Digital Health Technologies in the Treatment of Chronic Pelvic Pain Syndromes: A Systematic Review of Randomized Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Methodological Quality of Included Studies
2.6. Risk of Bias Assessment
3. Results
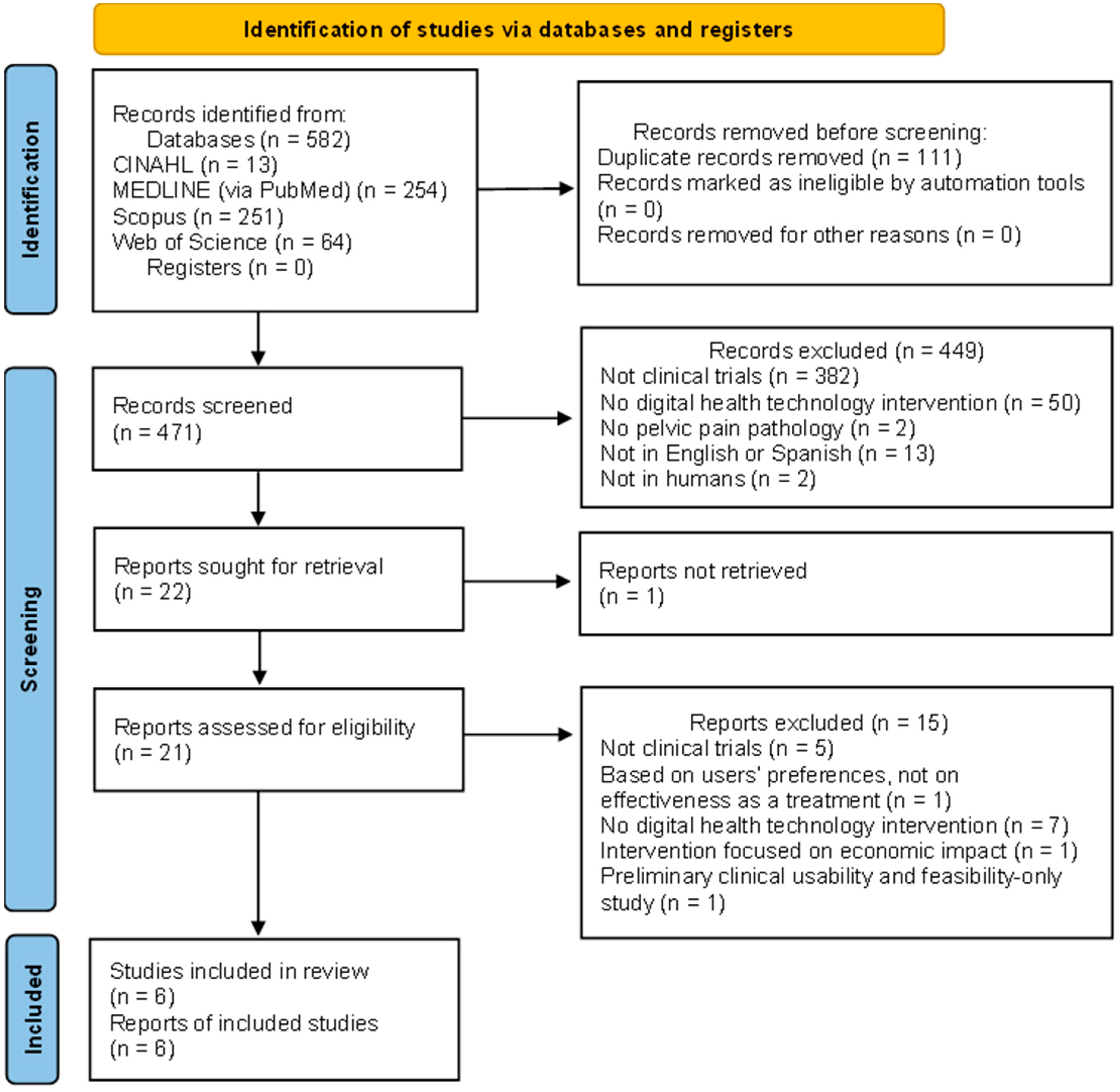
3.1. Search Selection
3.2. Characteristics of Included Studies
3.3. Characteristics of Interventions
3.4. Methodological Quality of Studies
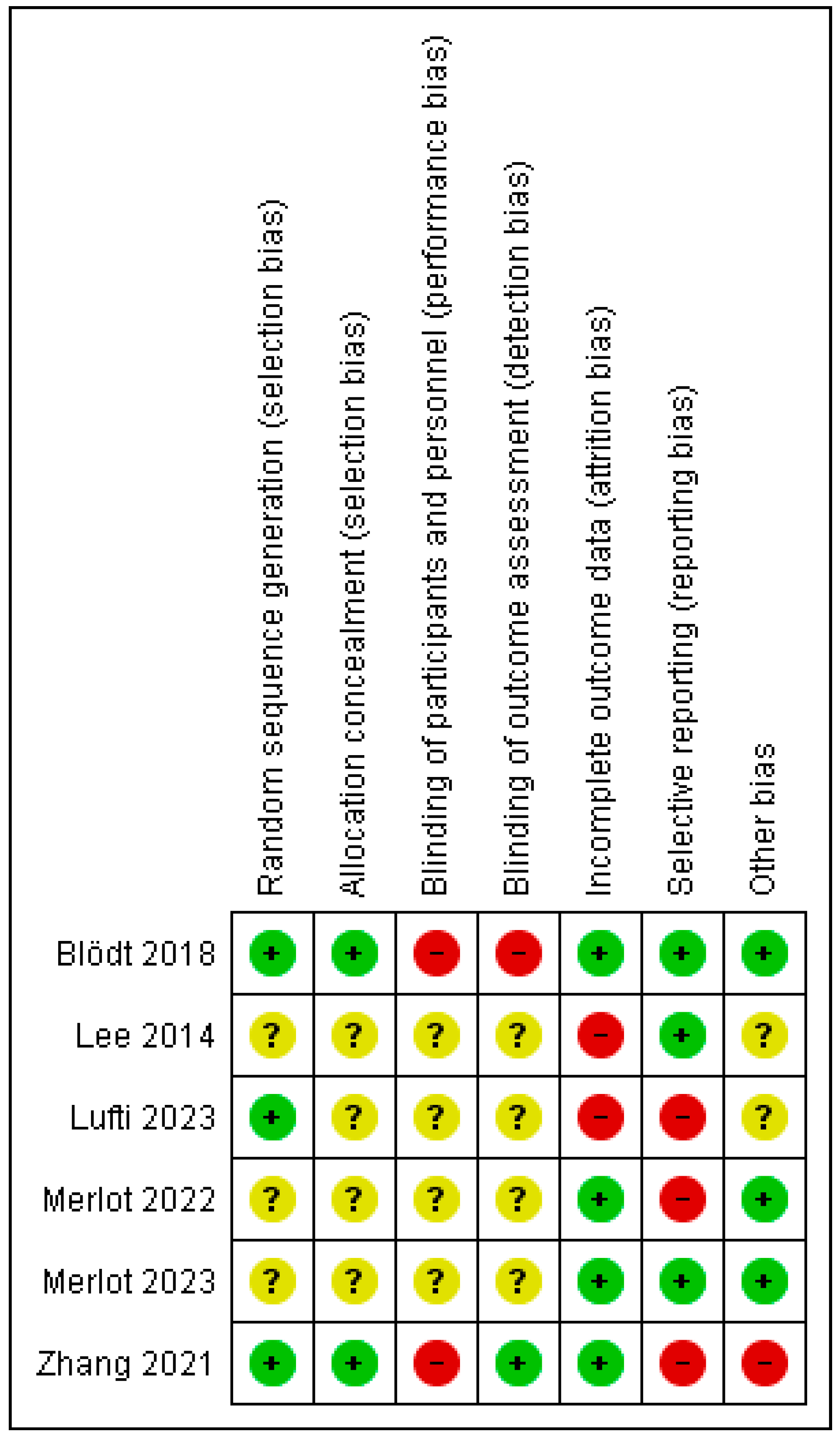
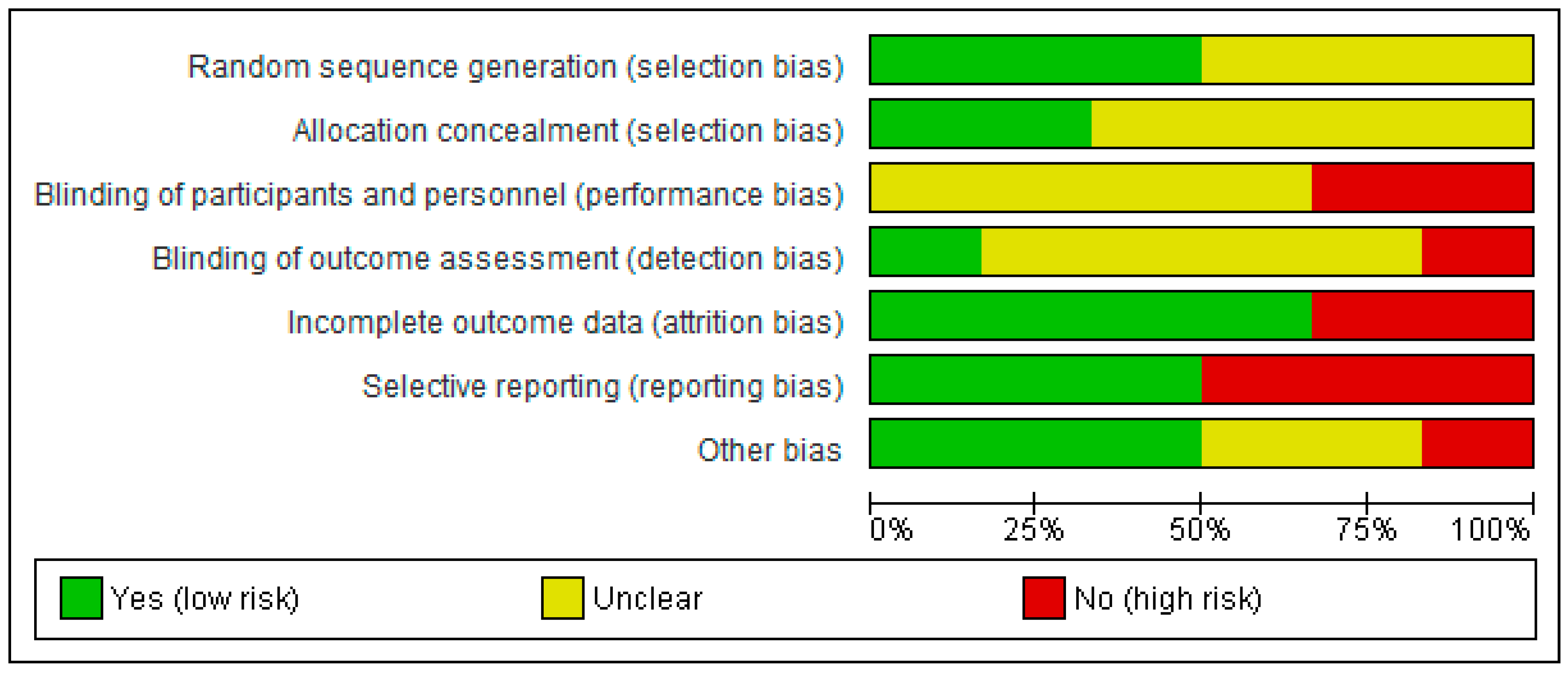
3.5. Risk of Bias of Studies
4. Discussion
4.1. Main Findings
4.1.1. Virtual Reality
4.1.2. Other Types of Digital Health
4.2. Study Strengths and Limitations
4.3. Implications for Clinical Practice
4.4. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EAU Guidelines. Edn. presented at the EAU Annual Congress, Madrid 2025. ISBN 978-94-92671-29-5.
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19. [Google Scholar] [CrossRef] [PubMed]
- Quaghebeur, J.; Wyndaele, J.J. Prevalence of lower urinary tract symptoms and level of quality of life in men and women with chronic pelvic pain. Scand. J. Urol. 2015, 49, 242–249. [Google Scholar] [CrossRef]
- Kaya, S.; Hermans, L.; Willems, T.; Roussel, N.; Meeus, M. Central sensitization in urogynecological chronic pelvic pain: A systematic literature review. Pain Physician 2013, 16, 291–308. [Google Scholar] [CrossRef]
- Hoffman, D. Central and peripheral pain generators in women with chronic pelvic pain: Patient centered assessment and treatment. Curr. Rheumatol. Rev. 2015, 11, 146–166. [Google Scholar] [CrossRef]
