Artificial Intelligence in Nutrition and Dietetics: A Comprehensive Review of Current Research
Abstract
1. Introduction
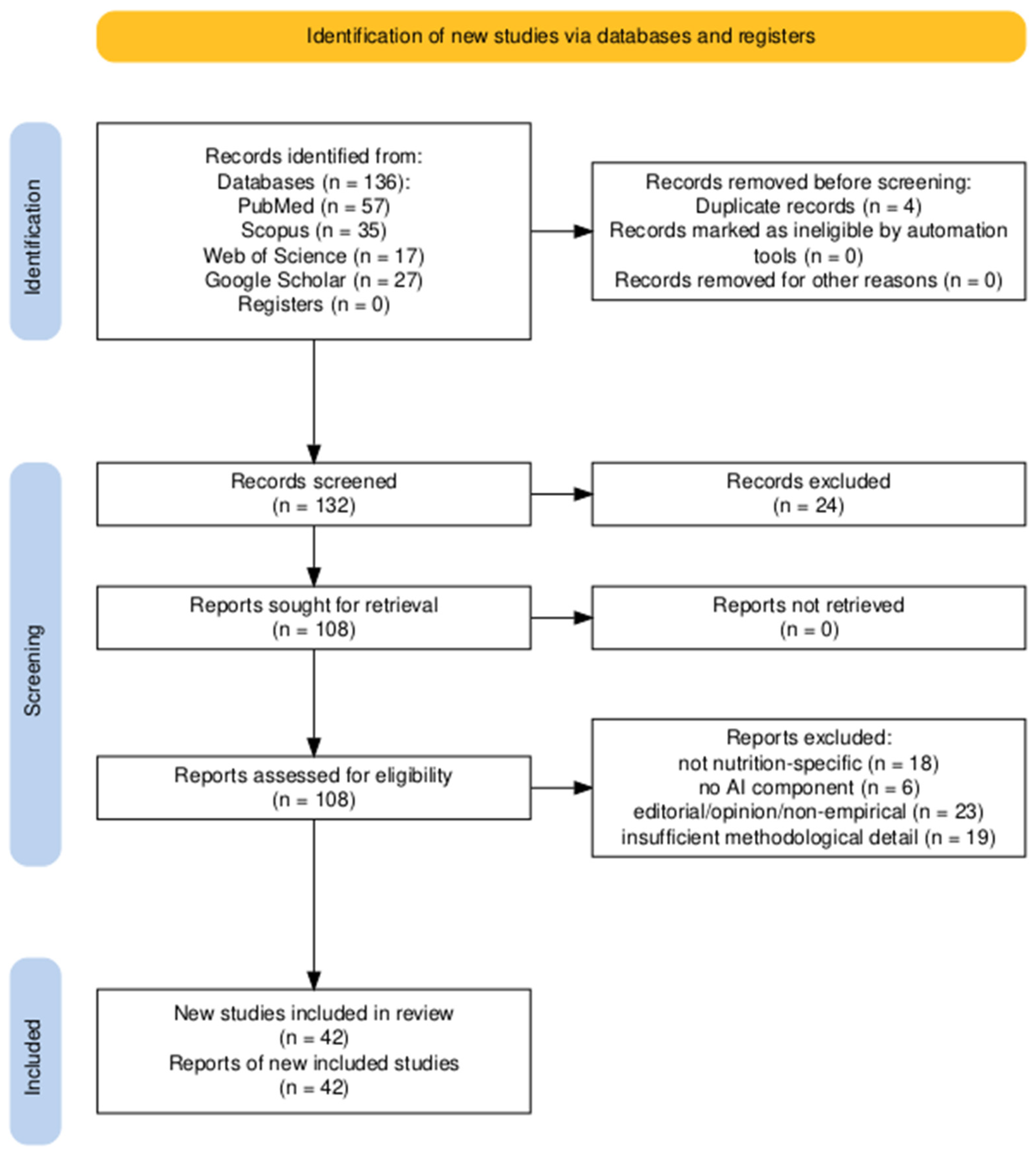
2. Materials and Methods
2.1. Objective and Scope
- AI-driven dietary assessment and food recognition;
- Personalized nutrition planning and metabolic prediction;
- Clinical decision support in chronic disease contexts;
- Generative AI and conversational agents in patient education;
- Mobile health applications and remote coaching;
- Ethical, regulatory, and professional practice considerations.
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
- Peer-reviewed journal articles, conference papers, and systematic reviews;
- Studies focused on the development, validation, or application of AI in nutrition science, dietetics, or public health nutrition;
- Articles written in English;
- Both clinical and non-clinical settings.
- Editorials, opinion pieces, or commentaries without empirical data;
- Articles that addressed general AI in healthcare without specific mention of nutrition or dietetics;
- Studies with poor methodological quality (e.g., no evaluation/validation methods reported).
