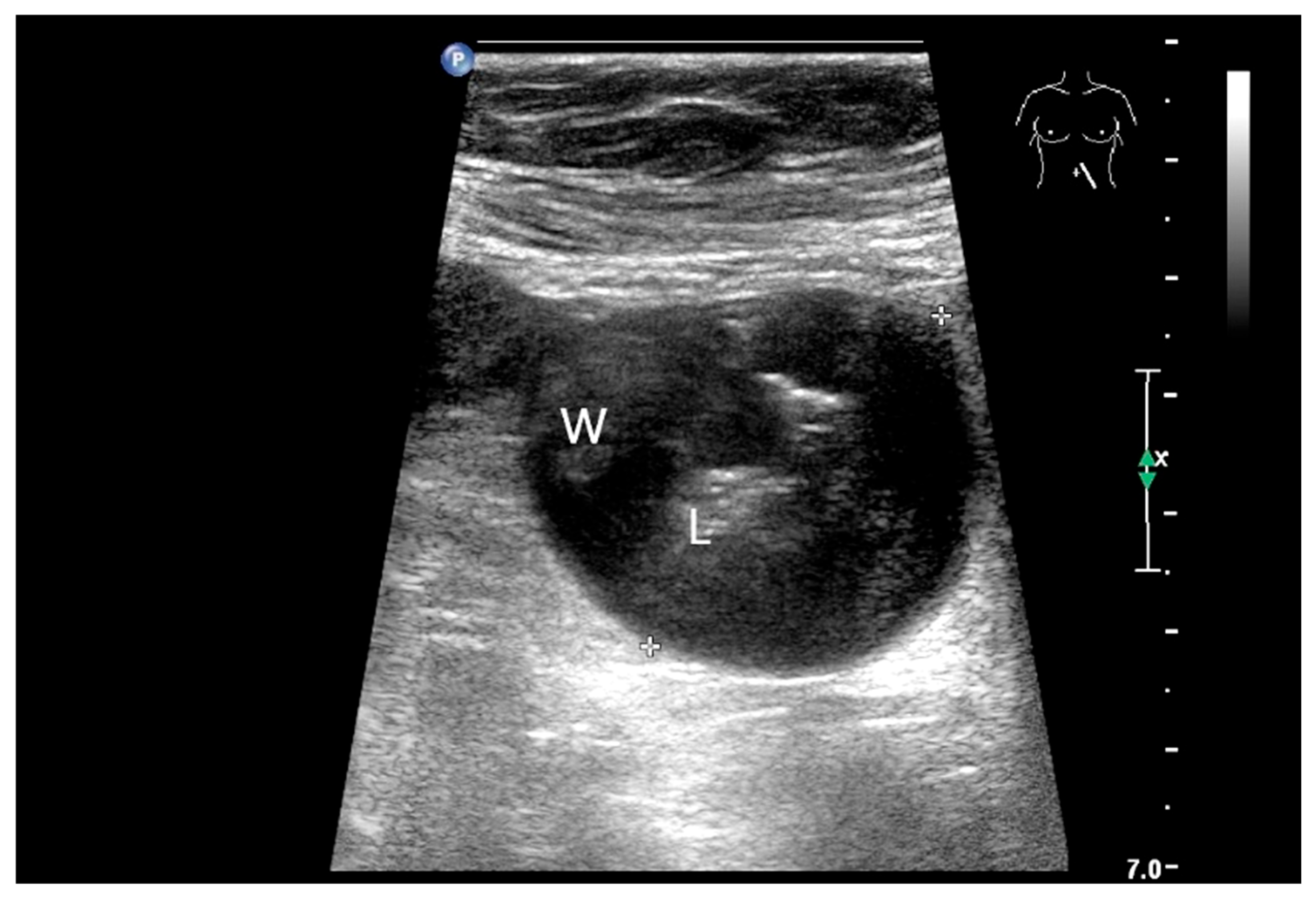
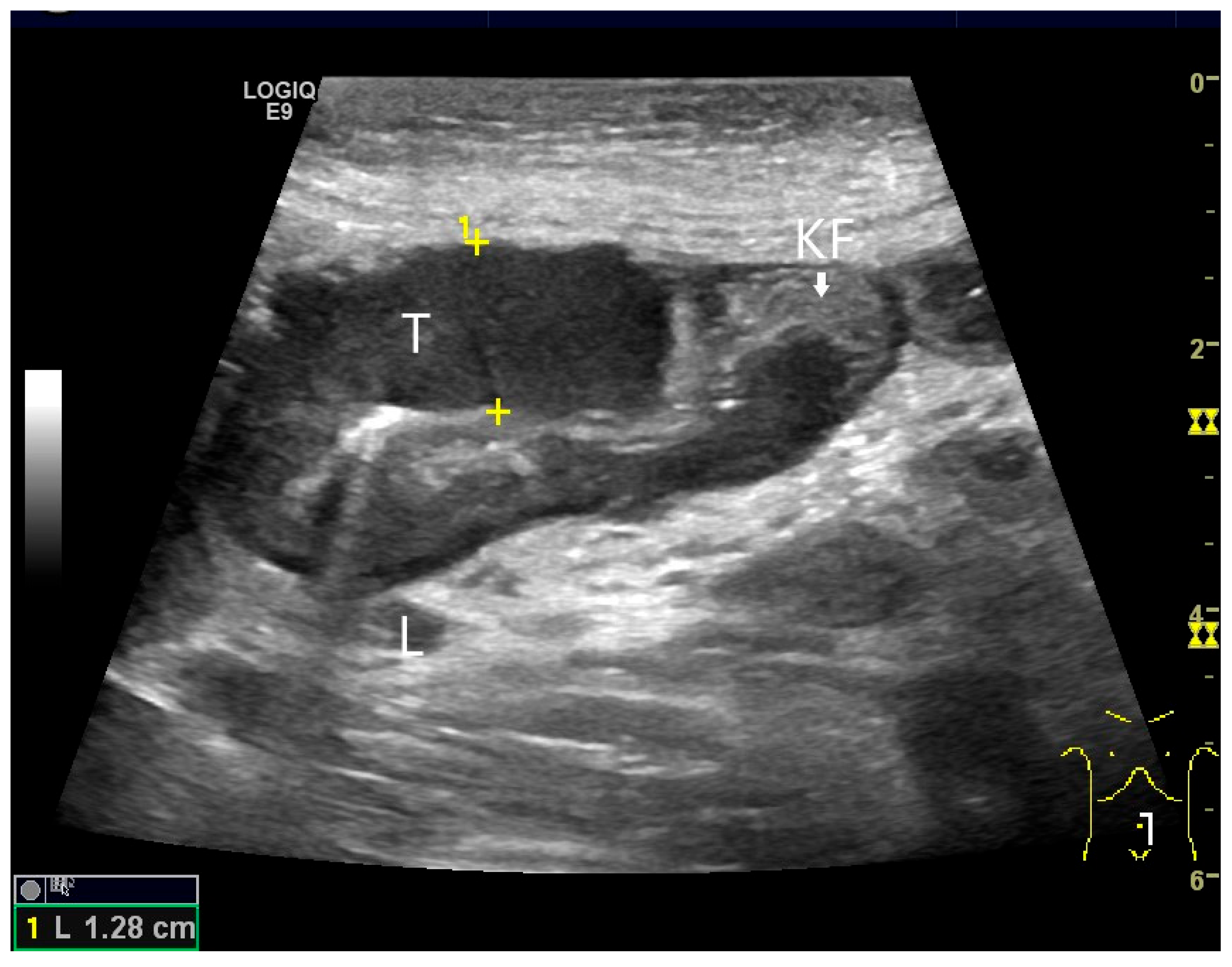
Figure 4.
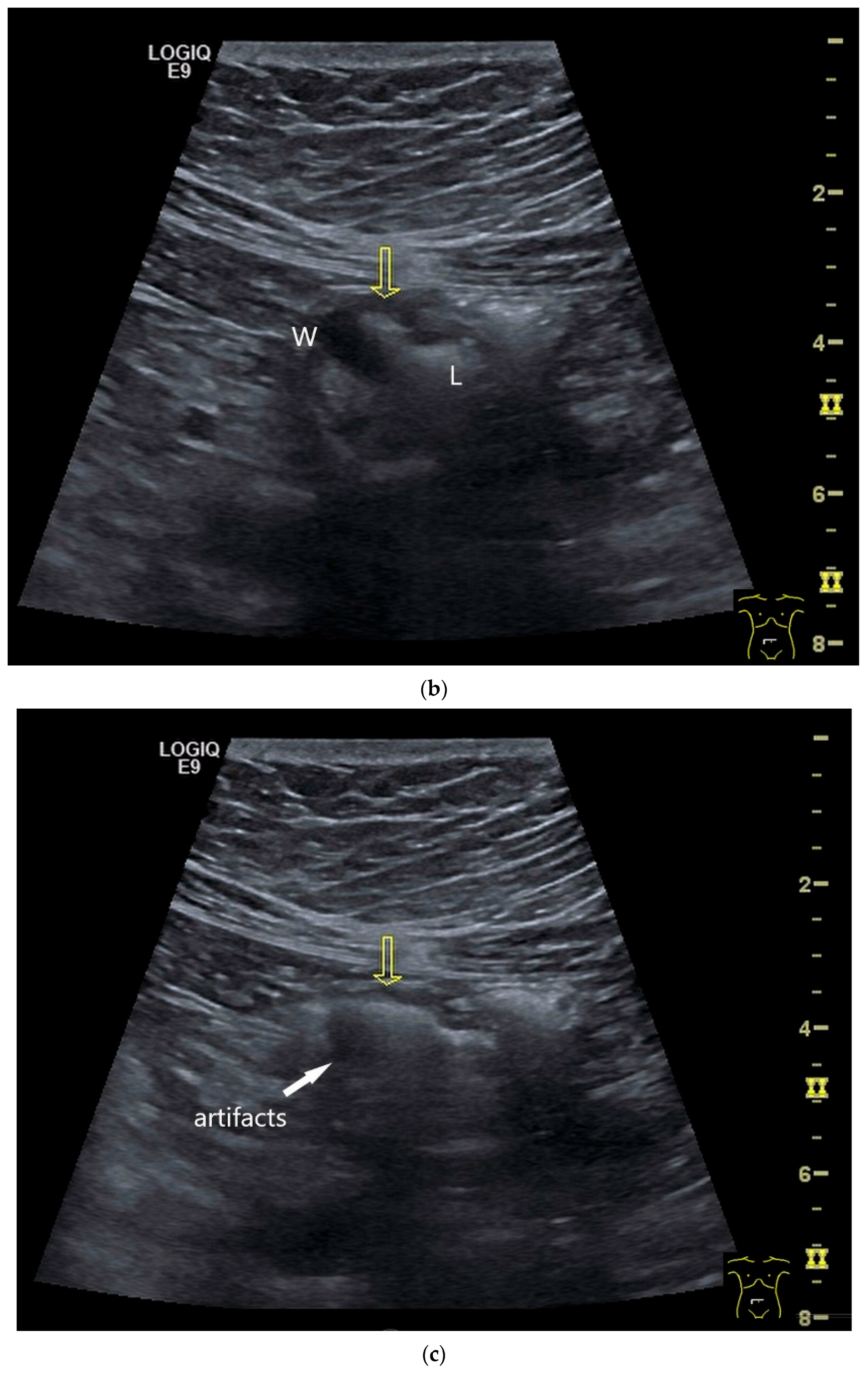
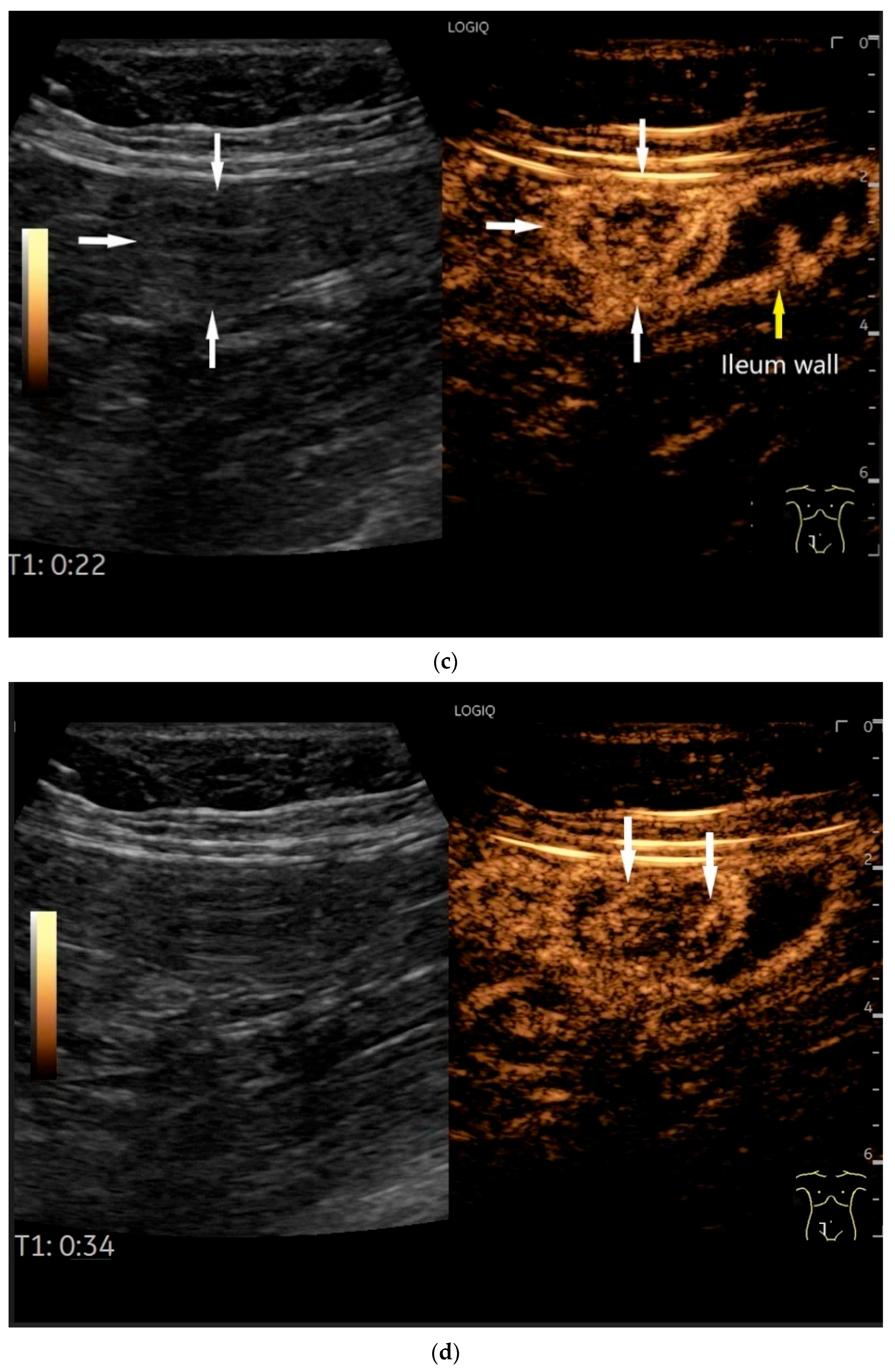
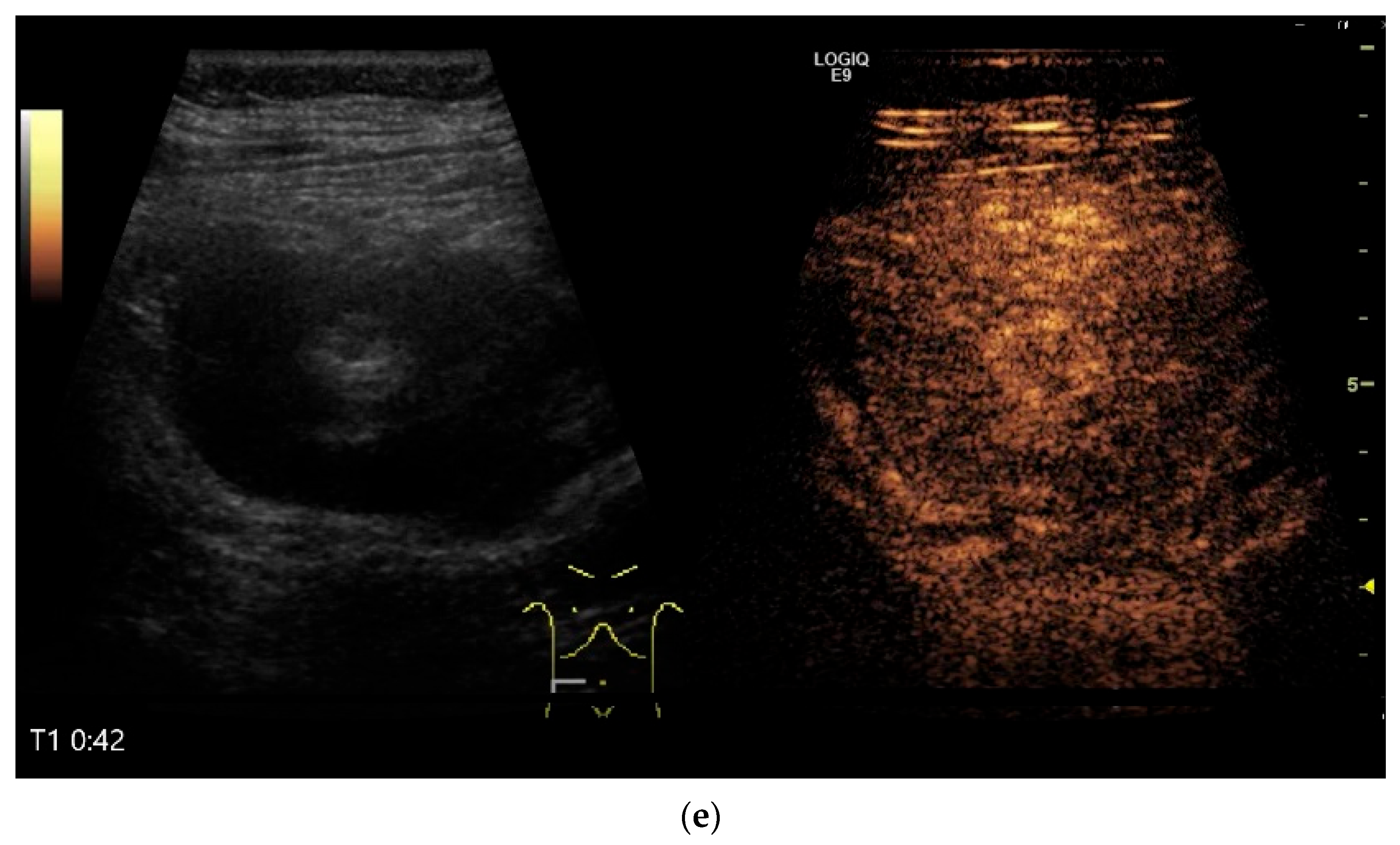
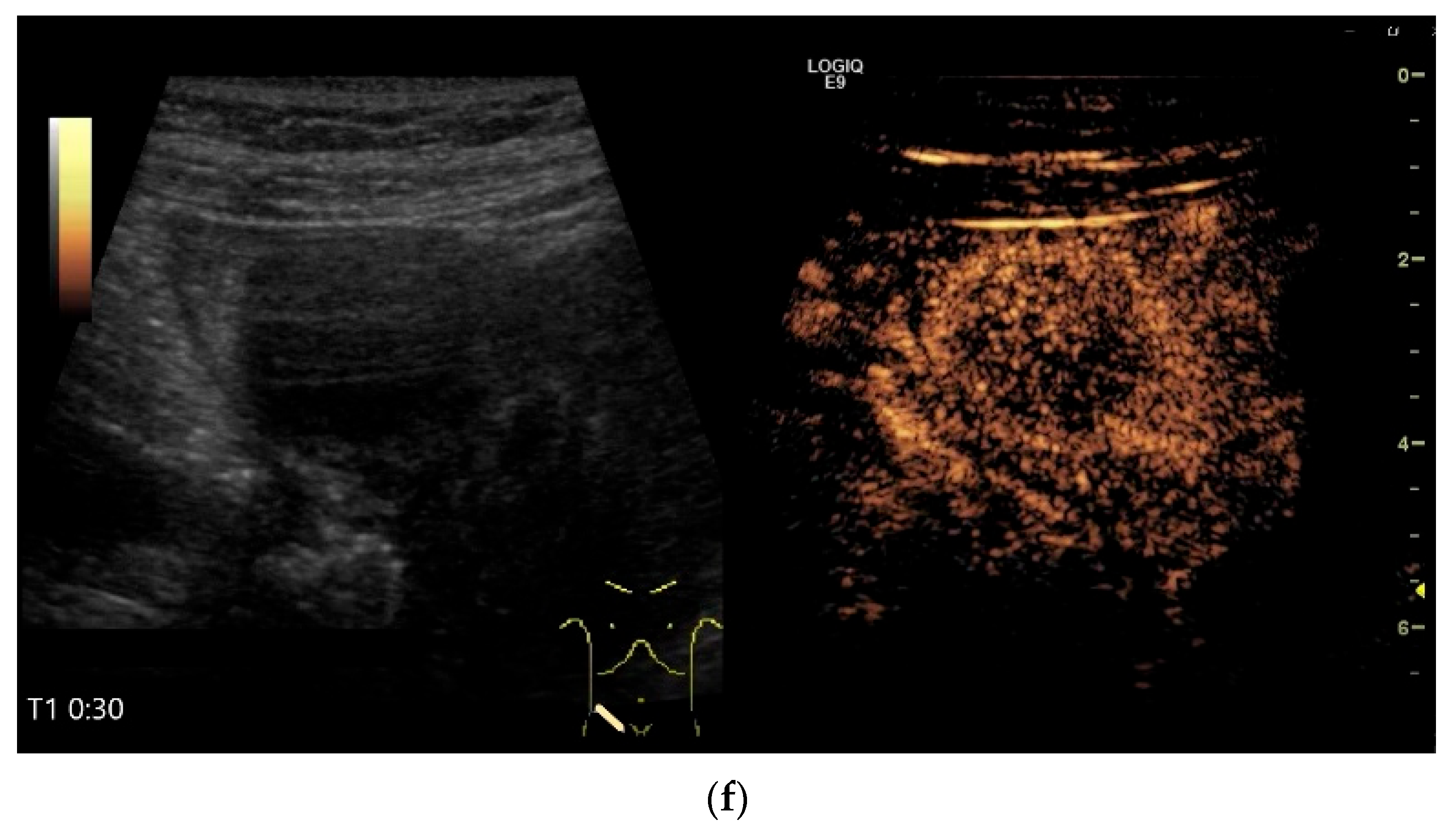
NEN in the ileum: an approximately 14 mm hypoechoic nodular tumor is visible (a). Subsequently, peristalsis reveals a hypoechoic wall thickening (W) and hyperechoic luminal reflex (L) (b) and, finally, causes the tumor to be obscured by the luminal contents and dorsal artifacts (c). The region of the tumor is marked with a yellow arrow in all three images.
In a study of Fukumoto et al. [41] in 2009, all 371 patients in 2003–2008 were consistently screened with 3.5–7.0 MHz ultrasound transducers prior to VCE and/or double balloon enteroscopy (DBE). 92 had a small intestine tumor on VCE and/or DBE. The overall lesion detection rate by US was 25.0% (sensitivity 26.4%; specificity 98.6%). While only 1.8% of tumors < 20 mm were detected by US, this was possible for 59.5% of tumors ≥ 20 mm. Five lesions were detected in US without correlation in VCE and DBE. None of the laterally spreading tumors (granular type) were detected in the US, but all circular ulcerating tumors. One GIST in the lesser pelvis could also not be detected, as the lesser pelvis is not or only partially visible on the US. The authors concluded that US is considered a useful method for detecting small bowel lesions of 20 mm or more and for avoiding unnecessary VCE examinations, especially when associated with the risk of capsule retention.
In a retrospective study by Fujita et al. [42] in 2015, 558 patients with 97 small intestine tumors underwent US with 3.5–7.5 MHz transducers before VCE and/or BAE in 2007–2013. Sensitivity and specificity of US in detecting small intestine tumors were 53.1% and 100%, respectively. For tumors larger than 20 mm, the detection rate was higher (91.7%): The detection rate of submucosal tumors larger than 20 mm was 85.7% and that of partial and circumferential ulcerative tumors larger than 20 mm was 96.9%. There were significant restrictions for tumors < 10 mm. US was able to detect only 2.6% of small intestine tumors < 10 mm. The small intestine tumors detected by US (mean 33.2 mm) were significantly larger than those undetected by US (mean 8.7 mm). Of the small intestine tumors that remained undetected by US, 91.3% were benign tumors [42]. Table 1 summarizes the factors that influence the detection of small intestine tumors.

Table 1.
Factors influencing the detection of small intestine tumors in US.
However, ultrasound technology has evolved with better B-mode resolution and high-resolution linear transducers. Color Doppler imaging (CDI) and contrast enhanced US (CEUS) are helpful in the description and differentiation of lesions.
Examination of the small intestine requires experience, a systematic approach, the consistent use of high-resolution, high-frequency linear transducers, and sufficient time. Table 2 compares the strengths and weaknesses of ultrasound with those of CT and CT/MR enterography.

Table 2.
Strengths and weaknesses of US compared to CT/MRI procedures, VCE, and DAET.
4.6. CEUS
Contrast-enhanced ultrasound (CEUS) is performed as a low mechanical index (MI) procedure using special ultrasound contrast agents (UCA) (SonoVue®, Bracco Suisse SA, Geneva, Switzerland; Sonazoid® GE Healthcare AS, Oslo, Norway, Daiichi-Sankyo), [44,45]. When CEUS is used extrahepatically, an arterial phase (0–30 s) and a venous phase (30–120 s) are differentiated. The first contrast signals are usually visible in the gastrointestinal tract 10–20 s after injection, predominantly in the submucosa. The maximum enhancement is achieved after 30–40 s [44]. Linear higher-frequency transducers require higher UCA dosages than conventional abdominal sector transducers. Typical applications of CEUS in the gastrointestinal tract include the diagnosis of abscesses versus phlegmons, the assessment of inflammatory activity and detection of intestinal strictures, fistulas and abscesses in Crohn’s disease [46,47,48]. The detection and differential diagnosis of intestinal tumors is not regarded as a standard indication for CEUS. However, in EUS, assessment of vascularization is important for the differential diagnosis of subepithelial tumors and treatment decisions. Study data from contrast-enhanced low MI-EUS showed that GIST and neuroendocrine tumors are hyperenhanced, while lipomas and the majority of leiomyomas are hypoenhanced [49,50,51,52,53,54,55]. Indications for CEUS include the differentiation of non-enhanced lesions (mesenteric cysts, lymphangiomas) from solid tumors. In cases of doubt, CEUS can be used to distinguish between solid tumors and other intestinal contents in the intestinal lumen. CEUS can be used to differentiate between well-hyperenhanced mesenchymal tumors such as GIST and hypoenhanced tumors such as leiomyomas. In cases of very pronounced hypoechogenicity, it can be demonstrated that the tissue is vital. In percutaneous US-guided sampling, CEUS is helpful in distinguishing between vital and necrotic tissue and in obtaining evaluable histological material [56,57,58]. In addition, the intestinal wall and Interenteric vessels can be better differentiated if necessary.