- Grinberg, K.; Granot, M.; Lowenstein, L.; Abramov, L.; Weissman-Fogel, I. A common pronociceptive pain modulation profile typifying subgroups of chronic pelvic pain syndromes is interrelated with enhanced clinical pain. Pain 2017, 158, 1021–1029. [Google Scholar] [CrossRef]
- Ness, T.J.; Lloyd, L.K.; Fillingim, R.B. An endogenous pain control system is altered in subjects with interstitial cystitis. J. Urol. 2014, 191, 364–370. [Google Scholar] [CrossRef]
- Levesque, A.; Riant, T.; Ploteau, S.; Rigaud, J.; Labat, J.-J.; Network, C.P.; Amarenco, G.; Attal, N.; Bautrant, E.; Marc, B.G.; et al. Clinical Criteria of Central Sensitization in Chronic Pelvic and Perineal Pain (Convergences PP Criteria): Elaboration of a Clinical Evaluation Tool Based on Formal Expert Consensus. Pain Med. 2018, 19, 2009–2015. [Google Scholar] [CrossRef]
- Smart, K.M.; Blake, C.; Staines, A.; Doody, C. Clinical indicators of ‘nociceptive’, ‘peripheral neuropathic’ and ‘central’ mechanisms of musculoskeletal pain. A Delphi survey of expert clinicians. Man. Ther. 2010, 15, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Le, A.L.; Rogers, R.G.; Lemos, N.; Jeppson, P.C. The cost of chronic pelvic pain in women: A systematic review of the literature. J. Obstet. Gynaecol. Can. 2021, 43, 655–656. [Google Scholar] [CrossRef]
