Quantitative MRCP as Part of Primary Sclerosing Cholangitis Standard of Care in the National Health Service in England: A Feasibility Assessment Among Hepatologists
Abstract
1. Introduction
2. Methods and Materials
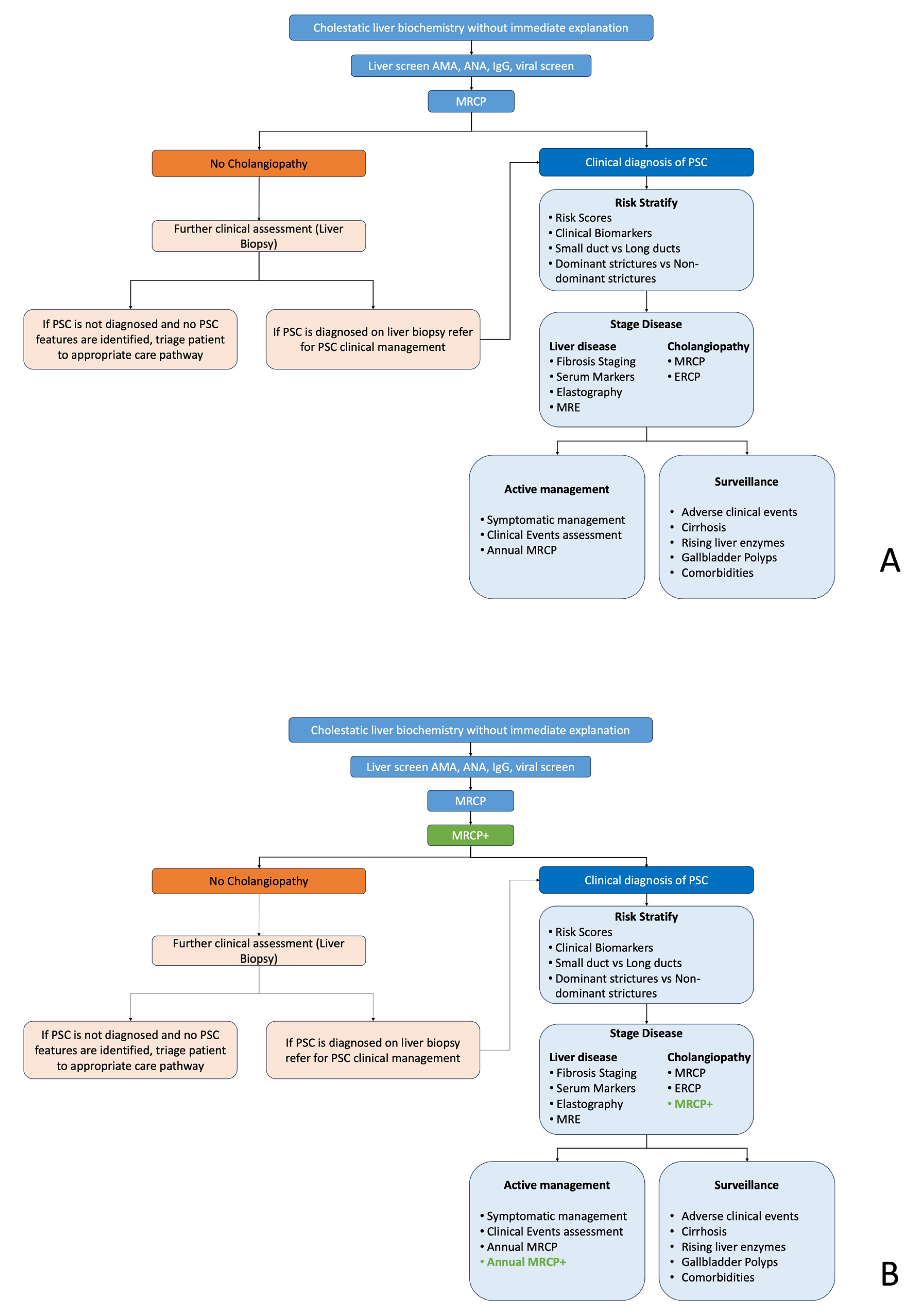
2.1. Standard Care Pathways
2.2. Feasibility Assessment and Stakeholder Identification
2.3. Lean Assessment Process Methodology
2.4. Methodology and Research Hypothesis
- Ease of Use (EoU)—the degree to which a person believes that using a system would be free of effort plays an important role in the usage and adoption of a technology. When users perceive a technology to have a positive impact on their tasks and activities, they will have the desire to use it as they will find it convenient and of (clinical) benefit.
