Effect of Plantar Sensory Stimulation on Sensorimotor Organization in General Joint Hypermobility: A Randomized Controlled Study
Abstract
1. Introduction
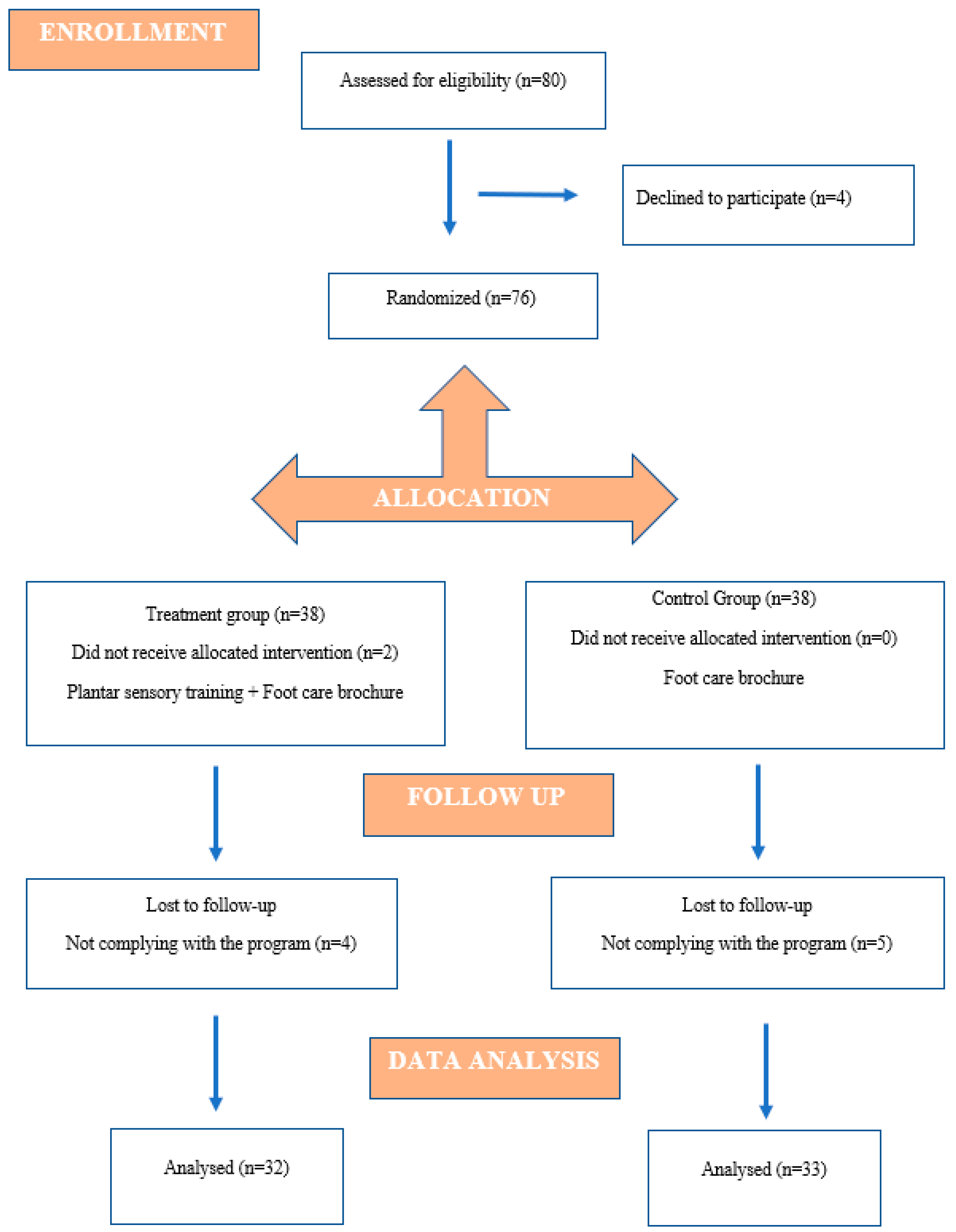
2. Materials and Methods
2.1. Participants
2.2. Power Analysis
2.3. Outcome Measures
2.4. Intervention
- Materials: Participants in the treatment group were provided with a spiky proprioceptive ball and three carpet pieces with different hardness levels to ensure program standardization. Additionally, a 500 mL water bottle and a chair were used for specific exercises. Warm water and moisturizing cream were recommended for foot preparation.
- Provider: A physiotherapist with 10 years of clinical experience initially demonstrated and instructed the intervention program. Participants also received written guidelines and an informative brochure to support the home application.