- Riegel, B.; Bruenahl, C.A.; Ahyai, S.; Bingel, U.; Fisch, M.; Löwe, B. Assessing psychological factors, social aspects and psychiatric co-morbidity associated with Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) in men—A systematic review. J. Psychosom. Res. 2014, 77, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, B. Physiotherapy for pelvic pain and female sexual dysfunction: An untapped resource. Int. Urogynecology J. 2018, 29, 631–638. [Google Scholar] [CrossRef]
- Pandey, M.; Shrivastava, V.; Patidar, V.; Dias, S.; Trivedi, S. Pelvic-floor relaxation techniques using biofeedback—More effective therapy for chronic prostatitis/chronic pelvic pain syndrome. J. Clin. Urol. 2020, 13, 454–459. [Google Scholar] [CrossRef]
- Zoorob, D.; South, M.; Karram, M.; Sroga, J.; Maxwell, R.; Shah, A.; Whiteside, J. A pilot randomized trial of levator injections versus physical therapy for treatment of pelvic floor myalgia and sexual pain. Int. Urogynecology J. 2015, 26, 845–852. [Google Scholar] [CrossRef]
- Pan, J.; Jin, S.; Xie, Q.; Wang, Y.; Wu, Z.; Sun, J.; Guo, T.P.; Zhang, D. Acupuncture for Chronic Prostatitis or Chronic Pelvic Pain Syndrome: An Updated Systematic Review and Meta-Analysis. Pain Res. Manag. 2023, 2023, 7754876. [Google Scholar] [CrossRef] [PubMed]
- Labetov, I.; Vaganova, A.; Kovalev, G.; Shkarupa, D. Extracorporeal shockwave therapy in treatment of chronic prostatitis/chronic pelvic pain syndrome: Systematic review and meta-analyses. Neurourol. Urodyn. 2024, 43, 1924–1937. [Google Scholar] [CrossRef]
