Inter-Finger Variability of SpO2 During Hypoxemia and Step Resaturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
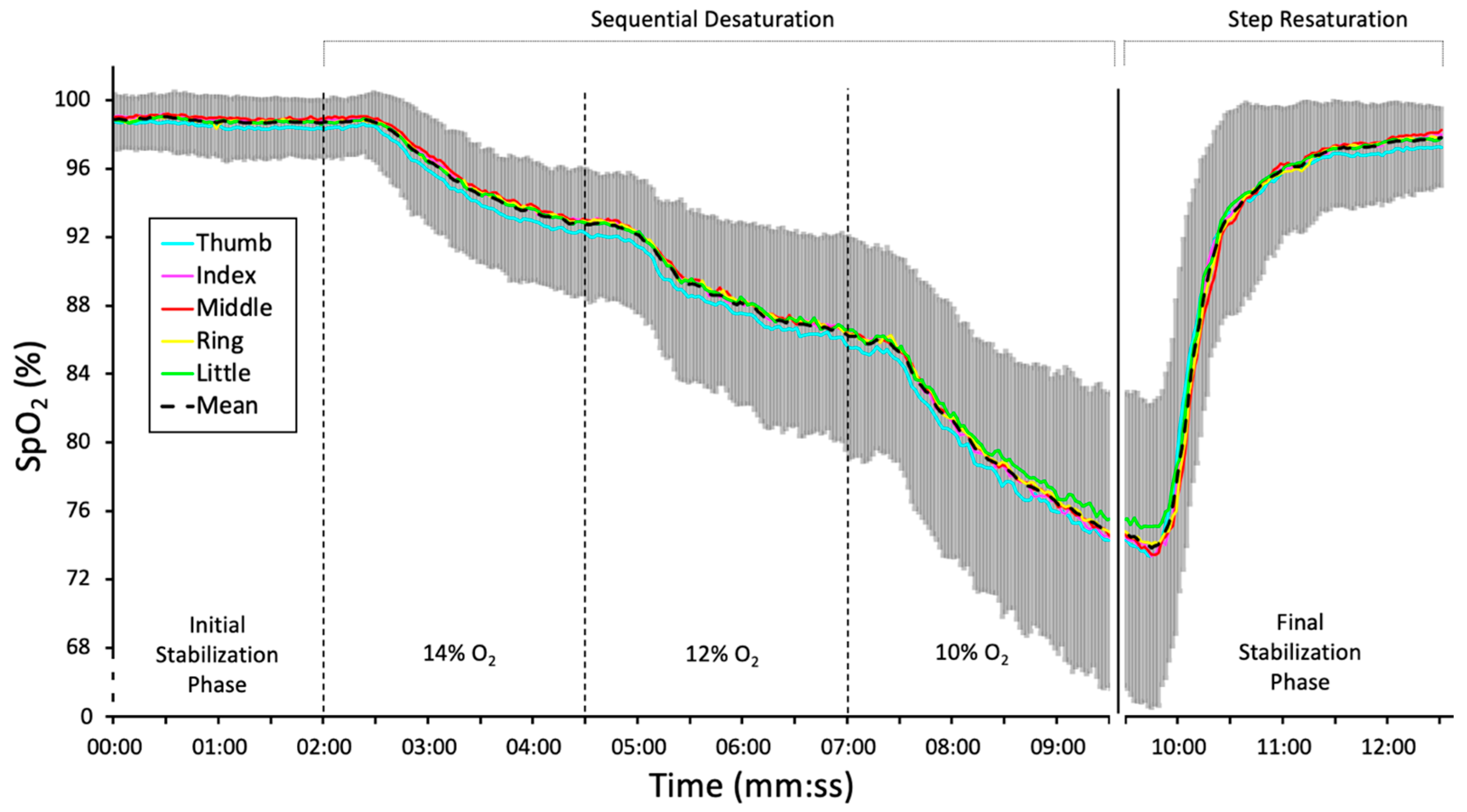
2.2. Experimental Setup and Protocol
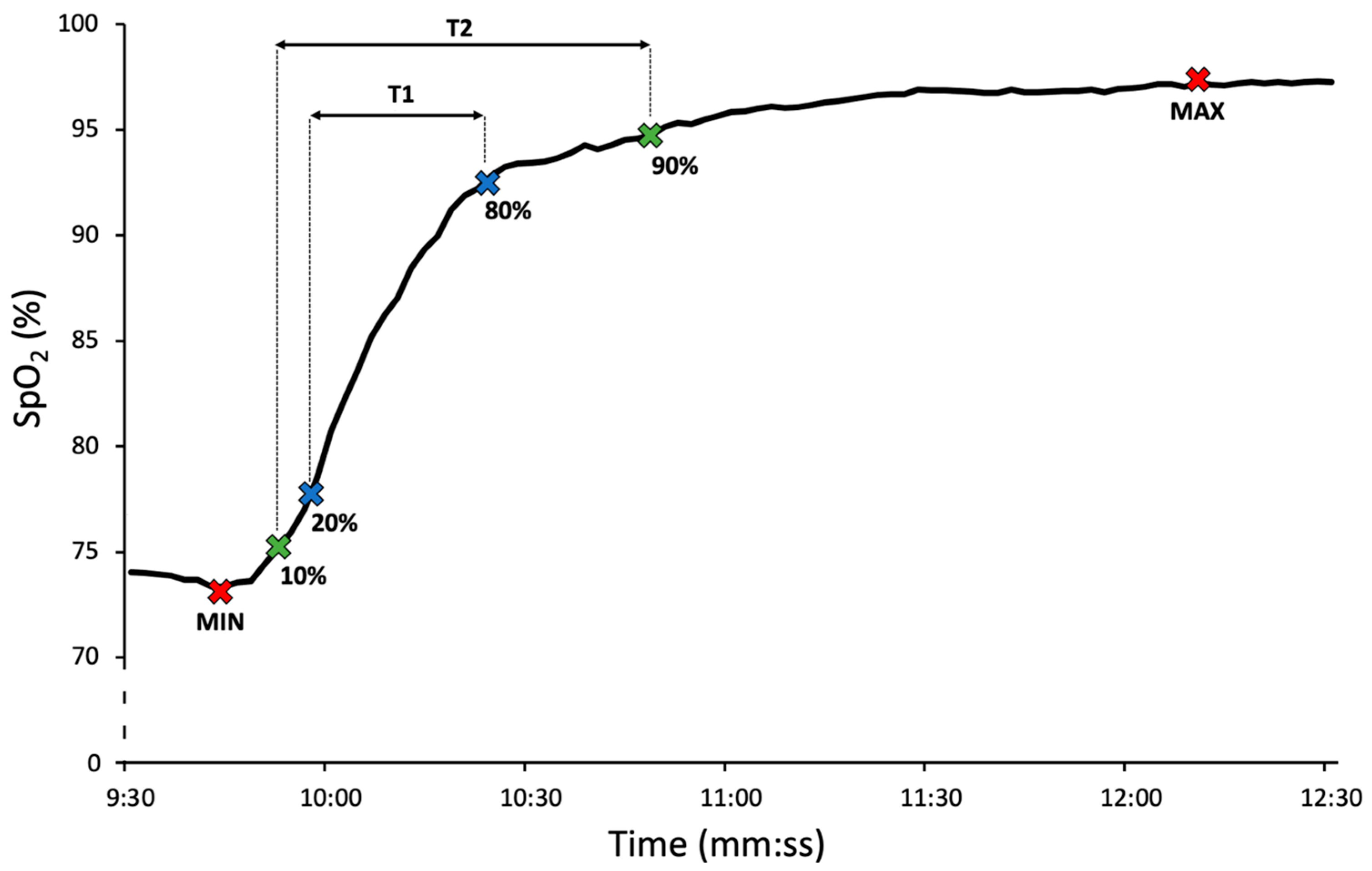
2.3. Data Extraction and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SpO2 | Peripheral blood oxygen saturation |
| PI | Perfusion index |
| BMI | Body mass index |
| ISO | International Organization for Standardization |
| ANOVA | Analysis of variance |
| HSD | Honestly significant difference |
| ICU | Intensive care unit |
| RMSE | Root mean square error |
| FDA | Food and Drug Administration |
| SaO2 | Arterial oxygen saturation |
References
- McMorrow, R.C.N.; Mythen, M.G. Pulse oximetry. Curr. Opin. Crit. Care 2006, 12, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, H.S.; Gümüş, S.; Deniz, Ö.; Yildiz, Ş.; Açikel, C.H.; Çakir, E.; Tozkoparan, E.; Uçar, E.; Bilgiç, H. Effect of nail polish and henna on oxygen saturation determined by pulse oximetry in healthy young adult females. Emerg. Med. J. 2011, 28, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Yönt, G.H.; Korhan, E.A.; Dizer, B. The effect of nail polish on pulse oximetry readings. Intensive Crit. Care Nurs. 2014, 30, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Foglia, E.E.; Whyte, R.K.; Chaudhary, A.; Mott, A.; Chen, J.; Propert, K.J.; Schmidt, B. The effect of skin pigmentation on the accuracy of pulse oximetry in infants with hypoxemia. J. Pediatr. 2017, 182, 375–377. [Google Scholar] [CrossRef]
