Acceptability, Usability, and Effectiveness of a Music Video Game for Pain Management: A Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
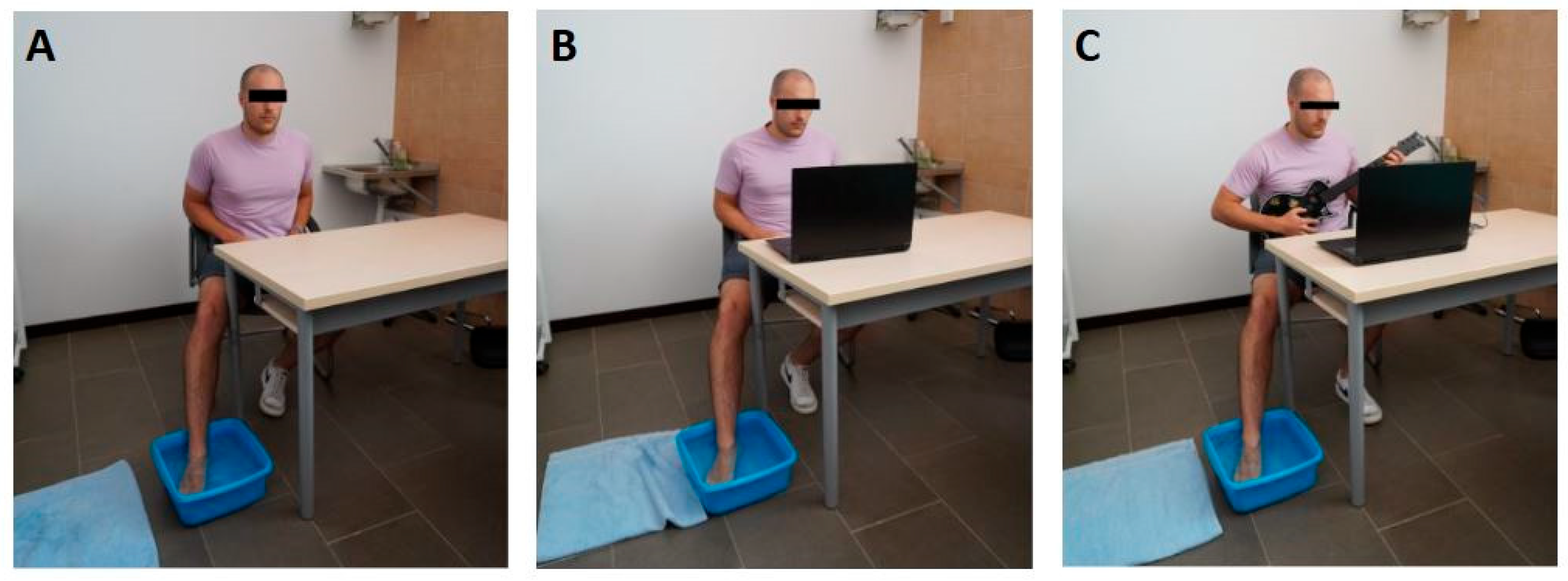
2.3. Interventions
2.3.1. Musical Video Game Setup
2.3.2. Equipment and Materials
Hardware and Software Specifications
2.4. Pain Induction Protocol
2.5. Outcomes Measures
2.5.1. Primary Outcomes
Usability and Acceptability Assessment
Pain
2.5.2. Secondary Outcomes
Physical Activity
Adverse Effects Monitoring
2.6. Sample Size
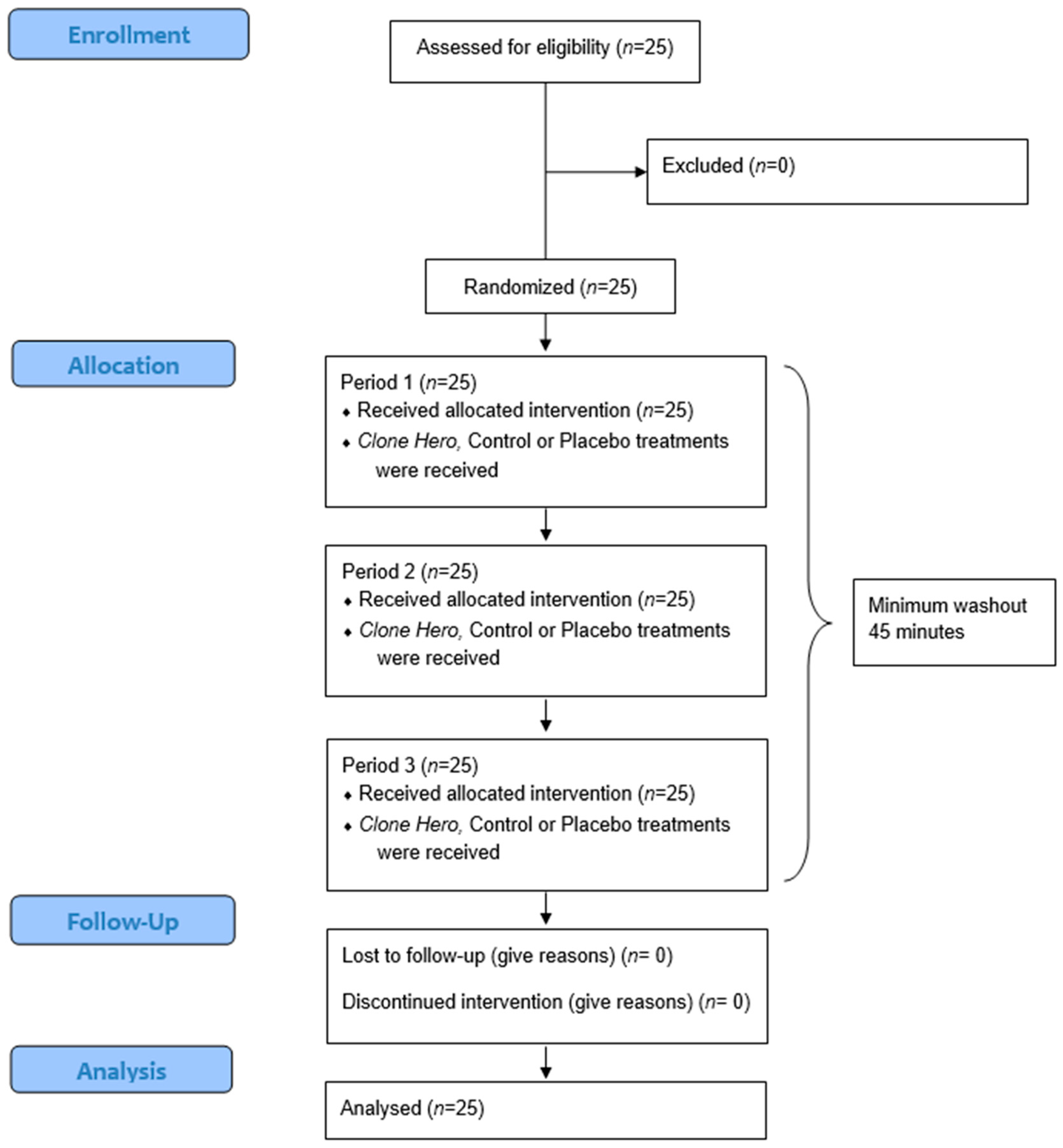
2.7. Randomization
2.8. Procedure
2.9. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Primary Results
3.2.1. Usability and Acceptability Assessment
Pre-Intervention Questions
- Prior Experience
Post-Intervention Questions
- Concentration and Distraction as Pain Modulators
- Cold Tolerance
- Environmental Conditions
- Prior Knowledge of Distraction Techniques and User Perception of Innovative Methods
- Overall Satisfaction
3.2.2. Pain
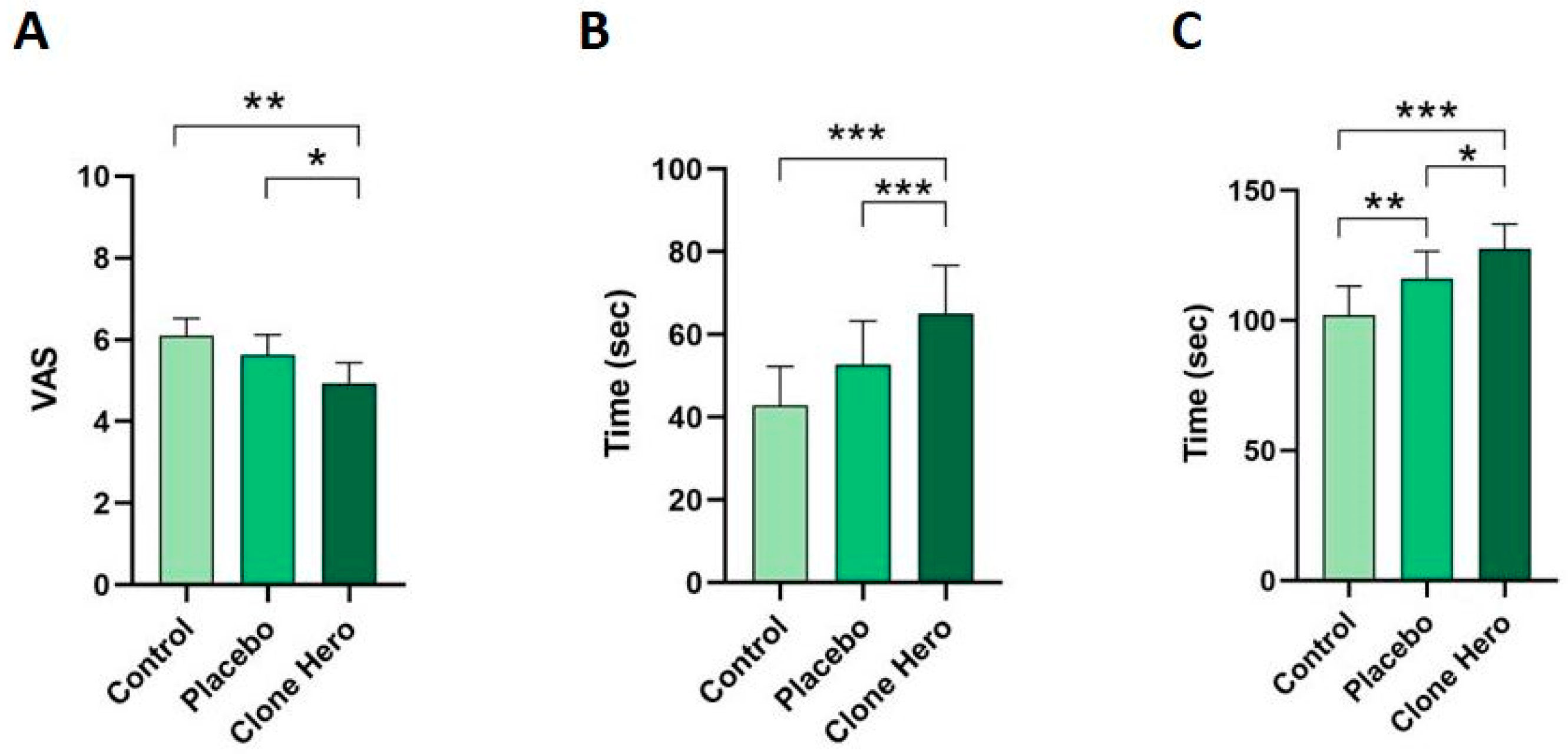
Pain Intensity (VAS)
Pain Threshold
Pain Tolerance
3.3. Secondary Results
3.3.1. Physical Activity (IPAQ)
3.3.2. Adverse Effects
4. Discussion
Study Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTP | Cold Pressor Test |
| IPAQ | International Physical Activity Questionnaire |
| VAS | Visual Analog Scale |
| VR | Virtual Reality |
References
- Malloy, K.M.; Milling, L.S. The Effectiveness of Virtual Reality Distraction for Pain Reduction: A Systematic Review. Clin. Psychol. Rev. 2010, 30, 1011–1018. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Richards, T.L.; Coda, B.; Bills, A.R.; Blough, D.; Richards, A.L.; Sharar, S.R. Modulation of Thermal Pain-Related Brain Activity with Virtual Reality: Evidence from FMRI. Neuroreport 2004, 15, 1245–1248. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Rodriguez, R.A.; Gonzalez, M.; Bernardy, M.; Peña, R.; Beck, W.; Patterson, D.R.; Meyer, W.J. Immersive Virtual Reality as an Adjunctive Non-Opioid Analgesic for Pre-Dominantly Latin American Children with Large Severe Burn Wounds During Burn Wound Cleaning in the Intensive Care Unit: A Pilot Study. Front. Hum. Neurosci. 2019, 13, 262. [Google Scholar] [CrossRef]
