Federated Learning in Public Health: A Systematic Review of Decentralized, Equitable, and Secure Disease Prevention Approaches
Abstract
1. Introduction
1.1. Background: The Role of AI in Public Health Policy
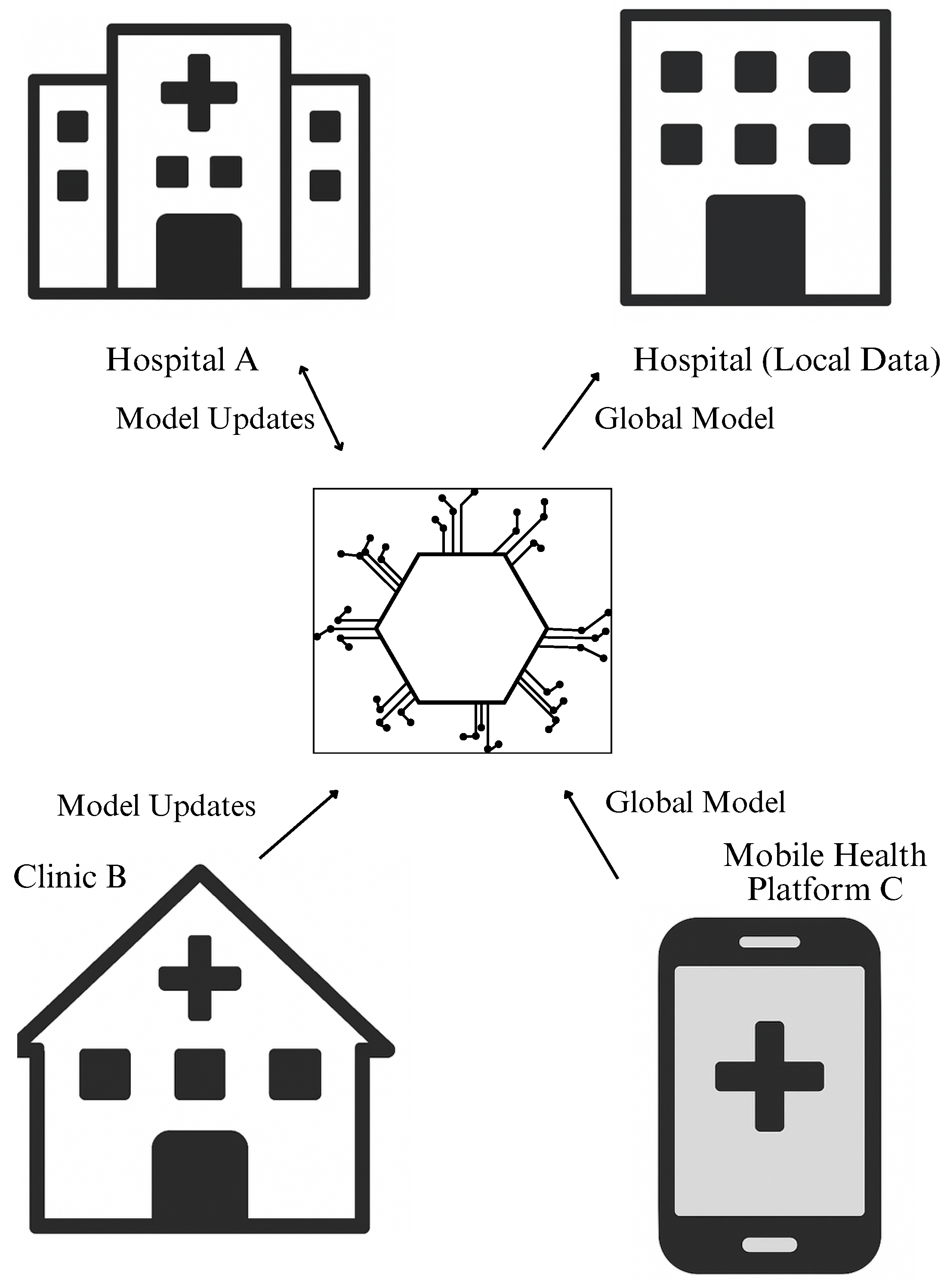
1.2. The Rise of Federated Learning in Healthcare
1.3. Disease Prevention and the Need for Privacy-Preserving Models
1.4. Rationale for the Review
- Lack of synthesized evidence on FL’s role in shaping public health policy across communicable and non-communicable disease domains;
- Limited understanding of FL’s capacity to overcome data-sharing barriers in multi jurisdictional public health environments;
- Insufficient attention to the socio-technical challenges of FL deployment, including interoperability, fairness, and regulatory harmonization;
- A need to map current FL applications to ethical frameworks, equity goals, and health systems readiness for AI integration.
1.5. Objectives and Research Questions
1.5.1. Research Objectives
- To systematically examine how FL has been applied in public health contexts for disease prevention;
- To identify the opportunities offered by FL in supporting equitable, privacy-preserving, and scalable public health policy;
- To explore the challenges, technical, infrastructural, ethical, and regulatory associated with implementing FL in diverse health systems;
- To evaluate how FL aligns with or supports existing public health goals, such as health equity, inclusion, data sovereignty, and policy responsiveness.
1.5.2. Research Questions
- What is the current scope and distribution of FL applications in public health-related disease prevention?
- What specific opportunities does FL offer for enhancing public health policy formulation and implementation?
- What are the major technical, ethical, and governance-related challenges associated with applying FL in public health settings?
- How well do existing FL applications address critical public health concerns such as data privacy, equity, and system interoperability?
- What gaps exist in the literature that may hinder the operationalization of FL in national and global health policy?
1.6. Scope and Purpose
2. Methods
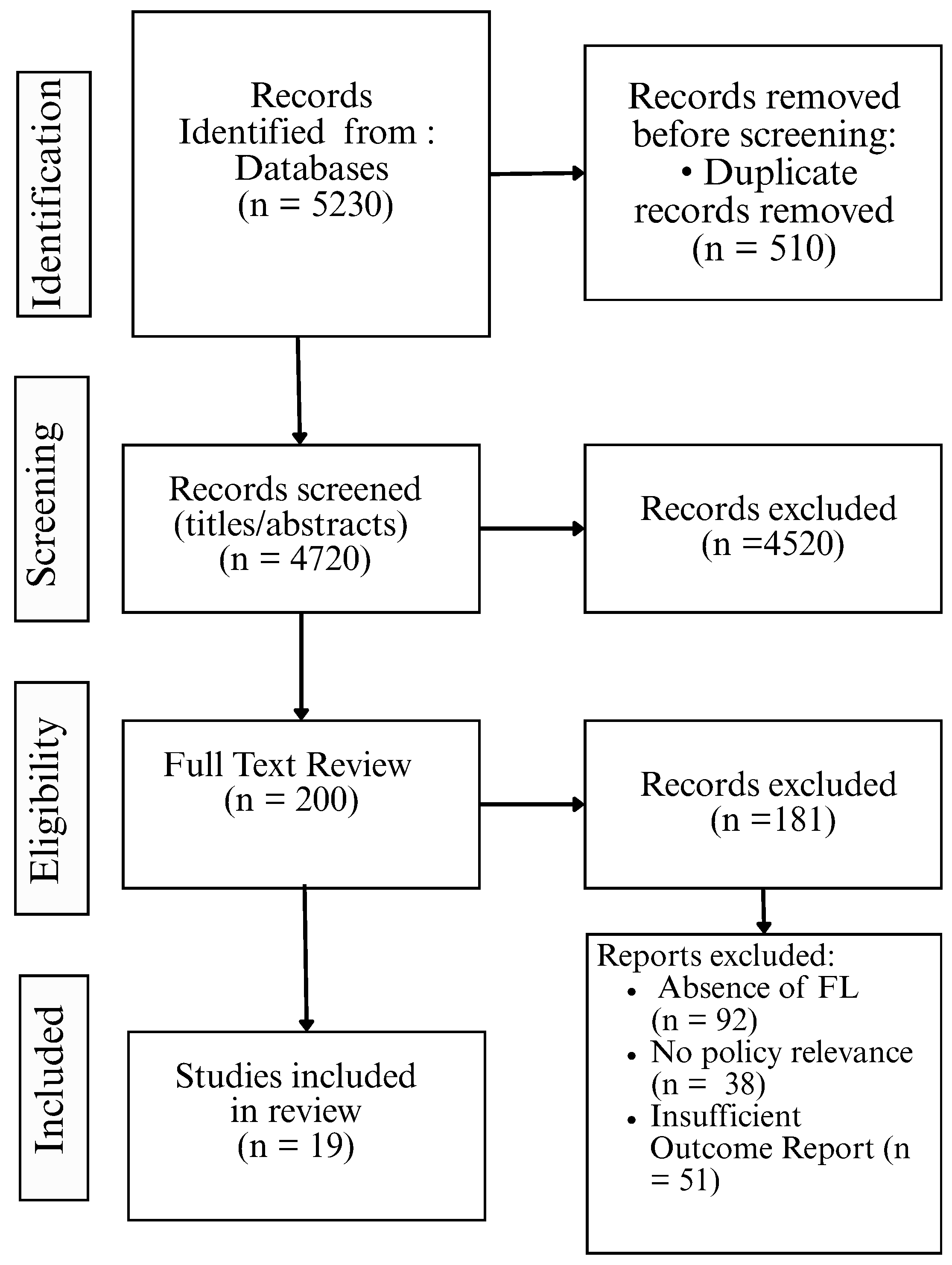
2.1. Review Protocol and Registration
- Clearly formulated research questions using a modified PICO framework suitable for technology-policy evaluations;
- Prespecified eligibility criteria regarding population (public health systems or institutions), intervention (FL), comparator (not required), and outcomes (policy relevance, privacy, equity, and technical performance);
- A comprehensive multi-database search strategy;
- Dual reviewer-based study screening and data extraction procedures;
- Quality assessment of included studies based on relevance, methodological rigor, and transparency;
- Descriptive synthesis and thematic analysis of study findings.
