Impact of the COVID-19 Pandemic on Hemato-Oncology Services: A Retrospective Dual-Center Cohort Study in Kazakhstan
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Collection
2.2. Study Setting
2.3. Measures
2.4. Statistical Analysis
3. Results
3.1. Socio-Demographic
3.2. Referral Source
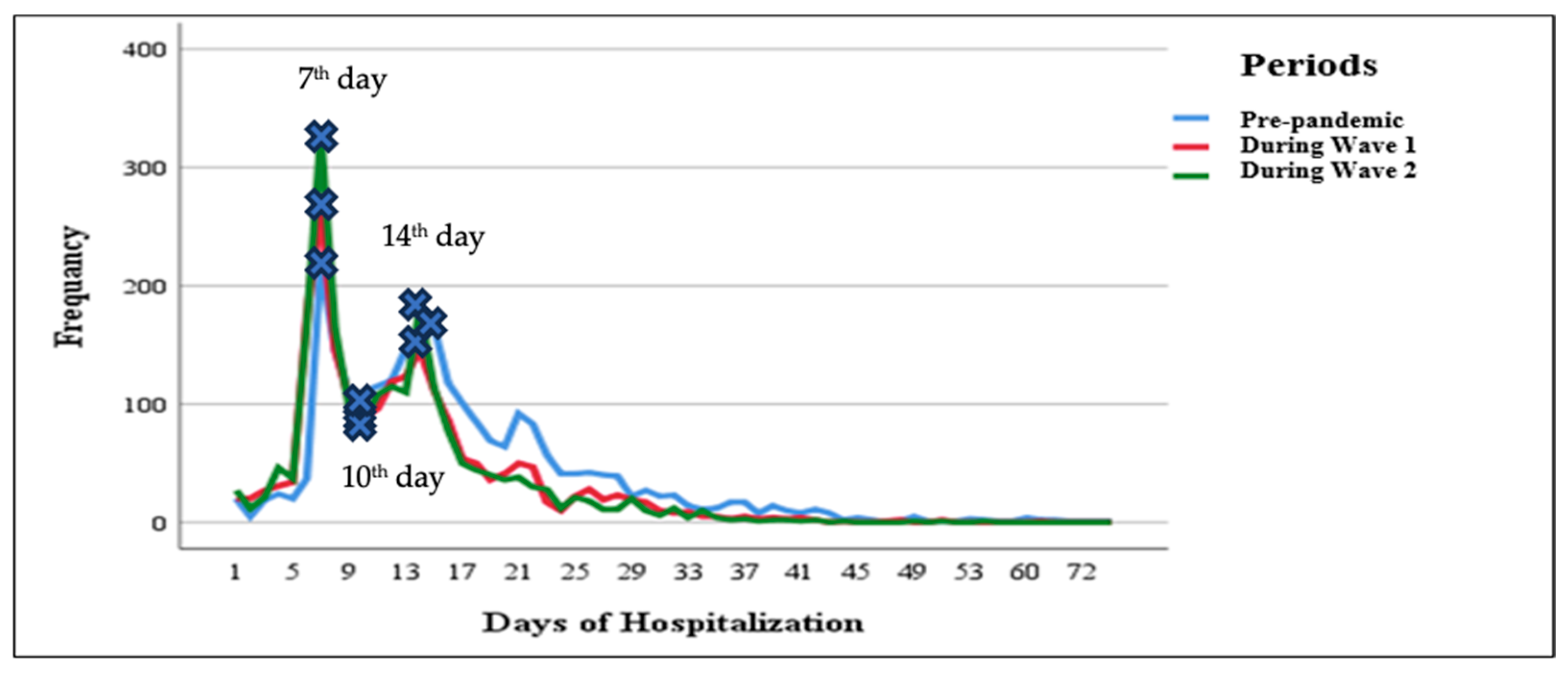
3.3. Hospitalization Days and Number of Visits
3.4. Hospitalization Outcomes
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoo, S.H.; Sim, J.A.; Shin, J.; Keam, B.; Park, J.B.; Shin, A. The impact of COVID-19 on cancer care in a tertiary hospital in Korea: Possible collateral damage to emergency care. Epidemiol. Health 2022, 44, e2022044. [Google Scholar] [CrossRef]
- Ranganathan, P.; Sengar, M.; Chinnaswamy, G.; Agrawal, G.; Arumugham, R.; Bhatt, R.; Bilimagga, R.; Chakrabarti, J.; Chandrasekharan, A.; Chaturvedi, H.K.; et al. Impact of COVID-19 on cancer care in India: A cohort study. Lancet Oncol. 2021, 22, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Emlen, M.F.; Shore, T.; Mayer, S.; Leonard, J.P.; Rossi, A.; Martin, P.; Ritchie, E.; Niesvizky, R.; Pastore, R.; et al. Hematology and oncology clinical care during the coronavirus disease 2019 pandemic. CA Cancer J. Clin. 2020, 70, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Crisan, C.; Cainap, C.; Deac, A.; Havasi, A.; Balacescu, O.; Balacescu, L.; Bochis, O.; Vlad, C.; Cainap, S. Decrease of oncological patients’ hospital visits during the COVID-19 pandemic; the experience of a tertiary Romanian center. J. BUON 2021, 26, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Borah, P.; Mirgh, S.; Sharma, S.K.; Bansal, S.; Dixit, A.; Dolai, T.K.; Lunkad, S.; Gupta, N.; Singh, G.; Jain, A.; et al. Effect of age, comorbidity and remission status on outcome of COVID-19 in patients with hematological malignancies. Blood Cells Mol. Dis. 2021, 87, 102525. [Google Scholar] [CrossRef]