- Delivery: The program was delivered as a home-based, individually performed exercise protocol. Participants applied the exercises once daily, independently, after receiving initial training. Standardization was supported through a face-to-face demonstration session held before the program began.
- Location: The intervention was conducted in the participants’ home environment.
- Dosage (Frequency, Intensity, Duration)
- Duration of program: 2 weeks.
- Frequency: 1 session per day.
- Session length: Approximately 20–25 min.
- Exercises included: Washing the feet with warm water and applying moisturizing cream, Static stretching of the gastro-soleus and plantar fascia, Picking up a sheet with the toes, Rolling a 500 mL water bottle back and forth under the sole, Distinguishing three different textured surfaces without visual cues, Circular rolling of the spiky ball on the sole.
- Tailoring (Individualization/Progression): The same exercise set was given to all participants. However, minor adjustments were allowed for difficulty, such as modifying the surface hardness used during sensory discrimination tasks according to participants’ tolerance levels.
- Compliance/Adherence: The intervention was performed in the participants’ home environment. To ensure standardization, a physiotherapist provided a face-to-face demonstration at the start of the program, accompanied by a brochure and written instructions. Each participant was given an exercise log to record daily adherence, and reminder messages were sent once per day.
2.5. Statistical Analysis
3. Results
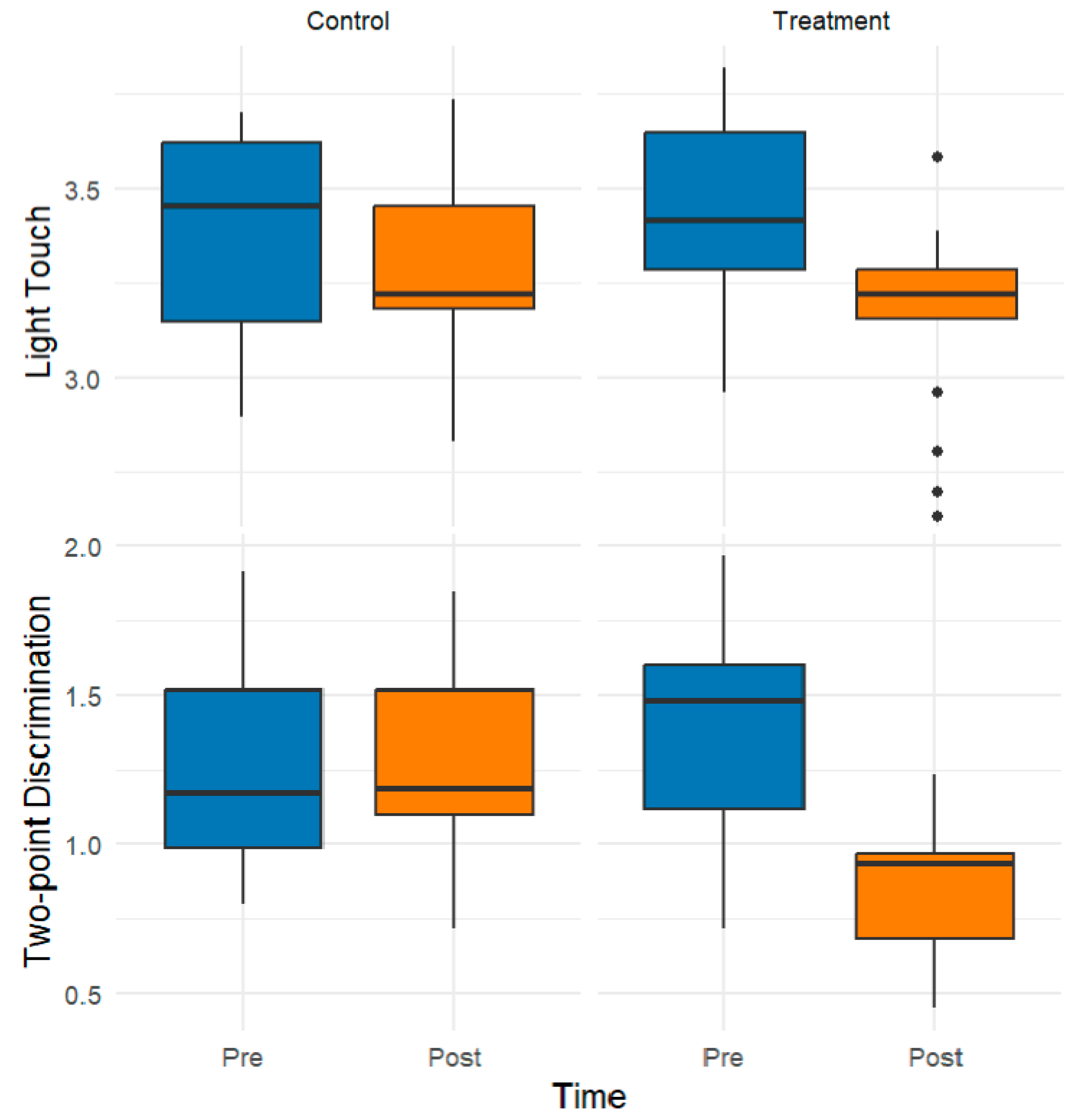
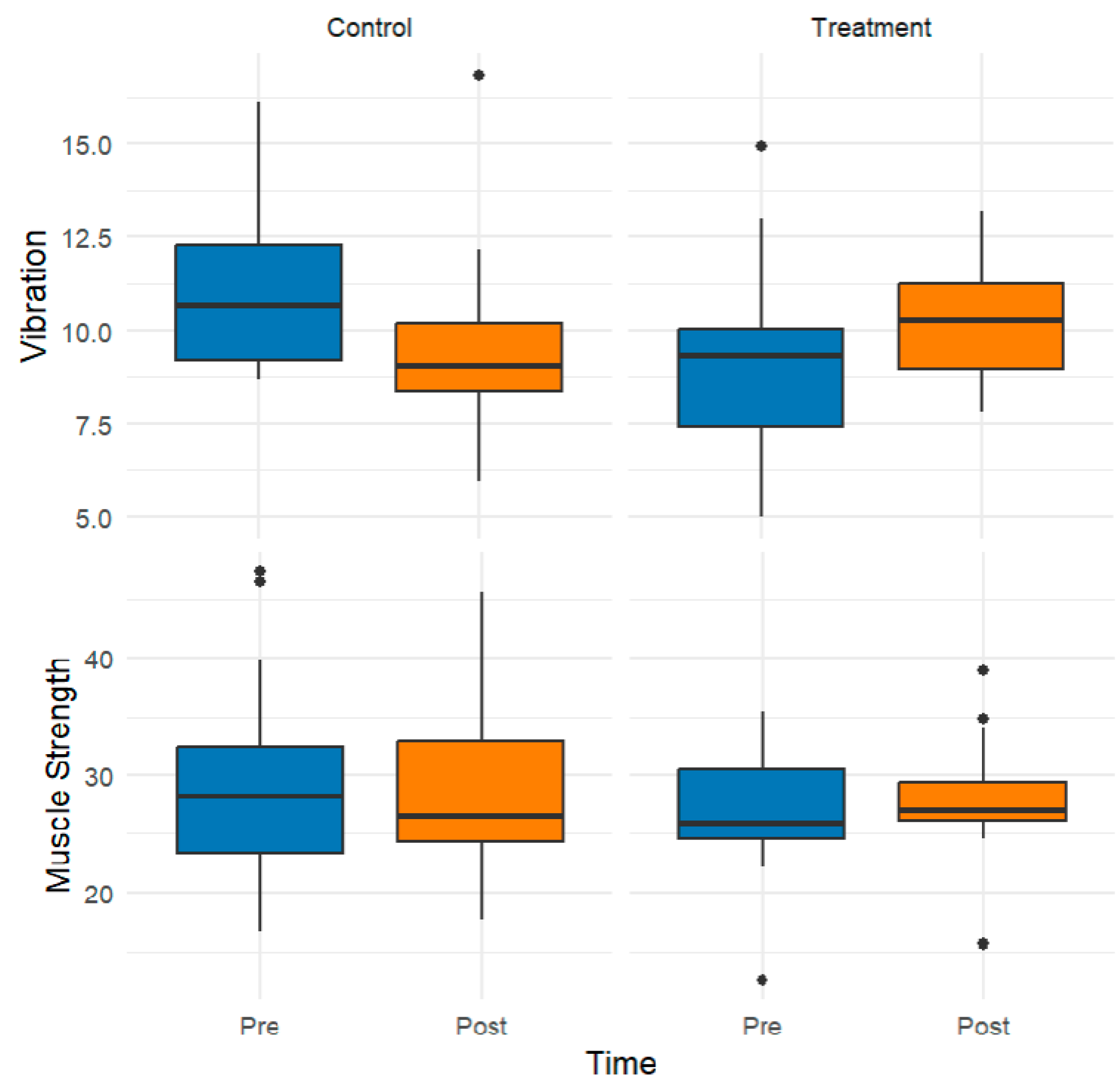
3.1. Exploratory Data Analysis
3.2. Inferential Findings from Rank-Based Mixed ANOVA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GJH | Generalized Joint Hypermobility |
| EDS | Ehlers–Danlos Syndrome |
| hEDS | Hypermobile Ehlers–Danlos Syndrome |
| HSDs | Hypermobility Spectrum Disorders |
| CONSORT | Consolidated Standards of Reporting Trials |
| IPAQ | International Physical Activity Questionnaire |
| SWM | Semmes–Weinstein Monofilament |
| JPS | Joint Position Sense |
| EDA | Exploratory Data Analysis |
| ANOVA | Analysis of Variance |
| TUBITAK | Türkiye Bilimsel ve Teknolojik Araştırma Kurumu |
References
- Castori, M.; Tinkle, B.; Levy, H.; Grahame, R.; Malfait, F.; Hakim, A. A framework for the classification of joint hypermobility and related conditions. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 148–157. [Google Scholar] [CrossRef]
- Remvig, L.; Jensen, D.V.; Ward, R.C. Epidemiology of general joint hypermobility and basis for the proposed criteria for benign joint hypermobility syndrome: Review of the literature. J. Rheumatol. 2007, 34, 804–809. [Google Scholar]
