Associations Between Sedentary Behaviors and Sedentary Patterns with Metabolic Syndrome in Children and Adolescents: The UP&DOWN Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
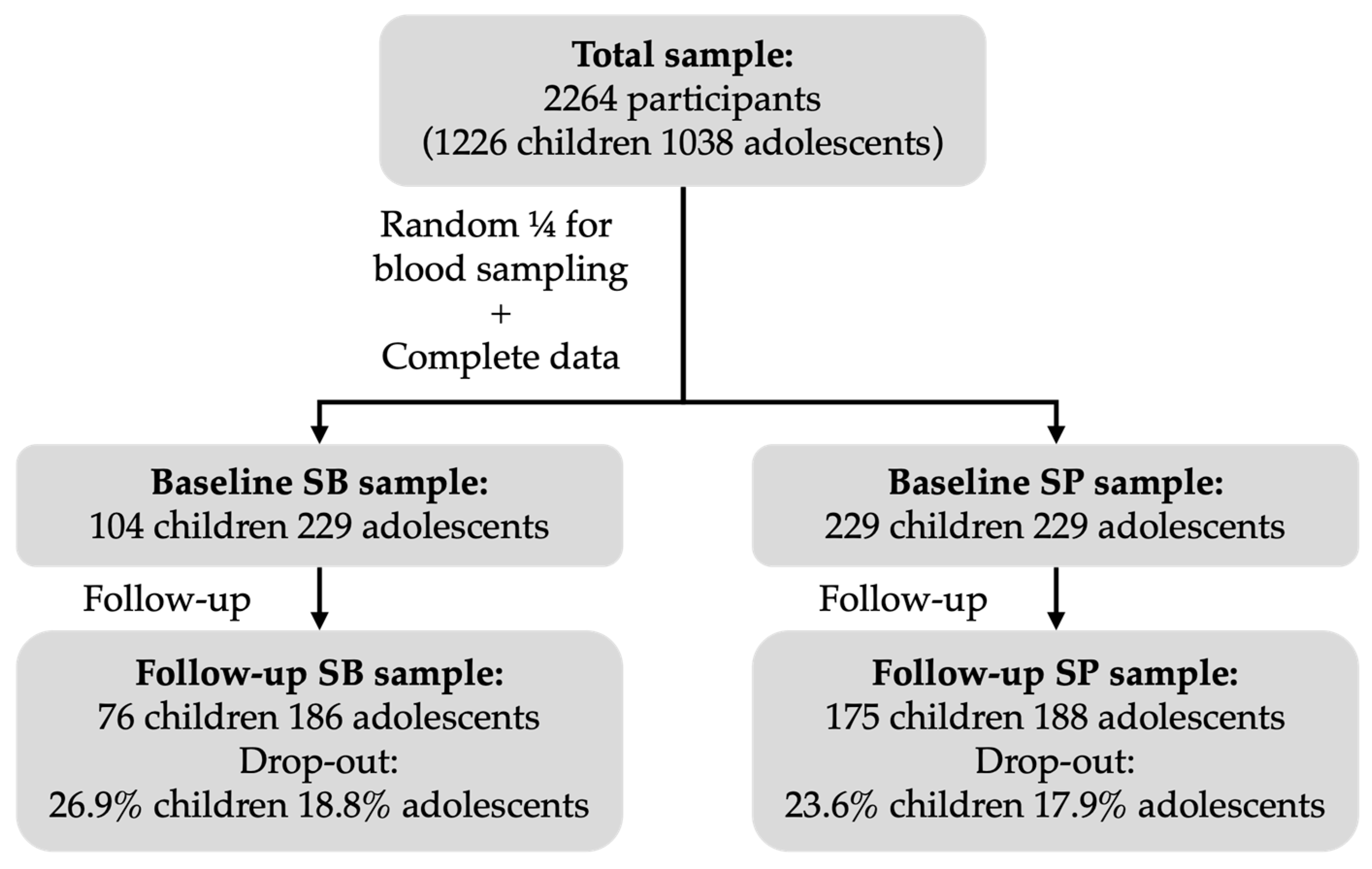
2.1. Study Design and Population
2.2. Tanner Stage
2.3. Blood Pressure
2.4. Blood Sampling
2.5. Body Composition
2.6. Metabolic Syndrome
2.7. Sedentary Behaviors
2.8. Sedentary Time and Sedentary Patterns
2.9. Moderate to Vigorous Physical Activity
2.10. Data Analyses
3. Results
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindstrom, M.; Decleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; Legrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Turco, V.; et al. Summary of Global Burden of Disease Study Methods. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Andersen, L.B.; Wedderkopp, N.; Hansen, H.S.; Cooper, A.R.; Froberg, K. Biological Cardiovascular Risk Factors Cluster in Danish Children and Adolescents: The European Youth Heart Study. Prev. Med. 2003, 37, 363–367. [Google Scholar] [CrossRef]
- Bugge, A.; El-Naaman, B.; Mcmurray, R.G.; Froberg, K.; Andersen, L.B. Tracking of Clustered Cardiovascular Disease Risk Factors from Childhood to Adolescence. Pediatr. Res. 2013, 73, 245–249. [Google Scholar] [CrossRef]
- Andersen, L.B.; Hasselstrøm, H.; Grønfeldt, V.; Hansen, S.E.; Karsten, F. The Relationship between Physical Fitness and Clustered Risk, and Tracking of Clustered Risk from Adolescence to Young Adulthood: Eight Years Follow-up in the Danish Youth and Sport Study. Int. J. Behav. Nutr. Phys. Act. 2004, 1, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic Syndrome—A New World-Wide Definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Dhondge, R.H.; Agrawal, S.; Patil, R.; Kadu, A.; Kothari, M. A Comprehensive Review of Metabolic Syndrome and Its Role in Cardiovascular Disease and Type 2 Diabetes Mellitus: Mechanisms, Risk Factors, and Management. Cureus 2024, 16, e67428. [Google Scholar] [CrossRef]
- Magge, S.N.; Goodman, E.; Armstrong, S.C.; Daniels, S.; Corkins, M.; De Ferranti, S.; Golden, N.H.; Kim, J.H.; Schwarzenberg, S.J.; Assar, C.L.; et al. The Metabolic Syndrome in Children and Adolescents: Shifting the Focus to Cardiometabolic Risk Factor Clustering. Pediatrics 2017, 140, e20171603. [Google Scholar] [CrossRef]
- Eisenmann, J.C. On the Use of a Continuous Metabolic Syndrome Score in Pediatric Research. Cardiovasc. Diabetol. 2008, 7, 17. [Google Scholar] [CrossRef]
- Khazdouz, M.; Hasani, M.; Mehranfar, S.; Ejtahed, H.S.; Djalalinia, S.; Mahdavi Gorabi, A.; Esmaeili-Abdar, M.; Karbalahi Saleh, S.; Arzaghi, S.M.; Zahedi, H.; et al. Validity of Continuous Metabolic Syndrome Score for Predicting Metabolic Syndrome; a Systematic Review and Meta-Analysis. J. Diabetes Metab. Disord. 2021, 20, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, Á.; Perona, J.S.; Schmidt-RioValle, J.; González-Jiménez, E. Concordance among Diagnostic Criteria for Metabolic Syndrome Is Inconsistent in Spanish Adolescents. Eur. J. Clin. Investig. 2021, 51, e13384. [Google Scholar] [CrossRef] [PubMed]
- Haapala, E.A.; Leppänen, M.H.; Lee, E.; Savonen, K.; Laukkanen, J.A.; Kähönen, M.; Brage, S.; Lakka, T.A. Accumulating Sedentary Time and Physical Activity From Childhood to Adolescence and Cardiac Function in Adolescence. J. Am. Heart Assoc. 2024, 13, e031837. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; Aminian, S.; et al. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project Process and Outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef]
- Carson, V.; Hunter, S.; Kuzik, N.; Gray, C.E.; Poitras, V.J.; Chaput, J.P.; Saunders, T.J.; Katzmarzyk, P.T.; Okely, A.D.; Connor Gorber, S.; et al. Systematic Review of Sedentary Behaviour and Health Indicators in School-Aged Children and Youth: An Update. Appl. Physiol. Nutr. Metab. 2016, 41, S240–S265. [Google Scholar] [CrossRef]
- van Ekris, E.; Altenburg, T.M.; Singh, A.S.; Proper, K.I.; Heymans, M.W.; Chinapaw, M.J.M. An Evidence-Update on the Prospective Relationship between Childhood Sedentary Behaviour and Biomedical Health Indicators: A Systematic Review and Meta-Analysis. Obes. Rev. 2016, 17, 833–849. [Google Scholar] [CrossRef] [PubMed]
- Biddle, S.J.H.; García Bengoechea, E.; Wiesner, G. Sedentary Behaviour and Adiposity in Youth: A Systematic Review of Reviews and Analysis of Causality. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 43. [Google Scholar] [CrossRef]
- Rey-López, J.P.; Vicente-Rodriguez, G.; Ortega, F.B.; Ruiz, J.R.; Martinez-Gómez, D.; De Henauw, S.; Manios, Y.; Molnar, D.; Polito, A.; Verloigne, M.; et al. Sedentary Patterns and Media Availability in European Adolescents: The HELENA Study. Prev. Med. 2010, 51, 50–55. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Aparicio-Ugarriza, R.; Castillo, A.; Ruiz, E.; Avila, J.M.; Aranceta-Bartrina, J.; Gil, A.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; et al. Sedentary Behavior among Spanish Children and Adolescents: Findings from the ANIBES Study. BMC Public Health 2017, 17, 94. [Google Scholar] [CrossRef]