2.4. Data Extraction and Synthesis
- AI technique used (e.g., supervised learning, deep learning, LLMs);
- Nutrition-related application (e.g., assessment, education, clinical support);
- Target population or health condition;
- Study design and setting;
- Key findings and performance metrics (e.g., accuracy, precision, user satisfaction);
- Limitations and future research suggestions.
- AI in dietary assessment;
- AI in personalized and clinical nutrition;
- Generative AI and conversational agents in nutrition advice;
- Mobile apps and virtual coaching;
- AI in global nutrition and public health;
- Ethical and professional implications of AI in dietetics.
3. AI Applications in Nutrition and Dietetics
3.1. AI for Dietary Assessment and Nutrient Tracking
- Variability in image quality affecting recognition accuracy;
- Insufficient database coverage for regional or homemade dishes;
- Difficulty estimating mixed meals or hidden ingredients;
- User compliance, especially in consistently photographing meals.
3.2. AI-Driven Personalized Nutrition and Disease Management
3.2.1. AI Models in T2DM, Obesity, and Cardiovascular Health
3.2.2. Dietitian-Assistive Chatbots and Virtual Coaches
3.2.3. Machine Learning for Dietary Plan Creation and Metabolic Prediction
3.3. Generative AI and Conversational Agents in Nutrition
3.3.1. ChatGPT and LLMs for Dietary Advice
3.3.2. Comparison of Chatbot Accuracy, Consistency, and Safety
3.3.3. Use in Education and Patient Communication
3.4. AI in Public and Global Health Nutrition
3.4.1. AI Tools for Malnutrition Screening in Resource-Limited Settings
3.4.2. Global Health Implications and Policy Integration
3.5. AI for Sensory Science and Food Innovation
4. Ethical, Practical, and Professional Considerations
4.1. Ethical Challenges
4.2. Professional Roles and AI Integration
5. Evaluation of AI Tools in Practice
5.1. Usability and Acceptance
5.2. Validity, Accuracy, and Reproducibility
5.3. Mixed-Methods and RCT Evidence
6. Research Gaps and Limitations in the Current Literature
6.1. Lack of Standardized Validation Protocols
6.2. Poor Reporting of AI Model Architectures
6.3. Limited Generalizability Across Populations and Diets
6.4. Underrepresentation in Low- and Middle-Income Countries (LMICs)
6.5. Policy and Practice Implications
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| LLMs | Large language models |
| ML | Machine learning |
| DL | Deep learning |
| NLP | Natural language processing |
| T2DM | Type 2 Diabetes Mellitus |
| WHO | World Health Organization |
| CNNs | Convolutional Neural Networks |
| RL | Reinforcement Learning |
| IoT | Internet of Things |
| HRV | Heart Rate Variability |
| XAI | Explainable artificial intelligence |
| GDPR | General Data Protection Regulation |
| HIPAA | Health Insurance Portability and Accountability Act |
| RCTs | Randomized controlled trials |
| CONSORT-AI | Consolidated Standards of Reporting Trials—Artificial Intelligence |
| MINIMAR | MINimum Information for Medical Artificial intelligence Reporting |
| LMIC | Low- and Middle-Income Country |
References
- Alyafei, A.; Daley, S.F. The Role of Dietary Lifestyle Modification in Chronic Disease Prevention and Management. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2025. [Google Scholar]
- Verma, M.; Hontecillas, R.; Tubau-Juni, N.; Abedi, V.; Bassaganya-Riera, J. Challenges in Personalized Nutrition and Health. Front. Nutr. 2018, 5, 117. [Google Scholar] [CrossRef]
- Defraeye, T.; Bahrami, F.; Kowatsch, T.; Annaheim, S.; Bragt, M.C.; Rossi, R.M.; Greger, M. Advances in Food-As-Medicine Interventions and Their Impact on Future Food Production, Processing, and Supply Chains. Adv. Nutr. 2025, 16, 100421. [Google Scholar] [CrossRef]
- Bajwa, J.; Munir, U.; Nori, A.; Williams, B. Artificial Intelligence in Healthcare: Transforming the Practice of Medicine. Future Healthc. J. 2021, 8, e188–e194. [Google Scholar] [CrossRef]
- Ruthsatz, M.; Candeias, V. Non-Communicable Disease Prevention, Nutrition and Aging. Acta Biomed. 2020, 91, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Bacaloni, S.; Agrawal, D.K. Nutrition, Gut Microbiota, and Epigenetics in the Modulation of Immune Response and Metabolic Health. Cardiol. Cardiovasc. Med. 2025, 9, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Stanescu, C.; Chiscop, I.; Mihalache, D.; Boev, M.; Tamas, C.; Stoleriu, G. The Roles of Micronutrition and Nutraceuticals in Enhancing Wound Healing and Tissue Regeneration: A Systematic Review. Molecules 2025, 30, 3568. [Google Scholar] [CrossRef] [PubMed]