2.2. Search Strategy and Information Sources
(“federated learning” OR “distributed learning” OR “collaborative AI”) AND (“public health” OR “health policy” OR “population health”) AND (“disease prevention” OR “epidemiology” OR “surveillance”)
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
- Study Type: Peer-reviewed original research articles, including experimental, observational, implementation, or modeling studies.
- Technology Focus: Studies that applied, proposed, or evaluated FL or closely related decentralized ML approaches in a public health context.
- Policy Relevance: Studies that linked FL outputs to decision-making, policy formulation, population-level interventions, or disease prevention strategies.
- Health Focus: Studies addressing both communicable and non-communicable diseases in human populations.
- Setting: Global studies including high-, middle-, or low-income settings.
- Language: Articles published in English.
- Publication Year: Studies published between 1 January 2020 and 30 June 2025.
2.3.2. Exclusion Criteria
- Non-peer-reviewed literature, including preprints, white papers, editorial pieces, and conference abstracts without full-text peer review.
- Purely technical papers focusing on algorithmic development without public or population health relevance.
- Studies limited to clinical decision support systems at the individual level without connection to population-wide health policy or prevention.
- Papers discussing only centralized AI or non-decentralized architectures without reference to federated or privacy-preserving frameworks.
- Animal studies, simulated populations, or lab-only test environments unrelated to public health systems.
- Studies not available in English.
2.4. PRISMA Flow Diagram and Study Selection Summary
2.5. Data Extraction and Management
- Bibliographic Information: Author(s), year of publication, journal, and country/region of study.
- Study Design and Type: Experimental, observational, modeling, or implementation study
- Federated Learning Approach: Type of FL architecture (horizontal, vertical, or hybrid), algorithm used, and model type.
- Health Focus: Disease type (communicable or non-communicable) and public health application domain
- Policy Relevance: Linkage to public health policy outcomes (e.g., health equity, resource allocation, outbreak control).
- Technical Details: Data sources, number of participating nodes/institutions, and privacy-preserving techniques employed.
- Evaluation Metrics: Model performance (accuracy, AUC, recall, and F1), fairness indicators, and data heterogeneity handling.
- Key Findings: Summary of main outcomes, challenges, and reported benefits.
- Limitations: Study-specific technical, ethical, or practical limitations.
2.6. Quality Assessment of Included Studies
- Clarity of Research Objectives: Whether the study clearly articulated its objectives in relation to FL and public health.
- Technical Soundness: Appropriateness of the FL method used, data description, algorithm choice, and handling of heterogeneity or bias.
- Policy Relevance and Public Health Impact: Degree to which the study addressed population-level outcomes, public health planning, equity, or disease prevention.
- Ethical and Privacy Considerations: Explicit reference to data privacy techniques (e.g., differential privacy), ethical compliance, and protection of sensitive data.
- Transparency and Reproducibility: Availability of code, datasets, model parameters, or documentation of reproducibility practices.
2.7. Data Synthesis Approach
2.7.1. Stage 1: Thematic Coding and Grouping
- Health domain: communicable vs. non-communicable diseases;
- Public health function: early detection, surveillance, risk prediction, resource allocation, behavioral prevention, etc.;
- Type of FL architecture: horizontal, vertical, hybrid, or cross-device FL;
- Policy relevance: direct vs. indirect contributions to policy formulation or implementation.
2.7.2. Stage 2: Cross-Comparative Analysis
- Model generalizability across settings;
- Ethical and privacy-preserving mechanisms adopted;
- Data and system heterogeneity challenges;
- Real-world integration into public health governance.
2.7.3. Stage 3: Pattern Identification and Synthesis
- Opportunities for Federated Learning in Disease Prevention Policy
- Challenges to Federated Learning Deployment in Public Health
- Policy and System-Level Implications
3. Result
3.1. Overview of Included Studies
3.1.1. Geographic Distribution
- Asia (n = 7): Studies were conducted in China, India, South Korea, and Singapore, with a focus on infectious disease prediction, COVID-19 outbreak control, and chronic disease surveillance using FL frameworks deployed across hospitals, mobile platforms, and community health centers [2,5,20,34,35,36,37].
- Africa (n = 1): One study from sub-Saharan Africa explored FL deployment for tuberculosis screening in under-resourced clinics, emphasizing the potential of FL in promoting health equity [30].
- High-Income Countries (HICs): 12 studies
- Upper-Middle-Income Countries (UMICs): 3 studies
- Lower-Middle-Income Countries (LMICs): 3 studies
- Low-Income Countries (LICs): 1 study
- Academic: Public health authority partnerships;
- Healthcare: Technology consortia;
- Interdisciplinary: AI research networks.
3.1.2. Disease Domains (e.g., Infectious vs. Chronic Diseases)
- Predict and detect outbreaks;
- Enable cross-institutional surveillance using real-time or historical EHR data;
- Train predictive models for epidemic modeling across regions or countries;
- Maintain patient privacy during collaborative surveillance or diagnostics.
- Diabetes mellitus (Type 2);
- Cardiovascular diseases;
- Cancer;
- Neurological and mental health conditions.
3.1.3. Types of Federated Learning Architectures Used
- Multi-hospital collaborations using electronic health records (EHRs) with similar structure;
- Population-level modeling with aligned variables across regions or clinics;
- Real-time epidemic surveillance systems aggregating FL models across districts or provinces.
- Linking insurance databases with hospital records for policy impact modeling;
- Combining lifestyle data from wearable devices with clinical indicators;
- Integration of socioeconomic and behavioral data into chronic disease risk stratification.
- Cross-silo + cross-device FL: Used in smart surveillance with data from both institutions and edge devices;
- Hierarchical FL models: Where intermediate aggregators first aggregate local updates before sending to national-level servers;
- Asynchronous FL: For low-bandwidth or heterogeneous environments.
3.2. Opportunities of Federated Learning in Disease Prevention
3.2.1. Early Warning and Outbreak Detection
3.2.2. Predictive Modeling and Risk Stratification
3.2.3. Data Sovereignty and Equity in Policy Design
- Lack of data standardization;
- Inability to share data due to infrastructure or political constraints;
- Lower digital capacity and research visibility.
- Federated health systems with regional autonomy (e.g., India, Brazil);
- International collaborations across public health agencies (e.g., WHO, CDC networks);
- Crisis response planning in politically fragmented or decentralized states.
3.3. Challenges in Applying Federated Learning to Public Health Policy
- Demographic variation: Different regions exhibit varying age distributions, socioeconomic statuses, comorbidity profiles, and health behaviors [66].
- Clinical practice patterns: Institutions may differ in diagnostic criteria, treatment protocols, and reporting standards [67].
- Technological infrastructure: Variations in electronic health record (EHR) systems, data granularity, and missingness rates [68].
- Geographical and environmental factors: Local climate, pollution levels, population density, and access to care impact disease patterns [44].
- Model Divergence: Local models trained on vastly different distributions may produce gradients that cancel each other during aggregation, reducing learning efficiency [65].
- Bias and Inaccuracy: The global model may disproportionately reflect data from dominant or well-resourced institutions, neglecting minority or rural populations [6].
- Reduced Generalizability: If the aggregated model is skewed toward specific cohorts, its performance across the entire population diminishes specially for subgroups not well represented in training data [44].
- Overfitting Local Trends: Local updates may overfit regional idiosyncrasies, hindering the model’s ability to extract general patterns across nodes [66].
- A nationwide FL model for diabetes prediction showed significantly higher accuracy in urban hospitals than in rural clinics [64].
- In a COVID-19 surveillance system, local data from under-resourced regions had high missingness, resulting in poorly updated local models and limited global model contribution [44].
- Attempts to integrate behavioral data from mobile apps with hospital EHRs faced feature misalignment and inconsistent data semantics [39].
- Personalized Federated Learning:Each node receives a customized model tailored to its local distribution, improving performance without fully sacrificing global generalization [69].
- Clustered Federated Learning: Nodes are grouped into clusters based on similarity, and separate models are trained per cluster before optionally aggregating [70].
- Data Harmonization and Preprocessing: Aligning data schemas and applying imputation or normalization techniques can reduce inter node variance [71].
- Adaptive Weighting: Contributions to the global model are weighted based on update reliability or data representativeness [72].
- Model Architecture Adaptation: Using modular or hierarchical models that can adapt parts of the architecture to local distributions while keeping shared layers globally synchronized [73].
- Supporting standardization of health data collection and coding practices [74];
- Investing in digital infrastructure to improve data quality in underrepresented regions [39];
- Establishing fairness metrics for evaluating FL-based health policy tools [6];
- Encouraging transparency and documentation in FL model reporting [71].
3.3.1. Resource Constraints and Technical Complexity
- Synchronization of updates across nodes with different computing speeds or schedules [63].
- Standardization of data schemas across diverse EHRs [68].
- Establishing secure communication protocols and legal agreements between participating institutions [71].
- Monitoring model performance and error propagation across the network [67].
- Investment in digital health infrastructure and capacity-building [75].
- Development of lightweight and energy-efficient FL algorithms [64].
- Creation of national FL frameworks tailored to public sector constraints [39].
- Public–private partnerships to support open-source FL tools and decentralized platforms [71].
- Incentivizing collaborative networks that include rural and low-resource participants [80].
3.3.2. Governance, Ethics, and Regulatory Barriers
- Unclear liability in cases of model failure or misdiagnosis due to federated updates [82].
- Data ownership ambiguity even though raw data is not shared; model updates may still leak information and trigger jurisdictional disputes [57].
- Regulatory gaps in defining what constitutes “data sharing” under laws like the GDPR, HIPAA (USA), or India’s Data Protection Bill [83].
- Inconsistent institutional policies on data security, retention, and usage rights [84].
- Lack of unified oversight bodies to ensure ethical model deployment and compliance monitoring [57].
- Fragmentation of legal accountability, especially when federated models are deployed in real-time for outbreak detection or policy support [6].
- Informed consent: Many FL deployments are built atop EHRs or registries where patients have not explicitly consented to their data being used for decentralized AI training [85].
