A Multidimensional Assessment of Sleep Disorders in Long COVID Using the Alliance Sleep Questionnaire
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables
- Demographic Information: We extracted demographic information (sex at birth, age, Body Mass Index (BMI), race/ethnicity).
- Vaccination Status: We considered vaccination, defining it as people who received at least two doses of the SARS-CoV-2 vaccine, regardless of whether the vaccine was mRNA (Moderna, Cambridge, MA, USA or Pfizer, New York, NY, USA) or a virus vector vaccine (Johnson & Johnson, New Brunswick, NJ, USA or AstraZeneca/Oxford, Cambridge, UK). There is a well-documented benefit of SARS-CoV-2 vaccination, such as on severity, emergency room visits, hospitalization, ICU admission, mortality, cardiovascular events, and Long COVID [41,42,43]. We planned to evaluate in our study population whether vaccination has any impact on sleep disorders; however, this population was highly vaccinated (173/200, 86.5%), and we consider the difference sufficient to conduct this kind of analysis.
- Sleep Complaint History: We defined individuals with “sleep complaints before SARS-CoV2 infection” as those who had a recorded history of any sleep complaint in their medical charts following a detailed manual chart review performed by one of four reviewers.
- COVID-19 History: We collected information on days from the initial COVID-19 infection and acute COVID-19 infection treatment (hospitalized/ambulatory) until completion of the ASQ. When assessing the number of hospitalized patients for this study, we considered each instance of SARS-CoV-2 acute infection for individuals who experienced COVID-19 multiple times.
2.3. Sleep Symptoms
2.4. Statistical Analysis
3. Results
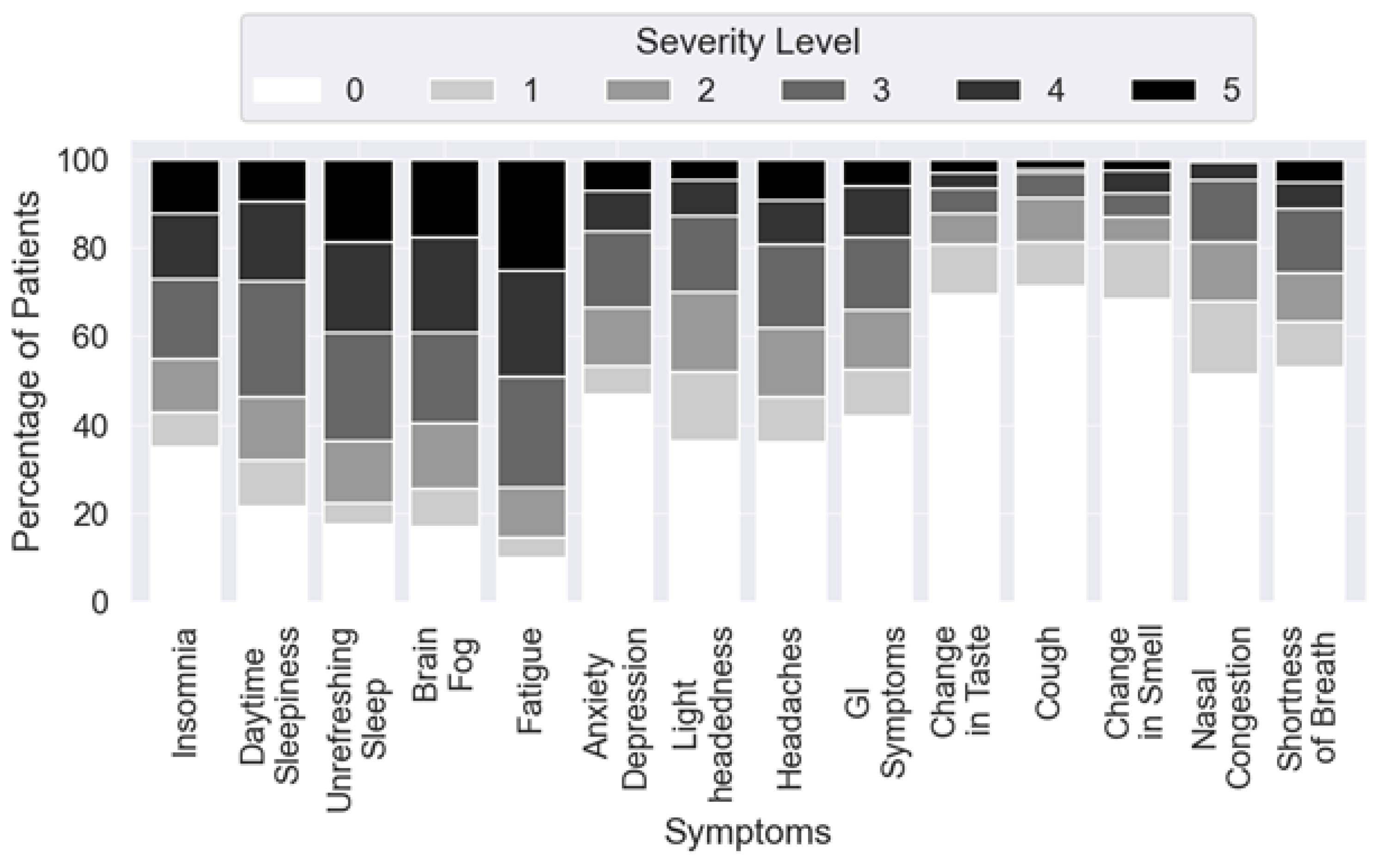
3.1. Prevalence of Specific Long COVID Sleep Complaints
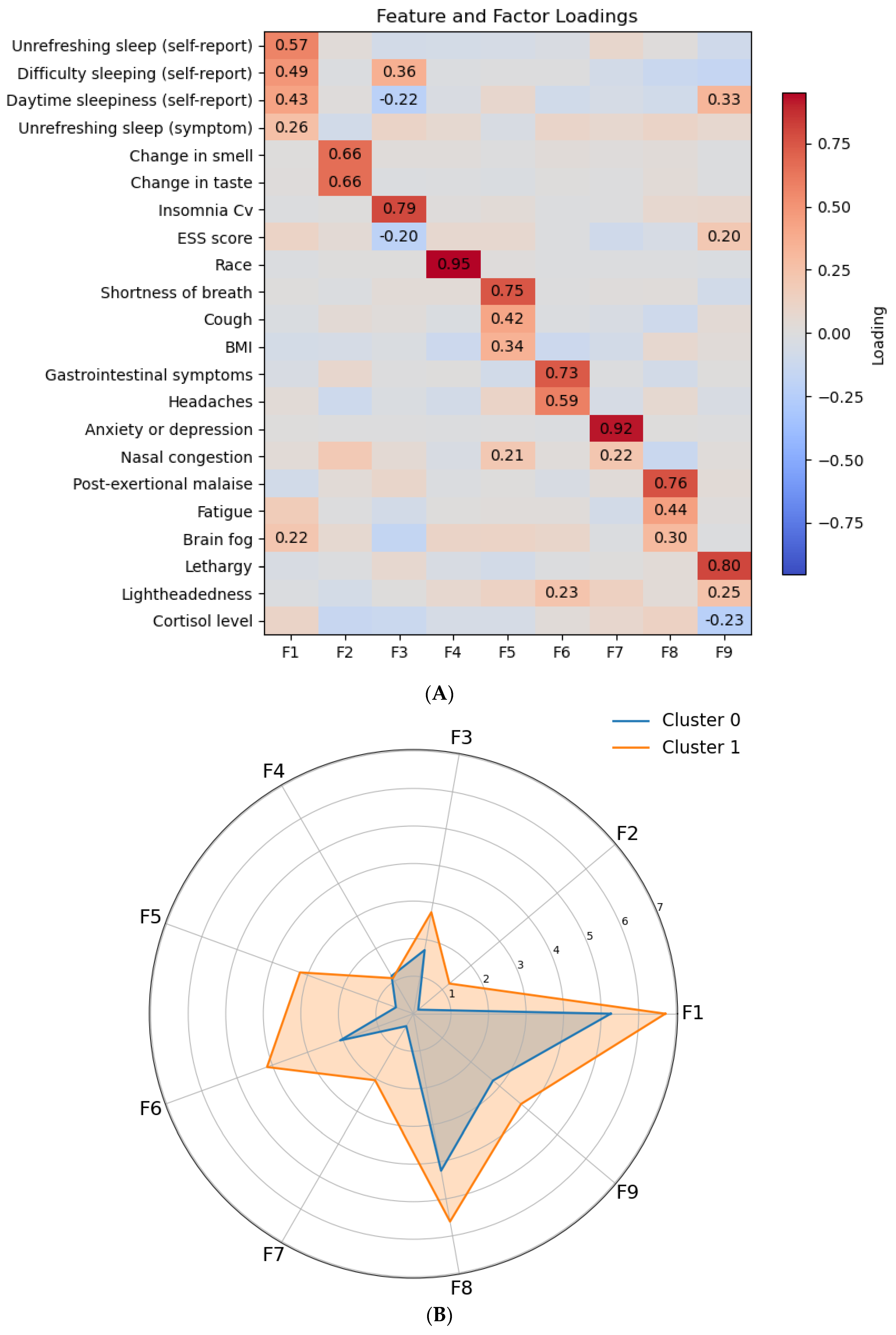
3.2. Long COVID Symptom Clusters
3.3. Predictors of Sleep Complaints in Long COVID Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASQ | Alliance Sleep Questionnaire |
| BIC | Bayesian Information Criterion |
| BMI | Body Mass Index |
| EHR | Electronic Health Record |
| ESS | Epworth Sleepiness Scale |
| FDR | False Discovery Rate |
| ISI | Insomnia Severity Index |
| NAAT | Nucleic Acid Amplification Tests |
| PCA | Principal Component Analysis |
| PASC | Post-Acute Sequelae of SARS-CoV-2 infection |
| RLS | Restless Legs Syndrome |
| rMEQ | reduced Morningness-Eveningness Questionnaire |
References
- Committee on Examining the Working Definition for Long COVID; Board on Health Sciences Policy; Board on Global Health; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. A Long COVID Definition: A Chronic, Systemic Disease State with Profound Consequences; Fineberg, H.V., Brown, L., Worku, T., Goldowitz, I., Eds.; National Academies Press: Washington, DC, USA, 2024; Available online: https://www.nap.edu/catalog/27768 (accessed on 14 May 2025).