- Trust (T)—the degree to which a person is willing to believe that their expectations will be met when using a particular system. Studies evaluating the usefulness and implementation of technologies have shown that the level of trust users have in a new technology can significantly impact their desire to use it.
- 1.
- Technology definition: undertaken by the manufacturer in collaboration with the AHSN.
- 2.
- Questionnaire design: AHSN developed a questionnaire that including statements related to PU including the extent to which users perceive the technology as useful in achieving their goals or fulfilling their needs. A 7-point Likert scale was used to measure responses (e.g., strongly agree, agree, neutral, disagree, strongly disagree).
- 3.
- Pilot testing: A pilot test of the questionnaire with an individual from the manufacturer and another from the AHSN was performed to identify any potential issues with the questionnaire’s clarity, wording, or response options.
- 4.
- Questionnaire administration and data collection: 16 interview questions were circulated to the stakeholders, and semi-structured recorded interviews were conducted.
3. Results
3.1. Questionnaire Results
3.2. Baseline Evaluation of Current Landscape
3.3. Feasibility Assessment
3.4. Perceived Usefulness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapman, M.H.; Thorburn, D.; Hirschfield, G.M.; Webster, G.G.J.; Rushbrook, S.M.; Alexander, G.; Collier, J.; Dyson, J.K.; Jones, D.E.; Patanwala, I.; et al. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut 2019, 68, 1356–1378. [Google Scholar] [CrossRef]
- Bowlus, C.L.; Arrive, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2023, 77, 659–702. [Google Scholar] [CrossRef]
- Trivedi, P.; Hirschfield, G. Recent advances in clinical practice: Epidemiology of autoimmune liver diseases. Gut 2021, 70, 1989–2003. [Google Scholar] [CrossRef]
- Crothers, H.; Ferguson, J.; Quraishi, M.N.; Cooney, R.; Iqbal, T.H.; Trivedi, P.J. Past, current, and future trends in the prevalence of primary sclerosing cholangitis and inflammatory bowel disease across England (2015–2027): A nationwide, population-based study. Lancet Reg. Health Eur. 2024, 44, 101002. [Google Scholar] [CrossRef]
- Marcus, E.; Stone, P.; Krooupa, A.M.; Thorburn, D.; Vivat, B. Quality of life in primary sclerosing cholangitis: A systematic review. Health Qual. Life Outcomes 2021, 19, 100. [Google Scholar] [CrossRef]