- Fairness and representation: FL networks that exclude low-resource regions due to lack of infrastructure inadvertently introduce bias, resulting in policies that overlook marginalized populations [86].
- Opacity of model decisions: The decentralized and encrypted nature of FL complicates auditing and explaining model behavior, reducing stakeholder trust in public health contexts [39].
- Develop FL-specific data governance frameworks involving regulators, ethicists, clinicians, and technologists [57].
- Adopt standardized legal templates for inter-institutional FL collaborations that clarify data rights and model liability and update ownership [88].
- Establish regulatory sandboxes to test FL models under controlled conditions while evolving compliance policies [75].
- Implement ethical auditing protocols that include fairness checks, bias audits, and explainability tools for FL systems [6].
- Incorporate federated IRB models to oversee multi-institutional FL research ethically and efficiently [30].
4. Discussion
4.1. Principal Findings and Interpretation
4.1.1. Summary of Key Findings
- Adoption Across Disease Domains: FL is being actively explored in both infectious disease control and chronic disease management, diabetes risk prediction, and cancer stratification. Its ability to integrate geographically and demographically dispersed datasets without direct data exchange has made it particularly attractive in multi-jurisdictional public health collaborations [44,67].
- Predominance of Horizontal FL Architectures: Most reviewed studies implemented horizontal FL where datasets across institutions share the same features but differ in patient samples. Vertical FL and hybrid architectures were underrepresented, highlighting a current research gap in modeling across heterogeneous feature spaces [65].
- Opportunities Identified: FL presents several high-value opportunities in public health policy:
- Early warning systems using distributed real-time data;
- Predictive analytics tailored to local epidemiology;
- Risk stratification models that preserve population diversity;
- Support for data sovereignty, enabling regions to retain control over their data while contributing to global models.
- Challenges Reported: Despite these advantages [39], critical barriers remain:
- Non-IID data significantly hamper model performance and generalizability;
- Infrastructure disparity limits equitable participation from low-resource regions;
- Ethical and regulatory ambiguity undermines confidence in implementation;
- Lack of model interpretability and fairness auditing, especially in policy-sensitive domains like vaccine distribution.
- Underrepresentation of LMICs: The majority of FL deployments and studies were conducted in high income countries or technologically advanced institutions. This reveals a substantial equity gap and calls for urgent attention to supporting FL readiness in underrepresented regions [64].
4.1.2. Interpretation of Findings in Public Health Context
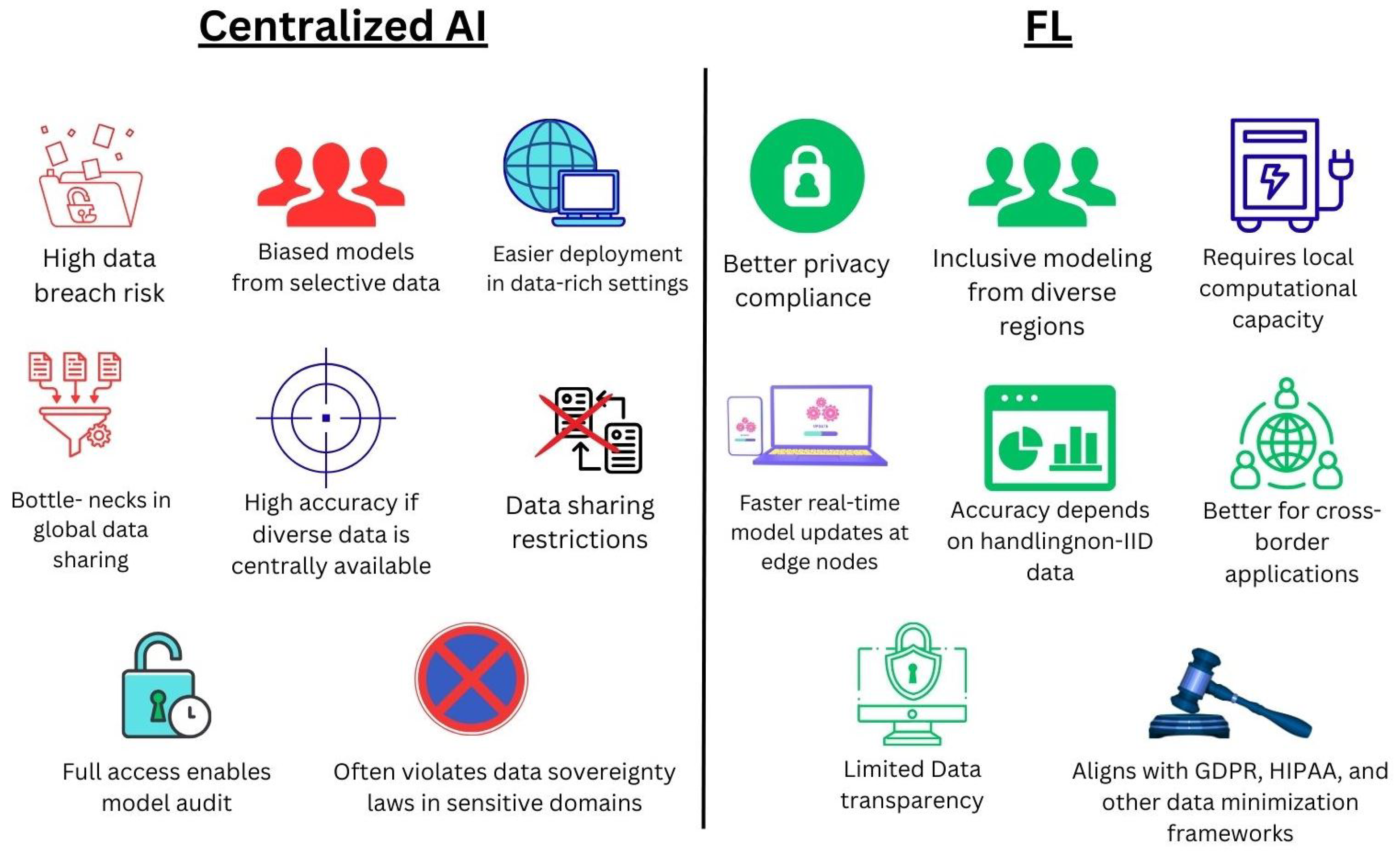
4.2. Comparison with Centralized AI Models
4.2.1. Data Governance and Privacy
4.2.2. Equity and Inclusivity
4.2.3. Technical and Infrastructural Requirements
- Non-IID data across institutions;
- Computational disparities (e.g., slow clients, weak hardware);
- Communication delays in synchronous updates.
4.2.4. Adaptability in Emergency and Outbreak Scenarios
- Outbreak detection;
- Syndromic surveillance;
- Border-crossing epidemiological collaboration.
4.2.5. Interpretability and Transparency
4.2.6. Cost and Sustainability
4.3. Policy Implications and Framework Alignment
4.3.1. Enabling Privacy-by-Design in Public Health Systems
4.3.2. Promoting Equitable Participation in Global Health Initiatives
4.3.3. Strengthening Cross-Border Collaboration in Epidemic Intelligence
4.3.4. Adapting Legal Frameworks and Procurement Policies
- Health IT procurement guidelines to include edge-computing requirements;
- Cybersecurity and medical device laws to support decentralized learning;
- Data residency laws to recognize encrypted parameter exchange as compliant.
4.3.5. Establishing Public–Private Partnerships
- Incentivize private sector FL tools aligned with public objectives;
- Use regulatory sandboxes to test FL for health applications;
- Create FL consortia under existing health innovation programs.
4.4. Addressing Data and Infrastructure Inequality
4.4.1. Unequal Access to Digital Infrastructure
- Limited broadband access and unreliable electricity supply;
- Outdated or no local computing hardware;
- Inadequate cybersecurity frameworks;
- Shortage of trained IT and data personnel.
4.4.2. Disparities in Data Quantity and Quality
- Data volume: Smaller institutions may have insufficient case numbers.
- Data completeness: Underfunded systems often contain missing or low-quality data.
- Semantic heterogeneity: Inconsistent labeling or coding across regions.
- Bias and underrepresentation: Marginalized groups may be absent from datasets.
4.4.3. Algorithmic and Architectural Strategies for Mitigation
- Weighted Aggregation, FedAvgM and FedProx: Adjusts influence based on data quality or size.
- Personalized FL: Allows local fine-tuning for contextual relevance.
- Data Augmentation at the Edge: Synthetic data generation for low sample clients.
- Knowledge Distillation and Transfer Learning: Share global insights with weaker nodes.
- Edge Resource Optimization: Deploy lightweight models using efficient runtimes (e.g., TensorFlow Lite).
4.4.4. Policy and Investment Considerations
- Funding schemes for FL infrastructure in LMICs.
- Digital literacy and workforce training programs.
- Inclusion of FL in health system strengthening by donors.
- National data standardization for interoperability.
4.5. Ethical and Legal Considerations in FL Deployment
4.5.1. Respect for Autonomy and Informed Consent
- Whether data subjects can meaningfully opt in or opt out of FL participation;
- If existing consent agreements cover federated model training;
- How dynamic consent models can be integrated into federated architectures.
- Provide transparent documentation on data usage;
- Support institutional and community-level governance;
- Explore user-controlled data access and federated consent mechanisms.
4.5.2. Privacy, Confidentiality, and Data Minimization
- Model inversion attacks;
- Gradient leakage of sensitive attributes;
- Reduced visibility into system-wide data misuse or bias.
- Differential privacy;
- Secure multi-party computation and encryption protocols;
- Regular third-party audits and adversarial robustness testing.
4.5.3. Algorithmic Fairness and Non-Discrimination
- Skewed disease predictions across demographic or geographic groups;
- Unequal allocation of health resources.
- Weighted aggregation to account for diversity;
- Performance auditing across subgroups;
- Use of fairness-aware FL methods (e.g., FairFed, FedAUX).
4.5.4. Accountability and Transparency
- Traceability of model errors;
- Assignment of responsibility;
- Post hoc explainability and legal liability [110].