- Sánchez-Oliva, D.; Leech, R.M.; Grao-Cruces, A.; Esteban-Cornejo, I.; Padilla-Moledo, C.; Veiga, O.L.; Cabanas-Sánchez, V.; Castro-Piñero, J. Does Modality Matter? A Latent Profile and Transition Analysis of Sedentary Behaviours among School-Aged Youth: The UP&DOWN Study: Profile Transitions of Sedentary Behaviours. J. Sports Sci. 2020, 38, 1062–1069. [Google Scholar] [CrossRef]
- Santos, D.A.; Magalhães, J.P.; Júdice, P.B.; Correia, I.R.; Minderico, C.S.; Ekelund, U.; Sardinha, L.B. Fitness Mediates Activity and Sedentary Patterns Associations with Adiposity in Youth. Med. Sci. Sports Exerc. 2019, 51, 323–329. [Google Scholar] [CrossRef]
- Bailey, D.P.; Charman, S.J.; Ploetz, T.; Savory, L.A.; Kerr, C.J. Associations between Prolonged Sedentary Time and Breaks in Sedentary Time with Cardiometabolic Risk in 10–14-Year-Old Children: The HAPPY Study. J. Sports Sci. 2017, 35, 2164–2171. [Google Scholar] [CrossRef]
- Verloigne, M.; Ridgers, N.D.; Chinapaw, M.; Altenburg, T.M.; Bere, E.; Van Lippevelde, W.; Cardon, G.; Brug, J.; De Bourdeaudhuij, I. Patterns of Objectively Measured Sedentary Time in 10- to 12-Year-Old Belgian Children: An Observational Study within the ENERGYproject. BMC Pediatr. 2017, 17, 147. [Google Scholar] [CrossRef]
- Chinapaw, M.; Klakk, H.; Møller, N.C.; Andersen, L.B.; Altenburg, T.; Wedderkopp, N. Total Volume versus Bouts: Prospective Relationship of Physical Activity and Sedentary Time with Cardiometabolic Risk in Children. Int. J. Obes. 2018, 42, 1733–1742. [Google Scholar] [CrossRef]
- Júdice, P.B.; Hetherington-Rauth, M.; Northstone, K.; Andersen, L.B.; Wedderkopp, N.; Ekelund, U.; Sardinha, L.B. Changes in Physical Activity and Sedentary Patterns on Cardiometabolic Outcomes in the Transition to Adolescence: International Children’s Accelerometry Database 2.0. J. Pediatr. 2020, 225, 166–173.e1. [Google Scholar] [CrossRef] [PubMed]
- Skrede, T.; Steene-Johannessen, J.; Anderssen, S.A.; Resaland, G.K.; Ekelund, U. The Prospective Association between Objectively Measured Sedentary Time, Moderate-to-Vigorous Physical Activity and Cardiometabolic Risk Factors in Youth: A Systematic Review and Meta-Analysis. Obes. Rev. 2019, 20, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Grao-Cruces, A.; Sánchez-Oliva, D.; Padilla-Moledo, C.; Izquierdo-Gómez, R.; Cabanas-Sánchez, V.; Castro-Piñero, J. Changes in the School and Non-School Sedentary Time in Youth: The UP&DOWN Longitudinal Study. J. Sports Sci. 2020, 38, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Brazo-Sayavera, J.; Aubert, S.; Barnes, J.D.; González, S.A.; Tremblay, M.S. Gender Differences in Physical Activity and Sedentary Behavior: Results from over 200,000 Latin-American Children and Adolescents. PLoS ONE 2021, 16, e0255353. [Google Scholar] [CrossRef]
- Botelho, G.; Ferrão, A.; Aguiar, M. Gender and Age Differences in Physical Activity and Sedentary Behaviour among Portuguese Adolescents. J. Phys. Educ. Sport 2013, 13, 184–194. [Google Scholar] [CrossRef]
- Castro-Piñero, J.; Carbonell-Baeza, A.; Martinez-Gomez, D.; Gómez-Martínez, S.; Cabanas-Sánchez, V.; Santiago, C.; Veses, A.M.; Bandrés, F.; Gonzalez-Galo, A.; Gomez-Gallego, F.; et al. Follow-up in Healthy Schoolchildren and in Adolescents with DOWN Syndrome: Psycho-Environmental and Genetic Determinants of Physical Activity and Its Impact on Fitness, Cardiovascular Diseases, Inflammatory Biomarkers and Mental Health; The UP&DOWN Study. BMC Public Health 2014, 14, 400. [Google Scholar] [CrossRef]
- Tanner, J.M.; Whitehouse, R.H. Clinical Longitudinal Standards for Height, Weight, Height Velocity, Weight Velocity, and Stages of Puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef]
- Topouchian, J.; El Assaad, M.; Orobinskaia, L.V.; El Feghali, R.N.; Asmar, R.G. Validation of Two Automatic Devices for Self-Measurement of Blood Pressure According to the International Protocol of the European Society of Hypertension: The Omron M6 (HEM-7001-E) and the Omron R7 (HEM 637-IT). Blood Press. Monit. 2006, 11, 165–171. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The Metabolic Syndrome in Children and Adolescents. Lancet 2007, 369, 2059–2061. [Google Scholar] [CrossRef] [PubMed]
- Cabanas-Sánchez, V.; Martínez-Gómez, D.; Esteban-Cornejo, I.; Castro-Piñero, J.; Conde-Caveda, J.; Veiga, Ó.L. Reliability and Validity of the Youth Leisure-Time Sedentary Behavior Questionnaire (YLSBQ). J. Sci. Med. Sport. 2018, 21, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Vanhelst, J.; Mikulovic, J.; Bui-Xuan, G.; Dieu, O.; Blondeau, T.; Fardy, P.; Béghin, L. Comparison of Two ActiGraph Accelerometer Generations in the Assessment of Physical Activity in Free Living Conditions. BMC Res. Notes 2012, 5, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Robusto, K.M.; Trost, S.G. Comparison of Three Generations of ActiGraphTM Activity Monitors in Children and Adolescents. J. Sports Sci. 2012, 30, 1429–1435. [Google Scholar] [CrossRef]
