Negative Healthcare Impacts of Management of Presumed Early-Onset Sepsis in Moderate to Late Preterm Infants on Feeding, Jaundice, and Hospital Length of Stay
Abstract
1. Introduction
2. Methods
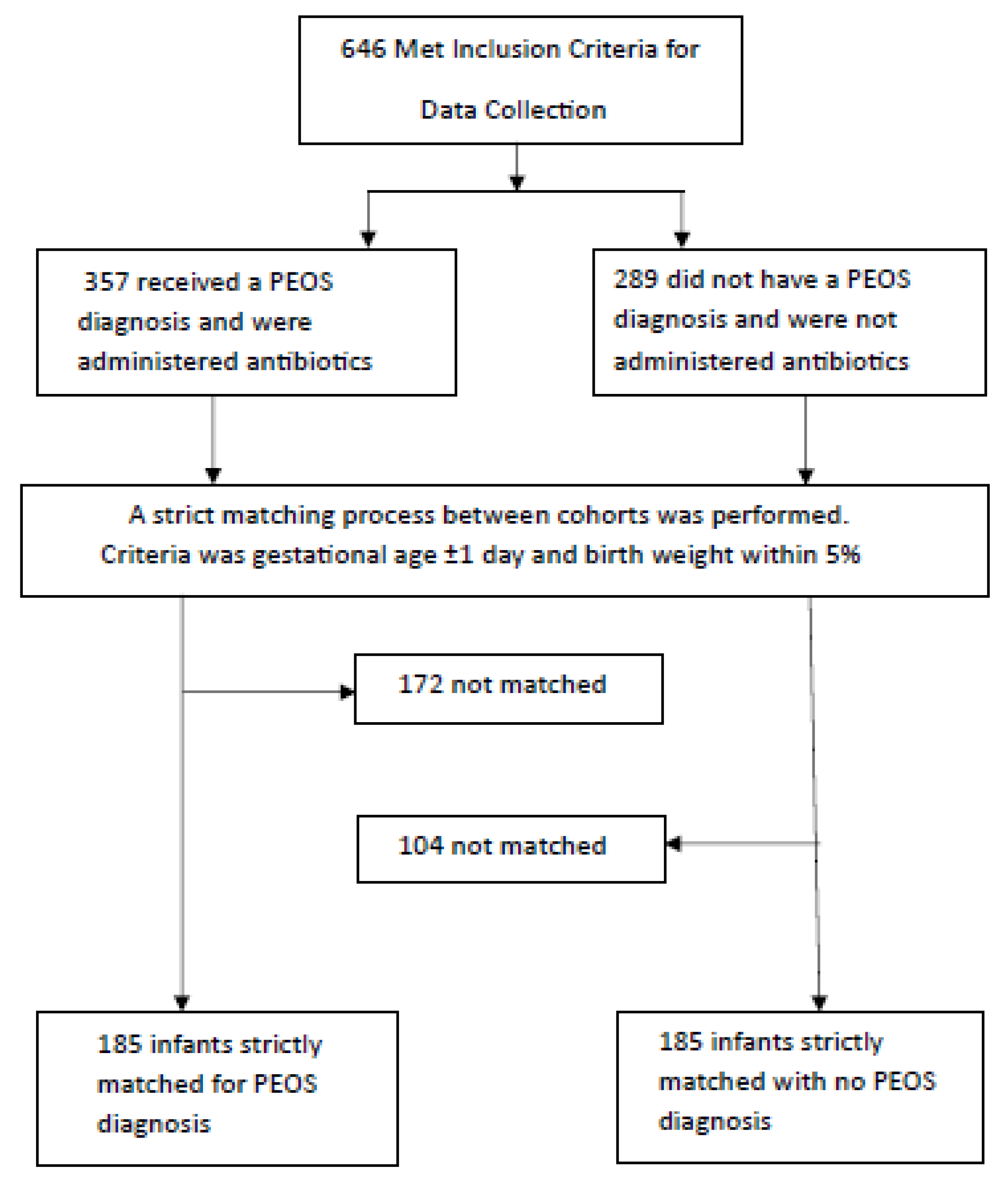
2.1. Study Design and Participants
2.2. Ethical Approval
2.3. Data Collection and Outcomes
2.4. Definitions
2.5. Sample Size Calculations
2.6. Statistical Analysis
3. Results
3.1. Overview
3.2. Antibiotic Therapy
3.3. Baseline Characteristics—Table 1
3.4. Jaundice Outcomes—Table 2
3.5. Feeding Tolerance and Secondary Results—Table 3
3.6. Multivariable Analysis—Table 4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmoud, H.A.H.; Parekh, R.; Dhandibhotla, S.; Sai, T.; Pradhan, A.; Alugula, S.; Cevallos-Cueva, M.; Hayes, B.K.; Athanti, S.; Abdin, Z.; et al. Insight Into Neonatal Sepsis: An Overview. Cureus 2023, 15, e45530. [Google Scholar] [CrossRef]
- Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Davies, H.D. Early-onset neonatal sepsis. Clin. Microbiol. Rev. 2014, 27, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.S. Diagnosis and management of bacterial infections in the neonate. Pediatr. Clin. N. Am. 2004, 51, 939–959. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Dimopoulou, V.; Klingenberg, C.; Navér, L.; Nordberg, V.; Berardi, A.; El Helou, S.; Fusch, G.; Bliss, J.M.; Lehnick, D.; et al. AENEAS Study Group. Analysis of Antibiotic Exposure and Early-Onset Neonatal Sepsis in Europe, North America, and Australia. JAMA Netw. Open 2022, 5, e2243691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, S.H.; Chang, Y.J.; Chen, L.J.; Lee, C.H.; Chen, H.N.; Chen, J.Y.; Hsiao, C.C. Relationship between Maternal Fever and Neonatal Sepsis: A Retrospective Study at a Medical Center. Biomedicines 2022, 10, 2222. [Google Scholar] [CrossRef]
- Ershad, M.; Mostafa, A.; Cruz, M.D.; Vearrier, D. Neonatal Sepsis. Curr. Emerg. Hosp. Med. Rep. 2019, 7, 83–90. [Google Scholar] [CrossRef]
- McMullan, B.; Cooper, C.; Spotswood, N.; James, R.; Jones, C.; Konecny, P.; Blyth, C.; Karen, T. Antibiotic prescribing in neonatal sepsis: An Australian nationwide survey. BMJ Paediatr. Open 2020, 4, e000643. [Google Scholar] [CrossRef]
- Garrido, F.; Allegaert, K.; Arribas, C.; Villamor, E.; Raffaeli, G.; Paniagua, M.; Cavallaro, G. Variations in Antibiotic Use and Sepsis Management in Neonatal Intensive Care Units: A European Survey. Antibiotics 2021, 10, 1046. [Google Scholar] [CrossRef]