- Log participation, updates, and training metrics;
- Use federated explainability tools (e.g., SHAP, LIME);
- Formalize roles and responsibilities via contracts or MoUs.
4.5.5. Regulatory and Jurisdictional Challenges
- Conflicting data residency laws;
- Ambiguous classification of model updates as data processing;
- Lack of unified AI regulation for decentralized systems [111].
- Creating FL-specific compliance toolkits;
- Establishing AI regulatory sandboxes;
- Supporting cross-border FL consortia and standards.
4.6. Limitations of the Review
4.6.1. Limited Number of Empirical Studies in Public Health
- Limited our ability to conduct meta-analysis or robust subgroup comparisons;
- Required inclusion of proof-of-concept or pilot studies;
- May have led to an overrepresentation of institutional healthcare settings versus national or population-based policy implementations.
4.6.2. Heterogeneity in Study Designs and Reporting
- Application domains (e.g., communicable vs. non-communicable diseases);
- FL architectures (horizontal, vertical, or hybrid);
- Outcome metrics and evaluation criteria.
4.6.3. Language and Publication Bias
- Non-English studies and gray literature (e.g., NGO reports, government documents) were excluded;
- FL applications in LMICs and low-resource settings may have been underrepresented.
4.6.4. Absence of Quantitative Meta-Analysis
4.6.5. Timeframe Constraints and Rapid Field Evolution
4.6.6. Potential Reviewer Subjectivity
- Interpretation of FL implementations;
- Categorization of disease domains and outcomes;
- Inclusion/exclusion decisions during full-text screening.
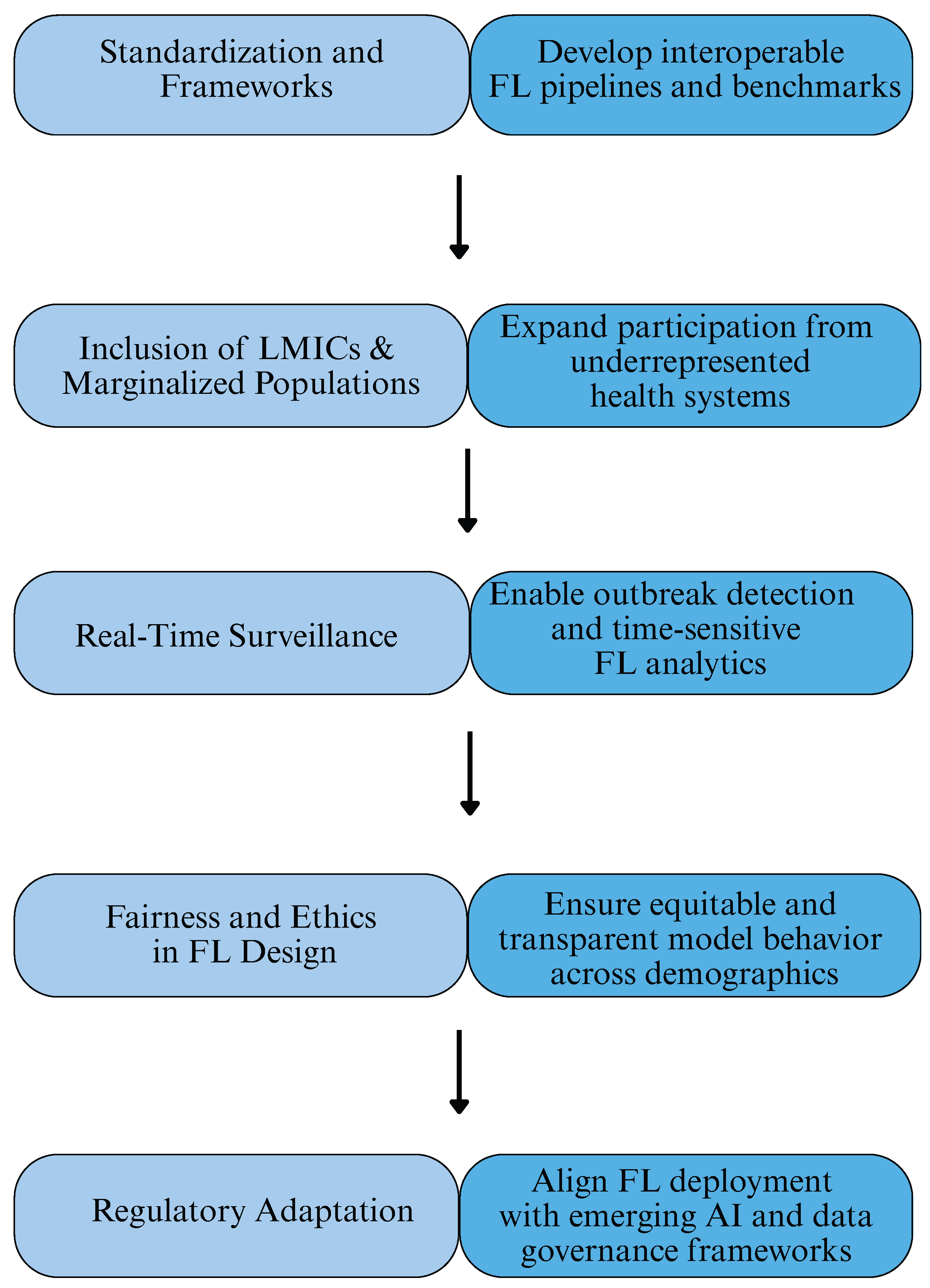
4.7. Future Directions
4.7.1. Development of Standardized FL Frameworks and Protocols
- Unified FL pipelines: Including reference architectures, data schemas, and secure aggregation protocols tailored for public health datasets (e.g., surveillance, EHRs, immunization records).
- Benchmark datasets and evaluation metrics: Publicly accessible FL ready datasets and fairness-aware benchmarks for assessing model robustness across diverse population groups.
- Modular open-source platforms: Frameworks such as TensorFlow Federated and Flower should be extended with plug-ins for health specific model evaluation, fairness metrics, and privacy risk audits.
4.7.2. Inclusion of Underrepresented and Low-Resource Settings
- Edge device optimization: Lightweight FL algorithms suitable for deployment in bandwidth-constrained environments.
- Synthetic data generation: Enabling augmentation of sparse datasets to improve training stability.
- Policy co-design with local stakeholders: Incorporating local health priorities and data stewardship norms into FL objectives and reward structures.
4.7.3. Federated Learning for Real-Time Disease Surveillance
- Time series FL models: Capable of adapting in real-time to changing epidemiological patterns.
- Cross jurisdictional coordination platforms: Secure multinode governance models for international epidemic intelligence.
- Integration with digital contact tracing and syndromic surveillance tools: To enhance early warning systems while preserving data sovereignty.
4.7.4. Federated Fairness and Ethical Algorithm Design
- Personalized FL and subgroup modeling: Algorithms that maintain both global performance and subgroup-specific accuracy.
- Federated explainability: Development of model interpretation tools compatible with decentralized architectures.
- Ethical auditing frameworks: Independent oversight of FL system impact on population level health equity.
4.7.5. Adaptive Regulatory and Legal Frameworks
- Federated data protection standards: Legal definitions for encrypted gradient sharing, local processing accountability, and data fiduciary responsibilities.
- AI-specific public health guidance: Harmonized FL regulations within broader digital health strategies.
- Regulatory sandboxes: Allowing experimentation with FL applications in controlled, low-risk settings before full-scale implementation.
4.7.6. Capacity Building and Workforce Training
- Training public health professionals: In FL concepts, privacy engineering, and ethical AI deployment.
- Building cross disciplinary teams: Integrating epidemiologists, data scientists, ethicists, and legal experts.
- Promoting global south participation: Through fellowships, open-source contributions, and institutional partnerships.
4.8. Policy Recommendations
- Adopt FL pilots for priority use-cases surveillance, early warning, and risk stratification with clear success metrics like timeliness, accuracy, and fairness.
- Publish procurement and governance templates like data-processing agreements, model update contracts, and audit clauses compatible with GDPR/HIPAA and cross-border deployments.
- Standardize data and metadata using open schemas and controlled vocabularies to enable cross site learning without custom plumbing.
- Mandate privacy by design, such as secure aggregation; implement differential privacy where feasible and report fairness metrics by region, facility type, and population group.
- Invest in capacity-building computing, networking, MLOps, and workforce training to include low-resource settings, reducing bias and improving generalizability.