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar]
- Al-Aly, Z.; Topol, E. Solving the puzzle of Long Covid. Science 2024, 383, 830–832. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Mandel, H.; Yoo, Y.J.; Allen, A.J.; Abedian, S.; Verzani, Z.; Karlson, E.W.; Kleinman, L.C.; Mudumbi, P.C.; Oliveira, C.R.; Muszynski, J.A.; et al. Long COVID Incidence Proportion in Adults and Children Between 2020 and 2024: An Electronic Health Record-Based Study from the RECOVER Initiative. Clin. Infect. Dis. 2025, 80, 1247–1261. [Google Scholar] [CrossRef]
- Long COVID—Household Pulse Survey—COVID-19. 2025. Available online: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (accessed on 28 January 2025).
- Products—Data Briefs, Number 480, September 2023. 2023. Available online: https://www.cdc.gov/nchs/products/databriefs/db480.htm (accessed on 28 January 2025).
- Products—Data Briefs Number 479, September 2023. 2023. Available online: https://www.cdc.gov/nchs/products/databriefs/db479.htm (accessed on 28 January 2025).
- Fang, Z.; Ahrnsbrak, R.; Rekito, A. Evidence Mounts That About 7% of US Adults Have Had Long COVID. JAMA 2024, 332, 5. [Google Scholar] [CrossRef]
- Prevalence of Ongoing Symptoms Following Coronavirus (COVID-19) Infection in the UK—Office for National Statistics. 2025. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/30march2023 (accessed on 28 January 2025).
