Healthcare 5.0-Driven Clinical Intelligence: The Learn-Predict-Monitor-Detect-Correct Framework for Systematic Artificial Intelligence Integration in Critical Care
Abstract
1. Introduction
2. Methods
2.1. Framework Development Methodology
2.2. Literature Synthesis Strategy
3. The LPMDC Framework Architecture
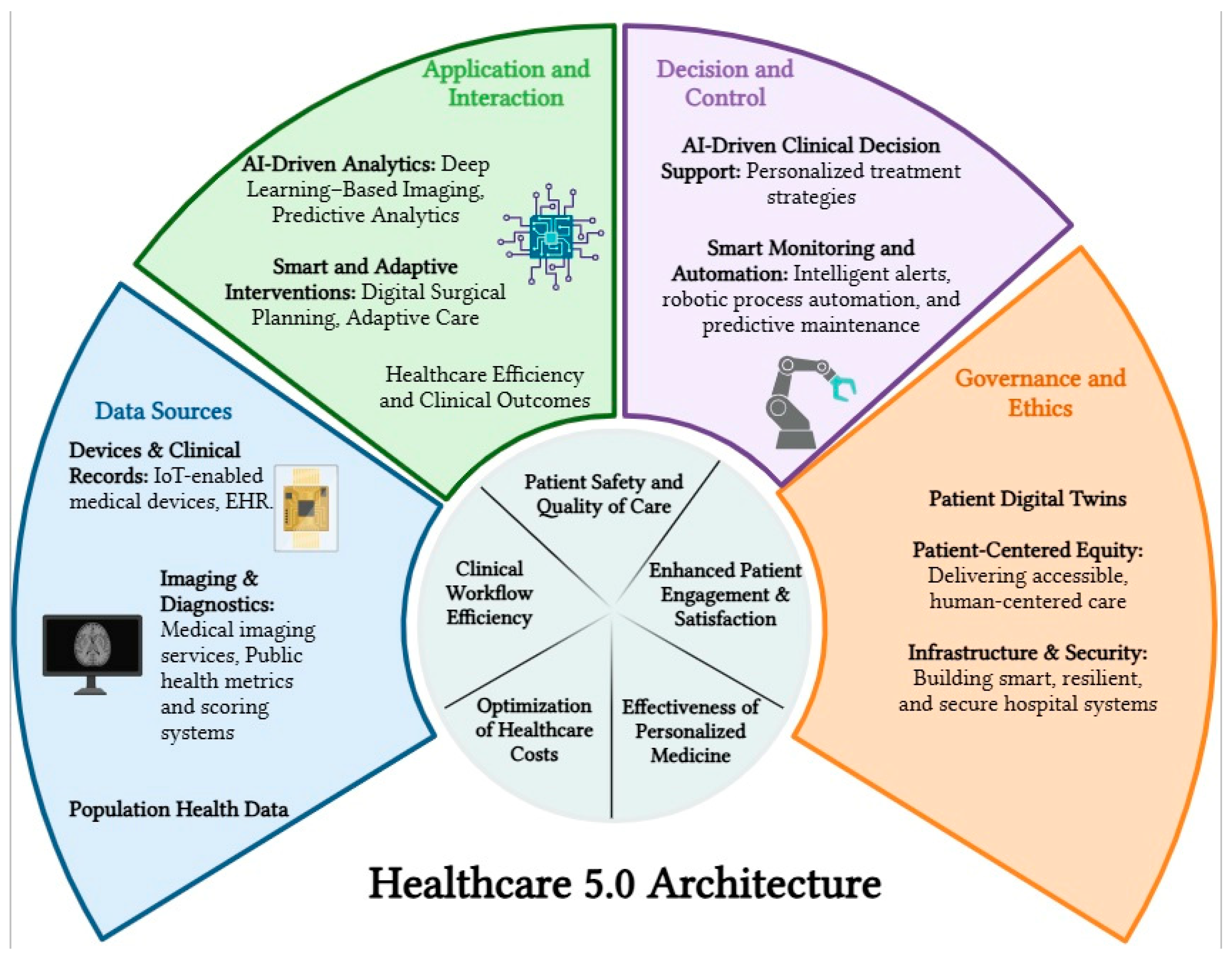
3.1. Healthcare 5.0—Compliant Framework Structure
3.2. Phase I: Learn—Advanced Data Integration and Pattern Recognition
3.3. Phase II: Predict—Sophisticated Risk Stratification and Early Warning
3.4. Phase III: Monitor—Comprehensive Continuous Surveillance
3.5. Phase IV: Detect—Real-Time Anomaly Recognition and Alert Management
3.6. Phase V: Correct—Intelligent Therapeutic Decision Support
4. Clinical Applications and Implementation Evidence
4.1. Sepsis Management and Early Detection
4.2. Respiratory Failure Prediction and Ventilator Management
4.3. Cardiovascular Monitoring and Crisis Prevention
5. Clinical Outcomes and Performance Metrics
5.1. Mortality Reduction and Clinical Effectiveness
5.2. Operational Efficiency and Resource Utilization
5.3. Staff Satisfaction and Workflow Optimization
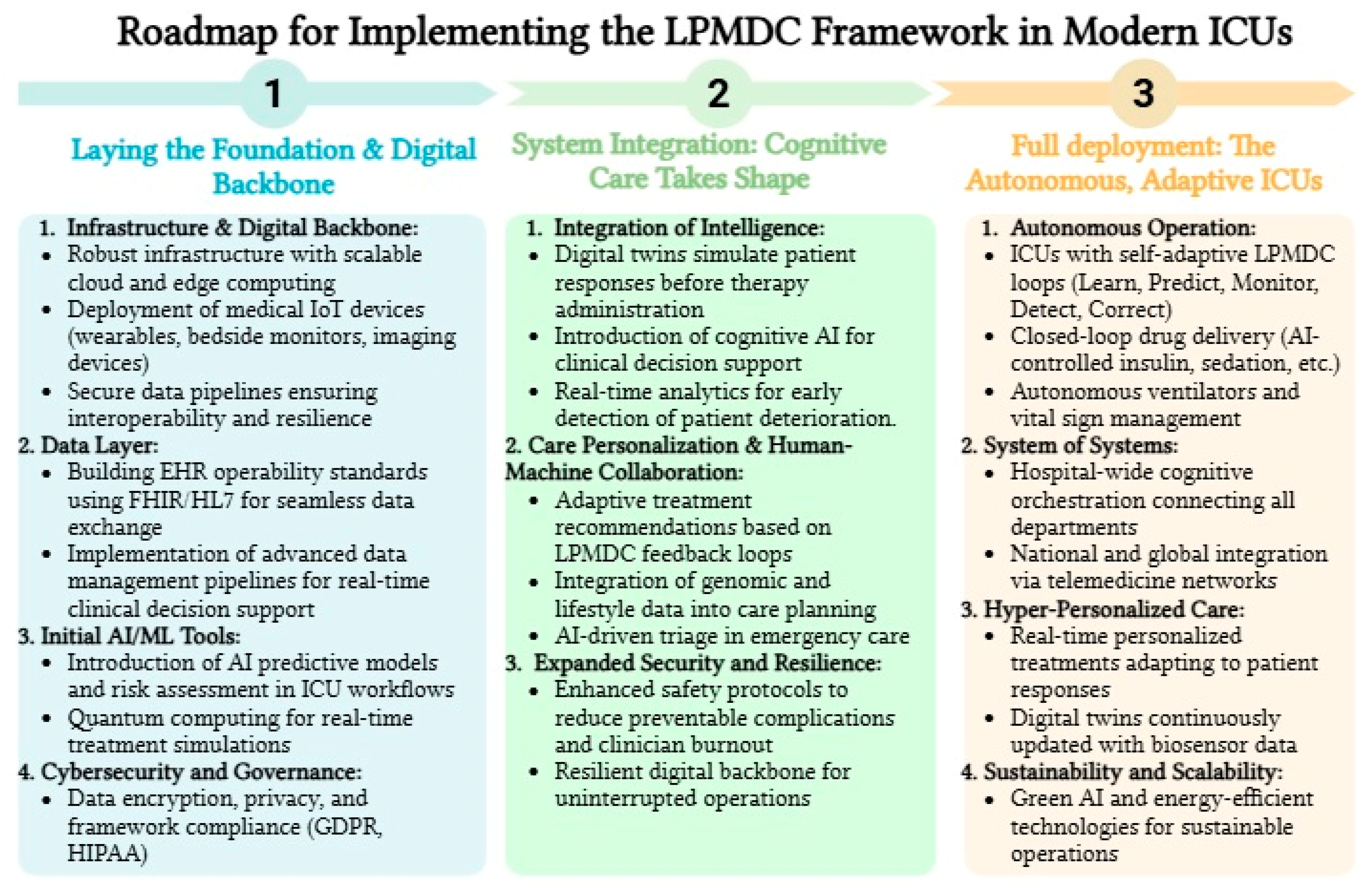
6. Implementation Challenges and Strategic Solutions
6.1. Technical and Infrastructure Requirements
6.2. Organizational and Cultural Adaptation
6.3. Existing Clinical AI Frameworks vs. LPMDC
6.4. Cybersecurity Imperatives for AI-Driven ICUs
6.5. Regulatory and Compliance Framework
6.6. Ethical Safeguards in AI-Enhanced Intensive Care
7. Future Directions and Research Priorities
7.1. Emerging Healthcare 5.0 Technologies
7.2. Clinical Research Priorities
8. Limitations of the LPMDC Framework
8.1. Causal Attribution Limitations
8.2. Limitations of Digital Twins Technology
8.3. Need for Prospective Evaluation
8.4. Regulatory and Medico-Legal Limitations
8.5. Economic Limitations and Concerns
9. Conclusions and Practical Implications
9.1. Healthcare 5.0 Impact on Critical Care Practice
9.2. Practical Implementation Recommendations
9.3. Economic and Healthcare System Impact
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolasa, K. The Digital Transformation of the Healthcare System: Healthcare 5.0; Taylor & Francis: Abingdon, UK, 2023. [Google Scholar]
- Turchi, T.; Prencipe, G.; Malizia, A.; Filogna, S.; Latrofa, F.; Sgandurra, G. Pathways to democratized healthcare: Envisioning human-centered AI-as-a-service for customized diagnosis and rehabilitation. Artif. Intell. Med. 2024, 151, 102850. [Google Scholar] [CrossRef]
- Gomathi, L.; Mishra, A.K.; Tyagi, A.K. Industry 5.0 for healthcare 5.0: Opportunities, challenges and future research possibilities. In Proceedings of the 2023 7th IEEE International Conference on Trends in Electronics and Informatics (ICOEI), Tamil Nadu, India, 11–13 April 2023. [Google Scholar]
- Pinsky, M.R.; Bedoya, A.; Bihorac, A.; Celi, L.; Churpek, M.; Economou-Zavlanos, N.J.; Elbers, P.; Saria, S.; Liu, V.; Lyons, P.G.; et al. Use of artificial intelligence in critical care: Opportunities and obstacles. Crit. Care 2024, 28, 113. [Google Scholar] [CrossRef]
- Davoudi, A.; Malhotra, K.R.; Shickel, B.; Siegel, S.; Williams, S.; Ruppert, M.; Bihorac, E.; Ozrazgat-Baslanti, T.; Tighe, P.J.; Bihorac, A.; et al. Intelligent ICU for Autonomous Patient Monitoring Using Pervasive Sensing and Deep Learning. Sci. Rep. 2019, 9, 8020. [Google Scholar] [CrossRef]
- Cheng, C.W.; Chanani, N.; Venugopalan, J.; Maher, K.; Wang, M.D. icuARM-An ICU clinical decision support system using association rule mining. IEEE J. Transl. Eng. Health Med. 2013, 1, 4400110. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Pesenti, A.; Cecconi, M.J.J. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA 2020, 323, 1545–1546. [Google Scholar] [CrossRef] [PubMed]
- Moor, M.; Rieck, B.; Horn, M.; Jutzeler, C.R.; Borgwardt, K. Early prediction of sepsis in the ICU using machine learning: A systematic review. Front. Med. 2021, 8, 607952. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Singh, K.K.; Vaish, E.; Gurjar, M.; Nambi, A.A.; Khulbe, Y.; Muzaffar, S.; Muzaffar, S.N.J.C. Artificial intelligence in the intensive care unit: Current evidence on an inevitable future tool. Cureus 2024, 16, e59797. [Google Scholar] [CrossRef]
- Johnson, A.E.W.; Pollard, T.J.; Shen, L.; Lehman, L.-W.H.; Feng, M.; Ghassemi, M.; Moody, B.; Szolovits, P.; Celi, L.A.; Mark, R.G. MIMIC-III, a freely accessible critical care database. Sci. Data 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Pang, K.; Li, L.; Ouyang, W.; Liu, X.; Tang, Y.J.D. Establishment of ICU mortality risk prediction models with machine learning algorithm using MIMIC-IV database. Diagnostics 2022, 12, 1068. [Google Scholar] [CrossRef]
- Vallée, A. Digital twin for healthcare systems. J. Front. Digit. Health 2023, 5, 1253050. [Google Scholar] [CrossRef]
- Ghassemi, M.; Oakden-Rayner, L.; Beam, A.L. The false hope of current approaches to explainable artificial intelligence in health care. J. Lancet Digit. Health 2021, 3, e745–e750. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Spooner, B.; Isherwood, J.; Lane, M.; Orrock, E.; Dennison, A. A systematic review of the barriers to the implementation of artificial intelligence in healthcare. J. Cureus 2023, 15, e46454. [Google Scholar] [CrossRef] [PubMed]
- Gleiss, A.; Lewandowski, S. Removing barriers for digital health through organizing ambidexterity in hospitals. J. Public. Health 2022, 30, 21–35. [Google Scholar] [CrossRef]
- Drew, B.J.; Harris, P.; Zègre-Hemsey, J.K.; Mammone, T.; Schindler, D.; Salas-Boni, R.; Bai, Y.; Tinoco, A.; Ding, Q.; Hu, X. Insights into the problem of alarm fatigue with physiologic monitor devices: A comprehensive observational study of consecutive intensive care unit patients. PLoS ONE 2014, 9, e110274. [Google Scholar] [CrossRef] [PubMed]
- Young, L.B.; Chan, P.S.; Lu, X.; Nallamothu, B.K.; Sasson, C.; Cram, P.M. Impact of telemedicine intensive care unit coverage on patient outcomes: A systematic review and meta-analysis. Arch. Intern. Med. 2011, 171, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Barbaria, S.; Mahjoubi, H.; Rahmouni, H.B. A novel blockchain-based architectural modal for healthcare data integrity: Covid19 screening laboratory use-case. Procedia Comput. Sci. 2023, 219, 1436–1443. [Google Scholar] [CrossRef]
- Smith, J.; Alcock, G.; Monk, B.; Jones, R. Concordance of temperature measurements on preterm and term infants using oesophageal (core), axilla & skin methods in a neonatal intensive care unit. J. Neonatal Nurs. 2022, 28, 445–451. [Google Scholar] [CrossRef]
- Chase, J.G.; Zhou, C.; Knopp, J.L.; Moeller, K.; Benyo, B.; Desaive, T.; Wong, J.H.K.; Malinen, S.; Naswall, K.; Shaw, G.M.; et al. Digital Twins and Automation of Care in the Intensive Care Unit. In Cyber-Physical-Human Systems: Fundamentals and Applications; IEEE: New York, NY, USA, 2023; pp. 457–489. [Google Scholar]
- Walinjkar, A. A composite and wearable sensor kit for location-aware healthcare monitoring and real-time trauma scoring for survival prediction. J. Appl. Syst. Innov. 2018, 1, 35. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Wang, G.; Lu, H.; Wang, W. Contactless Patient Care Using Hospital IoT: CCTV-Camera-Based Physiological Monitoring in ICU. IEEE Internet Things J. 2024, 11, 5781–5797. [Google Scholar] [CrossRef]