- Russek, L.N.; Errico, D.M. Prevalence, injury rate and, symptom frequency in generalized joint laxity and joint hypermobility syndrome in a “healthy” college population. Clin. Rheumatol. 2016, 35, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Zeng, X.; Xie, Y.; Lai, J.; Wu, J.; Xu, H.; Lin, C.; Li, H.; Cui, C.; Ma, L. Prevalence and dynamic characteristics of generalized joint hypermobility in college students. Gait Posture 2021, 84, 254–259. [Google Scholar] [CrossRef]
- Blajwajs, L.; Williams, J.; Timmons, W.; Sproule, J. Hypermobility prevalence, measurements, and outcomes in childhood, adolescence, and emerging adulthood: A systematic review. Rheumatol. Int. 2023, 43, 1423–1444. [Google Scholar] [CrossRef] [PubMed]
- Lamari, M.M.; Lamari, N.M.; de Medeiros, M.P.; Giacomini, M.G.; Santos, A.B.; de Araújo Filho, G.M.; Goloni-Bertollo, E.M.; Pavarino, É.C. Generalized Joint Hypermobility: A Statistical Analysis Identifies Non-Axial Involvement in Most Cases. Children 2024, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.; Briggs, J.; Hakim, A.; Grahame, R. Joint laxity and the benign joint hypermobility syndrome in student and professional ballet dancers. J. Rheumatol. 2004, 31, 173–178. [Google Scholar]
- Pacey, V.; Nicholson, L.L.; Adams, R.D.; Munn, J.; Munns, C.F. Generalized joint hypermobility and risk of lower limb joint injury during sport: A systematic review with meta-analysis. Am. J. Sports Med. 2010, 38, 1487–1497. [Google Scholar] [CrossRef]
- Diaz, M.A.; Estevez, E.C.; Guijo, P.S. Joint hyperlaxity and musculoligamentous lesions: Study of a population of homogeneous age, sex and physical exertion. Rheumatology 1993, 32, 120–122. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Testa, G.; Russo, A.; Buccheri, E.; Milana, M.; Prezioso, R.; Pavone, V.; Vecchio, M. Damage for gain: The useful damage of the Pitcher’s paradox. Heliyon 2024, 10, e25401. [Google Scholar] [CrossRef]
- Castori, M.; Morlino, S.; Pascolini, G.; Blundo, C.; Grammatico, P. Gastrointestinal and nutritional issues in joint hypermobility syndrome/Ehlers–Danlos syndrome, hypermobility type. Am. J. Med. Genet. Part C Semin. Med. Genet. 2015, 169, 54–75. [Google Scholar] [CrossRef]
- Bulbena, A.; Pailhez, G.; Bulbena-Cabré, A.; Mallorquí-Bagué, N.; Baeza-Velasco, C. Joint hypermobility, anxiety and psychosomatics: Two and a half decades of progress toward a new phenotype. Adv. Psychosom. Med. 2015, 34, 143–157. [Google Scholar]
- Luder, G.; Aeberli, D.; Mebes, C.M.; Haupt-Bertschy, B.; Baeyens, J.-P.; Verra, M.L. Effect of resistance training on muscle properties and function in women with generalized joint hypermobility: A single-blind pragmatic randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2021, 13, 1–12. [Google Scholar] [CrossRef]