- Pagano, L.; Salmanton-García, J.; Marchesi, F.; Busca, A.; Corradini, P.; Hoenigl, M.; Klimko, N.; Koehler, P.; Pagliuca, A.; Passamonti, F.; et al. COVID-19 infection in adult patients with hematological malignancies: A European Hematology Association Survey (EPICOVIDEHA). J. Hematol. Oncol. 2021, 14, 168. [Google Scholar] [CrossRef]
- Ishkinin, Y.; Kaidarova, D.; Nazarbek, S.; Zhylkaidarova, A.; Ossikbayeva, S.; Mussina, K.; Omarbayeva, N. COVID-19 pandemic shifted epidemiology for cancer screening sites: Breast, cervix, colon, and rectum. Front. Oncol. 2025, 14, 1481242. [Google Scholar] [CrossRef]
- Doubova, S.V.; Terreros-Muñoz, E.; Delgado-Lòpez, N.; Montaño-Figueroa, E.H.; Infante-Castañeda, C.; Pérez-Cuevas, R. Experiences with health care and health-related quality of life of patients with hematologic malignancies in Mexico. BMC Health Serv. Res. 2020, 20, 644. [Google Scholar] [CrossRef]
- Giesen, N.; Busch, E.; Schalk, E.; Beutel, G.; Rüthrich, M.M.; Hentrich, M.; Hertenstein, B.; Hirsch, H.H.; Karthaus, M.; Khodamoradi, Y.; et al. AGIHO guideline on evidence-based management of COVID-19 in cancer patients: 2022 update on vaccination, pharmacological prophylaxis and therapy in light of the omicron variants. Eur. J. Cancer 2023, 181, 102–118. [Google Scholar] [CrossRef]
- Jafari, M.; Dastgheib, S.A.; Ferdosian, F.; Mirjalili, H.; Aarafi, H.; Noorishadkam, M.; Mazaheri, M.; Neamatzadeh, H. Proportion of hematological cancer patients with SARS-CoV-2 infection during the COVID-19 pandemic: A systematic review and meta-analysis. Hematol. Transfus. Cell. Ther. 2022, 44, 225–234. [Google Scholar] [CrossRef]
- Total Coronavirus Cases in Kazakhstan. Kazakhstan COVID-19 Coronavirus Statistics-Worldometer. Available online: https://www.worldometers.info (accessed on 25 May 2024).