- Lundberg Bave, A.; Bergquist, A.; Bottai, M.; Warnqvist, A.; von Seth, E.; Nordenvall, C. Increased risk of cancer in patients with primary sclerosing cholangitis. Hepatol. Int. 2021, 15, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Sen, A.; Norat, T.; Riboli, E.; Folseraas, T. Primary sclerosing cholangitis and the risk of cancer, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 10646. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.J.; Crothers, H.; Mytton, J.; Bosch, S.; Iqbal, T.; Ferguson, J.; Hirschfield, G.M. Effects of Primary Sclerosing Cholangitis on Risks of Cancer and Death in People with Inflammatory Bowel Disease, Based on Sex, Race, and Age. Gastroenterology 2020, 159, 915–928. [Google Scholar] [CrossRef]
- Mol, B.; Werner, E.; Culver, E.L.; van der Meer, A.J.; Bogaards, J.A.; Ponsioen, C.Y. Epidemiological and economical burden of cholestatic liver disease. Hepatology 2025, 82, 813–833. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- van Munster, K.N.; Mol, B.; Goet, J.C.; van Munster, S.N.; Weersma, R.K.; de Vries, A.C.; van der Meer, A.J.; Inderson, A.; Drenth, J.P.; van Erpecum, K.J.; et al. Disease burden in primary sclerosing cholangitis in the Netherlands: A long-term follow-up study. Liver Int. 2023, 43, 639–648. [Google Scholar] [CrossRef]
- Saner, F.H.; Frey, A.; Stuben, B.O.; Hoyer, D.P.; Willuweit, K.; Daniel, M.; Rashidi-Alavieh, J.; Treckmann, J.W.; Schmidt, H.H. Transplantation for Primary Sclerosing Cholangitis: Outcomes and Recurrence. J. Clin. Med. 2023, 12, 3405. [Google Scholar] [CrossRef]
- Ueda, Y.; Kaido, T.; Okajima, H.; Hata, K.; Anazawa, T.; Yoshizawa, A.; Yagi, S.; Taura, K.; Masui, T.; Yamashiki, N.; et al. Long-term Prognosis and Recurrence of Primary Sclerosing Cholangitis After Liver Transplantation: A Single-Center Experience. Transpl. Direct 2017, 3, e334. [Google Scholar] [CrossRef] [PubMed]
- Visseren, T.; Erler, N.S.; Polak, W.G.; Adam, R.; Karam, V.; Vondran, F.W.R.; Ericzon, B.-G.; Thorburn, D.; Ijzermans, J.N.M.; Paul, A.; et al. Recurrence of primary sclerosing cholangitis after liver transplantation—Analysing the European Liver Transplant Registry and beyond. Transpl. Int. 2021, 34, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Ponsioen, C.Y. Endpoints in the design of clinical trials for primary sclerosing cholangitis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt B, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Ponsioen, C.Y.; Lindor, K.D.; Mehta, R.; Dimick-Santos, L. Design and Endpoints for Clinical Trials in Primary Sclerosing Cholangitis. Hepatology 2018, 68, 1174–1188. [Google Scholar] [CrossRef]
- Cornillet, M.; Villard, C.; Rorsman, F.; Molinaro, A.; Nilsson, E.; Kechagias, S.; von Seth, E.; Bergquist, A. The Swedish initiative for the study of Primary sclerosing cholangitis (SUPRIM). EClinicalMedicine 2024, 70, 102526. [Google Scholar] [CrossRef]