- Dumoulin, S.; Bouchard, S.; Loranger, C.; Quintana, P.; Gougeon, V.; Lavoie, K.L. Are Cognitive Load and Focus of Attention Differentially Involved in Pain Management: An Experimental Study Using a Cold Pressor Test and Virtual Reality. J. Pain Res. 2020, 13, 2213–2222. [Google Scholar] [CrossRef]
- Dahlquist, L.M.; Herbert, L.J.; Weiss, K.E.; Jimeno, M. Virtual-Reality Distraction and Cold-Pressor Pain Tolerance: Does Avatar Point of View Matter? Cyberpsychol. Behav. Soc. Netw. 2010, 13, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, S.H.; Stamp, K.; Dobbins, C.; Poole, H.M. Computer Games as Distraction from PAIN: Effects of Hardware and Difficulty on Pain Tolerance and Subjective IMMERSION. Int. J. Hum. Comput. Stud. 2020, 139, 102427. [Google Scholar] [CrossRef]
- Eijlers, R.; Utens, E.M.W.J.; Staals, L.M.; de Nijs, P.F.A.; Berghmans, J.M.; Wijnen, R.M.H.; Hillegers, M.H.J.; Dierckx, B.; Legerstee, J.S. Systematic Review and Meta-Analysis of Virtual Reality in Pediatrics: Effects on Pain and Anxiety. Anesth. Analg. 2019, 129, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Raudenbush, B.; Koon, J.; Cessna, T.; McCombs, K. Effects of Playing Video Games on Pain Response during a Cold Pressor Task. Percept. Mot. Skills 2009, 108, 439–448. [Google Scholar] [CrossRef]
- Bani Mohammad, E.; Ahmad, M. Virtual Reality as a Distraction Technique for Pain and Anxiety among Patients with Breast Cancer: A Randomized Control Trial. Palliat. Support. Care 2019, 17, 29–34. [Google Scholar] [CrossRef]
- Milgram, P.; Takemura, H. Augmented Reality: A Class of Displays on the Reality-Virtuality Continuum. In Telemanipulator and Telepresence Technologies; Spie: Bellingham, WA, USA, 1994; Volume 2351. [Google Scholar]
- Cairns, P.; Cox, A.; Imran Nordin, A. Immersion in Digital Games: Review of Gaming Experience Research. In Handbook of Digital Games; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Cairns, P.; Li, J.; Wang, W.; Nordin, A.I. The Influence of Controllers on Immersion in Mobile Games; ACM Digital Library: New York, NY, USA, 2014. [Google Scholar]