- Fragasso, T.; Ricci, Z.; Grutter, G.; Albanese, S.; Varano, C.; Amodeo, A.; Cogo, P. Incidence of healthcare-associated infections in a pediatric population with an extracorporeal ventricular assist device. Artif. Organs 2011, 35, 1110–1114. [Google Scholar] [CrossRef]
- Rais-Bahrami, K.; Rivera, O.; Mikesell, G.T.; Short, B.L. Continuous blood gas monitoring using an in-dwelling optode method: Clinical evaluation of the Neotrend sensor using a luer stub adaptor to access the umbilical artery catheter. J. Perinatol. Off. Calif. Perinat. Assoc. 2002, 22, 367–369. [Google Scholar] [CrossRef]
- Matey-Sanz, M.; Gonzalez-Perez, A.; Casteleyn, S.; Granell, C. Implementing and Evaluating the Timed Up and Go Test Automation Using Smartphones and Smartwatches. IEEE J. Biomed. Health Inform. 2024, 28, 6594–6605. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Tai, J.W.M.; Ho, S.K.Y.; Chan, J.F.W.; Hung, K.N.; Ho, P.L.; Yuen, K.Y. Introduction of an electronic monitoring system for monitoring compliance with Moments 1 and 4 of the WHO “My 5 Moments for Hand Hygiene” methodology. BMC Infect. Dis. 2011, 11, 151. [Google Scholar] [CrossRef]
- Cheng, S.M.; Chan, J.J.I.; Tan, C.W.; Lu, E.; Sultana, R.; Sng, B.L. Use of wireless respiratory rate sensor monitoring during opioid patient-controlled analgesia after gynaecological surgery: A prospective cohort study. Indian J. Anaesth. 2021, 65, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Marik, P.E.; Sibole, S.; Grooms, D.; Levitov, A. Changes in end-tidal carbon dioxide and volumetric carbon dioxide as predictors of volume responsiveness in hemodynamically unstable patients. J. Cardiothorac. Vasc. Anesth. 2013, 27, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Rana, M.M.; Curry, M.A.; Krishnakumar, A.; Rahimi, R. Sticker-Type Remote Monitoring System for Early Risk Detection of Catheter Associated Urinary Tract Infections. IEEE Trans. Bio-Med. Eng. 2024, 71, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yun, J.; Li, X.; Kim, M.; Li, J.; Lee, D.; Wu, A.; Lee, S.W. Power-free contact lens for glucose sensing. J. Adv. Funct. Mater. 2023, 33, 2304647. [Google Scholar] [CrossRef]
- Breteler, M.J.; KleinJan, E.J.; Dohmen, D.A.; Leenen, L.P.; van Hillegersberg, R.; Ruurda, J.P.; van Loon, K.; Blokhuis, T.J.; Kalkman, C.J. Vital signs monitoring with wearable sensors in high-risk surgical patients: A clinical validation study. J. Anesthesiol. 2020, 132, 424–439. [Google Scholar] [CrossRef]
- Capp, N.; Fauveau, V.; Au, Y.; Glasser, P.; Muqeem, T.; Hassen, G.; Cardenas, A. Strados labs: An efficient process to acquire and characterize clinically validated respiratory system information using a non-invasive bio-sensor. In Proceedings of the 2019 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 7 December 2019; pp. 1–3. [Google Scholar]
- Chou, Y.A.; Wang, Z.Y.; Chang, H.C.; Liu, Y.C.; Su, P.F.; Huang, Y.T.; Yang, C.T.; Lai, C.H. Indoor CO2 monitoring in a surgical intensive care unit under visitation restrictions during the COVID-19 pandemic. Front. Med. 2023, 10, 1052452. [Google Scholar] [CrossRef]
- Fries, J.; Segre, A.M.; Thomas, G.; Herman, T.; Ellingson, K.; Polgreen, P.M. Monitoring hand hygiene via human observers: How should we be sampling? Infect. Control Hosp. Epidemiol. 2012, 33, 689–695. [Google Scholar] [CrossRef]
- Mariani, S.; Schöde, A.; Homann, K.; Feueriegel, S.; Nöth, S.; Warnke, K.; Bounader, K.; Andreeva, A.; Li, T.; Dogan, G.; et al. Telemonitoring and Care Program for Left Ventricular Assist Device Patients During COVID-19 Outbreak: A European Experience. ASAIO J. 2021, 67, 973–981. [Google Scholar] [CrossRef]
- Ortiz-Barrios, M.; Arias-Fonseca, S.; Ishizaka, A.; Barbati, M.; Avendaño-Collante, B.; Navarro-Jiménez, E. Artificial intelligence and discrete-event simulation for capacity management of intensive care units during the COVID-19 pandemic: A case study. J. Bus. Res. 2023, 160, 113806. [Google Scholar] [CrossRef] [PubMed]
- Roncancio-Clavijo, A.; Gorostidi-Aicua, M.; Alberro, A.; Iribarren-Lopez, A.; Butler, R.; Lopez, R.; Iribarren, J.A.; Clemente, D.; Marimon, J.M.; Basterrechea, J.; et al. Early biochemical analysis of COVID-19 patients helps severity prediction. PLoS ONE 2023, 18, e0283469. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, A.; Tagliente, E.; Pasquini, L.; Cipriano, E.; Pietrantonio, F.; Ortis, P.; Curti, S.; Boellis, A.; Stefanini, T.; Bernardini, A.; et al. 3D CT-Inclusive Deep-Learning Model to Predict Mortality, ICU Admittance, and Intubation in COVID-19 Patients. J. Digit. Imaging 2023, 36, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.I.; Ali, T.E.; Al-Dahan, Z.T. Private Backend Server Software-Based Telehealthcare Tracking and Monitoring System. Int. J. Online Biomed. Eng. 2023, 19, 119–134. [Google Scholar] [CrossRef]
- Sharma, S.; Rawal, R.; Shah, D. Addressing the challenges of AI-based telemedicine: Best practices and lessons learned. J. Educ. Health Promot. 2023, 12, 338. [Google Scholar] [CrossRef]
- Guarrasi, V.; Soda, P. Multi-objective optimization determines when, which and how to fuse deep networks: An application to predict COVID-19 outcomes. J. Comput. Biol. Med. 2023, 154, 106625. [Google Scholar] [CrossRef]
- Bartenschlager, C.C.; Ebel, S.S.; Kling, S.; Vehreschild, J.; Zabel, L.T.; Spinner, C.D.; Schuler, A.; Heller, A.R.; Borgmann, S.; Hoffmann, R. COVIDAL: A machine learning classifier for digital COVID-19 diagnosis in German hospitals. J. ACM Trans. Manag. Inf. Syst. 2023, 14, 1–16. [Google Scholar] [CrossRef]
- Tasnim, N.; Al Mamun, S.; Shahidul Islam, M.; Kaiser, M.S.; Mahmud, M. Explainable Mortality Prediction Model for Congestive Heart Failure with Nature-Based Feature Selection Method. Appl. Sci. 2023, 13, 6138. [Google Scholar] [CrossRef]
- Kołodziejczak, M.M.; Sierakowska, K.; Tkachenko, Y.; Kowalski, P. Artificial Intelligence in the Intensive Care Unit: Present and Future in the COVID-19 Era. J. Pers. Med. 2023, 13, 891. [Google Scholar] [CrossRef]
- Agrimi, E.; Diko, A.; Carlotti, D.; Ciardiello, A.; Borthakur, M.; Giagu, S.; Melchionna, S.; Voena, C. COVID-19 therapy optimization by AI-driven biomechanical simulations. Eur. Phys. J. Plus 2023, 138, 182. [Google Scholar] [CrossRef]
- AlShehhi, A.; Almansoori, T.M.; Alsuwaidi, A.R.; Alblooshi, H. Utilizing machine learning for survival analysis to identify risk factors for COVID-19 intensive care unit admission: A retrospective cohort study from the United Arab Emirates. PLoS ONE 2024, 19, e0291373. [Google Scholar] [CrossRef]
- Genc, A.; Cekic, D.; Issever, K.; Genc, F.; Genc, A.; Toçoğlu, A.; Durmaz, Y.; Özkök, H.; Nalbant, A.; Yaylaci, S. Can artificial intelligence predict COVID-19 mortality? J. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 9866–9871. [Google Scholar]
- Charkoftaki, G.; Aalizadeh, R.; Santos-Neto, A.; Tan, W.Y.; Davidson, E.A.; Nikolopoulou, V.; Wang, Y.; Thompson, B.; Furnary, T.; Chen, Y. An AI-powered patient triage platform for future viral outbreaks using COVID-19 as a disease model. J. Human. Genom. 2023, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Guevarra, K.; Greenstein, Y. Point-of-Care Ultrasonography in the Critical Care Unit: An Update. Curr. Cardiol. Rep. 2025, 27, 54. [Google Scholar] [CrossRef]
- Niles, D.; Sutton, R.M.; Donoghue, A.; Kalsi, M.S.; Roberts, K.; Boyle, L.; Nishisaki, A.; Arbogast, K.B.; Helfaer, M.; Nadkarni, V. “Rolling Refreshers”: A novel approach to maintain CPR psychomotor skill competence. Resuscitation 2009, 80, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Liu, Y.; Peng, L. How to develop machine learning models for healthcare. Nat. Mater. 2019, 18, 410–414. [Google Scholar] [CrossRef]