- Takakura, W.R.; Tabibian, J.H.; Bowlus, C.L. The evolution of natural history of primary sclerosing cholangitis. Curr. Opin. Gastroenterol. 2017, 33, 71–77. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Kaasen Jorgensen, K.; Bergquist, A. Medical treatment of primary sclerosing cholangitis: What have we learned and where are we going? Hepatology 2025, 82, 927–948. [Google Scholar] [CrossRef]
- Ponsioen, C.Y.; Assis, D.N.; Boberg, K.M.; Bowlus, C.L.; Deneau, M.; Thorburn, D.; Aabakken, L.; Färkkilä, M.; Petersen, B.; Rupp, C.; et al. Defining Primary Sclerosing Cholangitis: Results from an International Primary Sclerosing Cholangitis Study Group Consensus Process. Gastroenterology 2021, 161, 1764–1775 e5. [Google Scholar] [CrossRef]
- Tan, N.; Ngu, N.; Worland, T.; Lee, T.; Abrahams, T.; Pandya, K.; Freeman, E.; Hannah, N.; Gazelakis, K.; Madden, R.G.; et al. Epidemiology and outcomes of primary sclerosing cholangitis: An Australian multicentre retrospective cohort study. Hepatol. Int. 2022, 16, 1094–1104. [Google Scholar] [CrossRef]
- Selvaraj, E.A.; Culver, E.L.; Bungay, H.; Bailey, A.; Chapman, R.W.; Pavlides, M. Evolving role of magnetic resonance techniques in primary sclerosing cholangitis. World J. Gastroenterol. 2019, 25, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Vitellas, K.M.; El-Dieb, A.; Vaswani, K.K.; Bennett, W.F.; Tzalonikou, M.; Mabee, C.; Kirkpatrick, R.; Bova, J.G. MR Cholangiopancreatography in Patients with Primary Sclerosing Cholangitis: Interobserver Variability and Comparison with Endoscopic Retrograde Cholangiopancreatography. Am. Roentgen Ray Soc. 2002, 179, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Zenouzi, R.; Welle, C.L.; Venkatesh, S.K.; Schramm, C.; Eaton, J.E. Magnetic Resonance Imaging in Primary Sclerosing Cholangitis-Current State and Future Directions. Semin. Liver Dis. 2019, 39, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Schramm, C.; Eaton, J.; Ringe, K.I.; Venkatesh, S.; Yamamura, J. Recommendations on the use of magnetic resonance imaging in PSC-A position statement from the International PSC Study Group. Hepatology 2017, 66, 1675–1688. [Google Scholar] [CrossRef]
- Venkatesh, S.K.; Welle, C.L.; Miller, F.H.; Jhaveri, K.; Ringe, K.I.; Eaton, J.E.; Bungay, H.; Arrivé, L.; Ba-Ssalamah, A.; Grigoriadis, A.; et al. Reporting standards for primary sclerosing cholangitis using MRI and MR cholangiopancreatography: Guidelines from MR Working Group of the International Primary Sclerosing Cholangitis Study Group. Eur. Radiol. 2022, 32, 923–937. [Google Scholar] [CrossRef]