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Panteli, D.; Adib, K.; Buttigieg, S.; Goiana-da Silva, F.; Ladewig, K.; Azzopardi-Muscat, N.; McKee, M. Artificial intelligence in public health: Promises, challenges, and an agenda for policy makers and public health institutions. Lancet Public Health 2025, 10, e428–e432. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.; Rosella, L.C. Priorities for successful use of artificial intelligence by public health organizations: A literature review. BMC Public Health 2022, 22, 2146. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J. Artificial intelligence empowering public health education: Prospects and challenges. Front. Public Health 2024, 12, 1389026. [Google Scholar] [CrossRef]
- Vargas-Santiago, M.; León-Velasco, D.A.; Maldonado-Sifuentes, C.E.; Chanona-Hernandez, L. A State-of-the-Art Review of Artificial Intelligence (AI) Applications in Healthcare: Advances in Diabetes, Cancer, Epidemiology, and Mortality Prediction. Computers 2025, 14, 143. [Google Scholar] [CrossRef]
- Abbas, S.R.; Abbas, Z.; Zahir, A.; Lee, S.W. Federated learning in smart healthcare: A comprehensive review on privacy, security, and predictive analytics with IoT integration. Healthcare 2024, 12, 2587. [Google Scholar] [CrossRef]
- Teo, Z.L.; Jin, L.; Liu, N.; Li, S.; Miao, D.; Zhang, X.; Ng, W.Y.; Tan, T.F.; Lee, D.M.; Chua, K.J.; et al. Federated machine learning in healthcare: A systematic review on clinical applications and technical architecture. Cell Rep. Med. 2024, 5, 101419. [Google Scholar] [CrossRef]
- Olawade, D.B.; Wada, O.J.; David-Olawade, A.C.; Kunonga, E.; Abaire, O.; Ling, J. Using artificial intelligence to improve public health: A narrative review. Front. Public Health 2023, 11, 1196397. [Google Scholar] [CrossRef]
- Choudhury, A.; Volmer, L.; Martin, F.; Fijten, R.; Wee, L.; Dekker, A.; van Soest, J. Advancing privacy-preserving health care analytics and implementation of the personal health train: Federated deep learning study. JMIR AI 2025, 4, e60847. [Google Scholar] [CrossRef]
- Azimaee, M.; Lix, L.M. Federated Learning for cross-jurisdictional analyses: A case study. Int. J. Popul. Data Sci. 2022, 7, 2026. [Google Scholar] [CrossRef]
- Shin, J.; Kim, J.Y. Customized quality assessment of healthcare data. Ann. Lab. Med. 2024, 44, 472–477. [Google Scholar] [CrossRef]
- Casaletto, J.; Bernier, A.; McDougall, R.; Cline, M.S. Federated analysis for privacy-preserving data sharing: A technical and legal primer. Annu. Rev. Genom. Hum. Genet. 2023, 24, 347–368. [Google Scholar] [CrossRef]
- Nazir, S.; Kaleem, M. Federated learning for medical image analysis with deep neural networks. Diagnostics 2023, 13, 1532. [Google Scholar] [CrossRef]
- Chetoui, M.; Akhloufi, M.A. Federated learning for diabetic retinopathy detection using vision transformers. BioMedInformatics 2023, 3, 948–961. [Google Scholar] [CrossRef]
- Turner, K.; Hohman, K.H. Demonstrated Progress and Future Promise of Chronic Disease Data Modernization. Prev. Chronic Dis. 2024, 21, E86. [Google Scholar] [CrossRef]
- Abbas, S.R.; Abbas, Z.; Zahir, A.; Lee, S.W. Advancing genome-based precision medicine: A review on machine learning applications for rare genetic disorders. Briefings Bioinform. 2025, 26, bbaf329. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, X. Integration of soft and hard laws: Profiling legal protection for “AI for all”. Int. J. Digit. Law Gov. 2025, 2. [Google Scholar] [CrossRef]
- Williamson, S.M.; Prybutok, V. Balancing privacy and progress: A review of privacy challenges, systemic oversight, and patient perceptions in AI-driven healthcare. Appl. Sci. 2024, 14, 675. [Google Scholar] [CrossRef]
- Zwiers, L.C.; Grobbee, D.E.; Uijl, A.; Ong, D.S. Federated learning as a smart tool for research on infectious diseases. BMC Infect. Dis. 2024, 24, 1327. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.R.; Seol, H.; Abbas, Z.; Lee, S.W. Exploring the Role of Artificial Intelligence in Smart Healthcare: A Capability and Function-Oriented Review. Healthcare 2025, 13, 1642. [Google Scholar] [CrossRef]
- Li, M.; Xu, P.; Hu, J.; Tang, Z.; Yang, G. From challenges and pitfalls to recommendations and opportunities: Implementing federated learning in healthcare. Med. Image Anal. 2025, 101, 103497. [Google Scholar] [CrossRef]
- Choudhury, A.; Sarma, K.K.; Gulvanskii, V.; Kaplun, D.; Dutta, L. Leveraging federated learning and edge computing for pandemic-resilient healthcare. Sci. Rep. 2025, 15, 20497. [Google Scholar] [CrossRef] [PubMed]
- Eden, R.; Chukwudi, I.; Bain, C.; Barbieri, S.; Callaway, L.; de Jersey, S.; Sullivan, C. A scoping review of the governance of federated learning in healthcare. npj Digit. Med. 2025, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Madathil, N.T.; Dankar, F.K.; Gergely, M.; Belkacem, A.N.; Alrabaee, S. Revolutionizing healthcare data analytics with federated learning: A comprehensive survey of applications, systems, and future directions. Comput. Struct. Biotechnol. J. 2025, 28, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Ahsan, M.M.; Tasnim, L.; Afrin, S.; Biswas, K.; Hossain, M.M.; Raman, S. Federated Learning in Healthcare: Model Misconducts, Security, Challenges, Applications, and Future Research Directions—A Systematic Review. arXiv 2024, arXiv:2405.13832. [Google Scholar]
- Xu, J.; Glicksberg, B.S.; Su, C.; Walker, P.; Bian, J.; Wang, F. Federated learning for healthcare informatics. J. Healthc. Inform. Res. 2021, 5, 1–19. [Google Scholar] [CrossRef]
- Ng, D.; Lan, X.; Yao, M.M.S.; Chan, W.P.; Feng, M. Federated learning: A collaborative effort to achieve better medical imaging models for individual sites that have small labelled datasets. Quant. Imaging Med. Surg. 2021, 11, 852. [Google Scholar] [CrossRef]
- Guan, H.; Yap, P.T.; Bozoki, A.; Liu, M. Federated learning for medical image analysis: A survey. Pattern Recognit. 2024, 151, 110424. [Google Scholar] [CrossRef]
- González-Sánchez, R.; Alonso-Munoz, S.; Kocollari, U. Exploring the supply chain’s transformation to achieve the sustainable development goals in the post-pandemic scenario: A review and a research agenda. Int. J. Logist. Manag. 2025, 36, 137–177. [Google Scholar] [CrossRef]
- Li, J.; He, J.; Hu, L.; Wu, C. A Federated Learning Framework via Decentralized Data Valuation for Chronic Disease Healthcare. In Proceedings of the 2024 6th International Conference on Intelligent Medicine and Image Processing, Bali, Indonesia, 26–29 April 2024. [Google Scholar]
- Fabila, J.; Garrucho, L.; Campello, V.M.; Martín-Isla, C.; Lekadir, K. Federated learning in low-resource settings: A chest imaging study in Africa–Challenges and lessons learned. arXiv 2025, arXiv:2505.14217. [Google Scholar]
- Akande, O.A. Integrating blockchain with federated learning for privacy-preserving data analytics across decentralized governmental health information systems. Int. J. Comput. Appl. Technol. Res. 2022, 11, 622–637. [Google Scholar]
- Benmalek, M.; Seddiki, A. Bias in Federated Learning: Factors, Effects, Mitigations, and Open Issues. Rev. Sci. Technol. Inf. Sér. ISI Ing. Syst. Inf. 2024, 29, 2137–2160. [Google Scholar] [CrossRef]
- Karargyris, A.; Umeton, R.; Sheller, M.J.; Aristizabal, A.; George, J.; Wuest, A.; Pati, S.; Kassem, H.; Zenk, M.; Baid, U.; et al. Federated benchmarking of medical artificial intelligence with MedPerf. Nat. Mach. Intell. 2023, 5, 799–810. [Google Scholar] [CrossRef]
- Tang, G.; Black, J.E.; Williamson, T.S.; Drew, S.H. Federated diabetes prediction in Canadian adults using real-world cross-province primary care data. AMIA Annu. Symp. Proc. 2025, 2024, 1099–1108. [Google Scholar] [PubMed]
- Li, S.; Liu, P.; Nascimento, G.G.; Wang, X.; Leite, F.R.M.; Chakraborty, B.; Hong, C.; Ning, Y.; Xie, F.; Teo, Z.L.; et al. Federated and distributed learning applications for electronic health records and structured medical data: A scoping review. J. Am. Med. Inform. Assoc. 2023, 30, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Linardos, A.; Kushibar, K.; Walsh, S.; Gkontra, P.; Lekadir, K. Federated learning for multi-center imaging diagnostics: A simulation study in cardiovascular disease. Sci. Rep. 2022, 12, 3551. [Google Scholar] [CrossRef] [PubMed]
- Bebortta, S.; Tripathy, S.S.; Basheer, S.; Chowdhary, C.L. Fedehr: A federated learning approach towards the prediction of heart diseases in IoT-based electronic health records. Diagnostics 2023, 13, 3166. [Google Scholar] [CrossRef]
- Lieftink, N.; dos Santos Ribeiro, C.; Kroon, M.; Haringhuizen, G.B.; Wong, A.; van de Burgwal, L.H.M. The potential of federated learning for public health purposes: A qualitative analysis of GDPR compliance, Europe, 2021. Eurosurveillance 2024, 29, 2300695. [Google Scholar] [CrossRef]
- Rieke, N.; Hancox, J.; Li, W.; Milletari, F.; Roth, H.R.; Albarqouni, S.; Cardoso, M.J. The future of digital health with federated learning. npj Digit. Med. 2020, 3, 119. [Google Scholar] [CrossRef]
- Huang, L.; Shea, A.L.; Qian, H.; Masurkar, A.; Deng, H.; Liu, D. Patient clustering improves efficiency of federated machine learning to predict mortality and hospital stay time using distributed electronic medical records. J. Biomed. Inform. 2019, 99, 103291. [Google Scholar] [CrossRef]
- Reddy, C.K.K.; Kaza, V.S.; Mohana, R.M.; Alhameed, M.; Jeribi, F.; Alam, S.; Shuaib, M. Detecting anomalies in smart wearables for hypertension: A deep learning mechanism. Front. Public Health 2025, 12, 1426168. [Google Scholar] [CrossRef]
