Neurodevelopmental Pathways from Maternal Obesity to Offspring Outcomes: An Umbrella Review of Cognitive and Behavioral Consequences Across Development
Abstract
1. Introduction
2. Contemporary State of the Art
2.1. Understanding Maternal Obesity and Neurodevelopmental Programming
2.2. Cognitive and Executive Function Outcomes
2.3. Behavioral and Emotional Outcomes
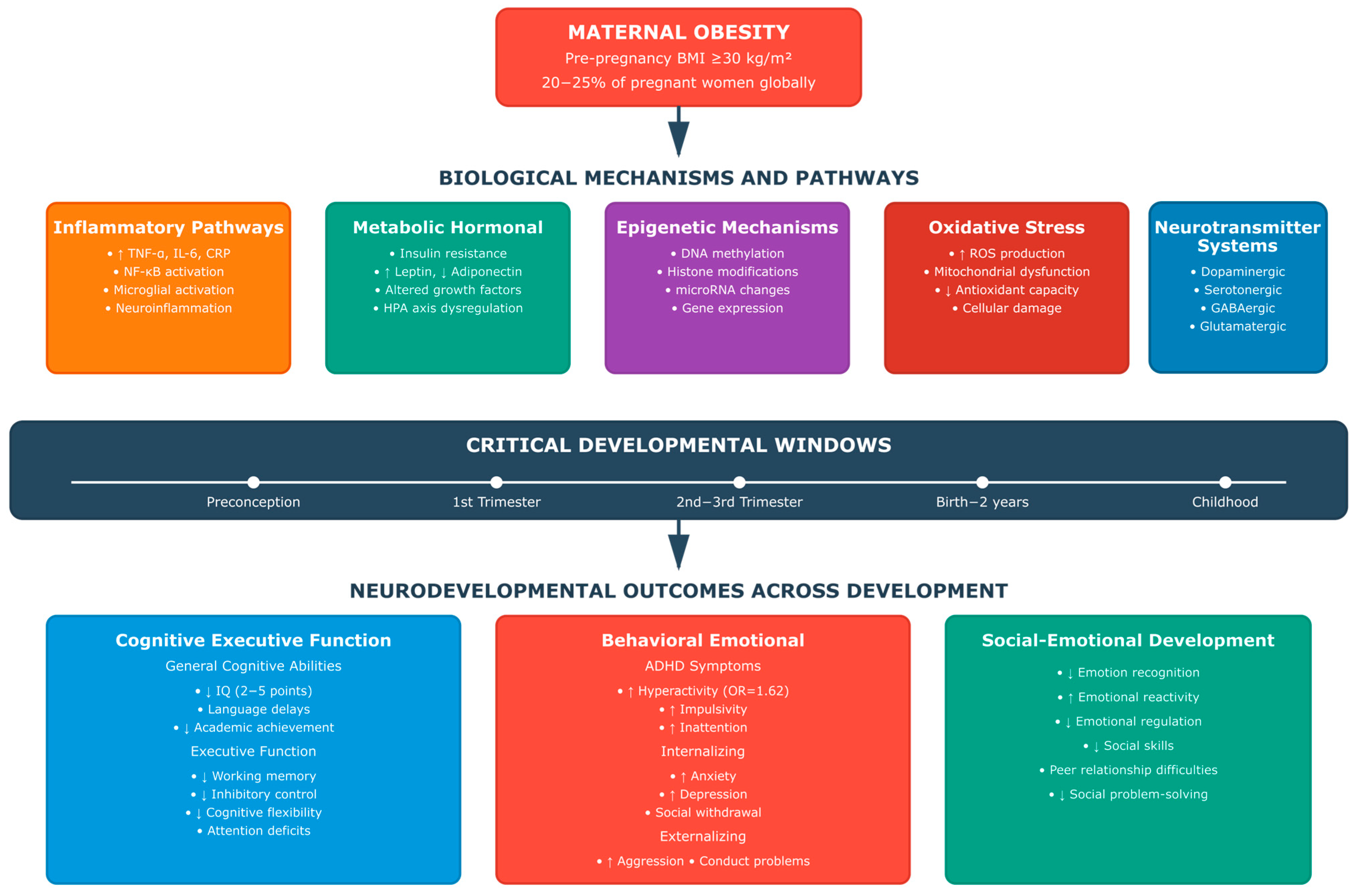
2.4. Biological Mechanisms and Pathways
2.5. Developmental Timing and Critical Periods
3. Materials and Methods
3.1. Research Questions
3.2. Search Strategy
3.3. Inclusion and Exclusion Criteria
3.3.1. Inclusion Criteria
3.3.2. Exclusion Criteria
3.4. Risk of Bias Assessment
3.5. Analytical Search Process
3.6. Data Synthesis
3.7. Software Tools
3.8. Study Classification and Methodological Overview
4. Results
4.1. [RQ1] Neurodevelopmental Outcomes Across the Developmental Spectrum: What Are the Associations Between Maternal Pre-Pregnancy Obesity and Offspring Neurodevelopmental Outcomes from the Prenatal Period Through Childhood and Adolescence, and How Do These Relationships Vary Across Different Developmental Stages?
4.1.1. Prenatal and Early Developmental Programming
4.1.2. Infancy and Toddlerhood (0–2 Years)
4.1.3. Preschool Period (3–5 Years)
4.1.4. School-Age Period (6–11 Years)
4.1.5. Adolescence (12+ Years)
4.1.6. Longitudinal Developmental Trajectories
4.1.7. Developmental Stage-Specific Synthesis
4.2. [RQ2] Specific Cognitive, Executive, and Behavioral Domains: How Does Maternal Obesity Specifically Affect Offspring Cognitive Abilities, Executive Function, and Behavioral Outcomes, and What Are the Relative Effect Sizes and Clinical Significance of These Associations Across Different Functional Domains?
4.2.1. Cognitive Abilities and Academic Achievement
4.2.2. Executive Function and Attention Regulation
4.2.3. Behavioral and Emotional Outcomes
4.2.4. Emotional Regulation and Internalizing Symptoms
4.2.5. Comparative Effect Sizes and Clinical Significance
4.3. [RQ3] Biological Mechanisms and Pathways: What Are the Underlying Biological Mechanisms and Pathways Through Which Maternal Obesity Influences Offspring Neurodevelopment, and How Do Inflammatory, Metabolic, Epigenetic, and Neurotransmitter-Related Mechanisms Interact to Produce Observed Outcomes?
4.4. [RQ4] Dose–Response Relationships and Critical Exposure Windows: How Do Different Degrees of Maternal Weight Status (Overweight vs. Obesity vs. Severe Obesity) and Timing of Exposure Affect the Magnitude and Pattern of Offspring Neurodevelopmental Outcomes, and What Are the Critical Windows of Vulnerability?
4.4.1. Dose–Response Patterns Across Weight Categories
4.4.2. Domain-Specific Vulnerability and Mediation Pathways
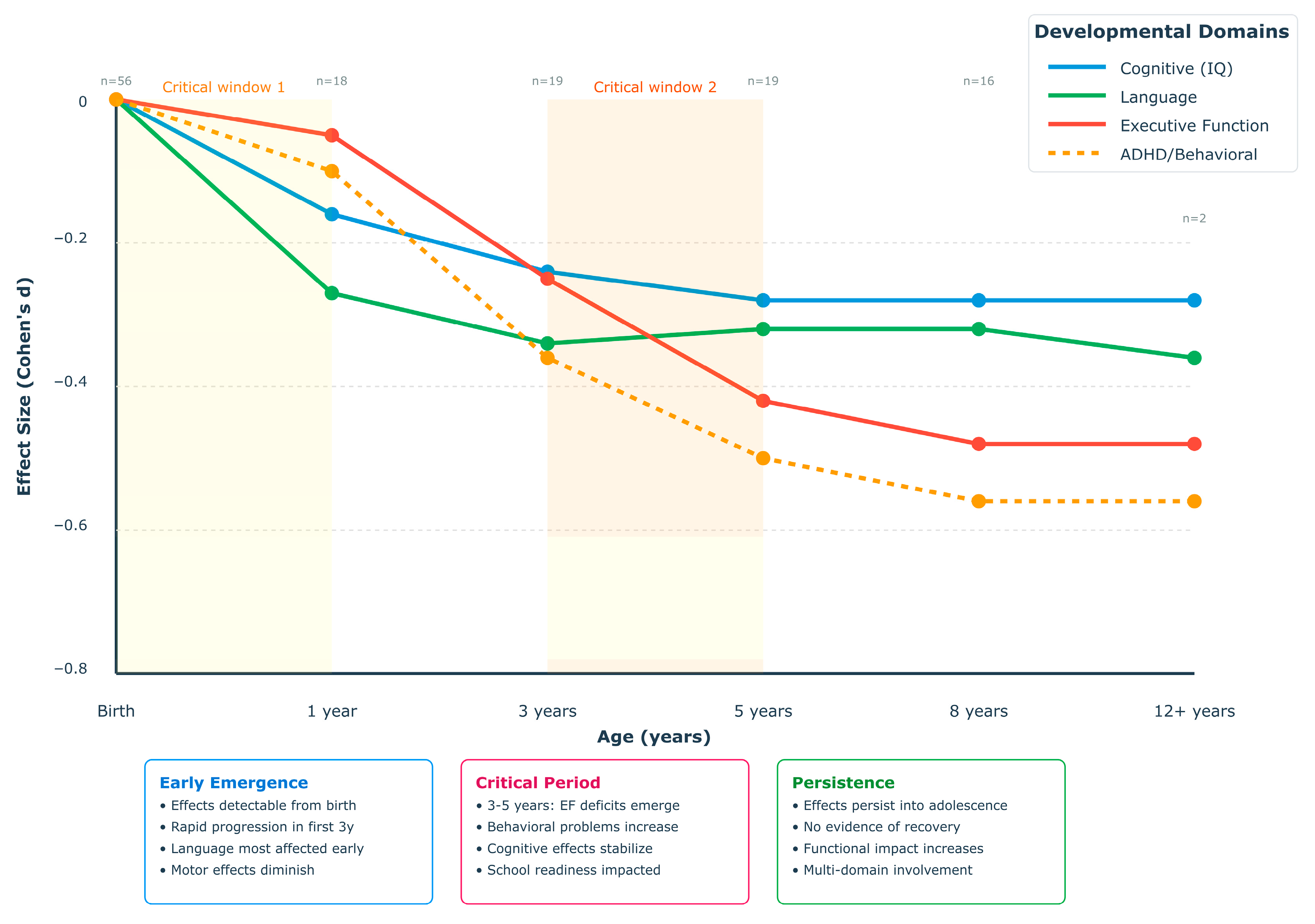
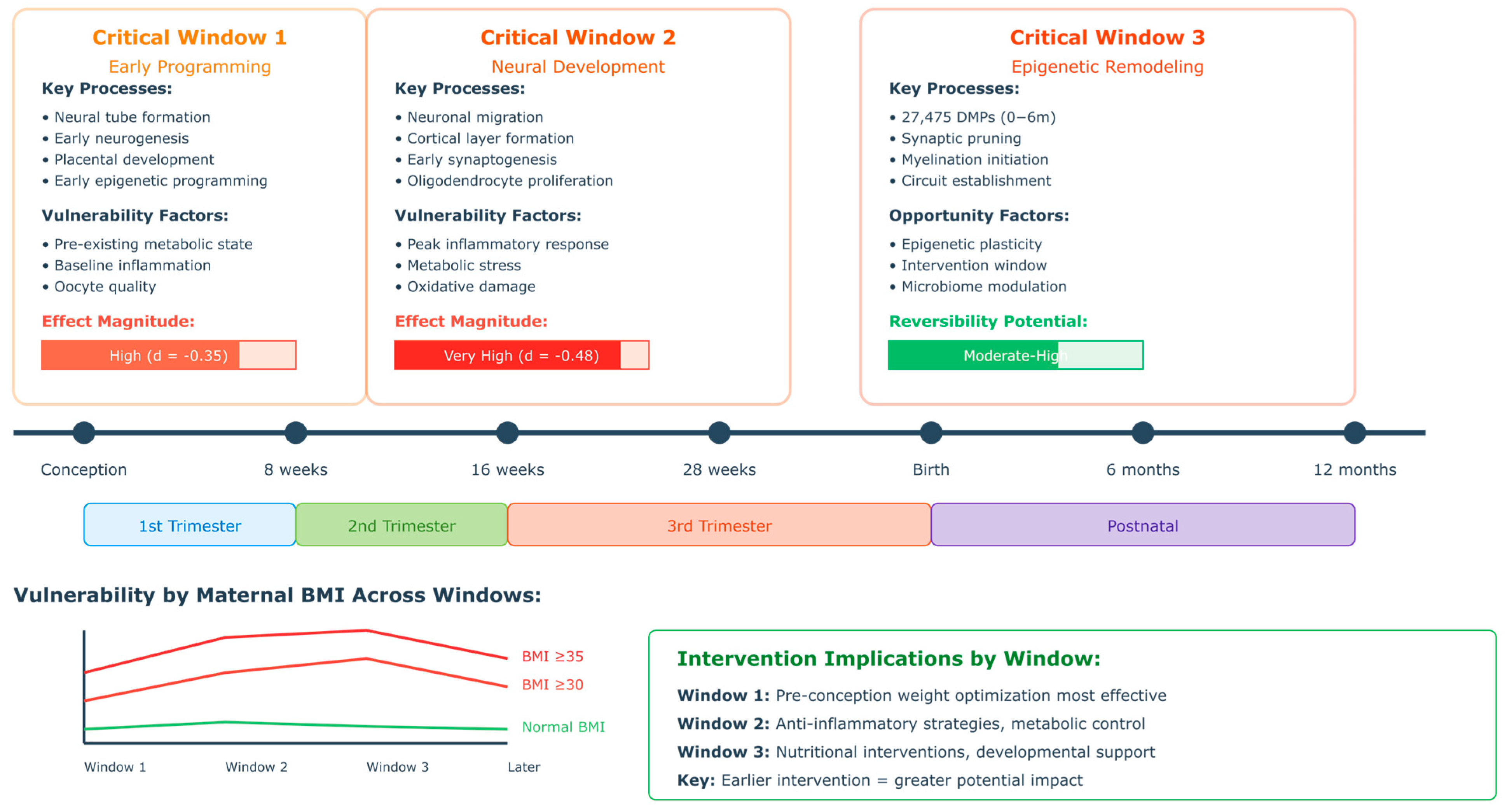
4.4.3. Critical Developmental Windows and Timing Effects
4.4.4. Sex-Specific Dose–Response Relationships
4.4.5. Gestational Weight Gain Interactions and Metabolic Modifiers
4.4.6. Non-Linear Thresholds and Accelerating Risks
4.4.7. Age-Dependent Effect Trajectories
4.4.8. Implications for Risk Stratification and Intervention
5. Discussion
5.1. Biological Mechanisms Linking Maternal Obesity to Offspring Neurodevelopment: A Critical Analysis
5.1.1. Inflammatory Pathways: Beyond Simple Association
Sex-Specific Inflammatory Programming and Biological Mechanisms
5.1.2. Metabolic Programming: Questioning the Insulin-Centric Model
Sex-Specific Metabolic Programming Mechanisms
5.1.3. Epigenetic Mechanisms: Reversibility Versus Permanence Debate
5.2. Critical Developmental Windows: Challenging the Trimester-Based Model
5.3. Heterogeneity and Precision Medicine: Moving Beyond Population Averages
5.4. Research Gaps and Limitations
5.4.1. Methodological Limitations
5.4.2. Knowledge Gaps
5.5. Future Research Directions
5.5.1. Advancing Measurement and Methodology
5.5.2. Mechanistic Research Priorities
5.5.3. Targeted Intervention Development
5.5.4. Implementation and Translation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grieger, J.A.; Hutchesson, M.J.; Cooray, S.D.; Khomami, M.B.; Zaman, S.; Segan, L.; Teede, H.; Moran, L.J. A review of maternal overweight and obesity and its impact on cardiometabolic outcomes during pregnancy and postpartum. Ther. Adv. Reprod. Health 2021, 15, 2633494120986544. [Google Scholar] [CrossRef]
- Lourenço, J.; Guedes-Martins, L. Pathophysiology of maternal obesity and hypertension in pregnancy. J. Cardiovasc. Dev. Dis. 2025, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Keramat, S.A.; Rahman, S.M.; Mustafa, M.U.R.; Alam, K. Association of maternal obesity with fetal and neonatal death: Evidence from South and South-East Asian countries. PLoS ONE 2021, 16, e0256725. [Google Scholar] [CrossRef] [PubMed]
- Reichetzeder, C. Overweight and obesity in pregnancy: Their impact on epigenetics. Eur. J. Clin. Nutr. 2021, 75, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Case, S.; Rijhsinghani, A. Maternal obesity: Perinatal implications. SAGE Open Med. 2023, 11, 20503121231176128. [Google Scholar] [CrossRef]
- Daliry, A.; Pereira, E.N.G.d.S. Role of Maternal Microbiota and Nutrition in Early-Life Neurodevelopmental Disorders. Nutrients 2021, 13, 3533. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Highlighting the trajectory from intrauterine growth restriction to future obesity. Front. Endocrinol. 2022, 13, 1041718. [Google Scholar] [CrossRef]
- Lubrano, C.; Parisi, F.; Cetin, I. Impact of maternal environment and inflammation on fetal neurodevelopment. Antioxidants 2024, 13, 453. [Google Scholar] [CrossRef]
- Papachatzi, E.; Papadopoulos, V.; Dimitriou, G.; Paparrodopoulos, S.; Papadimitriou-Olivgeris, M.; Vantarakis, A. Prepregnancy maternal obesity and fetal-perinatal death in a Mediterranean country. JPME 2014, 43, 291–298. [Google Scholar] [CrossRef]
- Denizli, M.; Capitano, M.L.; Kua, K.L. Maternal obesity and the impact of associated early-life inflammation on long-term health of offspring. Front. Cell. Infect. Microbiol. 2022, 12, 940937. [Google Scholar] [CrossRef]
- Apostolopoulou, A.; Tranidou, A.; Tsakiridis, I.; Magriplis, E.; Dagklis, T.; Chourdakis, M. Effects of Nutrition on Maternal Health, Fetal Development, and Perinatal Outcomes. Nutrients 2024, 16, 375. [Google Scholar] [CrossRef] [PubMed]
- Gkintoni, E.; Panagioti, M.; Vassilopoulos, S.P.; Nikolaou, G.; Boutsinas, B.; Vantarakis, A. Leveraging AI-Driven Neuroimaging Biomarkers for Early Detection and Social Function Prediction in Autism Spectrum Disorders: A Systematic Review. Healthcare 2025, 13, 1776. [Google Scholar] [CrossRef] [PubMed]
- Rosberg, A.; Merisaari, H.; Lewis, J.D.; Hashempour, N.; Lukkarinen, M.; Rasmussen, J.M.; Scheinin, N.M.; Karlsson, L.; Karlsson, H.; Tuulari, J.J. Associations between maternal pre-pregnancy BMI and infant striatal mean diffusivity. BMC Med. 2024, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Na, X.; Mackean, P.P.; Cape, G.A.; Johnson, J.W.; Ou, X. Maternal nutrition during pregnancy and offspring brain development: Insights from neuroimaging. Nutrients 2024, 16, 3337. [Google Scholar] [CrossRef]
- Hasebe, K.; Kendig, M.D.; Morris, M.J. Mechanisms underlying the cognitive and behavioural effects of maternal obesity. Nutrients 2021, 13, 240. [Google Scholar] [CrossRef]
- Tong, L.; Kalish, B.T. The impact of maternal obesity on childhood neurodevelopment. J. Perinatol. 2020, 41, 928–939. [Google Scholar] [CrossRef]
- Saeed, S.; Bonnefond, A.; Froguel, P. Obesity: Exploring its connection to brain function through genetic and genomic perspectives. Mol. Psychiatry 2024, 30, 651–658. [Google Scholar] [CrossRef]
- Norr, M.E.; Hect, J.L.; Lenniger, C.J.; Heuvel, M.V.D.; Thomason, M.E. An examination of maternal prenatal BMI and human fetal brain development. J. Child Psychol. Psychiatry 2020, 62, 458–469. [Google Scholar] [CrossRef]
- Laughlin, M.; Cooke, B.; Boutelle, K.; Savage, C.R.; Kravitz, A.; Small, D.; Arvanitakis, Z.; Martin, A.; Stoeckel, L.E. Neuroimaging and modulation in obesity and diabetes research: 10th anniversary meeting. Int. J. Obes. 2021, 46, 718–725. [Google Scholar] [CrossRef]
- Kong, L.; Chen, X.; Gissler, M.; Lavebratt, C. Relationship of prenatal maternal obesity and diabetes to offspring neurodevelopmental and psychiatric disorders: A narrative review. Int. J. Obes. 2020, 44, 1981–2000. [Google Scholar] [CrossRef]
- Eleftheriades, A.; Koulouraki, S.; Belegrinos, A.; Eleftheriades, M.; Pervanidou, P. Maternal obesity and neurodevelopment of the offspring. Nutrients 2025, 17, 891. [Google Scholar] [CrossRef]
- Chen, S.; Fan, M.; Lee, B.K.; Dalman, C.; Karlsson, H.; Gardner, R.M. Rates of maternal weight gain over the course of pregnancy and offspring risk of neurodevelopmental disorders. BMC Med. 2023, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: A systematic review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Mire, E. Maternal obesity and developmental programming of neuropsychiatric disorders: An inflammatory hypothesis. Brain Neurosci. Adv. 2021, 5, 23982128211003484. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Osorio, A.S.; Carreón-Torres, E.; Correa-Solís, E.; Ángel-García, J.; Arias-Rico, J.; Jiménez-Garza, O.; Morales-Castillejos, L.; Díaz-Zuleta, H.A.; Baltazar-Tellez, R.M.; Sánchez-Padilla, M.L.; et al. Inflammation and Oxidative Stress Induced by Obesity, Gestational Diabetes, and Preeclampsia in Pregnancy: Role of High-Density Lipoproteins as Vectors for Bioactive Compounds. Antioxidants 2023, 12, 1894. [Google Scholar] [CrossRef]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-Van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal nutrition and neurodevelopment: A scoping review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef]
- Gkintoni, E.; Halkiopoulos, C. Digital Twin Cognition: AI-Biomarker Integration in Biomimetic Neuropsychology. Biomimetics 2025, 10, 640. [Google Scholar] [CrossRef]
- Kankowski, L.; Ardissino, M.; McCracken, C.; Lewandowski, A.J.; Leeson, P.; Neubauer, S.; Harvey, N.C.; Petersen, S.E.; Raisi-Estabragh, Z. The impact of maternal obesity on offspring cardiovascular health: A systematic literature review. Front. Endocrinol. 2022, 13, 868441. [Google Scholar] [CrossRef]
- Mannino, A.; Sarapis, K.; Moschonis, G. The effect of maternal overweight and obesity pre-pregnancy and during childhood in the development of obesity in children and adolescents: A systematic literature review. Nutrients 2022, 14, 5125. [Google Scholar] [CrossRef]
- Berrigan, D.; Arteaga, S.S.; Colón-Ramos, U.; Rosas, L.G.; Monge-Rojas, R.; O’Connor, T.M.; Pérez-Escamilla, R.; Roberts, E.F.S.; Sanchez, B.; Téllez-Rojo, M.M.; et al. Measurement challenges for childhood obesity research within and between Latin America and the United States. Obes. Rev. 2021, 22, e13242. [Google Scholar] [CrossRef]
- Rodriguez, A.C.I.; Nagpal, T.S. The WOMBS framework: A review and new theoretical model for investigating pregnancy-related weight stigma and its intergenerational implications. Obes. Rev. 2021, 22, e13322. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.M.; Louise, J.; Deussen, A.R.; Mitchell, M.; Poston, L. Rethinking causal assumptions about maternal BMI, gestational weight gain, and adverse pregnancy outcomes. BMC Med. 2024, 22, 197. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, M.; Qian, G.; Yue, S.; Ossowski, Z.; Szumilewicz, A. A systematic review and Bayesian network meta-analysis comparing in-person, remote, and blended interventions in physical activity, diet, education, and behavioral modification on gestational weight gain among overweight or obese pregnant individuals. Adv. Nutr. Int. Rev. J. 2024, 15, 100253. [Google Scholar] [CrossRef] [PubMed]
- Nagi, M.A.; Ahmed, H.; Rezq, M.A.A.; Sangroongruangsri, S.; Chaikledkaew, U.; Almalki, Z.; Thavorncharoensap, M. Economic costs of obesity: A systematic review. Int. J. Obes. 2023, 48, 33–43. [Google Scholar] [CrossRef]
- Indarti, J.; Susilo, S.A.; Hyawicaksono, P.; Berguna, J.S.N.; Tyagitha, G.A.; Ikhsan, M. Maternal and perinatal outcome of maternal obesity at RSCM in 2014–2019. Obstet. Gynecol. Int. 2021, 2021, 6039565. [Google Scholar] [CrossRef]
- Escobar, M.B.C.; Contreras, J.O.; Bertoglia, M.P.; Bannout, M.A. Pregestational obesity, maternal morbidity and risk of caesarean delivery in a country in an advanced stage of obstetric transition. Obes. Res. Clin. Pract. 2021, 15, 73–77. [Google Scholar] [CrossRef]
- Fakhraei, R.; Denize, K.; Simon, A.; Sharif, A.; Zhu-Pawlowsky, J.; Dingwall-Harvey, A.L.J.; Hutton, B.; Pratt, M.; Skidmore, B.; Ahmadzai, N.; et al. Predictors of adverse pregnancy outcomes in pregnant women living with obesity: A systematic review. Int. J. Environ. Res. Public Health 2022, 19, 2063. [Google Scholar] [CrossRef]
- Khalifa, E.; El-Sateh, A.; Zeeneldin, M.; Abdelghany, A.M.; Hosni, M.; Abdallah, A.; Salama, S.; Abdel-Rasheed, M.; Mohammad, H. Effect of maternal BMI on labor outcomes in primigravida pregnant women. BMC Pregnancy Childbirth 2021, 21, 753. [Google Scholar] [CrossRef]
- Strauss, A.; Rochow, N.; Kunze, M.; Hesse, V.; Dudenhausen, J.W.; Voigt, M. Obesity in pregnant women: A 20-year analysis of the German experience. Eur. J. Clin. Nutr. 2021, 75, 1757–1763. [Google Scholar] [CrossRef]
- Van Lieshout, R.J.; Taylor, V.H.; Boyle, M.H. Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: A systematic review. Obes Rev. 2011, 12, e548–e559. [Google Scholar] [CrossRef]
- Bordeleau, M.; de Cossío, L.F.; Chakravarty, M.M.; Tremblay, M. From maternal diet to neurodevelopmental disorders: A story of neuroinflammation. Front. Cell. Neurosci. 2021, 14, 612705. [Google Scholar] [CrossRef] [PubMed]
- Rubini, E.; Schenkelaars, N.; Rousian, M.; Sinclair, K.D.; Wekema, L.; Faas, M.M.; Steegers-Theunissen, R.P.; Schoenmakers, S. Maternal obesity during pregnancy leads to derangements in one-carbon metabolism and the gut microbiota: Implications for fetal development and offspring wellbeing. Am. J. Obstet. Gynecol. 2022, 227, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Maternal obesity and gut microbiota are associated with fetal brain development. Nutrients 2022, 14, 4515. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, A.; Dearden, L.; Fernandez-Twinn, D.S.; Ozanne, S.E. Programming of cardiometabolic health: The role of maternal and fetal hyperinsulinaemia. J. Endocrinol. 2022, 253, R47–R63. [Google Scholar] [CrossRef]