- Shickel, B.; Tighe, P.J.; Bihorac, A.; Rashidi, P. Deep EHR: A Survey of Recent Advances in Deep Learning Techniques for Electronic Health Record (EHR) Analysis. IEEE J. Biomed. Health Inform. 2018, 22, 1589–1604. [Google Scholar] [CrossRef]
- Miotto, R.; Wang, F.; Wang, S.; Jiang, X.; Dudley, J.T. Deep learning for healthcare: Review, opportunities and challenges. Brief. Bioinform. 2018, 19, 1236–1246. [Google Scholar] [CrossRef]
- Kruse, C.S.; Stein, A.; Thomas, H.; Kaur, H. The use of Electronic Health Records to Support Population Health: A Systematic Review of the Literature. J. Med. Syst. 2018, 42, 214. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Rastegar-Mojarad, M.; Moon, S.; Shen, F.; Afzal, N.; Liu, S.; Zeng, Y.; Mehrabi, S.; Sohn, S.; et al. Clinical information extraction applications: A literature review. J. Biomed. Inform. 2018, 77, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.; Alderson, P.O.; Austin, J.H.; Cimino, J.J.; Johnson, S.B. A general natural-language text processor for clinical radiology. J. Am. Med. Inform. Assoc. JAMIA 1994, 1, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.D.; Long, W.J.; Moody, G.B.; Szolovits, P. Robust parameter extraction for decision support using multimodal intensive care data. Philos. Transactions. Ser. A Math. Phys. Eng. Sci. 2009, 367, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Villarroel, M.; Reisner, A.T.; Clifford, G.; Lehman, L.W.; Moody, G.; Heldt, T.; Kyaw, T.H.; Moody, B.; Mark, R.G. Multiparameter Intelligent Monitoring in Intensive Care II: A public-access intensive care unit database. Crit. Care Med. 2011, 39, 952–960. [Google Scholar] [CrossRef]
- Hravnak, M.; Chen, L.; Dubrawski, A.; Bose, E.; Clermont, G.; Pinsky, M.R. Real alerts and artifact classification in archived multi-signal vital sign monitoring data: Implications for mining big data. J. Clin. Monit. Comput. 2016, 30, 875–888. [Google Scholar] [CrossRef]
- Shickel, B.; Loftus, T.J.; Adhikari, L.; Ozrazgat-Baslanti, T.; Bihorac, A.; Rashidi, P. DeepSOFA: A Continuous Acuity Score for Critically Ill Patients using Clinically Interpretable Deep Learning. Sci. Rep. 2019, 9, 1879. [Google Scholar] [CrossRef]
- Purushotham, S.; Meng, C.; Che, Z.; Liu, Y. Benchmarking deep learning models on large healthcare datasets. J. Biomed. Inform. 2018, 83, 112–134. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef]
- Johnson, A.E.; Stone, D.J.; Celi, L.A.; Pollard, T.J. The MIMIC Code Repository: Enabling reproducibility in critical care research. J. Am. Med. Inform. Assoc. JAMIA 2018, 25, 32–39. [Google Scholar] [CrossRef]
- Pirracchio, R.; Petersen, M.L.; Carone, M.; Rigon, M.R.; Chevret, S.; van der Laan, M.J. Mortality prediction in intensive care units with the Super ICU Learner Algorithm (SICULA): A population-based study. Lancet. Respir. Med. 2015, 3, 42–52. [Google Scholar] [CrossRef]
- Awad, A.; Bader-El-Den, M.; McNicholas, J.; Briggs, J. Early hospital mortality prediction of intensive care unit patients using an ensemble learning approach. Int. J. Med. Inform. 2017, 108, 185–195. [Google Scholar] [CrossRef]
- Ashrafi, N.; Abdollahi, A.; Zhang, J.; Pishgar, M. Optimizing mortality prediction for icu heart failure patients: Leveraging xgboost and advanced machine learning with the mimic-iii database. arXiv 2024, arXiv:2409.01685. [Google Scholar] [CrossRef]
- Li, F.; Xin, H.; Zhang, J.; Fu, M.; Zhou, J.; Lian, Z. Prediction model of in-hospital mortality in intensive care unit patients with heart failure: Machine learning-based, retrospective analysis of the MIMIC-III database. BMJ Open 2021, 11, e044779. [Google Scholar] [CrossRef]
- Katsoulakis, E.; Wang, Q.; Wu, H.; Shahriyari, L.; Fletcher, R.; Liu, J.; Achenie, L.; Liu, H.; Jackson, P.; Xiao, Y.; et al. Digital twins for health: A scoping review. NPJ Digit. Med. 2024, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Babaie Sarijaloo, F.; Prakash, A.; Park, J.; Huang, C.; Barwise, A.; Herasevich, V.; Gajic, O.; Pickering, B.; Dong, Y.J.I.J.o.P.R. A multidisciplinary approach to the development of digital twin models of critical care delivery in intensive care units. Int. J. Prod. Res. 2022, 60, 4197–4213. [Google Scholar] [CrossRef]
- Rovati, L.; Gary, P.J.; Cubro, E.; Dong, Y.; Kilickaya, O.; Schulte, P.J.; Zhong, X.; Wörster, M.; Kelm, D.J.; Gajic, O.; et al. Development and usability testing of a patient digital twin for critical care education: A mixed methods study. Front. Med. 2023, 10, 1336897. [Google Scholar] [CrossRef] [PubMed]
- Sel, K.; Hawkins-Daarud, A.; Chaudhuri, A.; Osman, D.; Bahai, A.; Paydarfar, D.; Willcox, K.; Chung, C.; Jafari, R. Survey and perspective on verification, validation, and uncertainty quantification of digital twins for precision medicine. NPJ Digit. Med. 2025, 8, 40. [Google Scholar] [CrossRef]
- Desautels, T.; Calvert, J.; Hoffman, J.; Jay, M.; Kerem, Y.; Shieh, L.; Shimabukuro, D.; Chettipally, U.; Feldman, M.D.; Barton, C.; et al. Prediction of Sepsis in the Intensive Care Unit With Minimal Electronic Health Record Data: A Machine Learning Approach. JMIR Med. Inform. 2016, 4, e28. [Google Scholar] [CrossRef]
- Fleuren, L.M.; Klausch, T.L.T.; Zwager, C.L.; Schoonmade, L.J.; Guo, T.; Roggeveen, L.F.; Swart, E.L.; Girbes, A.R.J.; Thoral, P.; Ercole, A.; et al. Machine learning for the prediction of sepsis: A systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. 2020, 46, 383–400. [Google Scholar] [CrossRef]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Persson, I.; Macura, A.; Becedas, D.; Sjövall, F. Early prediction of sepsis in intensive care patients using the machine learning algorithm NAVOY® Sepsis, a prospective randomized clinical validation study. J. Crit. Care 2024, 80, 154400. [Google Scholar] [CrossRef]
- Persson, I.; Östling, A.; Arlbrandt, M.; Söderberg, J.; Becedas, D. A machine learning sepsis prediction algorithm for intended intensive care unit use (NAVOY Sepsis): Proof-of-concept study. JMIR Form. Res. 2021, 5, e28000. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Winslow, C.; Meltzer, D.O.; Kattan, M.W.M.; Edelson, D.P. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit. Care Med. 2016, 44, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Cai, Y.; Liu, J.; Liu, X.; Zhao, J.; Yang, Z.; Wen, M.; Liu, L. Mortality prediction in cerebral hemorrhage patients using machine learning algorithms in intensive care units. Front. Neurol. 2021, 11, 610531. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, M.; Celi, L.A.; Badawi, O.; Gordon, A.C.; Faisal, A.A. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat. Med. 2018, 24, 1716–1720. [Google Scholar] [CrossRef]
- Yang, Z.; Cui, X.; Song, Z. Predicting sepsis onset in ICU using machine learning models: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 635. [Google Scholar] [CrossRef]
- Mao, Q.; Jay, M.; Hoffman, J.L.; Calvert, J.; Barton, C.; Shimabukuro, D.; Shieh, L.; Chettipally, U.; Fletcher, G.; Kerem, Y.; et al. Multicentre validation of a sepsis prediction algorithm using only vital sign data in the emergency department, general ward and ICU. BMJ Open 2018, 8, e017833. [Google Scholar] [CrossRef]