- Hornsby, E.A.; Johnston, L.M. Impact of a Pilates intervention on physical function in children with generalised joint hypermobility and chronic musculoskeletal pain: A single-case experimental design. J. Bodyw. Mov. Ther. 2024, 40, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Chivate, S. Effect of Somatosensory Intervention on Joint Hypermobility and Proprioception in Young Dancers and Nondancers: A Clinical Trial. Indian J. Phys. Ther. Res. 2022, 4, 122–126. [Google Scholar] [CrossRef]
- Kennedy, P.M.; Inglis, J.T. Distribution and behaviour of glabrous cutaneous receptors in the human foot sole. J. Physiol. 2002, 538, 995–1002. [Google Scholar] [CrossRef]
- Bao, S.; Lei, Y. Modulating motor cortex plasticity via cortical and peripheral somatosensory stimulation. J. Neurophysiol. 2025, 133, 1955–1966. [Google Scholar] [CrossRef]
- Aries, A.M.; Downing, P.; Sim, J.; Hunter, S.M. Effectiveness of somatosensory stimulation for the lower limb and foot to improve balance and gait after stroke: A systematic review. Brain Sci. 2022, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.Y.; Hurry, M.; Reed, T.; Quek, J.X.; Strutton, P.H. Cortical contributions to anticipatory postural adjustments in the trunk. J. Physiol. 2018, 596, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Fabre, M.; Chavet, P.; Fornerone, T.; Juan, B.; Abossolo, O.; Pardo, F.; Blouin, J.; Dany, L.; Mouchnino, L. Somatosensory cortical facilitation during step preparation restored by an improved body representation in obese patients. Gait Posture 2020, 80, 246–252. [Google Scholar] [CrossRef]
- Perry, S.D. Evaluation of age-related plantar-surface insensitivity and onset age of advanced insensitivity in older adults using vibratory and touch sensation tests. Neurosci. Lett. 2006, 392, 62–67. [Google Scholar] [CrossRef]
- Hu, X.; Liao, J.; Hu, X.; Zeng, Z.; Wang, L. Effects of plantar-sensory treatments on postural control in chronic ankle instability: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0287689. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Suriyaamarit, D.; Siriphorn, A. Plantar sensory stimulation and its impact on gait and lower limb motor function in individuals with stroke: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0315097. [Google Scholar] [CrossRef]
- Qiu, F.; Cole, M.H.; Davids, K.; Hennig, E.; Silburn, P.; Netscher, H.; Kerr, G. Enhanced somatosensory information decreases postural sway in older people. Gait Posture 2012, 35, 630–635. [Google Scholar] [CrossRef]