- Cottrell, A.M.; Schneider, M.P.; Goonewardene, S.; Yuan, Y.; Baranowski, A.P.; Engeler, D.S.; Borovicka, J.; Dinis-Oliveira, P.; Elneil, S.; Hughes, J.; et al. Benefits and Harms of Electrical Neuromodulation for Chronic Pelvic Pain: A Systematic Review. Eur. Urol. Focus 2020, 6, 559–571. [Google Scholar] [CrossRef]
- Tutolo, M.; Ammirati, E.; Heesakkers, J.; Kessler, T.M.; Peters, K.M.; Rashid, T.; Sievert, K.-D.; Spinelli, M.; Novara, G.; Van der Aa, F.; et al. Efficacy and Safety of Sacral and Percutaneous Tibial Neuromodulation in Non-neurogenic Lower Urinary Tract Dysfunction and Chronic Pelvic Pain: A Systematic Review of the Literature. Eur. Urol. 2018, 73, 406–418. [Google Scholar] [CrossRef]
- Windgassen, S.; McKernan, L. Cognition, Emotion, and the Bladder: Psychosocial Factors in bladder pain syndrome and interstitial cystitis (BPS/IC). Curr. Bladder Dysfunct. Rep. 2020, 15, 9–14. [Google Scholar] [CrossRef]
- Kanter, G.; Komesu, Y.M.; Qaedan, F.; Jeppson, P.C.; Dunivan, G.C.; Cichowski, S.B.; Rogers, R.G. Mindfulness-based stress reduction as a novel treatment for interstitial cystitis/bladder pain syndrome: A randomized controlled trial. Int. Urogynecology J. 2016, 27, 1705–1711. [Google Scholar] [CrossRef]
- Bowering, K.J.; O’Connell, N.E.; Tabor, A.; Catley, M.J.; Leake, H.B.; Moseley, G.L.; Stanton, T.R. The effects of graded motor imagery and its components on chronic pain: A systematic review and meta-analysis. J. Pain 2013, 14, 3–13. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition of Digital Health. Available online: https://www.who.int/europe/health-topics/digital-health (accessed on 1 August 2024).