- Skenderidou, I.; Leontopoulos, S.; Skenderidis, P. Functional Food Ingredients Enhancing Immune Health. Int. J. Mol. Sci. 2025, 26, 8408. [Google Scholar] [CrossRef]
- del Carmen Alvarez-Nuncio, M.; Ziegler, T.R. Micronutrient Status and Protein-Energy Malnutrition in Free-Living Older Adults: A Current Perspective. Curr. Opin. Gastroenterol. 2024, 40, 99–105. [Google Scholar] [CrossRef]
- Badham, J. Ensuring Optimal Breastfeeding and Improvements in Complementary Feeding to Improve Infant and Young Child Nutrition in Developing Countries. Matern. Child Nutr. 2012, 9, 1–5. [Google Scholar] [CrossRef]
- Tabish, S.A. Complementary and Alternative Healthcare: Is It Evidence-Based? Int. J. Health Sci. 2008, 2, V–IX. [Google Scholar]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing Healthcare: The Role of Artificial Intelligence in Clinical Practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Sosa-Holwerda, A.; Park, O.-H.; Albracht-Schulte, K.; Niraula, S.; Thompson, L.; Oldewage-Theron, W. The Role of Artificial Intelligence in Nutrition Research: A Scoping Review. Nutrients 2024, 16, 2066. [Google Scholar] [CrossRef]
- Theodore Armand, T.P.; Nfor, K.A.; Kim, J.-I.; Kim, H.-C. Applications of Artificial Intelligence, Machine Learning, and Deep Learning in Nutrition: A Systematic Review. Nutrients 2024, 16, 1073. [Google Scholar] [CrossRef]
- Kassem, H.; Beevi, A.A.; Basheer, S.; Lutfi, G.; Cheikh Ismail, L.; Papandreou, D. Investigation and Assessment of AI’s Role in Nutrition-An Updated Narrative Review of the Evidence. Nutrients 2025, 17, 190. [Google Scholar] [CrossRef]
- Thacharodi, A.; Singh, P.; Meenatchi, R.; Tawfeeq Ahmed, Z.H.; Kumar, R.R.S.; V, N.; Kavish, S.; Maqbool, M.; Hassan, S. Revolutionizing Healthcare and Medicine: The Impact of Modern Technologies for a Healthier Future—A Comprehensive Review. Health Care Sci. 2024, 3, 329–349. [Google Scholar] [CrossRef]
- Parente, D.J. Generative Artificial Intelligence and Large Language Models in Primary Care Medical Education. Fam. Med. 2024, 56, 534–540. [Google Scholar] [CrossRef]
- Neha, F.; Bhati, D.; Shukla, D.K.; Amiruzzaman, M. ChatGPT: Transforming Healthcare with AI. AI 2024, 5, 2618–2650. [Google Scholar] [CrossRef]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef] [PubMed]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. Ethical and Regulatory Challenges of AI Technologies in Healthcare: A Narrative Review. Heliyon 2024, 10, e26297. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, J.; Shen, J.; An, R. Artificial Intelligence Applications to Measure Food and Nutrient Intakes: Scoping Review. J. Med. Internet Res. 2024, 26, e54557. [Google Scholar] [CrossRef]
- Vasiloglou, M.F.; van der Horst, K.; Stathopoulou, T.; Jaeggi, M.P.; Tedde, G.S.; Lu, Y.; Mougiakakou, S. The Human Factor in Automated Image-Based Nutrition Apps: Analysis of Common Mistakes Using the goFOOD Lite App. JMIR Mhealth Uhealth 2021, 9, e24467. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Oniani, D.; Shao, Z.; Arciero, P.; Sivarajkumar, S.; Hilsman, J.; Mohr, A.E.; Ibe, S.; Moharir, M.; Li, L.-J.; et al. A Scoping Review of Artificial Intelligence for Precision Nutrition. Adv. Nutr. 2025, 16, 100398. [Google Scholar] [CrossRef]
- Dias, S.B.; Oikonomidis, Y.; Diniz, J.A.; Baptista, F.; Carnide, F.; Bensenousi, A.; Botana, J.M.; Tsatsou, D.; Stefanidis, K.; Gymnopoulos, L.; et al. Users’ Perspective on the AI-Based Smartphone PROTEIN App for Personalized Nutrition and Healthy Living: A Modified Technology Acceptance Model (mTAM) Approach. Front. Nutr. 2022, 9, 898031. [Google Scholar] [CrossRef]
- Vasiloglou, M.F.; Marcano, I.; Lizama, S.; Papathanail, I.; Spanakis, E.K.; Mougiakakou, S. Multimedia Data-Based Mobile Applications for Dietary Assessment. J. Diabetes Sci. Technol. 2022, 17, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Z.; Xue, H.; An, R. Artificial Intelligence Applications to Personalized Dietary Recommendations: A Systematic Review. Healthcare 2025, 13, 1417. [Google Scholar] [CrossRef]