- Kim, J.; Hur, K.; Yang, S.; Choi, E. Universal EHR federated learning framework. arXiv 2022, arXiv:2211.07300. [Google Scholar] [CrossRef]
- Zhao, H.; Sui, D.; Wang, Y.; Ma, L.; Wang, L. Privacy-Preserving Federated Learning Framework for Multi-Source Electronic Health Records Prognosis Prediction. Sensors 2025, 25, 2374. [Google Scholar] [CrossRef]
- Dayan, I.; Roth, H.R.; Zhong, A.; Harouni, A.; Gentili, A.; Abidin, A.Z.; Li, Q. Federated learning for predicting clinical outcomes in patients with COVID-19. Nat. Med. 2021, 27, 1735–1743. [Google Scholar] [CrossRef]
- Subramanian, M.; Rajasekar, V.; VE, S.; Shanmugavadivel, K.; Nandhini, P.S. Effectiveness of decentralized federated learning algorithms in healthcare: A case study on cancer classification. Electronics 2022, 11, 4117. [Google Scholar] [CrossRef]
- Lyu, R.; Rosenfeld, R.; Wilder, B. Federated epidemic surveillance. PLoS Comput. Biol. 2025, 21, e1012907. [Google Scholar] [CrossRef] [PubMed]
- Almogadwy, B.; Alqarafi, A. Fused federated learning framework for secure and decentralized patient monitoring in healthcare 5.0 using IoMT. Sci. Rep. 2025, 15, 24263. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Li, T.; Chen, H.; Du, W.; He, Z. Towards the efficacy of federated prediction for epidemics on networks. arXiv 2024, arXiv:2412.02161. [Google Scholar] [CrossRef]
- van Rooden, S.M.; van der Werff, S.D.; van Mourik, M.S.; Lomholt, F.; Møller, K.L.; Valk, S.; Rinaldi, E. Federated systems for automated infection surveillance: A perspective. Antimicrob. Resist. Infect. Control 2024, 13, 113. [Google Scholar] [CrossRef]
- Alwakeel, M.M. AI-Assisted Real-Time Monitoring of Infectious Diseases in Urban Areas. Mathematics 2025, 13, 1911. [Google Scholar] [CrossRef]
- Qayyum, A.; Ahmad, K.; Ahsan, M.A.; Al-Fuqaha, A.; Qadir, J. Collaborative federated learning for healthcare: Multi-modal COVID-19 diagnosis at the edge. IEEE Open J. Comput. Soc. 2022, 3, 172–184. [Google Scholar] [CrossRef]
- Tahir, N.; Jung, C.R.; Lee, S.D.; Azizah, N.; Ho, W.C.; Li, T.C. Federated Learning-Based Model for Predicting Mortality: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2025, 27, e65708. [Google Scholar] [CrossRef]
- Rajendran, S.; Xu, Z.; Pan, W.; Ghosh, A.; Wang, F. Data heterogeneity in federated learning with Electronic Health Records: Case studies of risk prediction for acute kidney injury and sepsis diseases in critical care. PLoS Digit. Health 2023, 2, e0000117. [Google Scholar] [CrossRef]
- Pati, S.; Baid, U.; Edwards, B.; Sheller, M.; Wang, S.H.; Reina, G.A.; Poisson, L. Federated learning enables big data for rare cancer boundary detection. Nat. Commun. 2022, 13, 7346. [Google Scholar] [CrossRef]
- Torous, J.; Chan, S.R.; Tan, S.Y.-M.; Behrens, J.; Mathew, I.; Conrad, E.J.; Hinton, L.; Yellowlees, P.; Keshavan, M. Patient smartphone ownership and interest in mobile apps to monitor symptoms of mental health conditions: A survey in four geographically distinct psychiatric clinics. JMIR Mental Health 2014, 1, e4004. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bai, J.; Li, N.; Li, X.; Liu, D.; Buckeridge, D.L.; Li, Y. FedWeight: Mitigating covariate shift of federated learning on electronic health records data through patients re-weighting. npj Digit. Med. 2025, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kaiser, M.S.; Chaki, S.; Aloteibi, S.; Moni, M.A. Federated learning model with dynamic scoring-based client selection for diabetes diagnosis. Knowl. Based Syst. 2025, 320, 113662. [Google Scholar] [CrossRef]
- Li, S.; Wu, Q.; Li, X.; Miao, D.; Hong, C.; Gu, W.; Liu, N. FairFML: Fair federated machine learning with a case study on reducing gender disparities in cardiac arrest outcome prediction. arXiv 2024, arXiv:2410.17269. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, J.; Chen, Y.; Bandara, E.; Shetty, S. Architectural design of a blockchain-enabled, federated learning platform for algorithmic fairness in predictive health care: Design science study. J. Med. Internet Res. 2023, 25, e46547. [Google Scholar] [CrossRef]
- Jimenez, G.D.M.; Solans, D.; Heikkilä, M.; Vitaletti, A.; Kourtellis, N.; Anagnostopoulos, A.; Chatzigiannakis, I. Non-IID data in federated learning: A systematic review with taxonomy, metrics, methods, frameworks and future directions. arXiv 2024, arXiv:2411.12377. [Google Scholar] [CrossRef]
- Boscarino, N.; Cartwright, R.A.; Fox, K.; Tsosie, K.S. Federated learning and Indigenous genomic data sovereignty. Nat. Mach. Intell. 2022, 4, 909–911. [Google Scholar] [CrossRef]
- Kaya, M.; Shahid, H. Cross-Border Data Flows and Digital Sovereignty: Legal Dilemmas in Transnational Governance. Interdiscip. Stud. Soc. Law Politics 2025, 4, 219–233. [Google Scholar] [CrossRef]
- Kairouz, P.; McMahan, H.B.; Avent, B.; Bellet, A.; Bennis, M.; Bhagoji, A.N.; Bonawitz, K.; Charles, Z.; Cormode, G.; Cummings, R.; et al. Advances and open problems in federated learning. Found. Trends® Mach. Learn. 2021, 14, 1–210. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Pham, Q.V.; Pathirana, P.N.; Ding, M.; Seneviratne, A.; Lin, Z.; Dobre, O.; Hwang, W.-J. Federated learning for smart healthcare: A survey. ACM Comput. Surv. (Csur) 2022, 55, 1–37. [Google Scholar] [CrossRef]
- Li, T.; Sahu, A.K.; Zaheer, M.; Sanjabi, M.; Talwalkar, A.; Smith, V. Federated optimization in heterogeneous networks. Proc. Mach. Learn. Syst. 2020, 2, 429–450. [Google Scholar]
- Zhao, Y.; Li, M.; Lai, L.; Suda, N.; Civin, D.; Chandra, V. Federated learning with non-IID data. Found. Trends® Mach. Learn. 2021, 14, 1–100. [Google Scholar] [CrossRef]
- Sheller, M.J.; Edwards, B.; Reina, G.A.; Martin, J.; Pati, S.; Kotrotsou, A.; Milchenko, M.; Xu, W.; Marcus, D.; Colen, R.R.; et al. Federated learning in medicine: Facilitating multi-institutional collaborations without sharing patient data. Sci. Rep. 2020, 10, 12598. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, B.; Dong, Y.; Chen, C.; Li, J. Federated graph machine learning: A survey of concepts, techniques, and applications. ACM SIGKDD Explor. Newsl. 2022, 24, 32–47. [Google Scholar] [CrossRef]
- Fallah, A.; Mokhtari, A.; Ozdaglar, A. Personalized federated learning with theoretical guarantees: A model-agnostic meta-learning approach. Adv. Neural Inf. Process. Syst. 2020, 33, 3557–3568. [Google Scholar]
- Sattler, F.; Müller, K.R.; Samek, W. Clustered federated learning: Model-agnostic distributed multitask optimization under privacy constraints. IEEE Trans. Neural Netw. Learn. Syst. 2020, 32, 3710–3722. [Google Scholar] [CrossRef]
- Kaissis, G.A.; Makowski, M.R.; Rückert, D.; Braren, R.F. Secure, privacy-preserving and federated machine learning in medical imaging. Nat. Mach. Intell. 2020, 2, 305–311. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Liang, H.; Joshi, G.; Poor, H.V. Tackling the objective inconsistency problem in heterogeneous federated optimization. Adv. Neural Inf. Process. Syst. 2020, 33, 7611–7623. [Google Scholar]
- Ma, X.; Zhang, J.; Guo, S.; Xu, W. Layer-wised model aggregation for personalized federated learning. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, New Orleans, LA, USA, 19–24 June 2022; pp. 10092–10101. [Google Scholar]
- Johnson, A.E.; Bulgarelli, L.; Shen, L.; Gayles, A.; Shammout, A.; Horng, S.; Pollard, T.J.; Hao, S.; Moody, B.; Gow, B.; et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 2023, 10, 1. [Google Scholar] [CrossRef]
- Leslie, D.; Mazumder, A.; Peppin, A.; Wolters, M.K.; Hagerty, A. Does “AI” stand for augmenting inequality in the era of COVID-19 healthcare? BMJ 2021, 372, n304. [Google Scholar] [CrossRef]
- Salam, A. Internet of things for sustainable community development: Introduction and overview. In Internet of Things for Sustainable Community Development: Wireless Communications, Sensing, and Systems; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–31. [Google Scholar]
- Singh, P.; Singh, M.K.; Singh, R.; Singh, N. Federated learning: Challenges, methods, and future directions. In Federated Learning for IoT Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 199–214. [Google Scholar]
- Khan, S.; Khan, H.U.; Nazir, S. Systematic analysis of healthcare big data analytics for efficient care and disease diagnosing. Sci. Rep. 2022, 12, 22377. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Annavaram, M.; Avestimehr, S. Group knowledge transfer: Federated learning of large CNNs at the edge. Adv. Neural Inf. Process. Syst. 2020, 33, 14068–14080. [Google Scholar]
- Wahab, O.A.; Mourad, A.; Otrok, H.; Taleb, T. Federated machine learning: Survey, multi-level classification, desirable criteria and future directions in communication and networking systems. IEEE Commun. Surv. Tutor. 2021, 23, 1342–1397. [Google Scholar] [CrossRef]
- Strubell, E.; Ganesh, A.; McCallum, A. Energy and policy considerations for modern deep learning research. In Proc. AAAI Conf. Artif. Intell. 2020, 34, 13693–13696. [Google Scholar] [CrossRef]
- Morley, J.; Machado, C.C.; Burr, C.; Cowls, J.; Joshi, I.; Taddeo, M.; Floridi, L. The ethics of AI in health care: A mapping review. Soc. Sci. Med. 2020, 260, 113172. [Google Scholar] [CrossRef]
- Chalamala, S.R.; Kummari, N.K.; Singh, A.K.; Saibewar, A.; Chalavadi, K.M. Federated learning to comply with data protection regulations. CSI Trans. ICT 2022, 10, 47–60. [Google Scholar] [CrossRef]
- Dang, T.K.; Lan, X.; Weng, J.; Feng, M. Federated learning for electronic health records. ACM Trans. Intell. Syst. Technol. (TIST) 2022, 13, 72. [Google Scholar] [CrossRef]