- Bickler, P.E.; Feiner, J.R.; Severinghaus, J.W. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology 2005, 102, 715–719. [Google Scholar] [CrossRef]
- Trivedi, N.S.; Ghouri, A.F.; Shah, N.K.; Lai, E.; Barker, S.J. Effects of motion, ambient light, and hypoperfusion on pulse oximeter function. J. Clin. Anesth. 1997, 9, 179–183. [Google Scholar] [CrossRef]
- Radical 7–Masimo. Operators’ Manual. 2012. Available online: https://techdocs.masimo.com/globalassets/techdocs/pdf/lab-5475e.pdf (accessed on 13 September 2025).
- Louie, A.; Feiner, J.R.; Bickler, P.E.; Rhodes, L.; Bernstein, M.; Lucero, J. Four types of pulse oximeters accurately detect hypoxia during low perfusion and motion. Anesthesiology 2018, 128, 520–530. [Google Scholar] [CrossRef]
- Gehring, H.; Hornberger, C.; Matz, H.; Konecny, E.; Schmucker, P. The effects of motion artifact and low perfusion on the performance of a new generation of pulse oximeters in volunteers undergoing hypoxemia. Respir. Care 2002, 47, 48–60. [Google Scholar] [PubMed]
- Harskamp, R.E.; Bekker, L.; Himmelreich, J.C.; De Clercq, L.; Karregat, E.P.; Sleeswijk, M.E.; Lucassen, W.A.M. Performance of popular pulse oximeters compared with simultaneous arterial oxygen saturation or clinical-grade pulse oximetry: A cross-sectional validation study in intensive care patients. BMJ Open Respir. Res. 2021, 8, e000939. [Google Scholar] [CrossRef]