- Maher, C.A.; Davis, C.R.; Curtis, R.G.; Short, C.E.; Murphy, K.J. A Physical Activity and Diet Program Delivered by Artificially Intelligent Virtual Health Coach: Proof-of-Concept Study. JMIR Mhealth Uhealth 2020, 8, e17558. [Google Scholar] [CrossRef]
- Meder, B.; Asselbergs, F.W.; Ashley, E. Artificial Intelligence to Improve Cardiovascular Population Health. Eur. Heart J. 2025, 46, 1907–1916. [Google Scholar] [CrossRef]
- Kaya Kaçar, H.; Kaçar, Ö.F.; Avery, A. Diet Quality and Caloric Accuracy in AI-Generated Diet Plans: A Comparative Study Across Chatbots. Nutrients 2025, 17, 206. [Google Scholar] [CrossRef]
- Kim, D.W.; Park, J.S.; Sharma, K.; Velazquez, A.; Li, L.; Ostrominski, J.W.; Tran, T.; Seitter Peréz, R.H.; Shin, J.-H. Qualitative Evaluation of Artificial Intelligence-Generated Weight Management Diet Plans. Front. Nutr. 2024, 11, 1374834. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, V.; Rosato, R.; Scigliano, M.C.; Onida, M.; Cossai, S.; De Vecchi, M.; Devecchi, A.; Goitre, I.; Favaro, E.; Merlo, F.D.; et al. Comparison of the Accuracy, Completeness, Reproducibility, and Consistency of Different AI Chatbots in Providing Nutritional Advice: An Exploratory Study. J. Clin. Med. 2024, 13, 7810. [Google Scholar] [CrossRef]
- Patra, E.; Kokkinopoulou, A.; Wilson-Barnes, S.; Hart, K.; Gymnopoulos, L.P.; Tsatsou, D.; Solachidis, V.; Dimitropoulos, K.; Rouskas, K.; Argiriou, A.; et al. Personal Goals, User Engagement, and Meal Adherence within a Personalised AI-Based Mobile Application for Nutrition and Physical Activity. Life 2024, 14, 1238. [Google Scholar] [CrossRef]
- Li, X.; Yin, A.; Choi, H.Y.; Chan, V.; Allman-Farinelli, M.; Chen, J. Evaluating the Quality and Comparative Validity of Manual Food Logging and Artificial Intelligence-Enabled Food Image Recognition in Apps for Nutrition Care. Nutrients 2024, 16, 2573. [Google Scholar] [CrossRef]
- Ponzo, V.; Goitre, I.; Favaro, E.; Merlo, F.D.; Mancino, M.V.; Riso, S.; Bo, S. Is ChatGPT an Effective Tool for Providing Dietary Advice? Nutrients 2024, 16, 469. [Google Scholar] [CrossRef]
- Generative Artificial Intelligence ChatGPT in Clinical Nutrition—Advances and Challenges. Available online: https://www.researchgate.net/publication/389373505_Generative_artificial_intelligence_ChatGPT_in_clinical_nutrition_-_A-vances_and_challenges (accessed on 22 July 2025).
- Chen, P.-J.; Liou, W.-K. ChatGPT-Driven Interactive Virtual Reality Communication Simulation in Obstetric Nursing: A Mixed-Methods Study. Nurse Educ. Pract. 2025, 85, 104383. [Google Scholar] [CrossRef] [PubMed]
- Aster, A.; Ragaller, S.V.; Raupach, T.; Marx, A. ChatGPT as a Virtual Patient: Written Empathic Expressions During Medical History Taking. Med. Sci. Educ. 2025, 35, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Tudor Car, L.; Dhinagaran, D.A.; Kyaw, B.M.; Kowatsch, T.; Joty, S.; Theng, Y.-L.; Atun, R. Conversational Agents in Health Care: Scoping Review and Conceptual Analysis. J. Med. Internet Res. 2020, 22, e17158. [Google Scholar] [CrossRef] [PubMed]
- Hinostroza Fuentes, V.G.; Karim, H.A.; Tan, M.J.T.; AlDahoul, N. AI with Agency: A Vision for Adaptive, Efficient, and Ethical Healthcare. Front. Digit. Health 2025, 7, 1600216. [Google Scholar] [CrossRef]
- Janssen, S.M.; Bouzembrak, Y.; Tekinerdogan, B. Artificial Intelligence in Malnutrition: A Systematic Literature Review. Adv. Nutr. 2024, 15, 100264. [Google Scholar] [CrossRef]
- Kumar, Y.; Koul, A.; Singla, R.; Ijaz, M.F. Artificial Intelligence in Disease Diagnosis: A Systematic Literature Review, Synthesizing Framework and Future Research Agenda. J. Ambient. Intell. Humaniz. Comput. 2023, 14, 8459–8486. [Google Scholar] [CrossRef]
- Maleki Varnosfaderani, S.; Forouzanfar, M. The Role of AI in Hospitals and Clinics: Transforming Healthcare in the 21st Century. Bioengineering 2024, 11, 337. [Google Scholar] [CrossRef]
- Ramezani, M.; Takian, A.; Bakhtiari, A.; Rabiee, H.R.; Ghazanfari, S.; Mostafavi, H. The Application of Artificial Intelligence in Health Policy: A Scoping Review. BMC Health Serv. Res. 2023, 23, 1416. [Google Scholar] [CrossRef] [PubMed]