- Strain, J.; Spaans, F.; Serhan, M.; Davidge, S.T.; Connor, K.L. Programming of weight and obesity across the lifecourse by the maternal metabolic exposome: A systematic review. Mol. Asp. Med. 2022, 87, 100986. [Google Scholar] [CrossRef]
- Schoonejans, J.M.; Ozanne, S.E. Developmental programming by maternal obesity: Lessons from animal models. Diabet. Med. 2021, 38, e14694. [Google Scholar] [CrossRef]
- Rivera, H.M.; Christiansen, K.J.; Sullivan, E.L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 2015, 9, 194. [Google Scholar] [CrossRef]
- Alum, E.U. Metabolic Memory in Obesity: Can Early-Life Interventions Reverse Lifelong Risks? Obes. Med. 2025, 55, 100610. [Google Scholar] [CrossRef]
- Rodrigo, N.; Saad, S.; Pollock, C.; Glastras, S.J. Diet Modification before or during Pregnancy on Maternal and Foetal Outcomes in Rodent Models of Maternal Obesity. Nutrients 2022, 14, 2154. [Google Scholar] [CrossRef]
- Brunner, K.; Linder, T.; Klaritsch, P.; Tura, A.; Windsperger, K.; Göbl, C. The impact of overweight and obesity on pregnancy: A narrative review of physiological consequences, risks and challenges in prenatal care, and early intervention strategies. Curr. Diabetes Rep. 2025, 25, 30. [Google Scholar] [CrossRef]
- Picó, C.; Reis, F.; Egas, C.; Mathias, P.; Matafome, P. Lactation as a programming window for metabolic syndrome. Eur. J. Clin. Investig. 2020, 51, e13482. [Google Scholar] [CrossRef]
- Rousseau-Ralliard, D.; Chavatte-Palmer, P.; Couturier-Tarrade, A. The effect of maternal exposure to a diet high in fats and cholesterol on the placental function and phenotype of the offspring in a rabbit model: A summary review of about 15 years of research. Int. J. Mol. Sci. 2023, 24, 14547. [Google Scholar] [CrossRef]
- Radford-Smith, D.E.; Anthony, D.C. Mechanisms of maternal diet-induced obesity affecting the offspring brain and development of affective disorders. Metabolites 2023, 13, 455. [Google Scholar] [CrossRef]
- Samà, M.; Musillo, C.; Cirulli, F. Counteracting the effects of maternal obesity on offspring neurodevelopment through Omega-3-based nutritional strategies. Neuroscience 2024, 566, 142–148. [Google Scholar] [CrossRef]
- Contu, L.; Hawkes, C. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. Int. J. Mol. Sci. 2017, 18, 1093. [Google Scholar] [CrossRef] [PubMed]
- Mortaji, N.; Krzeczkowski, J.E.; Boylan, K.; Booij, L.; Perreault, M.; Van Lieshout, R.J. Maternal pregnancy diet, postnatal home environment and executive function and behavior in 3-to 4-y-olds. Am. J. Clin. Nutr. 2021, 114, 1418–1427. [Google Scholar] [CrossRef]
- Ahmed, S.; Cano, M.Á.; Sánchez, M.; Hu, N.; Ibañez, G. Effect of exposure to maternal diabetes during pregnancy on offspring’s brain cortical thickness and neurocognitive functioning. Child Neuropsychol. 2022, 29, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, V.; González-Palacios, P.; Baca, M.A.; González-Domenech, P.J.; Fernández-Cabezas, M.; Álvarez-Cubero, M.J.; Rodrigo, L.; Rivas, A. Effect of exposure to endocrine disrupting chemicals in obesity and neurodevelopment: The genetic and microbiota link. Sci. Total Environ. 2022, 852, 158219. [Google Scholar] [CrossRef] [PubMed]
- Yonatan, E.; Shukha, O.N.; Golani, I.; Abu-Ata, S.; Awad-Igbaria, Y.; Khatib, N.; Ginsberg, Y.; Palzur, E.; Beloosesky, R.; Shamir, A. Maternal N-acetylcysteine supplementation in lactation ameliorates metabolic and cognitive deficits in adult offspring exposed to maternal obesity. Neuropharmacology 2025, 271, 110390. [Google Scholar] [CrossRef]
- Dawson, S.L.; O’Hely, M.; Jacka, F.N.; Ponsonby, A.L.; Symeonides, C.; Loughman, A.; Collier, F.; Moreno-Betancur, M.; Sly, P.; Burgner, D.; et al. Maternal prenatal gut microbiota composition predicts child behaviour. EBioMedicine 2021, 68, 103400. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef]
- Volqvartz, T.; Andersen, H.H.B.; Pedersen, L.H.; Larsen, A. Obesity in pregnancy—Long-term effects on offspring hypothalamic-pituitary-adrenal axis and associations with placental cortisol metabolism: A systematic review. Eur. J. Neurosci. 2023, 58, 4393–4422. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.; Lorthe, E.; Bernard, J.Y.; Galera, C.; Marchand-Martin, L.; Tafflet, M.; Ancel, P.; Charles, M.; Heude, B. Maternal prepregnancy obesity and offspring intelligence quotient at 5 years: A multicohort analysis. Paediatr. Perinat. Epidemiol. 2025, 39, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Schneider, L.; Baresi, S.; Bodini, F.; Bona, F.; Chillemi, C.; De Silvestri, A.; Zanelli, S.; Zuccotti, G. Specific learning disorders in children and adolescents with obesity. Children 2023, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Machle, C.J.; Mokhtari, P.; González, J.O.; Schmidt, K.A.; Alderete, T.L.; Adise, S.; Peterson, B.S.; Goran, M.I. Pre-pregnancy maternal obesity and infant neurodevelopmental outcomes in Latino infants. Obesity 2024, 32, 979–988. [Google Scholar] [CrossRef]
- Hung, L.Y.; Margolis, K.G. Autism spectrum disorders and the gastrointestinal tract: Insights into mechanisms and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 2023, 21, 142–163. [Google Scholar] [CrossRef]
- Rodolaki, K.; Pergialiotis, V.; Iakovidou, N.; Boutsikou, T.; Iliodromiti, Z.; Kanaka-Gantenbein, C. The impact of maternal diabetes on the future health and neurodevelopment of the offspring: A review of the evidence. Front. Endocrinol. 2023, 14, 1125628. [Google Scholar] [CrossRef]
- Likhitweerawong, N.; Khorana, J.; Boonchooduang, N.; Phinyo, P.; Patumanond, J.; Louthrenoo, O. Association between executive function and excess weight in pre-school children. PLoS ONE 2022, 17, e0275711. [Google Scholar] [CrossRef]
- Creese, H.; Hope, S.; Christie, D.; Goddings, A.; Viner, R. Is earlier obesity associated with poorer executive functioning later in childhood? Findings from the Millennium Cohort Study. Pediatr. Obes. 2021, 16, e12785. [Google Scholar] [CrossRef]
- Smith, B.L. Improving translational relevance: The need for combined exposure models for studying prenatal adversity. Brain Behav. Immun. Health 2021, 16, 100294. [Google Scholar] [CrossRef]
- Musillo, C.; Creutzberg, K.C.; Collacchi, B.; Ajmone-Cat, M.A.; De Simone, R.; Lepre, M.; Amrein, I.; Riva, M.A.; Berry, A.; Cirulli, F. Bdnf-Nrf-2 crosstalk and emotional behavior are disrupted in a sex-dependent fashion in adolescent mice exposed to maternal stress or maternal obesity. Transl. Psychiatry 2023, 13, 399. [Google Scholar] [CrossRef]
- Cernigliaro, F.; Santangelo, A.; Nardello, R.; Cascio, S.L.; D’agostino, S.; Correnti, E.; Marchese, F.; Pitino, R.; Valdese, S.; Rizzo, C.; et al. Prenatal nutritional factors and neurodevelopmental disorders: A narrative review. Life 2024, 14, 1084. [Google Scholar] [CrossRef]
- Shen, F.; Zhou, H. Advances in the etiology and neuroimaging of children with attention deficit hyperactivity disorder. Front. Pediatr. 2024, 12, 1400468. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vantarakis, A.; Gourzis, P. Neuroimaging Insights into the Public Health Burden of Neuropsychiatric Disorders: A Systematic Review of Electroencephalography-Based Cognitive Biomarkers. Medicina 2025, 61, 1003. [Google Scholar] [CrossRef]
- Hao, X.; Lu, J.; Yan, S.; Tao, F.; Huang, K. Maternal pre-pregnancy body mass index, gestational weight gain and children’s cognitive development: A birth cohort study. Nutrients 2022, 14, 4613. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Schilder, N.; Adolphus, K.; Berry, A.; Musillo, C.; Dye, L.; Cirulli, F.; Korosi, A.; Thuret, S. The dynamic influence of nutrition on prolonged cognitive healthspan across the life course: A perspective review. Neurosci. Appl. 2024, 3, 104072. [Google Scholar] [CrossRef] [PubMed]
- Girchenko, P.; Lahti-Pulkkinen, M.; Lipsanen, J.; Heinonen, K.; Lahti, J.; Rantalainen, V.; Hämäläinen, E.; Laivuori, H.; Villa, P.M.; Kajantie, E.; et al. Maternal early-pregnancy body mass index-associated metabolomic component and mental and behavioral disorders in children. Mol. Psychiatry 2022, 27, 4653–4661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, T.; Zhang, Y.; Liu, X.; Huang, H. Effects of parental overweight and obesity on offspring’s mental health: A meta-analysis of observational studies. PLoS ONE 2022, 17, e0276469. [Google Scholar] [CrossRef]
- Karhunen, V.; Bond, T.A.; Zuber, V.; Hurtig, T.; Moilanen, I.; Järvelin, M.-R.; Evangelou, M.; Rodriguez, A. The link between attention deficit hyperactivity disorder (ADHD) symptoms and obesity-related traits: Genetic and prenatal explanations. Transl. Psychiatry 2021, 11, 455. [Google Scholar] [CrossRef]
- Thorsheim, C.; Khan, S.; Lu, Y.; Kauffman, R.P. Maternal exacerbating and protective factors that shape the prevalence and severity of child attention-deficit hyperactivity disorder: A narrative review. Front. Psychiatry 2025, 16, 1577707. [Google Scholar] [CrossRef]
- Havdahl, A.; Wootton, R.E.; Leppert, B.; Riglin, L.; Ask, H.; Tesli, M.; Askeland, R.B.; Hannigan, L.J.; Corfield, E.; Øyen, A.-S.; et al. Associations between pregnancy-related predisposing factors for offspring neurodevelopmental conditions and Parental genetic liability to attention-deficit/hyperactivity disorder, autism, and schizophrenia. JAMA Psychiatry 2022, 79, 799–810. [Google Scholar] [CrossRef]
- Li, L.; Lagerberg, T.; Chang, Z.; Cortese, S.; Rosenqvist, M.A.; Almqvist, C.; D’Onofrio, B.M.; Hegvik, T.A.; Hartman, C.; Chen, Q.; et al. Maternal pre-pregnancy overweight/obesity and the risk of attention-deficit/hyperactivity disorder in offspring: A systematic review, meta-analysis and quasi-experimental family-based study. Int. J. Epidemiol. 2020, 49, 857–875. [Google Scholar] [CrossRef]
- Nieto-Ruiz, A.; Cerdó, T.; Jordano, B.; Torres-Espínola, F.J.; Escudero-Marín, M.; García-Ricobaraza, M.; Bermúdez, M.G.; García-Santos, J.A.; Suárez, A.; Campoy, C. Maternal weight, gut microbiota, and the association with early childhood behavior: The PREOBE follow-up study. Child Adolesc. Psychiatry Ment. Health 2023, 17, 41. [Google Scholar] [CrossRef]
- Shahin, S.; Medley, E.A.; Naidu, M.; Trasande, L.; Ghassabian, A. Exposure to organophosphate esters and maternal-child health. Environ. Res. 2024, 252, 118955. [Google Scholar] [CrossRef]
- Kacperska, M.; Mizera, J.; Pilecki, M.; Pomierny-Chamioło, L. The impact of excessive maternal weight on the risk of neuropsychiatric disorders in offspring—A narrative review of clinical studies. Pharmacol. Rep. 2024, 76, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Shuffrey, L.C.; Morales, S.; Jacobson, M.H.; Enlow, M.B.; Ghassabian, A.; Margolis, A.E.; Lucchini, M.; Carroll, K.N.; Crum, R.M.; Dabelea, D.; et al. Association of Gestational Diabetes Mellitus and perinatal maternal depression with early childhood behavioral problems: An Environmental Influences on Child Health Outcomes (ECHO) study. Child Dev. 2023, 94, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
- Gunter-Rahman, F.; Mallett, S.; White, F.; Jacques, P.; Raju, R.M.; Hivert, M.-F.; Lee, E.A. Hypoxia in extravillous trophoblasts links maternal obesity and offspring neurobehavior. iScience 2025, 28, 112636. [Google Scholar] [CrossRef] [PubMed]
- Wallander, J.L.; Berry, S.; Carr, P.A.; Peterson, E.R.; Waldie, K.E.; Marks, E.; D’Souza, S.; Morton, S.M.B. Patterns of risk exposure in first 1000 days of life and health, behavior, and education-related problems at age 4.5: Evidence from Growing Up in New Zealand, a longitudinal cohort study. BMC Pediatr. 2021, 21, 285. [Google Scholar] [CrossRef]
- Maitre, L.; Julvez, J.; López-Vicente, M.; Warembourg, C.; Tamayo-Uria, I.; Philippat, C.; Gützkow, K.B.; Guxens, M.; Andrusaityte, S.; Basagaña, X.; et al. Early-life environmental exposure determinants of child behavior in Europe: A longitudinal, population-based study. Environ. Int. 2021, 153, 106523. [Google Scholar] [CrossRef]
- Niu, L.; Hanson, S.; Preciado-Becerra, J.; Eskandarani, A.; Lei, X.; Le, M.; Niu, Z.; Xie, B. Psychosocial adjustment as a mediator in the relationship between childhood exposure to maternal depression and subsequent BMI and overweight risk. Children 2024, 11, 441. [Google Scholar] [CrossRef]
- Moog, N.K.; Cummings, P.D.; Jackson, K.L.; Aschner, J.L.; Barrett, E.S.; Bastain, T.M.; Blackwell, C.K.; Enlow, M.B.; Breton, C.V.; Bush, N.R.; et al. Intergenerational transmission of the effects of maternal exposure to childhood maltreatment in the USA: A retrospective cohort study. Lancet Public Health 2023, 8, e226–e237. [Google Scholar] [CrossRef]
- Mattila, I.; Nolvi, S.; Kataja, E.-L.; Tuulari, J.J.; Korja, R.; Scheinin, N.M.; Kaaja, R.; Karlsson, H.; Ekholm, E.; Karlsson, L. Gestational diabetes mellitus and children’s social-emotional development, behavioral problems, and psychological adjustment. Pediatr. Res. 2025, 1–8. [Google Scholar] [CrossRef]
- Karakitsiou, G.; Plakias, S.; Christidi, F.; Tsiakiri, A. Unraveling childhood obesity: A grounded theory approach to psychological, social, parental, and biological factors. Children 2024, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Luo, D.; Guan, K.; Luo, X. Meeting 24-h movement behavior guidelines is associated with academic engagement, social-emotional functioning in obese/overweight youth. Complement. Ther. Clin. Pract. 2024, 56, 101863. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Hao, X.; Zhu, L.; Guo, Y.; Wu, X.; Hao, J.; Tao, F.; Huang, K. Non-linear and sex-specific effect of maternal pre-pregnancy BMI on emotional and behavioral development of preschool children: A population-based cohort study. Int. J. Environ. Res. Public Health 2022, 19, 13414. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zhang, Y.; Liu, J.; Zeng, C.; Qin, J.; Liu, Z.; Zhang, X. The long-term development outcomes of the offspring born to patients with systemic lupus erythematosus: A cross-sectional study. Eur. Child Adolesc. Psychiatry 2025, 34, 2359–2368. [Google Scholar] [CrossRef]
- Gao, R.; Liu, X.; Li, X.; Zhang, Y.; Wei, M.; Sun, P.; Zhang, J.; Cai, L. Association between maternal sugar-sweetened beverage consumption and the social-emotional development of child before 1 year old: A prospective cohort study. Front. Nutr. 2022, 9, 966271. [Google Scholar] [CrossRef]
- Wu, Y.; De Asis-Cruz, J.; Limperopoulos, C. Brain structural and functional outcomes in the offspring of women experiencing psychological distress during pregnancy. Mol. Psychiatry 2024, 29, 2223–2240. [Google Scholar] [CrossRef]
- Mentzelou, M.; Papadopoulou, S.K.; Psara, E.; Alexatou, O.; Koimtsidis, T.; Giaginis, C. Exploring the impact of emotional eating in children: A narrative review. Pediatr. Rep. 2025, 17, 66. [Google Scholar] [CrossRef]