- Al-Aly, Z.; Davis, H.; McCorkell, L.; Soares, L.; Wulf-Hanson, S.; Iwasaki, A.; Topol, E.J. Long COVID science, research and policy. Nat. Med. 2024, 30, 2148–2164. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-term neurologic outcomes of COVID-19. Nat. Med. 2022, 28, 2406–2415. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Al-Aly, Z. Risks of mental health outcomes in people with covid-19: Cohort study. BMJ 2022, 376, e068993. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Rosen, C.J. Long Covid and Impaired Cognition—More Evidence and More Work to Do. N. Engl. J. Med. 2024, 390, 858–860. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Lee, J.; Lee, S.W.; Kwon, R.; Kim, M.S.; Koyanagi, A.; Smith, L.; Fond, G.; Boyer, L.; et al. Short- and long-term neuropsychiatric outcomes in long COVID in South Korea and Japan. Nat. Hum. Behav. 2024, 8, 1530–1544. [Google Scholar] [CrossRef]
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: A cohort study. The Lancet Diabetes & Endocrinology. Lancet Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar]
- Xu, E.; Xie, Y.; Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2023, 11, 120–128. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Xu, E.; Al-Aly, Z. Kidney Outcomes in Long COVID. J. Am. Soc. Nephrol. 2021, 32, 2851–2862. [Google Scholar] [CrossRef]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.G.; Neidleman, J.; Andrew, A.; Young, K.C.; Ma, T.; Hoh, R.; et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 2024, 25, 218–225. [Google Scholar] [CrossRef]
- Peluso, M.J.; Abdel-Mohsen, M.; Henrich, T.J.; Roan, N.R. Systems analysis of innate and adaptive immunity in Long COVID. Semin. Immunol. 2024, 72, 101873. [Google Scholar] [CrossRef]
- Pollack, B.; von Saltza, E.; McCorkell, L.; Santos, L.; Hultman, A.; Cohen, A.K.; Soares, L. Female reproductive health impacts of Long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: A literature review. Front. Rehabil. Sci. 2023, 4, 1122673. [Google Scholar] [CrossRef]
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 2023, 14, 983. [Google Scholar] [CrossRef]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Brain Basics: Understanding Sleep|National Institute of Neurological Disorders and Stroke. 2025. Available online: https://www.ninds.nih.gov/health-information/public-education/brain-basics/brain-basics-understanding-sleep (accessed on 28 January 2025).
- Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory from the American Heart Association|Circulation. 2025. Available online: https://www.ahajournals.org/doi/10.1161/CIR.0000000000001078 (accessed on 28 January 2025).
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; text revision; American Academy of Sleep Medicine: Rochester, MN, USA, 2023. [Google Scholar]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar]
- Tedjasukmana, R.; Budikayanti, A.; Islamiyah, W.R.; Witjaksono, A.M.A.L.; Hakim, M. Sleep disturbance in post COVID-19 conditions: Prevalence and quality of life. Front. Neurol. 2023, 13, 1095606. [Google Scholar] [CrossRef]
- Geng, L.N.; Erlandson, K.M.; Hornig, M.; Letts, R.; Selvaggi, C.; Ashktorab, H.; Atieh, O.; Bartram, L.; Brim, H.; Brosnahan, S.B.; et al. 2024 Update of the RECOVER-Adult Long COVID Research Index. JAMA 2025, 333, 694. [Google Scholar] [CrossRef]
- Tański, W.; Tomasiewicz, A.; Jankowska-Polańska, B. Sleep Disturbances as a Consequence of Long COVID-19: Insights from Actigraphy and Clinimetric Examinations—An Uncontrolled Prospective Observational Pilot Study. J. Clin. Med. 2024, 13, 839. [Google Scholar] [CrossRef]
- Miaskowski, C.; Barsevick, A.; Berger, A.; Casagrande, R.; Grady, P.A.; Jacobsen, P.; Kutner, J.; Patrick, D.; Zimmerman, L.; Xiao, C.; et al. Advancing Symptom Science Through Symptom Cluster Research: Expert Panel Proceedings and Recommendations. J. Natl. Cancer Inst. 2017, 109, djw253. [Google Scholar]
- Bonilla, H.; Quach, T.C.; Tiwari, A.; Bonilla, A.E.; Miglis, M.; Yang, P.C.; Eggert, L.E.; Sharifi, H.; Horomanski, A.; Subramanian, A.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): Results from a post-COVID-19 multidisciplinary clinic. Front. Neurol. 2023, 14, 1090747. [Google Scholar]
- Leary, E.; Joergensen, S.; Malunjkar, S.; Kim, S.; Mignot, E.; Barwick, F. 0332 Validation of the Alliance Sleep Questionnaire (Asq) Insomnia Module in Sleep Disordered Patients. Sleep 2017, 40 (Suppl. S1), A123. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Santosa, A.; Wettermark, B.; Fall, T.; Björk, J.; Börjesson, M.; Gisslén, M.; Nyberg, F. Cardiovascular events following coronavirus disease 2019 vaccination in adults: A nationwide Swedish study. Eur. Hear. J. 2024, 46, 147–157. [Google Scholar] [CrossRef]
- Marra, A.R.; Kobayashi, T.; Callado, G.Y.; Pardo, I.; Gutfreund, M.C.; Hsieh, M.K.; Lin, V.; Alsuhaibani, M.; Hasegawa, S.; Tholany, J.; et al. The effectiveness of COVID-19 vaccine in the prevention of post-COVID conditions: A systematic literature review and meta-analysis of the latest research. Antimicrob. Steward. Health Epidemiol. 2023, 3, e168. [Google Scholar] [CrossRef]
- CDC. Benefits of Getting Vaccinated. COVID-19. 2025. Available online: https://www.cdc.gov/covid/vaccines/benefits.html (accessed on 13 September 2025).