- Zuhair, V.; Babar, A.; Ali, R.; Oduoye, M.O.; Noor, Z.; Chris, K.; Okon, I.I.; Rehman, L.U. Exploring the Impact of Artificial Intelligence on Global Health and Enhancing Healthcare in Developing Nations. J. Prim. Care Community Health 2024, 15, 21501319241245847. [Google Scholar] [CrossRef]
- Kuhl, E. AI for Food: Accelerating and Democratizing Discovery and Innovation. NPJ Sci. Food 2025, 9, 82. [Google Scholar] [CrossRef]
- Gunning, M.; Tagkopoulos, I. A Systematic Review of Data and Models for Predicting Food Flavor and Texture. Curr. Res. Food Sci. 2025, 11, 101127. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, L.P.; Nogueira, I.B.R.; Ribeiro, A.M. Flavor Engineering: A Comprehensive Review of Biological Foundations, AI Integration, Industrial Development, and Socio-Cultural Dynamics. Food Res. Int. 2024, 196, 115100. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.d.S.; Wazed, M.A.; Asha, S.; Hossen, M.A.; Fime, S.k.N.M.; Teeya, S.T.; Jenny, L.Y.; Dash, D.; Shimul, I.M. Flavor and Well-Being: A Comprehensive Review of Food Choices, Nutrition, and Health Interactions. Food Sci. Nutr. 2025, 13, e70276. [Google Scholar] [CrossRef]
- Weiner, E.B.; Dankwa-Mullan, I.; Nelson, W.A.; Hassanpour, S. Ethical Challenges and Evolving Strategies in the Integration of Artificial Intelligence into Clinical Practice. PLoS Digit. Health 2025, 4, e0000810. [Google Scholar] [CrossRef]
- Nazer, L.H.; Zatarah, R.; Waldrip, S.; Ke, J.X.C.; Moukheiber, M.; Khanna, A.K.; Hicklen, R.S.; Moukheiber, L.; Moukheiber, D.; Ma, H.; et al. Bias in Artificial Intelligence Algorithms and Recommendations for Mitigation. PLoS Digit. Health 2023, 2, e0000278. [Google Scholar] [CrossRef]
- Ennab, M.; Mcheick, H. Enhancing Interpretability and Accuracy of AI Models in Healthcare: A Comprehensive Review on Challenges and Future Directions. Front. Robot. AI 2024, 11, 1444763. [Google Scholar] [CrossRef]
- Alkhanbouli, R.; Matar Abdulla Almadhaani, H.; Alhosani, F.; Simsekler, M.C.E. The Role of Explainable Artificial Intelligence in Disease Prediction: A Systematic Literature Review and Future Research Directions. BMC Med. Inform. Decis. Mak. 2025, 25, 110. [Google Scholar] [CrossRef]
- Murdoch, B. Privacy and Artificial Intelligence: Challenges for Protecting Health Information in a New Era. BMC Med. Ethics 2021, 22, 122. [Google Scholar] [CrossRef]
- Khalid, M.I.; Ahmed, M.; Kim, J. Enhancing Data Protection in Dynamic Consent Management Systems: Formalizing Privacy and Security Definitions with Differential Privacy, Decentralization, and Zero-Knowledge Proofs. Sensors 2023, 23, 7604. [Google Scholar] [CrossRef]
- Esmaeilzadeh, P.; Maddah, M.; Mirzaei, T. Using AI Chatbots (e.g., CHATGPT) in Seeking Health-Related Information Online: The Case of a Common Ailment. Comput. Hum. Behav. Artif. Hum. 2025, 3, 100127. [Google Scholar] [CrossRef]
- Chew, H.S.J.; Achananuparp, P. Perceptions and Needs of Artificial Intelligence in Health Care to Increase Adoption: Scoping Review. J. Med. Internet Res. 2022, 24, e32939. [Google Scholar] [CrossRef] [PubMed]
- Tammets, K.; Ley, T. Integrating AI Tools in Teacher Professional Learning: A Conceptual Model and Illustrative Case. Front. Artif. Intell. 2023, 6, 1255089. [Google Scholar] [CrossRef] [PubMed]
- Mohd Johari, N.F.; Mohamad Ali, N.; Mhd Salim, M.H.; Abdullah, N.A. Factors Driving the Use of Mobile Health App: Insights from a Survey. Mhealth 2025, 11, 12. [Google Scholar] [CrossRef]
- Berger, M.; Jung, C. Gamification Preferences in Nutrition Apps: Toward Healthier Diets and Food Choices. Digit. Health 2024, 10, 20552076241260482. [Google Scholar] [CrossRef]
- Vasiloglou, M.F.; Christodoulidis, S.; Reber, E.; Stathopoulou, T.; Lu, Y.; Stanga, Z.; Mougiakakou, S. Perspectives and Preferences of Adult Smartphone Users Regarding Nutrition and Diet Apps: Web-Based Survey Study. JMIR Mhealth Uhealth 2021, 9, e27885. [Google Scholar] [CrossRef]
- Chew, H.S.J.; Chew, N.W.; Loong, S.S.E.; Lim, S.L.; Tam, W.S.W.; Chin, Y.H.; Chao, A.M.; Dimitriadish, G.K.; Gao, Y.; So, J.B.Y.; et al. Effectiveness of an Artificial Intelligence-Assisted App for Improving Eating Behaviors: Mixed Methods Evaluation. J. Med. Internet Res. 2024, 26, e46036. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.; Zare, Z.; Mojtabaeian, S.M.; Izadi, R. Artificial Intelligence and Decision-Making in Healthcare: A Thematic Analysis of a Systematic Review of Reviews. Health Serv. Res. Manag. Epidemiol. 2024, 11, 23333928241234863. [Google Scholar] [CrossRef]