- Goldfinger, M.H.; Ridgway, G.R.; Ferreira, C.; Langford, C.R.; Cheng, L.; Kazimianec, A.; Borghetto, A.; Wright, T.G.; Woodward, G.; Hassanali, N.; et al. Quantitative MRCP Imaging: Accuracy, Repeatability, Reproducibility, and Cohort-Derived Normative Ranges. J. Magn. Reson. Imaging 2020, 52, 807–820. [Google Scholar] [CrossRef]
- Mahalingam, G.; Torres, R.; Kapner, D.; Trautman, E.T.; Fliss, T.; Seshamani, S.; Perlman, E.; Young, R.; Kinn, S.; Buchanan, J.; et al. A scalable and modular automated pipeline for stitching of large electron microscopy datasets. Elife 2022, 11, e76354. [Google Scholar] [CrossRef]
- Nanashima, A.; Komi, M.; Mavar, M.; Ferreira, C.; O’Donoghue, P.; Goldfinger, M.; Langford, C.; Imamura, N. A 3D Quantitative MRC Modeling Images Detected Case of Intrahepatic Biliary Stricture Diseases. Case Rep. Gastroenterol. 2021, 15, 680–688. [Google Scholar] [CrossRef]
- Gilligan, L.A.; Trout, A.T.; Lam, S.; Singh, R.; Tkach, J.A.; Serai, S.D.; Miethke, A.G.; Dillman, J.R. Differentiating pediatric autoimmune liver diseases by quantitative magnetic resonance cholangiopancreatography. Abdom. Radiol. 2020, 45, 168–176. [Google Scholar] [CrossRef]
- Ismail, M.F.; Hirschfield, G.M.; Hansen, B.; Tafur, M.; Elbanna, K.Y.; Goldfinger, M.H.; Ridgway, G.R.; Jhaveri, K.S. Evaluation of quantitative MRCP (MRCP+) for risk stratification of primary sclerosing cholangitis: Comparison with morphological MRCP, MR elastography, and biochemical risk scores. Eur. Radiol. 2022, 32, 67–77. [Google Scholar] [CrossRef] [PubMed]
- McCrary, J.; Trout, A.T.; Mahalingam, N.; Singh, R.; Rojas, C.C.; Miethke, A.G.; Dillman, J.R. Associations Between Quantitative MRI Metrics and Clinical Risk Scores in Children and Young Adults with Autoimmune Liver Disease. Am. J. Roentgenol. 2022, 219, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, E.A.; Ba-Ssalamah, A.; Poetter-Lang, S.; Ridgway, G.R.; Brady, J.M.; Collier, J.; Culver, E.L.; Bailey, A.; Pavlides, M. A Quantitative Magnetic Resonance Cholangiopancreatography Metric of Intrahepatic Biliary Dilatation Severity Detects High-Risk Primary Sclerosing Cholangitis. Hepatol. Commun. 2022, 6, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Eurboonyanun, K.; Promsorn, J.; Sa-Ngiamwibool, P.; Eurboonyanun, C.; Finnegan, S.; Ferreira, C.; Herlihy, A.; Shumbayawonda, E.; Lahoud, R.M.; Atre, I.; et al. Quantitative MRCP metrics as imaging biomarkers to differentiate benign from malignant bile duct obstructions. Front. Oncol. 2025, 15, 1576163. [Google Scholar] [CrossRef]