- Dursun, Ö.; Mavuş, A.B. Effect of plantar vibration on ankle proprioception in chronic stroke patients: A randomized controlled trial. Physiother. Theory Pract. 2025, 1–11. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J. Pharmacol. Pharmacother. 2010, 1, 100–107. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Citaker, S.; Gunduz, A.G.; Guclu, M.B.; Nazliel, B.; Irkec, C.; Kaya, D. Relationship between foot sensation and standing balance in patients with multiple sclerosis. Gait Posture 2011, 34, 275–278. [Google Scholar] [CrossRef]
- Jerosch-Herold, C. Assessment of sensibility after nerve injury and repair: A systematic review of evidence for validity, reliability and responsiveness of tests. J. Hand Surg. 2005, 30, 252–264. [Google Scholar] [CrossRef]
- Dellon, A.L.; Mackinnon, S.E.; Crosby, P.M. Reliability of two-point discrimination measurements. J. Hand Surg. 1987, 12, 693–696. [Google Scholar] [CrossRef]
- Temlett, J. An assessment of vibration threshold using a biothesiometer compared to a C128-Hz tuning fork. J. Clin. Neurosci. 2009, 16, 1435–1438. [Google Scholar] [CrossRef]
- Duzgun, I.; Kanbur, N.O.; Baltaci, G.; Aydin, T. Effect of Tanner stage on proprioception accuracy. J. Foot Ankle Surg. 2011, 50, 11–15. [Google Scholar] [CrossRef]
- Stark, T.; Walker, B.; Phillips, J.K.; Fejer, R.; Beck, R. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: A systematic review. Pm&r 2011, 3, 472–479. [Google Scholar]
- Plisky, P.J.; Gorman, P.P.; Butler, R.J.; Kiesel, K.B.; Underwood, F.B.; Elkins, B. The reliability of an instrumented device for measuring components of the star excursion balance test. North Am. J. Sports Phys. Ther. NAJSPT 2009, 4, 92. [Google Scholar]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R.; Beck, B.; Bennell, K.; Brosseau, L.; Costa, L.; Cramp, F.; Cup, E. Consensus on exercise reporting template (CERT): Modified Delphi study. Phys. Ther. 2016, 96, 1514–1524. [Google Scholar] [CrossRef]
- Goodall, R.J.; Ellauzi, J.; Tan, M.K.; Onida, S.; Davies, A.H.; Shalhoub, J. A systematic review of the impact of foot care education on self efficacy and self care in patients with diabetes. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 282–292. [Google Scholar] [CrossRef]
- R core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Scheper, M.C.; Juul-Kristensen, B.; Rombaut, L.; Rameckers, E.A.; Verbunt, J.; Engelbert, R.H. Disability in adolescents and adults diagnosed with hypermobility-related disorders: A meta-analysis. Arch. Phys. Med. Rehabil. 2016, 97, 2174–2187. [Google Scholar] [CrossRef]
- Verma, C.; Walankar, P.; Patkar, D. Influence of generalized joint hypermobility on knee joint proprioception in asymptomatic healthy women. Int. J. Phys. Educ. Sports Health 2018, 5, 151–153. [Google Scholar]