- Nikolinakos, N.T. Ethical principles for trustworthy AI. In EU Policy and Legal Framework for Artificial Intelligence, Robotics and Related Technologies—The AI Act; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Chen, H.; Zhu, T.; Zhang, T.; Zhou, W.; Yu, P.S. Privacy and fairness in federated learning: On the perspective of tradeoff. ACM Comput. Surv. 2023, 56, 1–37. [Google Scholar] [CrossRef]
- Gasser, U.; Ienca, M.; Scheibner, J.; Sleigh, J.; Vayena, E. Digital tools against COVID-19: Framing the ethical challenges and how to address them. arXiv 2020, arXiv:2004.10236. [Google Scholar] [CrossRef]
- Sharma, T.; Bashir, M.; Kesarwani, V. Federated learning and privacy: A regulatory perspective. Comput. Law Secur. Rev. 2021, 41, 105545. [Google Scholar]
- Liu, C.; Luo, Y.; Xu, Y.; Du, B. Foundation models matter: Federated learning for multi-center tuberculosis diagnosis via adaptive regularization and model-contrastive learning. World Wide Web 2024, 27, 30. [Google Scholar] [CrossRef]
- Lyu, L.; Yu, H.; Yang, Q. Threats to federated learning: A survey. arXiv 2020, arXiv:2003.02133. [Google Scholar] [CrossRef]
- Pumplun, L.; Peters, F.; Gawlitza, J.F.; Buxmann, P. Bringing machine learning systems into clinical practice: A design science approach to explainable machine learning-based clinical decision support systems. J. Assoc. Inf. Syst. 2023, 24, 953–979. [Google Scholar] [CrossRef]
- Zhang, F.; Kreuter, D.; Chen, Y.; Dittmer, S.; Tull, S.; Shadbahr, T.; Schönlieb, C.B. Recent methodological advances in federated learning for healthcare. Patterns 2024, 5, 101006. [Google Scholar] [CrossRef]
- Bárcena, J.L.C.; Daole, M.; Ducange, P.; Marcelloni, F.; Renda, A.; Ruffini, F.; Schiavo, A. Fed-XAI: Federated learning of explainable artificial intelligence models. In Proceedings of the 3rd Italian Workshop on Explainable Artificial Intelligence, co-located with AI*IA 2022, Udine, Italy, 28 November–2 December 2022; pp. 104–117. [Google Scholar]
- Holzinger, A.; Dehmer, M.; Emmert-Streib, F.; Cucchiara, R.; Augenstein, I.; Del Ser, J.; Samek, W.; Jurisica, I.; Rodríguez, N.D. Information fusion as an integrative cross-cutting enabler to achieve robust, explainable, and trustworthy medical artificial intelligence. Inf. Fusion 2022, 79, 263–278. [Google Scholar] [CrossRef]
- Silva, T.; Lima, J.; Dias, L.; Costa, L.; Nascimento, I. Equity challenges in federated learning for healthcare: A scoping review. J. Biomed. Inform. 2023, 138, 104309. [Google Scholar]
- Parampottupadam, S.; Coşğun, M.; Pati, S.; Zenk, M.; Roy, S.; Bounias, D.; Hamm, B.; Sav, S.; Floca, R.; Maier-Hein, K. Inclusive, differentially private federated learning for clinical data. arXiv 2025, arXiv:2505.22108. [Google Scholar] [CrossRef]
- Wu, H.; Wang, P. Node selection toward faster convergence for federated learning on non-iid data. IEEE Trans. Netw. Sci. Eng. 2022, 9, 3099–3111. [Google Scholar] [CrossRef]
- Zeng, Y.; Mu, Y.; Yuan, J.; Teng, S.; Zhang, J.; Wan, J.; Ren, Y.; Zhang, Y. Adaptive federated learning with non-IID data. Comput. J. 2023, 66, 2758–2772. [Google Scholar] [CrossRef]
- Antunes, R.S.; André da Costa, C.; Küderle, A.; Yari, I.A.; Eskofier, B. Federated learning for healthcare: Systematic review and architecture proposal. ACM Trans. Intell. Syst. Technol. (TIST) 2022, 13, 54. [Google Scholar] [CrossRef]
- Prosperi, M.; Guo, Y.; Sperrin, M.; Koopman, J.S.; Min, J.S.; He, X.; Rich, S.; Wang, M.; Buchan, I.E.; Bian, J. Causal inference and counterfactual prediction in machine learning for actionable healthcare. Nat. Mach. Intell. 2020, 2, 369–375. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, Q.; Lei, L.; Zheng, K. Federated reinforcement learning: Techniques, applications, and open challenges. arXiv 2021, arXiv:2108.11887. [Google Scholar] [CrossRef]
- Morley, J.; Kinsey, L.; Elhalal, A.; Garcia, F.; Ziosi, M.; Floridi, L. Operationalising AI ethics: Barriers, enablers and next steps. AI Soc. 2023, 38, 411–423. [Google Scholar] [CrossRef]
- Kuo, T.T.; Gabriel, R.A.; Koola, J.; Schooley, R.T.; Ohno-Machado, L. Distributed cross-learning for equitable federated models: Privacy-preserving prediction on data from five California hospitals. Nat. Commun. 2025, 16, 1371. [Google Scholar] [CrossRef]
- Thakur, A.; Molaei, S.; Nganjimi, P.C.; Liu, F.; Soltan, A.; Schwab, P.; Clifton, D.A. Knowledge abstraction and filtering based federated learning over heterogeneous data views in healthcare. npj Digit. Med. 2024, 7, 283. [Google Scholar] [CrossRef]
- Kostick-Quenet, K.M.; Compagnucci, M.C.; Aboy, M.; Minssen, T. Patient-centric federated learning: Automating meaningful consent to health data sharing with smart contracts. J. Law Biosci. 2025, 12, lsaf003. [Google Scholar] [CrossRef]
- Moulaei, K.; Akhlaghpour, S.; Fatehi, F. Patient consent for the secondary use of health data in artificial intelligence (AI) models: A scoping review. Int. J. Med. Inform. 2025, 198, 105872. [Google Scholar] [CrossRef]
- Scheliga, D.; Mäder, P.; Seeland, M. Privacy preserving federated learning with convolutional variational bottlenecks. Cybersecurity 2025, 8, 33. [Google Scholar] [CrossRef]
- Ezzeldin, Y.H.; Yan, S.; He, C.; Ferrara, E.; Avestimehr, A.S. FairFed: Enabling group fairness in federated learning. Proc. AAAI Conf. Artif. Intell. 2023, 37, 7494–7502. [Google Scholar] [CrossRef]
- Zhang, F.; Shuai, Z.; Kuang, K.; Wu, F.; Zhuang, Y.; Xiao, J. Unified fair federated learning for digital healthcare. Patterns 2024, 5, 100907. [Google Scholar] [CrossRef] [PubMed]
- Alshkeili, H.M.H.A.; Almheiri, S.J.; Khan, M.A. Privacy-Preserving Interpretability: An Explainable Federated Learning Model for Predictive Maintenance in Sustainable Manufacturing and Industry 4.0. AI 2025, 6, 117. [Google Scholar] [CrossRef]
- Alekseenko, J.; Stieltjes, B.; Bach, M.; Boerries, M.; Opitz, O.; Karargyris, A.; Padoy, N. Clinnova federated learning proof of concept: Key takeaways from a cross-border collaboration. arXiv 2024, arXiv:2410.02443. [Google Scholar] [CrossRef]
- Crowson, M.G.; Moukheiber, D.; Arévalo, A.R.; Lam, B.D.; Mantena, S.; Rana, A.; Celi, L.A. A systematic review of federated learning applications for biomedical data. PLoS Digit. Health 2022, 1, e0000033. [Google Scholar] [CrossRef]
- Qu, L.; Balachandar, N.; Rubin, D.L. An experimental study of data heterogeneity in federated learning methods for medical imaging. arXiv 2021, arXiv:2107.08371. [Google Scholar] [CrossRef]
- Mheissen, S.; Spineli, L.M.; Daraqel, B.; Alsafadi, A.S. Language bias in orthodontic systematic reviews: A meta-epidemiological study. PLoS ONE 2024, 19, e0300881. [Google Scholar] [CrossRef]
- Uttley, L.; Quintana, D.S.; Montgomery, P.; Carroll, C.; Page, M.J.; Falzon, L.; Moher, D. The problems with systematic reviews: A living systematic review. J. Clin. Epidemiol. 2023, 156, 30–41. [Google Scholar] [CrossRef]
- Wu, X.; Gao, J.; Bilal, M.; Dai, F.; Xu, X.; Qi, L.; Dou, W. Federated learning-based private medical knowledge graph for epidemic surveillance in Internet of Things. Expert Syst. 2025, 42, e13372. [Google Scholar] [CrossRef]
| Scope | Methodology | Focus Area | Target Health System Level | Identified Gap | AI Methodology Challenge | Disease Focus | Reference |
|---|---|---|---|---|---|---|---|
| FL in public health and disease prevention | Systematic review (PRISMA) | Policy design, equity, ethical FL governance | National and cross-border public health systems | Lack of synthesis for policy; cross-border frameworks; fairness; national integration; benchmarking | Heterogeneity-robust FL; secure aggregation; standards | Both | This Review (2025) |
| FL in smart healthcare networks | Scoping review | Wearables, IoT in hospital environments | Institutional | [25] | |||
| FL for COVID-19 detection | Empirical study | Mobile symptom data, federated analysis | Regional healthcare systems | Infectious | [26] | ||
| Federated analytics for disease forecasting | Prototype framework | Pandemic prediction and response | Regional/ national public health agencies | Need for cross-border, privacy-preserving forecasting frameworks | Interoperability; model update security | Infectious | [27,28] |
| Ethical and regulatory aspects of FL | Conceptual review | Consent, fairness, explainability | Cross-level (individual to national) | Limited fairness/ethics integration in deployment | Fairness-aware training and auditing | Both | [21] |
| FL performance for chronic disease care | Experimental pipeline | Diabetes/CVD with EHRs | Institutional (hospital networks) | Underrepre sentation of chronic disease prevention | Longitudinal/ behavioral FL design | Chronic | [29] |
| System level optimization of FL | Technical survey | Communication efficiency, compression | Cross-domain | Underrepre sentation of chronic prevention; efficiency constraints | Communication-efficient, non-IID FL | Both | [5] |
| FL in LMIC rural settings | Field-based framework | Low-resource deployment | Regional clinics, rural LMICs | Limited evaluation in low-resource settings | Stable training under non-IID + weak networks | Infectious | [30] |
| FL for public health synthesis | Review/note | Policy-oriented evidence synthesis | Policy bodies | Lack of synthesis on FL for policymaking | Generalizability across heterogeneous data | Both | [6] |
| Cross-border data sharing for surveillance | Perspective/ framework | International collaboration | National/regional cross-border | Minimal analysis of cross-border frameworks; weak national integration | Interoperability; jurisdictional limits | Infectious | [31] |