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Adan, A.; Almirall, H. Horne & Östberg morningness-eveningness questionnaire: A reduced scale. Personal. Individ. Differ. 1991, 12, 241–253. [Google Scholar]
- Abdi, H. Factor rotations in factor analyses. In Encyclopedia for Research Methods for the Social Sciences; Sage: Thousand Oaks, CA, USA, 2003; pp. 792–795. [Google Scholar]
- McLachlan, G.J.; Peel, D. Finite Mixture Models; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Hubert, L.; Arabie, P. Comparing partitions. J. Classif. 1985, 2, 193–218. [Google Scholar] [CrossRef]
- McInnes, L.; Healy, J.; Melville, J. Umap: Uniform manifold approximation and projection for dimension reduction. arXiv 2018, arXiv:180203426. [Google Scholar]
- Austin, P.C.; Steyerberg, E.W. Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Stat. Methods Med. Res. 2017, 26, 796–808. [Google Scholar] [CrossRef]
- Ricciardiello, G. Analysis on Long Covid Dataset to Assess Sleep Complaints. Available online: https://github.com/GiorgioRicciardiello/ProlongSleepCovid (accessed on 13 September 2025).
- Pena-Orbea, C.; Lapin, B.; Li, Y.; Englund, K.; Heinzinger, C.; Foldvary-Schaefer, N.; Mehra, R. Sleep Disturbance Severity and Correlates in Post-acute Sequelae of COVID-19 (PASC). J. Gen. Intern. Med. 2023, 38, 2015–2017. [Google Scholar] [CrossRef]
- Nowakowski, S.; Kokonda, M.; Sultana, R.; Duong, B.B.; Nagy, S.E.; Zaidan, M.F.; Baig, M.M.; Grigg, B.V.; Seashore, J.; Deer, R.R. Association between Sleep Quality and Mental Health among Patients at a Post-COVID-19 Recovery Clinic. Brain Sci. 2022, 12, 586. [Google Scholar] [CrossRef]
- Guezguez, F.; Romdhani, M.; Boutaleb-Joutei, A.; Chamari, K.; Ben Saad, H. Management of long-COVID-19 patients with sleep disorders: Practical advice to general practitioners. Libyan J. Med. 2023, 18, 2182704. [Google Scholar] [CrossRef]
- Pellitteri, G.; Surcinelli, A.; De Martino, M.; Fabris, M.; Janes, F.; Bax, F.; Marini, A.; Milanic, R.; Piani, A.; Isola, M.; et al. Sleep alterations following COVID-19 are associated with both neuroinflammation and psychological disorders, although at different times. Front. Neurol. 2022, 13, 929480. [Google Scholar] [CrossRef]
- Percze, A.R.; Nagy, A.; Polivka, L.; Barczi, E.; Czaller, I.; Kovats, Z.; Varga, J.T.; Ballai, J.H.; Muller, V.; Horvath, G. Fatigue, sleepiness and sleep quality are SARS-CoV-2 variant independent in patients with long COVID symptoms. Inflammopharmacology 2023, 31, 2819–2825. [Google Scholar] [CrossRef]
- Sunada, N.; Nakano, Y.; Otsuka, Y.; Tokumasu, K.; Honda, H.; Sakurada, Y.; Matsuda, Y.; Hasegawa, T.; Omura, D.; Ochi, K.; et al. Characteristics of Sleep Disturbance in Patients with Long COVID: A Retrospective Observational Study in Japan. J. Clin. Med. 2022, 11, 7332. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Yue, Y.; Yuan, C.; Kang, J.H.; Chavarro, J.E.; Bhupathiraju, S.N.; Roberts, A.L. Adherence to Healthy Lifestyle Prior to Infection and Risk of Post–COVID-19 Condition. JAMA Intern. Med. 2023, 183, 232–241. [Google Scholar] [CrossRef]