- Akkaya, K.U.; Burak, M.; Yildiz, R.; Yildiz, A.; Elbasan, B. Examination of foot sensations in children with generalized joint hypermobility. Early Hum. Dev. 2023, 180, 105755. [Google Scholar] [CrossRef]
- Hijmans, J.M.; Geertzen, J.; Zijlstra, W.; Hof, A.L.; Postema, K. Effects of vibrating insoles on standing balance in diabetic neuropathy. J. Rehabil. Res. Dev. 2008, 45, 1442–1450. [Google Scholar] [CrossRef]
- Viseux, F.; Lemaire, A.; Barbier, F.; Charpentier, P.; Leteneur, S.; Villeneuve, P. How can the stimulation of plantar cutaneous receptors improve postural control? Review and clinical commentary. Neurophysiol. Clin. 2019, 49, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Ratamess, N.A.; Alvar, B.A.; Evetoch, T.K.; Housh, T.J.; Kibler, W.B.; Kraemer, W.J.; Triplett, N.T.; American College of Sports Medicine. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. Off. J. Am. Coll. Sports Med. 2009, 41, 687–708. [Google Scholar]
- Suda, M.; Kawakami, M.; Okuyama, K.; Ishii, R.; Oshima, O.; Hijikata, N.; Nakamura, T.; Oka, A.; Kondo, K.; Liu, M. Validity and reliability of the Semmes-Weinstein Monofilament test and the thumb localizing test in patients with stroke. Front. Neurol. 2021, 11, 625917. [Google Scholar] [CrossRef]
- Zimney, K.; Dendinger, G.; Engel, M.; Mitzel, J. Comparison of reliability and efficiency of two modified two-point discrimination tests and two-point estimation tactile acuity test. Physiother. Theory Pract. 2022, 38, 235–244. [Google Scholar] [CrossRef]
- Suganuma, J.; Ikeda, Y.; Chidori, K. Establishing Reference Values and Detectable Changes for Ankle Joint Position Sense among Healthy Young Adults: Assessment of Sensory Precision. J. Asian Rehabil. Sci. 2024, 7, 8–14. [Google Scholar]
- Zheng, Y.; Feng, R.; Hu, W.; Huang, P. Investigation of inter-rater and test-retest reliability of Y balance test in college students with flexible flatfoot. BMC Sports Sci. Med. Rehabil. 2024, 16, 40. [Google Scholar] [CrossRef]
- Norman, G.R.; Sloan, J.A.; Wyrwich, K.W. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med. Care 2003, 41, 582–592. [Google Scholar] [CrossRef]

| Variable | Treatment Median (IQR) | Control Median (IQR) | p * | p ** |
|---|---|---|---|---|
| Age (years) | 20.00 (2.00) | 20.00 (1.25) | <0.001 | 0.633 |
| Weight (kg) | 65.00 (16.00) | 55.00 (20.00) | <0.001 | 0.137 |
| Height (cm) | 164.00 (8.00) | 164.00 (5.00) | <0.001 | 0.859 |
| Beighton Score | 8.50 (2.00) | 8.00 (0.75) | <0.001 | 0.688 |
| n (%) | n (%) | Total | p *** | |
| Gender | 0.105 | |||
| Female | 27 (84.37) | 32 (96.97) | 59 (90.76) | |
| Male | 5 (15.62) | 1 (3.03) | 6 (9.23) | |
| IPAQ Level | ||||
| Inactive | 25 (78.13) | 27 (81.82) | 52 (80) | |
| Minimally active | 4 (12.5) | 6 (18.18) | 10 (15.38) | 0.209 |
| Very active | 3 (9.38) | 0 (0) | 3 (4.62) |