4.7. US-Guided Biopsy
Indications and procedures for transabdominal US-guided sampling of lesions of the gastrointestinal tract or abdominal lymphomas are outlined in the EFSUMB INVUS II guidelines [59]. Indications for US-guided sampling of lesions of the gastrointestinal tract are lesions in the small intestine that are not easily accessible by endoscopy, e.g., GIST or lymphomas. Contraindications are operable lesions of the gastrointestinal tract with a high suspicion of malignancy or lesions where surgery is highly likely to be the first-line treatment [59]. The prerequisite is that the lesion can be visualized with light transducer compression. Passage through sections of the stomach and small intestine with 18-gauge needles is generally safe [60]. Nevertheless, intestinal transit should be avoided. Passage through the colon is contraindicated [59]. With CDI, abdominal vessels can be visualized and avoided in the puncture access [58]. In retrospective studies, sensitivity and accuracy for gastrointestinal tract biopsies using core needles ranged between 81 and 99% [59]. Fine needles performed less well, with sensitivity values of 41 to 50% [61]. We prefer the 18-gauge needle. If lymphoma is suspected, the 16-gauge needle is used as an alternative, considering the risk-benefit ratio.
A percutaneous ultrasound-guided biopsy is always justified if immediate surgery is not indicated, a lesion type is expected that does require neoadjuvant (e.g., tyrosine kinase inhibitor pre-treatment with Imatinib in GISTs) or non-surgical treatment (e.g., chemo- and immunotherapy in malignant lymphoma) and if endoscopic biopsy is not feasible. In a study involving 19 children, 20 percutaneous ultrasound-guided biopsies were performed on small intestine masses. Sixteen were lymphomas, mostly large bulky masses resulting from Burkitt lymphoma (55%). Diagnostic adequacy was 100% [62]. In a study involving percutaneous US-guided biopsies of various areas of the GIT in 45 adults, sensitivity, specificity, PPV, NPV, and diagnostic accuracy, including an inadequate biopsy specimen, were 95.1%, 100%, 100%, 66.7%, and 95.6%, respectively [63].
Normal coagulation is a prerequisite for US-guided sampling. In a study of 36 percutaneous US guided sampling of small bowel lesions, there were no complications [64]. However, potentially complications include bleeding and infections due to perforations [65].
5. Small Intestine Adenomas
Small intestine adenomas can occur in the entire small intestine, but mostly in the duodenum and the ampulla Vateri. They are divided into non-ampullary adenomas and ampullary adenomas, the latter being not subject of this review. Non-ampullary adenomas are small intestine polyps of dysplastic glandular epithelium with intestinal or pyloric glandular differentiation.
They occur in any part of the small intestine, but mostly in the proximal duodenum. Around 60% of adenomas occur in patients with Familial Adenomatous Polyposis [66]. Sporadic duodenal polyps are rare and mostly incidental findings on gastroscopy, but account for only 5% of patients at most [67]. They are small and often solitary and can be removed endoscopically. In the remaining small intestine, the diagnosis is often only made when larger adenomas cause lumen obstruction. Adenomas are usually flat or small and are easily overlooked during US. In a study of 50,114 patients, 1.02% had duodenal polyps and 0.3% were duodenal adenomas [68]. The detection of adenomas is a domain of endoscopy.
6. Intestinal Carcinoma
Adenocarcinomas account for 30–40% of small intestine tumors [1]. They are most often localized in the duodenum and duodenojejunal junction (>50%), followed by the jejunum and ileum. In the distal small intestine, NEN, lymphomas, or metastatic disease should also be considered
Nevertheless, they are rare and account for <3% of cancers of the entire gastrointestinal tract [1]. Ampullary adenocarcinomas are not discussed in this review.
In Europe, 3600 new cases of adenocarcinoma of the small intestine are diagnosed every year and 5300 in the USA [1]. In contrast to colorectal carcinomas, small intestine adenocarcinomas have a higher proportion of poorly differentiated carcinomas [1].
Chronic inflammatory diseases (Crohn’s disease and celiac disease) are associated with an increased risk of adenocarcinomas in the small intestine. The same applies to some genetic syndromes such as familial adenomatous polyposis, Lynch syndrome and Peutz-Jeghers syndrome [1]. In addition, the possibility of multiple small intestine tumors [69] occurring, sometimes in association with multiple primary malignancies (MPMs) [37], should be considered.
Imaging: Tumors in the jejunum and ileum are usually circular ring-shaped and lumen-stenosing. In the duodenum, about one third have a polypoid component, but a proportion of cases show plaque-like growth [70]. On US, the adenocarcinoma presents as hypoechoic wall thickening with lumen narrowing. The infiltrative wall process may show further tongue-shaped hypoechoic infiltrations into the surrounding tissue. In the case of lymph node involvement, the mesenteric lymph nodes at the level of the tumor may be enlarged and/or morphologically conspicuous [71,72] (Figure 5 and Figure 6).
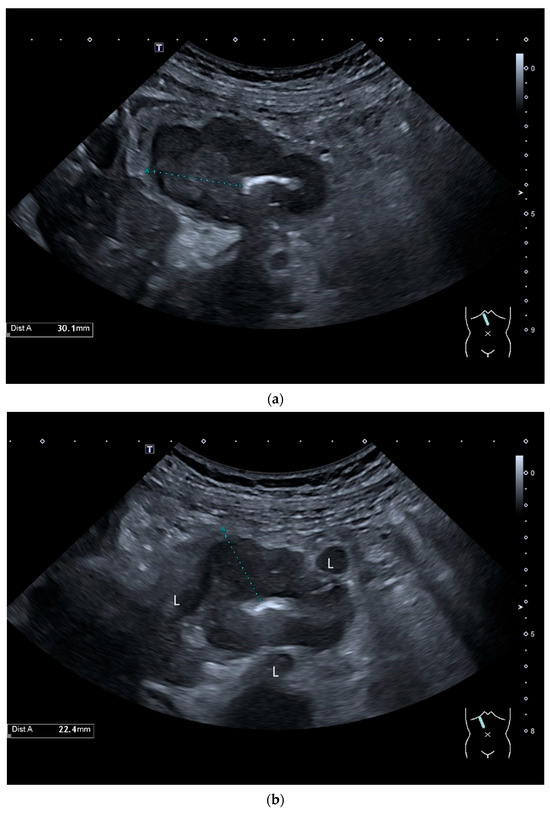
Figure 5.
Adenocarcinoma in the duodenum. Significant hypoechoic wall thickening (between the markers) with narrowed lumen reflexes (a). The tumor has a slightly polycyclic border on the outside (a,b). Several tumor-suspicious, round, hypoechoic lymph nodes (L) are visible in the paraduodenal region (b).
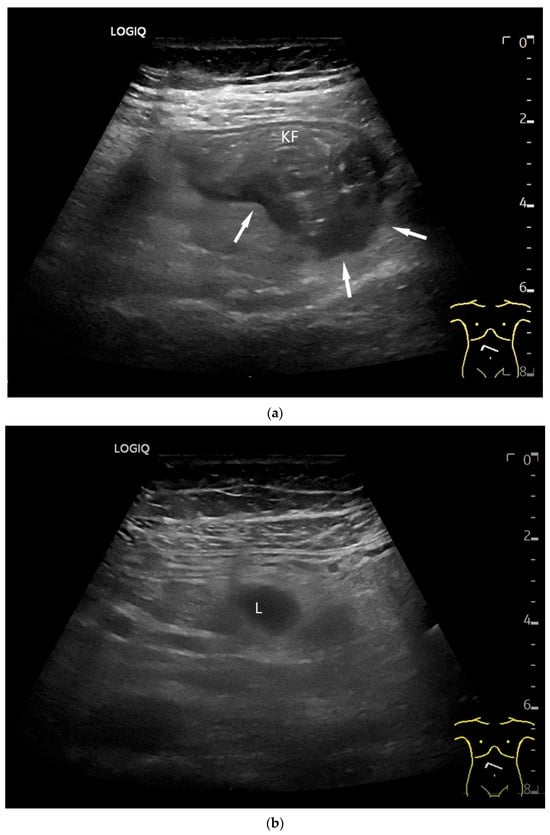
Figure 6.
Jejunal Adenocarcinoma. Segmental hypoechoic wall thickening (arrows) in the jejunum discovered during anemia diagnostics. Kerckring folds are faintly visible (KF) (a). Adjacent to this is a large round hypoechoic lymph node (L). The surrounding area shows hyperechoic changes (b).
Wu et al. [72] described a single adenocarcinoma in the jejunum using CEUS. The tumor is hypoechoic in B-mode, causing wall thickening and irregular borders. In CEUS, the tumor is hypoenhanced. Wu et al. also performed oral contrast enhancement with UCA [72]. The adenocarcinoma in Crohn’s disease is usually located in the distal jejunum or ileum. It is difficult to distinguish a pathological cockade due to a tumor from a transmural inflammatory wall thickening of Crohn’s disease. For diagnosis, clinical, imaging and laboratory data must be combined with a high degree of experience.
7. Neuroendocrine Neoplasms (NEN)
NEN of the small intestine belong to the gastroenteropancreatic NEN (GEP-NEN) [73]. The incidence rate for small intestine NEN was 0.78–1.7/100,000 [74,75,76]. The distribution of GEP-NEN varies regionally: in North America small intestine, colon and rectal NEN are most frequent, in Asia, rectal and pancreatic NEN are most common while in Europe, small bowel and pancreatic NEN are most prevalent [73]. The proportion of small intestine NEN among the GEP-NEN in the various studies was 5.6–29% [77,78,79,80,81,82,83,84].
Small intestine NEN can occur in the small intestine and the ampulla Vateri.
Functionally active NET include gastrinoma, somatostatinoma and paraganglioma. Somatostatin-expressing NET and gangliocytic paragangliomas occur almost exclusively in the ampullary region and in the appendix. The vast majority of jejunoileal NET are located in the distal ileum, only about 11% in the jejunum. Occasionally, enterochromaffin cell (EC cell) tumors develop in Meckel’s diverticula. NEC appear in the small intestine almost exclusively in the ampullary region [1,85].
Duodenal and periampullary NEN are usually small (<2 cm) subepithelial lesions in the submucosa. Jejunoileal NEN may be multifocal in about one third of patients. Upper jejunal NEN can be large and locally invasive. Lower jejunal and ileal NEN are mucosal/submucosal nodules with intact or slightly eroded mucosa. Deep infiltration of the muscular wall and subserosa is common in jejunal and ileal NEN [1]. Most duodenal NEN are diagnosed incidentally during gastroscopy as impressions due to submucosal lesions. First and foremost, they must be differentiated from GIST.
Non-functional NETs can be asymptomatic and be detected incidentally on imaging. However, they can also cause abdominal symptoms due to lumen obstruction. Gastrinoma occurs sporadically or as part of multiple endocrine neoplasia type I. 70% are located in the duodenum, with most of the remaining cases occurring in the pancreas [86]. There are isolated case reports from the jejunum [87]. Somatostatinoma is also very rare and occurs predominantly in the duodenum, the ampullary region, and the pancreas [88], while jejunal localization is reported only in single cases [89].
Ultrasound Imaging of Small Intestine NEN
On US, small intestine NEN appear as small focal hypoechoic wall thickenings with a pseudo nodular appearance or as hypoechoic nodular lesion. They are usually small tumors. Adjacent pathological lymph nodes may appear in the mesentery. The tumors extend from the submucosa and may extend into the mesentery via the lamina muscularis propria [90,91,92,93,94,95,96]. NEN are usually well vascularized. Color Doppler imaging (CDI) can show irregular macrovessels in the tumor. Case reports showed that small intestine NETs are hyperenhanced on CEUS [91,92,94,95,96,97] (Figure 7 and Figure 8). Further diagnostics include CT and DOTATOC-PET.
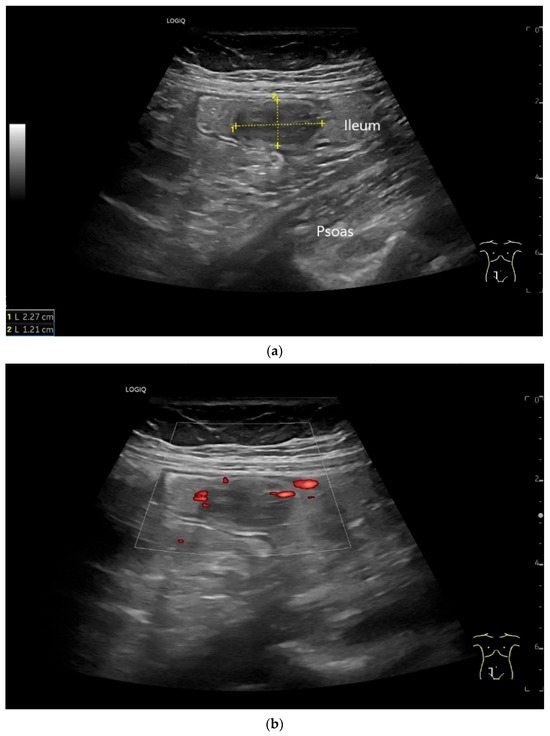
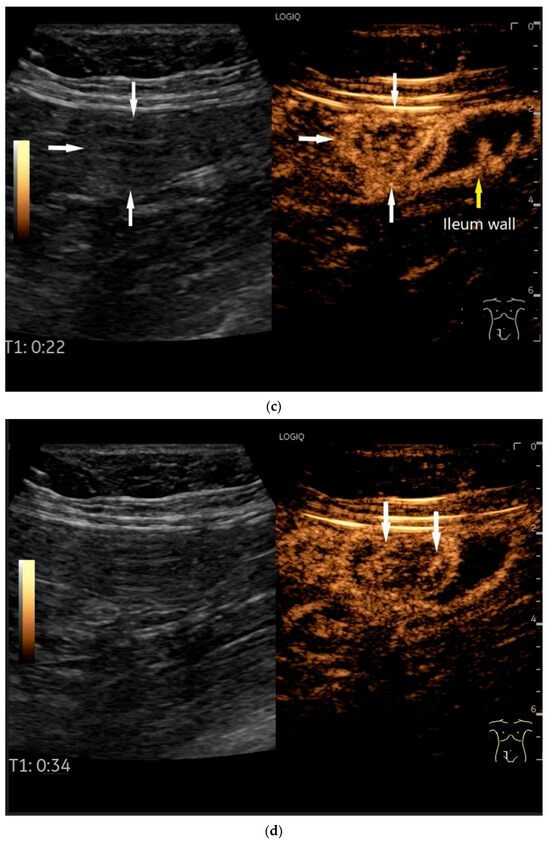
Figure 7.
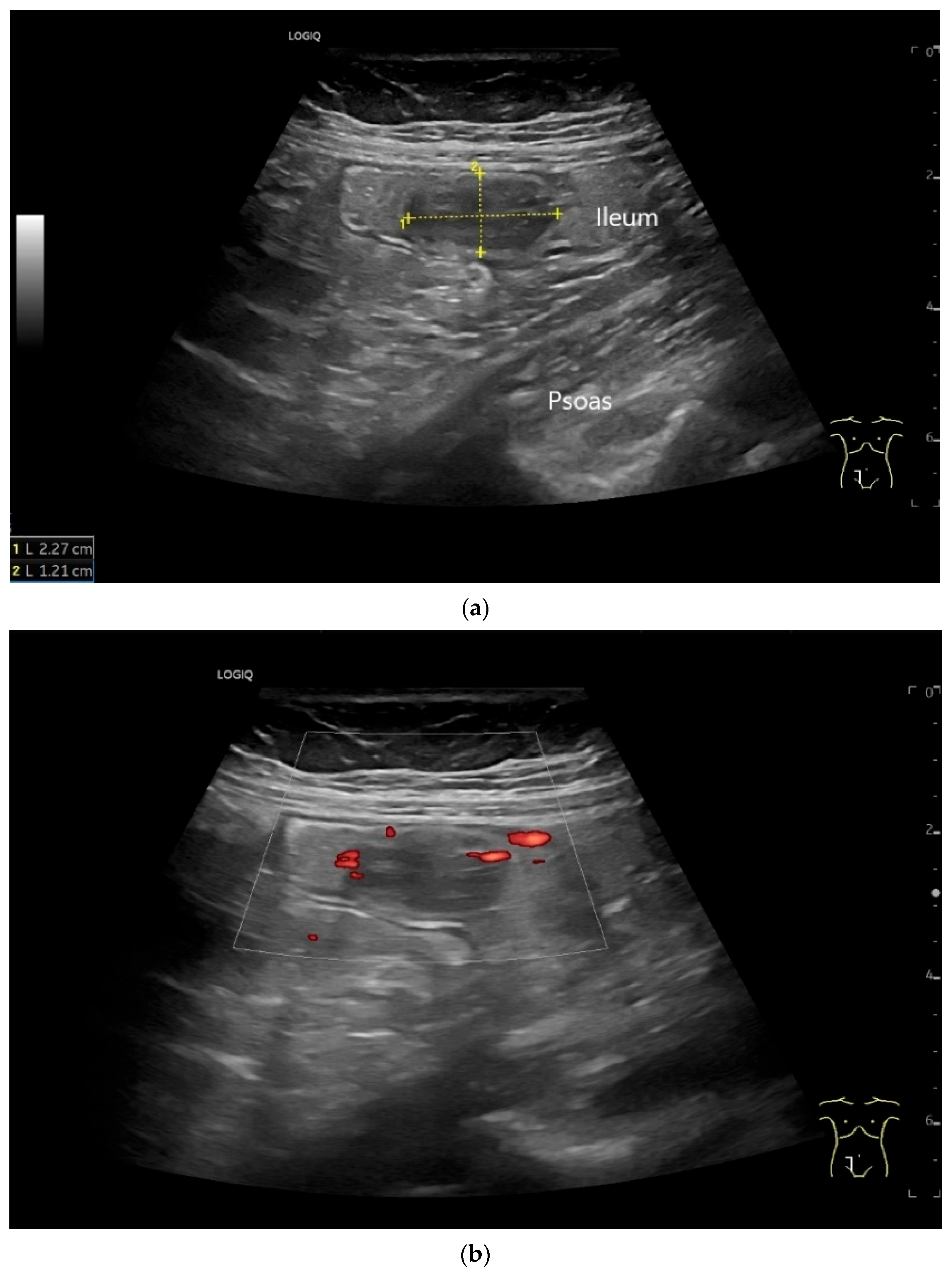
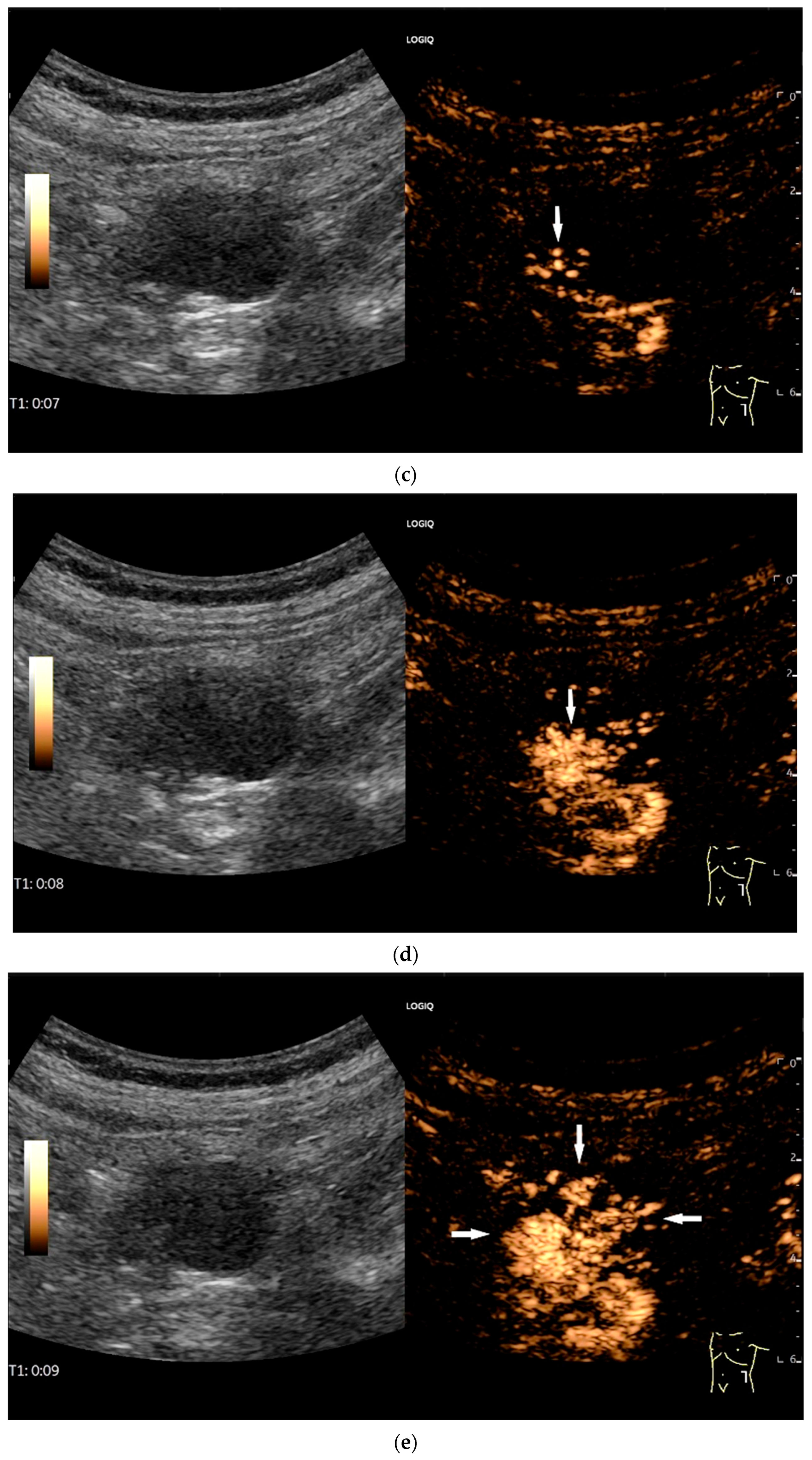
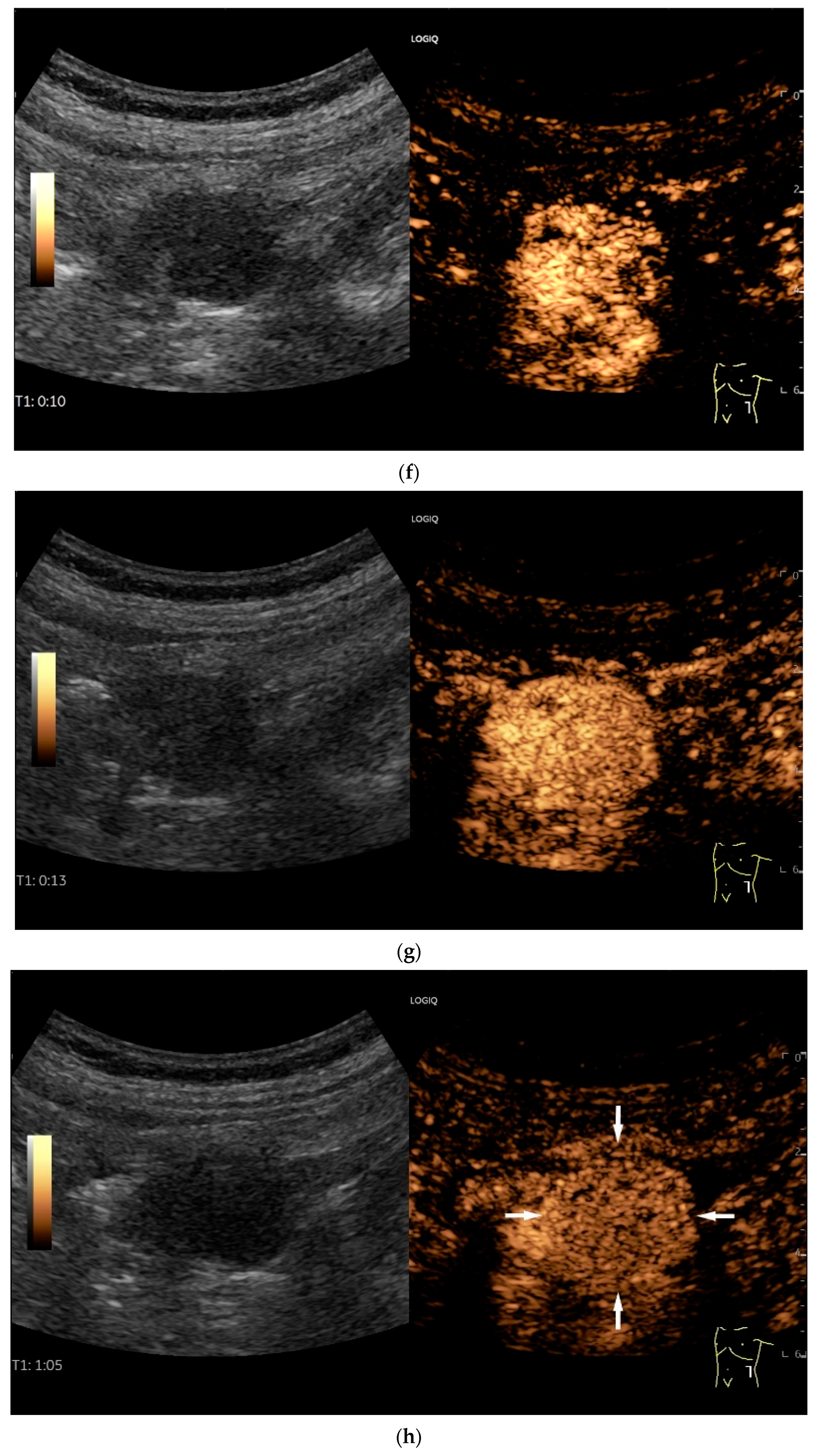
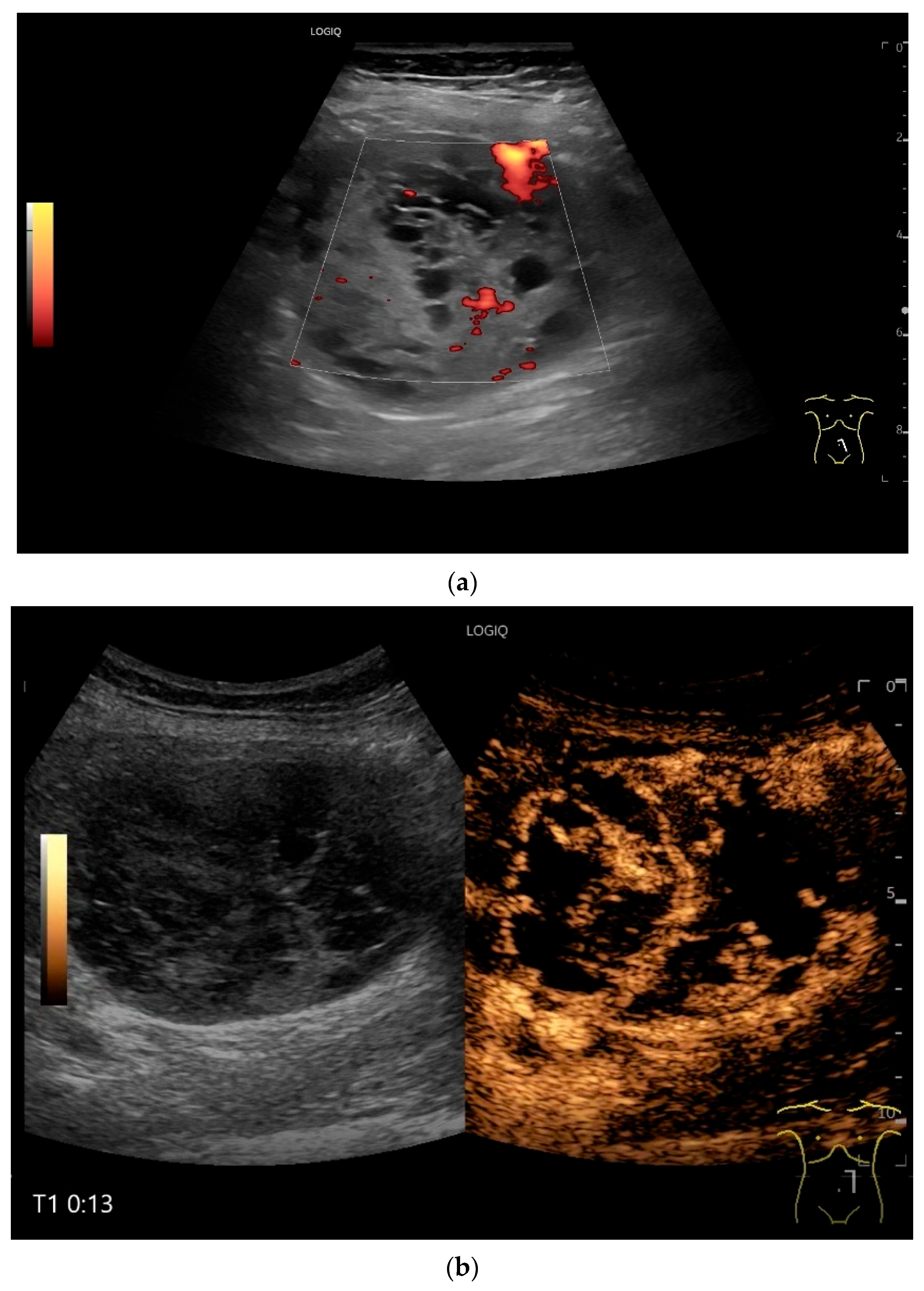
NET in the ileum. An oval polyp-like lesion is visible in the ileum (a). Small vessels are visible on power Doppler, indicating that the tissue is solid (b). In CEUS with 2.4 mL SonoVue (linear transducer 2–9 MHz), the tumor capsule shows enhancement. The tumor is marked with white arrows. At 22 s, vessels can be distinguished in the tumor, but there is no complete enhancement of the tumor. The ileum wall is also enhanced (yellow arrow) (c). Even at the end of the arterial phase at 34 s, the tumor is not completely enhanced. Two macro vessels are marked with an arrow. Distally, they show irregular vessel branches (d). The tumor was not located directly behind the ileocecal valve. Based on the US findings, the endoscope was inserted 20 cm into the terminal ileum, where the tumor was then discovered.