- Pujolar, G.; Oliver-Anglès, A.; Vargas, I.; Vázquez, M.L. Changes in Access to Health Services during the COVID-19 Pandemic: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1749. [Google Scholar]
- Mergenova, G.; Rosenthal, S.L.; Myrkassymova, A.; Bukharbayeva, A.; Iskakova, B.; Izekenova, A.; Izekenova, A.; Alekesheva, L.; Yerdenova, M.; Karibayev, K.; et al. The COVID-19 pandemic and mental health in Kazakhstan. Glob. Ment. Health 2023, 10, e52. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [PubMed]
- Indicators of the Oncology Service of the Republic of Kazakhstan. 2022. Available online: https://onco.kz/pokazateli-onkologicheskoj-sluzhby (accessed on 25 May 2024).
- Damumed.kz. Available online: https://damumed.kz/ (accessed on 25 April 2025).
- Egov.kz. Available online: https://egov.kz/cms/en/articles/health_care/Health-care-financing (accessed on 14 May 2025).
- Passamonti, F.; Cattaneo, C.; Arcaini, L.; Bruna, R.; Cavo, M.; Merli, F.; Angelucci, E.; Krampera, M.; Cairoli, R.; Della Porta, M.G.; et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: A retrospective, multicentre, cohort study. Lancet Haematol. 2020, 7, e737–e745. [Google Scholar] [CrossRef] [PubMed]
- Raad, I.I.; Hachem, R.; Masayuki, N.; Datoguia, T.; Dagher, H.; Jiang, Y.; Subbiah, V.; Siddiqui, B.; Bayle, A.; Somer, R.; et al. An international multicenter study comparing COVID-19 in patients with cancer to patients without cancer: Impact of risk factors and treatment modalities on survivorship. eLife 2023, 12, e81127. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Koo, H.Y.; Lee, J.R.; Lee, H.; Lee, J.Y. COVID-19 Mortality and Severity in Cancer Patients and Cancer Survivors. J. Korean Med. Sci. 2024, 39, e6. [Google Scholar] [CrossRef]
- Khatrawi, E.M.; Sayed, A.A. Assessing the Dynamics of COVID-19 Morbidity and Mortality in Response to Mass Vaccination: A Comparative Study Between Saudi Arabia and the United Kingdom. Cureus 2022, 14, e33042. [Google Scholar] [CrossRef]
- Riera, R.; Bagattini, Â.M.; Pacheco, R.L.; Pachito, D.V.; Roitberg, F.; Ilbawi, A. Delays and Disruptions in Cancer Health Care Due to COVID-19 Pandemic: Systematic Review. JCO Glob. Oncol. 2021, 7, 311–323. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Nair, R.K.S.; Goel, G.; Ramanan, V.; Chandy, M.; Nair, R. COVID-19 and haematology services in a cancer centre from a middle-income country: Adapting service delivery, balancing the known and unknown during the pandemic. Ecancermedicalscience 2020, 14, 1110. [Google Scholar] [CrossRef]
- AlSaleh, K. Effect of COVID-19 Pandemic on Oncology Services and the Impact of Specific Measures in Reducing the Delays. J. Appl. Hematol. 2021, 12, 74–78. [Google Scholar] [CrossRef]
- Chen, J.; Gu, J.; Ru, Y.; Wang, J.; Hu, Y.; Liu, K.; Liu, Q.; Zhang, X.; Xiao, Z.; Zhao, W.; et al. Chinese Society of Hematology, Chinese Society of Hematologist and Chinese Society of Hematology Youth Committee. Hematologic health services and practical characteristics: Report of a nationwide survey among Chinese hematologists. BMC Health Serv. Res. 2024, 24, 326. [Google Scholar] [CrossRef]