- Trivedi, N.S.; Ghouri, A.F.; Shah, N.K.; Lai, E.; Barker, S.J. Pulse oximeter performance during desaturation and resaturation: A comparison of seven models. J. Clin. Anesth. 1997, 9, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Horakova, L.; Roubik, K. Pulse oximeter performance during rapid desaturation. Sensors 2022, 22, 4236. [Google Scholar] [CrossRef]
- Brugger, H.; Sumann, G.; Meister, R.; Adler-Kastner, L.; Mair, P.; Gunga, H.C.; Schobersberger, W.; Falk, M. Hypoxia and hypercapnia during respiration into an artificial air pocket in snow: Implications for avalanche survival. Resuscitation 2003, 58, 81–88. [Google Scholar] [CrossRef]
- Strapazzon, G.; Paal, P.; Schweizer, J.; Falk, M.; Reuter, B.; Schenk, K.; Gatterer, H.; Grasegger, K.; Dal Cappello, T.; Malacrida, S.; et al. Effects of snow properties on humans breathing into an artificial air pocket–an experimental field study. Sci. Rep. 2017, 7, 17675. [Google Scholar] [CrossRef]
- Horakova, L.; Roubik, K. Performance of different pulse oximeters can affect the duration of field breathing experiments. In Proceedings of the 2019 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 21–23 November 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Mizukoshi, K.; Shibasaki, M.; Amaya, F.; Mizobe, T.; Tanaka, Y. Which finger do you attach pulse oximetry to? Index finger or not. Eur. J. Anesthesiol. 2009, 26, 3AP1–3AP5. Available online: https://www.masimo.co.jp/pdf/clinical/set/mizukoshi-which-finger-do-you-attach-pulse-oximetry-to-may-2009.pdf (accessed on 13 September 2025).