- World Health Organization. Global Strategy on Digital Health 2020–2025. 2021. Available online: https://iris.who.int/handle/10665/344249 (accessed on 1 August 2024).
- FDA. Digital Health Innovation Action Plan. U.S. Food and Drug Administration. Available online: https://www.fda.gov/media/106331/download (accessed on 5 August 2024).
- Dang, A.; Arora, D.; Rane, P. Role of digital therapeutics and the changing future of healthcare. J. Fam. Med. Prim. Care 2020, 9, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Digital Therapeutics Alliance. DTA Digital Therapeutics Ecosystem Categorization. Available online: https://dtxalliance.org/wp-content/uploads/2023/06/DTA_FS_DHT-Ecosystem-Categorization.pdf (accessed on 10 August 2024).
- Brea-Gómez, B.; Torres-Sánchez, I.; Ortiz-Rubio, A.; Calvache-Mateo, A.; Cabrera-Martos, I.; López-López, L.; Valenza, M.C. Virtual Reality in the Treatment of Adults with Chronic Low Back Pain: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int. J. Environ. Res. Public Health 2021, 18, 11806. [Google Scholar] [CrossRef]
- Gandy, M.; Pang, S.T.Y.; Scott, A.J.; Heriseanu, A.I.; Bisby, M.A.; Dudeney, J.; Karin, E.; Titov, N.; Dear, B.F. Internet-delivered cognitive and behavioural based interventions for adults with chronic pain: A systematic review and meta-analysis of randomized controlled trials. Pain 2022, 163, 1041–1053. [Google Scholar] [CrossRef]
- Martínez-Pérez, B.; de la Torre-Díez, I.; López-Coronado, M. Mobile health applications for the most prevalent conditions by the World Health Organization: Review and analysis. J. Med. Internet Res. 2013, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Kernebeck, S.; Busse, T.S.; Ehlers, J.P.; Vollmar, H.C. Adherence to digital health interventions: Definitions, methods, and open questions. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2021, 64, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0); The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, 5928. [Google Scholar] [CrossRef]
- Merlot, B.; Elie, V.; Perigord, A.; Husson, Z.; Jubert, A.; Chanavaz-Lacheray, I.; Dennis, T.; Cotty-Eslous, M.; Roman, H. Pain Reduction with an Immersive Digital Therapeutic in Women Living with Endometriosis-Related Pelvic Pain: At-Home Self-Administered Randomized Controlled Trial. J. Med. Internet Res. 2023, 25, 47869. [Google Scholar] [CrossRef]
- Blödt, S.; Pach, D.; von Eisenhart-Rothe, S.; Lotz, F.; Roll, S.; Icke, K.; Witt, C.M. Effectiveness of app-based self-acupressure for women with menstrual pain compared to usual care: A randomized pragmatic trial. Am. J. Obstet. Gynecol. 2018, 218, 227e1–227e9. [Google Scholar] [CrossRef]
- Lee, M.H.; Wu, H.C.; Lin, J.Y.; Tan, T.; Chan, P.; Chen, Y. Development and evaluation of an E-health system to care for patients with bladder pain syndrome/interstitial cystitis. Int. J. Urol. 2014, 21, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.R.; Tu, H.Y.; Wang, Y.; Xia, S.-L.; Zhao, G.-Y.; Yang, T.; Li, Y.-K. Effectiveness and Safety of Moxibustion Robots on Primary Dysmenorrhea: A Randomized Controlled Pilot Trial. Chin. J. Integr. Med. 2021, 27, 578–584. [Google Scholar] [CrossRef]
- Merlot, B.; Dispersyn, G.; Husson, Z.; Chanavaz-Lacheray, I.; Dennis, T.; Greco-Vuilloud, J.; Fougère, M.; Potvin, S.; Cotty-Eslous, M.; Roman, H.; et al. Pain Reduction with an Immersive Digital Therapeutic Tool in Women Living With Endometriosis-Related Pelvic Pain: Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, 39531. [Google Scholar] [CrossRef]
- Lutfi, M.; Dalleck, L.C.; Drummond, C.; Drummond, M.; Paparella, L.; Keith, C.E.; Kirton, M.; Falconer, L.; Gebremichael, L.; Phelan, C.; et al. A Single Session of a Digital Health Tool-Delivered Exercise Intervention May Provide Immediate Relief from Pelvic Pain in Women with Endometriosis: A Pilot Randomized Controlled Study. Int. J. Environ. Res. Public Health 2023, 20, 1665. [Google Scholar] [CrossRef]