- Wong, A.; Otles, E.; Donnelly, J.P.; Krumm, A.; McCullough, J.; DeTroyer-Cooley, O.; Pestrue, J.; Phillips, M.; Konye, J.; Penoza, C.; et al. External Validation of a Widely Implemented Proprietary Sepsis Prediction Model in Hospitalized Patients. JAMA Intern. Med. 2021, 181, 1065–1070. [Google Scholar] [CrossRef]
- Boussina, A.; Shashikumar, S.P.; Malhotra, A.; Owens, R.L.; El-Kareh, R.; Longhurst, C.A.; Quintero, K.; Donahue, A.; Chan, T.C.; Nemati, S.; et al. Impact of a deep learning sepsis prediction model on quality of care and survival. NPJ Digit. Med. 2024, 7, 14. [Google Scholar] [CrossRef]
- Shimabukuro, D.W.; Barton, C.W.; Feldman, M.D.; Mataraso, S.J.; Das, R. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: A randomised clinical trial. BMJ Open Respir. Res. 2017, 4, e000234. [Google Scholar] [CrossRef]
- Islam, S.M.R.; Kwak, D.; Kabir, M.H.; Hossain, M.; Kwak, K.S. The Internet of Things for Health Care: A Comprehensive Survey. IEEE Access 2015, 3, 678–708. [Google Scholar] [CrossRef]
- Darwish, A.; Hassanien, A.E.; Elhoseny, M.; Sangaiah, A.K.; Muhammad, K. The impact of the hybrid platform of internet of things and cloud computing on healthcare systems: Opportunities, challenges, and open problems. J. Ambient. Intell. Humaniz. Comput. 2019, 10, 4151–4166. [Google Scholar] [CrossRef]
- Mamdiwar, S.D.; R, A.; Shakruwala, Z.; Chadha, U.; Srinivasan, K.; Chang, C.-Y.J.B. Recent advances on IoT-assisted wearable sensor systems for healthcare monitoring. Biosensors 2021, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Hilty, D.M.; Armstrong, C.M.; Edwards-Stewart, A.; Gentry, M.T.; Luxton, D.D.; Krupinski, E.A. Sensor, wearable, and remote patient monitoring competencies for clinical care and training: Scoping review. J. Technol. Behav. Sci. 2021, 6, 252–277. [Google Scholar] [CrossRef] [PubMed]
- Nunes, T.; Gaspar, L.; Faria, J.N.; Portugal, D.; Lopes, T.; Fernandes, P.; Tavakoli, M. Deployment and validation of a smart bed architecture for untethered patients with wireless biomonitoring stickers. Med. Biol. Eng. Comput. 2024, 62, 3815–3840. [Google Scholar] [CrossRef]
- Saheb, T.; Izadi, L. Paradigm of IoT big data analytics in the healthcare industry: A review of scientific literature and mapping of research trends. J. Telemat. Inform. 2019, 41, 70–85. [Google Scholar] [CrossRef]
- Tarassenko, L.; Hann, A.; Young, D. Integrated monitoring and analysis for early warning of patient deterioration. Br. J. Anaesth. 2006, 97, 64–68. [Google Scholar] [CrossRef]
- Filho, I.d.M.B.; Aquino, G.; Malaquias, R.S.; Girão, G.; Melo, S.R.M. An IoT-Based Healthcare Platform for Patients in ICU Beds During the COVID-19 Outbreak. IEEE Access 2021, 9, 27262–27277. [Google Scholar] [CrossRef]
- Verderber, S.; Gray, S.; Suresh-Kumar, S.; Kercz, D.; Parshuram, C.J.H.H.E.R.; Journal, D. Intensive care unit built environments: A comprehensive literature review (2005–2020). HERD Health Environ. Res. Des. J. 2021, 14, 368–415. [Google Scholar] [CrossRef]
- Bani Younis, M.; Hayajneh, F.; Alshraideh, J.A. Effect of noise and light levels on sleep of intensive care unit patients. J. Nurs. Crit. Care 2021, 26, 73–78. [Google Scholar] [CrossRef]
- Pisani, M.A.; Friese, R.S.; Gehlbach, B.K.; Schwab, R.J.; Weinhouse, G.L.; Jones, S.F. Sleep in the intensive care unit. Am. J. Respir. Crit. Care Med. 2015, 191, 731–738. [Google Scholar] [CrossRef]
- Ellis, R.J.; Sander, R.M.; Limon, A. Twelve key challenges in medical machine learning and solutions. J. Intell. Based Med. 2022, 6, 100068. [Google Scholar] [CrossRef]
- Jandoubi, B.; Akhloufi, M.A. Multimodal Artificial Intelligence in Medical Diagnostics. J. Inf. 2025, 16, 591. [Google Scholar] [CrossRef]
- Spijkerboer, F.L.; Overdyk, F.J.; Dahan, A. A machine learning algorithm for detecting abnormal patterns in continuous capnography and pulse oximetry monitoring. J. Clin. Monit. Comput. 2024, 38, 915–925. [Google Scholar] [CrossRef]
- Hayyolalam, V.; Aloqaily, M.; Özkasap, Ö.; Guizani, M. Edge intelligence for empowering IoT-based healthcare systems. J. IEEE Wirel. Commun. 2021, 28, 6–14. [Google Scholar] [CrossRef]
- Karri, C.; Garg, L.; Prakash, V.; Pawar, B.D. Chapter 9. Healthcare 5.0 opportunities and challenges: A literature review. In Intelligent Biomedical Technologies and Applications for Healthcare 5.0; Garg, L., Mirajkar, G., Misra, S., Chattu, V.K., Eds.; Academic Press: Cambridge, MA, USA, 2025; Volume 16, pp. 133–146. [Google Scholar]
- Khude, H.; Shende, P. AI-driven clinical decision support systems: Revolutionizing medication selection and personalized drug therapy. Adv. Integr. Med. 2025, 12, 100529. [Google Scholar] [CrossRef]
- Bates, D.W.; Gawande, A.A. Improving safety with information technology. N. Engl. J. Med. 2003, 348, 2526–2534. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; He, J.; Zhu, N.; Wajahat, A.; Nazir, A.; Qureshi, S.; Pathan, M.S.; Dev, S. Blockchain-enabled EHR access auditing: Enhancing healthcare data security. Heliyon 2024, 10, e34407. [Google Scholar] [CrossRef]
- Said, A.; Yahyaoui, A.; Abdellatif, T. HIPAA and GDPR compliance in IoT healthcare systems. In Proceedings of the International Conference on Model and Data Engineering, Sousse, Tunisia, 2–4 November 2023; pp. 198–209. [Google Scholar]
- Neubauer, R.; Schröttner, J.; Baumgartner, C. Safety requirements for medical devices in compliance with European standards. In Medical Devices and In Vitro Diagnostics: Requirements in Europe; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–30. [Google Scholar]
- Santos, P.; Nazaré, I. The doctor and patient of tomorrow: Exploring the intersection of artificial intelligence, preventive medicine, and ethical challenges in future healthcare. Front. Digit. Health 2025, 7, 1588479. [Google Scholar] [CrossRef] [PubMed]
- Lekadir, K.; Frangi, A.F.; Porras, A.R.; Glocker, B.; Cintas, C.; Langlotz, C.P.; Weicken, E.; Asselbergs, F.W.; Prior, F.; Collins, G.S.; et al. FUTURE-AI: International consensus guideline for trustworthy and deployable artificial intelligence in healthcare. BMJ 2025, 388, e081554. [Google Scholar] [CrossRef]
- Bertl, M.; Piho, G.; Draheim, D.; Ross, P.; Pechmann, L.; Bucciarelli, N.; Sharma, R. Future Opportunities for Systematic AI Support in Healthcare. In International Conference on Bridging the Gap Between AI and Reality; Springer Nature: Cham, Switzerland, 2023; pp. 203–224. [Google Scholar]
- Pavuluri, S.; Sangal, R.; Sather, J.; Taylor, R.A. Balancing act: The complex role of artificial intelligence in addressing burnout and healthcare workforce dynamics. BMJ Health Care Inform. 2024, 31, e101120. [Google Scholar] [CrossRef]
- Pundkar, A.; Gadkari, C.; Patel, A.; Kumar, A. Transforming emergency medicine with artificial intelligence: From triage to clinical decision support. J. Multidiscip. Rev. 2025, 8, 2025285. [Google Scholar]
- Veldhuis, L.I.; Woittiez, N.J.C.; Nanayakkara, P.W.B.; Ludikhuize, J. Artificial Intelligence for the Prediction of In-Hospital Clinical Deterioration: A Systematic Review. Crit. Care Explor. 2022, 4, e0744. [Google Scholar] [CrossRef] [PubMed]
- Muralitharan, S.; Nelson, W.; Di, S.; McGillion, M.; Devereaux, P.J.; Barr, N.G.; Petch, J. Machine Learning-Based Early Warning Systems for Clinical Deterioration: Systematic Scoping Review. J. Med. Internet Res. 2021, 23, e25187. [Google Scholar] [CrossRef] [PubMed]