- Franklin, J.; Robinson, C.; Ferreira, C.; Shumbayawonda, E.; Jhaveri, K. Exploring the Role of MRCP+ for Enhancing Detection of High-Grade Strictures in Primary Sclerosing Cholangitis. J. Clin. Med. 2025, 14, 5530. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Arndtz, K.; Abbas, N.; Telford, A.; Young, L.; Banerjee, R.; Eddowes, P.; Jhaveri, K.S.; Hirschfield, G.M. Quantitative MRCP and metrics of bile duct disease over time in patients with primary sclerosing cholangitis: A prospective study. Aliment. Pharmacol. Ther. 2024, 59, 1366–1375. [Google Scholar] [CrossRef]
- Cazzagon, N.; El Mouhadi, S.; Vanderbecq, Q.; Ferreira, C.; Finnegan, S.; Lemoinne, S.; Corpechot, C.; Chazouillères, O.; Arrivé, L. Quantitative magnetic resonance cholangiopancreatography metrics are associated with disease severity and outcomes in people with primary sclerosing cholangitis. JHEP Rep. 2022, 4, 100577. [Google Scholar] [CrossRef]
- Cristoferi, L.; Porta, M.; Bernasconi, D.P.; Leonardi, F.; Gerussi, A.; Mulinacci, G.; Palermo, A.; Gallo, C.; Scaravaglio, M.; Stucchi, E.; et al. A quantitative MRCP-derived score for medium-term outcome prediction in primary sclerosing cholangitis. Dig. Liver Dis. 2023, 55, 373–380. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Are, V.; Telford, A.; Young, L.; Mouchti, S.; Ferreira, C.; Kettler, C.; Gromski, M.; Akisik, F.; Chalasani, N. A composite score using quantitative magnetic resonance cholangiopancreatography predicts clinical outcomes in primary sclerosing cholangitis. JHEP Rep. 2023, 5, 100834. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on sclerosing cholangitis. J. Hepatol. 2022, 77, 761–806. [Google Scholar] [CrossRef]
- Kelly, S.; Kaye, S.-A.; Oviedo-Trespalacios, O. What factors contribute to the acceptance of artificial intelligence? A systematic review. Telemat. Inform. 2023, 77, 101925. [Google Scholar] [CrossRef]
- Davis, F.D. User acceptance of information technology: System characteristics, user perceptions and behavioural impacts. Int. J. Man-Mach. Stud. 1993, 38, 475–487. [Google Scholar] [CrossRef]
- Arkorful, V.E.; Shuliang, Z.; Muhideen, S.; Basiru, I.; Hammond, A. An Empirical Investigation of Health Practitioners Technology Adoption: The Mediating Role of Electronic Health. Int. J. Public Adm. 2019, 43, 1013–1028. [Google Scholar] [CrossRef]
- Alloghani, M.; Hussain, A.; Al-Jumeily, D.; Abuelma’atti, O. Technology Acceptance Model for the Use of M-Health Services among Health Related Users in UAE. In Proceedings of the International Conference on Developments of E-Systems Engineering (DeSE), Dubai, United Arab Emirates, 13–14 December 2015; pp. 213–217. [Google Scholar] [CrossRef]