- Lo, H.H.M.; Zhu, M.; Zou, Z.; Wong, C.L.; Lo, S.H.S.; Chung, V.C.-H.; Wong, S.Y.-S.; Sit, R.W.S. Immersive and Nonimmersive Virtual Reality–Assisted Active Training in Chronic Musculoskeletal Pain: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2024, 26, 48787. [Google Scholar] [CrossRef]
- Peláez-Vélez, F.J.; Eckert, M.; Gacto-Sánchez, M.; Martínez-Carrasco, Á. Use of Virtual Reality and Videogames in the Physiotherapy Treatment of Stroke Patients: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 4747. [Google Scholar] [CrossRef] [PubMed]
- Lisón, J.F.; Palomar, G.; Mensorio, M.S.; Baños, R.M.; Cebolla-Martí, A.; Botella, C.; Benavent-Caballer, V.; Rodilla, E. Impact of a Web-Based Exercise and Nutritional Education Intervention in Patients Who Are Obese With Hypertension: Randomized Wait-List Controlled Trial. J. Med. Internet Res. 2020, 22, e14196. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| P | Adults with any form of chronic pelvic pain syndromes | |
| I | Digital health technologies for treatment | |
| C | Control group, no intervention, intervention without digital health, digital health intervention, placebo, traditional treatment | |
| O | Pain | |
| S | RCTs in English, French and Spanish | Not RCTs, protocols, pilots, non-peer-reviewed publications, meeting abstracts or gray literature |
| Author (Year) [ref] | Country | Sample Size (n) | Age (Mean ± SD) | Disease | Outcome Measures | Measuring Instrument | Effects of the Intervention on Pain Intensity | Time Point Assessment | Methodological Quality |
|---|---|---|---|---|---|---|---|---|---|
| Merlot et al. (2022) [39] | France | 45 | Total = 32.7 ± 8.02 EG = 32.2 ± 8.02 CG = 33.2 ± 8.12 | Endometriosis | Pain intensity | 11-point NRS | ↓EG * > ↓CG | Pain intensity: baseline/pre-treatment, post-intervention (15 min, 30 min, 45 min, 60 min, 240 min). | 22 |
| Pain relief | 5-point CS | Pain relief: post-intervention (15 min, 30 min, 45 min, 60 min, 240 min). | |||||||
| Merlot et al. (2023) [35] | France | 102 | Total = 32.9 ± 6.9 EG = 33.7 ± 6.6 CG = 32.1 ± 7.3 | Endometriosis | Pain intensity | 11-point NRS | ↓EG * > ↓CG | Pain intensity: wakeup, pre-treatment, post-intervention (60 min, 120 min, 180 min), bedtime. | 21 |
| Pain relief | 5-CS | ||||||||
| Fatigue | Pichot scale | Pain relief: post-intervention (60 min, 120 min, 180 min). | |||||||
| Stress | VAS | Fatigue and stress: wakeup and bedtime. | |||||||
| Medication | Report in follow-up diary | Medications: during the study | |||||||
| Quality of life | EHP-5 | Quality of life: baseline, end of the study | |||||||
| Catastrophizing | PCS | Catastrophizing: baseline | |||||||
| Global change | PGIC | Global change: end of the study | |||||||
| Satisfaction | Global rate | Satisfaction: end of the study | |||||||
| Lutfi et al. (2023) [40] | Australia | 19 | EG 1 = 29 ± 7 EG 2 = 27 ± 7 CG = 25 ± 4 | Endometriosis | Acute pelvic pain | 100 mm VAS | ↑EG 1 ≈ ↑EG 2 ↑EG 1 < ↑CG ↑EG 2 < ↑CG | Baseline/pre-treatment | 19 |
| 48 h post-intervention | |||||||||
| Blödt et al. (2018) [36] | Germany | 221 | Total = 24 ± 3.6 EG = 24.4 ± 3.3 CG = 23.7 ± 3.9 | Dysmenorrhea | Pain intensity | 11-point NRS | ↓EG * > ↓CG | Pain intensity: on the days of pain during the 3rd menstrual cycle after therapy starts. | 24 |
| Worst pain intensity during menstruation | NRS | ||||||||
| Other outcomes: during and after the 1st, 2nd, 3rd and 6th menstrual cycles. | |||||||||
| Duration of pain | Number of days with pain | ||||||||
| Responder rates | 50% reduction in mean pain intensity on the days with pain compared to the corresponding baseline value | ||||||||
| Pain medication | Number of days with pain medication | ||||||||
| Sick leave | Days of absence from work or school | ||||||||
| Body efficiency expectations | Body efficacy expectation 5 item scale | ||||||||
| Lee et al. (2014) [37] | Taiwan | 65 | EG = 46.5 ± 10.2 CG = 49.5 ± 11.8 | BPS/IC | Pain and urgency | VAS | ↓EG * > ↓CG | Baseline/pre-treatment | 18 |
| 8 weeks post-intervention | |||||||||