- Hsiehchen, D.; Muquith, M.; Haque, W.; Espinoza, M.; Yopp, A.; Beg, M.S. Clinical Efficiency and Safety Outcomes of Virtual Care for Oncology Patients During the COVID-19 Pandemic. JCO Oncol. Pract. 2021, 17, e1327–e1332. [Google Scholar] [CrossRef]
- Alpert, J.M.; Hampton, C.N.; Campbell-Salome, G.; Paige, S.; Murphy, M.; Heffron, E.; Amin, T.B.; Harle, C.A.; Le, T.; Vasquez, T.S.; et al. Tele-Oncology Use During the COVID-19 Pandemic: Patient Experiences and Communication Behaviors with Clinicians. Telemed. J. E Health 2024, 30, e1954–e1962. [Google Scholar] [CrossRef]

| Variable | Pandemic Period | p * | |||
|---|---|---|---|---|---|
| Pre-Pandemic (n, %) | Wave 1 (n, %) | Wave 2 (n, %) | |||
| Age Categories | |||||
| <17 | 13 (0.5) | 4 (0.2) | 0 (0.0) | <0.001 | |
| 18–29 | 307 (12.0) | 190 (9.1) | 189 (8.9) | ||
| 30–44 | 494 (19.3) | 415 (19.8) | 389 (18.4) | ||
| 45–59 | 732 (28.7) | 613 (29.2) | 593 (28.1) | ||
| >60 | 1008 (39.5) | 875 (41.7) | 941 (44.6) | ||
| Gender | |||||
| Female | 1420 (55.6) | 1185 (56.5) | 1213 (57.4) | 0.450 | |
| Male | 1134 (44.4) | 912 (43.5) | 899 (42.6) | ||
| Area | |||||
| Rural | 177 (6.9) | 192 (9.2) | 202 (9.6) | 0.002 | |
| Urban | 2376 (93.1) | 1905 (90.8) | 1910 (90.4) | ||
| Hospitalization Type | |||||
| Emergency | 857 (33.6) | 667 (31.8) | 781 (37.0) | <0.001 | |
| Planned | 1697 (66.4) | 1430 (68.2) | 1331 (63.0) | ||
| Outcome | |||||
| Died | 79 (3.1) | 74 (3.5) | 57 (2.7) | 0.003 | |
| Discharged | 2455 (96.1) | 2020 (96.3) | 2040 (96.6) | ||
| Variable | Pandemic Period | p * | |||
|---|---|---|---|---|---|
| Pre-Pandemic (n, %) | Wave 1 (n, %) | Wave 2 (n, %) | |||
| Hospitalization Type | |||||
| Emergency | Female | 489 (57.1%) | 406 (60.9%) | 511 (65.4%) | 0.002 |
| Male | 368 (42.9%) | 261 (39.1%) | 270 (34.6%) | ||
| Planned | Female | 931 (54.9%) | 779 (54.5%) | 702 (52.7%) | 0.481 |
| Male | 766 (45.1%) | 651 (45.5%) | 629 (47.3%) | ||
| Referral Source | Pre-Pandemic | Wave 1 | Wave 2 | p * | ||
|---|---|---|---|---|---|---|
| N (%) | N (%) | (%Change) † | N (%) | (%Change) † | ||
| Ambulance | 374 (14.6) | 395 (18.8) | 5.61 | 396 (18.8) | 5.88 | 0.672 |
| Another hospital | 15 (0.6) | 274 (13.1) | 1726.67 | 250 (11.8) | 1566.67 | <0.001 |
| Consultative and diagnostic | 159 (6.2) | 797 (38.0) | 401.26 | 854 (40.4) | 437.11 | <0.001 |
| Primary healthcare | 508 (19.9) | 376 (17.9) | −25.98 | 351 (16.6) | −30.91 | <0.001 |
| Self-referral | 170 (6.7) | 97 (4.6) | −42.94 | 159 (7.5) | −6.47 | <0.001 |
| Referrals from hematologists’ offices | 1328 (52.0) | 158 (7.5) | −88.1 | 102 (4.8) | −92.32 | <0.001 |
| Final Diagnosis | Number of Visits (Mean) | Bed Days (Mean) | ||||
|---|---|---|---|---|---|---|
| Pre-Pandemic | Wave 1 | Wave 2 | Pre-Pandemic | Wave 1 | Wave 2 | |
| Acute Leukemia | 6 | 6 | 5 | 20 | 16 | 14 |