| Muscle Strength (kg) | Control | Treatment | |||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Knee extension | R | 35.00 (8.77) | 32.40 (13.10) | 27.50 (12.70) | 36.60 (4.50) |
| L | 34.55 (16.63) | 31.50 (15.90) | 32.00 (7.30) | 33.30 (9.70) | |
| Hip flexion | R | 30.00 (7.70) | 28.00 (8.90) | 28.80 (13.10) | 27.00 (8.10) |
| L | 27.70 (7.80) | 24.50 (6.40) | 28.10 (6.90) | 25.00 (14.30) | |
| Plantar flexion | R | 28.80 (11.90) | 26.30 (14.80) | 25.60 (5.60) | 28.40 (7.40) |
| L | 30.60 (3.20) | 31.60 (13.10) | 27.60 (2.50) | 21.40 (11.60) | |
| Dorsiflexion | R | 18.00 (12.20) | 22.50 (5.00) | 19.20 (8.30) | 22.50 (6.60) |
| L | 16.40 (13.70) | 20.20 (10.30) | 18.90 (7.50) | 24.00 (3.20) | |
| Light touch | |||||
| First metatarsal head | R | 3.20 (0.40) | 3.60 (0.40) | 3.20 (0.00) | 3.60 (0.40) |
| L | 3.20 (0.40) | 3.20 (0.40) | 3.20 (0.80) | 3.60 (0.60) | |
| Fifth metatarsal head | R | 3.20 (0.40) | 3.60 (0.40) | 3.60 (0.40) | 3.60 (0.40) |
| L | 3.20 (0.50) | 3.40 (0.40) | 3.60 (0.40) | 3.20 (0.00) | |
| Heel | R | 3.60 (0.60) | 3.20 (0.10) | 3.60 (0.60) | 3.20 (0.40) |
| L | 3.20 (0.40) | 3.20 (0.40) | 3.60 (0.60) | 3.60 (0.60) | |
| Two-point discrimination (mm) | |||||
| First metatarsal head | R | 1.20 (0.40) | 1.00 (0.60) | 1.40 (0.40) | 0.80 (0.40) |
| L | 1.10 (0.60) | 1.10 (0.30) | 1.40 (0.30) | 0.90 (0.40) | |
| Fifth metatarsal head | R | 1.30 (0.50) | 1.30 (0.40) | 1.40 (0.60) | 0.80 (0.40) |
| L | 1.00 (0.50) | 1.20 (0.60) | 1.30 (0.90) | 0.80 (0.40) | |
| Heel | R | 1.20 (0.20) | 1.10 (0.40) | 1.50 (0.60) | 1.00 (0.20) |
| L | 1.20 (0.30) | 1.20 (0.60) | 1.50 (0.20) | 1.00 (0.60) | |
| Vibration (sec) | |||||
| First metatarsal head | R | 9.60 (4.10) | 10.00 (3.40) | 10.60 (1.80) | 9.80 (4.10) |
| L | 9.40 (5.30) | 13.00 (3.20) | 9.70 (3.40) | 8.90 (2.30) | |
| Fifth metatarsal head | R | 8.10 (2.60) | 9.50 (2.30) | 10.30 (2.80) | 10.60 (2.40) |
| L | 9.40 (5.30) | 13.00 (3.20) | 9.70 (3.40) | 8.90 (2.30) | |
| Heel | R | 9.00 (3.10) | 12.10 (2.20) | 10.60 (1.90) | 8.00 (3.50) |
| L | 9.70 (3.10) | 9.80 (2.20) | 9.60 (1.90) | 9.20 (3.50) | |
| Proprioception (◦) | |||||
| Dorsiflexion | R | 2.10 (1.90) | 2.30 (1.40) | 2.60 (3.70) | 1.10 (1.70) |
| L | 2.00 (2.10) | 2.30 (2.30) | 1.10 (4.00) | 1.00 (0.10) | |
| Plantar flexion | R | 2.30 (1.00) | 4.00 (2.30) | 4.30 (5.30) | 1.30 (0.60) |
| L | 3.00 (3.30) | 5.00 (3.70) | 3.30 (2.00) | 1.60 (1.30) | |
| Balance (cm) | |||||
| Anterior | 61.60 (4.90) | 65.80 (11.00) | 66.00 (12.70) | 70.00 (7.40) | |
| Posterolateral | 74.60 (8.72) | 70.50 (11.70) | 72.00 (3.30) | 80.30 (12.00) | |
| Posteromedial | 71.00 (12.25) | 74.00 (15.47) | 72.00 (20.00) | 72.00 (8.00) | |
| Variable | p (Time) | p (Group) | p (Group × Time) | |