- Krishnan, G.; Singh, S.; Pathania, M.; Gosavi, S.; Abhishek, S.; Parchani, A.; Dhar, M. Artificial Intelligence in Clinical Medicine: Catalyzing a Sustainable Global Healthcare Paradigm. Front. Artif. Intell. 2023, 6, 1227091. [Google Scholar] [CrossRef]
- Lewis, K.H.; Hsu, F.-C.; Block, J.P.; Skelton, J.A.; Schwartz, M.B.; Krieger, J.; Hindel, L.R.; Ospino Sanchez, B.; Zoellner, J. A Technology-Driven, Healthcare-Based Intervention to Improve Family Beverage Choices: Results from a Pilot Randomized Trial in the United States. Nutrients 2023, 15, 2141. [Google Scholar] [CrossRef]
- Lee, B.Y.; Ordovás, J.M.; Parks, E.J.; Anderson, C.A.M.; Barabási, A.-L.; Clinton, S.K.; de la Haye, K.; Duffy, V.B.; Franks, P.W.; Ginexi, E.M.; et al. Research Gaps and Opportunities in Precision Nutrition: An NIH Workshop Report. Am. J. Clin. Nutr. 2022, 116, 1877–1900. [Google Scholar] [CrossRef]
- Tornero-Costa, R.; Martinez-Millana, A.; Azzopardi-Muscat, N.; Lazeri, L.; Traver, V.; Novillo-Ortiz, D. Methodological and Quality Flaws in the Use of Artificial Intelligence in Mental Health Research: Systematic Review. JMIR Ment. Health 2023, 10, e42045. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L.; Li, K. Large Language Models in Medical Chatbots: Opportunities, Challenges, and the Need to Address AI Risks. Information 2025, 16, 549. [Google Scholar] [CrossRef]
- Brereton, T.A.; Malik, M.M.; Lifson, M.; Greenwood, J.D.; Peterson, K.J.; Overgaard, S.M. The Role of Artificial Intelligence Model Documentation in Translational Science: Scoping Review. Interact. J. Med. Res. 2023, 12, e45903. [Google Scholar] [CrossRef] [PubMed]
- Galanty, M.; Luitse, D.; Noteboom, S.H.; Croon, P.; Vlaar, A.P.; Poell, T.; Sanchez, C.I.; Blanke, T.; Išgum, I. Assessing the Documentation of Publicly Available Medical Image and Signal Datasets and Their Impact on Bias Using the BEAMRAD Tool. Sci. Rep. 2024, 14, 31846. [Google Scholar] [CrossRef]
- Hernandez-Boussard, T.; Bozkurt, S.; Ioannidis, J.P.A.; Shah, N.H. MINIMAR (MINimum Information for Medical AI Reporting): Developing Reporting Standards for Artificial Intelligence in Health Care. J. Am. Med. Inform. Assoc. 2020, 27, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Krois, J. Better Reporting of Studies on Artificial Intelligence: CONSORT-AI and Beyond. J. Dent. Res. 2021, 100, 677–680. [Google Scholar] [CrossRef]
- Raducu, R.; Esteban, G.; Rodríguez Lera, F.J.; Fernández, C. Collecting Vulnerable Source Code from Open-Source Repositories for Dataset Generation. Appl. Sci. 2020, 10, 1270. [Google Scholar] [CrossRef]
- LeBlanc, K.E.; Baer-Sinnott, S.; Lancaster, K.J.; Campos, H.; Lau, K.H.K.; Tucker, K.L.; Kushi, L.H.; Willett, W.C. Perspective: Beyond the Mediterranean Diet—Exploring Latin American, Asian, and African Heritage Diets as Cultural Models of Healthy Eating. Adv. Nutr. 2024, 15, 100221. [Google Scholar] [CrossRef]
- Barreda, M.; Cantarero-Prieto, D.; Coca, D.; Delgado, A.; Lanza-León, P.; Lera, J.; Montalbán, R.; Pérez, F. Transforming Healthcare with Chatbots: Uses and Applications—A Scoping Review. Digit. Health 2025, 11, 20552076251319174. [Google Scholar] [CrossRef]
- Grande, F.; Vincent, A. The Importance of Food Composition Data for Estimating Micronutrient Intake: What Do We Know Now and into the Future? Nestle Nutr. Inst. Workshop Ser. 2020, 93, 39–50. [Google Scholar] [CrossRef]
- Quan, S.; Zhu, W. Measuring Global Dietary Diversity by Considering Nutritional Functional Dissimilarity and Dietary Guidelines. Foods 2025, 14, 1759. [Google Scholar] [CrossRef]
- Whitehead, L.; Talevski, J.; Fatehi, F.; Beauchamp, A. Barriers to and Facilitators of Digital Health Among Culturally and Linguistically Diverse Populations: Qualitative Systematic Review. J. Med. Internet Res. 2023, 25, e42719. [Google Scholar] [CrossRef]
- Young, M.; Smith, M.A. Standards and Evaluation of Healthcare Quality, Safety, and Person-Centered Care. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2025. [Google Scholar]