| Disease severity | O’Leary-Sant symptom and problem indices | ||||||||
| Quality of life | SF-36 | ||||||||
| Zhang et al. (2021) [38] | China | 62 | EG = 23.1 ± 2.8 CG = 22.6 ± 3.8 | PD | Pain degree | SF-MPQ | ↓EG * ≈ ↓CG * | Baseline | 24 |
| Symptoms of PD | CMSS | ||||||||
| AEs | Occurrence rate of AEs (cases with AEs/the total number of cases × 100%) | 3rd menstrual cycle post-intervention | |||||||
| 6th menstrual cycle post-intervention |
| Author (Year) [ref] | Type of Digital Health Technology | Brand/Model of Digital Health Technology | Interventions | Session Duration | Treatment Place | Frequency | Program Duration | Supervision | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|
| Merlot et al. (2022) [39] | VR | In EG: Software: “Endocare” Class I medical software. Hardware: VR headset (Oculus Quest) with high-quality headphones (APK K-240-MKII) | EG: Endocare software offers treatment consisting of a combination of auditory (e.g., alpha/theta binaural beats, nature-based sounds) and visual (e.g., bilateral alternative stimulations consisting of a sphere appearing and moving on a horizontal axis) therapeutic procedures integrated in a 3D VR environment. | 20 min | IFEMEndo, a clinic that specializes in endometriosis | Single session | Single session | Through the entire treatment | 7 (15%) mild-to-moderate cases. 4 (8%) probably unrelated cases. 3 (6%) possibly related to the Endocare treatment, described as a mild headache and nausea related to motion sickness. |
| CG: Digital control program displayed through a tablet (Samsung Galaxy Tab A) with same high-quality headphones. Same context, environment and duration as the Endocare treatment but without any immersive effects, nor the auditory and visual stimuli. A soundtrack composed of nature sounds related to the projected image. | |||||||||
| Merlot et al. (2023) [35] | VR | In EG and CG: Software: “Endocare” Class I medical software. Hardware: Oculus Quest 2 VR headsets and AKG K-240MKII audio headphones | EG: Treatment combining auditory and visual therapeutic stimulations integrated in a 3D VR environment, including binaural beats, verbal hypnotic injunction, nature-based sounds, distraction of attention, and bilateral alternative stimulations. | 20 min | At home | Up to twice a day (minimum of 3 h between exposure) for at least 2 days and up to 5 days from the 1st day of painful menstruation, of de next menstrual cycle after starting the study. | 5 days | No supervision. | None |
| CG: Same hardware as EG, 20 min audio-video composition similar to Endocare (same context, environment, and duration) with exposure to nature sounds, but without Endocare’s stimulations. | |||||||||
| Lutfi et al. (2023) [40] | VR and telehealth | In EG1: the exercise apps were: Dance Central, Beat Saber, The Thrill of the Fight, Space Pirate Trainer, Fruit Ninja, OhShape, Racket NX, Table Tennis VR, Racket Fury, Swords of Gargantua, BoxVR, Superhot VR, VZ Fit Play, and VZFit Explorer. | EG1 (VR exercises): A 10 min VR pain-distraction experience using a list of apps previously shown to reduce pain; and 50 min of exercise using one of the applications based on participants’ preferences and goals. EG2 (telehealth delivered exercises): The exercises included cardiorespiratory exercises and stretching and specific stabilization exercises of the muscles of the lumbopelvic area. | 60 min | Not specified | Single session | Single session | EG1: supervised EG2: unsupervised | None |
| CG: Were instructed to continue with their activities of daily living. | |||||||||