- Ahmadpour, N.; Randall, H.; Choksi, H.; Gao, A.; Vaughan, C.; Poronnik, P. Virtual Reality Interventions for Acute and Chronic Pain Management. Int. J. Biochem. Cell Biol. 2019, 114, 105568. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Donegan, T.; Bottiroli, S.; Sandrini, G.; Sanchez-Vives, M.V.; Tassorelli, C. Immersive Virtual Reality and Virtual Embodiment for Pain Relief. Front. Hum. Neurosci. 2019, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.I.; Belmont, K.A.; Thomas, D.A. The Neurobiology of Virtual Reality Pain Attenuation. Cyberpsychol. Behav. 2007, 10, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory. In Psychosocial Processes and Health; Cambridge University Press: Cambridge, UK, 1994; Volume 7. [Google Scholar] [CrossRef]
- Melzack, R.; Katz, J. The Gate Control Theory: Reaching for the Brain. In Pain: Psychological Perspectives; Psychology Press: East Sussex, UK, 2003. [Google Scholar]
- Walsh, N.E.; Schoenfeld, L.; Ramamurthy, S.; Hoffman, J. Normative Model for Cold Pressor Test. Am. J. Phys. Med. Rehabil. 1989, 68, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Neiman, N.R.; Falkson, S.R.; Rodriguez, S.T.; Wang, E.Y.; Hemphill, S.F.; Khoury, M.E.; Kist, M.N.; Jackson, C.D.; Caruso, T.J. Quantifying Virtual Reality Pain Modulation in Healthy Volunteers: A Randomized, Crossover Study. J. Clin. Anesth. 2022, 80, 110876. [Google Scholar] [CrossRef]
- Choi, S.; Park, S.G.; Lee, H.H. The Analgesic Effect of Music on Cold Pressor Pain Responses: The Influence of Anxiety and Attitude toward Pain. PLoS ONE 2018, 13, e0201897. [Google Scholar] [CrossRef]
- Chai, P.R.; Schwartz, E.; Hasdianda, M.A.; Azizoddin, D.R.; Kikut, A.; Jambaulikar, G.D.; Edwards, R.R.; Boyer, E.W.; Schreiber, K.L. A Brief Music App to Address Pain in the Emergency Department: Prospective Study. J. Med. Internet Res. 2020, 22, e18537. [Google Scholar] [CrossRef]