- Charan, G.S.; Charan, A.S.; Khurana, M.S.; Narang, G.S. Impact of Analytics Applying Artificial Intelligence and Machine Learning on Enhancing Intensive Care Unit: A Narrative Review. Galician Med. J. 2023, 30, e-GMJ2023-A2006. [Google Scholar] [CrossRef]
- Alnawafleh, K.A. The Impact of AI on Nursing Workload and Stress Levels in Critical Care Settings. J. Pak. J. Life Social. Sci. 2024, 22, 8529–8542. [Google Scholar] [CrossRef]
- Mehta, S.D.; Martin, K.; McGowan, N.; Dominick, C.L.; Madu, C.; Denkin, B.K.; Yehya, N. Ventilator-Weaning Pathway Associated With Decreased Ventilator Days in Pediatric Acute Respiratory Distress Syndrome. Crit. Care Med. 2021, 49, 302–310. [Google Scholar] [CrossRef]
- Liu, X.; Cruz Rivera, S.; Moher, D.; Calvert, M.J.; Denniston, A.K. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI extension. Nat. Med. 2020, 26, 1364–1374. [Google Scholar] [CrossRef]
- Rais-Bahrami, K.; Rivera, O.; Mikesell, G.T.; Short, B.L. Continuous blood gas monitoring using an in-dwelling optode method: Comparison to intermittent arterial blood gas sampling in ECMO patients. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2002, 22, 472–474. [Google Scholar] [CrossRef]
- Liu, Z.; Shu, W.; Li, T.; Zhang, X.; Chong, W. Interpretable machine learning for predicting sepsis risk in emergency triage patients. Sci. Rep. 2025, 15, 887. [Google Scholar] [CrossRef]
- Burdick, H.; Pino, E.; Gabel-Comeau, D.; McCoy, A.; Gu, C.; Roberts, J.; Le, S.; Slote, J.; Pellegrini, E.; Green-Saxena, A.; et al. Effect of a sepsis prediction algorithm on patient mortality, length of stay and readmission: A prospective multicentre clinical outcomes evaluation of real-world patient data from US hospitals. BMJ Health Care Inform. 2020, 27, e100109. [Google Scholar] [CrossRef]
- Bignami, E.G.; Berdini, M.; Panizzi, M.; Domenichetti, T.; Bezzi, F.; Allai, S.; Damiano, T.; Bellini, V. Artificial Intelligence in Sepsis Management: An Overview for Clinicians. J. Clin. Med. 2025, 14, 286. [Google Scholar] [CrossRef]
- Fritsch, S.J.; Cecconi, M. Setting the ventilator with AI support: Challenges and perspectives. Intensive Care Med. 2025, 51, 593–595. [Google Scholar] [CrossRef]
- Akella, P.; Voigt, L.P.; Chawla, S. To Wean or Not to Wean: A Practical Patient Focused Guide to Ventilator Weaning. J. Intensive Care Med. 2022, 37, 1417–1425. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, J.; Deng, Y.; Tu, L.; Ding, Y.; Zhang, Y.; Yuan, X.; Xu, K.; Guo, L.; Gao, N. Advances in Machine Learning for Mechanically Ventilated Patients. Int. J. Gen. Med. 2025, 18, 3301–3311. [Google Scholar] [CrossRef]
- Meza-Fuentes, G.; Delgado, I.; Barbé, M.; Sánchez-Barraza, I.; Retamal, M.A.; López, R. Machine learning-based identification of efficient and restrictive physiological subphenotypes in acute respiratory distress syndrome. Intensive Care Med. Exp. 2025, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yue, R.; Lu, S.; Luo, J.; Wu, X.; Zhang, Z.; Liu, M.; Fan, Y.; Zhang, Y.; Pan, C.; et al. Artificial intelligence and machine learning in acute respiratory distress syndrome management: Recent advances. Front. Med. 2025, 12, 1597556. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.-M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazik, A.; Eldesouky, M.; Antoun, I.; Lau, E.Y.M.; Koya, A.; Vali, Z.; Suleman, S.A.; Donaldson, J.; Ng, G.A. Wearable Devices for Arrhythmia Detection: Advancements and Clinical Implications. Sensors 2025, 25, 2848. [Google Scholar] [CrossRef]
- Soudan, B.; Dandachi, F.F.; Nassif, A.B. Attempting cardiac arrest prediction using artificial intelligence on vital signs from Electronic Health Records. Smart Health 2022, 25, 100294. [Google Scholar] [CrossRef]
- Michard, F.; Mulder, M.P.; Gonzalez, F.; Sanfilippo, F. AI for the hemodynamic assessment of critically ill and surgical patients: Focus on clinical applications. Ann. Intensive Care 2025, 15, 26. [Google Scholar] [CrossRef]
- Yuan, S.; Yang, Z.; Li, J.; Wu, C.; Liu, S. AI-Powered early warning systems for clinical deterioration significantly improve patient outcomes: A meta-analysis. BMC Med. Inform. Decis. Mak. 2025, 25, 203. [Google Scholar] [CrossRef]
- Barboi, C.; Tzavelis, A.; Muhammad, L.N. Comparison of Severity of Illness Scores and Artificial Intelligence Models That Are Predictive of Intensive Care Unit Mortality: Meta-analysis and Review of the Literature. JMIR Med. Inform. 2022, 10, e35293. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, H.; Zhou, Y.; Yang, Q.; Mu, W.; Xiao, H.; Zhuo, Z.; Liu, H.; Wang, H.; Qu, X.; et al. The accuracy of artificial intelligence in predicting COVID-19 patient mortality: A systematic review and meta-analysis. BMC Med. Inform. Decis. Mak. 2023, 23, 155. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Lash, M.T.; Nachimuthu, S.K. Optimal sepsis patient treatment using human-in-the-loop artificial intelligence. Expert. Syst. Appl. 2021, 169, 114476. [Google Scholar] [CrossRef]
- Choudhury, A. Toward an Ecologically Valid Conceptual Framework for the Use of Artificial Intelligence in Clinical Settings: Need for Systems Thinking, Accountability, Decision-making, Trust, and Patient Safety Considerations in Safeguarding the Technology and Clinicians. JMIR Hum. Factors 2022, 9, e35421. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chang, T.C.; Liu, C.F.; Lai, C.C.; Chen, C.M.; Chou, W. The intervention of artificial intelligence to improve the weaning outcomes of patients with mechanical ventilation: Practical applications in the medical intensive care unit and the COVID-19 intensive care unit: A retrospective study. Medicine 2024, 103, e37500. [Google Scholar] [CrossRef]
- Dixon, D.; Sattar, H.; Moros, N.; Kesireddy, S.R.; Ahsan, H.; Lakkimsetti, M.; Fatima, M.; Doshi, D.; Sadhu, K.; Junaid Hassan, M. Unveiling the Influence of AI Predictive Analytics on Patient Outcomes: A Comprehensive Narrative Review. Cureus 2024, 16, e59954. [Google Scholar] [CrossRef]
- Hadweh, P.; Niset, A.; Salvagno, M.; Al Barajraji, M.; El Hadwe, S.; Taccone, F.S.; Barrit, S. Machine Learning and Artificial Intelligence in Intensive Care Medicine: Critical Recalibrations from Rule-Based Systems to Frontier Models. J. Clin. Med. 2025, 14, 4026. [Google Scholar] [CrossRef]
- Flint, A.R.; Schaller, S.J.; Balzer, F. How AI can help in error detection and prevention in the ICU? Intensive Care Med. 2025, 51, 590–592. [Google Scholar] [CrossRef]
- Albrecht, M.; Shanks, D.; Shah, T.; Hudson, T.; Thompson, J.; Filardi, T.; Wright, K.; Ator, G.A.; Smith, T.R. Enhancing clinical documentation with ambient artificial intelligence: A quality improvement survey assessing clinician perspectives on work burden, burnout, and job satisfaction. JAMIA Open 2025, 8, ooaf013. [Google Scholar] [CrossRef]
- Collins, R. Clinician Cognitive Overload and Its Implications for Nurse Leaders. Nurse Lead. 2020, 18, 44–47. [Google Scholar] [CrossRef]
- Raisa, J.F.; Rahman, M.S.; Mahmud, I.; Kaiser, M.S.; Han, D.S. Transition toward Healthcare 5.0: Impact of COVID-19 in the healthcare industry. ICT Express 2025, 11, 371–389. [Google Scholar] [CrossRef]
- Voutsas, F.; Violos, J.; Leivadeas, A. Mitigating Alert Fatigue in Cloud Monitoring Systems: A Machine Learning Perspective. Comput. Netw. 2024, 250, 110543. [Google Scholar] [CrossRef]
- Gerlich, M. AI Tools in Society: Impacts on Cognitive Offloading and the Future of Critical Thinking. Societies 2025, 15, 6. [Google Scholar] [CrossRef]