- Kumar, M.; Jha, A.K. Adolescent brain development: Limbic system and emotions. In Encyclopedia of Religious Psychology and Behavior; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–10. [Google Scholar] [CrossRef]
- Stephens, K.; Silk, T.J.; Anderson, V.; Hazell, P.; Enticott, P.G.; Sciberras, E. Associations between limbic system white matter structure and socio-emotional functioning in children with ADHD + ASD. J. Autism Dev. Disord. 2020, 51, 2663–2672. [Google Scholar] [CrossRef]
- Lei, D.; Li, W.; Qin, K.; Ai, Y.; Tallman, M.J.; Patino, L.R.; Welge, J.A.; Blom, T.J.; Klein, C.C.; Fleck, D.E.; et al. Effects of short-term quetiapine and lithium therapy for acute manic or mixed episodes on the limbic system and emotion regulation circuitry in youth with bipolar disorder. Neuropsychopharmacology 2022, 48, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Halkiopoulos, C.; Gkintoni, E.; Aroutzidis, A.; Antonopoulou, H. Advances in Neuroimaging and Deep Learning for Emotion Detection: A Systematic Review of Cognitive Neuroscience and Algorithmic Innovations. Diagnostics 2025, 15, 456. [Google Scholar] [CrossRef] [PubMed]
- Sanaeifar, F.; Pourranjbar, S.; Pourranjbar, M.; Ramezani, S.; Mehr, S.R.; Wadan, A.-H.S.; Khazeifard, F. Beneficial effects of physical exercise on cognitive-behavioral impairments and brain-derived neurotrophic factor alteration in the limbic system induced by neurodegeneration. Exp. Gerontol. 2024, 195, 112539. [Google Scholar] [CrossRef] [PubMed]
- Gkintoni, E.; Aroutzidis, A.; Antonopoulou, H.; Halkiopoulos, C. From Neural Networks to Emotional Networks: A Systematic Review of EEG-Based Emotion Recognition in Cognitive Neuroscience and Real-World Applications. Brain Sci. 2025, 15, 220. [Google Scholar] [CrossRef]
- Samson, J.A.; Newkirk, T.R.; Teicher, M.H. Practitioner Review: Neurobiological consequences of childhood maltreatment—Clinical and therapeutic implications for practitioners. J. Child Psychol. Psychiatry 2023, 65, 369–380. [Google Scholar] [CrossRef]
- Xiao, Q.; Shen, L.; He, H.; Wang, X.; Fu, Y.; Ding, J.; Jiang, F.; Zhang, J.; Zhang, Z.; Grecucci, A.; et al. Alteration of prefrontal cortex and its associations with emotional and cognitive dysfunctions in adolescent borderline personality disorder. Eur. Child Adolesc. Psychiatry 2024, 33, 3937–3949. [Google Scholar] [CrossRef]
- Fujihara, H.; Matsunaga, M.; Ueda, E.; Kajiwara, T.; Takeda, A.K.; Watanabe, S.; Baba, K.; Hagihara, K.; Myowa, M. Altered gut microbiota composition is associated with difficulty in explicit emotion regulation in young children. Microorganisms 2023, 11, 2245. [Google Scholar] [CrossRef]
- Bangma, J.T.; Hartwell, H.; Santos, H.P.; O’sHea, T.M.; Fry, R.C. Placental programming, perinatal inflammation, and neurodevelopment impairment among those born extremely preterm. Pediatr. Res. 2020, 89, 326–335. [Google Scholar] [CrossRef]
- Han, V.X.; Jones, H.F.; Patel, S.; Mohammad, S.S.; Hofer, M.J.; Alshammery, S.; Maple-Brown, E.; Gold, W.; Brilot, F.; Dale, R.C. Emerging evidence of Toll-like receptors as a putative pathway linking maternal inflammation and neurodevelopmental disorders in human offspring: A systematic review. Brain Behav. Immun. 2022, 99, 91–105. [Google Scholar] [CrossRef]
- Louwen, F.; Kreis, N.-N.; Ritter, A.; Yuan, J. Maternal obesity and placental function: Impaired maternal–fetal axis. Arch. Gynecol. Obstet. 2024, 309, 2279–2288. [Google Scholar] [CrossRef]
- Monaco-Brown, M.; Lawrence, D.A. Obesity and maternal-placental-fetal immunology and health. Front. Pediatr. 2022, 10, 859885. [Google Scholar] [CrossRef] [PubMed]
- Gagnidze, K.; Pfaff, D.W. Epigenetic mechanisms: DNA methylation and histone protein modification. In Neuroscience in the 21st Century: From Basic to Clinical; Springer: Berlin/Heidelberg, Germany, 2022; pp. 2677–2716. [Google Scholar] [CrossRef]
- Kakoulidou, I.; Avramidou, E.V.; Baránek, M.; Brunel-Muguet, S.; Farrona, S.; Johannes, F.; Kaiserli, E.; Lieberman-Lazarovich, M.; Martinelli, F.; Mladenov, V.; et al. Epigenetics for crop improvement in times of global change. Biology 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Mancera, F.M.; Sarno, F.; Goubert, D.; Rots, M.G. Gene-targeted DNA methylation: Towards long-lasting reprogramming of gene expression? In DNA Methyltransferases—Role and Function; Springer: Berlin/Heidelberg, Germany, 2022; pp. 515–533. [Google Scholar] [CrossRef]
- Mauceri, D. Role of Epigenetic Mechanisms in Chronic Pain. Cells 2022, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Akhter, Z.; Bi, Z.; Ali, K.; Sun, C.; Fiaz, S.; Haider, F.U.; Bai, J. In response to abiotic stress, DNA methylation confers epigenetic changes in plants. Plants 2021, 10, 1096. [Google Scholar] [CrossRef]
- Klibaner-Schiff, E.; Simonin, E.M.; Akdis, C.A.; Cheong, A.; Johnson, M.M.; Karagas, M.R.; Kirsh, S.; Kline, O.; Mazumdar, M.; Oken, E.; et al. Environmental exposures influence multigenerational epigenetic transmission. Clin. Epigenet. 2024, 16, 145. [Google Scholar] [CrossRef]
- Yuan, M.; Yang, B.; Rothschild, G.; Mann, J.J.; Sanford, L.D.; Tang, X.; Huang, C.; Wang, C.; Zhang, W. Epigenetic regulation in major depression and other stress-related disorders: Molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduct. Target. Ther. 2023, 8, 309. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Alkaraki, A.K.; Gammoh, O.; Tambuwala, M.M.; Mishra, V.; Mishra, Y.; Hassan, S.S.; El-Tanani, M. Deciphering depression: Epigenetic mechanisms and treatment strategies. Biology 2024, 13, 638. [Google Scholar] [CrossRef]
- Kunysz, M.; Mora-Janiszewska, O.; Darmochwał-Kolarz, D. Epigenetic modifications associated with exposure to endocrine disrupting chemicals in patients with gestational diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 4693. [Google Scholar] [CrossRef]
- Blanco, A.L.-Y.; Díaz-López, K.M.; Vilchis-Gil, J.; Diaz-Garcia, H.; Gomez-Lopez, J.; Medina-Bravo, P.; Granados-Riveron, J.T.; Gallardo, J.M.; Klünder-Klünder, M.; Sánchez-Urbina, R. Diet and maternal obesity are associated with increased oxidative stress in newborns: A cross-sectional study. Nutrients 2022, 14, 746. [Google Scholar] [CrossRef]
- Hu, C.; Yan, Y.; Ji, F.; Zhou, H. Maternal Obesity increases oxidative stress in placenta and it is associated with intestinal microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 671347. [Google Scholar] [CrossRef]
- Elías-López, A.; Vázquez-Mena, O.; Sferruzzi-Perri, A. Mitochondrial dysfunction in the offspring of obese mothers and it’s transmission through damaged oocyte mitochondria: Integration of mechanisms. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2023, 1869, 166802. [Google Scholar] [CrossRef]
- Mandò, C.; Castiglioni, S.; Novielli, C.; Anelli, G.M.; Serati, A.; Parisi, F.; Lubrano, C.; Zocchi, M.; Ottria, R.; Giovarelli, M. Placental bioenergetics and antioxidant homeostasis in maternal obesity and gestational diabetes. Antioxidants 2024, 13, 858. [Google Scholar] [CrossRef]
- de Oliveira, M.P.; da Silva, L.E.; Fernandes, B.B.; Steiner, M.R.; Pistóia, D.G.; Cichella, T.d.S.; Jacinto, L.B.; Spuldaro, K.M.; Iser, B.P.M.; Rezin, G.T. The impact of obesity on mitochondrial dysfunction during pregnancy. Mol. Cell. Endocrinol. 2025, 598, 112463. [Google Scholar] [CrossRef]
- Zhang, C.X.; Candia, A.A.; Sferruzzi-Perri, A.N. Placental inflammation, oxidative stress, and fetal outcomes in maternal obesity. Trends Endocrinol. Metab. 2024, 35, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Napso, T.; Lean, S.C.; Lu, M.; Mort, E.J.; Desforges, M.; Moghimi, A.; Bartels, B.; El-Bacha, T.; Fowden, A.L.; Camm, E.J.; et al. Diet-induced maternal obesity impacts feto-placental growth and induces sex-specific alterations in placental morphology, mitochondrial bioenergetics, dynamics, lipid metabolism and oxidative stress in mice. Acta Physiol. 2022, 234, e13795. [Google Scholar] [CrossRef] [PubMed]
- Heldens, A.; Antwi, M.; Onghena, L.; Meese, T.; Gansemans, Y.; Smet, J.; Dupont, E.; Verhelst, X.; Raevens, S.; Van Vlierberghe, H.; et al. Mitochondrial dysfunction characterises the multigenerational effects of maternal obesity on MASLD. JHEP Rep. 2025, 7, 101404. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, K.L.D.O.; Salla, D.H.; de Oliveira, M.P.; da Silva, L.E.; Vedova, L.M.D.; Mendes, T.F.; Bressan, C.B.C.; Costa, A.B.; da Silva, M.R.; Réus, G.Z.; et al. The impact of obesity-related neuroinflammation on postpartum depression: A narrative review. Int. J. Dev. Neurosci. 2022, 82, 375–384. [Google Scholar] [CrossRef]
- Steiner, M.R.; de Mello, A.H.; Salla, D.H.; Bressan, C.B.C.; Mendes, R.L.; de Oliveira, M.P.; da Silva, L.E.; Fernandes, B.B.; Lima, I.R.; Zaccaron, R.P.; et al. The Impact of Maternal Obesity and Deprivation On Energy Metabolism, Oxidative Stress and Brain Antioxidant Defense in the Neurodevelopment of Offspring in the Short, Medium and Long Term. Mol. Neurobiol. 2025, 62, 12473–12487. [Google Scholar] [CrossRef]
- Lackovic, M.; Nikolic, D.; Milicic, B.; Dimitrijevic, D.; Jovanovic, I.; Radosavljevic, S.; Mihajlovic, S. Pre-pregnancy obesity and infants’ motor development within the first twelve months of life: Who is expected to be the ultimate carrier of the obesity burden? Nutrients 2024, 16, 1260. [Google Scholar] [CrossRef]
- Rasmussen, J.M.; Tuulari, J.J.; Nolvi, S.; Thompson, P.M.; Merisaari, H.; Lavonius, M.; Karlsson, L.; Entringer, S.; Wadhwa, P.D.; Karlsson, H.; et al. Maternal pre-pregnancy body mass index is associated with newborn offspring hypothalamic mean diffusivity: A prospective dual-cohort study. BMC Med. 2023, 21. [Google Scholar] [CrossRef]
- Rosberg, A.; Merisaari, H.; Lewis, J.D.; Hashempour, N.; Lukkarinen, M.; Rasmussen, J.M.; Scheinin, N.M.; Karlsson, L.; Karlsson, H.; Tuulari, J.J. Associations between maternal pre-pregnancy BMI and mean diffusivity of the hippocampus and amygdala in infants. Int. J. Obes. 2025, 49, 938–941. [Google Scholar] [CrossRef]
- Fileva, N.; Severino, M.; Tortora, D.; Ramaglia, A.; Paladini, D.; Rossi, A. Second trimester fetal MRI of the brain: Through the ground glass. J. Clin. Ultrasound 2023, 51, 283–299. [Google Scholar] [CrossRef]
- Leibovitz, Z.; Lerman-Sagie, T.; Haddad, L. Fetal Brain Development: Regulating Processes and Related Malformations. Life 2022, 12, 809. [Google Scholar] [CrossRef] [PubMed]
- Beretta, E.; Cuboni, G.; Deidda, G. Unveiling GABA and serotonin interactions during neurodevelopment to re-open adult critical periods for neuropsychiatric disorders. Int. J. Mol. Sci. 2025, 26, 5508. [Google Scholar] [CrossRef] [PubMed]
- Perumal, N.; Manji, K.P.; Darling, A.M.; Kisenge, R.R.; Kvestad, I.; Hysing, M.; Belinger, D.C.; Urassa, W.; Strand, T.A.; Duggan, C.P.; et al. gestational age, birth weight, and neurocognitive development in adolescents in tanzania. J. Pediatr. 2021, 236, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Barnett, A.L.; Lin, Y.; Guan, H.; Sun, Y.; Williams, G.J.; Fu, Y.; Zhou, Y.; Du, W. Association of gestational age at birth with subsequent neurodevelopment in early childhood: A national retrospective cohort study in China. Front. Pediatr. 2022, 10, 860192. [Google Scholar] [CrossRef]
- Marín, M.J.B.; Elena, J.A.B.; Clavijo, J.M.; López, J.J.; López, D.M.L.; Mesa, E.G. Neurodevelopment outcome in children with fetal growth restriction at six years of age: A retrospective cohort study. Int. J. Environ. Res. Public Health 2022, 19, 11043. [Google Scholar] [CrossRef]
- Jiang, N.; Ma, S.-S.; Zu, P.; Zhang, L.; Xu, M.; Bian, J.-F.; Xu, J.-R.; Luo, W.; Wang, H.-X.; Zhu, D.-M.; et al. Intimate partner violence during pregnancy and early offspring development: A prospective birth cohort study. Biol. Psychiatry 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Olsen, J.E.; Lee, K.J.; Spittle, A.J.; Anderson, P.J.; Doyle, L.W.; Cheong, J.L.Y.; the members of the Victorian Infant Collaborative Study Group. The causal effect of being born extremely preterm or extremely low birthweight on neurodevelopment and social-emotional development at 2 years. Acta Paediatr. 2021, 111, 107–114. [Google Scholar] [CrossRef]
- Xie, Y.; Xiao, H.; Zheng, D.; Mahai, G.; Li, Y.; Xia, W.; Xu, S.; Zhou, A. Associations of prenatal metal exposure with child neurodevelopment and mediation by perturbation of metabolic pathways. Nat. Commun. 2025, 16, 2089. [Google Scholar] [CrossRef]
- Fan, W.Q.; Molinaro, A. Maternal obesity adversely affects early breastfeeding in a multicultural, multi-socioeconomic Melbourne community. A. N. Z. J. Obstet. Gynaecol. 2020, 61, 78–85. [Google Scholar] [CrossRef]
- Mantzorou, M.; Papandreou, D.; Vasios, G.K.; Pavlidou, E.; Antasouras, G.; Psara, E.; Taha, Z.; Poulios, E.; Giaginis, C. Exclusive breastfeeding for at least four months is associated with a lower prevalence of overweight and obesity in mothers and their children after 2–5 years from delivery. Nutrients 2022, 14, 3599. [Google Scholar] [CrossRef]
- Froń, A.; Orczyk-Pawiłowicz, M. Understanding the immunological quality of breast milk in maternal overweight and obesity. Nutrients 2023, 15, 5016. [Google Scholar] [CrossRef] [PubMed]
- Mazur, D.; Satora, M.; Rekowska, A.K.; Kabała, Z.; Łomża, A.; Kimber-Trojnar, Ż.; Leszczyńska-Gorzelak, B. Influence of breastfeeding on the state of meta-inflammation in obesity—A narrative review. Curr. Issues Mol. Biol. 2023, 45, 9003–9018. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.F.; Vazquez, C.; Cubbin, C.; Nichols, A.R.; Rickman, R.R.; Widen, E.M. Breastfeeding, socioeconomic status, and long-term postpartum weight retention. Int. Breastfeed. J. 2023, 18, 1. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takami, M.; Misumi, T.; Kawakami, C.; Miyagi, E.; Ito, S.; Aoki, S.; Japan Environment and Children’s Study (JECS) Group. Effects of breastfeeding on postpartum weight change in Japanese women: The Japan Environment and Children’s Study (JECS). PLoS ONE 2022, 17, e0268046. [Google Scholar] [CrossRef]
- Calcaterra, V.; Cena, H.; Pirazzi, A.; Sottotetti, F.; Cordaro, E.; Cavallo, C.; Milanta, C.; El Masri, D.; Conti, M.V.; Vandoni, M.; et al. From pregnancy to breastfeeding: The role of maternal exercise in preventing childhood obesity. Nutrients 2025, 17, 660. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Akker, O.R.v.D.; Peters, G.-J.Y.; Bakker, C.J.; Carlsson, R.; Coles, N.A.; Corker, K.S.; Feldman, G.; Moreau, D.; Nordström, T.; Pickering, J.S.; et al. Increasing the transparency of systematic reviews: Presenting a generalized registration form. Syst. Rev. 2023, 12, 170. [Google Scholar] [CrossRef]