- Stocker, M.; Klingenberg, C.; Navér, L.; Nordberg, V.; Berardi, A.; El Helou, S.; Fusch, G.; Bliss, J.M.; Lehnick, D.; Dimopoulou, V.; et al. Less is more: Antibiotics at the beginning of life. Nat. Commun. 2023, 14, 2423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cotton, C.M. Adverse consequences of neonatal antibiotic exposure. Curr. Opin. Pediatr. 2016, 28, 141–149. [Google Scholar] [CrossRef]
- Kuppala, V.S.; Meinzen-Derr, J.; Morrow, A.L.; Schibler, K.R. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J. Pediatr. 2011, 159, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.E.; Ferri, W.A.G.; Leone, C.R.; de Almeida, M.F.B.; Guinsburg, R.; Meneses, J.D.A.; Vale, M.S.D.; Marba, S.T.M.; de Carvalho, W.B.; de Souza Rugolo, L.M.S.; et al. Early Empiric Antibiotic Use Is Associated with Delayed Feeding Tolerance in Preterm Infants: A Retrospective Analysis. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yan, J.; Wen, H.; Deng, X.; Li, X.; Su, S. Feeding intolerance alters the gut microbiota of preterm infants. PLoS ONE 2019, 14, e0210609. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Sánchez, B.; Milani, C.; Duranti, S.; Solís, G.; Fernández, N.; de los Reyes-Gavilán, C.G.; Ventura, M.; Margolles, A.; Gueimonde, M. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J. Pediatr. 2015, 166, 538–544. [Google Scholar] [CrossRef]
- Achten, N.B.; Klingenberg, C.; Benitz, W.E.; Stocker, M.; Schlapbach, L.J.; Giannoni, E.; Bokelaar, R.; Driessen, G.J.A.; Brodin, P.; Uthaya, S.; et al. Association, of Use of the Neonatal Early-Onset Sepsis Calculator with Reduction in Antibiotic Therapy and Safety: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 1032–1040. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health. 2020, 8, e000262. [Google Scholar] [CrossRef]
- Zhou, W. Association Between Broad-Spectrum Antibiotic Use at Different Stage and Neonatal Enteral Nutrition and Prognosis in Very Low-Birth-Weight Infants with Culture-Negative Sepsis. J. Pediatr. Child Health Care 2020, 5, 1033. [Google Scholar]
- Fouhy, F.; Guinane, C.M.; Hussey, S.; Wall, R.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C.; et al. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob. Agents Chemother. 2012, 56, 5811–5820. [Google Scholar] [CrossRef]