| Anemia | 6 | 6 | 3 | 13 | 12 | 10 |
| Hemolytic Anemia | 6 | 6 | 4 | 15 | 14 | 10 |
| Lymphoproliferative Disorders | 6 | 6 | 5 | 15 | 12 | 11 |
| Malignant Disorders | 16 | 5 | 4 | 9 | 9 | 8 |
| Myeloproliferative Disorders | 5 | 5 | 3 | 19 | 15 | 14 |
| Other Neoplasms | 5 | 5 | 4 | 16 | 9 | 13 |
| Other Peripheral Arterial Diseases | 3 | 15 | - | 17 | 11 | - |
| Pathology of Hemostasis | 6 | 6 | 3 | 16 | 13 | 12 |
| p * | <0.001 | 0.034 | ||||
| Final Diagnosis | Died, N (%) | Discharged, N (%) | ||||
|---|---|---|---|---|---|---|
| Pre-Pandemic | Wave 1 | Wave 2 | Pre-Pandemic | Wave 1 | Wave 2 | |
| Acute Leukemia | 45 (37.8) | 48 (40.3) | 26 (21.8) | 656 (39.2) | 497 (29.7) | 522 (31.2) |
| Anemia | 3 (37.5) | 1 (12.5) | 4 (50.0) | 101 (36.7) | 95 (34.5) | 79 (28.7) |
| Hemolytic Anemia | 2 (66.7) | 0 (0.0) | 1 (33.3) | 37 (44.0) | 20 (23.8) | 27 (32.1) |
| Lymphoproliferative Disorders | 16 (36.4) | 15 (34.1) | 13 (29.5) | 1301 (36.6) | 1144 (32.2) | 1109 (31.2) |
| Malignant Disorders | 1 (50.0) | 1 (50.0) | 0 (0.0) | 2 (25.0) | 4 (50.0) | 2 (25.0) |
| Myeloproliferative Disorders | 11 (44.0) | 4 (16.0) | 10 (40.0) | 195 (38.0) | 141 (27.5) | 177 (34.5) |
| Other Neoplasms | 0 (0.0) | 0 (0.0) | 0 (0.0) | 15 (53.6) | 7 (25.0) | 6 (21.4) |
| Other Peripheral Arterial Diseases | 0 (0.0) | 0 (0.0) | 0 (0.0) | 14 (100.0) | 0 (0.0) | 0 (0.0) |
| Pathology of Hemostasis | 1 (33.3) | 2 (66.7) | 0 (0.0) | 110 (34.1) | 108 (33.4) | 105 (32.5) |
| p * | 0.300 | <0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerdenova, M.; Izekenova, A.; Myrkassymova, A.; Mergenova, G.; Merzah, M.; Issenova, B.; Mamyrkul, M.; Atabayeva, A.; Kalibatas, V.; Nikolic, D.; et al. Impact of the COVID-19 Pandemic on Hemato-Oncology Services: A Retrospective Dual-Center Cohort Study in Kazakhstan. Healthcare 2025, 13, 2520. https://doi.org/10.3390/healthcare13192520
Yerdenova M, Izekenova A, Myrkassymova A, Mergenova G, Merzah M, Issenova B, Mamyrkul M, Atabayeva A, Kalibatas V, Nikolic D, et al. Impact of the COVID-19 Pandemic on Hemato-Oncology Services: A Retrospective Dual-Center Cohort Study in Kazakhstan. Healthcare. 2025; 13(19):2520. https://doi.org/10.3390/healthcare13192520
Chicago/Turabian StyleYerdenova, Maral, Aigulsum Izekenova, Akbope Myrkassymova, Gaukhar Mergenova, Mohammed Merzah, Balday Issenova, Maksat Mamyrkul, Aliya Atabayeva, Vytenis Kalibatas, Dejan Nikolic, and et al. 2025. "Impact of the COVID-19 Pandemic on Hemato-Oncology Services: A Retrospective Dual-Center Cohort Study in Kazakhstan" Healthcare 13, no. 19: 2520. https://doi.org/10.3390/healthcare13192520
APA StyleYerdenova, M., Izekenova, A., Myrkassymova, A., Mergenova, G., Merzah, M., Issenova, B., Mamyrkul, M., Atabayeva, A., Kalibatas, V., Nikolic, D., & Chen, Y. (2025). Impact of the COVID-19 Pandemic on Hemato-Oncology Services: A Retrospective Dual-Center Cohort Study in Kazakhstan. Healthcare, 13(19), 2520. https://doi.org/10.3390/healthcare13192520