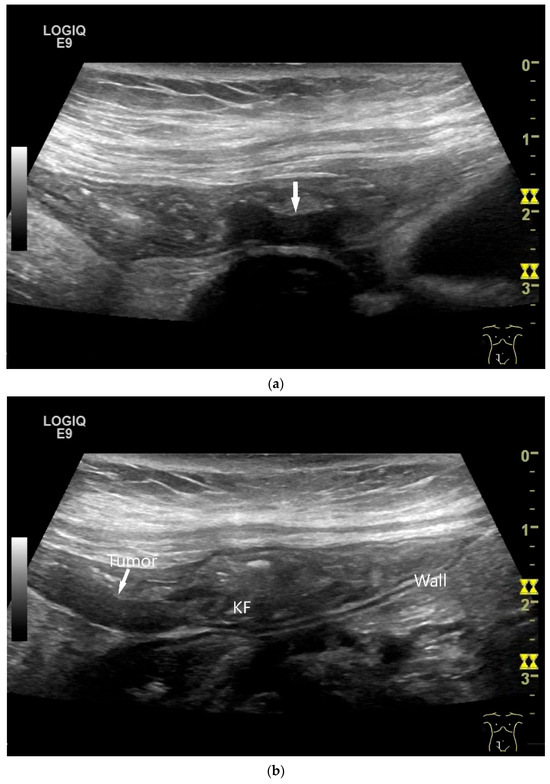
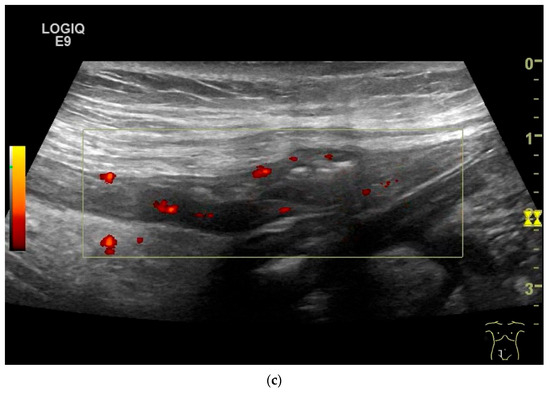
Figure 8.
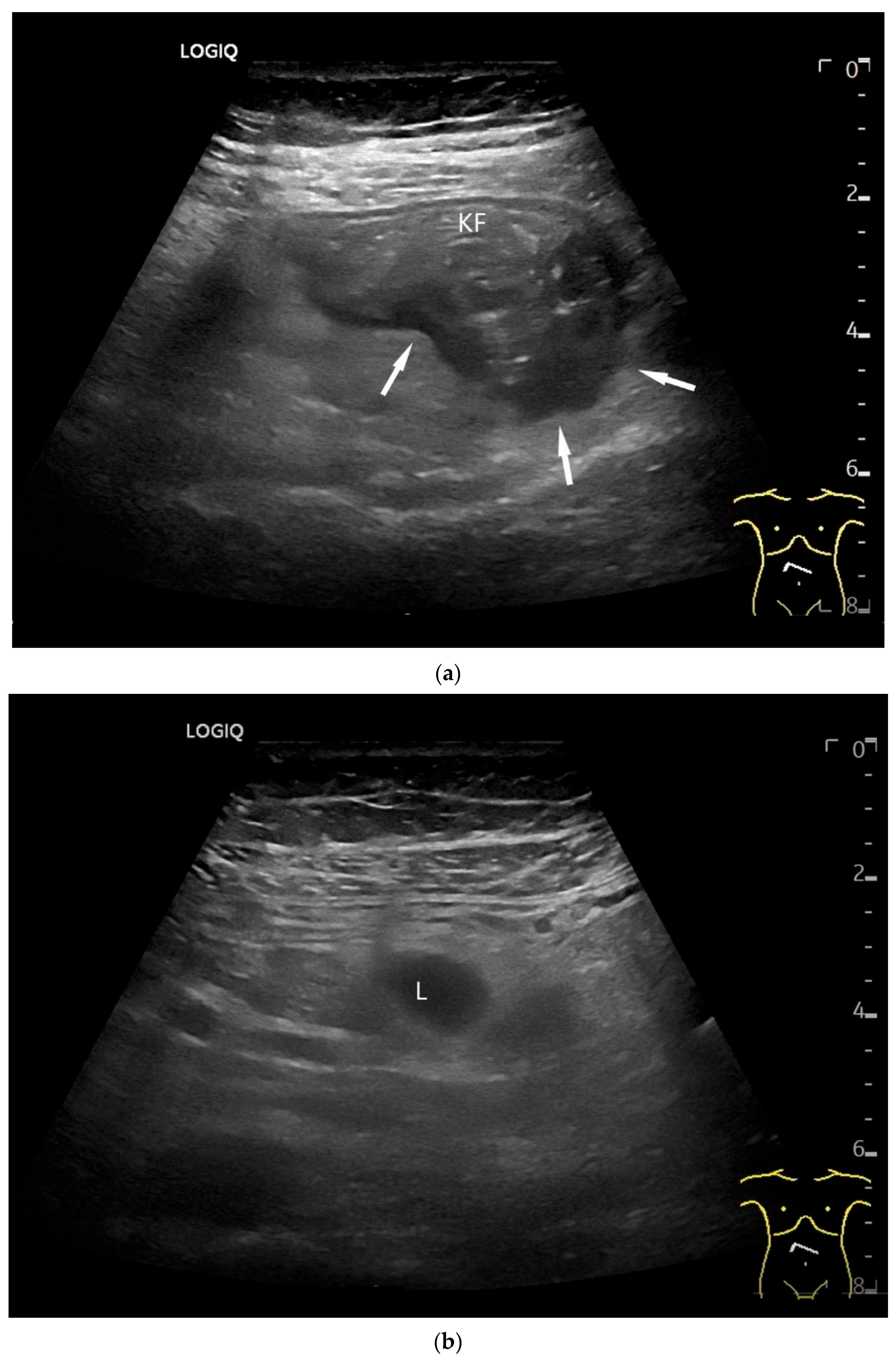
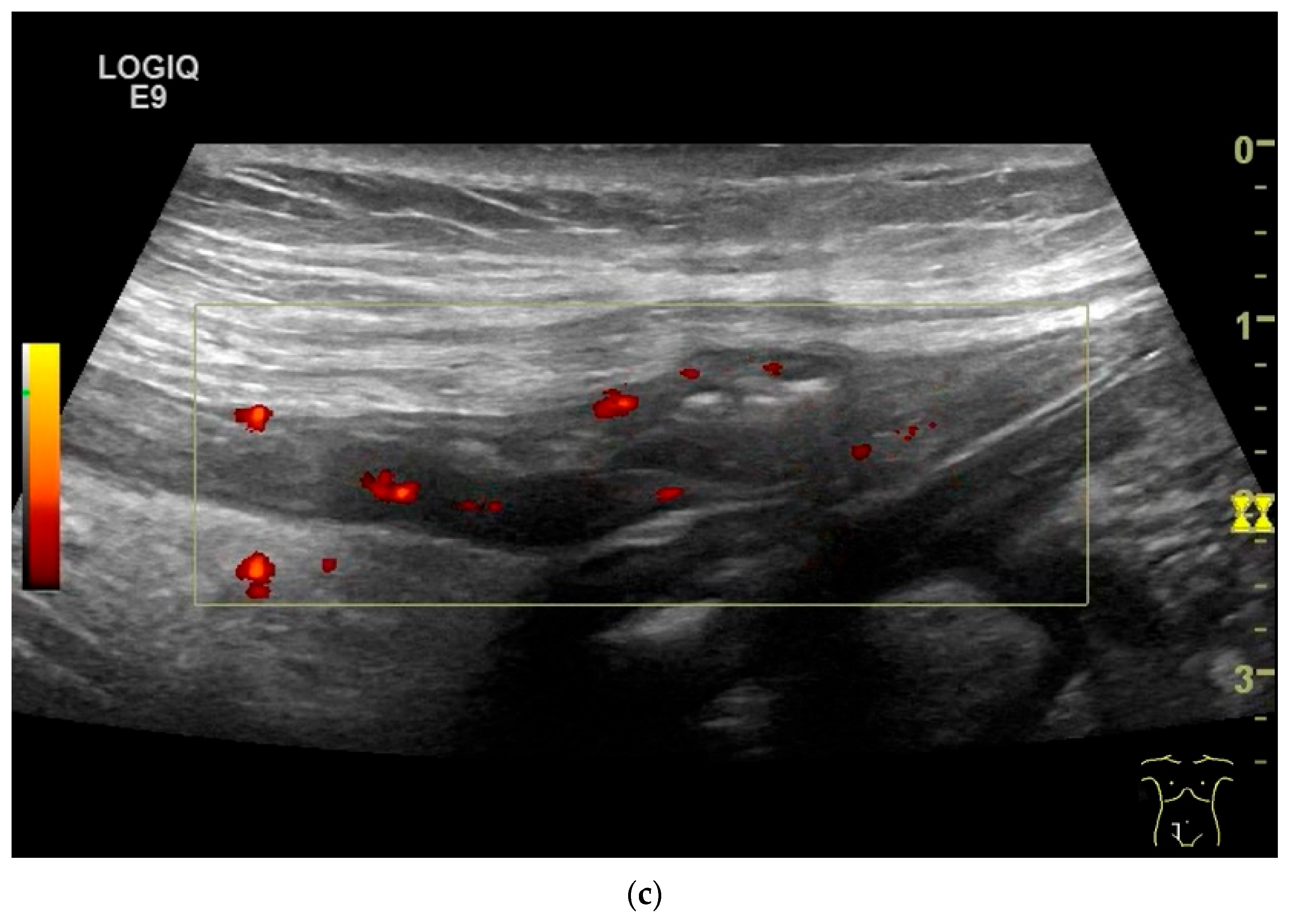
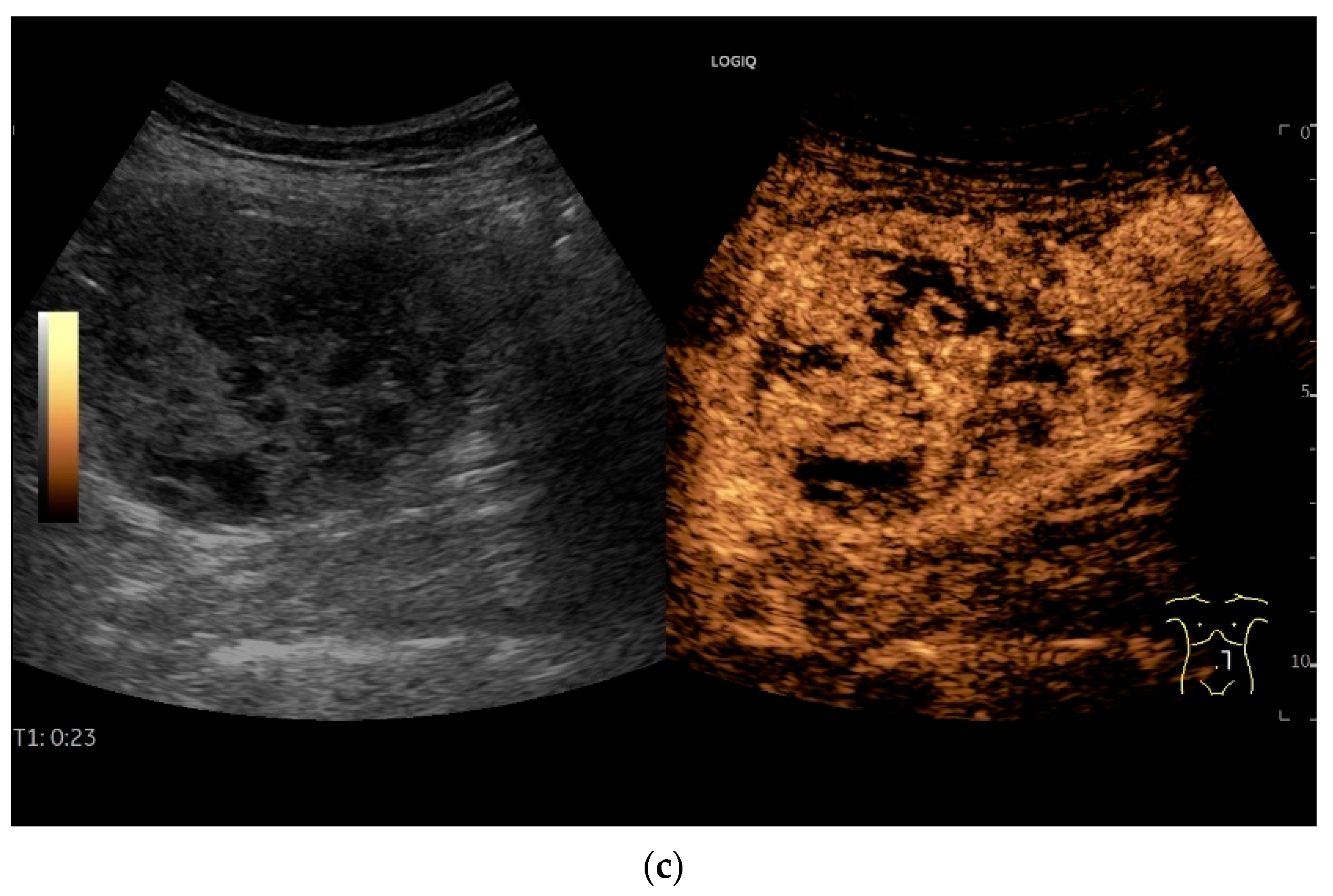
NET in the ileum. In the terminal ileum, there is a slight wall thickening <5 mm (arrow), but with loss of stratification (a). The tumor (arrow) changes position with peristalsis while the transducer position remains the same. With the 9 MHz linear transducer, the five layers of the unremarkable wall and Kerckring folds (KF) are visible (b). Small vessels can be distinguished in power Doppler (c).
8. Lymphoma, Hematolymphoid Tumors
Hematolymphoid tumors, and especially lymphomas, occur in the digestive system with varying frequency. They are either primary diseases or part of a systemic manifestation. A primary lymphoma of the digestive system has its origin and its main manifestation in the gastrointestinal tract (Table 3). Regional lymph nodes may be involved, but this is not obligatory. The gastrointestinal tract is the most common site for the occurrence of extranodal lymphomas accounting for 30–40% of all extranodal lymphomas. The small intestine is the second most common location in the gastrointestinal tract with 30% involvement after the stomach (50–60%) [2]. In the small intestine, the ileum and the ileocecal region are most frequently affected. Diffuse large B-cell lymphoma (DLBCL) is the most common type of intestinal lymphoma. Data on the other types of lymphoma vary in studies [2,98]. In the duodenum, follicular lymphoma is the most common subtype [99]. Lymphoid neoplasms (with the exception of histiocytic/dendritic cell neoplasms) are classified according to the Lugano classification [2,100].

Table 3.
Lymphomas with manifestation in the small intestine according to WHO classification 2019 [2].
8.1. Diffuse Large B-Cell Lymphoma (DLBCL)
Diffuse large B-cell lymphoma (DLBCL) is characterized by diffuse neoplastic proliferation of large B-lymphocytes. At 55–69%, DLBCL is the most common lymphoma in the gastrointestinal tract. It is the most common extranodal localization of DLBCL with approximately 40% [2,98]. The average age of onset is in the 6th decade of life. Patients typically present with pain, discomfort, or symptoms associated with luminal obstruction, perforation or gastrointestinal bleeding. B symptoms may be present. In cases of multilocular involvement, it may not be clear where the primary localization is. The lymph nodes may be affected [98].
8.2. Extranodal Marginal Zone Lymphoma of Mucosa-Associated Lymphoid Tissue (MALT Lymphoma)
Extranodal marginal zone lymphoma is an extranodal low-grade B-cell lymphoma that develops in mucosa or glandular tissue. It has the cytoarchitectural features of mucosa-associated lymphoid tissue (MALT). MALT lymphoma accounts for 7–8% of all B-cell lymphomas, 30–60% of primary gastric lymphomas and 2–28% of primary intestinal lymphomas [2,101,102]. The median age is in the sixth decade of life. Patients’ complaints are often non-specific or depend on the localization. Some patients are asymptomatic.
8.3. Follicular Lymphoma
Follicular lymphoma (FL) is a malignant lymphoid neoplasm of follicular-center B cells. Primary FLs of the gastrointestinal tract are rare and account for <4% of primary gastrointestinal lymphomas. The gastrointestinal tract is the most common extranodal manifestation of FLs. Primary digestive tract FLs often occur in the small intestine and colon [2,99]. Multiple nodular/polypoid lesions have been described endoscopically [103]. The mean age of onset is 50 years. FLs may be diagnosed incidentally, but non-specific abdominal symptoms, clinical symptoms of intestinal obstruction or gastrointestinal bleeding may also occur.
8.4. Duodenal-Type Follicular Lymphoma
Typical localization is the second portion of the duodenum. As a rule, these are incidental findings during gastroscopy. There are often further manifestations in the Jejunum and ileum [2,99]. This type of lymphoma is characterized by an indolent clinical course, and excellent outcome.
8.5. Mantle Cell Lymphoma
Mantle cell lymphoma (MCL) is a mature B-cell neoplasm. Subclinical tumor cell infiltration of the gastrointestinal tract is common. However, most patients present with stage III/IV disease, primarily with lymphadenopathy and bone marrow involvement [2]. Extranodal involvement usually occurs in conjunction with lymphadenopathy. In the gastrointestinal tract, superficial ulcers, large tumor masses and diffuse thickening of the gastrointestinal mucosa can be observed [104,105,106,107]. The median patient age is around 60 years.
8.6. Burkitt Lymphoma
Burkitt lymphoma (BL) is a highly aggressive B-cell lymphoma that often manifests itself in extranodal locations or as acute leukemia. The most common localization in the gastrointestinal tract is the ileocecal region. Typical symptoms are vomiting, abdominal pain, obstruction, jejunal intussusception, bleeding and anemia [2,62,108,109,110].
8.7. T-Cell Lymphoma
8.7.1. Enteropathy-Associated T-Cell Lymphoma
Enteropathy-associated T-cell lymphoma (EATL) is an intestinal T-cell lymphoma originating from intraepithelial lymphocytes in patients with celiac disease. EATL accounts for <5% of all gastrointestinal lymphomas. All patients have the genetic background of celiac disease [2,101,111]. As a rule, patients are over 50 years old. Symptoms include abdominal pain, diarrhea and weight loss. Manifestations with acute abdomen due to perforation or ileus have also been described.
8.7.2. Intestinal T-Cell Lymphoma NOS
Intestinal T-cell lymphoma (ITCL) NOS is an aggressive malignancy affecting mostly the colon and small intestine [2]. ITCL can be limited to one gastrointestinal organ or have multiple localizations. Dissemination to regional lymph nodes and extra gastrointestinal sites is also possible.
8.8. Imaging of Lymphomas
Due to the pronounced hypoechoic wall thickening with compressed central hyperechoic lumen reflex, there are comparative descriptions of the lymphoma manifestations in US as “doughnut sign” [112], “bull’s eye”, ‘target’ or “pseudo kidney” sign [110,113].
A common feature of small intestine lymphoma is a very pronounced wall thickening with marked hypoechogenicity, accompanied by enlarged lymph nodes. However, there are also focal circumscribed polypoid nodular changes or thickening of the mucosa and submucosa, which are mainly detectable endoscopically and are more difficult to delineate with cross-sectional imaging. The appearance of lymphomas in the small intestine depends on the extent of the different subtypes in the intestinal wall. The extent also depends on the stage of the disease (Figure 9 and Figure 10). Görg et al. [114] described the various manifestations of lymphoma in the gastrointestinal tract (including the small intestine) as mucosa-associated, transmural segmental, transmural circumferential, transmural nodular, and transmural bulky disease [114].