- Abuhammad, S.; Alzoubi, K.H.; Khabour, O.F.; Hamaideh, S.; Khasawneh, B. Sleep quality and sleep patterns among recovered individuals during post-COVID-19 among Jordanian: A cross-sectional national study. Medicine 2023, 102, e32737. [Google Scholar] [CrossRef]
- Henríquez-Beltrán, M.; Labarca, G.; Cigarroa, I.; Enos, D.; Lastra, J.; Nova-Lamperti, E.; Targa, A.; Barbe, F. Sleep health and the circadian rest-activity pattern four months after COVID-19. J. Bras. Pneumol. 2022, 48, e20210398. [Google Scholar] [CrossRef]
- Azambuja, P.; Bastos, L.S.; Batista-Da-Silva, A.A.; Ramos, G.V.; Kurtz, P.; Dias, C.M.; da Silva, E.P.; Arouca, L.E.; Soares, J.; Sejvar, J.J.; et al. Prevalence, risk factors, and impact of long COVID in a socially vulnerable community in Brazil: A prospective cohort study. Lancet Reg. Health Am. 2024, 37, 100839. [Google Scholar] [CrossRef]
- Cheetham, N.J.; Bowyer, V.; García, M.P.; Bowyer, R.C.; Carpentieri, J.D.; Guise, A.; Thompson, E.J.; Sudre, C.H.; Molteni, E.; Antonelli, M.; et al. Social determinants of recovery from ongoing symptoms following COVID-19 in two UK longitudinal studies: A prospective cohort study. BMJ Public Health 2025, 3, e001166. [Google Scholar] [CrossRef]
- Cohen, J.; Van Der Meulen Rodgers, Y. An intersectional analysis of long COVID prevalence. Int. J. Equity Health 2023, 22, 261. [Google Scholar] [CrossRef]
- Xiang, J.; Zheng, H.; Cai, Y.; Chen, S.; Wang, Y.; Chen, R. Cumulative social disadvantage and its impact on long COVID: Insights from a U.S. national survey. BMC Med. 2025, 23, 207. [Google Scholar] [CrossRef]
| Parameter | N (%) | Parameter | N (%) |
|---|---|---|---|
| Age (years) [mean (STD)] | 46.5 (16.9) | Functional status b | |
| Days since infection [mean (STD)] | 770 (392.61) | II | 23 (11.5%) |
| BMI (kg/m2) [mean (STD)] | 26.78 (6.59) | III | 94 (4.7%) |
| Sex a | IV | 59 (29.5%) | |
| Males | 67 (33.0%) | Did not respond | 8 (4.00%) |
| Females | 134 (67.0%) | Sleep Complaints Prior to COVID-19 Infection | |
| Race | Yes | 14 (7.00) | |
| White | 139 (69.50%) | No | 186 (93.0) |
| Black | 6 (3.00%) | Vaccination Status c | |
| American Indian/Native American | 3 (1.50%) | Vaccinated | 173 (86.5%) |
| Asian | 34 (17.0%) | Not Vaccinated | 27 (13.5%) |
| Pacific Islander | 1 (0.5%) | Initial COVID-19 Infection Hospitalization Status | |
| Multiple Races | 17 (8.50%) | Hospitalized | 14 (7.00%) |
| Ethnicity | Ambulatory | 186 (93.00%) | |
| Hispanic Origin | 22 (11.0%) | ||
| Non-Hispanic Origin | 178 (89.0%) | ||
| Highest Level of Education Attained | |||
| Elementary School | 2 (1.0) | ||
| High School | 27 (13.5) | ||
| Associate’s degree | 23 (11.5) | ||
| Bachelor’s Degree | 63 (32.5) | ||
| Graduate Degree | 77 (38.5) | ||
| Did Not Reply | 4 (2.0) | ||
| Complaint | Long COVID Cohort [N (%)] | |||
|---|---|---|---|---|
| Male (n = x) | Female (n = x) | Total (n = x) | p-Value * | |
| Insomnia ⧫ | 23 (34.8%) | 62 (46.3%) | 85 (42.5%) | 0.48960 |
| Excessive daytime sleepiness ◼ | 15 (54.0%) | 42 (31.3%) | 57(28.5%) | 0.48960 |