- Basaranoglu, G.; Bakan, M.; Umutoglu, T.; Zengin, S.U.; Idin, K.; Salihoglu, Z. Comparison of SpO2 values from different fingers of the hands. SpringerPlus 2015, 4, 561. [Google Scholar] [CrossRef]
- Sur, A.; Kundu, S.B. A study on inter-finger variation and hand dominance in peripheral capillary oxygen saturation values recorded from the different fingers of the hands by pulse oximetry. Natl. J. Physiol. Pharm. Pharmacol. 2021, 11, 1411–1415. [Google Scholar] [CrossRef]
- Juliana, N.; Azmani, S.; Idrose, A.; Amirfaiz, S.; Roslan, N.A.; Sulaiman, A.H.; Amin, N.A.; Fahmy, N.I.M.; Rahman, H.A. Reliable monitoring of oxygen saturation via pulse oximetry: Which site to choose? J. Fundam. Appl. Sci. 2017, 9, 122–130. [Google Scholar] [CrossRef]
- ISO 80601-2-61; Medical Electrical Equipment–Part 2-61: Particular Requirements for Basic Safety and Essential Performance of Pulse Oximeter Equipment. International Organization for Standardization: Geneva, Switzerland, 2017.
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 13 September 2025).
- Posit Team. RStudio: Integrated Development Environment for R. Posit Software; PBC: Boston, MA, USA, 2023; Available online: http://www.posit.co/ (accessed on 13 September 2025).
- U.S. Food and Drug Administration. Pulse Oximeters–Premarket Notification Submissions [510(k)s]: Guidance for Industry and Food and Drug Administration Staff; 2013. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pulse-oximeters-premarket-notification-submissions-510ks-guidance-industry-and-food-and-drug (accessed on 13 September 2025).
- Choi, S.J.; Ahn, H.J.; Yang, M.K.; Kim, C.S.; Sim, W.S.; Kim, J.A.; Kang, J.G.; Kim, J.K.; Kang, J.Y. Comparison of desaturation and resaturation response times between transmission and reflectance pulse oximeters. Acta Anaesthesiol. Scand. 2010, 54, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Hummler, H.D.; Engelmann, A.; Pohlandt, F.; Högel, J.; Franz, A.R. Decreased accuracy of pulse oximetry measurements during low perfusion caused by sepsis: Is the perfusion index of any value? Intensive Care Med. 2006, 32, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Johns, C.; Sorrell, L.; Healy, E.; Phull, M.; Olusanya, S.; Peters, M.; Fabes, J. Effect of skin tone on the accuracy of the estimation of arterial oxygen saturation by pulse oximetry: A systematic review. Br. J. Anaesth. 2024, 132, 945–956. [Google Scholar] [CrossRef] [PubMed]


| Parameter | All Participants (N = 45) |
|---|---|
| Age (years) | 23 ± 2 (20–28) |
| BMI (kg·m−2) | 24 ± 4 (19–37) |
| Heart rate (bpm) | 75 ± 13 (48–99) |
| Systolic pressure (mmHg) | 122 ± 10 (107–149) |
| Diastolic pressure (mmHg) | 72 ± 10 (57–88) |
| Finger | Circumference (cm) | PI (%) |
|---|---|---|
| Thumb | 5.9 ± 0.9 (4.5–8.5) | 2.6 (3.1) |
| Index | 5.0 ± 0.7 (3.5–7.0) | 2.1 (3.0) |
| Middle | 5.1 ± 0.8 (4.0–7.0) | 2.5 (3.5) |
| Ring | 4.9 ± 0.8 (4.0–8.0) | 2.1 (3.2) |