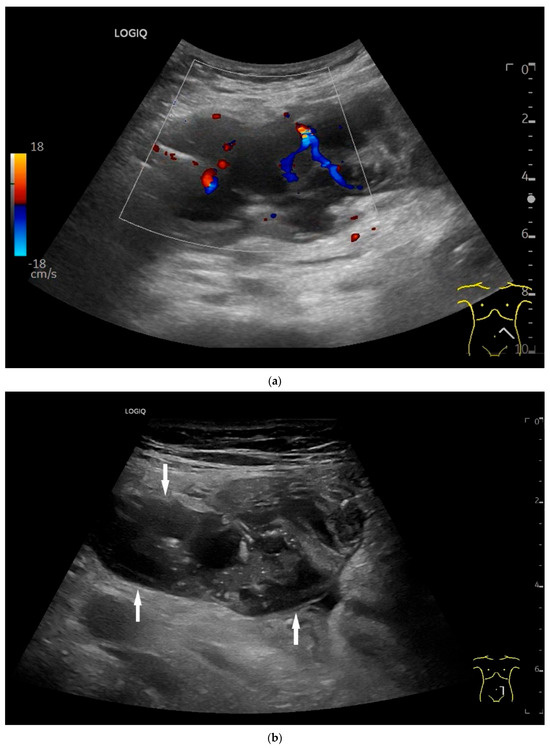
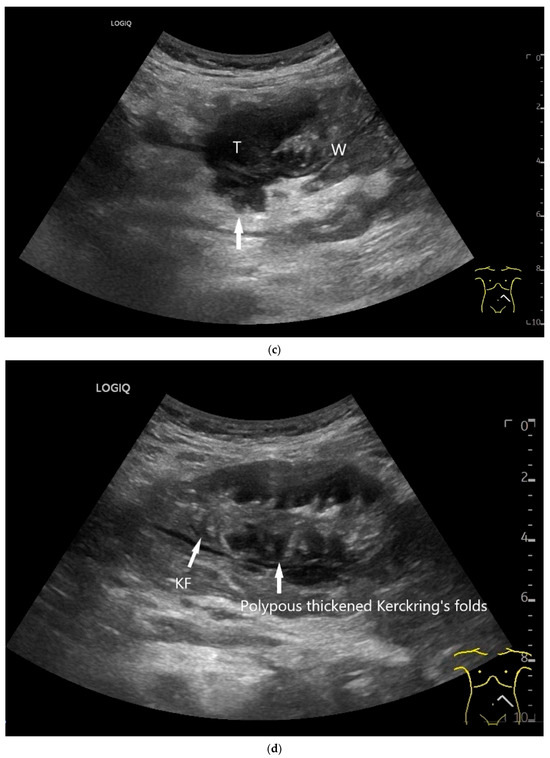
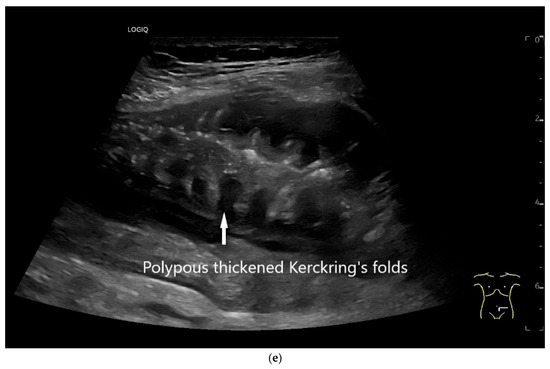
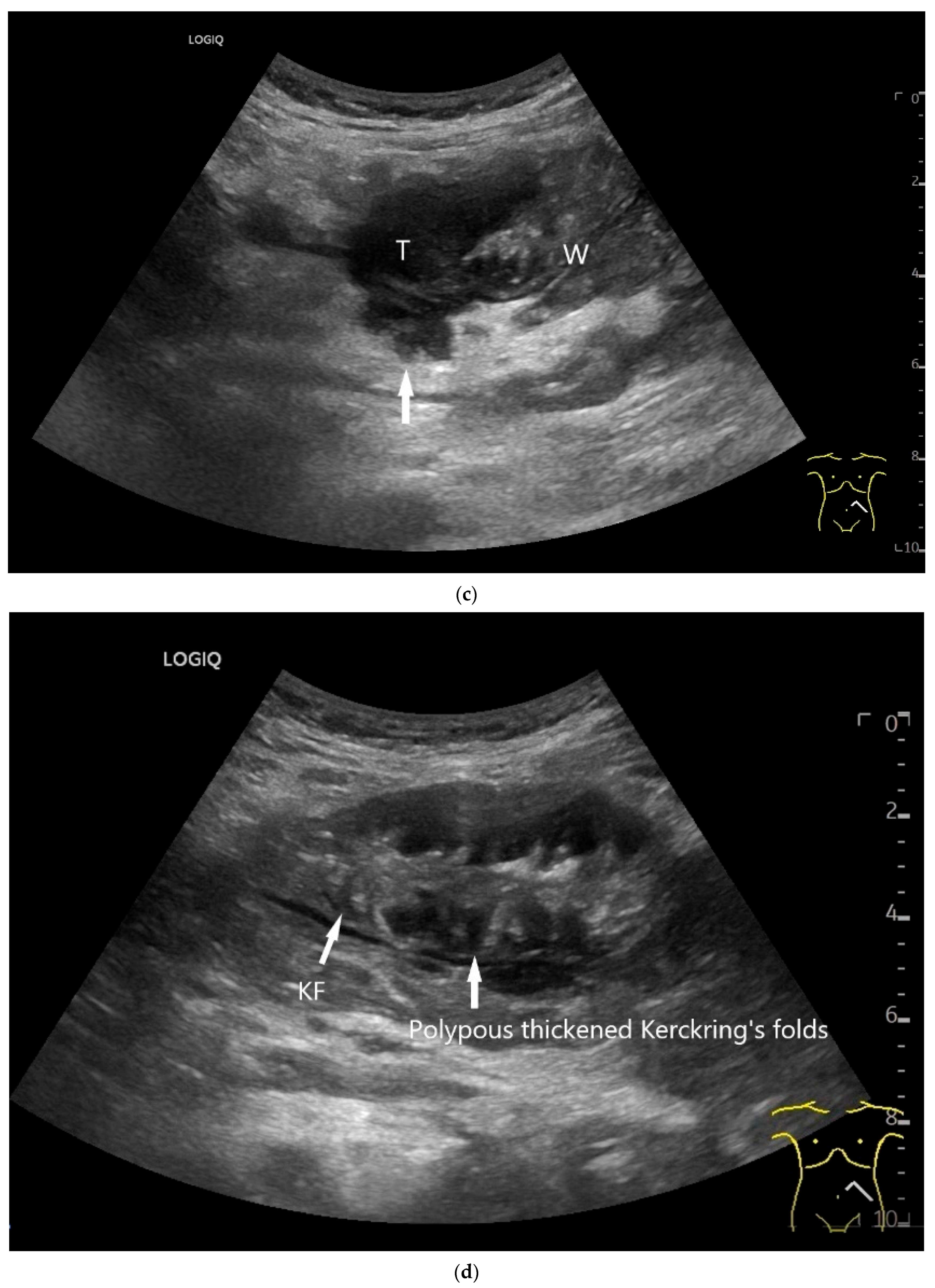
Figure 9.
Burkitt-Lymphoma. In a patient with weight loss, increased abdominal circumference, anemia, and physical weakness, the initial sonographic examination reveals an extensive tumor with intense hypoechogenicity and a connection to the jejunum in the left mid-abdomen. Despite its pronounced hypoechogenicity, a feeding vessel on CDI indicates a solid character (a). The tumor significantly thickens the wall and is intensely hypoechoic. The arrows point to the multisegmental hypoechoic wall thickenings (b). Normal wall structures are still visible (W), and the tumor extends beyond the wall (arrow) (c). In addition to delicate Kerckring folds (KF), there are significantly polypoid thickened KF with pronounced hypoechogenicity (d). The thickening of KF is very extensive (e). Wall thickening was found also in the stomach and colon and allowed endoscopic biopsy to establish the diagnosis.
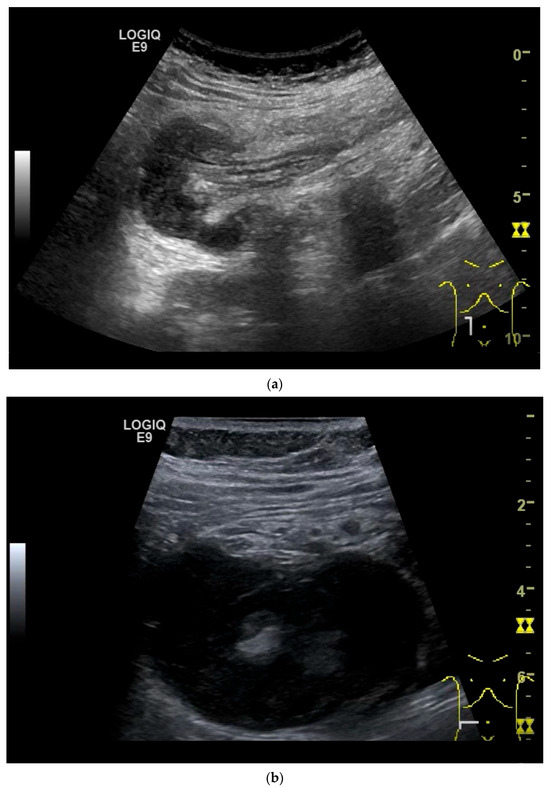
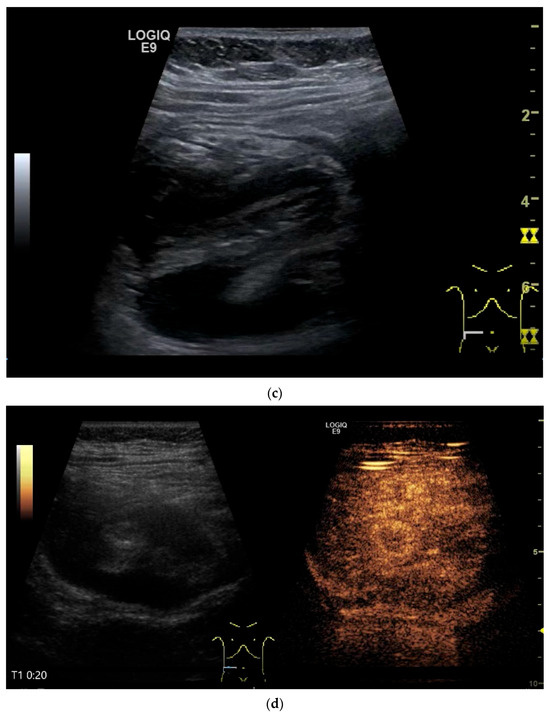
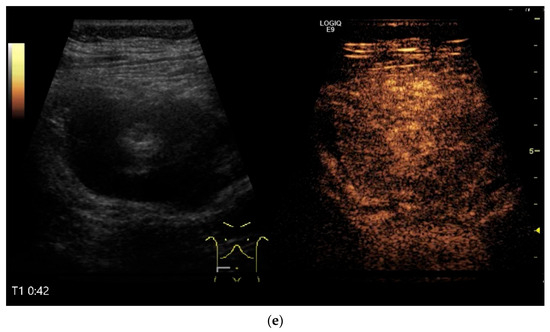
Figure 10.
Diffuse large B-cell lymphoma (DLBCL). In the right mid-lower abdomen, a large, smoothly circumscribed mass is present around the ileum (“pseudo kidney sign” (a)). Using a high-resolution linear transducer, the mass appears smoothly circumscribed and almost anechoic (b). The ileum runs centrally, and the echogenic wall is clearly defined (c). This wall does not merge into the mass. Since the mass is almost anechoic, it is also difficult in different transducer positions to distinguish whether it is a liquid lesion or a solid mass (b,c). CEUS with 2.4 mL SonoVue (9 MHz linear transducer) shows homogeneous arterial enhancement (d), but the intensity decreases with time in the venous phase (e). The ileal wall enhancement centrally within the mass is more intense than that of the tumor (d,e).
In a study of Hasaballah et al. [113] including 21 patients with small intestine lymphoma (90% DBLCL), marked wall thickening (15.6 ± 5.9 mm) with loss of stratification (76%) and high hypoechogenicity were the typical features of the lymphoma manifestations. In 85.7% the lymphoma was unilocular. Further features were dilatation of the intestinal lumen and periintestinal enlarged lymph nodes. US-guided biopsy was performed in 33% of patients and was successful in 100% [113].
Zhang et al. [115] distinguished the mass type, wall thickening type and non-specific signs in 19 intestine lymphomas. The mass type was characterized by solid and cystic-solid hypoechoic lesions with well-defined margins. Among them were pronounced hypoechoic lesions with dorsal enhanced cancellation. On CDI, abundant blood signals were visible in large masses.
The wall thickening type was characterized by thickening of the bowel walls with hypoechoic (including marked hypoechoic) foci and posterior acoustic enhancement. Nonspecific signs included dilatation of the bowel and enlargement of the mesenteric lymph nodes [115].
Cui et al. [116] described four sonographic patterns on B-mode US in 18 patients with small intestine lymphoma. The majority (61%) were DLBCL. The mass type (66.7%) presented as circumferential, marked intestinal wall thickening. The wall was highly hypoechoic with loss of stratification. The central luminal reflex was hyperechoic. The infiltrative type (5.6%) was a segmental thickening of the bowel wall without visible mass formation with a bowel wall thickness of 2.6 cm in a single case. The mesenteric type (22.2%) was characterized by multiple hyperechoic heterogeneous mesenteric masses with or without involvement of the adjacent bowel wall. The lesion sometimes encapsulated mesenteric vessels without stenosis of the vessel lumen. The mixed type (5.6%) showed a combination of two patterns [116].
On CEUS all small intestine lymphomas showed arterial enhancement (hyperenhancement in 17 of 18 cases) followed by venous washout. Tumor necrosis was observed in 61% of cases, which occurred more frequently in aggressive subtypes than in indolent subtypes. There was no correlation between tumor size and necrosis [116].
Standard imaging is performed using CT, MRI and PET-CT. US-guided sampling can be used for histological confirmation. Follow-up checks for treatment response can include US.
9. Mesenchymal Tumors
The most common mesenchymal tumor in the gastrointestinal tract is the gastrointestinal stromal tumor (GIST) with an overall incidence estimated at 15/100,000 people [117,118]. GIST amount to 1–3% of all gastrointestinal neoplasms [119,120]. The small intestine (30%) is the second most common location after the stomach (50–70%) [3,121]. All other mesenchymal tumors except GIST are very rare in the small intestine. According to the WHO classification of soft tissue and bone tumors, there are four categories of tumor behavior: benign, intermediate (locally aggressive), intermediate (rarely metastatic), and malignant [3,122] (Table 4).

Table 4.
Mesenchymal tumors with manifestation in the small intestine according to WHO-classification 2019 [3].
9.1. Gastrointestinal Stromal Tumor
GIST are the most common mesenchymal tumors in the gastrointestinal tract and in the small intestine. They are also referred to as SIST (small intestinal stromal tumor) [123]. In a study by Miettinen et al. of 906 small bowel GIST, the majority manifested with gastrointestinal bleeding followed by acute abdomen, only 18% were incidentalomas [121]. Gastrointestinal bleeding was also the most common clinical sign of GIST in other studies [123,124]. In rare cases, a GIST can also manifest itself with an upstream intussusception of the small intestine [125]. In a study of 75 small intestine GIST, the most common site was the jejunum (57%), followed by the ileum (32%) and duodenum (11%) [123].
GIST can be associated with several genetic syndromes. Among these, patients with neurofibromatosis type 1 may have multiple GIST in the small intestine [3,121,126].
In the study of 75 small intestine GISTs with a size of 0.9–20 cm, 51% of them were <5 cm, only 30% were detected by US. In contrast, dual-source CT diagnosed 87.3% of the tumors [123]. In another study of 32 patients with small intestine GIST and a mean tumor size of 5.3 cm, only 28% underwent an US examination. In eight of nine cases, the tumor could be identified but not assigned to an organ. This was very similar on MRI. An assignment to the intestinal wall was achieved by CT in 54% and by CT angiography in 71% [124].
GIST originate from the myenteric plexus in the muscularis propria and, therefore, are usually associated to the 4th wall layer (lamina muscularis propria). In an early US study [127] of cases with unexplained gastrointestinal bleeding, careful examination with 7.5 MHz transducers revealed mesenchymal tumors of a size of 3–6 cm. The positional variability caused by the motility of the small intestine was striking. Intermittent intussusceptions were also conspicuous [127].
Precise descriptions of subepithelial intramural masses including GIST are known from endoscopic ultrasound (EUS) [128]. Despite the lower resolution and proximity, the findings known from EUS can generally be transferred to US. However, in the small intestine, it is much more difficult to classify the layers, as the healthy wall is only up to 2 mm thick. GIST can develop into the lumen of the gastrointestinal tract or be located extraluminally. On EUS as well as on US, small GIST are generally round, homogeneous, and hypoechoic compared to submucosal layer, but isoechoic to the Lamina muscularis propria. On CDI, they show macrovessels. Larger GIST may be lobulated and heterogeneous, show necroses as hypoechoic or nonechoic liquid areas as well as calcifications. Air inclusions in the tumor are an indication of deep ulceration of the tumor surface with central necrosis and connection to the gastrointestinal tract lumen [55] (Figure 11 and Figure 12).
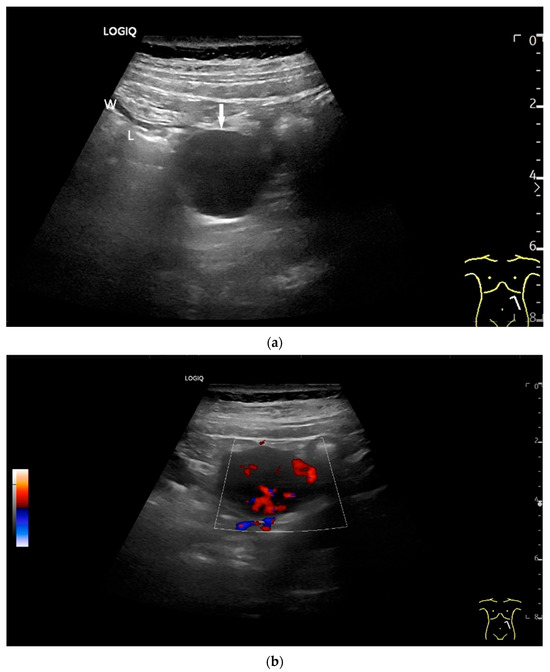
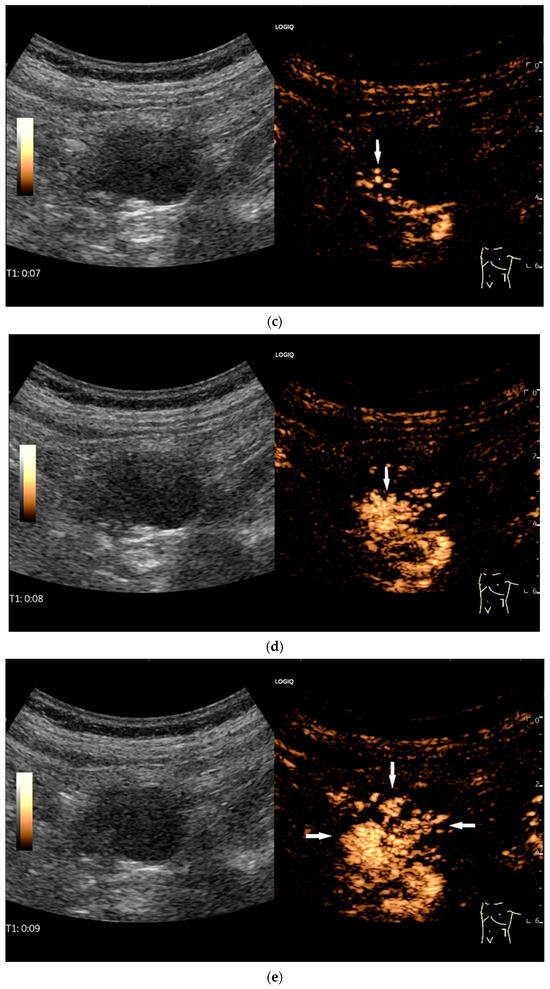
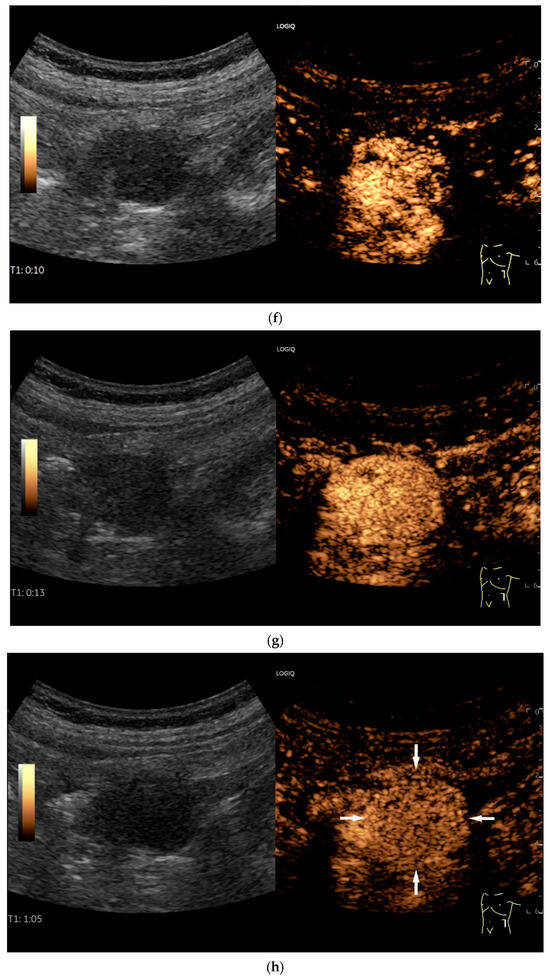
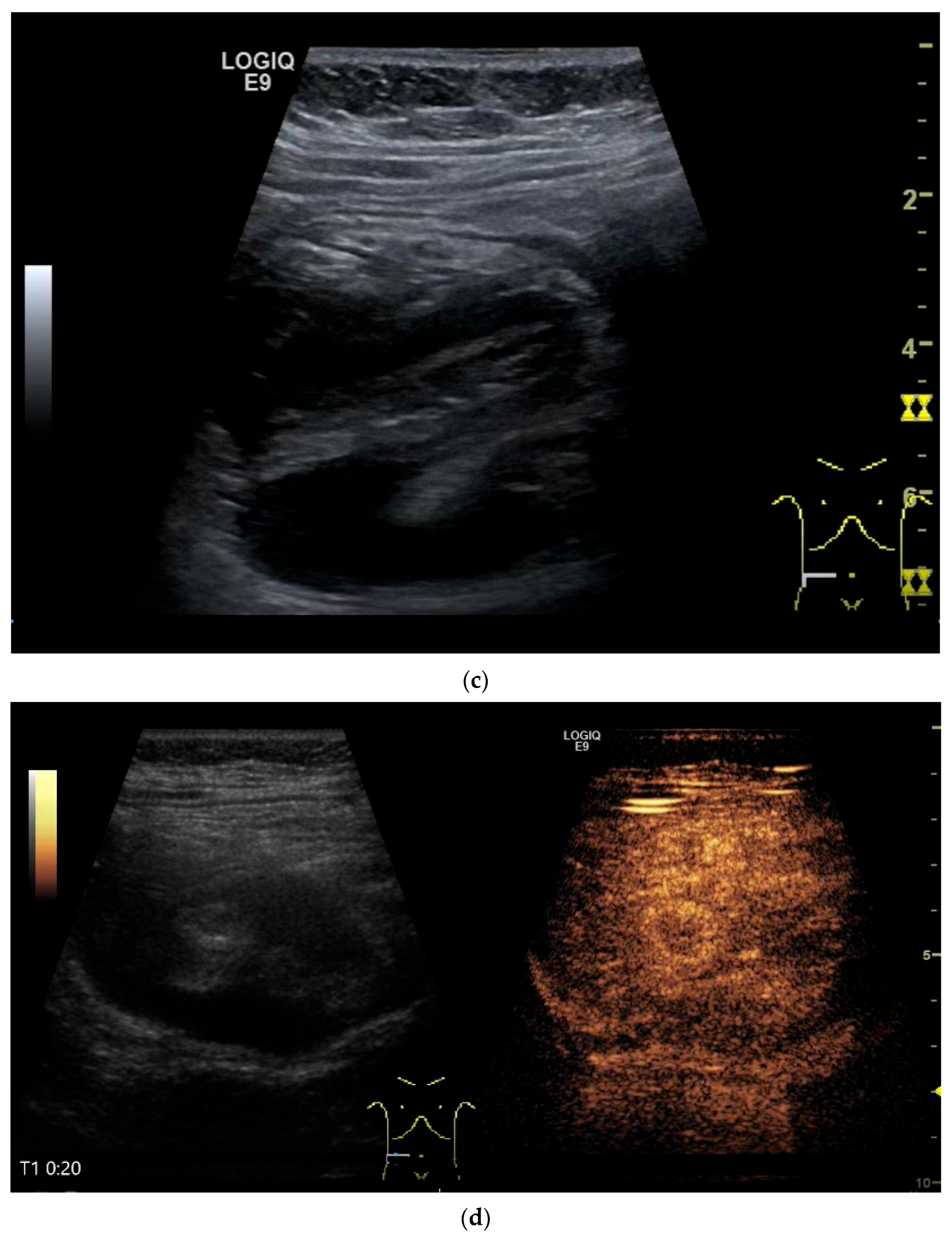
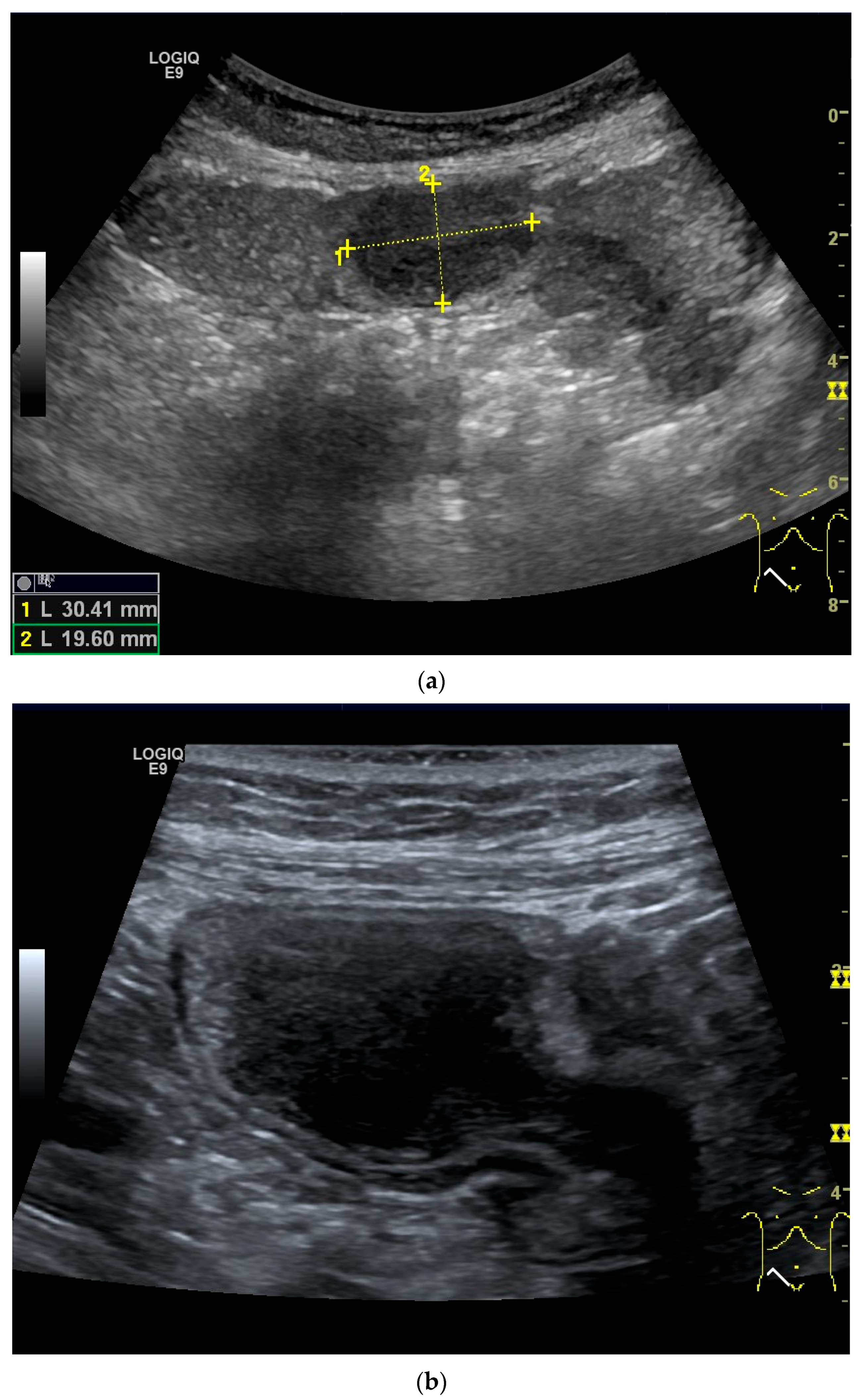
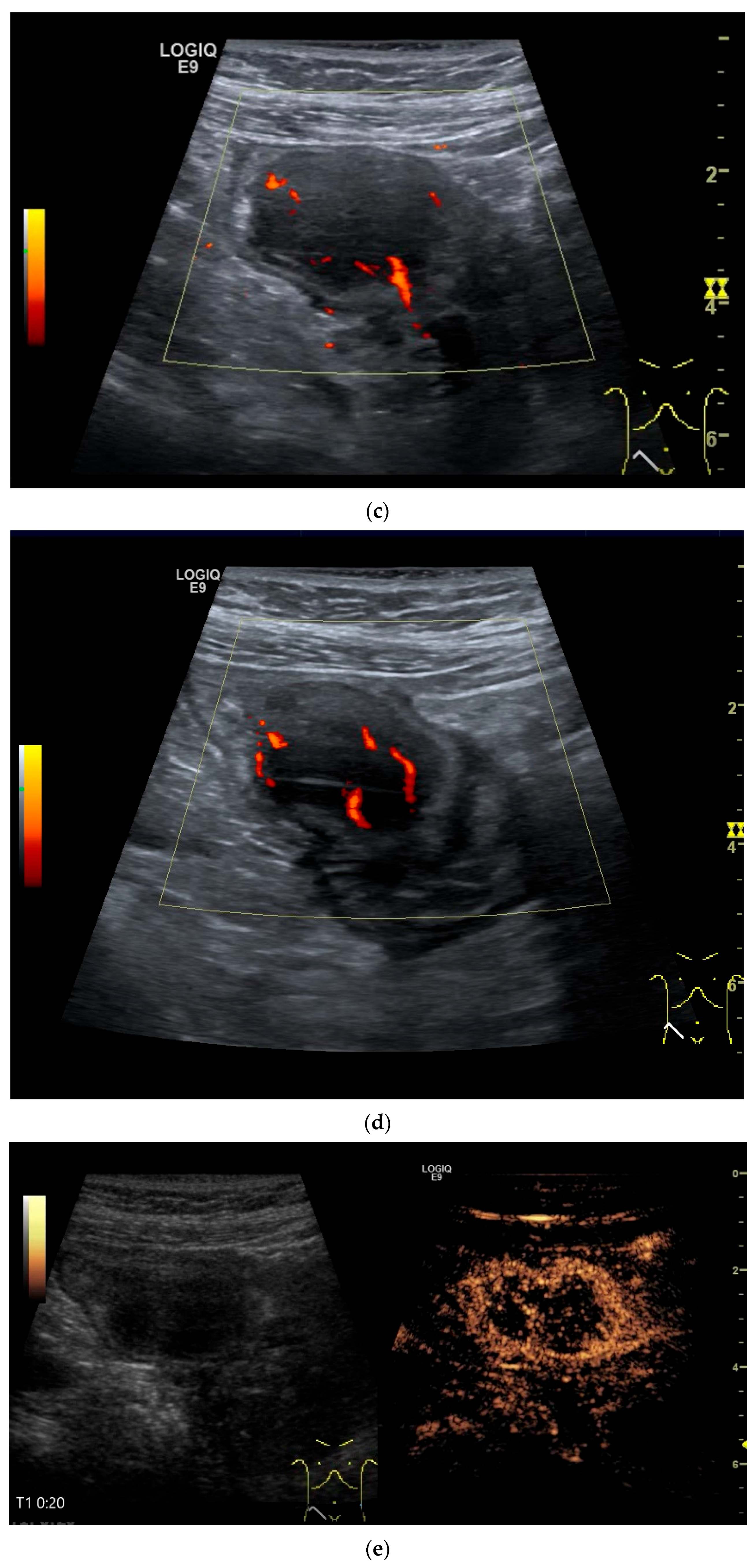
Figure 11.