- Nguyen, M.; Fujioka, J.; Wentlandt, K.; Onabajo, N.; Wong, I.; Bhatia, R.S.; Bhattacharyya, O.; Stamenova, V. Using the technology acceptance model to explore health provider and administrator perceptions of the usefulness and ease of using technology in palliative care. BMC Palliat. Care 2020, 19, 138. [Google Scholar] [CrossRef]
- Ni, M.; Borsci, S.; Buckle, M. The Lean Assessment Process (LAP)—Experiences of NIHR London IVD Cooperative Working with Early-Stage Medical Technologies; The International Federation for Medical and Biological Engineering: Houston, TX, USA, 2017. [Google Scholar]
- Bajre, M.; Rose, J.; Hart, J. The Lean Assessment Process (LAP) Methodology: An iterative and multi-disciplinary method for identifying clinical needs, assessing technology acceptance and a precursor for early economic evaluation and adoption strategies. ISPOR Eur. 2023, 26, S105. [Google Scholar] [CrossRef]
- NHS. National Tariff Payment System; National Health Service: London, UK, 2020. [Google Scholar]
- Harisinghani, M.; Davis, T.; Ralli, G.; Ferreira, C.; Paun, B.; Borghetto, A.; Dennis, A.; Jhaveri, K.; Del Grande, F.; Finnegan, S.; et al. Accuracy, repeatability, reproducibility and reference ranges of primary sclerosing cholangitis specific biomarkers from quantitative MRCP. Abdom. Radiol 2025. Online ahead of print. [Google Scholar] [CrossRef]

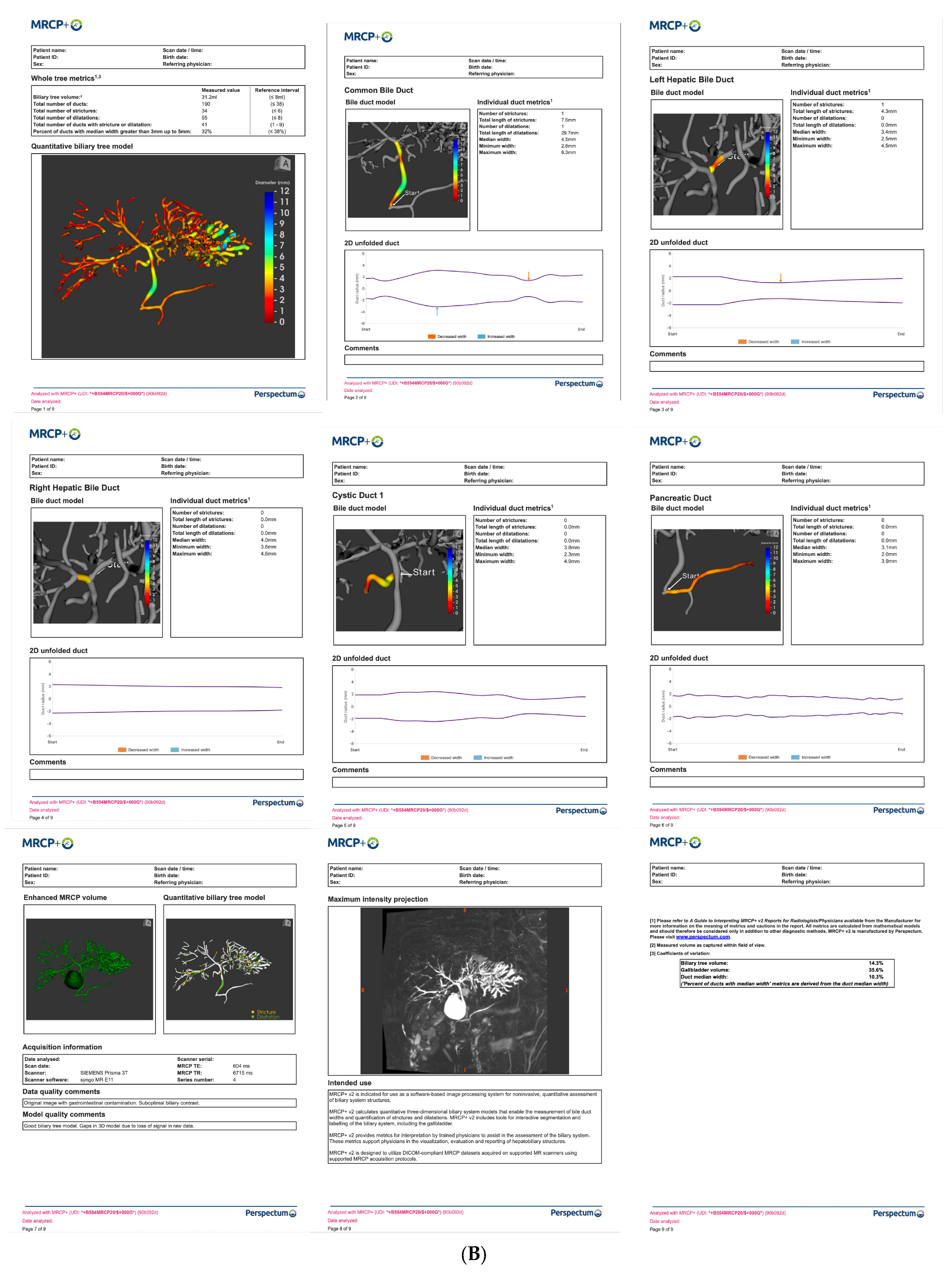
| Techniques | Mean Score | Pros | Cons |
|---|---|---|---|
| MRCP | 81% | Non-invasive and can spot changes in the bile duct. Preferred noninvasive route for diagnosis of PSC. | Reporting is subjective; therefore, smaller centres with less-experienced radiologists may be under- or over-interpreting images. There is no quantification of the biliary tree. Lacks the resolution to see intrahepatic duct. |
| ERCP | 50% | Enhanced diagnostic tool. Provides a good 3D view of the cholangiogram. | Rarely used for diagnosis in the UK to treat PSC patients. Only in therapy for strictures. Invasive with high risk of complications. |
| Liver biopsy | 68% | Considered to be more accurate than MRCP and ERCP as It shows the liver pathology. | Subject to sampling error. Small portion of liver is analysed. Risk of complications and mortality. |
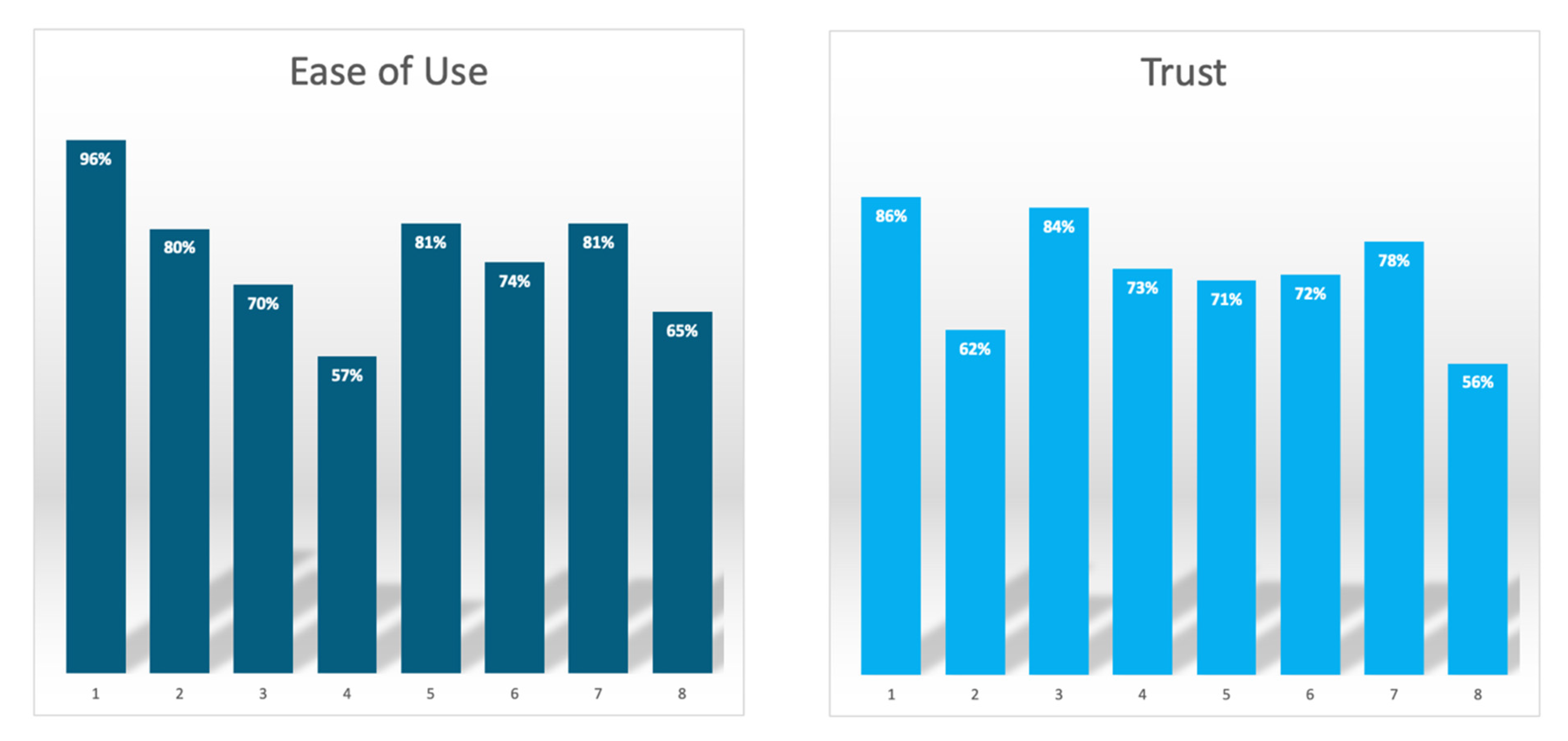
| Assessment of Perceptions of Using Quantitative MRCP | Mean Score |
|---|---|
| Perceived ease of use | |
| Quantitative MRCP is a noninvasive diagnostic technique | 96% |
| Quantitative MRCP facilitates the reporting of whole biliary tree metrics | 80% |
| Quantitative MRCP suppresses noise and provides quantitative, visually rich models of the biliary tree from routine 3D MRCP | 70% |
| Quantitative MRCP reports report whole tree metrics, such as duct number, biliary tree volume and gallbladder volume | 57% |