- Junaid, S.B.; Imam, A.A.; Balogun, A.O.; De Silva, L.C.; Surakat, Y.A.; Kumar, G.; Abdulkarim, M.; Shuaibu, A.N.; Garba, A.; Sahalu, Y.; et al. Recent Advancements in Emerging Technologies for Healthcare Management Systems: A Survey. Healthcare 2022, 10, 1940. [Google Scholar] [CrossRef]
- Woo, B.F.Y.; Song, J.; Middleton, E.; Fijačko, N.; Cato, K. Teaching Critical Thinking in the Age of AI: Safeguarding Clinical Reasoning in Healthcare Documentation. Int. Nurs. Rev. 2025, 72, e70102. [Google Scholar] [CrossRef]
- Sunday, O. Behavioral Impacts of AI Reliance in Diagnostics: Balancing Automation with Skill Retention. Epidemiol. Health Data Insights 2025, 1, ehdi014. [Google Scholar] [CrossRef]
- Torab-Miandoab, A.; Samad-Soltani, T.; Jodati, A.; Rezaei-Hachesu, P. Interoperability of heterogeneous health information systems: A systematic literature review. BMC Med. Inform. Decis. Mak. 2023, 23, 18. [Google Scholar] [CrossRef]
- Esper, A.M.; Arabi, Y.M.; Cecconi, M.; Du, B.; Giamarellos-Bourboulis, E.J.; Juffermans, N.; Machado, F.; Peake, S.; Phua, J.; Rowan, K.; et al. Systematized and efficient: Organization of critical care in the future. Crit. Care 2022, 26, 366. [Google Scholar] [CrossRef]
- Zhang, Y.; Callaghan-Koru, J.A.; Koru, G. The challenges and opportunities of continuous data quality improvement for healthcare administration data. JAMIA Open 2024, 7, ooae058. [Google Scholar] [CrossRef] [PubMed]
- Aldoseri, A.; Al-Khalifa, K.N.; Hamouda, A.M. Re-Thinking Data Strategy and Integration for Artificial Intelligence: Concepts, Opportunities, and Challenges. Appl. Sci. 2023, 13, 7082. [Google Scholar] [CrossRef]
- Feldman, K.; Johnson, R.A.; Chawla, N.V. The state of data in healthcare: Path towards standardization. J. Healthc. Inform. Res. 2018, 2, 248–271. [Google Scholar] [CrossRef] [PubMed]
- Rancea, A.; Anghel, I.; Cioara, T. Edge Computing in Healthcare: Innovations, Opportunities, and Challenges. Futur. Internet 2024, 16, 329. [Google Scholar] [CrossRef]
- Hennebelle, A.; Dieng, Q.; Ismail, L.; Buyya, R. SmartEdge: Smart Healthcare End-to-End Integrated Edge and Cloud Computing System for Diabetes Prediction Enabled by Ensemble Machine Learning. In Proceedings of the 2024 IEEE International Conference on Cloud Computing Technology and Science (CloudCom), Online, 9–11 December 2024; pp. 127–134. [Google Scholar]
- Lluch, M. Healthcare professionals’ organisational barriers to health information technologies—A literature review. Int. J. Med. Inform. 2011, 80, 849–862. [Google Scholar] [CrossRef]
- Tsai, C.H.; Eghdam, A.; Davoody, N.; Wright, G.; Flowerday, S.; Koch, S. Effects of Electronic Health Record Implementation and Barriers to Adoption and Use: A Scoping Review and Qualitative Analysis of the Content. Life 2020, 10, 327. [Google Scholar] [CrossRef]
- Laukka, E.; Pölkki, T.; Kanste, O. Leadership in the context of digital health services: A concept analysis. J. Nurs. Manag. 2022, 30, 2763–2780. [Google Scholar] [CrossRef]
- van der Vegt, A.H.; Scott, I.A.; Dermawan, K.; Schnetler, R.J.; Kalke, V.R.; Lane, P.J. Implementation frameworks for end-to-end clinical AI: Derivation of the SALIENT framework. J. Am. Med. Inform. Assoc. 2023, 30, 1503–1515. [Google Scholar] [CrossRef]
- Wells, B.J.; Nguyen, H.M.; McWilliams, A.; Pallini, M.; Bovi, A.; Kuzma, A.; Kramer, J.; Chou, S.-H.; Hetherington, T.; Corn, P.; et al. A practical framework for appropriate implementation and review of artificial intelligence (FAIR-AI) in healthcare. NPJ Digit. Med. 2025, 8, 514. [Google Scholar] [CrossRef]
- Nauka, P.C.; Kennedy, J.N.; Brant, E.B.; Komorowski, M.; Pirracchio, R.; Angus, D.C.; Seymour, C.W. Challenges with reinforcement learning model transportability for sepsis treatment in emergency care. NPJ Digit. Med. 2025, 8, 1–5. [Google Scholar] [CrossRef]
- Thawait, N.K. Enhancing IoT systems: Cybercrime prevention through security vulnerability management. Preprints 2024. [Google Scholar] [CrossRef]
- Firat Kilincer, I.; Ertam, F.; Sengur, A.; Tan, R.-S.; Rajendra Acharya, U. Automated detection of cybersecurity attacks in healthcare systems with recursive feature elimination and multilayer perceptron optimization. Biocybern. Biomed. Eng. 2023, 43, 30–41. [Google Scholar] [CrossRef]
- He, Y.; Huang, D.; Chen, L.; Ni, Y.; Ma, X. A survey on zero trust architecture: Challenges and future trends. J. Wirel. Commun. Mob. Comput. 2022, 2022, 6476274. [Google Scholar] [CrossRef]
- Dhinakaran, D.; Srinivasan, L.; Sankar, S.U.; Selvaraj, D. Quantum-based privacy-preserving techniques for secure and trustworthy internet of medical things an extensive analysis. J. Quantum Inf. Comput. 2024, 24, 227–266. [Google Scholar] [CrossRef]
- Balasubramani, M.; Srinivasan, M.; Jean, W.H.; Fan, S.Z.; Shieh, J.S. A Novel Framework for Quantum-Enhanced Federated Learning with Edge Computing for Advanced Pain Assessment Using ECG Signals via Continuous Wavelet Transform Images. Sensors 2025, 25, 1436. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, T.; Abbas, H.; Atiquzzaman, M. Security Vulnerabilities, Attacks, Countermeasures, and Regulations of Networked Medical Devices—A Review. IEEE Commun. Surv. Tutor. 2019, 21, 3723–3768. [Google Scholar] [CrossRef]
- Blauth, T.F.; Gstrein, O.J.; Zwitter, A. Artificial intelligence crime: An overview of malicious use and abuse of AI. J. IEEE Access 2022, 10, 77110–77122. [Google Scholar] [CrossRef]
- Wani, R.U.Z.; Thabit, F.; Can, O. Security and privacy challenges, issues, and enhancing techniques for Internet of Medical Things: A systematic review. J. Secur. Priv. 2024, 7, e409. [Google Scholar] [CrossRef]
- Hireche, R.; Mansouri, H.; Pathan, A.-S.K. Security and privacy management in Internet of Medical Things (IoMT): A synthesis. J. Cybersecur. Priv. 2022, 2, 640–661. [Google Scholar] [CrossRef]
- Kulothungan, V. Using Blockchain Ledgers to Record AI Decisions in IoT. IoT 2025, 6, 37. [Google Scholar] [CrossRef]
- Sylla, T.; Mendiboure, L.; Chalouf, M.A.; Krief, F. Blockchain-Based Context-Aware Authorization Management as a Service in IoT. Sensors 2021, 21, 7656. [Google Scholar] [CrossRef] [PubMed]
- Marsh-Armstrong, B.; Pacheco, F.; Dameff, C.; Tully, J. Design and Pilot Study of a High-Fidelity Medical Simulation of a Hospital-Wide Cybersecurity Attack. Preprint 2024. [Google Scholar] [CrossRef]
- Singh, V.; Cheng, S.; Kwan, A.C.; Ebinger, J. United States Food and Drug Administration Regulation of Clinical Software in the Era of Artificial Intelligence and Machine Learning. Mayo Clin. proceedings. Digit. Health 2025, 3, 100231. [Google Scholar] [CrossRef] [PubMed]
- Weiner, E.B.; Dankwa-Mullan, I.; Nelson, W.A.; Hassanpour, S. Ethical challenges and evolving strategies in the integration of artificial intelligence into clinical practice. PLoS Digit. Health 2025, 4, e0000810. [Google Scholar] [CrossRef]
- Baumbach, J. To share or not to share? Privacy-preserving AI in medicine. In Proceedings of the RExPO24 Conference, Munich, Germany, 3–5 July 2024. [Google Scholar]
- McCradden, M.D.; Anderson, J.A.; Stephenson, A.E.; Drysdale, E.; Erdman, L.; Goldenberg, A.; Zlotnik Shaul, R. A Research Ethics Framework for the Clinical Translation of Healthcare Machine Learning. Am. J. Bioeth. AJOB 2022, 22, 8–22. [Google Scholar] [CrossRef]
- U.S. FDA. Administration, Clinical Decision Support Software: Guidance for Industry and Food and Drug Administration Staff; U.S. FDA: Washington, DC, USA, 2022.