GIST. A 30 mm large, very hypoechoic, almost anechoic round mass is visible in the left upper abdomen. This is located in the jejunum; the wall (W) and lumen (L) are visible (a). Macro vessels can be distinguished on Power Doppler, demonstrating that the lesion is solid and not cystic (b). On CEUS with 2.4 mL SonoVue (linear transducer 9 MHz), a small wheel-spoke-like vascular branching is visible at the margin (arrow) (c) with centrifugal enhancement (arrow) (d). Hyperenhancement is heterogeneous in the early arterial phase (e,f) and becomes homogeneous in the later course of the arterial phase (g). The extent of the heterogeneously enhanced tumor is marked with arrows (e). The intensity of the enhancement decreases during the first minute. The tumor is marked with arrows (h). Jejunal segment resection revealed the histology of an epithelioid GIST.
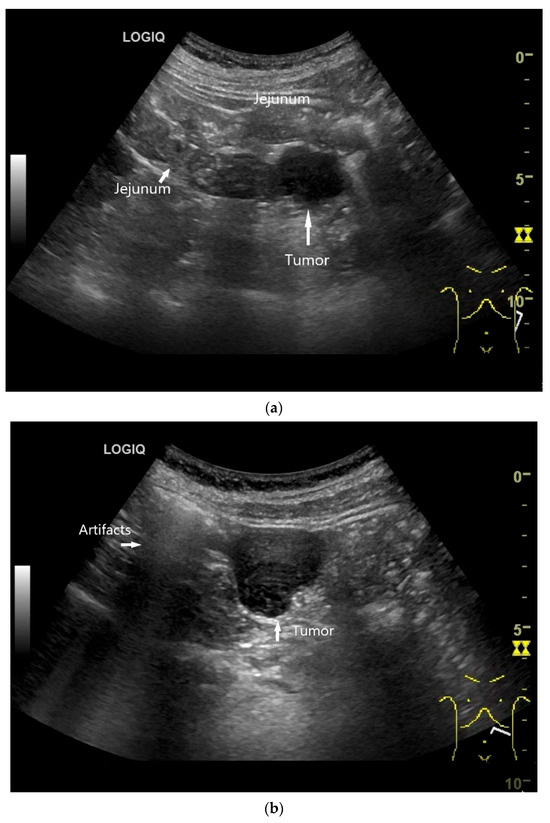
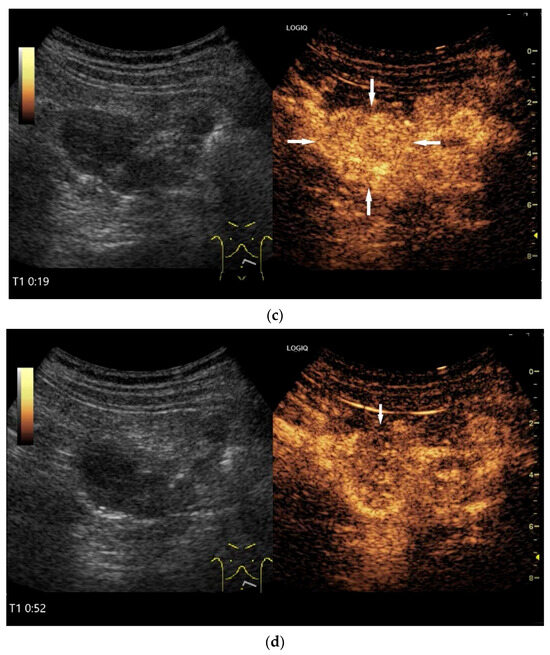
Figure 12.
GIST. Incidental finding of a 35 mm, slightly polycyclic, homogeneous hypoechoic mass in the left upper abdomen (a,b). This changes position with the movements of the small intestine. In CEUS with 1.2 mL SonoVue using the abdominal sector transducer (1–5 MHz), the mass shows homogeneous hyperenhancement in the arterial phase (c). The intensity then decreases (d). The tumor is marked with arrows in CEUS.
In a multicenter study, 98% of GIST of all locations, including the small intestine (no specific location), showed arterial hyperenhancement on contrast enhanced low mechanical index EUS (CELMI-EUS) with SonoVue. Some demonstrated non-enhanced areas as evidence of necrosis [55].
9.2. Inflammatory Myofibroblastic Tumor (IMFT)
On US, small intestine IMFT is described as well-defined and hypoechoic. The tumor may appear heterogeneous with hyperechoic parts and dorsal attenuation as well as small calcifications [129]. On CEUS with SonoVue, the IMFT demonstrated contrast enhancement in the arterial phase but slightly lower intensity than the adjacent small bowel wall, followed by central washout with marginal enhancement [129].
9.3. Inflammatory Fibroid Tumor (Vanek’s Tumor)
The only US description of an ileal IFP is as a clearly defined “target-like” lesion, which led to ileo-ileal intussusception [130] (Figure 13).
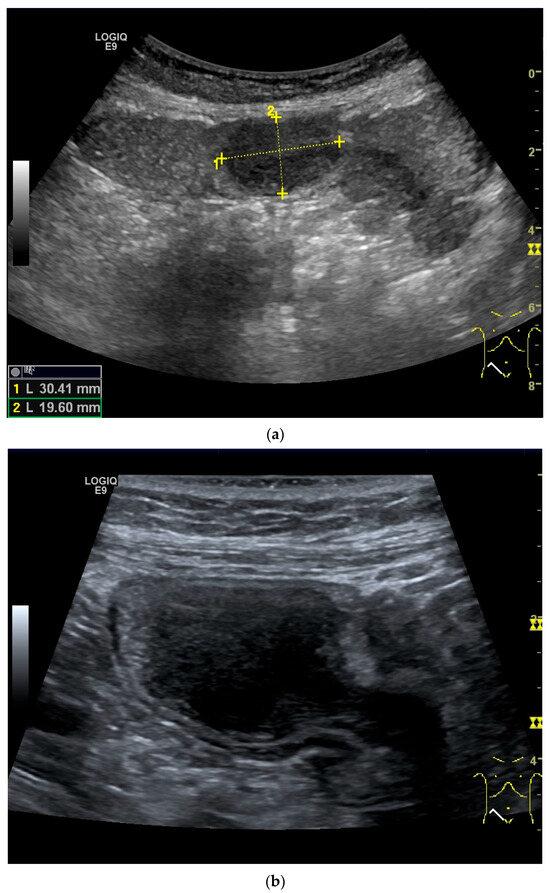
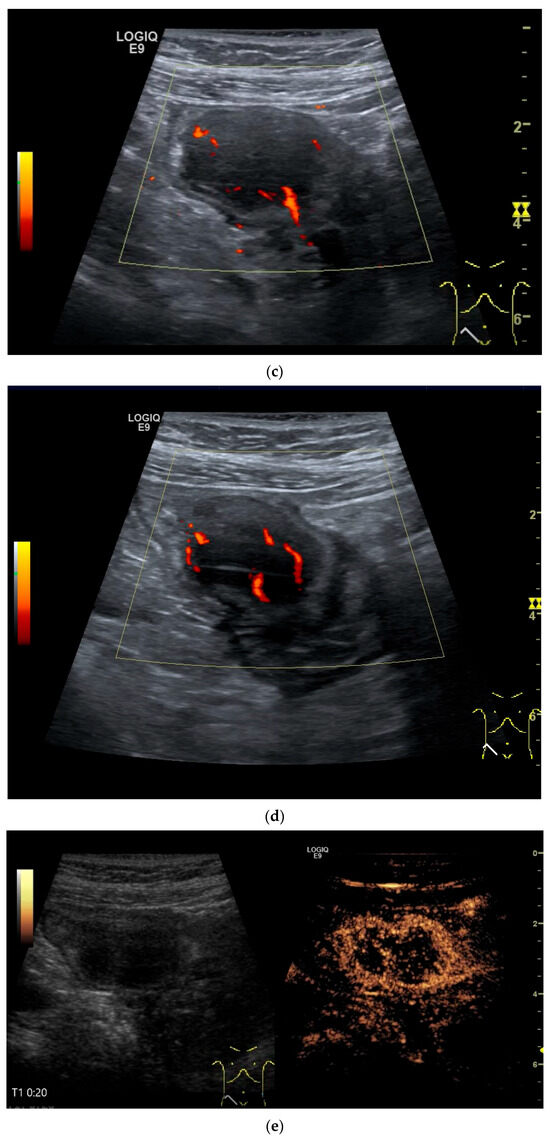
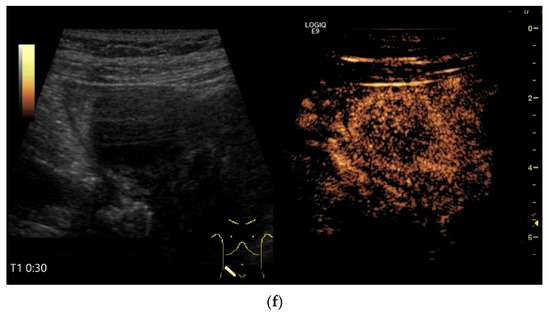
Figure 13.
Inflammatory fibroid tumor (Vanek’s tumor). In cases of unclear occult gastrointestinal bleeding, a smooth-edged oval hypoechoic mass measuring up to 30 mm in size can be seen in the terminal ileum. Examination with the abdominal sector transducer 1–5 MHz (a) and linear transducer 2–9 MHz (b). In power Doppler, a feeding vessel (c) and macro vessels in the mass (d) are visible. In CEUS with 2.4 mL SonoVue using a 9 MHz linear transducer, hyperenhancement is only visible at the edge in the arterial phase. Few vascular signals are visible in the lesion (e). The tumor remains hypoenhanced (f).
9.4. Desmoid Tumors
Desmoid tumors are locally aggressive mesenchymal tumors with a tendency to local recurrence. They are generally very rare. The incidence of desmoid tumors is 0.03% of all neoplasms and less than 3% of all soft tissue tumors [131]. There are associations with familial adenomatous polyposis, Gardner syndrome and mutations in the adenomatous polyposis coli (APC) gene [132]. Small intestine manifestations are very rare without any description of US or CEUS features in the few case reports [132] (Figure 14). Apart from musculoskeletal US descriptions, there are descriptions of US characteristics including CEUS and EUS of desmoid tumors in solid abdominal organs [133]. Desmoid tumors appear as well-circumscribed, homogeneous masses on CT [132].
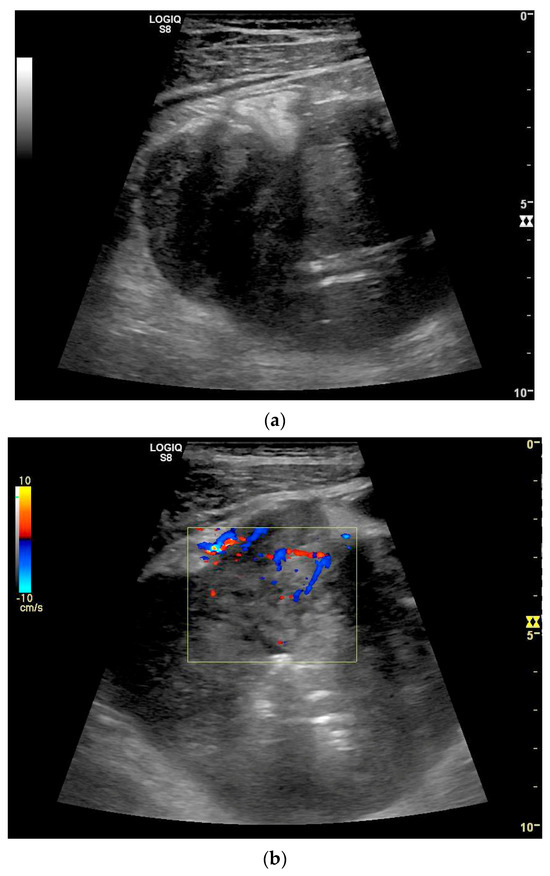
Figure 14.
Desmoid fibromatosis of the terminal ileum. Initial pain in the right lower abdomen and suspicion of acute appendicitis. US revealed a polygonal tumor measuring up to 9 cm. B-mode US with reference to the small intestine (a), marginal vessels in duplex (b). CT revealed a tumor presumably in the terminal ileum with low contrast uptake, central necrosis, and air pockets. In addition, covered perforation was suspected. Surgical ileocecal resection was performed, followed by histological diagnosis of desmoid fibromatosis.
9.5. Lymphangioma
Lymphangiomas are very rare benign tumors of the gastrointestinal tract and can occur in the intestine, the gall bladder and the pancreas [3]. However, they are more likely to be vascular malformations that are filled with lymph. Lymphangiomas consist of different sized spaces that contain lymph. They exist in three variants: capillary, cavernous and cystic variants [134]. Children are usually affected. However, lymphangiomas can also be diagnosed in adulthood if they cause intussusception or other acute obstructive symptoms due to the increase in size [135,136,137]. However, as with other intestinal tumors, lymphangioma can manifest with gastrointestinal bleeding. US can be used to detect and localize the most anechoic lesions and determine their cystic appearance [134,135] (Figure 15). On CT, the tumors appear as homogeneous, non-enhancing lesions with variable attenuation values depending on whether the fluid is chylous or serous [134].
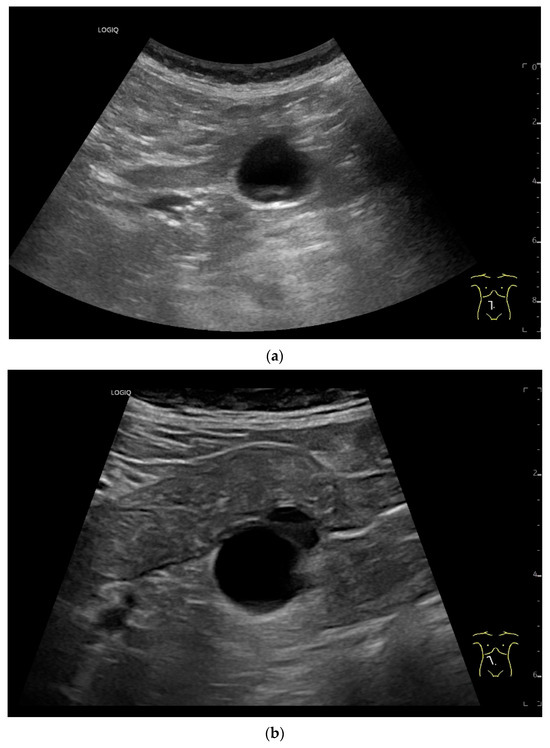
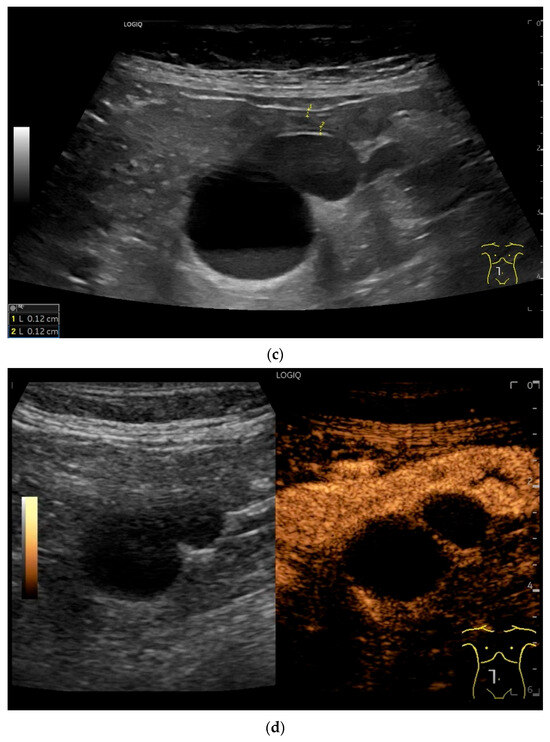
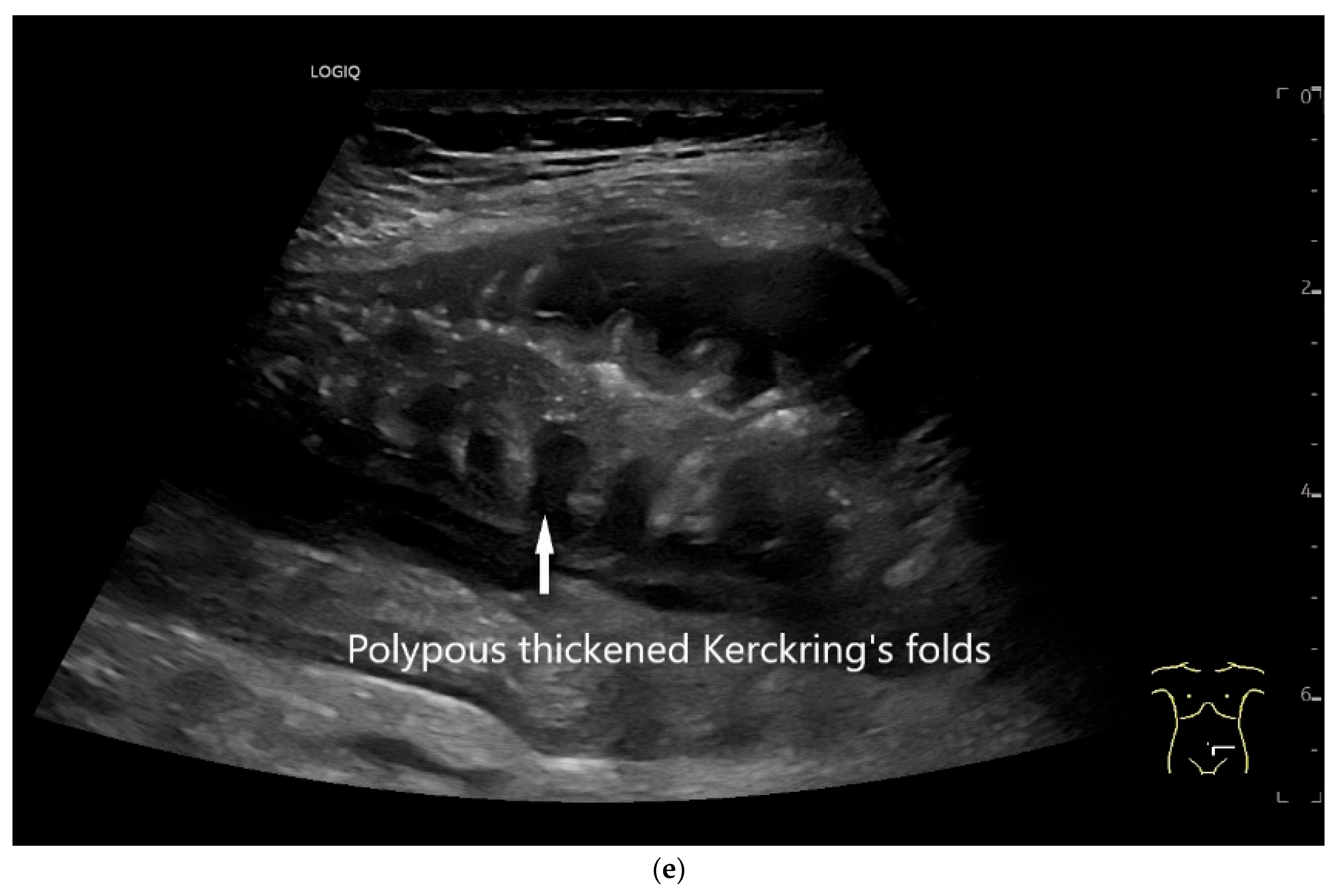
Figure 15.
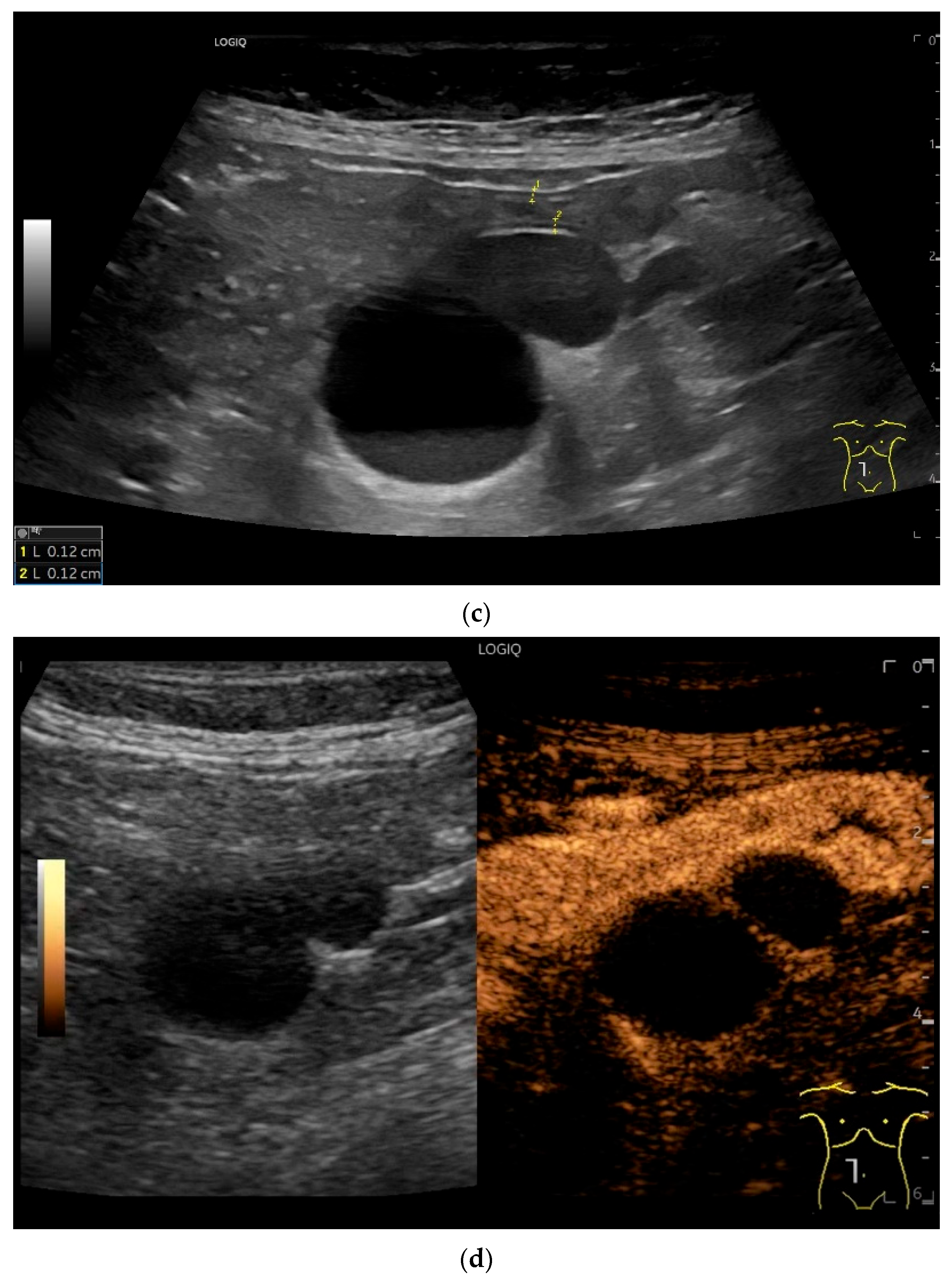
Cyst related to the serosa of the Jejunum. An incidental finding reveals a smoothly defined, non-echoic lesion in the right mid-abdomen using the abdominal sector transducer (a). Using the linear transducer, the cystic lesion can be visualized adjacent to the small intestine/jejunum (b). Kerckring folds are visible. The cystic lesion moves with the jejunum. The lesion is in contact with the small intestine wall (between the markers) (c). In CEUS with 2.0 mL SonoVue (linear transducer 9 MHz), the wall shows subtle enhancement. The lesion itself shows no enhancement and is therefore not solid, but liquid. The wall of the adjacent small intestine shows clear enhancement (d). Differential diagnoses include lymphangioma or mesothelial cyst. Since the elderly patient was completely symptom-free and showed no signs of gastrointestinal bleeding, no surgery was performed. The findings remained on follow-up.