- Trost, S.G.; Mciver, K.L.; Pate, R.R. Conducting Accelerometer-Based Activity Assessments in Field-Based Research. Med. Sci. Sports Exerc. 2005, 37, 531–543. [Google Scholar] [CrossRef]
- Cain, K.L.; Sallis, J.F.; Conway, T.L.; Van Dyck, D.; Calhoon, L. Using Accelerometers in Youth Physical Activity Studies: A Review of Methods. J. Phys. Act. Health 2013, 10, 437–450. [Google Scholar] [CrossRef]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of Accelerometer Wear and Nonwear Time Classification Algorithm. Med. Sci. Sports Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef]
- Evenson, K.R.; Catellier, D.J.; Gill, K.; Ondrak, K.S.; McMurray, R.G. Calibration of Two Objective Measures of Physical Activity for Children. J. Sports Sci. 2008, 26, 1557–1565. [Google Scholar] [CrossRef]
- Carson, V.; Stone, M.; Faulkner, G. Patterns of Sedentary Behavior and Weight Status among Children. Pediatr. Exerc. Sci. 2014, 26, 95–102. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Grøntved, A.; Ried-Larsen, M.; Møller, N.C.; Kristensen, P.L.; Wedderkopp, N.; Froberg, K.; Hu, F.B.; Ekelund, U.; Andersen, L.B. Youth Screen-Time Behaviour Is Associated with Cardiovascular Risk in Young Adulthood: The European Youth Heart Study. Eur. J. Prev. Cardiol. 2014, 21, 49–56. [Google Scholar] [CrossRef]
- Khan, M.A.; Shah, S.M.; Shehab, A.; Ghosal, S.; Muhairi, S.J.; Al-Rifai, R.H.; Al Maskari, F.; Alkaabi, J.; Nauman, J. Screen Time and Metabolic Syndrome among Expatriate Adolescents in the United Arab Emirates. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2565–2569. [Google Scholar] [CrossRef]
- Mark, A.E.; Janssen, I. Relationship between Screen Time and Metabolic Syndrome in Adolescents. J. Public Health 2008, 30, 153–160. [Google Scholar] [CrossRef]
- Tambalis, K.D.; Panagiotakos, D.B.; Psarra, G.; Sidossis, L.S. Screen Time and Its Effect on Dietary Habits and Lifestyle among Schoolchildren. Cent. Eur. J. Public Health 2020, 28, 260–266. [Google Scholar] [CrossRef]
- Stephens, C.R.; Easton, J.F.; Robles-Cabrera, A.; Fossion, R.; de la Cruz, L.; Martínez-Tapia, R.; Barajas-Martínez, A.; Hernández-Chávez, A.; López-Rivera, J.A.; Rivera, A.L. The Impact of Education and Age on Metabolic Disorders. Front. Public Health 2020, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Guo, Q.; Ju, L.; Gong, W.; Wei, X.; Xu, X.; Zhao, L.; Fang, H. Association between Sedentary Behavior, Screen Time and Metabolic Syndrome among Chinese Children and Adolescents. BMC Public Health 2024, 24, 1715. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.O.; Norde, M.M.; Vasques, A.C.; Zambom, M.P.; Antonio, M.A.R.d.G.M.; Rodrigues, A.M.D.B.; Geloneze, B. Association of Physical Activity and Sitting with Metabolic Syndrome and Hyperglycemic Clamp Parameters in Adolescents—BRAMS Pediatric Study. Front. Endocrinol. 2023, 14, 1191935. [Google Scholar] [CrossRef] [PubMed]
- Renninger, M.; Hansen, B.H.; Steene-Johannessen, J.; Kriemler, S.; Froberg, K.; Northstone, K.; Sardinha, L.; Anderssen, S.A.; Andersen, L.B.; Ekelund, U. Associations between Accelerometry Measured Physical Activity and Sedentary Time and the Metabolic Syndrome: A Meta-Analysis of More than 6000 Children and Adolescents. Pediatr. Obes. 2020, 15, e12578. [Google Scholar] [CrossRef]
- Sehn, A.P.; Silveira, J.F.d.C.; Brand, C.; Lemes, V.B.; Borfe, L.; Tornquist, L.; Pfeiffer, K.A.; Renner, J.D.P.; Andersen, L.B.; Burns, R.D.; et al. Screen Time, Sleep Duration, Leisure Physical Activity, Obesity, and Cardiometabolic Risk in Children and Adolescents: A Cross-Lagged 2-Year Study. BMC Cardiovasc. Disord. 2024, 24, 525. [Google Scholar] [CrossRef]
- Sánchez-Delgado, A.; Pérez-Bey, A.; Izquierdo-Gómez, R.; Jimenez-Iglesias, J.; Marcos, A.; Gómez-Martínez, S.; Girela-Rejón, M.J.; Veiga, O.L.; Castro-Piñero, J. Fitness, Body Composition, and Metabolic Risk Scores in Children and Adolescents: The UP&DOWN Study. Eur. J. Pediatr. 2023, 182, 669–687. [Google Scholar] [CrossRef]
- Wijndaele, K.; White, T.; Andersen, L.B.; Bugge, A.; Kolle, E.; Northstone, K.; Wedderkopp, N.; Ried-Larsen, M.; Kriemler, S.; Page, A.S.; et al. Substituting Prolonged Sedentary Time and Cardiovascular Risk in Children and Youth: A Meta-Analysis within the International Children’s Accelerometry Database (ICAD). Int. J. Behav. Nutr. Phys. Act. 2019, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Janssen, X.; Mann, K.D.; Basterfield, L.; Parkinson, K.N.; Pearce, M.S.; Reilly, J.K.; Adamson, A.J.; Reilly, J.J. Development of Sedentary Behavior across Childhood and Adolescence: Longitudinal Analysis of the Gateshead Millennium Study. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.G.; Guedes, D.P. Physical Activity, Sedentary Behavior, Cardiorespiratory Fitness and Metabolic Syndrome in Adolescents: Systematic Review and Meta-Analysis of Observational Evidence. PLoS ONE 2016, 11, e0168503. [Google Scholar] [CrossRef]