- Schumann, A.; Nutten, S.; Donnicola, D.; Comelli, E.M.; Mansourian, R.; Cherbut, C.; Corthesy-Theulaz, I.; Garcia-Rodenas, C. Neonatal antibiotic treatment alters gastrointestinal tract developmental gene expression and intestinal barrier transcriptome. Physiol. Genom. 2005, 23, 235–245. [Google Scholar] [CrossRef]
- Di Mauro, A.; Neu, J.; Riezzo, G.; Raimondi, F.; Martinelli, D.; Francavilla, R.; Indrio, F. Gastrointestinal function development and microbiota. Ital. J. Pediatr. 2013, 39, 15. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.K.; Dennery, P.A.; Hintz, S.R. Understanding newborn jaundice. J. Perinatol. 2001, 21 (Suppl. S1), S21–S39. [Google Scholar] [CrossRef] [PubMed]
- Lijana, R.C.; Williams, M.C. The effects of antibiotics on hemolytic behavior of red cells. Cell Biophys. 1986, 8, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagina, V.M. Deĭstvie penitsillina na membranu éritrotsitov [Action of; penicillin on the erythrocyte membrane]. Antibiotiki 1984, 29, 40–42. [Google Scholar]
- Ma, X.W.; Fan, W.Q. Earlier Nutrient Fortification of Breastmilk Fed LBW Infants Improves Jaundice Related Outcomes. Nutrients 2020, 12, 2116. [Google Scholar] [CrossRef]
- Gourley, G.R.; Kreamer, B.; Cohnen, M.; Kosorok, M.R. Neonatal jaundice and diet. Arch. Pediatr. Adolesc. Med. 1999, 153, 184–188. [Google Scholar] [CrossRef][Green Version]
- Tiribelli, C.; Ostrow, J.D. Intestinal flora and bilirubin. J. Hepatol. 2005, 42, 170–172. [Google Scholar] [CrossRef]
- Maisels, M.J.; McDonagh, A.F. Phototherapy for neonatal jaundice. N. Engl. J. Med. 2008, 358, 920–928. [Google Scholar] [CrossRef]
- Lieberman, E.; Lang, J.; Richardson, D.K.; Frigoletto, F.D.; Heffner, L.J.; Cohen, A. Intrapartum maternal fever and neonatal outcome. Pediatrics 2000, 105, 8–13. [Google Scholar] [CrossRef]
- Lieberman, E.; Lang, J.M.; Frigoletto, F., Jr.; Richardson, D.K.; Ringer, S.A.; Cohen, A. Epidural analgesia, intrapartum fever, and neonatal sepsis evaluation. Pediatrics 1997, 99, 415–419. [Google Scholar] [CrossRef]
- Safer Care Victoria. Sepsis in Neonates. Available online: https://www.safercare.vic.gov.au/best-practice-improvement/clinical-guidance/neonatal/sepsis-in-neonates (accessed on 9 December 2023).