| Sleep-related breathing complaint | 37 (56.1%) | 78 (58.2%) | 115 (57.5%) | 1 |
| Restless legs | 7 (10.6%) | 24 (17.9%) | 31 (15.5%) | 0.48960 |
| Parasomnia activity | 9 (13.6%) | 20 (14.9%) | 29 (14.5%) | 1 |
| Extreme circadian phenotype | 7 (10.6%) | 15 (11.2%) | 22 (11.0%) | 1 |
| Sleep Complaint of Interest [OR (95% CI)] | ||||||
|---|---|---|---|---|---|---|
| Variable | Breathing Symptoms | Excessive Daytime Sleepiness | Extreme Circadian | Insomnia | Parasomnia | Restless Legs |
| Age | 1.216 (0.90, 1.65) | 0.768 (0.55, 1.07) | 0.737 (0.48, 1.14) | 1.029 (0.76, 1.38) | 1.086 (0.70, 1.69) | 1.006 (0.66, 1.54) |
| Male | 0.967 (0.52, 1.78) | 0.531 (0.26, 1.11) | 0.899 (0.35, 2.30) | 0.761 (0.39, 1.47) | 0.836 (0.35, 1.99) | 0.547 (0.22, 1.38) |
| BMI | 1.216 (0.86, 1.72) | 1.214 (0.88, 1.67) | 1.150 (0.69, 1.90) | 0.741 (0.54, 1.03) | 1.246 (0.84, 1.84) | 0.963 (0.65, 1.43) |
| Vaccinated | 0.978 (0.42, 2.28) | 0.704 (0.27, 1.84) | 1.100 (0.29, 4.21) | 0.603 (0.25, 1.47) | 1.199 (0.34, 4.19) | 0.671 (0.23, 1.94) |
| Duration a | 1.274 (0.88, 1.84) | 2.574 (0.86, 7.70) | 1.063 (0.80, 1.41) | 0.902 (0.73, 1.12) | 1.031 (0.82, 1.29) | 1.103 (0.84, 1.44) |
| Hospitalized | 1.190 (0.30, 4.65) | 0.908 (0.28, 2.96) | 2.50 × 10−9 (9.74 × 10−10, 6.43 × 10−9) | 4.413 * (1.27, 15.36) | 0.618 (0.12, 3.08) | 3.151 (0.91, 10.92) |
| Race (White/Caucasian = reference category) | ||||||
| Asian | 1.250 (0.56, 2.78) | 2.111 (0.88, 5.08) | 0.405 (0.09, 1.81) | 1.584 (0.74, 3.39) | 2.071 (0.68, 6.29) | 0.494 (0.13, 1.87) |
| Other b | 2.934 (0.56, 15.38) | 2.603 (0.75, 9.06) | 1.160 (0.14, 9.91) | 1.315 (0.30, 5.78) | 3.298 (0.79, 13.73) | 0.772 (0.21, 2.80) |
| Multiple Races | 1.587 (0.53, 4.76) | 0.732 (0.22, 2.44) | 1.709 (0.43, 6.75) | 3.219 * (1.00, 10.34) | 1.047 (0.22, 5.06) | 0.207 (0.03, 1.66) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, A.; Ricciardiello Mejia, G.C.; Lomba, S.; Geng, L.N.; Malunjkar, S.; Bonilla, H.; Sum-Ping, O. A Multidimensional Assessment of Sleep Disorders in Long COVID Using the Alliance Sleep Questionnaire. Healthcare 2025, 13, 2611. https://doi.org/10.3390/healthcare13202611
Wilson A, Ricciardiello Mejia GC, Lomba S, Geng LN, Malunjkar S, Bonilla H, Sum-Ping O. A Multidimensional Assessment of Sleep Disorders in Long COVID Using the Alliance Sleep Questionnaire. Healthcare. 2025; 13(20):2611. https://doi.org/10.3390/healthcare13202611
Chicago/Turabian StyleWilson, Alina, Giorgio Camillo Ricciardiello Mejia, Sara Lomba, Linda N. Geng, Sanjay Malunjkar, Hector Bonilla, and Oliver Sum-Ping. 2025. "A Multidimensional Assessment of Sleep Disorders in Long COVID Using the Alliance Sleep Questionnaire" Healthcare 13, no. 20: 2611. https://doi.org/10.3390/healthcare13202611
APA StyleWilson, A., Ricciardiello Mejia, G. C., Lomba, S., Geng, L. N., Malunjkar, S., Bonilla, H., & Sum-Ping, O. (2025). A Multidimensional Assessment of Sleep Disorders in Long COVID Using the Alliance Sleep Questionnaire. Healthcare, 13(20), 2611. https://doi.org/10.3390/healthcare13202611