| Baseline | ||||||
| Male children | Female children | p value | Male adolescents | Female adolescents | p value | |
| Sedentary behaviors | (n = 59) | (n = 45) | (n = 119) | (n = 110) | ||
| Total DSB (min/day) | 306.77 (116.29) | 312.31 (132.20) | 0.821 | 416.85 (127.19) | 433.27 (127.73) | 0.331 |
| Screen DSB (min/day) | 101.63 (96.29) | 80.42 (91.69) | 0.259 | 156.95 (124.42) | 113.35 (106.36) | 0.005 |
| Educative DSB (min/day) | 142.24 (105.23) | 147.87 (110.85) | 0.792 | 162.18 (125.30) | 215.49 (135.18) | 0.002 |
| Social DSB (min/day) | 43.18 (42.43) | 66.99 (75.37) | 0.044 | 65.14 (94.12) | 76.78 (78.54) | 0.313 |
| Other DSB (min/day) | 19.72 (27.91) | 17.03 (25.86) | 0.616 | 32.59 (49.68) | 27.64 (51.22) | 0.459 |
| Total WSB (min/day) | 516.27 (158.62) | 484.79 (174.32) | 0.339 | 571.50 (188.12) | 577.85 (161.95) | 0.785 |
| Screen WSB (min/day) | 282.36 (160.44) | 228.97 (167.72) | 0.102 | 312.00 (180.89) | 238.66 (141.36) | 0.001 |
| Educative WSB (min/day) | 93.25 (82.34) | 99.06 (83.01) | 0.723 | 113.90 (101.18) | 150.62 (121.97) | 0.014 |
| Social WSB (min/day) | 90.68 (70.39) | 109.24 (82.30) | 0.219 | 93.90 (85.59) | 137.73 (115.19) | 0.001 |
| Other WSB (min/day) | 49.97 (57.54) | 47.52 (47.09) | 0.817 | 51.71 (75.29) | 50.85 (61.75) | 0.925 |
| Mean SB (min/day) | 366.63 (101.57) | 361.59 (129.40) | 0.824 | 461.04 (119.71) | 474.58 (109.36) | 0.374 |
| Sedentary pattern | (n = 123) | (n = 106) | (n = 119) | (n = 110) | ||
| Accelerometer wear time (h/day) | 13.65 (0.8) | 13.5 (0.74) | 0.398 | 14.27 (1.81) | 14.26 (1.52) | 0.947 |
| Sedentary time (min/day) | 520.60 (69.04) | 537.72 (72.85) | 0.069 | 650.22 (116.81) | 686.07 (100.40) | 0.014 |
| Bouts of 10 min (number/day) | 5.98 (3.14) | 6.71 (3.15) | 0.079 | 12.03 (5.06) | 14.26 (4.56) | 0.001 |
| Time in bouts of 10 min (min/day) | 104.91 (60.78) | 117.85 (59.06) | 0.105 | 218.97 (113.25) | 279.41 (110.48) | <0.001 |
| Bouts of 20 min (number/day) | 1.39 (1.08) | 1.61 (1.03) | 0.12 | 3.21 (2.32) | 4.75 (2.42) | <0.001 |
| Time in bouts of 20 min (min/day) | 43.80 (35.38) | 49.73 (33.84) | 0.198 | 99.48 (79.16) | 148.68 (82.86) | <0.001 |
| Bouts of 30 min (number/day) | 0.54 (0.54) | 0.58 (0.49) | 0.537 | 1.26 (1.18) | 1.98 (1.31) | <0.001 |
| Time in bouts of 30 min (min/day) | 23.30 (23.96) | 25.00 (22.71) | 0.584 | 52.46 (52.39) | 81.83 (57.30) | <0.001 |
| Bouts of 45 min (number/day) | 0.18 (0.25) | 0.19 (0.22) | 0.669 | 0.38 (0.51) | 0.59 (0.56) | 0.003 |
| Time in bouts of 45 min (min/day) | 10.35 (15.02) | 11.15 (13.95) | 0.678 | 20.68 (28.89) | 31.58 (31.10) | 0.006 |
| Physical activity | ||||||
| MVPA (min/day) | 69.00 (21.06) | 52.73 (18.61) | <0.001 | 61.11 (19.31) | 44.68 (16.83) | <0.001 |
| Tanner stage | 1.59 (0.63) | 1.45 (0.72) | 0.137 | 3.61 (0.92) | 3.42 (0.75) | 0.081 |
| Age (years) | 8.09 (1.52) | 8.05 (1.54) | 0.833 | 14.10 (1.64) | 13.87 (1.47) | 0.266 |
| Systolic blood pressure (mmHg) | 101.82 (10.74) | 99.25 (11.32) | 0.081 | 111.16 (13.84) | 106.27 (9.88) | 0.003 |
| Triglycerides (mg/dL) | 40.10 (18.75) | 45.67 (17.91) | 0.023 | 48.12 (19.17) | 53.99 (22.16) | 0.033 |
| HDL cholesterol (mg/dL) | 39.98 (16.30) | 41.63 (15.86) | 0.44 | 48.14 (15.34) | 49.55 (14.70) | 0.481 |
| Glucose (mg/dL) | 61.42 (17.86) | 62.83 (16.53) | 0.539 | 79.55 (15.90) | 77.58 (15.63) | 0.345 |
| Body composition | ||||||
| Weight (kg) | 30.54 (8.12) | 31.16 (11.07) | 0.626 | 55.22 (12.92) | 52.19 (9.39) | 0.044 |
| Height (cm) | 128.78 (9.96) | 129.23 (12.11) | 0.753 | 162.86 (12.23) | 157.85 (6.62) | <0.001 |
| Body mass index (kg/m2) | 18.14 (2.76) | 18.14 (3.63) | 0.996 | 20.57 (2.93) | 20.86 (3.01) | 0.462 |
| Waist circumference (cm) | 59.32 (6.81) | 58.15 (8.59) | 0.255 | 69.19 (7.05) | 66.06 (5.79) | <0.001 |
| Follow-up | ||||||
| Male children | Female children | p value | Male adolescents | Female adolescents | p value | |
| Sedentary behaviors | (n = 42) | (n = 34) | (n = 92) | (n = 94) | ||
| Total DSB (min/day) | 323.71 (131.78) | 276.82 (162.85) | 0.169 | 444.57 (133.05) | 481.87 (116.84) | 0.044 |
| Screen DSB (min/day) | 111.15 (114.07) | 83.18 (81.49) | 0.233 | 145.68 (132.10) | 102.24 (107.48) | 0.015 |
| Educative DSB (min/day) | 138.22 (110.18) | 136.29 (119.87) | 0.942 | 191.15 (134.53) | 256.79 (172.84) | 0.004 |
| Social DSB (min/day) | 44.50 (46.22) | 44.12 (49.70) | 0.973 | 79.97 (71.97) | 103.28 (78.10) | 0.036 |
| Other DSB (min/day) | 29.84 (58.60) | 13.23 (18.09) | 0.116 | 27.78 (35.62) | 19.56 (26.82) | 0.077 |
| Total WSB (min/day) | 554.61 (186.73) | 489.59 (232.67) | 0.181 | 617.31 (183.46) | 616.07 (161.73) | 0.961 |
| Screen WSB (min/day) | 314.51 (167.32) | 223.64 (195.26) | 0.032 | 297.34 (192.70) | 180.02 (137.74) | <0.001 |
| Educative WSB (min/day) | 109.66 (109.90) | 105.19 (84.50) | 0.846 | 159.34 (174.67) | 230.53 (197.24) | 0.010 |