- Koehle, H.; Kronk, C.; Lee, Y.J. Digital Health Equity: Addressing Power, Usability, and Trust to Strengthen Health Systems. Yearb. Med. Inform. 2022, 31, 20–32. [Google Scholar] [CrossRef]
- Singh, M.P.; Keche, Y.N. Ethical Integration of Artificial Intelligence in Healthcare: Narrative Review of Global Challenges and Strategic Solutions. Cureus 2025, 17, e84804. [Google Scholar] [CrossRef]
| AI Technique | Application Area | Examples | Strengths | Limitations |
|---|---|---|---|---|
| Machine Learning (ML) | Dietary assessment, predictive modeling | Prediction of nutrient intake patterns; risk stratification for T2DM/obesity | Learns from large datasets, identifies hidden patterns, adaptable to diverse contexts | Requires large, high-quality datasets; risk of bias from training data |
| Deep Learning (DL) | Food image recognition, portion estimation | CNN-based food recognition apps (e.g., FoodAI, DietCam) | High accuracy in visual classification; reduces self-report errors | Limited generalizability; depends on food image databases |
| Natural Language Processing (NLP) | Conversational agents, dietary advice | Chatbots simulating dietitians; LLM-based nutrition Q&A | Enables real-time dialogue, supports education, improves accessibility | Challenges in ensuring accuracy, potential for misinformation |
| Large Language Models (LLMs) | Virtual coaching, education, health literacy | ChatGPT-based nutrition assistants, personalized diet advice | Generates tailored responses, scalable, user-friendly | Explainability issues, prone to hallucinations, ethical concerns |
| Reinforcement Learning (RL) | Behavior change support, personalized recommendations | Adaptive diet plans based on user adherence | Learns dynamically from user feedback, supports habit formation | Computationally intensive; limited testing in nutrition contexts |
| Hybrid Models (ML + Sensors/IoT) | Continuous monitoring, precision nutrition | Wearables integrating HRV, glucose, activity with AI-driven diet feedback | Combines physiological + behavioral data; supports real-time personalized nutrition | Data privacy issues; requires interoperability of devices |
| Generative AI | Food innovation, flavor design | AI-assisted flavor compound generation, recipe development | Creative potential; accelerates product development in sensory science | Early stage; limited validation of consumer acceptance |
| Theme | Key Issues | Implications for Practice | Proposed Strategies |
|---|---|---|---|
| Bias in Training Data | Underrepresentation of certain populations, cultural food diversity not captured | Risk of inaccurate or inequitable recommendations for minority and low-income groups | Curate diverse datasets; conduct fairness audits; continuous model retraining |
| Transparency & Explainability | Black-box nature of deep learning and LLMs | Reduced trust among clinicians and patients; difficulty in verifying recommendations | Develop interpretable models; provide confidence scores; use explainable AI techniques |
| Data Privacy & Security | Sensitive dietary, medical, and biometric data at risk | Potential breaches of GDPR/HIPAA compliance; erosion of patient trust | Encryption, federated learning, anonymization, robust consent frameworks |
| Professional Roles | Concerns that AI might replace dietitians | Threat to professional identity; fear of devaluation of expertise | Promote AI as augmentation rather than replacement; emphasize collaboration |
| Task-Shifting | Delegation of routine tasks to AI systems | Risk of oversimplifying complex patient cases | Clear delineation of AI vs. human responsibilities; establish clinical oversight |
| Education & Training Needs | Lack of digital/AI literacy among dietitians and nutritionists | Risk of misuse or over-reliance on AI systems | Integrate AI literacy into curricula and continuing professional education programs |
| Accountability & Liability | Ambiguity about responsibility when AI advice causes harm | Legal and ethical uncertainty for clinicians and institutions | Define liability frameworks; establish shared accountability between developers & users |