- Gres, A.; Adcock, S.; Kuhlman, A.; Murphy, M.F. Including Patients and Healthy Volunteers in First-in-Human Clinical Trials: The Opportunities and Limitations of “Hybrid” Clinical Studies; Worldwide Clinical Trials: San Antonio, TX, USA, 2022. [Google Scholar]
- Dwan, K.; Li, T.; Altman, D.G.; Elbourne, D. CONSORT 2010 statement: Extension to randomised crossover trials. BMJ 2019, 366, l4378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mitchell, L.A.; MacDonald, R.A.R.; Brodie, E.E. Temperature and the Cold Pressor Test. J. Pain 2004, 5, 233–237. [Google Scholar] [CrossRef]
- Phelan, I.; Furness, P.J.; Fehily, O.; Thompson, A.R.; Babiker, N.T.; Lamb, M.A.; Lindley, S.A. A Mixed-Methods Investigation into the Acceptability, Usability, and Perceived Effectiveness of Active and Passive Virtual Reality Scenarios in Managing Pain under Experimental Conditions. J. Burn Care Res. 2019, 40, 85–90. [Google Scholar] [CrossRef]
- Dy, M.; Olazo, K.; Lyles, C.R.; Lisker, S.; Weinberg, J.; Lee, C.; Tarver, M.E.; Saha, A.; Kontson, K.; Araojo, R.; et al. Usability and Acceptability of Virtual Reality for Chronic Pain Management among Diverse Patients in a Safety-Net Setting: A Qualitative Analysis. JAMIA Open 2023, 6, ooad050. [Google Scholar] [CrossRef]
- Honzel, E.; Murthi, S.; Brawn-Cinani, B.; Colloca, G.; Kier, C.; Varshney, A.; Colloca, L. Virtual Reality, Music, and Pain: Developing the Premise for an Interdisciplinary Approach to Pain Management. Pain 2019, 160, 1909–1919. [Google Scholar] [CrossRef]
- Kucyi, A.; Davis, K.D. The Dynamic Pain Connectome. Trends Neurosci. 2015, 38, 86–95. [Google Scholar] [CrossRef]
- Kühn, S.; Romanowski, A.; Schilling, C.; Lorenz, R.; Mörsen, C.; Seiferth, N.; Banaschewski, T.; Barbot, A.; Barker, G.J.; Büchel, C.; et al. The Neural Basis of Video Gaming. Transl. Psychiatry 2011, 1, e53. [Google Scholar] [CrossRef]