| Social WSB (min/day) | 86.58 (98.48) | 117.20 (128.39) | 0.243 | 120.20 (94.83) | 174.64 (114.85) | 0.001 |
| Other WSB (min/day) | 43.85 (55.77) | 43.57 (62.93) | 0.984 | 40.43 (44.56) | 30.88 (30.85) | 0.090 |
| Mean SB (min/day) | 389.68 (127.86) | 337.62 (159.77) | 0.119 | 493.93 (120.87) | 520.21 (108.93) | 0.121 |
| Sedentary pattern | (n = 93) | (n = 82) | (n = 93) | (n = 95) | ||
| Accelerometer wear time (h/day) | 10.17 (1.78) | 10.35 (1.74) | 0.654 | 10.62 (2.7) | 11.51 (2.12) | 0.013 |
| Sedentary time (min/day) | 558.70 (64.50) | 577.73 (65.53) | 0.055 | 849.72 (426.81) | 877.48 (370.85) | 0.634 |
| Bouts of 10 min (number/day) | 8.07 (3.59) | 8.93 (3.45) | 0.11 | 14.60 (5.44) | 16.81 (4.70) | 0.003 |
| Time in bouts of 10 min (min/day) | 136.29 (70.88) | 161.38 (75.27) | 0.024 | 423.71 (392.71) | 489.49 (339.19) | 0.22 |
| Bouts of 20 min (number/day) | 1.75 (1.39) | 2.36 (1.58) | 0.007 | 4.63 (2.63) | 6.39 (2.79) | <0.001 |
| Time in bouts of 20 min (min/day) | 51.66 (43.02) | 72.12 (53.19) | 0.006 | 288.14 (380.88) | 345.62 (329.10) | 0.269 |
| Bouts of 30 min (number/day) | 0.58 (0.60) | 0.83 (0.80) | 0.017 | 1.98 (1.54) | 3.08 (1.81) | <0.001 |
| Time in bouts of 30 min (min/day) | 23.91 (25.77) | 35.19 (34.69) | 0.015 | 224.55 (371.97) | 265.80 (324.72) | 0.419 |
| Bouts of 45 min (number/day) | 0.16 (0.23) | 0.27 (0.35) | 0.009 | 0.80 (0.91) | 1.25 (1.16) | 0.004 |
| Time in bouts of 45 min (min/day) | 8.91 (13.62) | 15.26 (19.22) | 0.012 | 181.91 (365.67) | 199.57 (316.13) | 0.723 |
| Physical activity | ||||||
| MVPA (min/day) | 60.04 (18.77) | 53.30 (22.60) | 0.032 | 56.30 (27.37) | 42.15 (19.75) | <0.001 |
| Tanner stage | 2.31 (0.59) | 2.16 (1.06) | 0.232 | 4.45 (0.62) | 3.98 (0.62) | <0.001 |
| Age (years) | 10.14 (1.50) | 10.18 (1.50) | 0.865 | 16.00 (1.60) | 15.81 (1.43) | 0.393 |
| Systolic blood pressure (mmHg) | 105.92 (9.04) | 105.42 (10.55) | 0.734 | 113.57 (12.39) | 103.67 (9.49) | <0.001 |
| Triglycerides (mg/dL) | 40.73 (19.31) | 45.32 (24.66) | 0.17 | 63.53 (25.39) | 62.17 (22.98) | 0.701 |
| HDL cholesterol (mg/dL) | 39.95 (14.97) | 40.33 (16.52) | 0.872 | 52.02 (12.28) | 59.58 (11.48) | <0.001 |
| Glucose (mg/dL) | 71.57 (16.91) | 73.21 (17.08) | 0.526 | 86.94 (7.94) | 84.71 (8.07) | 0.058 |
| Body composition | ||||||
| Weight (kg) | 38.56 (10.23) | 40.18 (13.28) | 0.365 | 62.66 (10.88) | 56.26 (8.88) | <0.001 |
| Height (cm) | 140.69 (10.07) | 143.31 (12.29) | 0.123 | 171.83 (7.83) | 162.18 (5.17) | <0.001 |
| Body mass index (kg/m2) | 19.21 (3.32) | 19.10 (4.05) | 0.844 | 21.14 (2.98) | 21.40 (3.24) | 0.58 |
| Waist circumference (cm) | 63.37 (8.41) | 61.24 (9.23) | 0.112 | 71.80 (6.75) | 67.15 (6.61) | <0.001 |
| Sedentary behaviors | ||||||||||||
| Children (n = 104) | Adolescents (n = 229) | |||||||||||
| Male (n = 59) | Female (n = 45) | Male (n = 119) | Female (n = 110) | |||||||||
| Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | |
| Model 1 | ||||||||||||
| Total DSB | −0.023 | 0.001 | 0.175 | 0.292 | 0.000 | 0.824 | 0.264 | 0.001 | 0.134 | 0.039 | 0.000 | 0.303 |
| Screen DSB | −0.021 | 0.001 | 0.167 | 0.320 | −0.001 | 0.283 | 0.249 | 0.000 | 0.584 | 0.036 | 0.000 | 0.385 |
| Educative DSB | −0.070 | 0.000 | 0.924 | 0.309 | −0.001 | 0.403 | 0.253 | 0.000 | 0.362 | 0.028 | 0.000 | 0.885 |
| Social DSB | −0.070 | 0.000 | 0.897 | 0.362 | 0.002 | 0.090 | 0.267 | 0.001 | 0.104 | 0.032 | 0.000 | 0.523 |
| Other DSB | −0.070 | 0.000 | 0.850 | 0.301 | 0.003 | 0.537 | 0.261 | 0.001 | 0.173 | 0.029 | 0.000 | 0.750 |
| Total WSB | −0.070 | 0.000 | 0.868 | 0.334 | −0.001 | 0.190 | 0.259 | 0.000 | 0.215 | 0.035 | 0.000 | 0.430 |
| Screen WSB | −0.069 | 0.000 | 0.805 | 0.389 | −0.001 | 0.044 | 0.260 | 0.000 | 0.189 | 0.033 | 0.000 | 0.491 |
| Educative WSB | −0.029 | 0.001 | 0.206 | 0.292 | 0.000 | 0.899 | 0.253 | 0.000 | 0.393 | 0.054 | 0.001 | 0.118 |
| Social WSB | −0.010 | −0.002 | 0.124 | 0.307 | 0.001 | 0.426 | 0.247 | 0.000 | 0.933 | 0.040 | 0.000 | 0.279 |
| Other WSB | −0.037 | 0.001 | 0.255 | 0.300 | 0.001 | 0.551 | 0.257 | 0.001 | 0.260 | 0.031 | 0.000 | 0.611 |
| Mean SB | −0.035 | 0.001 | 0.240 | 0.304 | 0.000 | 0.484 | 0.270 | 0.001 | 0.081 | 0.043 | 0.001 | 0.230 |
| Model 2 | ||||||||||||
| Total DSB | 0.056 | 0.001 | 0.185 | 0.269 | 0.000 | 0.837 | 0.277 | 0.000 | 0.238 | 0.047 | 0.000 | 0.407 |
| Screen DSB | 0.069 | 0.001 | 0.130 | 0.297 | −0.001 | 0.305 | 0.269 | 0.000 | 0.616 | 0.052 | 0.001 | 0.274 |
| Educative DSB | 0.014 | 0.000 | 0.833 | 0.289 | −0.001 | 0.388 | 0.273 | 0.000 | 0.367 | 0.042 | 0.000 | 0.655 |
| Social DSB | 0.015 | 0.001 | 0.773 | 0.342 | 0.002 | 0.093 | 0.284 | 0.001 | 0.133 | 0.043 | 0.000 | 0.564 |
| Other DSB | 0.014 | 0.000 | 0.863 | 0.282 | 0.004 | 0.476 | 0.274 | 0.001 | 0.349 | 0.041 | 0.000 | 0.751 |
| Total WSB | 0.014 | 0.000 | 0.818 | 0.315 | −0.001 | 0.184 | 0.278 | 0.000 | 0.236 | 0.044 | 0.000 | 0.511 |
| Screen WSB | 0.013 | 0.000 | 0.934 | 0.368 | −0.001 | 0.049 | 0.279 | 0.000 | 0.202 | 0.045 | 0.000 | 0.477 |