- Group, M.D.C. MDCG 2021-24 Guidance on Classification of Medical Devices; European Commission: Brussels, Belgium, 2021.
- Dirgová Luptáková, I.; Pospíchal, J.; Huraj, L. Beyond Code and Algorithms: Navigating Ethical Complexities in Artificial Intelligence. In Proceedings of the Computational Methods in Systems and Software; Springer Nature: Cham, Switzerland, 2023; pp. 316–332. [Google Scholar]
- Chinta, S.V.; Wang, Z.; Palikhe, A.; Zhang, X.; Kashif, A.; Smith, M.A.; Liu, J.; Zhang, W. AI-driven healthcare: Fairness in AI healthcare: A survey. PLOS Digit. Health 2025, 4, e0000864. [Google Scholar] [CrossRef]
- Gupta, S.; Modgil, S.; Bhatt, P.C.; Chiappetta Jabbour, C.J.; Kamble, S. Quantum computing led innovation for achieving a more sustainable COVID-19 healthcare industry. Technovation 2023, 120, 102544. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef]
- Solenov, D.; Brieler, J.; Scherrer, J.F. The Potential of Quantum Computing and Machine Learning to Advance Clinical Research and Change the Practice of Medicine. Mo. Med. 2018, 115, 463–467. [Google Scholar]
- Yang, Q.; Liu, Y.; Chen, T.; Tong, Y. Federated machine learning: Concept and applications. J. ACM Trans. Intell. Syst. Technol. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Sheller, M.J.; Edwards, B.; Reina, G.A.; Martin, J.; Pati, S.; Kotrotsou, A.; Milchenko, M.; Xu, W.; Marcus, D.; Colen, R.R.; et al. Federated learning in medicine: Facilitating multi-institutional collaborations without sharing patient data. Sci. Rep. 2020, 10, 12598. [Google Scholar] [CrossRef]
- Holzinger, A.; Langs, G.; Denk, H.; Zatloukal, K.; Müller, H. Causability and explainability of artificial intelligence in medicine. J. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2019, 9, e1312. [Google Scholar] [CrossRef] [PubMed]
- Mienye, I.D.; Obaido, G.; Jere, N.; Mienye, E.; Aruleba, K.; Emmanuel, I.D.; Ogbuokiri, B. A survey of explainable artificial intelligence in healthcare: Concepts, applications, and challenges. Inform. Med. Unlocked 2024, 51, 101587. [Google Scholar] [CrossRef]
- Park, Y.; Jackson, G.P.; Foreman, M.A.; Gruen, D.; Hu, J.; Das, A.K. Evaluating artificial intelligence in medicine: Phases of clinical research. JAMIA Open 2020, 3, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef]
- El Arab, R.A.; Al Moosa, O.A. Systematic review of cost effectiveness and budget impact of artificial intelligence in healthcare. NPJ Digit. Med. 2025, 8, 548. [Google Scholar] [CrossRef]
- Rony, M.K.K.; Parvin, M.R.; Wahiduzzaman, M.; Debnath, M.; Bala, S.D.; Kayesh, I. “I Wonder if my Years of Training and Expertise Will be Devalued by Machines”: Concerns About the Replacement of Medical Professionals by Artificial Intelligence. SAGE Open Nurs. 2024, 10, 23779608241245220. [Google Scholar] [CrossRef]
- Wiens, J.; Saria, S.; Sendak, M.; Ghassemi, M.; Liu, V.X.; Doshi-Velez, F.; Jung, K.; Heller, K.; Kale, D.; Saeed, M.; et al. Do no harm: A roadmap for responsible machine learning for health care. Nat. Med. 2019, 25, 1337–1340. [Google Scholar] [CrossRef]
- Azadi, A.; García-Peñalvo, F.J. Optimizing Clinical Decision Support System Functionality by Leveraging Specific Human-Computer Interaction Elements: Insights From a Systematic Review. JMIR Hum. Factors 2025, 12, e69333. [Google Scholar] [CrossRef]
- Zhou, K.; Gattinger, G. The Evolving Regulatory Paradigm of AI in MedTech: A Review of Perspectives and Where We Are Today. Ther. Innov. Regul. Sci. 2024, 58, 456–464. [Google Scholar] [CrossRef]
- Hasanzadeh, F.; Josephson, C.B.; Waters, G.; Adedinsewo, D.; Azizi, Z.; White, J.A. Bias recognition and mitigation strategies in artificial intelligence healthcare applications. NPJ Digit. Med. 2025, 8, 154. [Google Scholar] [CrossRef]
- Schinkel, M.; Nanayakkara, P.W.B.; Wiersinga, W.J. Sepsis Performance Improvement Programs: From Evidence Toward Clinical Implementation. Crit. Care 2022, 26, 77. [Google Scholar] [CrossRef]
- Choi, S.; Son, J.; Oh, D.K.; Huh, J.W.; Lim, C.M.; Hong, S.B. Rapid Response System Improves Sepsis Bundle Compliances and Survival in Hospital Wards for 10 Years. J. Clin. Med. 2021, 10, 4244. [Google Scholar] [CrossRef]
- Siddique, S.M.; Tipton, K.; Leas, B.; Greysen, S.R.; Mull, N.K.; Lane-Fall, M.; McShea, K.; Tsou, A.Y. Interventions to Reduce Hospital Length of Stay in High-risk Populations: A Systematic Review. JAMA Netw. Open 2021, 4, e2125846. [Google Scholar] [CrossRef]

| Institution/Center | Service/Department | Validation Focus | Number of Theses/Dissertations |
|---|---|---|---|
| National Computer Center (CNI), Ministry of Health | Interoperability and Systems Department | Interoperability, technical integration, and AI deployment for clinical decision-making | 9 Masters |
| National Center for Organ Transplantation (CNPTO), Tunis | Transplant Coordination | Data flow management, post-operative monitoring, traceability | 4 Masters |
| Tunis Military Hospital | Intensive Care & Cardiology and a physician | Patient monitoring, AI for complication prediction | 2 PhD, 2 Masters |
| Al Matri Hospital | Colorectal Surgery | Surgical simulation and optimization of operative protocols | 2 Master |
| Memi University Hospital | Radiology | Medical imaging, PACS–AI integration | 1 Masters |
| Mongi Slim University Hospital | Intensive Care and a physician | Real-time monitoring, post-pandemic solutions | 1 PhD, 1 Master |
| Razi Hospital, Tunis | Neurology | Longitudinal follow-up, early detection of relapses | 1 PhD, 2 Master’s |
| Cross-cutting Projects (Pandemic & Post-pandemic) | Various hospital services | Tele-monitoring of COVID and post-COVID patients, AI integration for continuity of care | 1 PhD, 1 Master [18] |
| 1st Author, Years | Type of Technology | Clinical Applications | Limitations Compared to LPMDC | Journals (Quartile) |
|---|---|---|---|---|
| Smith, J., 2022 [19] | Wearable sensors (skin, axilla) and invasive core probe | Optimizing neonatal thermal monitoring in the ICU to detect early temperature variations | -Focused solely on temperature monitoring in neonates -Lacks predictive AI/ML integration, multi-metric outcomes | Journal of Neonatal Nursing (Q2) |
| Geoffrey Chase, J., 2023 [20] | Digital twins and AI-based prediction detection support | Developing digital twins in medicine: automating cyber-physical-human systems to improve treatment in the ICUs | -High computational and data requirements for real-time simulation. -Integration with patient-specific dosimetry is limited | Cyber–Physical–Human Systems: Fundamentals and Applications (Q2) |
| Walinjkar, A., 2018 [21] | Wearable sensors kit and smart monitoring system | Using wearable sensors to monitor in real-time by predicting trauma scores (National Early Warning Score, Revised Trauma Score, Trauma Score-Injury Severity Score) and Predicting Survival, using physiological data | Limited to physiological parameters; does not integrate multimodal patient data or predictive modeling at the clinical decision support level | Applied System Innovation (Q1) |
| Wang, H., 2023 [22] | Contactless sensor, IoT-based monitoring | Using optical sensors for non-contact physiological assessment and early detection in a remote patient monitoring system using IoT-enabled CCTV cameras | -Requires complex data processing and network bandwidth -Less portable and not wearable; not suitable for low-power, personal medical devices | IEEE Internet of Things Journal (Q1) |
| Fragasso, T., 2011 [23] | Wearable sensors, contactless sensors and mHealth app | Validation and optimization of thermal monitoring methods in the neonatal ICUs to ensure accurate and reliable monitoring, early detection of anomalies, and adaptive temperature management | Limited integration with multi-parameter data collection may provide less comprehensive physiological monitoring; potential data gaps due to sensor placement or signal interference may require frequent calibration to maintain accuracy in neonatal ICU settings | Artificial Organs (Q2) |
| Rais-Bahrami, K., 2002 [24] | Wearable sensors and continuous blood gas monitoring sensors | Implementation of a precise and less invasive continuous blood gas monitoring approach for optimal assessment and early detection of imbalances in newborns in the ICUs | Limited long-term monitoring in extremely low birth weight infants Less flexible for integration with multiple physiological parameters compared to LPMDC | Journal of Perinatology (Q1) |
| Matey-Sanz, M., 2024 [25] | Smartphone, smartwatch, mHealth app, AI, wearable sensor | Developing mHealth systems using AI and sensors for predicting and detecting motor disorders (as part of remote care management strategies) | Limited precision in capturing complex motor patterns compared to lab-based or high-fidelity LPMDC systems Dependency on user compliance | IEEE Journal of Biomedical and Health Informatics (Q1) |
| Cheng, V. C., 2011 [26] | IoT-based monitoring + AI prediction decision support | MedSense combines automated monitoring, predictive analytics, and feedback to improve hand hygiene compliance in the ICUs | Limited to hand hygiene monitoring; does not integrate multi-source patient data or real-time personalized clinical decision support | BMC Infectious Diseases (Q1) |
| Cheng, S. M., 2021 [27] | Wearable sensors: wireless respiratory rate sensor | Integrating wireless sensors to monitor respiratory rate, detect, and prevent postoperative respiratory depression in gynecological intensive care | The short duration of monitoring meant that long-term outcomes and complications were not assessed | Indian journal of anaesthesia (Q2) |