9.6. Lipoma
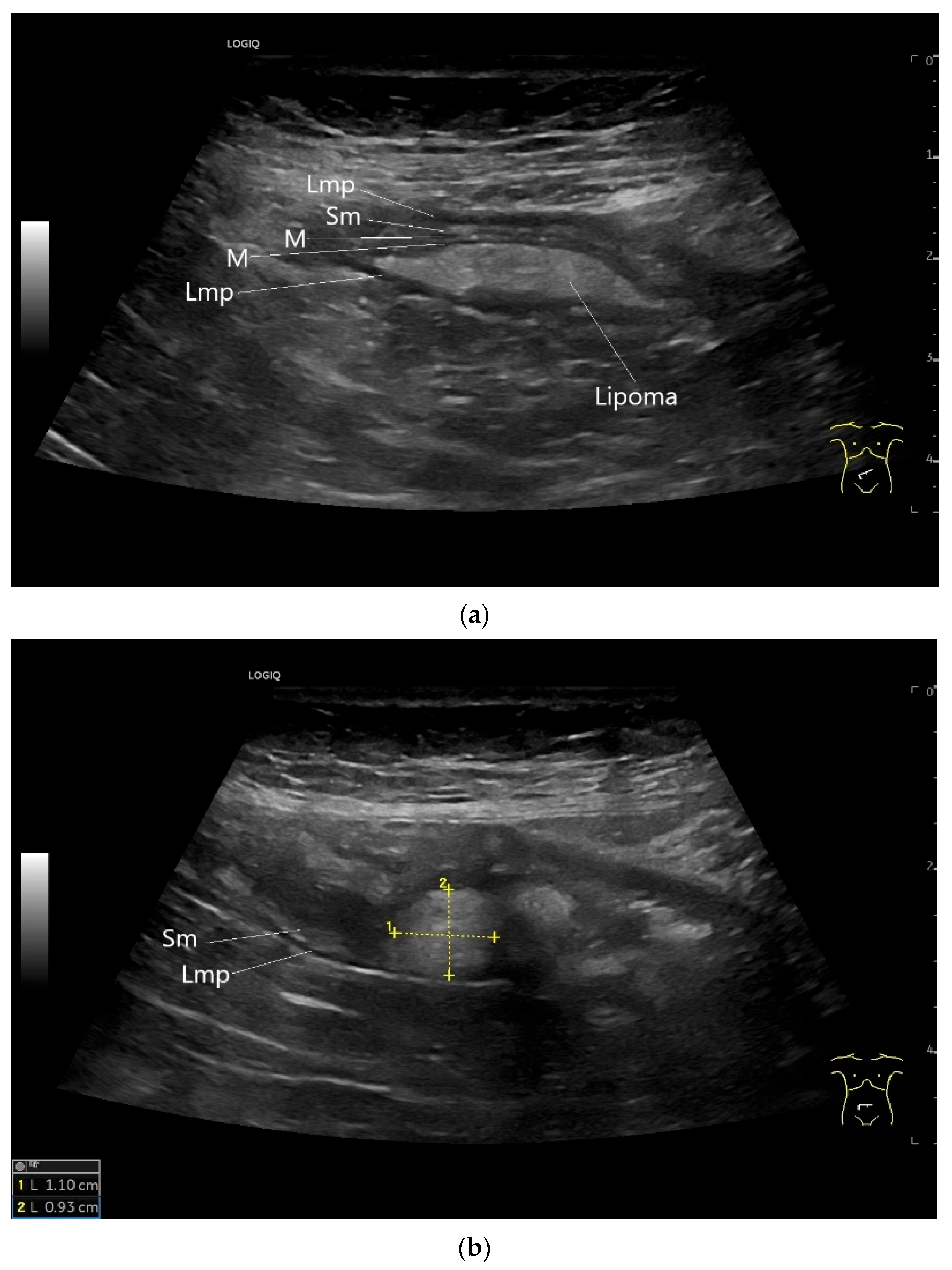
Lipomas are benign, smoothly bordered oval/round lipomatous lesions that usually originate in the submucosa. They appear homogeneous and hyperechoic on EUS and US (Figure 16). They are rare in the small intestine.
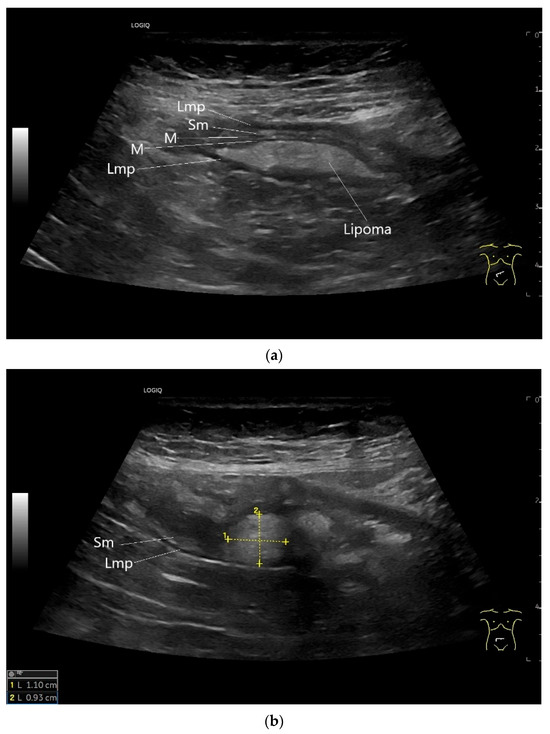
Figure 16.
Lipoma in the ileum. An incidental finding reveals a homogeneous hyperechoic lesion in the right lower abdomen. This can be attributed to the ileum. The mass is shown in longitudinal section (a) and cross-section (b). The longitudinal section (b) shows the layer classification. M—mucosa; Sm—submucosa; Lmp—muscularis propria layer.
9.7. Schwannoma
Schwannomas are tumors that develop from Schwann cells in the sheaths of peripheral nerves. They are very rare in the small intestine, and only a few reports on schwannomas of the small intestine have been published [138,139,140]. Schwannomas exhibit two microscopic growth patterns, Antoni A (hypercellular component) and Antoni B (hypocellular component). In contrast-enhanced CT scans, Antoni A areas show heterogeneous enhancement. Antoni B areas are hypocellular and show a homogeneous pseudocystic appearance on CT without significant contrast enhancement. In addition, true cystic degeneration may occur in Antoni B areas due to vascular thrombosis and necrosis. Due to the varying extent of Antoni A and B areas, the appearance of schwannomas in imaging can vary considerably [141]. Schwannomas can be solid, cystic-solid, encapsulated, and clearly defined (Figure 17). We are not aware of any descriptions of small intestine schwannomas in the US or CEUS. In other organs, schwannomas show enhancement of the capsule and septa in CEUS [142].
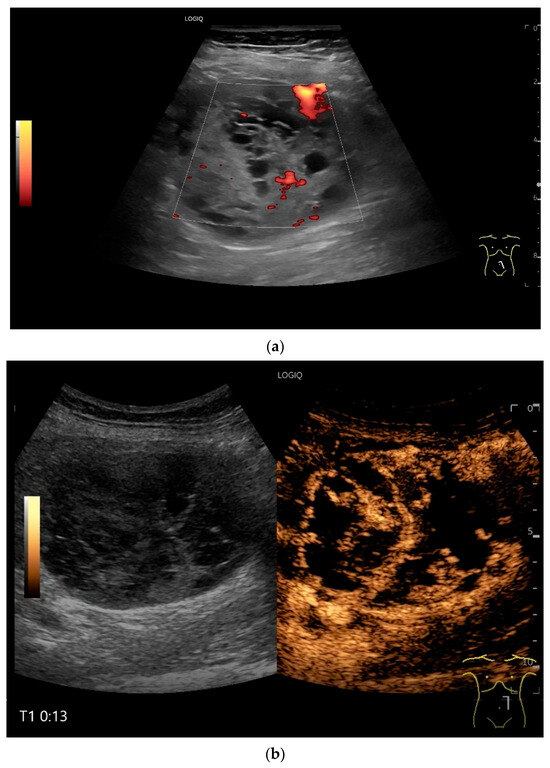
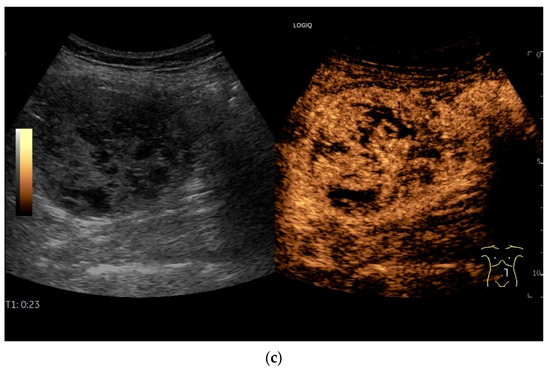
Figure 17.
Schwannoma. Incidental finding of a 60 mm solid mass with nonechoic cystic components (a). In CEUS after 13 s (b) and 23 s (c), the echogenic components are increasingly heterogeneous and irregularly enhanced, thus corresponding to solid vital tissue. The mass was located in the left upper abdomen in the middle of the intestinal loops. US suggested a connection to the jejunum, while CT showed a tumor in the duodenojejunal flexure. Intraoperatively, a tumor was seen to the left of the mesenteric root. The first jejunal loop was adherent. In summary, a tumor was diagnosed, which corresponded to a schwannoma on histology.
10. Metastases in the Small Intestines
Metastases of other tumors can occur in the small intestine. These include cutaneous melanomas, breast carcinomas, lung carcinomas, renal cell carcinoma, thyroid cancer, ovarian tumors, testicular tumors and head and neck squamous cell carcinoma [4,143,144,145,146,147,148]. In a study of more than 5000 patients, melanomas (66% of all small bowel metastases) were the most common secondary small intestine tumors [32]. In a study of 193 malignancies in the small intestine over a period of 20 years, 5.7% were metastases [146]. The metastases were located in both the jejunum and ileum [146]. Small bowel metastases of head and neck squamous cell carcinoma were mostly located in the ileum (58%), also jejunum (25%), and less frequently in the duodenum (15%) [143]. Clinical manifestations of small intestine metastases are gastrointestinal bleeding, abdominal discomfort up to acute abdomen in connection with intestinal lumen obstruction and also intussusception [146]. They manifest themselves as wall thickenings or intramural nodules in the wall of the small intestine (Figure 18 and Figure 19).
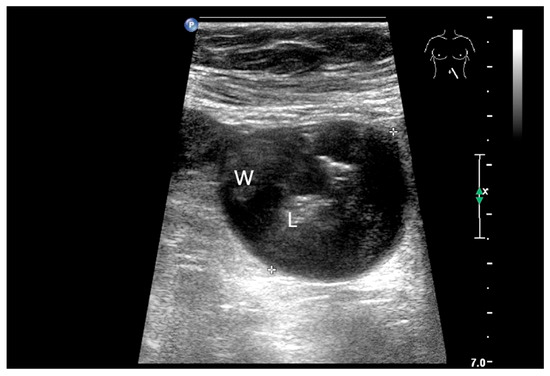
Figure 18.
Small intestine metastasis of a malignant melanoma (between the markers). Significant hypoechoic wall thickening (W) with narrowed lumen reflex (L) and lumen obstruction.
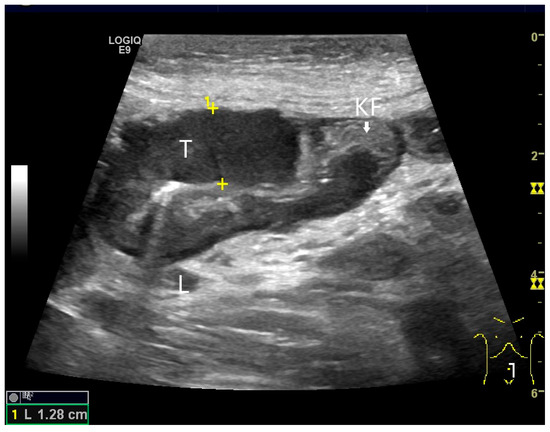
Figure 19.
Small intestine metastasis of pleural mesothelioma. Hypoechoic tumorous wall thickening (T) between the markers. The normal wall with Kerckring’s folds (KF) is visible adjacent to it. Next to the small intestine is a round hypoechoic tumor-suspicious lymph node (L).
The typical appearance of the various small intestine tumors and indirect indications are summarized in Table 5. Differential diagnoses of tumor-related wall thickening in the small intestine include inflammatory wall changes in Crohn’s disease and duodenal wall hematoma (Figure 20, Figure 21 and Figure 22). If wall thickening does not regress in long-standing Crohn’s disease under anti-inflammatory therapy and there are no explicit clinical and paraclinical signs of inflammation, the development of a tumor on the basis of Crohn’s disease must always be considered.

Table 5.
Small intestine tumors and their appearance on US and differential diagnoses.
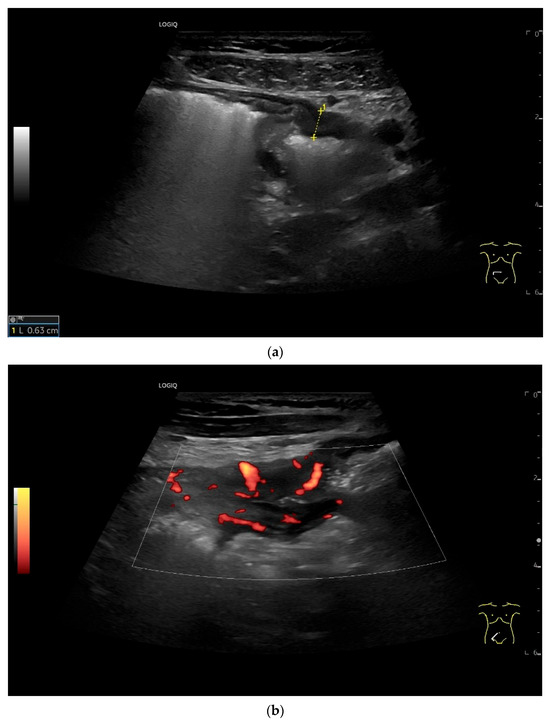
Figure 20.
Crohn’s disease under anti-inflammatory therapy, anastomosis after ileocecal resection. Hypoechoic wall thickening of the neoterminal ileum without definable stratification (a). Numerous vascular reflexes are visible in color Doppler (b), indicating ongoing inflammation.
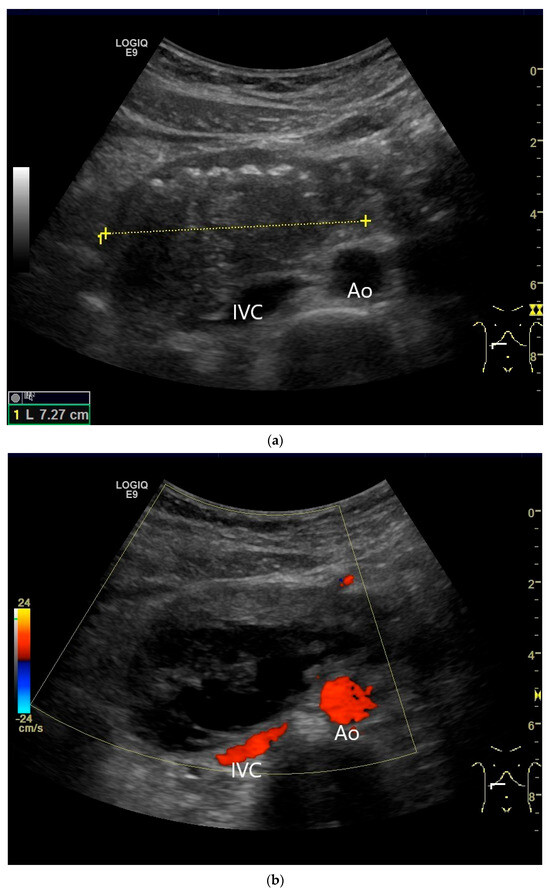
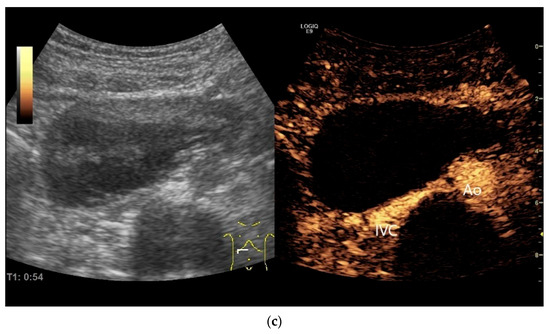
Figure 21.
Duodenal wall hematoma after gastroscopy with forceps biopsy under dual antiplatelet therapy. Clinically, there was pain and lumen obstruction. Evident focal hypoechoic wall thickening (between the markers) with lumen obstruction (a), for which there was no correlation in the previous gastroscopy. No evidence of macro vessels in CDI (b). In CEUS, the mass is smoothly defined and completely non-enhanced (c). IVC—inferior caval vein, Ao—aorta.
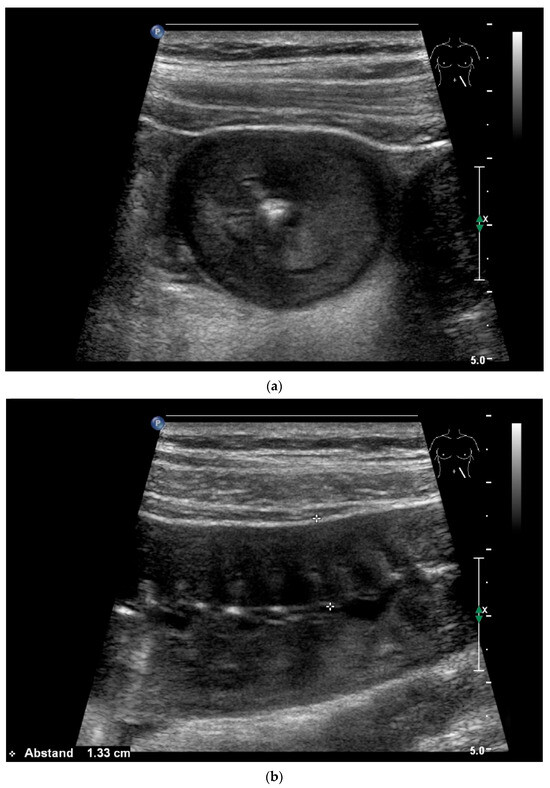
Figure 22.
Jejunal wall hematoma with a Quick ratio <5%. Smoothly defined, distinct wall thickening and lumen obstruction. The wall stratification is indistinct, slightly hyperechoic, and clearly blurred and unfocused (a). The Kerckring folds are thickened and also very blurred (b).
11. Future Directions and Artificial Intelligence (AI)
There is experience with the use of AI in US for diffuse liver disease and focal liver lesions [149,150]. The integration of CEUS in the differentiation of liver lesions has been investigated in studies [150,151]. The main focus of AI in gastroscopy is on cancer detection, determining the depth of cancer invasion, and predicting pathological diagnoses. In the field of VCE, the automated detection of bleeding, ulcers, tumors, and various small bowel diseases is being investigated. In colonoscopy, prospective studies are being conducted on the automated detection and classification of colon polyps and inflammation in IBD [152]. The primary objective of this publication was to raise awareness among examiners of the detection of small intestine tumors using US. This requires excellent training and experience in US, systematic and thorough examination of the small intestine, additional scheduling for the examination, and appropriate US technique. We would like to see AI assist in the detection of small intestinal tumors, similar to endoscopy. This would be the most important factor. Further steps would include the differential diagnosis of small intestinal tumors using multiparametric ultrasound, including B-mode, CDI, and, if necessary, elastography and CEUS. There is very little data available for CEUS of small intestine tumors. Therefore, data that reveal, for example, characteristic enhancement patterns should be evaluated in multicenter studies. In our opinion, there is insufficient experience with microvascular imaging for small intestinal tumors. A further step would be, for example, the differential diagnosis (before histology) by verifying vascularization using microvascular imaging, CEUS, and qualitative and qualitative dynamic CEUS.
12. Conclusions
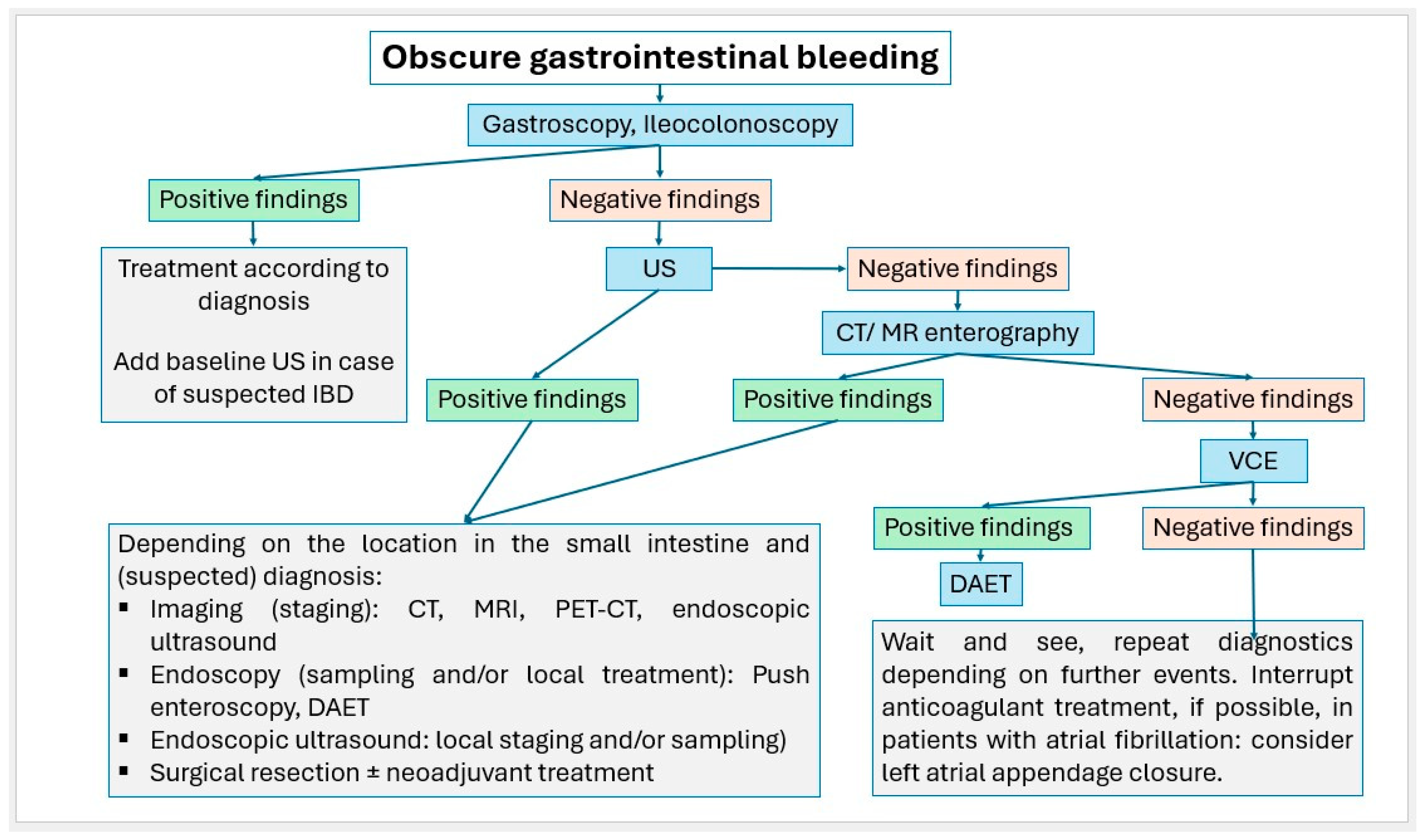
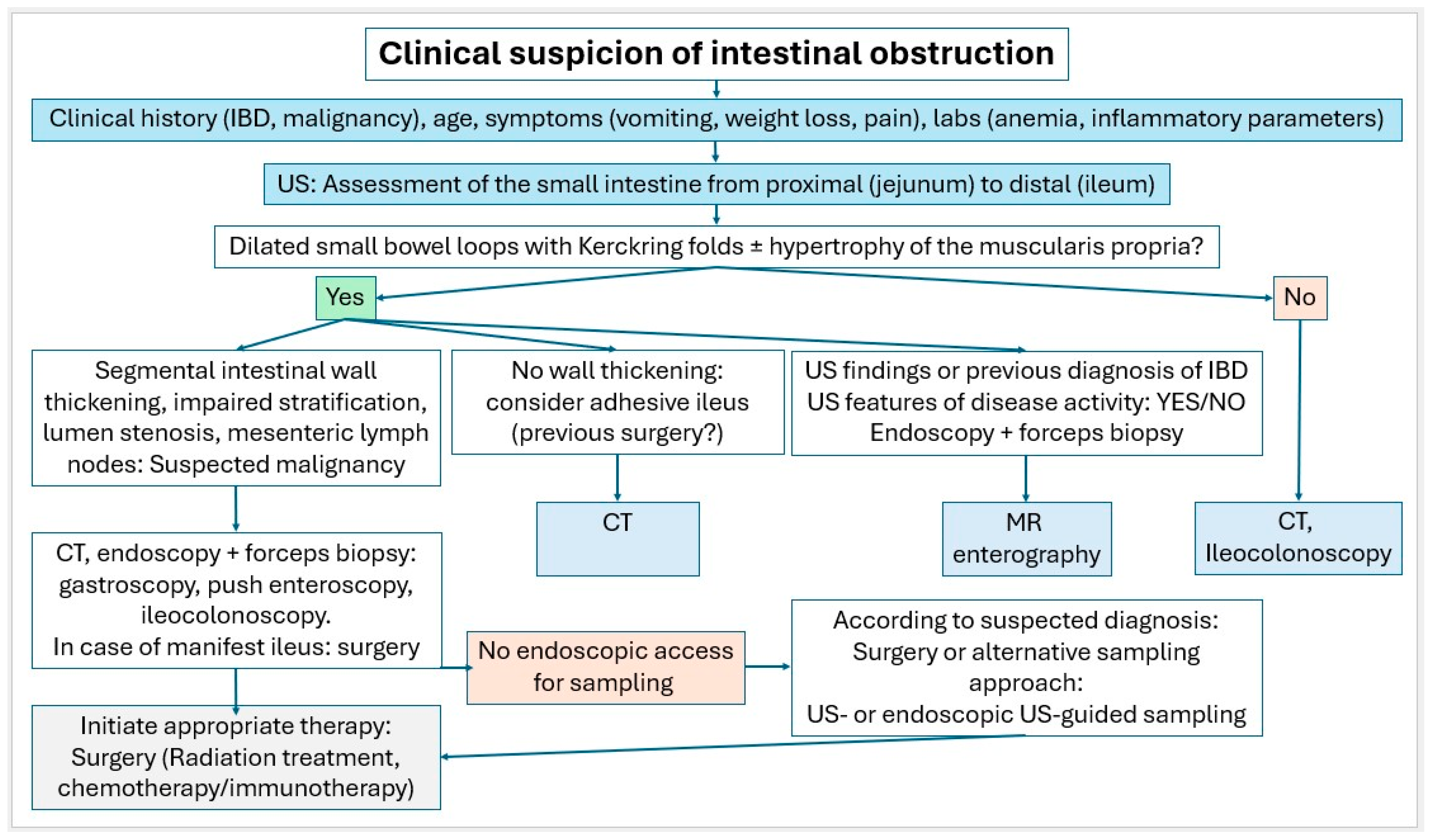
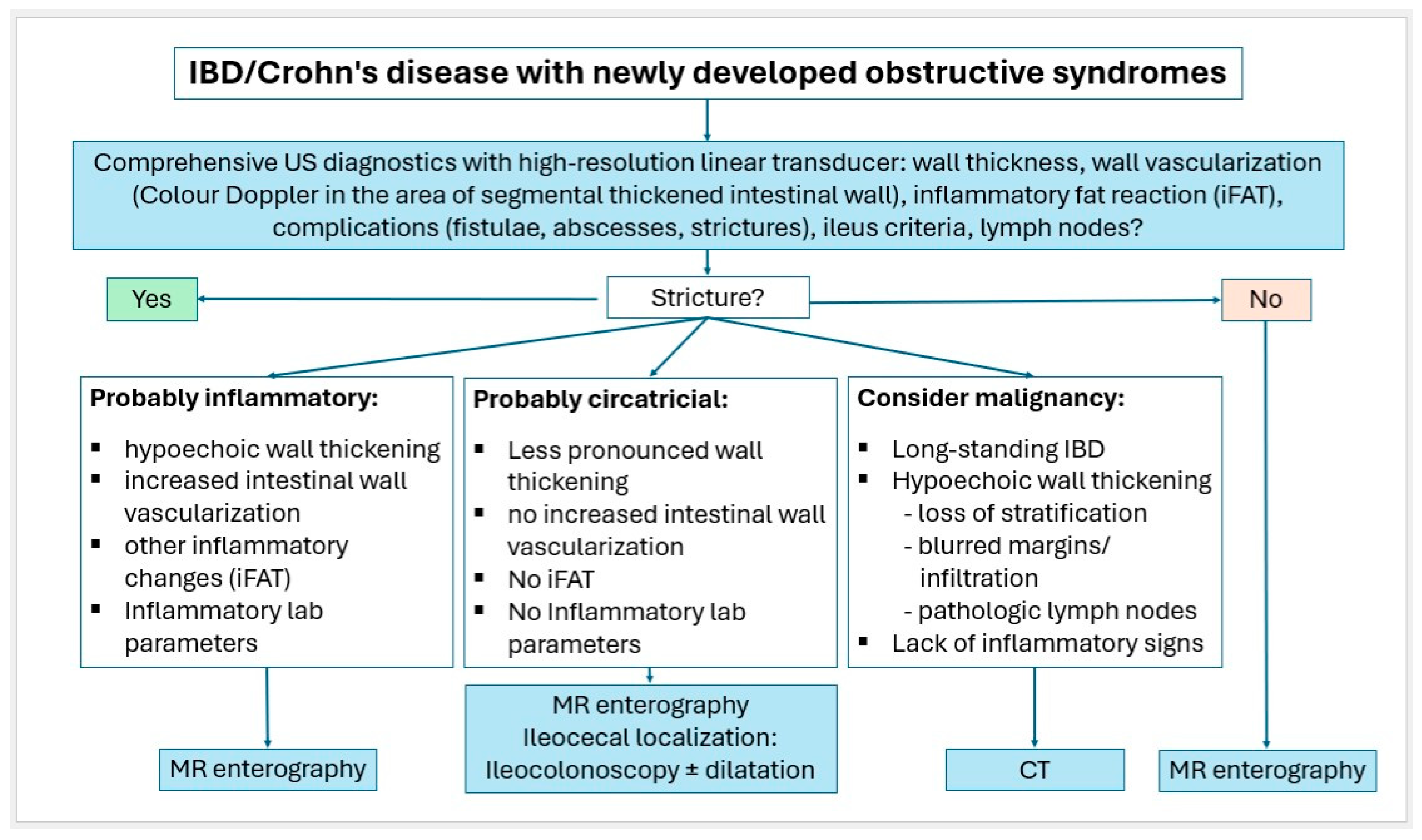
Small intestinal tumors are rare. Diagnostic management of patients with unexplained anemia, gastrointestinal bleeding, abdominal complaints or other symptoms indicating malignant disease that cannot be clarified by gastroscopy and ileocolonoscopy, should undergo examination for small intestine tumors. In Figure 23, Figure 24 and Figure 25, we suggest the potential diagnostic steps for the evaluation of occult bleeding, suspected small bowel obstruction, and suspected new obstruction in IBD/Crohn’s disease.