| Educative WSB | 0.053 | 0.001 | 0.202 | 0.269 | 0.000 | 0.867 | 0.269 | 0.000 | 0.661 | 0.058 | 0.000 | 0.188 |
| Social WSB | 0.058 | −0.001 | 0.174 | 0.286 | 0.001 | 0.419 | 0.267 | 0.000 | 0.834 | 0.052 | 0.000 | 0.287 |
| Other WSB | 0.024 | 0.001 | 0.508 | 0.275 | 0.001 | 0.629 | 0.272 | 0.001 | 0.395 | 0.041 | 0.000 | 0.683 |
| Mean SB | 0.047 | 0.001 | 0.239 | 0.281 | 0.000 | 0.489 | 0.283 | 0.001 | 0.139 | 0.050 | 0.000 | 0.327 |
| Sedentary patterns | ||||||||||||
| Children (n = 229) | Adolescents (n = 229) | |||||||||||
| Male (n = 123) | Female (n = 106) | Male (n = 119) | Female (n = 110) | |||||||||
| Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | |
| Model 1 | ||||||||||||
| Sedentary time | 0.099 | 0.000 | 0.726 | 0.154 | 0.000 | 0.157 | 0.249 | 0.000 | 0.611 | 0.036 | 0.000 | 0.375 |
| Bouts of 10 min | 0.120 | 0.003 | 0.114 | 0.181 | 0.006 | 0.029 | 0.248 | 0.000 | 0.764 | 0.056 | 0.002 | 0.103 |
| Time in bouts of 10 min | 0.113 | 0.000 | 0.187 | 0.169 | 0.000 | 0.060 | 0.250 | 0.000 | 0.523 | 0.050 | 0.000 | 0.146 |
| Bouts of 20 min | 0.114 | 0.008 | 0.175 | 0.147 | 0.009 | 0.262 | 0.250 | −0.002 | 0.547 | 0.041 | 0.003 | 0.277 |
| Time in bouts of 20 min | 0.108 | 0.000 | 0.291 | 0.146 | 0.000 | 0.291 | 0.253 | 0.000 | 0.382 | 0.041 | 0.000 | 0.277 |
| Bouts of 30 min | 0.111 | 0.014 | 0.228 | 0.136 | 0.006 | 0.700 | 0.259 | −0.008 | 0.208 | 0.046 | 0.007 | 0.192 |
| Time in bouts of 30 min | 0.105 | 0.000 | 0.401 | 0.139 | 0.000 | 0.517 | 0.261 | 0.000 | 0.167 | 0.044 | 0.000 | 0.216 |
| Bouts of 45 min | 0.099 | −0.007 | 0.759 | 0.141 | 0.027 | 0.434 | 0.269 | −0.024 | 0.089 | 0.034 | 0.009 | 0.452 |
| Time in bouts of 45 min | 0.099 | 0.000 | 0.747 | 0.144 | 0.001 | 0.319 | 0.270 | 0.000 | 0.082 | 0.034 | 0.000 | 0.451 |
| Model 2 | ||||||||||||
| Sedentary time | 0.112 | 0.000 | 0.851 | 0.158 | 0.000 | 0.180 | 0.269 | 0.000 | 0.594 | 0.052 | 0.000 | 0.272 |
| Bouts of 10 min | 0.127 | 0.003 | 0.179 | 0.176 | 0.005 | 0.053 | 0.270 | −0.001 | 0.561 | 0.071 | 0.002 | 0.084 |
| Time in bouts of 10 min | 0.123 | 0.000 | 0.253 | 0.166 | 0.000 | 0.105 | 0.273 | 0.000 | 0.375 | 0.067 | 0.000 | 0.105 |
| Bouts of 20 min | 0.125 | 0.007 | 0.210 | 0.147 | 0.008 | 0.380 | 0.272 | −0.002 | 0.414 | 0.057 | 0.004 | 0.206 |
| Time in bouts of 20 min | 0.120 | 0.000 | 0.319 | 0.146 | 0.000 | 0.410 | 0.275 | 0.000 | 0.288 | 0.058 | 0.000 | 0.190 |
| Bouts of 30 min | 0.122 | 0.012 | 0.269 | 0.140 | 0.003 | 0.841 | 0.281 | −0.008 | 0.165 | 0.067 | 0.008 | 0.109 |
| Time in bouts of 30 min | 0.117 | 0.000 | 0.420 | 0.142 | 0.000 | 0.644 | 0.284 | 0.000 | 0.134 | 0.065 | 0.000 | 0.122 |
| Bouts of 45 min | 0.112 | −0.003 | 0.891 | 0.144 | 0.022 | 0.524 | 0.289 | −0.024 | 0.087 | 0.051 | 0.013 | 0.300 |
| Time in bouts of 45 min | 0.112 | 0.000 | 0.874 | 0.147 | 0.000 | 0.399 | 0.290 | 0.000 | 0.077 | 0.052 | 0.000 | 0.287 |
| Sedentary behaviors | ||||||||||||
| Children (n = 76) | Adolescents (n = 186) | |||||||||||
| Male (n = 42) | Female (n = 34) | Male (n = 92) | Female (n = 94) | |||||||||
| Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | |
| Model 1 | ||||||||||||
| Total DSB | 0.275 | 0.000 | 0.760 | 0.219 | 0.000 | 0.686 | 0.587 | −0.001 | 0.006 | 0.442 | 0.000 | 0.593 |
| Screen DSB | 0.273 | 0.000 | 0.849 | 0.211 | 0.000 | 0.936 | 0.542 | 0.000 | 0.980 | 0.443 | 0.000 | 0.502 |
| Educative DSB | 0.277 | 0.000 | 0.678 | 0.293 | −0.001 | 0.179 | 0.581 | −0.001 | 0.011 | 0.456 | 0.001 | 0.141 |
| Social DSB | 0.296 | −0.002 | 0.372 | 0.336 | 0.003 | 0.091 | 0.546 | −0.001 | 0.393 | 0.441 | 0.000 | 0.632 |
| Other DSB | 0.288 | −0.002 | 0.473 | 0.220 | −0.003 | 0.655 | 0.550 | 0.001 | 0.261 | 0.471 | −0.002 | 0.040 |
| Total WSB | 0.278 | 0.000 | 0.654 | 0.225 | 0.000 | 0.584 | 0.542 | 0.000 | 0.982 | 0.444 | 0.000 | 0.425 |
| Screen WSB | 0.356 | 0.001 | 0.090 | 0.224 | 0.000 | 0.598 | 0.545 | 0.000 | 0.456 | 0.440 | 0.000 | 0.862 |
| Educative WSB | 0.304 | −0.001 | 0.300 | 0.212 | 0.000 | 0.910 | 0.561 | −0.001 | 0.081 | 0.441 | 0.000 | 0.629 |
| Social WSB | 0.387 | −0.002 | 0.044 | 0.239 | 0.001 | 0.439 | 0.542 | 0.000 | 0.783 | 0.443 | 0.000 | 0.494 |
| Other WSB | 0.273 | 0.000 | 0.867 | 0.271 | −0.002 | 0.251 | 0.549 | 0.001 | 0.288 | 0.440 | 0.000 | 0.846 |
| Mean SB | 0.272 | 0.000 | 0.973 | 0.211 | 0.000 | 0.966 | 0.568 | −0.001 | 0.038 | 0.444 | 0.000 | 0.426 |
| Model 2 | ||||||||||||
| Total DSB | 0.250 | 0.000 | 0.692 | 0.170 | 0.000 | 0.695 | 0.592 | −0.001 | 0.004 | 0.439 | 0.000 | 0.668 |
| Screen DSB | 0.247 | 0.000 | 0.777 | 0.162 | 0.000 | 0.936 | 0.542 | 0.000 | 0.976 | 0.440 | 0.000 | 0.585 |
| Educative DSB | 0.251 | 0.000 | 0.670 | 0.250 | −0.001 | 0.188 | 0.582 | −0.001 | 0.011 | 0.451 | 0.000 | 0.184 |
| Social DSB | 0.274 | −0.002 | 0.341 | 0.295 | 0.003 | 0.101 | 0.548 | −0.001 | 0.358 | 0.439 | 0.000 | 0.666 |
| Other DSB | 0.260 | −0.002 | 0.496 | 0.172 | −0.003 | 0.662 | 0.547 | 0.001 | 0.376 | 0.468 | −0.002 | 0.042 |