- Alba-Linares, J.J.; Pérez, R.F.; Tejedor, J.R.; Bastante-Rodríguez, D.; Ponce, F.; Carbonell, N.G.; Zafra, R.G.; Fernández, A.F.; Fraga, M.F.; Lurbe, E. Maternal obesity and gestational diabetes reprogram the methylome of offspring beyond birth by inducing epigenetic signatures in metabolic and developmental pathways. Cardiovasc. Diabetol. 2023, 22, 44. [Google Scholar] [CrossRef]
- Álvarez-Bueno, C.; Cavero-Redondo, I.; la Cruz, L.L.-D.; Notario-Pacheco, B.; Martínez-Vizcaíno, V. Association between pre-pregnancy overweight and obesity and children’s neurocognitive development: A systematic review and meta-analysis of observational studies. Leuk. Res. 2017, 46, 1653–1666. [Google Scholar] [CrossRef]
- Alves, J.M.; Luo, S.; Chow, T.; Herting, M.; Xiang, A.H.; Page, K.A. Sex differences in the association between prenatal exposure to maternal obesity and hippocampal volume in children. Brain Behav. 2020, 10, e01522. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.R.; Patinkin, Z.; Shapiro, A.L.; De La Houssaye, B.A.; Woontner, M.; Boyle, K.E.; Vanderlinden, L.; Dabelea, D.; Friedman, J.E. Maternal obesity and increased neonatal adiposity correspond with altered infant mesenchymal stem cell metabolism. J. Clin. Investig. 2017, 2, e94200. [Google Scholar] [CrossRef] [PubMed]
- Basatemur, E.; Gardiner, J.; Williams, C.; Melhuish, E.; Barnes, J.; Sutcliffe, A. Maternal Prepregnancy BMI and Child Cognition: A Longitudinal Cohort Study. Pediatrics 2013, 131, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.C.C.; Moreno, B.; González-Santos, L.; Concha, L.; Barquera, S.; Barrios, F.A. Child overweight and obesity are associated with reduced executive cognitive performance and brain alterations: A magnetic resonance imaging study in Mexican children. Pediatr. Obes. 2014, 10, 196–204. [Google Scholar] [CrossRef]
- Boyle, K.E.; Patinkin, Z.W.; Shapiro, A.L.; Bader, C.; Vanderlinden, L.; Kechris, K.; Janssen, R.C.; Ford, R.J.; Smith, B.K.; Steinberg, G.R.; et al. Maternal obesity alters fatty acid oxidation, AMPK activity, and associated DNA methylation in mesenchymal stem cells from human infants. Mol. Metab. 2017, 6, 1503–1516. [Google Scholar] [CrossRef]
- van der Burg, J.W.; Sen, S.; Chomitz, V.R.; Seidell, J.C.; Leviton, A.; Dammann, O. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr. Res. 2015, 79, 3–12. [Google Scholar] [CrossRef]
- Buss, C.; Entringer, S.; Davis, E.P.; Hobel, C.J.; Swanson, J.M.; Wadhwa, P.D.; Sandman, C.A. Impaired Executive Function Mediates the Association between Maternal Pre-Pregnancy Body Mass Index and Child ADHD Symptoms. PLoS ONE 2012, 7, e37758. [Google Scholar] [CrossRef]
- Buss, C.; Tuulari, J.J.; Nolvi, S.; Thompson, P.M.; Merisaari, H.; Lavonius, M.; Karlsson, L.; Gyllenhammer, L.E.; Lindsay, K.L.; O’Connor, T.G.; et al. Intergenerational transmission of obesity risk by fetal programming of hypothalamus development. Psychoneuroendocrinology 2024, 160, 106744. [Google Scholar] [CrossRef]
- Cáceres, A.; Carreras-Gallo, N.; Andrusaityte, S.; Bustamante, M.; Carracedo, Á.; Chatzi, L.; Dwaraka, V.B.; Grazuleviciene, R.; Gutzkow, K.B.; Lepeule, J.; et al. Prenatal environmental exposures associated with sex differences in childhood obesity and neurodevelopment. BMC Med. 2023, 21, 142. [Google Scholar] [CrossRef]
- Camargos, A.C.R.; Mendonça, V.A.; Oliveira, K.S.; de Andrade, C.A.; Leite, H.R.; da Fonseca, S.F.; Vieira, E.L.M.; Júnior, A.L.T.; Lacerda, A.C.R. Association between obesity-related biomarkers and cognitive and motor development in infants. Behav. Brain Res. 2017, 325, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.; Chatzi, L.; Carsin, A.-E.; Amiano, P.; Guxens, M.; Kogevinas, M.; Koutra, K.; Lertxundi, N.; Murcia, M.; Rebagliato, M.; et al. Maternal pre-pregnancy overweight and obesity, and child neuropsychological development: Two Southern European birth cohort studies. Leuk. Res. 2013, 42, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Project, O.B.O.T.I.; Casas, M.; Forns, J.; Martínez, D.; Guxens, M.; Fernandez-Somoano, A.; Ibarluzea, J.; Lertxundi, N.; Murcia, M.; Rebagliato, M.; et al. Maternal pre-pregnancy obesity and neuropsychological development in pre-school children: A prospective cohort study. Pediatr. Res. 2017, 82, 596–606. [Google Scholar] [CrossRef]
- Cirulli, F.; De Simone, R.; Musillo, C.; Ajmone-Cat, M.A.; Berry, A. Inflammatory Signatures of Maternal Obesity as Risk Factors for Neurodevelopmental Disorders: Role of Maternal Microbiota and Nutritional Intervention Strategies. Nutrients 2022, 14, 3150. [Google Scholar] [CrossRef]
- Cirulli, F.; Musillo, C.; Berry, A. Maternal Obesity as a Risk Factor for Brain Development and Mental Health in the Offspring. Neuroscience 2020, 447, 122–135. [Google Scholar] [CrossRef]
- Dearden, L.; Ozanne, S.E. Early life origins of metabolic disease: Developmental programming of hypothalamic pathways controlling energy homeostasis. Front. Neuroendocr. 2015, 39, 3–16. [Google Scholar] [CrossRef]
- Dearden, L.; Buller, S.; Furigo, I.; Fernandez-Twinn, D.; Ozanne, S. Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways. Mol. Metab. 2020, 42, 101079. [Google Scholar] [CrossRef]
- Desai, M.; Han, G.; Ross, M.G. Programmed hyperphagia in offspring of obese dams: Altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite 2016, 99, 193–199. [Google Scholar] [CrossRef]
- Duko, B.; Mengistu, T.S.; Stacey, D.; Moran, L.J.; Tessema, G.; Pereira, G.; Bedaso, A.; Gebremedhin, A.T.; Alati, R.; Ayonrinde, O.T.; et al. Associations between maternal preconception and pregnancy adiposity and neuropsychiatric and behavioral outcomes in the offspring: A systematic review and meta-analysis. Psychiatry Res. 2024, 342, 116149. [Google Scholar] [CrossRef]
- Edlow, A.G.; Vora, N.L.; Hui, L.; Wick, H.C.; Cowan, J.M.; Bianchi, D.W. Maternal Obesity Affects Fetal Neurodevelopmental and Metabolic Gene Expression: A Pilot Study. PLoS ONE 2014, 9, e88661. [Google Scholar] [CrossRef]
- Edlow, A.G.; Guedj, F.; Pennings, J.L.; Sverdlov, D.; Neri, C.; Bianchi, D.W. Males are from Mars, and females are from Venus: Sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity. Am. J. Obstet. Gynecol. 2016, 214, 623.e1–623.e10. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Grayton, H.; Poston, L.; Samuelsson, A.-M.; Taylor, P.D.; Collier, D.A.; Rodriguez, A. Prenatal exposure to maternal obesity leads to hyperactivity in offspring. Mol. Psychiatry 2011, 17, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Francis, E.C.; Kechris, K.; Jansson, T.; Dabelea, D.; Perng, W. Novel Metabolic Subtypes in Pregnant Women and Risk of Early Childhood Obesity in Offspring. JAMA Netw. Open 2023, 6, e237030. [Google Scholar] [CrossRef] [PubMed]
- Fuemmeler, B.F.; Zucker, N.; Sheng, Y.; Sanchez, C.E.; Maguire, R.; Murphy, S.K.; Kollins, S.H.; Hoyo, C. Pre-Pregnancy Weight and Symptoms of Attention Deficit Hyperactivity Disorder and Executive Functioning Behaviors in Preschool Children. Int. J. Environ. Res. Public Health 2019, 16, 667. [Google Scholar] [CrossRef]
- Furigo, I.C.; Dearden, L. Mechanisms mediating the impact of maternal obesity on offspring hypothalamic development and later function. Front. Endocrinol. 2022, 13, 1078955. [Google Scholar] [CrossRef]
- Gaillard, R.; Rifas-Shiman, S.L.; Perng, W.; Oken, E.; Gillman, M.W. Maternal inflammation during pregnancy and childhood adiposity. Obesity 2016, 24, 1320–1327. [Google Scholar] [CrossRef]
- Galley, J.D.; Bailey, M.; Dush, C.K.; Schoppe-Sullivan, S.; Christian, L.M. Maternal Obesity Is Associated with Alterations in the Gut Microbiome in Toddlers. PLoS ONE 2014, 9, e113026. [Google Scholar] [CrossRef]
- Grissom, N.M.; Herdt, C.T.; Desilets, J.; Lidsky-Everson, J.; Reyes, T.M. Dissociable Deficits of Executive Function Caused by Gestational Adversity are Linked to Specific Transcriptional Changes in the Prefrontal Cortex. Neuropsychopharmacology 2014, 40, 1353–1363. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Ederveen, T.H.; Rizzo, F.; Weisz, A.; Collado, M.C.; Muratori, F.; Gross, G.; Alkema, W.; Iozzo, P. Maternal pre-pregnancy overweight and neonatal gut bacterial colonization are associated with cognitive development and gut microbiota composition in pre-school-age offspring. Brain Behav. Immun. 2022, 100, 311–320. [Google Scholar] [CrossRef]
- Harmancıoğlu, B.; Kabaran, S. Maternal high fat diets: Impacts on offspring obesity and epigenetic hypothalamic programming. Front. Genet. 2023, 14, 1158089. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Zhang, Z.; Taha, A.Y.; Capitanio, J.P.; Bauman, M.D.; Golub, M.S.; Van de Water, J.; VandeVoort, C.A.; Walker, C.K.; Slupsky, C.M. Impact of Maternal Obesity on the Gestational Metabolome and Infant Metabolome, Brain, and Behavioral Development in Rhesus Macaques. Metabolites 2022, 12, 764. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, S.N.; Schieve, L.A.; Stein, A.D.; Swan, D.W.; Ramakrishnan, U.; Sharma, A.J. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int. J. Obes. 2012, 36, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, X.; Keim, S.; Li, L.; Zhang, L.; Zhang, J. Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project. Leuk. Res. 2014, 43, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Keimpema, E.; Calvigioni, D.; Harkany, T. Endocannabinoid signals in the developmental programming of delayed-onset neuropsychiatric and metabolic illnesses. Biochem. Soc. Trans. 2013, 41, 1569–1576. [Google Scholar] [CrossRef]
- Kim, T.-W.; Park, H.-S. Physical exercise improves cognitive function by enhancing hippocampal neurogenesis and inhibiting apoptosis in male offspring born to obese mother. Behav. Brain Res. 2018, 347, 360–367. [Google Scholar] [CrossRef]
- Krakowiak, P.; Walker, C.K.; Bremer, A.A.; Baker, A.S.; Ozonoff, S.; Hansen, R.L.; Hertz-Picciotto, I. Maternal Metabolic Conditions and Risk for Autism and Other Neurodevelopmental Disorders. Pediatrics 2012, 129, e1121–e1128. [Google Scholar] [CrossRef]
- Krzeczkowski, J.E.; Boylan, K.; Arbuckle, T.E.; Dodds, L.; Muckle, G.; Fraser, W.; Favotto, L.A.; Van Lieshout, R.J. Neurodevelopment in 3–4 year old children exposed to maternal hyperglycemia or adiposity in utero. Early Hum. Dev. 2018, 125, 8–16. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, H.J.; Jang, Y.H.; Kim, H.; Im, K.; Yang, S.; Hoh, J.-K.; Ahn, J.-H. Maternal pre-pregnancy obesity affects the uncinate fasciculus white matter tract in preterm infants. Front. Pediatr. 2023, 11, 1225960. [Google Scholar] [CrossRef]
- Levin, B.E. Interaction of perinatal and pre-pubertal factors with genetic predisposition in the development of neural pathways involved in the regulation of energy homeostasis. Brain Res. 2010, 1350, 10–17. [Google Scholar] [CrossRef]
- Li, X.; Andres, A.; Shankar, K.; Pivik, R.T.; Glasier, C.M.; Ramakrishnaiah, R.H.; Zhang, Y.; Badger, T.M.; Ou, X. Differences in brain functional connectivity at resting state in neonates born to healthy obese or normal-weight mothers. Int. J. Obes. 2016, 40, 1931–1934. [Google Scholar] [CrossRef]
- Lippert, R.N.; Brüning, J.C. Maternal Metabolic Programming of the Developing Central Nervous System: Unified Pathways to Metabolic and Psychiatric Disorders. Biol. Psychiatry 2022, 91, 898–906. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Xia, B.; Jin, X.; Zou, Q.; Zeng, Z.; Zhao, W.; Yan, S.; Li, L.; Yuan, S.; et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021, 33, 923–938.e6. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Angelo, B.; Chow, T.; Monterosso, J.R.; Xiang, A.H.; Thompson, P.M.; Page, K.A. The role of maternal BMI on brain food cue reactivity in children: A preliminary study. Brain Imaging Behav. 2021, 15, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Menting, M.D.; van de Beek, C.; de Rooij, S.R.; Painter, R.C.; Vrijkotte, T.G.; Roseboom, T.J. The association between pre-pregnancy overweight/obesity and offspring’s behavioral problems and executive functioning. Early Hum. Dev. 2018, 122, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Mina, T.H.; Lahti, M.; Drake, A.J.; Denison, F.C.; Räikkönen, K.; Norman, J.E.; Reynolds, R.M. Prenatal exposure to maternal very severe obesity is associated with impaired neurodevelopment and executive functioning in children. Pediatr. Res. 2017, 82, 47–54. [Google Scholar] [CrossRef]
- Mina, T.H.; Lahti, M.; Drake, A.J.; Räikkönen, K.; Minnis, H.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Prenatal exposure to very severe maternal obesity is associated with adverse neuropsychiatric outcomes in children. Psychol. Med. 2016, 47, 353–362. [Google Scholar] [CrossRef]
- Monthé-Drèze, C.; Rifas-Shiman, S.L.; Gold, D.R.; Oken, E.; Sen, S. Maternal obesity and offspring cognition: The role of inflammation. Pediatr. Res. 2018, 85, 799–806. [Google Scholar] [CrossRef]
- Morgan, J.E.; Lee, S.S.; Mahrer, N.E.; Guardino, C.M.; Davis, E.P.; Shalowitz, M.U.; Ramey, S.L.; Schetter, C.D. Prenatal maternal C-reactive protein prospectively predicts child executive functioning at ages 4–6 years. Dev. Psychobiol. 2020, 62, 1111–1123. [Google Scholar] [CrossRef]
- Na, X.; Phelan, N.; Tadros, M.; Wu, Z.; Andres, A.; Badger, T.; Glasier, C.; Ramakrishnaiah, R.; Rowell, A.; Wang, L.; et al. Maternal Obesity during Pregnancy is Associated with Lower Cortical Thickness in the Neonate Brain. Am. J. Neuroradiol. 2021, 42, 2238–2244. [Google Scholar] [CrossRef]
- Ou, X.; Thakali, K.M.; Shankar, K.; Andres, A.; Badger, T.M. Maternal adiposity negatively influences infant brain white matter development. Obesity 2015, 23, 1047–1054. [Google Scholar] [CrossRef]
- Page, K.A.; Luo, S.; Wang, X.; Chow, T.; Alves, J.; Buchanan, T.A.; Xiang, A.H. Children Exposed to Maternal Obesity or Gestational Diabetes Mellitus During Early Fetal Development Have Hypothalamic Alterations That Predict Future Weight Gain. Diabetes Care 2019, 42, 1473–1480. [Google Scholar] [CrossRef]
- Panagos, P.G.; Vishwanathan, R.; Penfield-Cyr, A.; Matthan, N.R.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Sen, S. Breastmilk from obese mothers has pro-inflammatory properties and decreased neuroprotective factors. J. Perinatol. 2016, 36, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jang, A.; Bouret, S.G. Maternal obesity-induced endoplasmic reticulum stress causes metabolic alterations and abnormal hypothalamic development in the offspring. PLoS Biol. 2020, 18, e3000296. [Google Scholar] [CrossRef] [PubMed]