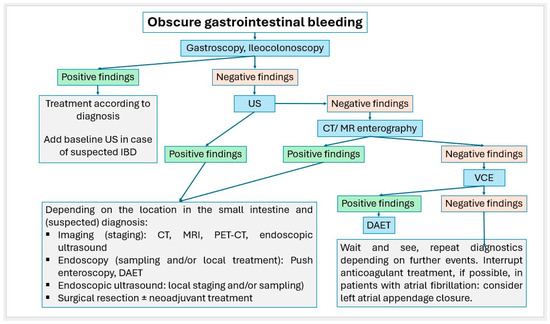
Figure 23.
Algorithm for the stepwise approach to obscure gastrointestinal bleeding.
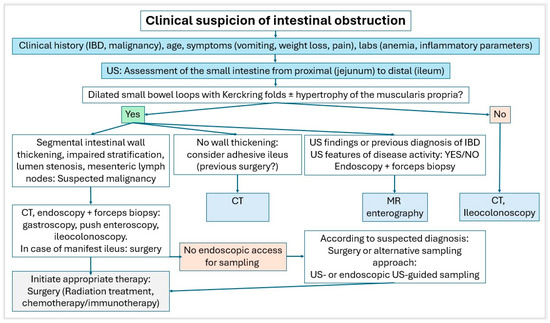
Figure 24.
Algorithm in case of clinical suspicion of intestinal obstruction.
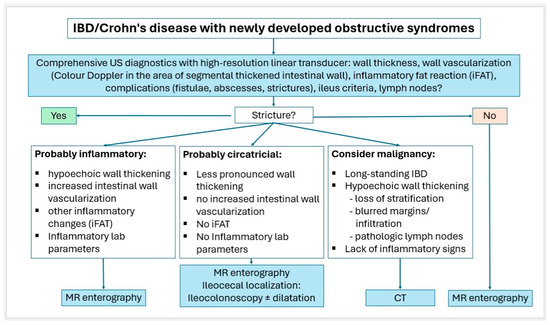
Figure 25.
Algorithm for IBD/Crohn’s disease with newly developed obstructive syndromes.
In literature, initial diagnosis of small intestine tumors is most often made by CT. An endoscopic examination of the jejunum and ileum is extensive and not completely possible and only intended for specific problems. CT enterography and MR enterography are more complex examinations, and CT is associated with radiation exposure. VCE is expensive and associated with false negatives in the context of subepithelial lesions.
While small intestine tumors < 10 mm tend to be overlooked, gastrointestinal US has a good detection rate for larger tumors > 20 mm. In our opinion, it is important to allow sufficient time for an examination of the small intestine and to perform the examination systematically using high-resolution transducers. US can be used to detect wall thickening, surrounding infiltrations, and lymph nodes. Indirect signs such as luminal dilation of the proximal intestinal segment, muscular hypertrophy, or intussusception can be visualized with US. With CEUS, additional information can be obtained about vascularization, and necrotic tumor areas can be delineated. Detection and characterization of small intestine tumors requires special knowledge and training in gastrointestinal ultrasound applications. The detection of small intestine tumors on ultrasound allows the targeted use of further complex diagnostic procedures for further characterization and to obtain a histologic diagnosis, occasionally also through US-guided sampling.
13. Take Home Messages
- Small intestine tumors are rare but clinically significant, especially in patients with unexplained anemia, GI bleeding, or abdominal pain not clarified by endoscopy.
- Transabdominal US is an underutilized but valuable tool for the detection of small bowel tumors, particularly those ≥20 mm in size.
- High-resolution transducers and systematic scanning techniques are essential to visualize bowel wall stratification, segmental thickening, and indirect signs like proximal dilation or intussusception.
- Multiparametric US, including CDI and CEUS, enhances lesion characterization by assessing vascularity and necrosis, especially in GISTs and neuroendocrine tumors.
- Ultrasound-guided biopsy is a reliable diagnostic option for accessible tumors not amenable to endoscopic sampling, particularly in lymphomas and large stromal tumors.
- US can guide further diagnostic decisions, reducing unnecessary CT, MRI, or capsule endoscopy in selected clinical scenarios—provided sufficient time, expertise, and high-quality equipment are available.
- We advocate for a broader use of multiparametric ultrasound in the diagnostic workup of patients with suspected small intestinal tumors.
Author Contributions
Conceptualization, C.F.D. and K.M.; methodology, C.F.D. and K.M.; validation, all authors (K.M., C.J., K.D., A.H., H.G., S.F. and C.F.D.); formal analysis, all authors (K.M., C.J., K.D., A.H., H.G., S.F. and C.F.D.); investigation, all authors (K.M., C.J., K.D., A.H., H.G., S.F. and C.F.D.); data curation, all authors (K.M., C.J., K.D., A.H., H.G., S.F. and C.F.D.); writing—original draft preparation, K.M.; writing—review and editing, all authors (K.M., C.J., K.D., A.H., H.G., S.F. and C.F.D.); supervision, C.F.D. and K.M.; project administration, C.F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. Some of the data originates from articles of other authors (see references). The data are not publicly accessible, as the personal rights of the patients involved must be respected.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Klimstra, D.S.N.I.; Rugge, M.; Salto-Tellez, M. Tumours of the small intestine and ampulla; Chapter 4. In WHO Classification of Tumours, 5th ed.; Digestive System Tumors; WHO Classification of Tumours Editorial Board, Ed.; WHO: Geneva, Switzerland, 2019; pp. 111–134. [Google Scholar]
- Chan, J.; Fukayama, M. Haematolymphoid tumours of the digestive system; chapter 11. In WHO Classification of Tumours, 5th ed.; Digestive System Tumours; WHO Classification of Tumours Editorial Board, Ed.; WHO: Geneva, Switzerland, 2019; pp. 372–431. [Google Scholar]
- Fukayama, M.G.J.; Miettinen, M.; Lazar, A.J. Mesenchymal tumours of the digestive system; Chapter 12. In WHO Classification of Tumours, 5th ed.; Digestive Tumours; WHO Classification of Tumours Editorial Board, Ed.; WHO: Geneva, Switzerland, 2019; pp. 432–498. [Google Scholar]
- Paradis, V.S.P.; Singh, R. Other tumours of the digestive system. Chapter 13. In WHO Classification of Tumours, 5th ed.; Digestive System Tumors; WHO Classification of Tumours Editorial Board, Ed.; WHO: Geneva, Switzerland, 2019; pp. 499–510. [Google Scholar]
- Schottenfeld, D.; Beebe-Dimmer, J.L.; Vigneau, F.D. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann. Epidemiol. 2009, 19, 58–69. [Google Scholar] [CrossRef]
- Hatzaras, I.; Palesty, J.A.; Abir, F.; Sullivan, P.; Kozol, R.A.; Dudrick, S.J.; Longo, W.E. Small-bowel tumors: Epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch. Surg. 2007, 142, 229–235. [Google Scholar] [CrossRef]
- Pan, S.Y.; Morrison, H. Epidemiology of cancer of the small intestine. World J. Gastrointest. Oncol. 2011, 3, 33–42. [Google Scholar] [CrossRef]
- Barsouk, A.; Rawla, P.; Barsouk, A.; Thandra, K.C. Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention. Med. Sci. 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.E.; Ilaghi, M.; Mahdavizadeh, V.; Ebrahimi, R.; Aslani, A.; Yekta, Z.; Nejadghaderi, S.A. A population-based study on incidence trends of small intestine cancer in the United States from 2000 to 2020. PLoS ONE 2024, 19, e0307019. [Google Scholar] [CrossRef] [PubMed]
- Nylund, K.; Maconi, G.; Hollerweger, A.; Ripolles, T.; Pallotta, N.; Higginson, A.; Serra, C.; Dietrich, C.F.; Sporea, I.; Saftoiu, A.; et al. EFSUMB Recommendations and Guidelines for Gastrointestinal Ultrasound. Ultraschall Med. 2017, 38, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Nylund, K.; Odegaard, S.; Hausken, T.; Folvik, G.; Lied, G.A.; Viola, I.; Hauser, H.; Gilja, O.H. Sonography of the small intestine. World J. Gastroenterol. 2009, 15, 1319–1330. [Google Scholar] [CrossRef]
- Nylund, K.; Hausken, T.; Odegaard, S.; Eide, G.E.; Gilja, O.H. Gastrointestinal wall thickness measured with transabdominal ultrasonography and its relationship to demographic factors in healthy subjects. Ultraschall Med. 2012, 33, E225–E232. [Google Scholar] [CrossRef]
- Möller, K.; Fischer, P.; Gilja, O.H.; Gottschall, H.; Jenssen, C.; Hollerweger, A.; Lucius, C.; Meier, J.; Rogler, G.; Misselwitz, B.; et al. Gastrointestinal Ultrasound: Measurements and Normal Findings—What Do You Need to Know? Dig. Dis. 2025, 43, 300–335. [Google Scholar] [CrossRef]
- Maconi, G.; Nylund, K.; Ripolles, T.; Calabrese, E.; Dirks, K.; Dietrich, C.F.; Hollerweger, A.; Sporea, I.; Saftoiu, A.; Maaser, C.; et al. EFSUMB Recommendations and Clinical Guidelines for Intestinal Ultrasound (GIUS) in Inflammatory Bowel Diseases. Ultraschall Med. 2018, 39, 304–317. [Google Scholar] [CrossRef]
- Dirks, K.; Calabrese, E.; Dietrich, C.F.; Gilja, O.H.; Hausken, T.; Higginson, A.; Hollerweger, A.; Maconi, G.; Maaser, C.; Nuernberg, D.; et al. EFSUMB Position Paper: Recommendations for Gastrointestinal Ultrasound (GIUS) in Acute Appendicitis and Diverticulitis. Ultraschall Med. 2019, 40, 163–175. [Google Scholar] [CrossRef]
- Hollerweger, A.; Maconi, G.; Ripolles, T.; Nylund, K.; Higginson, A.; Serra, C.; Dietrich, C.F.; Dirks, K.; Gilja, O.H. Gastrointestinal Ultrasound (GIUS) in Intestinal Emergencies—An EFSUMB Position Paper. Ultraschall Med. 2020, 41, 646–657. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Hollerweger, A.; Dirks, K.; Higginson, A.; Serra, C.; Calabrese, E.; Dong, Y.; Hausken, T.; Maconi, G.; Mihmanli, I.; et al. EFSUMB Gastrointestinal Ultrasound (GIUS) Task Force Group: Celiac sprue and other rare gastrointestinal diseases ultrasound features. Med. Ultrason. 2019, 21, 299–315. [Google Scholar] [CrossRef]
- Maconi, G.; Hausken, T.; Dietrich, C.F.; Pallotta, N.; Sporea, I.; Nurnberg, D.; Dirks, K.; Romanini, L.; Serra, C.; Braden, B.; et al. Gastrointestinal Ultrasound in Functional Disorders of the Gastrointestinal Tract—EFSUMB Consensus Statement. Ultrasound Int. Open 2021, 7, E14–E24. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Lembcke, B.; Jenssen, C.; Hocke, M.; Ignee, A.; Hollerweger, A. Intestinal Ultrasound in Rare Gastrointestinal Diseases, Update, Part 2. Ultraschall Med. 2015, 36, 428–456. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, K.; Takedatsu, H.; Yoshioka, S.; Yoshikai, M.; Tomiyasu, K.; Morita, M.; Kuwaki, K.; Mitsuyama, K.; Kawaguchi, T. Symptoms Contributing to the Diagnosis of Small Bowel Tumors. Digestion 2023, 104, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Nishie, H.; Suzuki, T.; Ichikawa, H.; Kataoka, H. Intestinal obstruction caused by small bowel adenocarcinoma misdiagnosed as psychogenic disorder. BMJ Case Rep. 2019, 12, bcr-2018-227326. [Google Scholar] [CrossRef]
- Xin, L.; Liao, Z.; Jiang, Y.P.; Li, Z.S. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: A systematic review of data over the first decade of use. Gastrointest. Endosc. 2011, 74, 563–570. [Google Scholar] [CrossRef]
- Messer, I.; May, A.; Manner, H.; Ell, C. Prospective, randomized, single-center trial comparing double-balloon enteroscopy and spiral enteroscopy in patients with suspected small-bowel disorders. Gastrointest. Endosc. 2013, 77, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yano, T.; Ohmiya, N.; Tanaka, S.; Tanaka, S.; Endo, Y.; Matsuda, T.; Matsui, T.; Iida, M.; Sugano, K. Double-balloon endoscopy is safe and effective for the diagnosis and treatment of small-bowel disorders: Prospective multicenter study carried out by expert and non-expert endoscopists in Japan. Dig. Endosc. 2015, 27, 331–337. [Google Scholar] [CrossRef]
- Beyna, T.; Arvanitakis, M.; Schneider, M.; Gerges, C.; Böing, D.; Devière, J.; Neuhaus, H. Motorised spiral enteroscopy: First prospective clinical feasibility study. Gut 2021, 70, 261–267. [Google Scholar] [CrossRef]
- Buchholz, H.; Mende, M.; Hornoff, S.; Faiss, S. Results of motorized spiral enteroscopy in 83 consecutive patients. Z. Gastroenterol. 2022, 60, 1635–1643. [Google Scholar] [CrossRef]
- Pal, P.; Ramchandani, M.; Banerjee, R.; Viswakarma, P.; Singh, A.P.; Reddy, M.; Rughwani, H.; Patel, R.; Sekaran, A.; Kanaganti, S.; et al. Technical performance and diagnostic yield of motorised spiral enteroscopy compared with single-balloon enteroscopy in suspected Crohn’s disease: A randomised controlled, open-label study (the MOTOR-CD trial). Gut 2023, 72, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.G.; Shan, G.D.; Zhang, H.; Li, L.; Yue, M.; Xiang, Z.; Cheng, Y.; Wu, C.J.; Fang, Y.; Chen, L.H. Double-balloon enteroscopy in small bowel tumors: A Chinese single-center study. World J. Gastroenterol. 2013, 19, 3665–3671. [Google Scholar] [CrossRef]
- Noujaim, M.G.; Dorsey, C.; Parish, A.; Raines, D.; Boudreaux, L.; Hanscom, M.; Cave, D.; Niedzwiecki, D.; Wild, D. Clinical Features and Management of Small Bowel Masses Detected During Device-Assisted Enteroscopy: A Multi-Center Experience. Gastroenterol. Res. 2022, 15, 353–363. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, L.; Zhang, T.; Sun, P.; Sun, J.; Zhou, J.; Wang, L.; Fan, R.; Wang, Z.; Cheng, S.; et al. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: Experience from a Chinese tertiary hospital. Turk. J. Gastroenterol. 2020, 31, 30–35. [Google Scholar] [CrossRef]
- Mitsui, K.; Tanaka, S.; Yamamoto, H.; Kobayashi, T.; Ehara, A.; Yano, T.; Goto, H.; Nakase, H.; Tanaka, S.; Matsui, T.; et al. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: The first Japanese multicenter study. Gastrointest. Endosc. 2009, 70, 498–504. [Google Scholar] [CrossRef]
- Rondonotti, E.; Pennazio, M.; Toth, E.; Menchen, P.; Riccioni, M.E.; De Palma, G.D.; Scotto, F.; De Looze, D.; Pachofsky, T.; Tacheci, I.; et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: A multicenter European study. Endoscopy 2008, 40, 488–495. [Google Scholar] [CrossRef]
- Lewis, B.S.; Eisen, G.M.; Friedman, S. A Pooled Analysis to Evaluate Results of Capsule Endoscopy Trials. Endoscopy 2005, 37, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Murino, A.; Nakamura, M.; Watanabe, O.; Yamamura, T.; Nagura, A.; Yoshimura, T.; Nakano, A.; Goto, H.; Hirooka, Y. Effectiveness of Endoscopic Ultrasonography during Double Balloon Enteroscopy for characterization and management of small bowel submucosal tumours. Dig. Liver Dis. 2016, 48, 1187–1193. [Google Scholar] [CrossRef]
- Zhongcheng, L.; Dongting, C.; Biyao, W.; Xinyu, L.; Qin, G. Application value of small intestinal endoscopic ultrasonography for protruding lesions of the small intestine. Surg. Endosc. 2025, 39, 2902–2910. [Google Scholar] [CrossRef]
- Rangiah, D.S.; Cox, M.; Richardson, M.; Tompsett, E.; Crawford, M. Small bowel tumours: A 10 year experience in four Sydney teaching hospitals. ANZ J. Surg. 2004, 74, 788–792. [Google Scholar] [CrossRef]
- Corvino, A.; Setola, S.V.; Sandomenico, F.; Corvino, F.; Catalano, O. Synchronous tumours detected during cancer patient staging: Prevalence and patterns of occurrence in multidetector computed tomography. Pol. J. Radiol. 2020, 85, e261–e270. [Google Scholar] [CrossRef]
- Masselli, G.; Di Tola, M.; Casciani, E.; Polettini, E.; Laghi, F.; Monti, R.; Bernieri, M.G.; Gualdi, G. Diagnosis of Small-Bowel Diseases: Prospective Comparison of Multi-Detector Row CT Enterography with MR Enterography. Radiology 2016, 279, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Del Gaizo, A.J.; Fletcher, J.G.; Yu, L.; Paden, R.G.; Spencer, G.C.; Leng, S.; Silva, A.M.; Fidler, J.L.; Silva, A.C.; Hara, A.K. Reducing radiation dose in CT enterography. Radiographics 2013, 33, 1109–1124. [Google Scholar] [CrossRef]
- Wang, N.; Cui, X.Y.; Liu, Y.; Long, J.; Xu, Y.H.; Guo, R.X.; Guo, K.J. Adult intussusception: A retrospective review of 41 cases. World J. Gastroenterol. 2009, 15, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, A.; Tanaka, S.; Imagawa, H.; Shishido, T.; Oka, S.; Yoshida, S.; Yamada, H.; Chayama, K. Usefulness and limitations of transabdominal ultrasonography for detecting small-bowel tumors. Scand. J. Gastroenterol. 2009, 44, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Manabe, N.; Honda, K.; Murao, T.; Osawa, M.; Kawai, R.; Akiyama, T.; Shiotani, A.; Haruma, K.; Hata, J. Usefulness of Ultrasonography for Diagnosis of Small Bowel Tumors: A Comparison Between Ultrasonography and Endoscopic Modalities. Medicine 2015, 94, e1464. [Google Scholar] [CrossRef]
- Masselli, G.; Polettini, E.; Casciani, E.; Bertini, L.; Vecchioli, A.; Gualdi, G. Small-bowel neoplasms: Prospective evaluation of MR enteroclysis. Radiology 2009, 251, 743–750. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version). Ultraschall Med. 2018, 39, 154–180. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.A.; et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2018, 4, E2–E15. [Google Scholar] [CrossRef]
- Petersen, F.; Hopfner, M.H.; Jenssen, C.; Nurnberg, D.; Strobel, D.; Vogelpohl, J.; Dietrich, C.F. Why is ultrasound needed in inflammatory bowel disease? Med. Ultrason. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Jenssen, C.; Petersen, F.; Hopfner, M.; Nurnberg, D.; Strobel, D.; Vogelpohl, J.; Dietrich, C.F. Why and when are activity scores and advanced ultrasound techniques needed in inflammatory bowel disease (IBD)? Med. Ultrason. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Petersen, F.; Hopfner, M.; Nurnberg, D.; Strobel, D.; Vogelpohl, J.; Jenssen, C. Ultrasound for detection of complications and evaluation of treatment response in inflammatory bowel disease (IBD)? Med. Ultrason. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Matsui, S.; Kamata, K.; Komaki, T.; Imai, H.; Dote, K.; Kudo, M. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest. Endosc. 2011, 73, 227–237. [Google Scholar] [CrossRef]
- Kannengiesser, K.; Mahlke, R.; Petersen, F.; Peters, A.; Ross, M.; Kucharzik, T.; Maaser, C. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand. J. Gastroenterol. 2012, 47, 1515–1520. [Google Scholar] [CrossRef]
- Kamata, K.; Takenaka, M.; Kitano, M.; Omoto, S.; Miyata, T.; Minaga, K.; Yamao, K.; Imai, H.; Sakurai, T.; Watanabe, T.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of submucosal tumors of the upper gastrointestinal tract. J. Gastroenterol. Hepatol. 2017, 32, 1686–1692. [Google Scholar] [CrossRef]
- Tamura, T.; Kitano, M. Contrast Enhanced EUS Imaging for Gastrointestinal Subepithelial Tumors. Clin. Endosc. 2019, 52, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Saftoiu, A.; Napoleon, B.; Arcidiacono, P.G.; Braden, B.; Burmeister, S.; Carrara, S.; Cui, X.W.; Fusaroli, P.; Gottschalk, U.; Hocke, M.; et al. Do we need contrast agents for EUS? Endosc. Ultrasound 2020, 9, 361–368. [Google Scholar] [CrossRef]
- Yang, Y.T.; Shen, N.; Ao, F.; Chen, W.Q. Diagnostic value of contrast-enhanced harmonic endoscopic ultrasonography in predicting the malignancy potential of submucosal tumors: A systematic review and meta-analysis. Surg. Endosc. 2020, 34, 3754–3765. [Google Scholar] [CrossRef] [PubMed]
- Ignee, A.; Jenssen, C.; Hocke, M.; Dong, Y.; Wang, W.P.; Cui, X.W.; Woenckhaus, M.; Iordache, S.; Saftoiu, A.; Schuessler, G.; et al. Contrast-enhanced (endoscopic) ultrasound and endoscopic ultrasound elastography in gastrointestinal stromal tumors. Endosc. Ultrasound 2017, 6, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Sparchez, Z.; Radu, P.; Zaharia, T.; Kacso, G.; Grigorescu, I.; Badea, R. Contrast enhanced ultrasound guidance: A new tool to improve accuracy in percutaneous biopsies. Med. Ultrason. 2010, 12, 133–138. [Google Scholar]
- Sparchez, Z.; Mocan, T.; Craciun, R.; Sparchez, M.; Nolsoe, C. Contrast enhancement for ultrasound-guided interventions: When to use it and what to expect? Ultrasonography 2022, 41, 263–278. [Google Scholar] [CrossRef]
- Moller, K.; Dietrich, C.F.; Faiss, S.; Mutze, S.; Goelz, L. Alternatives of histological material collection—When and how is histological confirmation by ultrasound (US), computer tomography (CT) or endosonography (EUS) useful? Z. Gastroenterol. 2022, 60, 937–958. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Brabrand, K.; Cantisani, V.; Correas, J.M.; Cui, X.W.; D’Onofrio, M.; Essig, M.; Freeman, S.; Gilja, O.H.; Gritzmann, N. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part II. Diagnostic Ultrasound-Guided Interventional Procedures (Long Version). Ultraschall Med. 2015, 36, E15–E35. [Google Scholar]
- Tudor, G.R.; Rodgers, P.M.; West, K.P. Bowel lesions: Percutaneous US-guided 18-gauge needle biopsy--preliminary experience. Radiology 1999, 212, 594–597. [Google Scholar] [CrossRef]
- Marco-Domenech, S.F.; Gil-Sanchez, S.; Fernandez-Garcia, P.; De La Iglesia-Carrena, P.; Gonzalez-Anon, M.; Arenas-Jimenez, J.J.; Alonso-Charterina, S.; Piqueras-Olmeda, R.M. Sonographically guided percutaneous biopsy of gastrointestinal tract lesions. Am. J. Roentgenol. 2001, 176, 147–151. [Google Scholar] [CrossRef]
- Minhas, K.; Roebuck, D.J.; Barnacle, A.; De Coppi, P.; Sebire, N.; Patel, P.A. Diagnostic yield and safety of ultrasound-guided bowel mass biopsies in children. Pediatr. Radiol. 2019, 49, 1809–1815. [Google Scholar] [CrossRef]
- Tombesi, P.; Postorivo, S.; Catellani, M.; Tassinari, D.; Abbasciano, V.; Sartori, S. Percutaneous ultrasonography-guided core needle biopsy of gastrointestinal lesions: What’s its actual role in clinical practice? A retrospective study for safety and effectiveness. Ultraschall Med. 2011, 32 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef]
- de Sio, I.; Funaro, A.; Vitale, L.M.; Niosi, M.; Francica, G.; Federico, A.; Sgambato, D.; Loguercio, C.; Romano, M. Ultrasound-guided percutaneous biopsy for diagnosis of gastrointestinal lesions. Dig. Liver Dis. 2013, 45, 816–819. [Google Scholar] [CrossRef]