- Zubieta, J.K.; Smith, Y.R.; Bueller, J.A.; Xu, Y.; Kilbourn, M.R.; Jewett, D.M.; Meyer, C.R.; Koeppe, R.A.; Stohler, C.S. Regional Mu Opioid Receptor Regulation of Sensory and Affective Dimensions of Pain. Science 2001, 293, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Guétin, S.; Giniès, P.; Siou, D.K.A.; Picot, M.C.; Pommié, C.; Guldner, E.; Gosp, A.M.; Ostyn, K.; Coudeyre, E.; Touchon, J. The Effects of Music Intervention in the Management of Chronic Pain: A Single-Blind, Randomized, Controlled Trial. Clin. J. Pain 2012, 28, 329–337. [Google Scholar] [CrossRef]
- Lee, J.H. The Effects of Music on Pain: A Meta-Analysis. J. Music. Ther. 2016, 53, 430–477. [Google Scholar] [CrossRef]
- Gupta, A.; Scott, K.; Dukewich, M. Innovative Technology Using Virtual Reality in the Treatment of Pain: Does It Reduce Pain via Distraction, or Is There More to It? Pain Med. 2018, 19, 151–159. [Google Scholar] [CrossRef]
- Xiang, H.; Shen, J.; Wheeler, K.K.; Patterson, J.; Lever, K.; Armstrong, M.; Shi, J.; Thakkar, R.K.; Groner, J.I.; Noffsinger, D.; et al. Efficacy of Smartphone Active and Passive Virtual Reality Distraction vs Standard Care on Burn Pain among Pediatric Patients a Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2112082. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, A.; Davis, S.; Marchak, A.; Whiteside, T.; Sikka, N. Virtual Reality as a Clinical Tool for Pain Management. Curr. Pain Headache Rep. 2018, 22, 53. [Google Scholar] [CrossRef]
- Chirico, A.; Lucidi, F.; De Laurentiis, M.; Milanese, C.; Napoli, A.; Giordano, A. Virtual Reality in Health System: Beyond Entertainment. A Mini-Review on the Efficacy of VR During Cancer Treatment. J. Cell. Physiol. 2016, 231, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Grassini, S. Virtual Reality Assisted Non-Pharmacological Treatments in Chronic Pain Management: A Systematic Review and Quantitative Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4071. [Google Scholar] [CrossRef] [PubMed]
| Outcome | Value |
|---|---|
| Age (mean ± SD) | 45.9 ± 8.4 |
| Sex | Male 12 (48%)/Female 13 (52%) |
| Height (mean ± SD) | 168.8 ± 9.5 |
| Weight (mean ± SD) | 75.2 ± 15.6 |
| Smoker (yes/no) | Yes: 6 (24%)/No: 19 (76%) |
| Physical Activity (IPAQ) | Low: 5 (20%), Moderate: 12 (48%), High activity: 8 (32%) |