- Pettinger, K.J.; Mayers, K.; McKechnie, L.; Phillips, B. Sensitivity of the Kaiser Permanente early-onset sepsis calculator: A systematic review and meta-analysis. eClinicalMedicine 2019, 19, 100227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grace, E.; Jayakumar, N.; Cooper, C.; Andersen, C.; Callander, E.; Gomersall, J.; Rumbold, A.; Keir, A. Reducing intravenous antibiotics in neonates born ≥35 weeks’ gestation: A quality improvement study. J. Paediatr. Child Health 2024, 60, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.A.; Lai, M.; Inglis, G.D.T.; Davies, M.W. Neonatal early-onset sepsis calculator safety in an Australian tertiary perinatal centre. J. Paediatr. Child Health 2022, 58, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Cuoco, M.P.; Parbhoo, D.; D’Aprano, A. Introduction of the neonatal sepsis calculator at a low-dependency special care nursery in Australia. J. Matern. Fetal Neonatal Med. 2022, 35, 7532–7535. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.A.; Jager, K.J.; Zoccali, C.; Dekker, F.W. Matching, an appealing method to avoid confounding? Nephron. Clin. Pract. 2011, 118, c315–c318. [Google Scholar] [CrossRef]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E.; committee on fetus and newborn; committee on infectious diseases. Management of Neonates Born at ≤34 6/7 Weeks’ Gestation with Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182896. [Google Scholar] [CrossRef]
- Wood, B.; Culley, P.; Roginski, C.; Waterhouse, J. Factors affecting neonatal jaundice. Arch. Dis. Child. 1979, 54, 111–115. [Google Scholar] [CrossRef]
- Billing, B.H.; Cole, P.G.; Lathe, G.H. Increased plasma bilirubin in newborn infants in relation to birth weight. Br. Med. J. 1954, 2, 1263–1265. [Google Scholar] [CrossRef][Green Version]
- Wrotniak, B.H.; Stettler, N.; Medoff-Cooper, B. The relationship between birth weight and feeding maturation in preterm infants. Acta Paediatr. 2009, 98, 286–290. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Baghestani, A.R.; Vahedi, M. How to control confounding effects by statistical analysis. Gastroenterol. Hepatol. Bed Bench 2012, 5, 79–83. [Google Scholar]
| NPEOS (n = 185) | PEOS (n = 185) | p-Value | |
|---|---|---|---|
| Maternal characteristics | |||
| Age median (IQR) (years) (paired) | 30 (7) | 30 (7) | 0.288 |
| Region of birth, % | |||
| Australia, New Zealand vs. other (paired) | 51.9% | 54.1% | 0.755 |
| Gravida, median (IQR) (paired) | 2 (2) | 2 (2) | 0.658 |
| Para, median (IQR) | 1 (1) | 1 (1) | 0.790 |
| GBS-positive status | 22.0% | 25.2% | 0.645 |
| Maternal intrapartum fever | 0% | 5.9% | 0.001 |
| Maternal intrapartum antibiotics | 14.1% | 20.5% | 0.130 |
| WCC between 6 and 15 × 109/L | 82.4% | 84.8% | 0.571 |
| Maternal CRP < 5, mg/L | 38.5% | 25.1% | 0.112 |
| Neonatal characteristics | |||
| Gestational age, median (IQR) (weeks) (paired) | 35.5 (1.3) | 35.4 (1.3) | 0.194 |
| Birthweight, mean (SD) (grams) (paired) | 2364 (454) | 2365 (451) | 0.958 |
| Male, % | 53.5% | 58.4% | 0.402 |
| Apgar 5 min, median (IQR) (unpaired) | 9 (0) | 9 (0) | 0.030 |
| Foetal distress, % | 35.7% | 35.7% | 1.000 |
| Delivery mode, % | |||
| Normal vaginal delivery | 34.1% | 63.8% | <0.001 |
| Emergency Caesarean | 43.8% | 30.8% | 0.013 |
| Elective Caesarean | 22.2% | 5.4% | <0.001 |
| Instrumentation at delivery, % | 11.4% | 14.1% | 0.533 |
| Neonatal birth trauma, % | 2.7% | 14.1% | <0.001 |
| Neonatal resuscitation, % | 28.6% | 42.2% | 0.009 |
| Neonatal respiratory distress, % | 29.7% | 53.5% | <0.001 |
| Neonatal hypoglycaemia, % | 35.7% | 31.4% | 0.441 |
| Haemolysis present, % | 3.2% | 2.3% | 0.716 |