- Chiorean, L.; Cantisani, V.; Jenssen, C.; Sidhu, P.S.; Baum, U.; Dietrich, C.F. Focal masses in a non-cirrhotic liver: The additional benefit of CEUS over baseline imaging. Eur. J. Radiol. 2015, 84, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Mackey, R.; Brown, N.; Church, J.; Burke, C.; Walsh, R.M. Outcome based on management for duodenal adenomas: Sporadic versus familial disease. J. Gastrointest. Surg. 2010, 14, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Culver, E.L.; McIntyre, A.S. Sporadic duodenal polyps: Classification, investigation, and management. Endoscopy 2011, 43, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Chung, W.C.; Kim, E.J.; Kim, S.H.; Paik, C.N.; Lee, B.I.; Cho, Y.S.; Lee, K.M. Evaluation of non-ampullary duodenal polyps: Comparison of non-neoplastic and neoplastic lesions. World J. Gastroenterol. 2010, 16, 5474–5480. [Google Scholar] [CrossRef]
- Corvino, A.; Corvino, F.; Radice, L.; Catalano, O. Synchronous mucinous colonic adenocarcinoma and multiple small intestinal adenocarcinomas: Report of a case and review of literature. Clin. Imaging 2015, 39, 538–542. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, H.C.; Oh, J.; Won, K.Y.; Park, S.J.; Yang, D.M. Tumors of the jejunum and ileum: A pattern-based imaging approach on CT. Abdom. Radiol. 2019, 44, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Capela, T.; Sousa, P.; Caldeira, A.; Pereira, E. Intestinal Obstruction of Uncommon Cause and Point-of-Care Ultrasonography—Where Do We Stand? GE Port. J. Gastroenterol. 2018, 25, 38–41. [Google Scholar] [CrossRef]
- Wu, S.; Yu, H.; Liu, Y.; Zhou, H.; Zhou, Y. Small bowel adenocarcinoma of the jejunum detected by double contrast enhanced ultrasound: A case report of a novel ultrasound modality. Front. Oncol. 2024, 14, 1288041. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Snorradottir, S.; Asgeirsdottir, A.; Rögnvaldsson, S.; Jonasson, J.G.; Björnsson, E.S. Incidence and prognosis of patients with small intestinal neuroendocrine tumors in a population based nationwide study. Cancer Epidemiol. 2022, 79, 102197. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Sandvik, O.M.; Søreide, K.; Gudlaugsson, E.; Kvaløy, J.T.; Søreide, J.A. Epidemiology and classification of gastroenteropancreatic neuroendocrine neoplasms using current coding criteria. Br. J. Surg. 2016, 103, 226–232. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C.H.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef]
- Palepu, J.; Shrikhande, S.V.; Bhaduri, D.; Shah, R.C.; Sirohi, B.; Chhabra, V.; Dhar, P.; Sastry, R.; Sikora, S. Trends in diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in India: A report of multicenter data from a web-based registry. Indian. J. Gastroenterol. 2017, 36, 445–451. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, L.; Bao, H.; Zhang, J.; Wang, Z.; Gong, P. Clinical, pathological and prognostic characteristics of gastroenteropancreatic neuroendocrine neoplasms in China: A retrospective study. BMC Endocr. Disord. 2014, 14, 54. [Google Scholar] [CrossRef]
- Fan, J.H.; Zhang, Y.Q.; Shi, S.S.; Chen, Y.J.; Yuan, X.H.; Jiang, L.M.; Wang, S.M.; Ma, L.; He, Y.T.; Feng, C.Y.; et al. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in china. Oncotarget 2017, 8, 71699–71708. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.Y.; Kim, J.M.; Sohn, J.H.; Kim, M.J.; Kim, K.M.; Kim, W.H.; Kim, H.; Kook, M.C.; Park, D.Y.; Lee, J.H.; et al. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res. Treat. 2012, 44, 157–165. [Google Scholar] [CrossRef]
- Tsai, H.J.; Wu, C.C.; Tsai, C.R.; Lin, S.F.; Chen, L.T.; Chang, J.S. The epidemiology of neuroendocrine tumors in Taiwan: A nation-wide cancer registry-based study. PLoS ONE 2013, 8, e62487. [Google Scholar] [CrossRef]
- Ellis, L.; Shale, M.J.; Coleman, M.P. Carcinoid tumors of the gastrointestinal tract: Trends in incidence in England since 1971. Am. J. Gastroenterol. 2010, 105, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
- Genus, T.S.E.; Bouvier, C.; Wong, K.F.; Srirajaskanthan, R.; Rous, B.A.; Talbot, D.C.; Valle, J.W.; Khan, M.; Pearce, N.; Elshafie, M.; et al. Impact of neuroendocrine morphology on cancer outcomes and stage at diagnosis: A UK nationwide cohort study 2013–2015. Br. J. Cancer 2019, 121, 966–972. [Google Scholar] [CrossRef]
- Milione, M.; Parente, P.; Grillo, F.; Zamboni, G.; Mastracci, L.; Capella, C.; Fassan, M.; Vanoli, A. Neuroendocrine neoplasms of the duodenum, ampullary region, jejunum and ileum. Pathologica 2021, 113, 12–18. [Google Scholar] [CrossRef]
- Klöppel, G.; Anlauf, M. Gastrinoma--morphological aspects. Wien. Klin. Wochenschr. 2007, 119, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.A.; Chou, Y.H.; Huang, S.H.; Chen, T.J. Primary jejunal gastrinoma: A case report and review of the literature. World J. Surg. Oncol. 2015, 13, 313. [Google Scholar] [CrossRef]
- Sandru, F.; Carsote, M.; Valea, A.; Albu, S.E.; Petca, R.C.; Dumitrascu, M.C. Somatostatinoma: Beyond neurofibromatosis type 1 (Review). Exp. Ther. Med. 2020, 20, 3383–3388. [Google Scholar] [CrossRef]
- Constantinoiu, S.; Constantin, A.; Predescu, D.; Iosif, C.; Hoara, P.; Achim, F.; Surugiu, P.; Bacanu, F.; Cociu, L. Somatostatinoma of the first jejunal loop in a patient with neurofibromatosis von Recklinghausen and bilateral pheochromocytoma. Hepatogastroenterology 2012, 59, 1874–1878. [Google Scholar]
- Maccioni, F.; Rossi, P.; Gourtsoyiannis, N.; Bezzi, M.; Di Nardo, L.; Broglia, L. US and CT findings of small bowel neoplasms. Eur. Radiol. 1997, 7, 1398–1409. [Google Scholar] [CrossRef]
- Giannetti, A.; Randisi, P.; Stumpo, M.; Coratti, F. Diagnosis of one small bowel tumor: The role of conventional ultrasound and elastography. J. Ultrasound 2016, 19, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Grad, S.; Dumitraşcu, D.; Badea, R.; Iobagiu, S.; Pascu, O. Ileal neuroendocrine tumor—Ultrasonographic and capsule endoscopy appearance: A case report. Med. Ultrason. 2010, 12, 245–248. [Google Scholar]
- Tsujimura, K.; Takushi, Y.; Teruya, T.; Iha, K.; Ota, M.; Nakachi, A.; Gakiya, A. Neuroendocrine tumor of the small intestine diagnosed with trans-abdominal ultrasonography: A case report. Int. J. Surg. Case Rep. 2017, 31, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Rioux, M.; Langis, P.; Naud, F. Sonographic appearance of primary small bowel carcinoid tumor. Abdom. Imaging 1995, 20, 37–43. [Google Scholar] [CrossRef]
- Schwarze, V.; Marschner, C.; Grosu, S.; Rübenthaler, J.; Knösel, T.; Clevert, D.A. Modern sonographic imaging of abdominal neuroendocrine tumors. Radiologe 2019, 59, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Smereczyński, A.; Starzyńska, T.; Kołaczyk, K. Mesenteric changes in an ultrasound examination can facilitate the diagnosis of neuroendocrine tumors of the small intestine. J. Ultrason. 2015, 15, 274–282. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Zhuang, H.; Luo, Y.; Su, M.G.; Xiong, M.L.; Wu, Y.T. Double contrast-enhanced ultrasonography of a small intestinal neuroendocrine tumor: A case report of a recommendable imaging modality. Precis. Clin. Med. 2020, 3, 147–152. [Google Scholar] [CrossRef]
- Yin, L.; Chen, C.Q.; Peng, C.H.; Chen, G.M.; Zhou, H.J.; Han, B.S.; Li, H.W. Primary small-bowel non-Hodgkin’s lymphoma: A study of clinical features, pathology, management and prognosis. J. Int. Med. Res. 2007, 35, 406–415. [Google Scholar] [CrossRef]
- Iwamuro, M.; Tanaka, T.; Okada, H. Review of lymphoma in the duodenum: An update of diagnosis and management. World J. Gastroenterol. 2023, 29, 1852–1862. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Olszewska-Szopa, M.; Wróbel, T. Gastrointestinal non-Hodgkin lymphomas. Adv. Clin. Exp. Med. 2019, 28, 1119–1124. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.; Deshmukh, A.; Chandrashekhara, S.H. Imaging of Bowel Lymphoma: A Pictorial Review. Dig. Dis. Sci. 2022, 67, 1187–1199. [Google Scholar] [CrossRef]
- Chowdhury, M.; Endo, M.; Chiba, T.; Kudara, N.; Oana, S.; Sato, K.; Akasaka, R.; Tomita, K.; Fujiwara, S.; Mizutani, T.; et al. Characterization of follicular lymphoma in the small intestine using double-balloon endoscopy. Gastroenterol. Res. Pract. 2009, 2009, 835258. [Google Scholar] [CrossRef]
- Tormane, M.A.; Laamiri, G.; Beji, H.; Gazzeh, H.; Bouassida, M.; Touinsi, H. Primary mantle-cell lymphoma of small intestine presenting with intussusceptions: A case report and review of the literature. Int. J. Surg. Case Rep. 2024, 121, 109963. [Google Scholar] [CrossRef]
- Kella, V.K.; Constantine, R.; Parikh, N.S.; Reed, M.; Cosgrove, J.M.; Abo, S.M.; King, S. Mantle cell lymphoma of the gastrointestinal tract presenting with multiple intussusceptions--case report and review of literature. World J. Surg. Oncol. 2009, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.G.; Xu, X.M.; Wei, S.M. Multiple Lymphomatous Polyposis of the Intestine with Ileocecal Intussusception Due to Mantle Cell Lymphoma: A Case Report of a 34-Year-Old Man. Am. J. Case Rep. 2018, 19, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Aukhojee, V.; Gilong, C.M.; Seewoogoolam, G.; Strauss, P.N. Rare presentation of ileocolic intussusception secondary to mantle cell lymphoma. BMJ Case Rep. 2019, 12, e229425. [Google Scholar] [CrossRef] [PubMed]
- Kasparian, S.; Burns, E.; Shehabeldin, A.; Awar, M.; Pingali, S.R. Recurrent small bowel obstruction caused by Burkitt lymphoma in an elderly man: A case report and review of the literature. J. Med. Case Rep. 2020, 14, 127. [Google Scholar] [CrossRef]
- Takayama, Y.; Saito, M.; Ichida, K.; Muto, Y.; Tanaka, A.; Rikiyama, T. Intestinal perforation secondary to intestinal Burkitt lymphoma. Int. J. Surg. Case Rep. 2021, 79, 417–420. [Google Scholar] [CrossRef]
- Grajo, J.R.; Kayton, M.L.; Steffensen, T.S.; Dragicevic, N.; Guidi, C.B. Presentation of ileal Burkitt lymphoma in children. J. Radiol. Case Rep. 2012, 6, 27–38. [Google Scholar] [CrossRef]
- Mago, S.; Mavilia, M.; Forouhar, F.; Vaziri, H. Small bowel T-cell lymphoma: A MEITL-ing diagnosis. Clin. J. Gastroenterol. 2021, 14, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Sumimura, J.; Mizutani, S.; Tazuke, Y.; Okuda, S.; Dezawa, T. The doughnut sign: An ultrasound finding in pediatric intestinal Burkitt’s lymphoma. Pediatr. Surg. Int. 1998, 13, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Hasaballah, M.; Abdel-Malek, R.; Zakaria, Z.; Marie, M.S.; Naguib, M.S. Transabdominal ultrasonographic features in the diagnosis of gastrointestinal lymphoma. J. Gastrointest. Oncol. 2018, 9, 1190–1197. [Google Scholar] [CrossRef]
- Goerg, C.; Schwerk, W.B.; Goerg, K. Gastrointestinal lymphoma: Sonographic findings in 54 patients. Am. J. Roentgenol. 1990, 155, 795–798. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, B.; Cai, S.; Jiang, Y.X.; Li, W.B.; Yang, X.; Zhao, R.N. Ultrasonographic and general pathologic features assessment of small intestinal lymphoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2013, 35, 318–321. [Google Scholar] [CrossRef]
- Cui, N.Y.; Gong, X.T.; Tian, Y.T.; Wang, Y.; Zhang, R.; Liu, M.J.; Han, J.; Wang, B.; Yang, D. Contrast-enhanced ultrasound imaging for intestinal lymphoma. World J. Gastroenterol. 2021, 27, 5438–5447. [Google Scholar] [CrossRef]
- Starczewska Amelio, J.M.; Cid Ruzafa, J.; Desai, K.; Tzivelekis, S.; Muston, D.; Khalid, J.M.; Ashman, P.; Maguire, A. Prevalence of gastrointestinal stromal tumour (GIST) in the United Kingdom at different therapeutic lines: An epidemiologic model. BMC Cancer 2014, 14, 364. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Ota, S.; Yamasaki, M.; Batsaikhan, B.; Furukawa, A.; Watanabe, Y. Gastrointestinal stromal tumors: A comprehensive radiological review. Jpn. J. Radiol. 2022, 40, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Tryggvason, G.; Gíslason, H.G.; Magnússon, M.K.; Jónasson, J.G. Gastrointestinal stromal tumors in Iceland, 1990–2003: The icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int. J. Cancer 2005, 117, 289–293. [Google Scholar] [CrossRef]
- Nilsson, B.; Bümming, P.; Meis-Kindblom, J.M.; Odén, A.; Dortok, A.; Gustavsson, B.; Sablinska, K.; Kindblom, L.G. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005, 103, 821–829. [Google Scholar] [CrossRef]
- Miettinen, M.; Makhlouf, H.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors of the jejunum and ileum: A clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am. J. Surg. Pathol. 2006, 30, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Z.; Wang, W.; Zhao, W.; Tuo, S.; Shi, Y.; Zhang, W.; Chen, L.; Hong, L.; Yang, J.; et al. Current characteristics on small intestinal stromal tumor-a case control study. Ann. Palliat. Med. 2020, 9, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liao, Y.; Wu, J.; Yang, J.; Zhang, H.; Wang, X.; Sun, S. Small bowel gastrointestinal stromal tumor: A retrospective study of 32 cases at a single center and review of the literature. Ther. Clin. Risk Manag. 2018, 14, 1467–1481. [Google Scholar] [CrossRef]
- Basukala, S.; Karki, S.; Maharjan, S.; Banmala, S.; Shrestha, M.; Jayswal, M.; Shrestha, K. Small bowel intussusception in an adult secondary to gastrointestinal stromal tumor: A rare case report. Ann. Med. Surg. 2023, 85, 1952–1955. [Google Scholar] [CrossRef]
- Mishra, A.; Gyawali, S.; Kharel, S.; Mishra, A.; Pathak, N.; Subedi, N.; Gaire, P. Multiple jejunal gastrointestinal stromal tumors and Neurofibromatosis type 1: A rare association. Int. J. Surg. Case Rep. 2021, 85, 106178. [Google Scholar] [CrossRef]
- Bozkurt, T.; Butsch, B.; Schmiegelow, P.; Lux, G. Sonographische Darstellung mesenchymaler Dünndarmtumoren in der Diagnostik von unklaren, gastrointestinalen Blutungen. Ultraschall Med. 1993, 14, 264–268. [Google Scholar] [CrossRef]
- Hocke, M.; Braden, B.; Jenssen, C.; Dietrich, C.F. Present status and perspectives of endosonography 2017 in gastroenterology. Korean J. Intern. Med. 2018, 33, 36–63. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Wu, T.; Qin, L. Case report: Ultrasound and contrast-enhanced ultrasound findings of pediatric small intestinal inflammatory myofibroblastic tumor. Front. Oncol. 2025, 15, 1512402. [Google Scholar] [CrossRef]
- Rispo, A.; De Sire, R.; D’Armiento, M.; De Bonis, L.; Tropeano, F.P.; Ricciolino, S.; Nardone, G.; Luglio, G. Ultrasonographic diagnosis of ileo-ileal intussusception secondary to Vanek’s tumor. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 350–353. [Google Scholar] [CrossRef]
- Shinagare, A.B.; Ramaiya, N.H.; Jagannathan, J.P.; Krajewski, K.M.; Giardino, A.A.; Butrynski, J.E.; Raut, C.P. A to Z of desmoid tumors. Am. J. Roentgenol. 2011, 197, W1008–W1014. [Google Scholar] [CrossRef]
- Eswaravaka, S.K.; Deshpande, S.H.; Chiranjeev, R.; Pandya, J.S. Intra-abdominal small intestinal desmoid tumour mimicking GIST. BMJ Case Rep. 2021, 14, e237032. [Google Scholar] [CrossRef]
- Möller, K.; Holz, T.; Jenssen, C.; Braden, B.; Hocke, M.; On, W.; Everett, S.M.; Dong, Y.; Ge, N.; Sun, S.; et al. Comments and illustrations of the European Federation of Societies for Ultrasound in Medicine contrast-enhanced ultrasonography guidelines: Multiparametric imaging and EUS-guided sampling in rare pancreatic tumors. Mesenchymal pancreatic tumors of intermediate biological behaviour. Endosc. Ultrasound 2024, 13, 145–153. [Google Scholar] [CrossRef]
- Al-Obeed, O.A.; Abdulla, M.H. Lymphangioma of the ileocecal valve clinically masquerading as a submucosal small intestinal GIST: Report of a case and literature review. Saudi J. Gastroenterol. 2014, 20, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.-L.; Lee, T.-Y.; Hung, C.-F.; Ng, K.-K. Ultrasound and CT findings of a cecal lymphangioma presenting with intussusception. Eur. J. Radiol. 1998, 27, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Limaiem, F.; Khalfallah, T.; Marsaoui, L.; Bouraoui, S.; Lahmar, A.; Mzabi, S. Jejunal lymphangioma: An unusual cause of intussusception in an adult patient. Pathologica 2015, 107, 19–21. [Google Scholar]
- Zhao, N.; Fu, Y.; Wang, Z.; An, Q.; Jia, W.-Z. Case report: Submucosal cavernous lymphangioma causing jejuno-jejunal intussusception in an adult. Front. Surg. 2022, 9, 953840. [Google Scholar] [CrossRef]
- Fukushima, N.; Aoki, H.; Fukazawa, N.; Ogawa, M.; Yoshida, K.; Yanaga, K. Schwannoma of the Small Intestine. Case Rep. Gastroenterol. 2019, 13, 294–298. [Google Scholar] [CrossRef]
- Onishi, S.; Ibuka, T.; Uno, Y.; Kojima, K.; Takada, J.; Kubota, M.; Shimizu, M. A rare case of schwannoma of the small intestine discovered during intussusception. JGH Open 2024, 8, e13078. [Google Scholar] [CrossRef] [PubMed]
- Jezovit, M.; Bakirli, H.; Bakirov, I.; Hureibi, K.; Bakirova, G.; Okolicany, R.; Janac, P.; Meciarova, I.; Alhwaymel, N.; Bakirli, I.; et al. Ileal Schwannoma: A Rare Cause of Pelvic Mass. Case Rep. Surg. 2024, 2024, 5572087. [Google Scholar] [CrossRef]
- Wippold, F.J., 2nd; Lubner, M.; Perrin, R.J.; Lammle, M.; Perry, A. Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns. Am. J. Neuroradiol. 2007, 28, 1633–1638. [Google Scholar] [CrossRef]
- Möller, K.; Batali, A.; Jenssen, C.; Braden, B.; Hocke, M.; On, W.; Everett, S.M.; Dong, Y.; Ge, N.; Sun, S.; et al. Comments and illustrations of the European Federation of Societies for Ultrasound in Medicine contrast-enhanced ultrasound guidelines: Multiparametric imaging and EUS-guided sampling in rare pancreatic tumors. Benign mesenchymal pancreatic tumors. Endosc. Ultrasound 2024, 13, 218–231. [Google Scholar] [CrossRef]
- Dwivedi, R.C.; Kazi, R.; Agrawal, N.; Chisholm, E.; St Rose, S.; Elmiyeh, B.; Rennie, C.; Pepper, C.; Clarke, P.M.; Kerawala, C.J.; et al. Comprehensive review of small bowel metastasis from head and neck squamous cell carcinoma. Oral. Oncol. 2010, 46, 330–335. [Google Scholar] [CrossRef]
- Di, J.Z.; Peng, J.Y.; Wang, Z.G. Prevalence, clinicopathological characteristics, treatment, and prognosis of intestinal metastasis of primary lung cancer: A comprehensive review. Surg. Oncol. 2014, 23, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Gakuhara, A.; Kitani, K.; Terashita, D.; Tomihara, H.; Fukuda, S.; Ota, K.; Hashimoto, K.; Ishikawa, H.; Hida, J.; Wakasa, T.; et al. Small Bowel Metastasis of Breast Cancer-A Case Report. Gan To Kagaku Ryoho 2021, 48, 1737–1739. [Google Scholar] [PubMed]
- Zhang, Z.; Liu, J.; Yu, P.F.; Yang, H.R.; Li, J.Y.; Dong, Z.W.; Shi, W.; Gu, G.L. Clinicopathological analysis of small intestinal metastasis from extra-abdominal/extra-pelvic malignancy. World J. Gastrointest. Oncol. 2024, 16, 4138–4145. [Google Scholar] [CrossRef]
- Đokić, M.; Badovinac, D.; Petrič, M.; Trotovšek, B. An unusual presentation of metastatic malignant melanoma causing jejuno-jejunal intussusception: A case report. J. Med. Case Rep. 2018, 12, 337. [Google Scholar] [CrossRef]
- Hsu, Y.F.; Huang, C.Y.; Chen, T.J.; Chou, Y.H. Small bowel intussusception due to metastatic intestinal carcinosarcoma from a pulmonary primary. Int. Med. Case Rep. J. 2011, 4, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.Q.; Ren, J.Y.; Xu, X.L.; Xiong, L.Y.; Peng, Y.X.; Pan, X.F.; Dietrich, C.F.; Cui, X.W. Ultrasound-based artificial intelligence in gastroenterology and hepatology. World J. Gastroenterol. 2022, 28, 5530–5546. [Google Scholar] [CrossRef]
- Graumann, O.; Cui Xin, W.; Goudie, A.; Blaivas, M.; Braden, B.; Campbell Westerway, S.; Chammas, M.C.; Dong, Y.; Gilja, O.H.; Hsieh, P.C.; et al. Artificial Intelligence in Abdominal, Gynecological, Obstetric, Musculoskeletal, Vascular and Interventional Ultrasound. Ultrasound Med. Biol. 2025, 51, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.A.; Kallenbach, M.; Radu, I.P.; Berzigotti, A.; Dietrich, C.F.; Kather, J.N.; Luedde, T.; Seraphin, T.P. Artificial Intelligence for Contrast-Enhanced Ultrasound of the Liver: A Systematic Review. Digestion 2025, 106, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Okagawa, Y.; Abe, S.; Yamada, M.; Oda, I.; Saito, Y. Artificial Intelligence in Endoscopy. Dig. Dis. Sci. 2022, 67, 1553–1572. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).