| Fairness/bias in healthcare FL | Review/ analysis | Equity and bias mitigation | Cross-level | Limited ethical/fairness discourse in deployment | Bias propagation; subgroup performance gaps | Both | [32] |
| Benchmarking in health FL | Survey/ framework | Standards and evaluation | Cross-ecosystem | Lack of standardized benchmarking | Open metrics and datasets for public health FL | Both | [33] |
| Criterion Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Study Type | Peer-reviewed original research articles (experimental, observational, implementation, or modeling studies) | Editorials, preprints, white papers, commentaries, or conference abstracts without full peer review |
| Technology Focus | Studies applying or evaluating FL or similar decentralized AI approaches | Studies using only centralized AI models or unrelated ML techniques |
| Policy Relevance | Addressing public health decision-making, disease prevention, or population-level policy impact | Focused only on individual-level clinical tools with no public health or policy connection |
| Health Focus | Studies involving communicable or non-communicable diseases in human populations | Simulated, laboratory, or animal studies with no relevance to public health systems |
| Geographic Scope | Global scope, including low-, middle-, and high-income settings | Not applicable (no exclusions based on location) |
| Language | English only | Any language other than English |
| Publication Period | Published between 1 January 2020 and 30 June 2025 | Published before 2020 or after June 2025 |
| Synthesis Theme | Description | Representative Examples |
|---|---|---|
| Opportunities for FL in Disease Prevention Policy | Highlights the technical and operational advantages of FL in public health, such as secure data sharing, real-time collaboration, and modeling across diverse populations. | Early COVID-19 outbreak detection across hospitals; FL models for diabetes risk prediction using distributed primary care data. |
| Challenges to FL Deployment in Public Health | Documents technical, ethical, and infrastructural barriers including data heterogeneity, interoperability issues, governance gaps, and unequal access to computing resources. | Non-IID data across regions; lack of FL support in low-resource settings; absence of regulatory frameworks for decentralized AI. |
| Policy and System-Level Implications | Explores how FL contributes to health equity, privacy preservation, and local policymaking autonomy, while emphasizing the need for cross-sector collaboration. | Privacy-preserving modeling in Europe under GDPR; use of FL to guide local vaccination resource planning; partnerships between ministries of health and tech firms. |
| FL Architecture Type | No. of Studies (n = 19) | Percentage (%) | Key Applications | Representative Use Cases |
|---|---|---|---|---|
| Horizontal Federated Learning (HFL) | 11 | 57.9% | Epidemic modeling, chronic disease screening, multi-hospital EHR aggregation | COVID-19 prediction across provinces; diabetes screening via regional health centers |
| Vertical Federated Learning (VFL) | 4 | 21.1% | Socio-clinical integration, cross-sector modeling, personalized risk assessment | Merging insurance and hospital data; combining wearables with EHRs for cancer risk |
| Hybrid/Customized Architectures | 3 | 15.8% | Smart surveillance, edge-to-cloud FL, hierarchical governance | FL with mobile apps and hospitals; city-level FL aggregation for vaccine allocation |
| Unspecified/Not Reported | 1 | 5.2% | General policy modeling with no defined architecture | Public health early-warning model with unclear system structure |
| Limitation | Impact on Public Health Modeling | Mitigation Strategy | Method Type | Application Area | Reference |
|---|---|---|---|---|---|
| Non-IID Data Distribution | Model divergence; poor generalization across populations | Clustered FL; FedProx; personalization layers | Algorithmic | Disease risk prediction, surveillance | [65,66] |
| Data Schema Heterogeneity | Feature misalignment between nodes | Data harmonization; standard ontologies | Preprocessing | Multi-site EHR integration | [39,71] |
| Resource Imbalance Across Nodes | Slower nodes delay training; accuracy skewed by high-resource institutions | Adaptive model aggregation; asynchronous FL | Infrastructure-aware FL | Rural vs. urban health centers | [63] |
| Privacy Leakage from Gradients | Inference of sensitive data from model updates | Differential privacy; secure aggregation | Security-enhancing | Sensitive patient data | [5] |
| Lack of Model Explainability | Difficulty interpreting risk scores; mistrust by clinicians | Use of interpretable models; SHAP in FL | Explainable AI | Clinical decision support | [35] |
| Bias Toward Dominant Institutions | Unequal influence on global model | Fair weighting schemes; participation normalization | Fairness-aware FL | Multi-region model development | [26] |
| Slow Convergence in Heterogeneous Settings | Extended training time and communication costs | FedAvg optimization; learning rate tuning | Algorithm optimization | Pandemic-time model deployment | [72] |
| Aspect | Centralized AI | Federated Learning |
|---|---|---|
| Raw Data Movement | Required | Not required |
| Privacy Risk | High | Low |
| Legal Compliance | Challenging in multi- jurisdiction settings | More compliant due to data locality |
| Aspect | Centralized AI | FL | Advantage | Challenges | Reference(s) |
|---|---|---|---|---|---|
| Data Privacy and Security | Requires raw data transfer to central server | Keeps data local and shares only encrypted model updates | FL, Better privacy compliance | Centralized AI— High breach risk | [68,71] |
| Equity and Representation | Often excludes under-resourced or data-restricted institutions | Allows wider participation regardless of location or resources | FL, Inclusive modeling from diverse regions | Centralized, Biased models from selective data | [95,96] |
| Infrastructure Dependence | Requires high-capacity central server | Distributed computing required across all clients | Centralized, Easier deployment in data-rich settings | FL, Requires local computational capacity | [63,65] |
| Speed of Deployment | Slower in multi-region scenarios due to regulatory friction | Faster real-time model updates at edge nodes | FL, Suited for outbreaks and emergency response | Centralized, Bottlenecks in global data sharing | [21,67] |
| Model Accuracy and Robustness | High accuracy if diverse data are centrally available | Accuracy depends on handling non-IID data | Centralized, When full data is accessible | FL, Requires optimization for statistical heterogeneity | [97,98] |
| Scalability | Limited scalability in international or multi-hospital projects | High scalability via federated nodes and asynchronous updates | FL, Better for cross-border applications | Centralized, Data sharing restrictions | [65,99] |
| Explainability | Easier due to full data access | Requires development of remote XAI tools | Centralized, Full access enables model audit | FL, Limited transparency | [100] |
| Legal and Ethical Governance | Often violates data sovereignty laws in sensitive domains | Aligns with GDPR, HIPAA, and other data minimization frameworks | FL, Privacy by design | Centralized, May breach consent frameworks | [39,101] |
| Limitation Category | Description | Implication |
|---|---|---|
| Limited Evidence Base | Few empirical FL studies applied directly to public health policy; inclusion of conceptual or pilot frameworks | Limits generalizability and robustness of conclusions |
| Study Heterogeneity | Wide variation in FL architecture, disease domains, and outcome metrics | Hinders direct comparison or synthesis |
| Language and Publication Bias | Exclusion of non-English, gray literature, and LMIC pilot programs | Skews findings toward English-speaking, high-income settings |
| No Meta-Analysis Conducted | Lack of standardized metrics across studies prevented pooled statistical analysis | Limits quantification of effect sizes and accuracy |
| Rapid Field Evolution | Review cutoff may exclude recent advances post-2024 | Limits inclusion of latest tools, models, and policies |
| Reviewer Subjectivity | Some subjectivity in categorization, eligibility, and thematic coding | Potential influence on thematic synthesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.T.; Ali, Z.; Waqar, M.; Kim, A. Federated Learning in Public Health: A Systematic Review of Decentralized, Equitable, and Secure Disease Prevention Approaches. Healthcare 2025, 13, 2760. https://doi.org/10.3390/healthcare13212760
Shah ST, Ali Z, Waqar M, Kim A. Federated Learning in Public Health: A Systematic Review of Decentralized, Equitable, and Secure Disease Prevention Approaches. Healthcare. 2025; 13(21):2760. https://doi.org/10.3390/healthcare13212760
Chicago/Turabian StyleShah, Sayed Tariq, Zulfiqar Ali, Muhammad Waqar, and Ajung Kim. 2025. "Federated Learning in Public Health: A Systematic Review of Decentralized, Equitable, and Secure Disease Prevention Approaches" Healthcare 13, no. 21: 2760. https://doi.org/10.3390/healthcare13212760
APA StyleShah, S. T., Ali, Z., Waqar, M., & Kim, A. (2025). Federated Learning in Public Health: A Systematic Review of Decentralized, Equitable, and Secure Disease Prevention Approaches. Healthcare, 13(21), 2760. https://doi.org/10.3390/healthcare13212760