| Birth temperature, mean (SD) °C (unpaired) | 36.5 (0.37) | 36.6 (0.47) | 0.062 |
| WCC between 5 and 30 × 109/L | 2.2% | 2.0% | 1.000 |
| CRP < 10 mg/L | 96.6% | 91.7% | 0.166 |
| Neonatal blood culture positive | NIL | NIL | ------- |
| Antibiotic duration, median (IQR), hours | ------ | 36 (12) | ------- |
| NPEOS | PEOS | p-Value | |
|---|---|---|---|
| Neonatal jaundice outcomes | |||
| Any phototherapy | 75.2% | 76.5% | 0.806 |
| Maximum total bilirubin, mean (SD) (µmol/L) | 213 (45) | 214 (46) | 0.840 |
| Maximum direct bilirubin, mean (SD) (µmol/L) | 12 (4) | 11(4) | 0.398 |
| Age at maximum total bilirubin, median (IQR) (hours of life) | 72 (51) | 93 (55) | 0.002 |
| Phototherapy treatment duration, median (IQR) (hours) | 28 (22) | 38(26) | 0.016 |
| Phototherapy light dosage | Number of light dosages | ||
| Median (IQR) [paired, n = 20] | 0 (0) | 1.5 (1.25) | <0.001 |
| Median (IQR) [paired, n = 18] | 1 (0) | 2 (1.75) | 0.037 |
| Median (IQR) [paired, n = 27] | 2 (0) | 2 (3) | 0.085 |
| (NPEOS infants compared to PEOS gestation-matched infants) | |||
| NPEOS | PEOS | p-Value | |
|---|---|---|---|
| Feeding intolerance % | 10.8% | 10.8% | 1.000 |
| Enteral feed during admission | |||
| Breast milk: exclusive % | 4.3% | 8.6% | 0.138 |
| Mixed % | 87.0% | 82.2% | 0.249 |
| Enteral feed commencement, median (IQR) (hours) | 1.0 (0.6) | 2.0 (8.5) | <0.001 |
| Full enteral feed achievement, median (IQR) (days) | 6 (1.0) | 8(12) | 0.010 |
| Secondary outcomes | |||
| LoS, median (IQR) (days) | 8 (12) | 10 (11) | 0.002 |
| Readmission % | 13.0% | 13.0% | 1.000 |
| Weight gain from birth at discharge in g, mean (SD) | 81 (279) | 116 (271) | 0.031 |
| Outcome | Odds or Hazards Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Neonatal hyperbilirubinaemia/phototherapy * | 1.04 | 0.77–1.4 | 0.791 |
| Triple phototherapy * | 1.54 | 0.65–3.65 | 0.325 |
| Double phototherapy * | 0.92 | 0.39–2.19 | 0.853 |
| Duration of phototherapy * | 1.24 | 1.10–1.41 | 0.001 |
| Age at maximum bilirubin * | 1.24 | 1.12–1.37 | 0.001 |
| Feeding intolerance † | 1.00 | 0.52–1.93 | 1.000 |
| Time to commence enteral feeds † | 2.75 | 2.32–3.27 | <0.001 |
| Time to reach full enteral feeds † | 1.10 | 0.96–1.25 | 0.173 |
| Length of stay ‡ | 1.31 | 1.02–1.67 | 0.035 |
| Readmission ‡ | 1.65 | 0.65–4.16 | 0.290 |
| Weight gain on discharge ^ | 1.10 | 0.87–1.38 | 0.433 |
| Weight Z-score gain on discharge ^ | 0.96 | 0.89–1.04 | 0.303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, D.; Tran, D.; Subhi, R.; Fan, W.Q. Negative Healthcare Impacts of Management of Presumed Early-Onset Sepsis in Moderate to Late Preterm Infants on Feeding, Jaundice, and Hospital Length of Stay. Healthcare 2025, 13, 136. https://doi.org/10.3390/healthcare13020136
Ng D, Tran D, Subhi R, Fan WQ. Negative Healthcare Impacts of Management of Presumed Early-Onset Sepsis in Moderate to Late Preterm Infants on Feeding, Jaundice, and Hospital Length of Stay. Healthcare. 2025; 13(2):136. https://doi.org/10.3390/healthcare13020136
Chicago/Turabian StyleNg, Daniel, David Tran, Rami Subhi, and Wei Qi Fan. 2025. "Negative Healthcare Impacts of Management of Presumed Early-Onset Sepsis in Moderate to Late Preterm Infants on Feeding, Jaundice, and Hospital Length of Stay" Healthcare 13, no. 2: 136. https://doi.org/10.3390/healthcare13020136
APA StyleNg, D., Tran, D., Subhi, R., & Fan, W. Q. (2025). Negative Healthcare Impacts of Management of Presumed Early-Onset Sepsis in Moderate to Late Preterm Infants on Feeding, Jaundice, and Hospital Length of Stay. Healthcare, 13(2), 136. https://doi.org/10.3390/healthcare13020136