| Equity in Access | Limited availability in low- and middle-income countries | Risk of widening global health disparities | Promote open-source solutions; support infrastructure development; encourage global policy |
| Study | AI Technique | Application | Study Design | Key Outcomes |
|---|---|---|---|---|
| Vasiloglou, 2021 [23] | Deep learning (CNN) | Food image recognition (goFOODTM) | Validation against dietitian assessments | Moderate agreement achieved; main errors in mixed meals and lighting conditions |
| Wang, 2025 [27] | LLM + image recognition | Personalized meal planning for T2DM | Preclinical validation study | Accurate nutrient analysis and tailored diet recommendations |
| Maher, 2020 [28] | Virtual health coach (ML + chatbot) | Diet and physical activity counseling | Proof-of-concept RCT (12 weeks) | Significant improvements in fruit/vegetable intake and physical activity |
| Ponzo, 2024 [35] | AI chatbots (ChatGPT, Bard, Bing) | Dietary advice provision | Comparative evaluation study | ChatGPT most consistent, but all chatbots showed variable accuracy and reproducibility |
| Chew, 2024 [62] | AI-assisted food tracking app | Eating behavior modification | Mixed-methods evaluation | Improved adherence and user satisfaction with personalized feedback |
| Lewis, 2023 [65] | AI-enhanced nutrition app | Beverage choice improvement | Pilot RCT | Increased water intake, reduced sugary drink consumption over 3 months |
| Research Gap | Current Limitation | Future Direction |
|---|---|---|
| Lack of standardized validation protocols | Many studies use inconsistent metrics, making cross-comparison difficult | Develop unified validation frameworks; adopt reporting standards (e.g., CONSORT-AI) |
| Poor reporting of AI model architectures | Insufficient detail on algorithms, hyperparameters, and training datasets | Encourage transparent reporting; promote open science and model-sharing practices |
| Generalizability across populations | Most models trained on Western, high-income populations; poor adaptation to diverse diets | Expand datasets to include global populations; integrate cultural dietary variability |
| Underrepresentation of LMICs | Scarcity of studies and implementation in low- and middle-income countries | Support international collaborations; design resource-appropriate AI solutions |
| Limited real-world implementation evidence | Many tools tested only in pilot studies or controlled settings | Conduct pragmatic trials and long-term implementation studies in diverse contexts |
| Data privacy and ethical frameworks | Unclear accountability and uneven compliance with GDPR/HIPAA | Advance federated learning, anonymization, and robust governance frameworks |
| Integration with clinical workflows | Lack of seamless interoperability with electronic health records (EHRs) | Develop standards for interoperability; test integration in real-world health systems |
| Equity and access issues | Risk of AI widening health disparities | Design inclusive tools; subsidize access; ensure open-source and low-cost solutions |
| AI literacy among professionals | Many dietitians lack training in AI technologies | Include AI/ML modules in curricula; create continuing education opportunities |
| Evaluation of multimodal models | Few studies explore combined use of LLMs, images, and sensor data | Advance multimodal research; test integration with genomics, wearables, and imaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panayotova, G.G. Artificial Intelligence in Nutrition and Dietetics: A Comprehensive Review of Current Research. Healthcare 2025, 13, 2579. https://doi.org/10.3390/healthcare13202579
Panayotova GG. Artificial Intelligence in Nutrition and Dietetics: A Comprehensive Review of Current Research. Healthcare. 2025; 13(20):2579. https://doi.org/10.3390/healthcare13202579
Chicago/Turabian StylePanayotova, Gabriela Georgieva. 2025. "Artificial Intelligence in Nutrition and Dietetics: A Comprehensive Review of Current Research" Healthcare 13, no. 20: 2579. https://doi.org/10.3390/healthcare13202579
APA StylePanayotova, G. G. (2025). Artificial Intelligence in Nutrition and Dietetics: A Comprehensive Review of Current Research. Healthcare, 13(20), 2579. https://doi.org/10.3390/healthcare13202579