| Previous experience | 1. Previous experience with the Clone Hero game | Yes | 12% |
| No | 88% | ||
| 2. Ability to play Clone Hero | Good | 16% | |
| Regular | 40% | ||
| Bad | 44% | ||
| 3. Previous experience with guitar or string instrument | Yes | 20% | |
| No | 80% | ||
| Concentration and distraction as pain modulators | 4. Ease of concentrating on the game during cold exposure | Totally agree | 32% |
| Agree | 36% | ||
| Neutral | 16% | ||
| Disagree | 16% | ||
| Totally disagree | 0% | ||
| 5. Perceived influence of concentration on pain perception | Totally agree | 56% | |
| Agree | 36% | ||
| Neutral | 4% | ||
| Disagree | 4% | ||
| Totally disagree | 0% | ||
| 6. Impact of music and entertainment on pain perception | Totally agree | 60% | |
| Agree | 32% | ||
| Neutral | 0% | ||
| Disagree | 8% | ||
| Totally disagree | 0% | ||
| 7. Noticed distraction reduces pain intensity | Totally agree | 52% | |
| Agree | 28% | ||
| Neutral | 8% | ||
| Disagree | 12% | ||
| Totally disagree | 0% | ||
| Cold tolerance | 8. Prior experience of pain from cold exposure | Yes | 88% |
| No | 12% | ||
| 9. Self-rated cold tolerance (scale 1–10) | 0 | 0% | |
| 1 | 0% | ||
| 2 | 0% | ||
| 3 | 8% | ||
| 4 | 4% | ||
| 5 | 16% | ||
| 6 | 8% | ||
| 7 | 36% | ||
| 8 | 20% | ||
| 9 | 0% | ||
| 10 | 8% | ||
| Concentration and distraction as pain modulators | 10. Music and gameplay influencing cold response | Totally agree | 40% |
| Agree | 56% | ||
| Neutral | 0% | ||
| Disagree | 4% | ||
| Totally disagree | 0% | ||
| Environmental conditions | 11. Environmental factors affecting pain sensation | Totally agree | 12% |
| Agree | 20% | ||
| Neutral | 44% | ||
| Disagree | 24% | ||
| Totally disagree | 0% | ||
| 12. Suggested changes to the experimental setup | Yes | 8% | |
| No | 92% | ||
| Previous knowledge of distraction techniques | 13. Awareness of distraction techniques for pain before study | Yes | 44% |
| No | 56% | ||
| 14. Perceived innovation of combining music and gaming | Totally agree | 40% | |