- Parsaei, M.; Hashemi, S.M.; Moghaddam, H.S.; Peterson, B.S. A systematic review of MRI studies on the effects of maternal obesity on offspring brain structure and function. J. Neurosci. Res. 2024, 102, e25368. [Google Scholar] [CrossRef] [PubMed]
- Plucińska, K.; Barger, S.W. Maternal obesity reprograms offspring’s executive brain centers in a sex-specific manner? J. Neurochem. 2018, 145, 358–361. [Google Scholar] [CrossRef]
- Rafiq, T.; Stearns, J.C.; Shanmuganathan, M.; Azab, S.M.; Anand, S.S.; Thabane, L.; Beyene, J.; Williams, N.C.; Morrison, K.M.; Teo, K.K.; et al. Integrative multiomics analysis of infant gut microbiome and serum metabolome reveals key molecular biomarkers of early onset childhood obesity. Heliyon 2023, 9, e16651. [Google Scholar] [CrossRef]
- Ross, M.G.; Desai, M. Developmental Programming of Appetite/Satiety. Ann. Nutr. Metab. 2014, 64 (Suppl. S1), 36–44. [Google Scholar] [CrossRef]
- Salzwedel, A.P.; Gao, W.; Andres, A.; Badger, T.M.; Glasier, C.M.; Ramakrishnaiah, R.H.; Rowell, A.C.; Ou, X. Maternal Adiposity Influences Neonatal Brain Functional Connectivity. Front. Hum. Neurosci. 2019, 12, 514. [Google Scholar] [CrossRef]
- Samara, A.; Murphy, T.; Strain, J.; Rutlin, J.; Sun, P.; Neyman, O.; Sreevalsan, N.; Shimony, J.S.; Ances, B.M.; Song, S.-K.; et al. Neuroinflammation and White Matter Alterations in Obesity Assessed by Diffusion Basis Spectrum Imaging. Front. Hum. Neurosci. 2020, 13, 464. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2017, 19, 464–484. [Google Scholar] [CrossRef]
- Sanders, T.R.; Kim, D.W.; Glendining, K.A.; Jasoni, C.L. Maternal obesity and IL-6 lead to aberrant developmental gene expression and deregulated neurite growth in the fetal arcuate nucleus. Endocrinology 2014, 155, 2566–2577. [Google Scholar] [CrossRef]
- Sanguinetti, E.; Guzzardi, M.A.; Tripodi, M.; Panetta, D.; Selma-Royo, M.; Zega, A.; Telleschi, M.; Collado, M.C.; Iozzo, P. Microbiota signatures relating to reduced memory and exploratory behaviour in the offspring of overweight mothers in a murine model. Sci. Rep. 2019, 9, 12609. [Google Scholar] [CrossRef]
- Sarker, G.; Peleg-Raibstein, D. Maternal Overnutrition Induces Long-Term Cognitive Deficits across Several Generations. Nutrients 2018, 11, 7. [Google Scholar] [CrossRef]
- Saros, L.; Lind, A.; Setänen, S.; Tertti, K.; Koivuniemi, E.; Ahtola, A.; Haataja, L.; Shivappa, N.; Hébert, J.R.; Vahlberg, T.; et al. Maternal obesity, gestational diabetes mellitus, and diet in association with neurodevelopment of 2-year-old children. Pediatr. Res. 2023, 94, 280–289. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Liang, D.; Busgang, S.A.; Curtin, P.; Giulivi, C. Maternal Plasma Metabolic Profile Demarcates a Role for Neuroinflammation in Non-Typical Development of Children. Metabolites 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.L.B.; Moore, B.F.; Sutton, B.; Wilkening, G.; Stence, N.; Dabelea, D.; Tregellas, J.R. In Utero Exposure to Maternal Overweight or Obesity is Associated with Altered Offspring Brain Function in Middle Childhood. Obesity 2020, 28, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, A.A.; Leibel, R.L.; LeDuc, C.A. Neurodevelopmental Programming of Adiposity: Contributions to Obesity Risk. Endocr. Rev. 2023, 45, 253–280. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, E.K.; Oommen, S.; Vasu, V.T.; Srinivasan, M.; Stachowiak, M.; Gohil, K.; Patel, M.S. Maternal obesity affects gene expression and cellular development in fetal brains. Nutr. Neurosci. 2013, 16, 96–103. [Google Scholar] [CrossRef]
- Sullivan, E.L.; Riper, K.M.; Lockard, R.; Valleau, J.C. Maternal high-fat diet programming of the neuroendocrine system and behavior. Horm. Behav. 2015, 76, 153–161. [Google Scholar] [CrossRef]
- Tanda, R.; Salsberry, P.J.; Reagan, P.B.; Fang, M.Z. The Impact of Prepregnancy Obesity on Children’s Cognitive Test Scores. Matern. Child Health J. 2012, 17, 222–229. [Google Scholar] [CrossRef]
- Torres-Espinola, F.J.; Berglund, S.K.; García-Valdés, L.M.; Segura, M.T.; Jerez, A.; Campos, D.; Moreno-Torres, R.; Rueda, R.; Catena, A.; Pérez-García, M.; et al. Maternal Obesity, Overweight and Gestational Diabetes Affect the Offspring Neurodevelopment at 6 and 18 Months of Age—A Follow Up from the PREOBE Cohort. PLoS ONE 2015, 10, e0133010. [Google Scholar] [CrossRef]
- Urbonaite, G.; Knyzeliene, A.; Bunn, F.S.; Smalskys, A.; Neniskyte, U. The impact of maternal high-fat diet on offspring neurodevelopment. Front. Neurosci. 2022, 16, 909762. [Google Scholar] [CrossRef]
- Walker, C.; Naef, L.; D’Asti, E.; Long, H.; Xu, Z.; Moreau, A.; Azeddine, B. Perinatal Maternal Fat Intake Affects Metabolism and Hippocampal Function in the Offspring. Ann. N. Y. Acad. Sci. 2008, 1144, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Widen, E.M.; Nichols, A.R.; Kahn, L.G.; Factor-Litvak, P.; Insel, B.J.; Hoepner, L.; Dube, S.M.; Rauh, V.; Perera, F.; Rundle, A. Prepregnancy obesity is associated with cognitive outcomes in boys in a low-income, multiethnic birth cohort. BMC Pediatr. 2019, 19, 507. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Deng, S.; Li, W.-G.; Yu, Y.; Li, F.; Mao, M. Maternal Obesity Caused by Overnutrition Exposure Leads to Reversal Learning Deficits and Striatal Disturbance in Rats. PLoS ONE 2013, 8, e78876. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.H.; Sundaram, R.; Ghassabian, A.; Xie, Y.; Louis, G.B. Parental Obesity and Early Childhood Development. Pediatrics 2017, 139, e20161459. [Google Scholar] [CrossRef]
- Zhu, C.; Han, T.-L.; Zhao, Y.; Zhou, X.; Mao, X.; Qi, H.; Baker, P.N.; Zhang, H. A mouse model of pre-pregnancy maternal obesity combined with offspring exposure to a high-fat diet resulted in cognitive impairment in male offspring. Exp. Cell Res. 2018, 368, 159–166. [Google Scholar] [CrossRef]
- Halkiopoulos, C.; Gkintoni, E. The Role of Machine Learning in AR/VR-Based Cognitive Therapies: A Systematic Review for Mental Health Disorders. Electronics 2025, 14, 1110. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Mindfulness-Based Cognitive Therapy in Clinical Practice: A Systematic Review of Neurocognitive Outcomes and Applications for Mental Health and Well-Being. J. Clin. Med. 2025, 14, 1703. [Google Scholar] [CrossRef]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Miguel-Andrés, I.; Huerta-Franco, M.R.; García-González, S.B.; León-Rodríguez, M.; Barrera-Beltrán, K.; Ortiz-Lango, L.A. The Importance of the Kinematic Evaluation Methods of the Upper Limbs in Women with Breast Cancer Mastectomy: A Literature Review. Healthcare 2023, 11, 2064. [Google Scholar] [CrossRef]
- Crawford, A.; Serhal, E. Digital Health Equity and COVID-19: The Innovation Curve Cannot Reinforce the Social Gradient of Health. J. Med. Internet Res. 2020, 22, e19361. [Google Scholar] [CrossRef] [PubMed]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Next-Generation Cognitive-Behavioral Therapy for Depression: Integrating Digital Tools, Teletherapy, and Personalization for Enhanced Mental Health Outcomes. Medicina 2025, 61, 431. [Google Scholar] [CrossRef] [PubMed]
- Gkintoni, E.; Vantaraki, F.; Skoulidi, C.; Anastassopoulos, P.; Vantarakis, A. Gamified Health Promotion in Schools: The Integration of Neuropsychological Aspects and CBT—A Systematic Review. Medicina 2024, 60, 2085. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.L.; Hopkins, C.M.; Brooks, T.L.; Bennett, G.G. Comparing Self-Monitoring Strategies for Weight Loss in a Smartphone App: Randomized Controlled Trial. JMIR mHealth uHealth 2019, 7, e12209. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vantaraki, F.; Skoulidi, C.; Anastassopoulos, P.; Vantarakis, A. Promoting Physical and Mental Health among Children and Adolescents via Gamification—A Conceptual Systematic Review. Behav. Sci. 2024, 14, 102. [Google Scholar] [CrossRef]
- Daly, L.M.; Horey, D.; Middleton, P.F.; Boyle, F.M.; Flenady, V. The Effect of Mobile App Interventions on Influencing Healthy Maternal Behavior and Improving Perinatal Health Outcomes: Systematic Review. JMIR MHealth UHealth 2018, 6, e10012. [Google Scholar] [CrossRef]
- Johnson, D.; Deterding, S.; Kuhn, K.-A.; Staneva, A.; Stoyanov, S.; Hides, L. Gamification for health and wellbeing: A systematic review of the literature. Internet Interv. 2016, 6, 89–106. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Aki, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]



| Authors | Key Findings | Method |
|---|---|---|
| Alba-Linares et al. (2023) [153] | - The study identified significant DNA methylation changes in children from birth to 6 months, indicating a critical period for epigenetic remodelling. - DNA methylation biomarkers were found to distinguish children born to mothers with obesity or gestational diabetes, suggesting a link between maternal metabolic conditions and offspring epigenetics. - These biomarkers are associated with metabolic pathways, developmental processes, and mitochondrial bioenergetics, indicating potential long-term health implications. | - Used Illumina Infinium MethylationEPIC BeadChip arrays to profile DNA methylation in blood samples. - Collected blood samples at birth, 6 months, and 12 months. - Extracted genomic DNA using RealPure kit and bisulphite converted using EZ-96 DNA Methylation Kit. - Analyzed data using R software with minfi, ssNoob, and BMIQ for processing and normalization. - Predicted cell-type composition using Houseman algorithm. - Conducted differential methylation analyses using linear mixed models and empirical Bayes-moderated t-tests. - Performed pathway enrichment analyses using missMethyl package and MSigDB. |
| Álvarez-Bueno et al. (2017) [154] | - Pre-pregnancy obesity is associated with negative effects on children’s neurocognitive development. - The pooled effect size for obesity was −0.06 (95% CI: −0.09 to −0.03), indicating a significant negative impact. - Overweight status was not significantly associated with negative effects on neurocognitive development. | - Systematic search of MEDLINE, EMBASE, Cochrane Library, and Web of Science databases. - Mantel-Haenszel fixed-effects method and DerSimonian and Laird method for meta-analysis. - Sensitivity analysis and random-effects meta-regression.- Publication bias evaluation using Egger’s regression asymmetry test. - Independent data extraction by two researchers with inter-rater agreement calculation. - Methodological quality assessment using a standardized checklist. - Calculation of effect sizes using standardized mean difference scores or odds ratios. |
| Alves et al. (2020) [155] | - Boys but not girls showed significant associations between prenatal exposure to maternal obesity and reductions in hippocampal volume. - These sex-specific effects were consistently observed in the adolescent PING cohort and were replicated in the early childhood RANN cohort. - Smaller hippocampal volume in boys was associated with increased behavioral problems and ADHD symptoms. | - High-resolution structural MRI scans were conducted on 88 children. - FreeSurfer 6.0 was used to quantify total hippocampal volume and subfield volumes. - Maternal prepregnancy BMI was used to indicate prenatal exposure to maternal obesity. - Child Behavior Checklist (CBCL) scores were used to evaluate behavioral problems and ADHD symptoms. - Statistical analyses included linear regression models with adjustments for relevant covariates. - Replication analysis was performed using data from the PING cohort (n = 236) and the RANN cohort (n = 77). |
| Baker et al. (2017) [156] | - Biomarkers of incomplete β-oxidation were positively correlated with infant adiposity and maternal lipid levels in uMSC myocytes from offspring of obese mothers. - Metabolic and biosynthetic processes were enriched in differential gene expression analysis, with genes related to mitochondrial respiratory chain and mitochondrial biogenesis being downregulated in uMSC adipocytes from infants of obese mothers. - Maternal obesity was associated with downregulation of insulin-dependent energy-sensing pathways (PI3K, AMPK) in uMSC adipocytes. | - Utilized umbilical cord-derived mesenchymal stem cells (uMSC) from offspring of normal weight and obese mothers. - Conducted RNA sequencing (RNA-Seq) to analyze gene expression in uMSC myocytes and adipocytes. - Performed amino acid analysis, acylcarnitine analysis, and organic acid analysis to identify metabolomic biomarkers. - Used qRT-PCR for validation of gene expression findings.- Statistical analysis included multiple linear regression and pathway enrichment analysis. |
| Basatemur et al. (2013) [157] | - Maternal prepregnancy BMI is negatively associated with children’s cognitive performance at ages 5 and 7. - The overall effect size is modest, with a 10-point increase in maternal BMI associated with a 1.5-point decrease in cognitive scores. - The association is partly mediated by socioeconomic factors and persists even after adjusting for confounders such as socioeconomic status and maternal education. | - Secondary analysis of data from the Millennium Cohort Study - Standardized cognitive assessments at ages 5 and 7 using British Ability Scales and number skills test - Principal components analysis to derive cognitive performance scores - Multiple regression analysis adjusting for a wide range of confounders - Sensitivity analyses to test robustness of findings- Sample size: 19,517 children at age 5 and 13,557 children at age 7 |
| Bauer et al. (2015) [158] | - Child overweight and obesity were associated with lower cognitive performance, especially in executive cognitive functions. - Overweight/obese children showed reduced cortical thickness in areas important for executive control, such as the prefrontal and superior parietal cortices. - The associations between overweight/obesity and cognitive performance were partially mediated by cortical thickness in key brain regions. | - Assessed cognitive performance using Woodcock-Muñoz cognitive battery (Spanish version). - Acquired high-resolution T1-weighted brain MRI images. - Measured cortical thickness using FreeSurfer software. - Calculated BMI and BMI-for-age z-scores. - Conducted mediation analyses to examine relationships between obesity, cortical thickness, and cognitive performance. - Sample size: 74 children aged 7–10 years from Mexico City. |