|---|---|---|---|---|
| Muscle Strength (kg) | ||||
| Knee extension | R | 0.051 | 0.583 | 0.550 |
| L | 0.602 | 0.810 | 0.697 | |
| Hip flexion | R | <0.01 | 0.316 | 0.175 |
| L | 0.021 | 0.545 | 0.394 | |
| Plantar flexion | R | 0.767 | 0.442 | 0.961 |
| L | 0.030 | 0.003 | 0.831 | |
| Dorsiflexion | R | 0.102 | 0.847 | 0.101 |
| L | 0.001 | 0.151 | 0.584 | |
| Light touch | ||||
| First metatarsal head | R | <0.01 | 0.964 | <0.01 |
| L | <0.01 | 0.960 | 0.002 | |
| Fifth metatarsal head | R | <0.01 | 0.023 | 0.059 |
| L | 0.062 | 0.572 | <0.01 | |
| Heel | R | <0.01 | 0.451 | 0.501 |
| L | <0.01 | 0.694 | 0.902 | |
| Two-point discrimination (mm) | ||||
| First metatarsal head | R | <0.01 | 0.008 | <0.01 |
| L | <0.01 | 0.010 | <0.01 | |
| Fifth metatarsal head | R | <0.01 | 0.072 | <0.01 |
| L | <0.01 | 0.080 | 0.001 | |
| Heel | R | <0.01 | 0.424 | <0.01 |
| L | <0.01 | 0.399 | <0.01 | |
| Vibration (sec) | ||||
| First metatarsal head | R | 0.263 | 0.821 | <0.01 |
| L | 0.009 | 0.039 | <0.01 | |
| Fifth metatarsal head | R | 0.048 | 0.091 | 0.023 |
| L | 0.099 | 0.382 | 0.025 | |
| Heel | R | 0.410 | 0.121 | <0.01 |
| L | 0.326 | 0.427 | 0.028 | |
| Proprioception (◦) | ||||
| Dorsiflexion | R | <0.01 | 0.125 | <0.01 |
| L | 0.002 | <0.01 | 0.275 | |
| Plantar flexion | R | <0.01 | 0.012 | 0.007 |
| L | <0.01 | 0.005 | 0.016 | |
| Balance | ||||
| Anterior | <0.01 | 0.040 | 0.160 | |
| Posterolateral | <0.01 | 0.437 | <0.01 | |
| Posteromedial | 0.016 | 0.377 | 0.069 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildiz, R.; Yildiz, A.; Camli, O.; Akkaya, H.; Aydin, M.; Basaran, Z. Effect of Plantar Sensory Stimulation on Sensorimotor Organization in General Joint Hypermobility: A Randomized Controlled Study. Healthcare 2025, 13, 2572. https://doi.org/10.3390/healthcare13202572
Yildiz R, Yildiz A, Camli O, Akkaya H, Aydin M, Basaran Z. Effect of Plantar Sensory Stimulation on Sensorimotor Organization in General Joint Hypermobility: A Randomized Controlled Study. Healthcare. 2025; 13(20):2572. https://doi.org/10.3390/healthcare13202572
Chicago/Turabian StyleYildiz, Ramazan, Ayse Yildiz, Onur Camli, Hüseyin Akkaya, Mehmet Aydin, and Zekiye Basaran. 2025. "Effect of Plantar Sensory Stimulation on Sensorimotor Organization in General Joint Hypermobility: A Randomized Controlled Study" Healthcare 13, no. 20: 2572. https://doi.org/10.3390/healthcare13202572
APA StyleYildiz, R., Yildiz, A., Camli, O., Akkaya, H., Aydin, M., & Basaran, Z. (2025). Effect of Plantar Sensory Stimulation on Sensorimotor Organization in General Joint Hypermobility: A Randomized Controlled Study. Healthcare, 13(20), 2572. https://doi.org/10.3390/healthcare13202572


_MD__MPH_PhD.png)