| Quantitative MRCP reports report single duct metrics such a stricture and dilation length and number | 81% |
| Quantitative MRCP allows the production of 2D graph of unfolded ducts, enabling easier monitoring of stricture(s) | 74% |
| Quantitative MRCP allows for comprehensive assessment of the pancreaticobiliary tract morphology | 81% |
| Quantitative MRCP allows for 3D visualisation which can aid improved characterisation of gallstones | 65% |
| Perceived trust | |
| Quantitative MRCP is an accurate means for the early detection of PSC | 86% |
| Quantitative MRCP is a way to accurately determine the stage of PSC which can support a prognosis | 62% |
| Quantitative MRCP allows objective assessment of the biliary tract for monitoring of PSC progression | 84% |
| Quantitative MRCP allows improved diagnosis and monitoring of PSC | 73% |
| Quantitative MRCP is a clinically meaningful way to quantitatively define dominant bile stenosis | 71% |
| Quantitative MRCP allows for enhanced visualisation of the biliary tree to support pre-surgery planning | 72% |
| Quantitative MRCP is an effective tool for assessment of the biliary system pre- and post-liver transplant | 78% |
| Quantitative MRCP is an accurate way to detect the early stages of cholangiocarcinoma | 56% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumbayawonda, E.; Bajre, M.; Eadle, D.; Ferreira, C.; Pansini, M.; Banerjee, R. Quantitative MRCP as Part of Primary Sclerosing Cholangitis Standard of Care in the National Health Service in England: A Feasibility Assessment Among Hepatologists. Healthcare 2025, 13, 2630. https://doi.org/10.3390/healthcare13202630
Shumbayawonda E, Bajre M, Eadle D, Ferreira C, Pansini M, Banerjee R. Quantitative MRCP as Part of Primary Sclerosing Cholangitis Standard of Care in the National Health Service in England: A Feasibility Assessment Among Hepatologists. Healthcare. 2025; 13(20):2630. https://doi.org/10.3390/healthcare13202630
Chicago/Turabian StyleShumbayawonda, Elizabeth, Mamta Bajre, Daniel Eadle, Carlos Ferreira, Michele Pansini, and Rajarshi Banerjee. 2025. "Quantitative MRCP as Part of Primary Sclerosing Cholangitis Standard of Care in the National Health Service in England: A Feasibility Assessment Among Hepatologists" Healthcare 13, no. 20: 2630. https://doi.org/10.3390/healthcare13202630
APA StyleShumbayawonda, E., Bajre, M., Eadle, D., Ferreira, C., Pansini, M., & Banerjee, R. (2025). Quantitative MRCP as Part of Primary Sclerosing Cholangitis Standard of Care in the National Health Service in England: A Feasibility Assessment Among Hepatologists. Healthcare, 13(20), 2630. https://doi.org/10.3390/healthcare13202630