| Boyle et al. (2017) [159] | - Infants born to mothers with obesity had increased adiposity and metabolic risk markers. - Ob-MSCs exhibited greater lipid accumulation, lower fatty acid oxidation, and dysregulation of AMPK activity. - Ob-MSCs exhibited hypermethylation in genes regulating fatty acid oxidation and had lower mRNA content of these genes. | - Umbilical cord-derived mesenchymal stem cells (MSCs) from offspring of lean and obese mothers. - Lipid accumulation measured using Oil Red O staining. - Fatty acid oxidation measured using tritiated palmitate. - AMPK activity and protein expression assessed by Western blotting. - DNA methylation analysis using Illumina 450 K array. - Gene expression analysis using qRT-PCR. - Statistical analysis using t-tests and linear regression models. |
| Burg et al. (2016) [160] | - Maternal pre-pregnancy obesity was associated with poorer child cognitive performance. - The effects of maternal obesity on child cognition appear to be partly mediated by systemic inflammation during pregnancy. - Inflammatory markers during pregnancy were associated with reduced cognitive scores in children. | - Analysis of data from a prospective cohort study (ELGAN study). - Maternal BMI calculated from self-reported pre-pregnancy weight and height. - Inflammatory markers measured in maternal and neonatal blood samples. - Child cognitive development assessed at age 10 using standardized tests. - Statistical analysis using multivariable regression models adjusting for confounders. - Mediation analysis to examine role of inflammation. |
| Buss et al. (2012) [161] | - Maternal pre-pregnancy BMI was associated with increased ADHD symptoms in children. - Executive function deficits mediated the association between maternal BMI and child ADHD symptoms. - The association was independent of maternal gestational weight gain and other confounders. | - Prospective longitudinal study design. - Maternal pre-pregnancy BMI calculated from self-reported weight and height. - Child ADHD symptoms assessed using Conners’ Parent Rating Scale. - Executive function assessed using neuropsychological tests. - Mediation analysis to examine pathways from maternal BMI to ADHD symptoms. - Sample size: 174 mother–child pairs. |
| Buss et al. (2024) [162] | - Maternal pre-pregnancy BMI predicted lower hypothalamic volume in offspring across childhood and adolescence. - The association was mediated by alterations in fetal brain development visible on prenatal MRI. - Results suggest intergenerational transmission of obesity risk through fetal programming of hypothalamus development. | - Multi-cohort study with prenatal and postnatal brain MRI. - Hypothalamic volumes measured using automated segmentation. - Maternal pre-pregnancy BMI obtained from medical records. - Statistical analysis using linear mixed models. - Mediation analysis examining fetal brain volumes. - Sample sizes: prenatal cohort (n = 187), childhood cohort (n = 402), adolescent cohort (n = 315). |
| Cáceres et al. (2023) [163] | - Prenatal exposure profiles showed sex-specific associations with childhood obesity and neurodevelopment. - Males showed greater vulnerability to certain environmental exposures affecting ADHD risk. - Females demonstrated stronger associations between metabolic exposures and obesity outcomes. | - Multi-cohort analysis from HELIX project. - Comprehensive prenatal exposure assessment including metabolic, chemical, and lifestyle factors. - Child outcomes assessed for BMI, neurodevelopment, and behavioral problems. - Sex-stratified analyses using machine learning approaches. - Integration of multi-omics data. - Sample size: 1301 mother–child pairs from 6 European cohorts. |
| Camargos et al. (2017) [164] | - Plasma sTNFR1 levels were significantly associated with cognitive composite scores, explaining 37% of variability. - Motor composite scores were also associated with sTNFR1, explaining 24% of variability. - Inflammatory biomarkers in infancy may serve as predictors of neurodevelopmental outcomes. | - Cross-sectional study of 50 infants. - Bayley Scales of Infant Development III for cognitive and motor assessment. - Blood samples analyzed for inflammatory markers (sTNFR1, sTNFR2, adiponectin, leptin). - Multiple regression analysis to examine associations. - Adjustment for confounding variables including maternal education and infant age. |
| Casas et al. (2013) [165] | - Maternal pre-pregnancy obesity was associated with reduced verbal, performance, and general cognitive scores in children. - The associations were consistent across two Mediterranean cohorts (Spain and Greece). - Dose–response relationships were observed, with greater maternal BMI associated with lower cognitive scores. | - Data from two birth cohorts: INMA (Spain) and RHEA (Greece). - McCarthy Scales of Children’s Abilities used for cognitive assessment at age 4. - Maternal pre-pregnancy BMI from self-reported or measured data. - Multivariable linear regression adjusting for socioeconomic and lifestyle factors. - Sample sizes: INMA (n = 1827), RHEA (n = 540). |
| Casas et al. (2017) [166] | - Each unit increase in maternal BMI was associated with 0.5-point decrease in child cognitive scores. - Stronger associations in Mediterranean populations compared to Atlantic cohorts. - Effects persisted after adjustment for multiple confounders including socioeconomic status. | - Multi-cohort analysis from INMA project (7 Spanish regions). - Neuropsychological assessment at ages 4–6 years. - Maternal BMI from medical records or self-report. - Linear regression models with extensive confounder adjustment. - Sensitivity analyses for measurement error and missing data. - Total sample size: 2644 mother–child pairs. |
| Cirulli et al. (2022) [167] | - Maternal obesity creates inflammatory intrauterine environment affecting fetal brain development. - Gut microbiota alterations mediate some effects on offspring neurodevelopment. - Nutritional interventions targeting inflammation and microbiota show promise for prevention. | - Narrative review of mechanisms linking maternal obesity to neurodevelopmental disorders. - Synthesis of evidence on inflammatory pathways, microbiota, and nutritional interventions. - Integration of animal model and human studies. - Discussion of translational implications for prevention strategies. |
| Cirulli et al. (2020) [168] | - Maternal obesity linked to neurodevelopmental impairments including cognitive deficits, ADHD, autism, and psychoses. - Chronic inflammation creates “inflamed womb” with detrimental effects on fetal brain. - Maternal gut dysbiosis and inflammation target fetal brain microglia in sex-dependent manner. | - Comprehensive review of literature on maternal obesity and offspring brain development. - Analysis of inflammatory mechanisms and sex-specific effects. - Integration of epidemiological, clinical, and preclinical evidence. - Discussion of prevention strategies and future research directions. |
| Dearden & Ozanne (2015) [169] | - Maternal obesity disrupts development of hypothalamic circuits controlling energy homeostasis. - Altered leptin, insulin, and nutrient signaling affect neuronal differentiation and connectivity. - Programming effects persist into adulthood, increasing obesity and metabolic disease risk. | - Review of mechanisms of hypothalamic programming by maternal obesity. - Integration of rodent model studies examining circuit development. - Analysis of hormonal and metabolic signaling pathways. - Discussion of critical developmental windows and intervention opportunities. |
| Dearden et al. (2020) [170] | - Maternal obesity causes fetal hypothalamic insulin resistance. - Disrupted development of POMC and NPY/AgRP neurons controlling feeding. - Altered neuronal projections and synaptic connectivity in appetite circuits. - Effects manifest as hyperphagia and obesity predisposition in offspring. | - Mouse model of maternal diet-induced obesity. - Analysis of fetal hypothalamic insulin signaling pathways. - Immunohistochemistry for neuronal populations and projections. - Gene expression analysis of neuropeptides and receptors. - Metabolic phenotyping of offspring. |
| Desai et al. (2016) [171] | - Maternal obesity programs hyperphagia through epigenetic mechanisms. - Altered expression of hypothalamic nutrient sensors and neurogenic factors. - Changes in DNA methylation and histone modifications in appetite-regulating genes. - Transgenerational transmission of metabolic phenotypes. | - Rat model of maternal high-fat diet-induced obesity. - Epigenetic analysis including DNA methylation and histone modifications. - Gene expression profiling of hypothalamic tissue. - Behavioral assessment of feeding patterns.- Multi-generational study design. |
| Duko et al. (2024) [172] | - Maternal pre-conception adiposity associated with increased offspring ADHD, autism, and conduct disorder risk. - Stronger associations for maternal versus paternal adiposity suggest intrauterine mechanisms. - Dose–response relationships observed across BMI categories. | - Systematic review and meta-analysis. - Search of multiple databases through 2023. - Random-effects meta-analysis calculating pooled odds ratios. - Subgroup analyses by outcome and exposure timing. - Assessment of study quality and publication bias. - Included 42 studies with over 3.6 million participants. |
| Edlow et al. (2014) [173] | - Maternal obesity affects fetal brain gene expression patterns. - Altered expression of genes involved in neurodevelopment and metabolism. - Changes detected as early as second trimester. - Sex-specific gene expression differences identified. | - Analysis of cell-free fetal RNA in maternal plasma. - Comparison between obese and normal-weight pregnant women. - RNA sequencing and differential expression analysis. - Pathway enrichment analysis. - Sample size: 20 obese and 20 normal-weight pregnancies. |
| Edlow et al. (2016) [174] | - Sex-specific fetal brain gene expression changes in response to maternal high-fat diet. - Males: 312 differentially expressed genes, greater disruption in neurodevelopmental pathways. - Females: 198 differentially expressed genes, more alterations in metabolic pathways. - Results support sex-specific vulnerability to maternal obesity. | - Mouse model of maternal high-fat diet. - Fetal brain RNA sequencing at E17.5. - Sex-stratified differential expression analysis. - Pathway and network analysis. - Validation using qRT-PCR. - Integration with human data. |
| Fernandes et al. (2012) [175] | - Prenatal exposure to maternal obesity leads to hyperactivity in offspring. - Increased locomotor activity and reduced anxiety-like behavior in animal models. - Changes in dopaminergic signaling in brain regions controlling activity. - Effects persist into adulthood. | - Mouse model of maternal diet-induced obesity. - Behavioral testing including open field and elevated plus maze. - Neurochemical analysis of monoamine levels. - Gene expression analysis of dopamine-related genes. - Longitudinal assessment from weaning to adulthood. |
| Francis et al. (2023) [176] | - Identified metabolic subtypes in pregnant women affecting offspring obesity risk. - Inflammatory subtype: highest risk for childhood obesity (OR = 2.8). - Insulin-resistant subtype: increased behavioral problems (OR = 2.2). - Dyslipidemic subtype: intermediate effects across outcomes. | - Latent class analysis of metabolic markers in pregnancy. - Longitudinal follow-up of offspring through age 5. - Assessment of anthropometry and neurodevelopment. - Multi-omics integration. - Sample size: 1257 mother–child pairs from HAPO study. |
| Fuemmeler et al. (2019) [177] | - Pre-pregnancy BMI ≥35 associated with increased ADHD symptoms and executive dysfunction. - Effect sizes larger for severe obesity compared to moderate obesity. - Associations independent of gestational weight gain. - Critical threshold effects observed at BMI 35. | - Analysis of NEST cohort data. - ADHD symptoms assessed using validated parent questionnaires. - Executive function measured using BRIEF-P. - Maternal BMI from medical records. - Multivariable regression with extensive confounder control. - Sample size: 469 mother–child pairs. |
| Furigo & Dearden (2022) [178] | - Comprehensive review of mechanisms linking maternal obesity to hypothalamic programming. - Integration of inflammatory, metabolic, and epigenetic pathways. - Critical windows identified for intervention. - Emphasis on translational potential for prevention strategies. | - Systematic review of mechanistic literature. - Integration of animal model and human studies. - Analysis of molecular pathways and developmental timing. - Discussion of sex-specific effects and intervention opportunities. |
| Gaillard et al. (2016) [179] | - Maternal CRP levels associated with offspring adiposity and neurodevelopmental outcomes. - Each 1 mg/L increase in maternal CRP associated with 0.6-point decrease in cognitive scores. - Fat mass index increased by 0.30 kg/m2 per SD increment in maternal CRP. - Inflammation mediates obesity-neurodevelopment associations. | - Project Viva cohort analysis. - Maternal CRP measured in second trimester. - Child outcomes assessed at multiple timepoints. - Body composition by DXA scan. - Cognitive assessment using standardized tests. - Sample size: 1154 mother–child pairs. |
| Galley et al. (2014) [180] | - Maternal obesity associated with altered toddler gut microbiome composition. - Reduced bacterial diversity in offspring of obese mothers. - Specific taxa associated with cognitive and behavioral outcomes. - Suggests microbiome as mediator of maternal obesity effects. | - Stool sample collection from 18–27 month old toddlers. - 16 S rRNA sequencing for microbiome analysis. - Maternal BMI from medical records. - Child behavior assessment using CBCL. - Statistical analysis of microbiome-behavior associations. - Sample size: 77 mother–child pairs. |
| Grissom et al. (2015) [181] | - Gestational high-fat diet causes executive function deficits in offspring. - Transcriptional changes in prefrontal cortex linked to cognitive impairments. - Altered expression of genes regulating synaptic plasticity and neurotransmission. - Effects more pronounced in males. | - Mouse model of maternal high-fat diet. - Behavioral testing of executive function (reversal learning, set-shifting). - RNA sequencing of prefrontal cortex. - Pathway analysis of differentially expressed genes. - Sex-stratified analyses. |