| Total WSB | 0.251 | 0.000 | 0.665 | 0.177 | 0.000 | 0.588 | 0.542 | 0.000 | 0.960 | 0.441 | 0.000 | 0.476 |
| Screen WSB | 0.330 | 0.001 | 0.100 | 0.176 | 0.000 | 0.608 | 0.546 | 0.000 | 0.451 | 0.438 | 0.000 | 0.866 |
| Educative WSB | 0.285 | −0.001 | 0.266 | 0.162 | 0.000 | 0.909 | 0.557 | −0.001 | 0.129 | 0.438 | 0.000 | 0.723 |
| Social WSB | 0.363 | −0.002 | 0.050 | 0.192 | 0.001 | 0.451 | 0.544 | 0.000 | 0.681 | 0.441 | 0.000 | 0.515 |
| Other WSB | 0.245 | 0.000 | 0.951 | 0.242 | −0.003 | 0.210 | 0.548 | 0.001 | 0.332 | 0.438 | 0.000 | 0.781 |
| Mean SB | 0.245 | 0.000 | 0.918 | 0.162 | 0.000 | 0.968 | 0.571 | −0.001 | 0.034 | 0.441 | 0.000 | 0.502 |
| Sedentary patterns | ||||||||||||
| Children (n = 175) | Adolescents (n = 188) | |||||||||||
| Male (n = 93) | Female (n = 82) | Male (n = 93) | Female (n = 95) | |||||||||
| Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | |
| Model 1 | ||||||||||||
| Sedentary time | 0.472 | 0.000 | 0.472 | 0.527 | 0.000 | 0.405 | 0.537 | 0.000 | 0.785 | 0.457 | 0.000 | 0.482 |
| Bouts of 10 min | 0.469 | 0.001 | 0.677 | 0.522 | 0.000 | 0.845 | 0.536 | 0.000 | 0.814 | 0.453 | 0.000 | 0.992 |
| Time in bouts of 10 min | 0.468 | 0.000 | 0.855 | 0.523 | 0.000 | 0.634 | 0.536 | 0.000 | 0.967 | 0.453 | 0.000 | 0.826 |
| Bouts of 20 min | 0.468 | −0.001 | 0.822 | 0.524 | 0.005 | 0.552 | 0.536 | 0.000 | 0.986 | 0.453 | 0.000 | 0.930 |
| Time in bouts of 20 min | 0.468 | 0.000 | 0.804 | 0.527 | 0.000 | 0.383 | 0.536 | 0.000 | 0.909 | 0.454 | 0.000 | 0.764 |
| Bouts of 30 min | 0.468 | 0.001 | 0.915 | 0.528 | 0.016 | 0.358 | 0.536 | 0.000 | 0.956 | 0.454 | 0.001 | 0.813 |
| Time in bouts of 30 min | 0.468 | 0.000 | 0.928 | 0.532 | 0.000 | 0.239 | 0.536 | 0.000 | 0.936 | 0.455 | 0.000 | 0.617 |
| Bouts of 45 min | 0.468 | −0.003 | 0.907 | 0.530 | 0.039 | 0.283 | 0.537 | −0.004 | 0.757 | 0.457 | 0.008 | 0.464 |
| Time in bouts of 45 min | 0.469 | 0.000 | 0.775 | 0.534 | 0.001 | 0.199 | 0.537 | 0.000 | 0.683 | 0.459 | 0.000 | 0.389 |
| Model 2 | ||||||||||||
| Sedentary time | 0.465 | 0.000 | 0.471 | 0.544 | 0.000 | 0.604 | 0.538 | 0.000 | 0.732 | 0.456 | 0.000 | 0.427 |
| Bouts of 10 min | 0.462 | 0.001 | 0.676 | 0.543 | −0.001 | 0.635 | 0.537 | 0.000 | 0.954 | 0.451 | 0.000 | 0.977 |
| Time in bouts of 10 min | 0.461 | 0.000 | 0.856 | 0.542 | 0.000 | 0.806 | 0.537 | 0.000 | 0.903 | 0.452 | 0.000 | 0.768 |
| Bouts of 20 min | 0.461 | −0.001 | 0.819 | 0.542 | 0.000 | 0.980 | 0.538 | 0.000 | 0.882 | 0.451 | 0.000 | 0.860 |
| Time in bouts of 20 min | 0.461 | 0.000 | 0.800 | 0.542 | 0.000 | 0.822 | 0.538 | 0.000 | 0.808 | 0.452 | 0.000 | 0.679 |
| Bouts of 30 min | 0.461 | 0.001 | 0.918 | 0.543 | 0.006 | 0.752 | 0.537 | 0.000 | 0.957 | 0.452 | 0.002 | 0.697 |
| Time in bouts of 30 min | 0.461 | 0.000 | 0.924 | 0.544 | 0.000 | 0.581 | 0.538 | 0.000 | 0.849 | 0.454 | 0.000 | 0.509 |
| Bouts of 45 min | 0.461 | −0.003 | 0.896 | 0.544 | 0.021 | 0.578 | 0.538 | −0.004 | 0.714 | 0.457 | 0.010 | 0.374 |
| Time in bouts of 45 min | 0.462 | 0.000 | 0.762 | 0.546 | 0.000 | 0.466 | 0.539 | 0.000 | 0.631 | 0.459 | 0.000 | 0.301 |
| Change in sedentary behaviors | ||||||||||||
| Children (n = 76) | Adolescents (n = 186) | |||||||||||
| Male (n = 42) | Female (n = 34) | Male (n = 92) | Female (n = 94) | |||||||||
| Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | |
| Model 1 | ||||||||||||
| Total DSB | 0.273 | 0.000 | 0.858 | 0.220 | 0.000 | 0.665 | 0.553 | 0.000 | 0.190 | 0.440 | 0.000 | 0.847 |
| Screen DSB | 0.274 | 0.000 | 0.816 | 0.211 | 0.000 | 0.977 | 0.553 | 0.000 | 0.173 | 0.477 | 0.001 | 0.023 |
| Educative DSB | 0.272 | 0.000 | 0.971 | 0.222 | 0.000 | 0.626 | 0.548 | 0.000 | 0.313 | 0.473 | −0.001 | 0.033 |
| Social DSB | 0.272 | 0.000 | 0.966 | 0.212 | 0.000 | 0.908 | 0.553 | −0.001 | 0.176 | 0.440 | 0.000 | 0.771 |
| Other DSB | 0.272 | 0.000 | 0.917 | 0.245 | 0.005 | 0.396 | 0.543 | 0.000 | 0.639 | 0.471 | 0.002 | 0.037 |
| Total WSB | 0.278 | 0.000 | 0.645 | 0.212 | 0.000 | 0.871 | 0.543 | 0.000 | 0.614 | 0.448 | 0.000 | 0.282 |
| Screen WSB | 0.274 | 0.000 | 0.801 | 0.240 | 0.000 | 0.429 | 0.548 | 0.000 | 0.333 | 0.460 | 0.001 | 0.097 |
| Educative WSB | 0.277 | 0.000 | 0.688 | 0.235 | −0.001 | 0.474 | 0.546 | 0.000 | 0.395 | 0.441 | 0.000 | 0.655 |
| Social WSB | 0.289 | 0.000 | 0.460 | 0.287 | 0.001 | 0.197 | 0.545 | 0.000 | 0.476 | 0.440 | 0.000 | 0.881 |
| Other WSB | 0.342 | −0.002 | 0.122 | 0.252 | 0.001 | 0.345 | 0.553 | −0.001 | 0.189 | 0.443 | 0.001 | 0.470 |
| Mean SB | 0.275 | 0.000 | 0.742 | 0.218 | 0.000 | 0.695 | 0.546 | 0.000 | 0.438 | 0.442 | 0.000 | 0.534 |
| Model 2 | ||||||||||||
| Total DSB | 0.242 | 0.000 | 0.870 | 0.230 | 0.000 | 0.846 | 0.546 | 0.000 | 0.199 | 0.442 | 0.000 | 0.940 |
| Screen DSB | 0.243 | 0.000 | 0.851 | 0.245 | 0.001 | 0.560 | 0.547 | 0.000 | 0.181 | 0.475 | 0.001 | 0.033 |
| Educative DSB | 0.241 | 0.000 | 0.960 | 0.228 | 0.000 | 0.947 | 0.542 | 0.000 | 0.319 | 0.474 | −0.001 | 0.037 |