| Blödt et al. (2018) [36] | Mobile phone app | In EG and CG: Smartphone app AKUD (Software development: Smart Mobile Factory, Berlin, Germany) | EG: AKUD app with access to self-acupressure specific features: explanations of the acupressure procedure, drawings, videos and photos of the acupressure points and a timer to guide the 1 min acupressure of each point. The acupressure points SP6, LI4 and LR3 were used on both sides. | 6 min (1 min per point) | At home via smartphone | 5 days before period: at least once a day, twice if possible. During menstruation, on painful days: at least twice a day, up to 5 times. | 6 menstrual cycles (6 months) | No supervision | EG: 15 patients reported at least 1 suspected AEs: bruises (n = 5), deterioration (n = 3), pain in the hand (n = 1), pressure pain (n = 1), shift in menstruation cycle (n = 3), dizziness (n = 1), nausea (n = 1), pain in the legs (n = 1), tingling in a finger (n = 2). Two serious AEs occurred in each group. EG: hip surgery, hospitalization due to dizziness. CG: surgery of the nose, appendix surgery. None was considered related to the trial or the trial intervention. |
| CG: AKUD app without acupressure specific features, access to just menstrual cycle visualization, questionnaires and diary. | |||||||||
| Lee et al. (2014) [37] | E-Health (Internet and SMS) | In EG: Web service installed in the web server to respond to or communicate with the mobile phone by sending/receiving short messages through the Hinet message center. | EG: Health education through web service, for consolidating healthy dieting habits and lifestyle. Health education questions to check their compliance in following the suggestions of the provided educational materials. SMS server to handle the cases of flare symptoms + regular treatments. | No session duration, at-home self-management | At home via smartphone or through an Internet browser | Weekly | 8 weeks | No supervision | None |
| CG: Regular treatments. | |||||||||
| Zhang et al. (2021) [38] | Robots | In EG: MR composed of a 6-degree-of-freedom robot arm, a controller, an infrared temperature sensor (MLX90614ESF), a laser ranging sensor (ATK-VL530L0X), and a moxa stick propulsion device. | EG: Moxibustion treatment through a robot that monitors and adjusts the skin temperature of CV4 point and the distance along with the change in skin temperature. | 30 min | In the Acupuncture Department of Affiliated Hospital of Chengdu University of Traditional Chinese Medicine. | Once a day, 5 days a week. Starting 5 days before the beginning of menses until forthcoming menstruation | 3 menstrual cycles of treatment and 3 menstrual cycles of follow-up | Through the entire treatment | Total number of AEs: 37 cases (in 815 patients). Grading: 31 mild cases, 6 moderate cases, no severe cases. AEs in EG: 9 cases. Rate of 2.1% (9 in 424 sessions). Cases: itching (4), bowel changes (3), menstrual changes (1), menorrhagia (1). AEs in CG: 28 cases. Rate of 7.2% (28 in 391 sessions). Cases: first grade burns (2), second grade burns (4), itching (9), bowel changes (7), menstrual changes (3), menorrhagia (2), fatigue (1). |
| CG: Traditional manual moxibustion treatment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Sánchez, I.; Tejada-Vega, O.; Rebollo-Segovia, G.; López-López, L.; Díaz-Mohedo, E. Digital Health Technologies in the Treatment of Chronic Pelvic Pain Syndromes: A Systematic Review of Randomized Clinical Trials. Healthcare 2025, 13, 2665. https://doi.org/10.3390/healthcare13212665
Torres-Sánchez I, Tejada-Vega O, Rebollo-Segovia G, López-López L, Díaz-Mohedo E. Digital Health Technologies in the Treatment of Chronic Pelvic Pain Syndromes: A Systematic Review of Randomized Clinical Trials. Healthcare. 2025; 13(21):2665. https://doi.org/10.3390/healthcare13212665
Chicago/Turabian StyleTorres-Sánchez, Irene, Olga Tejada-Vega, Guadalupe Rebollo-Segovia, Laura López-López, and Esther Díaz-Mohedo. 2025. "Digital Health Technologies in the Treatment of Chronic Pelvic Pain Syndromes: A Systematic Review of Randomized Clinical Trials" Healthcare 13, no. 21: 2665. https://doi.org/10.3390/healthcare13212665
APA StyleTorres-Sánchez, I., Tejada-Vega, O., Rebollo-Segovia, G., López-López, L., & Díaz-Mohedo, E. (2025). Digital Health Technologies in the Treatment of Chronic Pelvic Pain Syndromes: A Systematic Review of Randomized Clinical Trials. Healthcare, 13(21), 2665. https://doi.org/10.3390/healthcare13212665