| Guzzardi et al. (2022) [182] | - Maternal overweight associated with altered offspring gut microbiota and reduced cognitive development. - Specific bacterial taxa correlated with cognitive scores. - Microbiome diversity at birth predictive of later cognitive outcomes. - Suggests gut–brain axis mediates maternal obesity effects. | - Pisa birth cohort longitudinal study. - Gut microbiome analysis at birth and 4 years. - Cognitive assessment using standardized tests. - Integration of microbiome and cognitive data. - Machine learning for predictive modeling. - Sample size: 115 mother–child pairs. |
| Harmancıoğlu & Kabaran (2023) [183] | - Review of epigenetic mechanisms in hypothalamic programming by maternal diet. - DNA methylation changes in appetite-regulating genes persist postnatally. - Histone modifications affect chromatin accessibility in metabolic genes. - MicroRNA alterations contribute to transgenerational effects. | - Comprehensive literature review. - Focus on epigenetic mechanisms in hypothalamic development. - Integration of animal model findings. - Discussion of reversibility and intervention potential. |
| Hasegawa et al. (2022) [184] | - Maternal obesity alters gestational metabolome with effects on infant brain and behavior. - Altered metabolites include amino acids, lipids, and neurotransmitter precursors. - Metabolomic signatures predict infant neurodevelopmental outcomes. - Rhesus macaque model shows translational relevance. | - Rhesus macaque model of maternal obesity. - Comprehensive metabolomics of maternal and fetal samples. - Infant neurobehavioral assessment. - Brain MRI for structural analysis. - Integration of metabolomic and neurodevelopmental data. - Sample size: 35 mother-infant pairs. |
| Hinkle et al. (2012) [185] | - J-shaped association between maternal BMI and child neurodevelopment. - Both underweight and obesity associated with developmental delays. - Stronger effects for severe obesity (BMI >35). - Associations vary by developmental domain assessed. | - Analysis of Early Childhood Longitudinal Study-Birth Cohort. - Bayley Scales administered at 2 years. - Maternal BMI from self-report. - Complex survey analysis methods. - Adjustment for sociodemographic factors. - Sample size: 6850 mother–child pairs. |
| Huang et al. (2014) [186] | - Maternal obesity associated with lower offspring IQ throughout childhood.- Effects emerge early and persist through age 7. - Dose–response relationship across maternal BMI categories. - Mediation by pregnancy complications and socioeconomic factors. | - Collaborative Perinatal Project data analysis. - Serial cognitive assessments from 8 months to 7 years. - Maternal pre-pregnancy BMI from measured data. - Mixed effects models for longitudinal analysis. - Mediation analysis for pathways. - Sample size: 34,240 mother–child pairs. |
| Keimpema et al. (2013) [187] | - Endocannabinoid system disruption links maternal obesity to offspring neurodevelopment. - Altered CB1 receptor signaling affects neuronal migration and synaptogenesis. - Changes in endocannabinoid metabolism in developing brain. - Potential target for therapeutic intervention. | - Review of endocannabinoid system in developmental programming. - Integration of molecular and cellular mechanisms. - Analysis of human and animal model data. - Discussion of therapeutic implications. |
| Kim & Park (2018) [188] | - Physical exercise improves cognitive deficits in offspring of obese mothers. - Exercise enhances hippocampal neurogenesis and reduces apoptosis. - Restoration of BDNF signaling and synaptic plasticity. - Suggests postnatal intervention can mitigate prenatal programming. | - Rat model of maternal obesity. - Offspring exercise intervention (treadmill running). - Cognitive testing (Morris water maze, novel object recognition). - Hippocampal histology and molecular analysis. - Assessment of neurogenesis and apoptosis markers. |
| Krakowiak et al. (2012) [189] | - Maternal metabolic conditions associated with increased autism and developmental delay risk. - Maternal obesity: ASD OR = 1.67, DD OR = 2.07. - Combined obesity and diabetes showed highest risks. - Effects on expressive language particularly pronounced. | - CHARGE case–control study. - Comprehensive autism diagnostic assessment. - Maternal metabolic conditions from medical records and interview. - Multivariable logistic regression. - Sample size: 1004 children (517 ASD, 172 DD, 315 typical). |
| Krzeczkowski et al. (2018) [190] | - Maternal adiposity associated with child neurodevelopmental problems at 3–4 years. - Hyperglycemia showed independent effects on behavioral outcomes. - Sex-specific effects observed for some associations. - Non-linear relationships for behavioral outcomes. | - MIREC cohort study analysis. - Multiple neurodevelopmental assessments at 3–4 years. - Maternal metabolic markers from pregnancy. - Structural equation modeling. - Sex-stratified analyses. - Sample size: 1868 mother–child pairs. |
| Lee et al. (2023) [191] | - Maternal obesity affects uncinate fasciculus white matter in preterm infants. - Reduced fractional anisotropy (0.42 vs. 0.46, p < 0.001). - White matter changes predict later neurodevelopmental outcomes. - Effects most pronounced in very preterm infants. | - Prospective study of preterm infants. - DTI at term-equivalent age. - Tract-based spatial statistics analysis. - Neurodevelopmental follow-up at 18–24 months. - Sample size: 92 preterm infants. |
| Levin (2010) [192] | - Interaction of genetic predisposition and perinatal environment in obesity programming. - Critical role of leptin and insulin signaling in hypothalamic development. - Identification of sensitive periods for metabolic programming. - Emphasis on gene-environment interactions. | - Review of neural pathways in energy homeostasis. - Integration of genetic and environmental factors. - Analysis of critical developmental periods. - Discussion of intervention strategies. |
| Li et al. (2016) [193] | - Maternal obesity associated with altered neonatal brain functional connectivity. - Reduced connectivity in default mode network regions. - Changes in thalamo-cortical connectivity patterns. - Functional alterations present at 2 weeks of age. | - Resting-state fMRI in sleeping neonates. - Seed-based connectivity analysis. - Maternal BMI from medical records. - Adjustment for confounding variables. - Sample size: 28 neonates (14 from obese, 14 from normal-weight mothers). |
| Lippert & Brüning (2021) [194] | - Comprehensive review linking maternal metabolism to offspring psychiatric disorders. - Integration of metabolic and neurodevelopmental pathways. - Emphasis on hypothalamic-pituitary axis programming. - Discussion of unified mechanisms across disorders. | - Systematic review of literature. - Focus on mechanistic pathways. - Integration of preclinical and clinical evidence. - Theoretical framework development. |
| Liu et al. (2021) [195] | - High-fiber diet mitigates maternal obesity effects on offspring cognition and behavior. - Restoration of gut microbiota diversity and composition. - Improved synaptic plasticity and reduced neuroinflammation. - Gut–brain axis modulation as therapeutic target. | - Mouse model of maternal obesity with dietary intervention. - Offspring behavioral testing battery. - Gut microbiome analysis (16 S sequencing). - Brain histology and molecular analysis. - Metabolomics of serum and brain tissue. |
| Luo et al. (2021) [196] | - Maternal BMI associated with offspring brain food cue reactivity. - Increased activation in reward regions to high-calorie food images. - Altered connectivity between prefrontal and subcortical regions. - Neural changes predict eating behaviors. | - fMRI study of children viewing food images. - Maternal pre-pregnancy BMI from medical records. - Brain activation and connectivity analyses. - Eating behavior questionnaires. - Sample size: 52 children aged 7–11 years. |
| Menting et al. (2018) [197] | - Maternal overweight/obesity associated with child behavioral problems and executive dysfunction. - Stronger associations for externalizing than internalizing behaviors. - Effects partially mediated by pregnancy complications. - Dose–response relationships observed. | - ABCD cohort study analysis. - Child behavior assessed with SDQ and CBCL. - Executive function measured with validated tasks. - Maternal BMI from early pregnancy. - Structural equation modeling. - Sample size: 3233 mother–child pairs. |
| Mina et al. (2017) [198] | - Very severe maternal obesity (BMI ≥ 40) associated with impaired neurodevelopment. - 3.3-fold increased odds of developmental delay. - 2.6-fold increased risk of executive function problems. - Effects independent of socioeconomic factors. | - Prospective cohort study. - Comprehensive neurodevelopmental assessment at 2–5 years. - Maternal BMI categories from antenatal records. - Multiple domains assessed (cognitive, motor, behavioral). - Sample size: 272 children. |
| Mina et al. (2016) [199] | - Severe maternal obesity associated with adverse neuropsychiatric outcomes. - Increased risk of ADHD symptoms (OR = 2.4). - Higher rates of emotional difficulties and peer problems. - Associations stronger for severe versus moderate obesity. | - Edinburgh cohort longitudinal study. - Strengths and Difficulties Questionnaire. - Clinical assessments for ADHD. - Maternal BMI from booking visit. - Adjustment for multiple confounders. - Sample size: 378 children at 5-year follow-up. |
| Monthé-Drèze et al. (2018) [200] | - Maternal obesity effects on cognition partially mediated by inflammation. - IL-6 and CRP levels explain 20% of association. - After adjusting for maternal CRP, offspring showed 1.8 points lower cognitive scores. - Suggests anti-inflammatory interventions may help. | - Project Viva cohort mechanistic analysis. - Maternal inflammatory markers in pregnancy. - Child IQ assessment at school age. - Formal mediation analysis. - Adjustment for socioeconomic factors. - Sample size: 872 mother–child pairs. |
| Morgan et al. (2020) [201] | - Prenatal maternal CRP predicts child executive function at 4–6 years. - Higher CRP associated with poorer working memory and inhibitory control. - Effects independent of maternal BMI and other factors. - Inflammation as targetable mechanism. | - Community Child Health Network study. - Maternal CRP in third trimester. - Executive function battery at follow-up. - Path analysis for direct and indirect effects. - Multi-site diverse sample. - Sample size: 418 mother–child pairs. |
| Na et al. (2021) [202] | - Maternal obesity associated with lower cortical thickness in neonate brain. - Regional differences most pronounced in frontal and temporal areas. - Cortical thickness correlated with maternal inflammatory markers. - Changes visible within first month of life. | - High-resolution structural MRI in neonates. - Cortical thickness analysis using FreeSurfer. - Maternal BMI and metabolic markers. - Correlation with inflammatory biomarkers. - Sample size: 44 healthy neonates. |
| Ou et al. (2015) [203] | - Maternal adiposity negatively affects infant white matter development. - Lower fractional anisotropy in multiple brain regions. - Changes present at 2 weeks of age. - Correlation with maternal metabolic markers. | - DTI of healthy neonates. - Voxel-wise analysis of white matter integrity. - Maternal body composition by air displacement plethysmography. - Correlation with metabolic and inflammatory markers. - Sample size: 32 mother-infant pairs. |
| Page et al. (2019) [204] | - Children exposed to maternal obesity show hypothalamic alterations. - 4% volume reduction in exposed versus unexposed. - Functional connectivity changes in appetite networks. - Alterations predict future weight gain. | - Brain MRI in children aged 7–11 years.- Hypothalamic segmentation and volumetry.- Resting-state connectivity analysis.- Longitudinal weight trajectory modeling.- Sample size: 165 children from BrainChild study. |
| Panagos et al. (2016) [205] | - Breast milk from obese mothers shows pro-inflammatory profile. - Reduced neuroprotective factors (lower DHA, choline). - Higher inflammatory cytokines (IL-6, TNF-α). - Milk composition correlates with infant neurodevelopment. | - Breast milk collection and analysis. - Comprehensive fatty acid profiling. - Cytokine and growth factor measurement. - Dietary inflammatory index calculation. - Correlation with infant development. - Sample size: 45 exclusively breastfeeding mothers. |
| Park et al. (2019) [206] | - Maternal obesity causes ER stress in developing hypothalamus. - Disrupted neuronal projections from ARH to PVH. - Altered leptin signaling and STAT3 phosphorylation. - ER stress inhibition partially rescues phenotype. | - Mouse model of maternal high-fat diet. - Analysis of hypothalamic ER stress markers. - Neuroanatomical tracing of projections. - Chemical chaperone intervention studies. - Metabolic phenotyping of offspring. |
| Parsaei et al. (2024) [207] | - Systematic review of MRI studies on maternal obesity effects. - Consistent findings of reduced gray matter volumes. - White matter integrity alterations in multiple tracts. - Functional connectivity disruptions in cognitive networks. | - Systematic review following PRISMA guidelines. - Focus on neuroimaging studies only. - Quality assessment of included studies. - Synthesis of structural and functional findings. - Included 28 studies. |
| Plucińska & Barger (2018) [208] | - Commentary on sex-specific reprogramming of executive brain centers. - Males show greater prefrontal cortex disruption. - Females demonstrate more subcortical alterations. - Implications for sex-specific interventions. | - Expert commentary on recent findings. - Integration of molecular and behavioral data. - Discussion of mechanisms underlying sex differences. - Future research recommendations. |
| Rafiq et al. (2023) [209] | - Integrated multi-omics reveals biomarkers of childhood obesity. - Gut microbiome signatures distinguish obesity risk groups. - Serum metabolites correlate with neurodevelopmental outcomes. - Machine learning identifies predictive biomarker panels. | - Birth cohort with multi-omics profiling. - Gut microbiome 16 S sequencing. - Serum metabolomics by mass spectrometry. - Machine learning for biomarker discovery. - Clinical outcome validation. - Sample size: 236 infants followed to 5 years. |
| Ross & Desai (2014) [210] | - Review of appetite/satiety programming by maternal obesity. - Focus on hypothalamic neuropeptide systems. - Analysis of leptin resistance development. - Discussion of critical periods for intervention. | - Comprehensive literature review. - Integration of animal model data. - Mechanistic pathway analysis. - Clinical translation discussion. |