| Agree | 52% | ||
| Neutral | 4% | ||
| Disagree | 4% | ||
| Totally disagree | 0% | ||
| 15. Openness to novel pain relief methods | Totally agree | 56% | |
| Agree | 36% | ||
| Neutral | 0% | ||
| Disagree | 8% | ||
| Totally disagree | 0% | ||
| 16. Willingness to recommend Clone Hero as pain strategy | Totally agree | 44% | |
| Agree | 40% | ||
| Neutral | 0% | ||
| Disagree | 16% | ||
| Totally disagree | 0% | ||
| Satisfaction | 17. Overall satisfaction with the experience | Totally satisfied | 60% |
| Satisfied | 32% | ||
| Neutral | 8% | ||
| Unsatisfied | 0% | ||
| Totally unsatisfied | 0% |
| IPAQ Level | Total Sample (%) | Female (%) | Male (%) |
|---|---|---|---|
| Low activity | 40% | 23.1% | 16.7% |
| Moderate Activity | 44% | 46.2% | 50% |
| High Activity | 16% | 30.8% | 33.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteban-Sopeña, J.; Bravo-Aparicio, J.; Trillo-Charlín, I.; Roldán-Ruiz, A.; Beltran-Alacreu, H.; García-Magro, N. Acceptability, Usability, and Effectiveness of a Music Video Game for Pain Management: A Crossover Study. Healthcare 2025, 13, 2439. https://doi.org/10.3390/healthcare13192439
Esteban-Sopeña J, Bravo-Aparicio J, Trillo-Charlín I, Roldán-Ruiz A, Beltran-Alacreu H, García-Magro N. Acceptability, Usability, and Effectiveness of a Music Video Game for Pain Management: A Crossover Study. Healthcare. 2025; 13(19):2439. https://doi.org/10.3390/healthcare13192439
Chicago/Turabian StyleEsteban-Sopeña, Jara, Javier Bravo-Aparicio, Iria Trillo-Charlín, Alberto Roldán-Ruiz, Hector Beltran-Alacreu, and Nuria García-Magro. 2025. "Acceptability, Usability, and Effectiveness of a Music Video Game for Pain Management: A Crossover Study" Healthcare 13, no. 19: 2439. https://doi.org/10.3390/healthcare13192439
APA StyleEsteban-Sopeña, J., Bravo-Aparicio, J., Trillo-Charlín, I., Roldán-Ruiz, A., Beltran-Alacreu, H., & García-Magro, N. (2025). Acceptability, Usability, and Effectiveness of a Music Video Game for Pain Management: A Crossover Study. Healthcare, 13(19), 2439. https://doi.org/10.3390/healthcare13192439