| Young, A., 2013 [28] | IoT-based sensor/physiological monitoring devices | Personalizing hemodynamic treatment in the ICUs by predicting the response to vascular filling | Small sample size and limited patient diversity; only evaluated in controlled ICU settings; does not employ advanced machine learning models or continuous long-term monitoring | Journal of cardiothoracic and vascular anesthesia (Q2) |
| Gopalakrishnan, S., 2024 [29] | Wearable sensors, mHealth app | The STARS system automates urinary catheter monitoring in the ICUs and predicts infections | Requires wearable sensors and app infrastructure, which might limit scalability in low-resource settings Lacks flexibility in capturing multi-source patient data beyond urinary catheters | IEEE Transactions on Biomedical Engineering (Q1) |
| Li, Z., 2023 [30] | Contactless/wearable sensors | Detection and management of metabolic imbalances in the ICUs using passive smart lenses for real-time blood glucose monitoring | Limited validation in diverse ICU patient populations; performance under variable physiological conditions remains uncertain | Advanced Functional Materials (Q1) |
| Breteler, M. J., 2020 [31] | Wearable sensors | Predicting and detecting postoperative deterioration in the ICUs using wearable sensors | Limited generalizability due to a single-center study and a small sample size, which may not capture the full variability of patient populations | Anesthesiology (Q1) |
| Capp, N., 2019 [32] | Contactless/wearable sensors, AI-based monitoring | Predicting and detecting acute decompensation in chronic obstructive pulmonary disease/asthma patients by using intelligent respiratory monitoring | Limited generalizability due to a small and homogeneous patient cohort | IEEE Signal Processing in Medicine and Biology Symposium (SPMB) (Q2) |
| Chou, Y. A., 2023 [33] | IoT (smart sensor) | Smart IoT monitoring of air quality in the ICUs to detect occupancy-related CO2 spikes to optimize health safety | Limited generalizability due to the study being conducted in a single ICU setting with specific COVID-19 visitation restrictions, which may not reflect typical ICU conditions | Frontiers in Medicine (Q1) |
| Fries, J., 2012 [34] | Modeling, smart system, AI-assisted monitoring | Modeling caregiver flows to predict and optimize hand hygiene monitoring in the ICUs | Relies on human observation and modeling, which may introduce observer bias and lack the real-time automated monitoring capability present in LPMDC | Infection Control & Hospital Epidemiology (Q1) |
| Mariani, S., 2021 [35] | Telemonitoring, mHealth app | Telemonitoring of left ventricular assist device patients for the early prediction and detection of complications and treatment adjustment in the ICUs during the COVID-19 pandemic | Limited sample size and short follow-up period, which may affect the generalizability of the findings | Asaio Journal (Q1) |
| Ortiz-Barrios, M., 2023 [36] | AI/simulation | AI is used to analyze patient data from the emergency department to predict the likelihood of ICU admission. These predictions are integrated into a discrete event simulation model to observe ICU bed occupancy in real-time and identify current bottlenecks | This study relies on AI predictions integrated into a simulation without validating the model against real-time ICU admission outcomes, which may limit the generalizability and accuracy of its capacity management insights | Journal of Business Research (Q1) |
| Roncancio-Clavijo, A., 2023 [37] | AI predictive modeling | Predict disease severity and detect ICU patients at risk of clinical deterioration based on AI predictive models using blood test data | Limited generalizability due to the relatively small sample size and single-center data | PLOS One (Q1) |
| Di Napoli, A., 2023 [38] | Deep Learning–based Predictive Analytics | Predict mortality, intubation, and ICU admission based on deep learning algorithms using 3D chest CT images and clinical data | The model requires high-quality 3D CT scans and extensive clinical data, which may limit its generalizability to settings where such data are not readily available | Journal of Digital Imaging (Q2) |
| Ali, F. I., 2023 [39] | IoT (monitoring system) | IoT-based health monitoring system in the ICUs: monitoring of vital signs and prompt detection of clinical changes | Limited integration with predictive models for patient deterioration | International journal of online and biomedical engineering (Q2) |
| Sharma, S., 2023 [40] | Telemedicine/Remote Patient Monitoring Technology | Telemedicine in the ICUs | Focuses on general AI telemedicine challenges but lacks patient-specific predictive modeling | Journal of education and health promotion (Q2) |
| Guarrasi, V., 2023 [41] | AI | AI-based models are utilized in ICUs to predict disease progression, identify high-risk cases, and monitor patient status using chest X-rays and clinical data | Lack of comprehensive integration of multi-modal patient data beyond imaging and basic clinical metrics | Computers in Biology and Medicine (Q1) |
| Bartenschlager, C. C., 2023 [42] | Machine Learning for Clinical Prediction | AI can predict infection status and detect symptomatic COVID-19 cases using laboratory data | This study is limited by its focus on laboratory data | ACM Transactions on Management Information Systems (Q1) |
| Tasnim, N., 2023 [43] | Explainable Artificial Intelligence for clinical risk prediction | Predict mortality risk accurately and identify clinical risk factors using AI to optimize ICU resource allocation | This study is limited by its focus on specific datasets, which affect the generalizability of the AI model to other populations or clinical settings | Applied Sciences (Q2) |
| Kołodziejczak, M. M., 2023 [44] | AI | Predict patient deterioration by monitoring ongoing conditions in the ICUs using AI models | A conventional AI approach, lacking continuous feedback and corrective capabilities | Journal of Personalized Medicine (Q2) |
| Agrimi, E., 2023 [45] | AI-driven biomechanical simulation modeling | AI-based biomechanical simulations can predict respiratory function decline in the ICUs using lung CT scans and arterial blood gas data | AI-based biomechanical simulations without integrating the continuous monitoring and adaptive correction capabilities | The European Physical Journal Plus (Q2) |
| AlShehhi, A., 2024 [46] | ML | AI-based models help monitor disease progression and detect early signs of deterioration in ICU patients by using EHR | Limited by its retrospective design and reliance on EHR data, which restricts real-time applicability and comprehensive Healthcare 5.0 integration | PLOS One (Q1) |
| Genc, A. C., 2023 [47] | AI | AI models forecast mortality risk at very early stages in the ICUs, monitor patients in critical states, and recognize those at the highest risk | Narrower scope, lacking continuous monitoring and real-time corrective feedback for ICU patients | European Review for Medical & Pharmacological Sciences (Q2) |
| Charkoftaki, G., 2023 [48] | AI | Predict disease severity and monitor patient status in real-time in the ICU. Detection of key biomarkers associated with serious complications (decrease in serotonin levels) to identify patients requiring intensive care early | This study is limited by its reactive, ICU-focused approach, which lacks continuous monitoring and corrective feedback loop | Human Genomics (Q1) |
| Guevarra, K., 2025 [49] | AI-based imaging analysis | Prediction of clinical deterioration, monitoring of hemodynamic status, and complication detection in the ICUs by an AI-based model using imaging data | Focuses primarily on ICU imaging data and lacks integrated prediction, monitoring, detection, and correction | Current Cardiology Reports (Q1) |
| Niles, D., 2009 [50] | Continuous learning and skill-monitoring technology | Cardiopulmonary resuscitation training in the ICU is based on continuous learning, with monitoring and immediate correction of techniques, allowing for rapid and lasting mastery of skills | Traditional ICU training methods lack integration with constant, data-driven monitoring and corrective feedback | Resuscitation (Q1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boussi Rahmouni, H.; Hassine, N.B.E.H.; Chouchen, M.; Ceylan, H.İ.; Muntean, R.I.; Bragazzi, N.L.; Dergaa, I. Healthcare 5.0-Driven Clinical Intelligence: The Learn-Predict-Monitor-Detect-Correct Framework for Systematic Artificial Intelligence Integration in Critical Care. Healthcare 2025, 13, 2553. https://doi.org/10.3390/healthcare13202553
Boussi Rahmouni H, Hassine NBEH, Chouchen M, Ceylan Hİ, Muntean RI, Bragazzi NL, Dergaa I. Healthcare 5.0-Driven Clinical Intelligence: The Learn-Predict-Monitor-Detect-Correct Framework for Systematic Artificial Intelligence Integration in Critical Care. Healthcare. 2025; 13(20):2553. https://doi.org/10.3390/healthcare13202553
Chicago/Turabian StyleBoussi Rahmouni, Hanene, Nesrine Ben El Hadj Hassine, Mariem Chouchen, Halil İbrahim Ceylan, Raul Ioan Muntean, Nicola Luigi Bragazzi, and Ismail Dergaa. 2025. "Healthcare 5.0-Driven Clinical Intelligence: The Learn-Predict-Monitor-Detect-Correct Framework for Systematic Artificial Intelligence Integration in Critical Care" Healthcare 13, no. 20: 2553. https://doi.org/10.3390/healthcare13202553
APA StyleBoussi Rahmouni, H., Hassine, N. B. E. H., Chouchen, M., Ceylan, H. İ., Muntean, R. I., Bragazzi, N. L., & Dergaa, I. (2025). Healthcare 5.0-Driven Clinical Intelligence: The Learn-Predict-Monitor-Detect-Correct Framework for Systematic Artificial Intelligence Integration in Critical Care. Healthcare, 13(20), 2553. https://doi.org/10.3390/healthcare13202553