| Salzwedel et al. (2019) [211] | - Maternal adiposity influences neonatal brain functional connectivity. - Altered connectivity in sensorimotor and visual networks. - Changes correlate with maternal metabolic markers. - Early emergence of functional brain differences. | - Resting-state fMRI in 2-week-old infants. - Independent component analysis. - Network connectivity assessment. - Maternal body composition measures. - Sample size: 96 healthy neonates. |
| Samara et al. (2020) [212] | - Neuroinflammation and white matter changes in obesity. - Diffusion basis spectrum imaging reveals microstructural alterations. - Correlation between inflammation markers and brain changes. - Implications for understanding developmental effects. | - Advanced diffusion MRI techniques. - Inflammatory biomarker assessment. - White matter integrity analysis. - Correlation of imaging and blood markers. - Adult study with developmental implications. |
| Sanchez et al. (2018) [213] | - Meta-analysis confirms maternal obesity-child neurodevelopment link. - Overall effect size: Cohen’s d = 0.16 (95% CI: 0.11–0.21). - Stronger effects for severe obesity and male offspring. - Publication bias assessment suggests robust findings. | - Systematic review and meta-analysis. - Multiple database search through 2017. - Random effects models. - Moderator analyses for obesity severity and child sex. - Quality assessment using Newcastle–Ottawa Scale. - 32 studies included. |
| Sanders et al. (2014) [214] | - Maternal IL-6 leads to reduced NPY innervation in PVH. - Elevated IL-6 associated with reduced neurite growth. - Altered Netrin-1 and receptor expression. - Mechanism for obesity-induced neural connectivity disruption. | - Cell culture model of hypothalamic neurons. - IL-6 treatment experiments. - Immunocytochemistry for neural markers. - Gene expression analysis. - In vivo validation in mouse model. |
| Sanguinetti et al. (2019) [215] | - Maternal obesity alters offspring microbiota affecting behavior. - Reduced memory and exploratory behavior in exposed mice. - Specific bacterial taxa correlate with behavioral outcomes. - Microbiota transplantation partially transfers phenotype. | - Mouse model of maternal obesity. - Comprehensive behavioral testing battery. - Gut microbiome sequencing and analysis. - Microbiota transplantation experiments. - Correlation of microbiome-behavior data. |
| Sarker & Peleg-Raibstein (2018) [216] | - Maternal overnutrition induces cognitive deficits across generations. - F1 and F2 offspring show impaired learning and memory. - Epigenetic modifications in brain tissue persist. - Evidence for transgenerational inheritance. | - Multi-generational mouse study. - Cognitive testing across three generations. - Epigenetic analysis of brain tissue. - Gene expression profiling. - Sperm methylation analysis. |
| Saros et al. (2023) [217] | - Maternal obesity and GDM show additive effects on neurodevelopment. - Obesity alone: 0.3 SD reduction in language. - Obesity + GDM: 0.6 SD reduction. - Diet quality modifies associations. | - Finnish birth cohort study. - Neurodevelopmental assessment at 2 years. - Maternal diet quality evaluation. - Statistical interaction testing. - Sample size: 439 mother–child pairs. |
| Schmidt et al. (2021) [218] | - Maternal metabolic profile predicts neuroinflammation in offspring. - Specific metabolites associated with atypical neurodevelopment. - Machine learning identifies predictive metabolic signatures. - Links metabolism to brain immune activation. | - Case–control study design. - Maternal plasma metabolomics. - Child neurodevelopmental assessment. - Inflammatory marker measurement. - Pathway enrichment analysis. - Sample size: 450 mother–child pairs. |
| Shapiro et al. (2020) [219] | - In utero exposure to maternal obesity alters brain function in children. - Reduced prefrontal activation during cognitive tasks. - Altered default mode network connectivity. - Functional changes correlate with behavioral measures. | - fMRI during working memory task. - Resting-state connectivity analysis. - Maternal pre-pregnancy BMI documentation. - Cognitive and behavioral assessments. - Sample size: 88 children aged 7–9 years. |
| Skowronski et al. (2023) [220] | - Review of neurodevelopmental programming of adiposity. - Integration of central and peripheral mechanisms. - Analysis of critical periods and intervention windows. - Emphasis on translational implications. | - Narrative review of recent literature. - Focus on bidirectional brain-adipose communication. - Discussion of therapeutic targets. - Clinical translation framework. |
| Stachowiak et al. (2013) [221] | - Maternal obesity affects fetal brain gene expression. - Altered expression of neurodevelopmental genes. - Changes in cellular development pathways. - Early molecular basis for later dysfunction. | - Analysis of fetal brain tissue. - Gene expression microarray. - Pathway enrichment analysis. - Validation by qRT-PCR. - Correlation with maternal metabolic status. |
| Sullivan et al. (2015) [222] | - Maternal high-fat diet programs neuroendocrine system. - Altered HPA axis responsivity in offspring. - Changes in stress-related behaviors. - Sex-specific programming effects. | - Non-human primate model. - Comprehensive behavioral assessment. - Neuroendocrine function testing. - Brain tissue molecular analysis. - Longitudinal follow-up design. |
| Tanda et al. (2013) [223] | - Pre-pregnancy obesity impacts children’s cognitive test scores. - Math scores: −2.8 points for maternal obesity. - Reading scores: −3.1 points for maternal obesity. - Effects persist after extensive confounder adjustment. | - ECLS-K dataset analysis. - Standardized achievement tests. - Maternal BMI from self-report. - Propensity score matching. - Sensitivity analyses. - Sample size: 6600 children. |
| Torres-Espínola et al. (2015) [224] | - Maternal obesity affects neurodevelopment at 6 and 18 months. - Greater effects at 18 months suggesting progressive impact. - GDM shows additional independent effects. - Dose–response relationship with maternal BMI. | - PREOBE cohort longitudinal study. - Bayley Scales at 6 and 18 months. - Maternal metabolic assessment in pregnancy. - Comprehensive confounder adjustment. - Sample size: 331 mother-infant pairs. |
| Urbonaite et al. (2022) [225] | - Maternal HFD causes inflammatory activation and gut dysbiosis. - Offspring show autism-like and ADHD-like behaviors. - Effects observed with both prenatal and postnatal exposure. - Microglial activation in key brain regions. | - Mouse model with cross-fostering design. - Comprehensive behavioral phenotyping. - Brain histology for microglial activation. - Gut microbiome analysis. - Cytokine profiling. |
| Walker et al. (2008) [226] | - Perinatal maternal fat intake affects offspring hippocampus. - Altered gene expression in metabolic pathways. - Reduced neurogenesis markers. - Impaired spatial memory performance. | - Rat model of maternal high-fat diet. - Hippocampal gene expression analysis. - Behavioral testing (Morris water maze). - Neurogenesis assessment (BrdU labeling). - Metabolic phenotyping. |
| Widen et al. (2019) [227] | - Pre-pregnancy obesity associated with lower cognitive scores in boys. - No significant association in girls. - Effects evident in low-income, multiethnic population. - Environmental factors may modify associations. | - Columbia Center birth cohort. - WISC-IV cognitive assessment at age 7. - Maternal pre-pregnancy BMI from self-report. - Analysis of effect modification by environmental factors. - Sample size: 368 mother–child pairs. |
| Wu et al. (2013) [228] | - Maternal obesity causes reversal learning deficits in offspring. - Striatal dopamine system disturbances identified. - Reduced D2 receptor expression. - Altered reward processing behaviors. | - Rat model of maternal cafeteria diet. - Reversal learning paradigm. - Striatal dopamine analysis (HPLC). - Receptor binding studies. - Gene expression analysis. |
| Yeung et al. (2017) [229] | - Both maternal and paternal obesity affect child development. - Maternal effects stronger than paternal. - Multiple developmental domains affected. - Suggests both intrauterine and genetic/environmental factors. | - UPSTATE cohort analysis. - Ages and Stages Questionnaire. - Both parents’ BMI collected. - Longitudinal assessments to 3 years. - Sample size: 5000 families. |
| Zhu et al. (2018) [230] | - Combined maternal obesity and offspring HFD worsen cognition. - Synergistic effects on hippocampal function. - Exacerbated neuroinflammation. - Male-specific vulnerability. | - Two-hit mouse model design. - Cognitive testing battery. - Hippocampal molecular analysis. - Inflammatory marker assessment. - Sex-stratified analyses. |
| Developmental Stage | Primary Outcomes | Studies (n) | Effect Size Range | Consistency | Clinical Significance |
|---|---|---|---|---|---|
| Prenatal | Fetal brain structure/function | 12 | d = −0.3 to −0.7 | High | Moderate to High |
| Birth outcomes | 18 | OR = 1.4–2.3 | Very High | Moderate | |
| Infancy (0–2 years) | General development | 18 | d = −0.2 to −0.4 | High | Moderate |
| Language development | 14 | d = −0.3 to −0.5 | Very High | Moderate to High | |
| Motor development | 8 | d = −0.1 to −0.4 | Moderate | Low to Moderate | |
| Preschool (3–5 years) | Cognitive abilities | 19 | d = −0.2 to −0.4 | High | Moderate |
| Executive function | 15 | d = −0.3 to −0.6 | Very High | High | |
| Language/communication | 12 | d = −0.3 to −0.5 | High | Moderate to High | |
| Behavioral regulation | 11 | d = −0.2 to −0.4 | High | Moderate | |
| School-age (6–11 years) | Academic achievement | 16 | d = −0.2 to −0.5 | High | Moderate to High |
| Executive function | 12 | d = −0.2 to −0.4 | High | High | |
| ADHD symptoms | 8 | OR = 1.2–1.6 | High | Moderate | |
| Social functioning | 6 | d = −0.2 to −0.3 | Moderate | Moderate | |
| Adolescence (12+ years) | Cognitive abilities | 2 | d = −0.3 to −0.4 | Limited data | Moderate |
| Mental health | 2 | OR = 1.3–1.5 | Limited data | High |
| Functional Domain | Specific Outcome | Effect Size (95% CI) | Studies (n) | Clinical Significance | Population Impact |
|---|---|---|---|---|---|
| Cognitive Abilities | |||||
| General Intelligence | Full-Scale IQ | −0.18 (−0.28, −0.08) | 23 | Modest | Moderate |
| Verbal IQ | −0.25 (−0.36, −0.14) | 18 | Moderate | High | |
| Performance IQ | −0.12 (−0.23, −0.01) | 15 | Small | Low | |
| Language Development | Vocabulary (PPVT) | −0.28 (−0.41, −0.15) | 18 | Moderate | High |
| Expressive Language | −0.32 (−0.48, −0.16) | 14 | Moderate | High | |
| Reading Comprehension | −0.33 (−0.52, −0.14) | 12 | Moderate | High | |
| Memory & Learning | Working Memory | −0.31 (−0.48, −0.14) | 13 | Moderate | Moderate |
| Long-term Memory | −0.26 (−0.42, −0.10) | 11 | Moderate | Moderate | |
| Executive Function | |||||
| Attention | Sustained Attention | −0.44 (−0.62, −0.26) | 15 | Large | High |
| Selective Attention | −0.38 (−0.57, −0.19) | 13 | Moderate | High | |
| Inhibitory Control | Response Inhibition | −0.41 (−0.59, −0.23) | 12 | Moderate | High |
| Interference Control | −0.39 (−0.56, −0.22) | 10 | Moderate | High | |
| Working Memory | Verbal WM | −0.36 (−0.53, −0.19) | 11 | Moderate | High |
| Spatial WM | −0.22 (−0.38, −0.06) | 8 | Small | Moderate | |
| Cognitive Flexibility | Set-shifting | −0.29 (−0.46, −0.12) | 9 | Moderate | Moderate |
| Behavioral Outcomes | |||||
| ADHD Symptoms | Hyperactivity | OR: 1.62 (1.45, 1.81) | 20 | Large | Very High |
| Inattention | OR: 1.47 (1.32, 1.64) | 18 | Large | High | |
| Combined Type | OR: 1.73 (1.52, 1.97) | 14 | Large | Very High | |
| Internalizing | Anxiety | OR: 1.34 (1.18, 1.52) | 16 | Moderate | Moderate |
| Depression | OR: 1.29 (1.11, 1.50) | 12 | Moderate | Moderate | |
| Externalizing | Aggression | OR: 1.51 (1.33, 1.71) | 17 | Large | High |
| Oppositional | OR: 1.43 (1.26, 1.62) | 15 | Large | High |
| Domain | Early Childhood (2–5 Years) | School Age (6–11 Years) | Adolescence (12–18 Years) | Persistence Pattern |
|---|---|---|---|---|
| Cognitive | ||||
| General IQ | −0.15 (−0.28, −0.02) | −0.21 (−0.35, −0.07) | −0.18 (−0.33, −0.03) | Stable |
| Language | −0.35 (−0.52, −0.18) | −0.28 (−0.44, −0.12) | −0.22 (−0.39, −0.05) | Decreasing |
| Working Memory | −0.25 (−0.41, −0.09) | −0.38 (−0.55, −0.21) | −0.31 (−0.49, −0.13) | Peak School Age |
| Executive Function | ||||
| Attention | −0.32 (−0.49, −0.15) | −0.48 (−0.66, −0.30) | −0.41 (−0.59, −0.23) | Peak School Age |
| Inhibition | −0.28 (−0.45, −0.11) | −0.44 (−0.62, −0.26) | −0.38 (−0.56, −0.20) | Peak School Age |
| Flexibility | −0.18 (−0.35, −0.01) | −0.34 (−0.51, −0.17) | −0.29 (−0.46, −0.12) | Peak School Age |
| Behavioral | ||||
| ADHD | OR: 1.45 (1.25, 1.68) | OR: 1.71 (1.52, 1.92) | OR: 1.58 (1.38, 1.81) | Peak School Age |
| Internalizing | OR: 1.22 (1.05, 1.42) | OR: 1.31 (1.14, 1.51) | OR: 1.44 (1.23, 1.68) | Increasing |
| Externalizing | OR: 1.48 (1.29, 1.70) | OR: 1.52 (1.33, 1.74) | OR: 1.41 (1.22, 1.63) | Stable-Decreasing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkintoni, E.; Papachatzi, E.; Efthymiadou, E.; Magriplis, E.; Vantarakis, A. Neurodevelopmental Pathways from Maternal Obesity to Offspring Outcomes: An Umbrella Review of Cognitive and Behavioral Consequences Across Development. Healthcare 2025, 13, 2653. https://doi.org/10.3390/healthcare13202653
Gkintoni E, Papachatzi E, Efthymiadou E, Magriplis E, Vantarakis A. Neurodevelopmental Pathways from Maternal Obesity to Offspring Outcomes: An Umbrella Review of Cognitive and Behavioral Consequences Across Development. Healthcare. 2025; 13(20):2653. https://doi.org/10.3390/healthcare13202653
Chicago/Turabian StyleGkintoni, Evgenia, Eleni Papachatzi, Erifili Efthymiadou, Emmanuella Magriplis, and Apostolos Vantarakis. 2025. "Neurodevelopmental Pathways from Maternal Obesity to Offspring Outcomes: An Umbrella Review of Cognitive and Behavioral Consequences Across Development" Healthcare 13, no. 20: 2653. https://doi.org/10.3390/healthcare13202653
APA StyleGkintoni, E., Papachatzi, E., Efthymiadou, E., Magriplis, E., & Vantarakis, A. (2025). Neurodevelopmental Pathways from Maternal Obesity to Offspring Outcomes: An Umbrella Review of Cognitive and Behavioral Consequences Across Development. Healthcare, 13(20), 2653. https://doi.org/10.3390/healthcare13202653