| Little | 4.3 ± 0.7 (3.0–6.0) | 1.7 (2.1) |
| Finger 1 | Finger 2 | SpO2 Mean Difference Finger 1–Finger 2 (%) | Standard. Error (%) | 95% Confidence Interval for SpO2 Mean Difference | p-Value | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Little | Ring | 0.07 | 0.09 | −0.18 | 0.33 | 0.939 |
| Little | Middle | 0.01 | 0.09 | −0.25 | 0.26 | 1.000 |
| Little | Index | 0.13 | 0.09 | −0.13 | 0.39 | 0.642 |
| Little | Thumb | 0.70 | 0.09 | 0.44 | 0.96 | <0.001 * |
| Ring | Middle | −0.06 | 0.09 | −0.32 | 0.19 | 0.961 |
| Ring | Index | 0.06 | 0.09 | −0.20 | 0.31 | 0.974 |
| Ring | Thumb | 0.63 | 0.09 | 0.37 | 0.88 | <0.001 * |
| Middle | Index | 0.12 | 0.09 | −0.14 | 0.38 | 0.701 |
| Middle | Thumb | 0.69 | 0.09 | 0.44 | 0.95 | <0.001 * |
| Index | Thumb | 0.57 | 0.09 | 0.31 | 0.83 | <0.001 * |
| Device 1 | Device 2 | SpO2 Mean Difference Device 1−Device 2 (%) | Standard. Error (%) | 95% Confidence Interval for SpO2 Mean Difference | p-Value | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Oximeter #1 | Oximeter #2 | 0.06 | 0.09 | −0.20 | 0.31 | 0.977 |
| Oximeter #1 | Oximeter #3 | −0.24 | 0.09 | −0.49 | 0.02 | 0.087 |
| Oximeter #1 | Oximeter #4 | 0.26 | 0.09 | 0.00 | 0.52 | 0.045 * |
| Oximeter #1 | Oximeter #5 | 1.45 | 0.09 | 1.20 | 1.71 | <0.001 * |
| Oximeter #2 | Oximeter #3 | −0.29 | 0.09 | −0.55 | −0.04 | 0.016 * |
| Oximeter #2 | Oximeter #4 | 0.20 | 0.09 | −0.05 | 0.46 | 0.188 |
| Oximeter #2 | Oximeter #5 | 1.40 | 0.09 | 1.14 | 1.65 | <0.001 * |
| Oximeter #3 | Oximeter #4 | 0.50 | 0.09 | 0.24 | 0.75 | <0.001 * |
| Oximeter #3 | Oximeter #5 | 1.69 | 0.09 | 1.43 | 1.95 | <0.001 * |
| Oximeter #4 | Oximeter #5 | 1.19 | 0.09 | 0.94 | 1.45 | <0.001 * |
| Thumb | Index | Middle | Ring | Little | p-Value | |
|---|---|---|---|---|---|---|
| T1 (s) | 19.2 ± 15.2 | 18.5 ± 14.6 | 17.9 ± 15.1 | 18.0 ± 14.3 | 19.1 ± 18.2 | 0.712 |
| T2 (s) | 33.3 ± 22.6 | 31.9 ± 22.5 | 32.0 ± 21.1 | 31.1 ± 22.1 | 32.4 ± 22.9 | 0.518 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walzel, S.; Rafl-Huttova, V.; Rozanek, M.; Kudrna, P.; Rybar, M.; Rafl, J. Inter-Finger Variability of SpO2 During Hypoxemia and Step Resaturation. Healthcare 2025, 13, 2648. https://doi.org/10.3390/healthcare13202648
Walzel S, Rafl-Huttova V, Rozanek M, Kudrna P, Rybar M, Rafl J. Inter-Finger Variability of SpO2 During Hypoxemia and Step Resaturation. Healthcare. 2025; 13(20):2648. https://doi.org/10.3390/healthcare13202648
Chicago/Turabian StyleWalzel, Simon, Veronika Rafl-Huttova, Martin Rozanek, Petr Kudrna, Marian Rybar, and Jakub Rafl. 2025. "Inter-Finger Variability of SpO2 During Hypoxemia and Step Resaturation" Healthcare 13, no. 20: 2648. https://doi.org/10.3390/healthcare13202648
APA StyleWalzel, S., Rafl-Huttova, V., Rozanek, M., Kudrna, P., Rybar, M., & Rafl, J. (2025). Inter-Finger Variability of SpO2 During Hypoxemia and Step Resaturation. Healthcare, 13(20), 2648. https://doi.org/10.3390/healthcare13202648