| Social DSB | 0.241 | 0.000 | 0.982 | 0.235 | −0.001 | 0.718 | 0.547 | −0.001 | 0.182 | 0.444 | 0.000 | 0.586 |
| Other DSB | 0.242 | 0.000 | 0.943 | 0.254 | 0.004 | 0.466 | 0.537 | 0.000 | 0.637 | 0.475 | 0.002 | 0.035 |
| Total WSB | 0.248 | 0.000 | 0.661 | 0.228 | 0.000 | 0.982 | 0.538 | 0.000 | 0.584 | 0.449 | 0.000 | 0.315 |
| Screen WSB | 0.243 | 0.000 | 0.815 | 0.246 | 0.000 | 0.548 | 0.541 | 0.000 | 0.347 | 0.457 | 0.000 | 0.153 |
| Educative WSB | 0.246 | 0.000 | 0.717 | 0.267 | −0.001 | 0.372 | 0.540 | 0.000 | 0.387 | 0.442 | 0.000 | 0.792 |
| Social WSB | 0.260 | 0.000 | 0.461 | 0.275 | 0.001 | 0.323 | 0.539 | 0.000 | 0.489 | 0.442 | 0.000 | 0.832 |
| Other WSB | 0.314 | −0.002 | 0.131 | 0.276 | 0.001 | 0.319 | 0.547 | −0.001 | 0.193 | 0.446 | 0.001 | 0.442 |
| Mean SB | 0.245 | 0.000 | 0.758 | 0.229 | 0.000 | 0.880 | 0.539 | 0.000 | 0.457 | 0.443 | 0.000 | 0.699 |
| Sedentary patterns | ||||||||||||
| Children (n = 175) | Adolescents (n = 188) | |||||||||||
| Male (n = 93) | Female (n = 82) | Male (n = 93) | Female (n = 95) | |||||||||
| Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | Adjusted R2 | β | p | |
| Model 1 | ||||||||||||
| Sedentary time | 0.469 | 0.000 | 0.695 | 0.523 | 0.000 | 0.706 | 0.537 | 0.000 | 0.782 | 0.461 | 0.000 | 0.290 |
| Bouts of 10 min | 0.468 | 0.000 | 0.876 | 0.534 | 0.003 | 0.202 | 0.536 | 0.000 | 0.925 | 0.456 | −0.001 | 0.534 |
| Time in bouts of 10 min | 0.468 | 0.000 | 0.930 | 0.532 | 0.000 | 0.238 | 0.536 | 0.000 | 0.855 | 0.457 | 0.000 | 0.469 |
| Bouts of 20 min | 0.469 | 0.001 | 0.769 | 0.529 | 0.005 | 0.327 | 0.536 | 0.000 | 0.986 | 0.455 | −0.001 | 0.639 |
| Time in bouts of 20 min | 0.468 | 0.000 | 0.867 | 0.527 | 0.000 | 0.387 | 0.536 | 0.000 | 0.821 | 0.456 | 0.000 | 0.544 |
| Bouts of 30 min | 0.470 | −0.005 | 0.600 | 0.525 | 0.006 | 0.503 | 0.537 | −0.002 | 0.763 | 0.454 | −0.001 | 0.806 |
| Time in bouts of 30 min | 0.469 | 0.000 | 0.785 | 0.525 | 0.000 | 0.524 | 0.536 | 0.000 | 0.861 | 0.455 | 0.000 | 0.620 |
| Bouts of 45 min | 0.468 | 0.001 | 0.952 | 0.522 | 0.003 | 0.867 | 0.536 | 0.000 | 0.956 | 0.454 | −0.002 | 0.819 |
| Time in bouts of 45 min | 0.468 | 0.000 | 0.871 | 0.522 | 0.000 | 0.864 | 0.537 | 0.000 | 0.797 | 0.454 | 0.000 | 0.708 |
| Model 2 | ||||||||||||
| Sedentary time | 0.462 | 0.000 | 0.742 | 0.515 | 0.000 | 0.754 | 0.531 | 0.000 | 0.728 | 0.459 | 0.000 | 0.506 |
| Bouts of 10 min | 0.462 | 0.000 | 0.844 | 0.526 | 0.003 | 0.213 | 0.530 | 0.000 | 0.957 | 0.456 | 0.000 | 0.732 |
| Time in bouts of 10 min | 0.461 | 0.000 | 0.908 | 0.525 | 0.000 | 0.247 | 0.531 | 0.000 | 0.833 | 0.457 | 0.000 | 0.722 |
| Bouts of 20 min | 0.462 | 0.001 | 0.756 | 0.522 | 0.005 | 0.331 | 0.530 | 0.000 | 0.998 | 0.456 | 0.000 | 0.909 |
| Time in bouts of 20 min | 0.462 | 0.000 | 0.857 | 0.520 | 0.000 | 0.387 | 0.531 | 0.000 | 0.807 | 0.456 | 0.000 | 0.835 |
| Bouts of 30 min | 0.463 | −0.005 | 0.599 | 0.518 | 0.007 | 0.497 | 0.531 | −0.001 | 0.790 | 0.456 | 0.001 | 0.910 |
| Time in bouts of 30 min | 0.462 | 0.000 | 0.785 | 0.518 | 0.000 | 0.514 | 0.531 | 0.000 | 0.845 | 0.456 | 0.000 | 0.893 |
| Bouts of 45 min | 0.461 | 0.001 | 0.944 | 0.515 | 0.004 | 0.835 | 0.530 | 0.000 | 0.984 | 0.456 | 0.001 | 0.913 |
| Time in bouts of 45 min | 0.461 | 0.000 | 0.865 | 0.515 | 0.000 | 0.834 | 0.531 | 0.000 | 0.785 | 0.456 | 0.000 | 0.935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Delgado, A.; Perez-Bey, A.; Conde-Caveda, J.; Izquierdo-Gómez, R.; Gómez-Martínez, S.; Veiga, O.L.; Marcos, A.; Castro-Piñero, J. Associations Between Sedentary Behaviors and Sedentary Patterns with Metabolic Syndrome in Children and Adolescents: The UP&DOWN Longitudinal Study. Healthcare 2025, 13, 2544. https://doi.org/10.3390/healthcare13192544
Sánchez-Delgado A, Perez-Bey A, Conde-Caveda J, Izquierdo-Gómez R, Gómez-Martínez S, Veiga OL, Marcos A, Castro-Piñero J. Associations Between Sedentary Behaviors and Sedentary Patterns with Metabolic Syndrome in Children and Adolescents: The UP&DOWN Longitudinal Study. Healthcare. 2025; 13(19):2544. https://doi.org/10.3390/healthcare13192544
Chicago/Turabian StyleSánchez-Delgado, Alejandro, Alejandro Perez-Bey, Julio Conde-Caveda, Rocío Izquierdo-Gómez, Sonia Gómez-Martínez, Oscar L. Veiga, Ascensión Marcos, and José Castro-Piñero. 2025. "Associations Between Sedentary Behaviors and Sedentary Patterns with Metabolic Syndrome in Children and Adolescents: The UP&DOWN Longitudinal Study" Healthcare 13, no. 19: 2544. https://doi.org/10.3390/healthcare13192544
APA StyleSánchez-Delgado, A., Perez-Bey, A., Conde-Caveda, J., Izquierdo-Gómez, R., Gómez-Martínez, S., Veiga, O. L., Marcos, A., & Castro-Piñero, J. (2025). Associations Between Sedentary Behaviors and Sedentary Patterns with Metabolic Syndrome in Children and Adolescents: The UP&DOWN Longitudinal Study. Healthcare, 13(19), 2544. https://doi.org/10.3390/healthcare13192544









